Extraordinary advances in cancer screening, therapeutics, and supportive care have contributed to substantial progress in survival rates among patients with cancer over the last 50 years.1, 2, 3, 4 Clinical cancer research trials represent the main avenue to foster this progress, allowing discoveries to be translated into patient benefit.
In a recent analysis of pivotal oncology trials from 2015 to 2017, the estimated median trial cost was $31.7 million.5 Costs per patient for oncology phase III trials have increased from an average of $3000 to $5000 in the early 1990s to up to $125 000 in 2013.6 While these figures include both operational and regulatory costs, Sertkaya et al., using aggregate clinical trial budget data provided by Medidata Solutions, found that administrative staff costs accounted for ∼11%-20% and site monitoring accounted for 9%-14% of overall study costs across phases I through III of clinical trials ranging from 2004 to 2012.7 Global phase III trials are often slow moving (e.g. a very successful large phase III trial operation involving 8381 patients took almost 4 years from trial submission to last patient inclusion)8 and time consuming (studies usually require >200 h of work per patient, with a third of time devoted to non-clinical tasks)9 which creates substantial burden for clinical sites and practitioners, and concentrates clinical trials in a selected number of equipped cancer centers. Additionally, <10% of patients with cancer enroll in clinical trials and current clinical trial workflows are non-inclusive; restrict participant access; and often exclude elderly patients, patients living in rural areas, and patients belonging to an ethnic minority or lower socioeconomic group.10,11 For example, although these demographic groups represent almost one-third of the cancer patient population in the United States, only 4%-6% of trial participants are black, and 3%-6% are Hispanic.12
The widespread use of digital technology may offer clinical cancer research an opportunity to innovate and move away from an often exceedingly expensive, slow-moving, burdensome, and unequal research apparatus.13
Cardiology has pioneered the use of digital technology to facilitate clinical trials. For example, a 2019 study led by the Stanford Division of Cardiovascular Medicine was conducted to assess the use of Apple smartwatches to identify atrial fibrillation using a fully decentralized and digitized pathway.14 For instance, researchers used an app to obtain consent and to educate and guide participants throughout the study, patient monitoring occurred through the use of the Apple Watch sensor, and patients were prompted to initiate a telemedicine visit directly from the app upon the receipt of an irregular pulse notification.14 The study was able to recruit 419 297 patients within 8 months from all 50 US states and found that among patients who received an irregular pulse notification, 34% had atrial fibrillation and 84% had electrocardiogram (ECG) patch readings that were concordant with atrial fibrillation.14
Benefits of digitization of clinical cancer trials
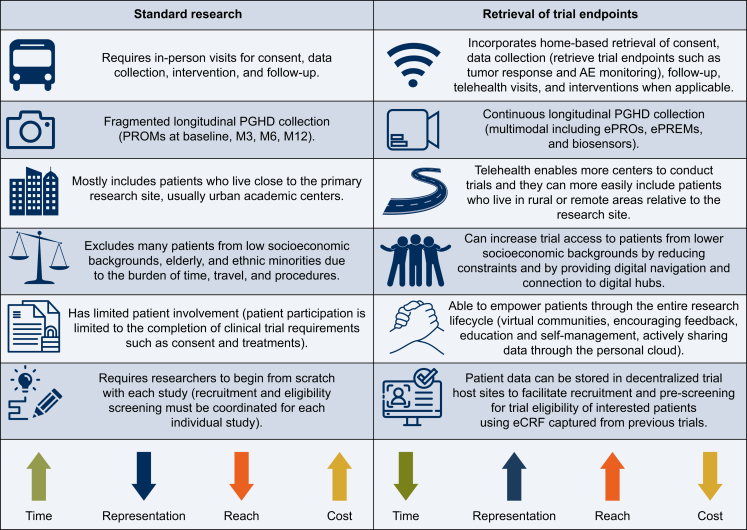
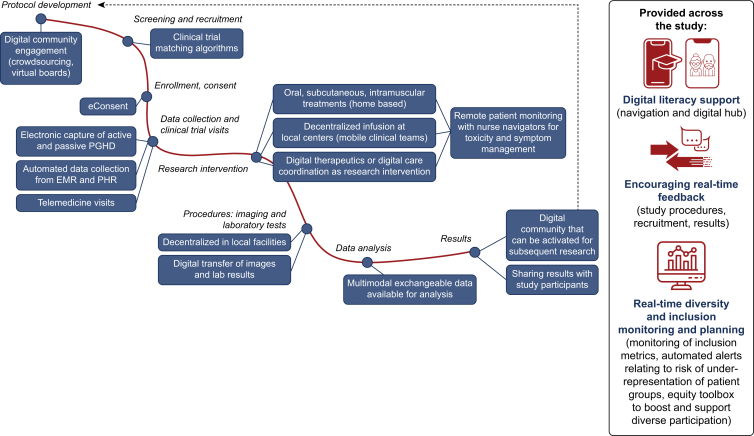
The potential benefits of comprehensive or partial digitization of clinical cancer trials can be wide-reaching and consequential (Figure 1). Practically, digital technology can simplify trial procedures, expedite and enhance data collection, promote a global and more equal reach, and foster patient empowerment and participation (Figure 2).
Figure 1.
Advantages of digitally enabled cancer research. AE, adverse event; eCRF, electronic case report form; ePREMs, electronic patient-reported experience measures; ePROs, electronic patient-reported outcomes; PGHD, patient-generated health data; PROMs, patient-reported outcome measures.
Figure 2.
A decentralized clinical research pathway. EMR, electronic medical record; PGHD, patient-generated health data; PHR, personal health record.
Simplifying clinical trial procedures
Complete or partial decentralization of the screening, recruitment, consent, and patient follow-up processes using digital tools is an effective way of reducing the burden, expense, and delay associated with clinical trial procedures.
Screening and recruitment
Artificial intelligence (AI)-developed software can be used to determine patient eligibility using natural language processing (NLP) and machine learning techniques with relative success and accuracy when compared to traditional pre-screening methods. A software can be trained to recognize eligibility requirements for systemic therapy trials and also match complex genomic characteristics for targeted therapies.15, 16, 17 For example, the use of such software in a community-based breast cancer care unit demonstrated agreement with manually generated eligibility assessments (>80%) and a reduction in the time burden of eligibility assessments from 110 min using manual review to 24 min using system-assisted eligibility determinations.15 Recently Klein et al. reported the use of the MatchMiner open source platform to computationally match genomically profiled cancer patients to precision medicine clinical trials in an academic large-volume center. In this study, patients identified by MatchMiner consented to clinical trials 55 days earlier than those included through traditional methods, demonstrating the ability of these tools to simplify inclusion workflows.16
AI software can also be designed to refine selection criteria for inclusion in clinical trials. Gustave Roussy in France, for example, piloted a study that used NLP directed to electronic medical records (EMRs) to automatically identify patients who were fit for successful screening and dose-limiting toxicity period completion (SSD) in phase I-II studies. In a retrospective analysis, the model used in this study could have reduced screen failure rates from 39.8% to 12.8% selecting patients who would benefit most from clinical trial participation.18 Overall, clinical trial matching algorithms are a valuable tool for facilitating and speeding up recruitment as well as eventually reducing the administrative burden of clinical trials.15,16
Conduct of the trial (consent and follow-up)
Investigators can facilitate the digital collection of remote or on-site consent for participation by deploying a decentralized clinical trial platform or electronic consent (e-consent)-specific technology.19,20 Studies have observed that e-consent platforms have high levels of completion and low rates of error compared to standard methods of consent (an error rate of 0.32% for e-consent compared to 7% for standard methods).21 Completion of e-consent can be encouraged by text and email reminders, according to patient preference, and patient training provided by researchers to help navigate the research interface.22,23 These same platforms and targeted technologies can also be used to enable telehealth follow-up visits and the administration of patient-reported outcome measures.24,25 Clinical visits are adaptable to a digital format by respecting telemedicine standards that are now available.26
Oncology research protocols often involve advanced imaging, blood tests, biopsies, infusion therapies, and close monitoring of treatment-related side-effects. Oral, subcutaneous, or intramuscular therapies with established safety profiles, however, can be transported to patient’s homes for self-administration and are particularly well suited for decentralized clinical trials.27 Technology may be leveraged for mapping and activating clinical teams close to the patient’s residency for local blood test collection and infusion therapies.28 In addition, home-based infusions have been successfully implemented in proof-of-concept studies for selected indications.29 Remote patient monitoring with electronic patient-reported outcomes (ePROs) can be activated to allow close monitoring and management of acute adverse events of both infusion and oral therapies while the patient is at home.30 Imaging exams may be carried out in local centers and digitally transferred to central teams.31 Furthermore, several supportive care and care coordination interventions have proven to be successful when delivered remotely.27,32,33 These technological opportunities create opportunities for remote participation in clinical trials. This is an important step to expand the reach of clinical trials because the unavailability of a suitable research trial at a patient’s primary care center is a barrier to trial enrollment 55.6% of the time.11
Clinical research trials have many non-clinical costs associated with the execution of the clinical trial including the cost of labor required to sufficiently screen and recruit patients, solicit consent, record, and centralize clinical trial data.6 Paper documents often need to be manually transcribed to digital files requiring more hours of work from research personnel and thus more budget resources. Using an online digital research platform to execute a study or using digital applications to send clinical trial invitations and retrieve and store patient consent reduces the administrative burden for researchers, ultimately reducing the time and expense required to complete a study. There will be less time devoted to organizing and executing in-person tasks when the patient has the autonomy to complete research tasks digitally. Less bureaucratic work may also encourage a more diversified group of health care professionals to pursue research activities, will allow proper time for discussions during clinical encounters of the pros and cons of a clinical trial, and may increase patient enrollment in research as literature demonstrates that physician–patient rapport and effective communication play an important role in patient decision making.34, 35, 36, 37
Expediting and enhancing data collection
Digital tools can also play a key role in the collection of data, both patient-generated health data (PGHD) and clinical data during the study conduct.
Patient-generated data
Patient-reported outcomes (PROs) are crucial measures for researchers when evaluating a clinical trial outcome.38 In fact, the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) have proposed standardized measures to evaluate clinical trial results (the Net Health Benefit and Magnitude of Clinical Benefit Scale, respectively) which include PRO data on quality of life (QoL) and toxicity.39 Many PRO questionnaires have been converted into a digital format that can be electronically administered (ePROs). Digital tools can improve the pragmatic inclusion of ePROs in clinical trials. A 2020 meta-analysis and systematic literature review determined some advantages of using ePROs versus paper questionnaires to be greater patient preference and acceptability, better response rates, higher data quality, and lower overall costs.40 The PROSPECT trial provides a recent example of the ability of ePROs to collect valuable PGHD. This study leveraged the use of ePROs at baseline, during neoadjuvant treatment, and at 12 months after surgery to understand the effect of neoadjuvant chemotherapy with fluorouracil and oxaliplatin (FOLFOX) versus neoadjuvant chemoradiation with fluorouracil (5FUCRT) on QoL to better inform treatment decisions. Out of the 1128 patients who initiated treatment, 940 contributed to the NCI Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) data and researchers were able to evaluate trends in QoL trajectories during and after treatment. This collection of PGHD enriched the clinical trial results beyond clinical endpoints providing researchers with enough data to create distinctive PRO profiles for the two treatment pathways, building the foundations for better treatment selection and shared decision making.41
In addition to the use of ePROs, technology may allow researchers to capture other types of PGHD that are not fully integrated into oncology research. There is potential for the collection of PGHD through the use of biometric facilities incorporated in technology used by patients in their everyday life such as smartphones, smartwatches, and smartscales.42 Data that can be retrieved by such devices include mobility data (step counts, GPS location, distance and elevation walked, but also indirect assessment of strength, gait, and balance), vital signs (heart rate, breathing frequency, SpO2, temperature), ECG, sleep and emotional distress patterns (mainly retrieved by heart rate variation), weight and body composition, UV exposition, and alcohol and tobacco consumption.43 In addition, retrieval and linkage with data of personal behavioral mobile apps used by the patient may also allow for more granular data collection of daily life health behaviors such as dietary patterns, physical exercise, and meditation practices for example.44 All these data may help researchers to objectively assess performance status, symptoms, and treatment-related toxicities, but also to investigate how daily life behaviors interfere with cancer and its treatment and vice versa. More innovative biosensors can also be leveraged for noninvasive continuous assessment of specific biomarkers of interest in oncology in human fluids such as the interstitial fluid similar to that used for glucose monitoring in diabetes. Other applications may include home-based blood drop assessment, smart toilets, houses, clothing, and pill dispensers.43
Technology can also facilitate equitable participation in formative qualitative research in oncology which is particularly relevant for intervention mapping45 and implementation science46 such as exploring the multi-faceted contexts where implementation takes place, the processes and steps involved in implementation, as well as the effectiveness of implementation strategies.47 Video conference may be used for virtual discussion rooms which hold a strong resemblance to traditional focus groups in their synchronous nature but also take advantage of text-based nonverbal communication and discussions.47
Collection of clinical data
Digital collection of research data is not limited to PGHD, but also encompasses clinical data from EMRs, adverse event (AE) monitoring, laboratory data, and imaging. Clinical research trials in oncology are often characterized by multi-site participation and manual data collection. For clinical and treatment data, automated data collection could substantially decrease the operational burden of clinical trials; however, since centers operate using different methods of data capture and storage with distinct structures, data transmission between sites can be difficult due to lack of interoperability, or the inability of two systems to connect and coordinate in an efficient and effective manner.48 In response, the FAIR principles, outlined to safeguard data management and stewardship, advocate for medical data to be Findable, Accessible, Interoperable, and Re-Usable.49
To ensure FAIR principles, interoperability standards were created such as the Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR) and the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM). Standards now also exist to help select a minimum set of variables to enable data collection according to HL7 FHIR standards at the provider level. Examples include the Minimal Common Oncology Data Elements (mCODE), launched by ASCO and its partners, including a set of 90 data elements ranging from patient, disease, laboratory, genomics, treatment, to outcomes data50 and the OSIRIS set of data piloted by UNICANCER network including 67 clinical and 65 omics items.51
Although initiatives advocate for upfront EMR data structuration52,53 (and ultimately standardization within interoperable systems which could facilitate automated data collection), the current reality is that clinical data are often recorded in an unstructured format and require conversion into a structured and centralized format. A feasible digital alternative to the standard manual structuration of free-text EMRs by research personnel (as demonstrated by the implementation of different structuration systems at many centers) is the use of NLP systems.54
Hong et al. developed an advanced FHIR-based clinical data normalization pipeline known as NLP2FHIR. NLP2FHIR’s multiple functionalities include: a module for NLP with an FHIR-based type system, an integrating structured data module, and a content normalization module.55 The researchers concluded that the NLP2FHIR model was acceptable for modeling EMR data and integrating structured elements into the model using FHIR parameters.55 The success of this model is significant because it demonstrates the ability to overcome interoperability restraints through the use of a normalizing model and thus greatly expands the potential use of clinical data in clinical research trials to collect large-scale data analytics.
While the availability of NLP technology and interoperability standards may vary by country, it is evident that the structuration of EMRs may unlock large-scale data collection, sharing, and analysis within the clinical research paradigm.56
Promoting a global and equal reach
Adesoye et al., in an analysis of clinical trials in the United States, highlighted that 76% of participants in clinical trials were white, 11% were Asian, 7% were black, and, when highlighted by ethnic distribution, 13% of participants were Hispanic or Latino.57 In terms of health care, lack of diversity compounds the problem of poor accrual rates, confirms mistrust in the research and medical enterprise among certain populations, and fortifies health disparities in general, particularly for patients suffering from rare diseases where participating in a clinical trial may be the only treatment option.35,57 This unequal representation of participants harms the development of new research by preventing researchers from unveiling biological processes that are truly generalizable as the research populations do not reflect the general population.
Participants may be excluded, or exclude themselves, from a trial for several reasons. One factor affecting many patient participants is geography and the associated constraints. Traditional trials are often based at urban academic centers and many potential participants are unwilling to travel for many hours for a traditional study and may withdraw if they experience long waits at trial sites.58 Potential participants may be reluctant to travel because of time and expense required to travel to the research site, reliance on caregivers, and also the obstacle of having to organize childcare.59 Decentralized research can address this geographical challenge through the implementation of aforementioned digital tools.
Patients may also be excluded because of implicit bias from the provider who may judge the patient not well suited for trial participation based on unfounded judgments (too old, uninterested, too vulnerable, not educated enough). Online training programs have been proposed to help research teams identify and address their own implicit biases.60 Matching algorithms, including those mentioned earlier, can also minimize the impact of human bias in the search for eligible clinical trial participants.61
The unequal access to digital technology, known as the digital divide,62 represents an outstanding challenge for the effective implementation of digitally enabled cancer research. Even accounting for this challenge, a trial enrolling 7904 participants found that research sites implementing video-based consent recruited more quickly and enrolled more patients who were nonwhite, older than 75 years, and who had lower levels of education when compared with standard methods of obtaining consent.63 In addition, a pragmatic clinical trial testing a remote monitoring strategy with ePROs across 52 US centers successfully enrolled 143 patients who had never used a computer, tablet, or phone.30 Paradoxically, these patients have been flagged as the ones most impacted by digitally enabled continuous symptom management interventions, demonstrating the practical, yet counterintuitive, value of decentralized research trials for patients in older and minority demographic groups.64
Strategies that are responsive to individual digital health literacy are highly encouraged such as using adaptive digital interfaces or providing digital hubs where patients with limited technological exposure and skills can receive personalized support.65, 66, 67 Care centers must implement digital literacy programs for both patients and health care providers to ensure no one is left behind during digital research transformation.68
Fostering patient empowerment and participation
In traditional clinical trials, participant retention is often facilitated through close contact with study coordinators. Digital technology is uniquely situated to encourage patient empowerment by helping patients to self-advocate and act in the course of their care. In the context of clinical trials, patient empowerment can generate patient-centered hypotheses to be tested and ensure patient-centered clinical trial procedures, but also increase participant engagement across research conduct.
Several reproducible empowerment interventions can be delivered entirely through digital platforms and do not require the same time, resources, and cost burden for providers to implement.69, 70, 71 These may include specific internet-delivered interventions such as supportive care strategies (pre/post-treatment-specific education targeting self-management advice for local and systemic therapies, cognitive behavioral therapies, adaptive physical activity, rehabilitation, etc.). These are examples of patient empowerment tools that can be the subject of the clinical research trial or used to empower patients during clinical trials investigating pharmacological agents.
In addition, technology may allow for patient and community input and engagement during early phases of clinical trial design. This may be achieved through digital crowdsourcing processes or digital community advisory boards that can inform specific research questions and help to co-design health programs and clinical trial materials adapted to patient’s needs.47
Engagement during the clinical trial conduct phase can be maximized by prompting real-time feedback upon completion of clinical trial procedures and by updating patients of recruitment status, preliminary, and final trial results.72 Furthermore, technology may also be a good tool for providing formal research training to patients.73
Personal health records (PHRs), or a centralized cloud-based collection of patient data, offer patients an unprecedented opportunity to control and share their health data with researchers. Including both clinical data (EHRs, AE reporting, laboratory results, imaging diagnostics) and patient-generated data (ePROs, biosensor data, user data from health-related apps such as meditation, exercise, or even GPS-based air quality metrics), PHRs can offer valuable and multi-faceted insights to researchers that are impossible to achieve using only clinical data or PGHD alone.
Other major challenges to deploy fully digitally enabled and decentralized research
Effectively implementing fully decentralized clinical trials comes with several other challenges that have not yet been addressed such as74: (i) identifier protocols, cybersecurity, and privacy concerns, including managing and authenticating identities of participants to prevent fake entries,75 and the need for strong encryption processes and access controls for data transmission and storage. According to this challenge, cybersecurity frameworks are being produced to provide the appropriate guidance.76 (ii) Data storage: multimodal data will require capacity of thousands of petabytes from internet service providers with associated energy expenses. (iii) Data quality: there is a need to ensure reliable digitally enabled data collection across various geographical locations and environments with different levels of technology usage and access.74 (iv) Regulatory and ethics concerns: regulatory bodies may be reluctant to accept and adopt a decentralized clinical trial model.74
Accelerating digitally enabled research in oncology
The initial setup and ongoing maintenance of a digitally enabled research decentralized infrastructure can be extremely costly and complex, calling for the need of organized initiatives to create federated infrastructures.
There are several commercial and academic organizations that have launched digital initiatives that can host and facilitate decentralized clinical trials including Medidata, Evidation, and Eureka in the United States and Climedo in Germany. We launched the WeSHARE program (https://weshare.unicancer.com/), in France, in 2021. WeSHARE is a consortium funded by the national government coordinated by UNICANCER that aims to accelerate research digitalization in oncology and provide all tools necessary to address contemporary research challenges in oncology specifically QoL and social issues. WeSHARE uses pilot studies to continuously evolve. It currently offers researchers and patients a digital toolbox for simplifying clinical trial procedures, collecting PGHD, and improving the research experience including specific guidance on fostering inclusion and diversity. Upcoming features include the automated exchange of data with EMR, partnership with digital literacy initiatives, and the empowerment of a virtual community of citizens and researchers allowing the co-creation of research interventions. Data generated through the WeSHARE platform follow FAIR principles by storing data for subsequent use in new research proposals and thereby expanding the research potential of existing datasets. In the future, WeSHARE hopes to launch interoperable exchange of data to a next level, by directly exchanging data through our platform between all stakeholders including the patient, physician, health care organizations, researchers, and industry partners to achieve a proactive, personalized, and participatory oncology care model.
Acknowledgments
Funding
MAF is funded by a Conquer Cancer—Breast Cancer Research Foundation Career Development Award for Diversity and Inclusion, supported by Breast Cancer Research Foundation. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer, or Breast Cancer Research Foundation. IVL declares that the WeShare project is funded by the French National Health Agency [grant number ANR-21-ESRE-0017].
Disclosure
MAF: research funding: Resilience Care (Inst); speaker honoraria: Novartis (Inst). IVL: honoraria: AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst), Novartis (Inst), Sandoz. Research funding: Resilience Care (Inst); Travel support: Novartis. EG has declared no conflicts of interest.
References
- 1.Bluethmann S.M., Mariotto A.B., Rowland J.H. Anticipating the ‘silver tsunami’: prevalence trajectories and co-morbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold M., Rutherford M.J., Bardot A., et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 4.Berry D.A., Cronin K.A., Plevritis S.K., et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 5.Hsiue E.H.-C., Moore T.J., Alexander G.C. Estimated costs of pivotal trials for U.S. Food and Drug Administration-approved cancer drugs, 2015-2017. Clin Trials. 2020;17:119–125. doi: 10.1177/1740774520907609. [DOI] [PubMed] [Google Scholar]
- 6.Steensma D.P., Kantarjian H.M. Impact of cancer research bureaucracy on innovation, costs, and patient care. J Clin Oncol. 2014;32:376–378. doi: 10.1200/JCO.2013.54.2548. [DOI] [PubMed] [Google Scholar]
- 7.Sertkaya A., Wong H.-H., Jessup A., Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials Lond Engl. 2016;13:117–126. doi: 10.1177/1740774515625964. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Aspitia A., McCormick Holmes E., Jackisch C., et al. Updated results from the phase III ALTTO trial (BIG 2-06; NCCTG (Alliance) N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L) or their combination (L+T) in the adjuvant treatment of HER2-positive early breast cancer. J Clin Oncol. 2017;35:502. [Google Scholar]
- 9.Emanuel E.J., Schnipper L.E., Kamin D.Y., Levinson J., Lichter A.S. The costs of conducting clinical research. J Clin Oncol. 2003;21:4145–4150. doi: 10.1200/JCO.2003.08.156. [DOI] [PubMed] [Google Scholar]
- 10.Guerra C.E., Fleury M.E., Byatt L.P., Lian T., Pierce L. Strategies to advance equity in cancer clinical trials. Am Soc Clin Oncol Educ Book. 2022;42:127–137. doi: 10.1200/EDBK_350565. [DOI] [PubMed] [Google Scholar]
- 11.Unger J.M., Vaidya R., Hershman D.L., Minasian L.M., Fleury M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111:245–255. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duma N., Vera Aguilera J., Paludo J., et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. 2018;14:e1–e10. doi: 10.1200/JOP.2017.025288. [DOI] [PubMed] [Google Scholar]
- 13.Inan O.T., Tenaerts P., Prindiville S.A., et al. Digitizing clinical trials. NPJ Digit Med. 2020;3 doi: 10.1038/s41746-020-0302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez M.V., Mahaffey K.W., Hedlin H., et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddad T.C., Helgeson J., Pomerleau K., et al. Impact of a cognitive computing clinical trial matching system in an ambulatory oncology practice. J Clin Oncol. 2018;36:6550. [Google Scholar]
- 16.Klein H., Mazor T., Siegel E., et al. MatchMiner: an open-source platform for cancer precision medicine. NPJ Precis Oncol. 2022;6:69. doi: 10.1038/s41698-022-00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain N.M., Culley A., Micheel C.M., Osterman T.J., Levy M.A. Learnings from precision clinical trial matching for oncology patients who received NGS testing. JCO Clin Cancer Inform. 2021;5:231–238. doi: 10.1200/CCI.20.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delorme J., Charvet V., Wartelle M., et al. Natural language processing for patient selection in phase I or II oncology clinical trials. JCO Clin Cancer Inform. 2021;5:709–718. doi: 10.1200/CCI.21.00003. [DOI] [PubMed] [Google Scholar]
- 19.Medidata eConsent in Clinical Trials. Medidata Solutions. https://www.medidata.com/en/clinical-trial-products/patient-centric-clinical-trials/econsent/ Available at.
- 20.Kadakia K.T., Asaad M., Adlakha E., Overman M.J., Checka C.M., Offodile A.C. Virtual clinical trials in oncology—overview, challenges, policy considerations, and future directions. JCO Clin Cancer Inform. 2021;5:421–425. doi: 10.1200/CCI.20.00169. [DOI] [PubMed] [Google Scholar]
- 21.Chhin V., Roussos J., Michaelson T., et al. Leveraging mobile technology to improve efficiency of the consent-to-treatment process. JCO Clin Cancer Inform. 2017;1:1–8. doi: 10.1200/CCI.17.00041. [DOI] [PubMed] [Google Scholar]
- 22.Patt D., Wilfong L., Hudson K.E., et al. Implementation of electronic patient-reported outcomes for symptom monitoring in a large multisite community oncology practice: dancing the Texas two-step through a pandemic. JCO Clin Cancer Inform. 2021;5:615–621. doi: 10.1200/CCI.21.00063. [DOI] [PubMed] [Google Scholar]
- 23.M.D. Anderson Cancer Center . 2023. Potential Impact of the COVID-19 Pandemic on Financial Toxicity in Breast Cancer Surgical Patients: The Impact on Out of Pocket Costs, Lost Wages and Economic Strain.https://clinicaltrials.gov/study/NCT04169542 Available at. [Google Scholar]
- 24.Meghiref Y., Parnot C., Duverger C., et al. The use of telemedicine in cancer clinical trials: connect-patient-to-doctor prospective study. JMIR Cancer. 2022;8 doi: 10.2196/31255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y.-N., Shin D.G., Park S., Lee C.H. Randomized clinical trial to assess the effectiveness of remote patient monitoring and physician care in reducing office blood pressure. Hypertens Res. 2015;38:491–497. doi: 10.1038/hr.2015.32. [DOI] [PubMed] [Google Scholar]
- 26.Zon R.T., Kennedy E.B., Adelson K., et al. Telehealth in oncology: ASCO standards and practice recommendations. JCO Oncol Pract. 2021;17:546–564. doi: 10.1200/OP.21.00438. [DOI] [PubMed] [Google Scholar]
- 27.Mir O., Ferrua M., Fourcade A., et al. Digital remote monitoring plus usual care versus usual care in patients treated with oral anticancer agents: the randomized phase 3 CAPRI trial. Nat Med. 2022;28:1224–1231. doi: 10.1038/s41591-022-01788-1. [DOI] [PubMed] [Google Scholar]
- 28.Jiang C.Y., El-Kouri N.T., Elliot D., et al. Telehealth for cancer care in veterans: opportunities and challenges revealed by COVID. JCO Oncol Pract. 2021;17:22–29. doi: 10.1200/OP.20.00520. [DOI] [PubMed] [Google Scholar]
- 29.Evans J.M., Qiu M., MacKinnon M., Green E., Peterson K., Kaizer L. A multi-method review of home-based chemotherapy. Eur J Cancer Care. 2016;25:883–902. doi: 10.1111/ecc.12408. [DOI] [PubMed] [Google Scholar]
- 30.Basch E., Schrag D., Henson S., et al. Effect of electronic symptom monitoring on patient-reported outcomes among patients with metastatic cancer. JAMA. 2022;327:2413–2422. doi: 10.1001/jama.2022.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B., Abbott A., Davidson S., Syrkin L., LeFever G., Van den Abbeele A.D. Centralized clinical trial imaging data management: practical guidance from a comprehensive cancer center’s experience. J Digit Imaging. 2019;32:849–854. doi: 10.1007/s10278-018-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akechi T., Yamaguchi T., Uchida M., et al. Smartphone psychotherapy reduces fear of cancer recurrence among breast cancer survivors: a fully decentralized randomized controlled clinical trial (J-SUPPORT 1703 Study) J Clin Oncol. 2023;41:1069–1078. doi: 10.1200/JCO.22.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummel S.B., van Lankveld J.J., Oldenburg H.S., Hahn D.E., Broomans E., Aaronson N.K. Internet-based cognitive behavioral therapy for sexual dysfunctions in women treated for breast cancer: design of a multicenter, randomized controlled trial. BMC Cancer. 2015;15:321. doi: 10.1186/s12885-015-1320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baer A.R., Michaels M., Good M.J., Schapira L. Engaging referring physicians in the clinical trial process. J Oncol Pract. 2012;8:e8–e10. doi: 10.1200/JOP.2011.000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nipp R.D., Hong K., Paskett E.D. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105–114. doi: 10.1200/EDBK_243729. [DOI] [PubMed] [Google Scholar]
- 36.Embi P.J., Jain A., Clark J., Bizjack S., Hornung R., Harris C.M. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005;165:2272–2277. doi: 10.1001/archinte.165.19.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregg J.R., Horn L., Davidson M.A., Gilbert J. Patient enrollment onto clinical trials: the role of physician knowledge. J. Cancer Educ. 2014;29:74–79. doi: 10.1007/s13187-013-0548-z. [DOI] [PubMed] [Google Scholar]
- 38.Basch E., Abernethy A.P., Mullins C.D., et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 39.Cherny N.I., de Vries E.G.E., Dafni U., et al. Comparative assessment of clinical benefit using the ESMO-Magnitude of Clinical Benefit Scale version 1.1 and the ASCO Value Framework Net Health Benefit Score. J Clin Oncol. 2019;37:336–349. doi: 10.1200/JCO.18.00729. [DOI] [PubMed] [Google Scholar]
- 40.Meirte J., Hellemans N., Anthonissen M., et al. Benefits and disadvantages of electronic patient-reported outcome measures: systematic review. JMIR Perioper Med. 2020;3 doi: 10.2196/15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basch E., Dueck A.C., Mitchell S.A., et al. Patient-reported outcomes during and after treatment for locally advanced rectal cancer in the PROSPECT trial (Alliance N1048) J Clin Oncol. 2023;41:3724–3734. doi: 10.1200/JCO.23.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low C.A. Harnessing consumer smartphone and wearable sensors for clinical cancer research. NPJ Digit Med. 2020;3:140. doi: 10.1038/s41746-020-00351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C., Ding S., Wang J. Digital health for aging populations. Nat Med. 2023;29:1623–1630. doi: 10.1038/s41591-023-02391-8. [DOI] [PubMed] [Google Scholar]
- 44.Jung S.Y., Kim J.W., Hwang H., et al. Development of comprehensive personal health records integrating patient-generated health data directly from Samsung S-Health and Apple Health apps: retrospective cross-sectional observational study. JMIR MHealth UHealth. 2019;7 doi: 10.2196/12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Daccache M., Bardus M. The Palgrave Encyclopedia of Social Marketing. Springer International Publishing; United Kingdom: 2020. Intervention mapping; pp. 1–4. [Google Scholar]
- 46.Glasgow R.E., Harden S.M., Gaglio B., et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan R.K.J., Wu D., Day S., et al. Digital approaches to enhancing community engagement in clinical trials. NPJ Digit Med. 2022;5:37. doi: 10.1038/s41746-022-00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.What is interoperability? Definition from TechTarget. App Architecture. https://www.techtarget.com/searchapparchitecture/definition/interoperability Available at.
- 49.Boeckhout M., Zielhuis G.A., Bredenoord A.L. The FAIR guiding principles for data stewardship: fair enough? Eur J Hum Genet. 2018;26:931–936. doi: 10.1038/s41431-018-0160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osterman T.J., Terry M., Miller R.S. Improving cancer data interoperability: the promise of the Minimal Common Oncology Data Elements (mCODE) initiative. JCO Clin Cancer Inform. 2020;4:993–1001. doi: 10.1200/CCI.20.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guérin J., Laizet Y., Le Texier V., et al. OSIRIS: a minimum data set for data sharing and interoperability in oncology. JCO Clin Cancer Inform. 2021;5:256–265. doi: 10.1200/CCI.20.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emamekhoo H., Carroll C.B., Stietz C., et al. Supporting structured data capture for patients with cancer: an initiative of the University of Wisconsin Carbone Cancer Center Survivorship Program to improve capture of malignant diagnosis and cancer staging data. JCO Clin Cancer Inform. 2022;6 doi: 10.1200/CCI.22.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cecchini M., Framski K., Lazette P., et al. Electronic intervention to improve structured cancer stage data capture. J Oncol Pract. 2016;12:e949–e956. doi: 10.1200/JOP.2016.013540. [DOI] [PubMed] [Google Scholar]
- 54.Kreimeyer K., Foster M., Pandey A., et al. Natural language processing systems for capturing and standardizing unstructured clinical information: a systematic review. J Biomed Inform. 2017;73:14–29. doi: 10.1016/j.jbi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong N., Wen A., Shen F., et al. Developing a scalable FHIR-based clinical data normalization pipeline for standardizing and integrating unstructured and structured electronic health record data. JAMIA Open. 2019;2:570–579. doi: 10.1093/jamiaopen/ooz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wulff A., Mast M., Hassler M., Montag S., Marschollek M., Jack T. Designing an openEHR-based pipeline for extracting and standardizing unstructured clinical data using natural language processing. Methods Inf Med. 2020;59:e64–e78. doi: 10.1055/s-0040-1716403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adesoye T., Katz M.H.G., Offodile A.C. Meeting trial participants where they are: decentralized clinical trials as a patient-centered paradigm for enhancing accrual and diversity in surgical and multidisciplinary trials in oncology. JCO Oncol Pract. 2023;19:317–321. doi: 10.1200/OP.22.00702. [DOI] [PubMed] [Google Scholar]
- 58.Goodson N., Wicks P., Morgan J., Hashem L., Callinan S., Reites J. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. NPJ Digit Med. 2022;5:58. doi: 10.1038/s41746-022-00603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Academies of Sciences, Engineering, and Medicine, Policy and Global Affairs, Committee on Women in Science, Engineering, and Medicine, Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research . In: Improving representation in clinical trials and research: building research equity for women and underrepresented groups. Bibbins-Domingo K., Helman A., editors. National Academies Press (US); Washington, DC: 2022. [PubMed] [Google Scholar]
- 60.Barrett N.J., Boehmer L., Schrag J., et al. An assessment of the feasibility and utility of an ACCC-ASCO implicit bias training program to enhance racial and ethnic diversity in cancer clinical trials. JCO Oncol Pract. 2023;19:e570–e580. doi: 10.1200/OP.22.00378. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz A.L., Alsan M., Morris A.A., Halpern S.D. Why diverse clinical trial participation matters. N Engl J Med. 2023;388:1252–1254. doi: 10.1056/NEJMp2215609. [DOI] [PubMed] [Google Scholar]
- 62.The Lancet Healthy Longevity. Tackling the digital divide. Lancet Healthy Longev. 2021;2 doi: 10.1016/S2666-7568(21)00233-6. [DOI] [PubMed] [Google Scholar]
- 63.Fanaroff A.C., Li S., Webb L.E., et al. An observational study of the association of video- versus text-based informed consent with multicenter trial enrollment: lessons from the PALM study (Patient and Provider Assessment of Lipid Management) Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Digital Health Hubs – 100% Digital Leeds. https://digitalinclusionleeds.com/our-work/key-initiatives/digital-health-hubs Available at.
- 66.Programme Société Numérique - Societé Numérique. https://societenumerique.gouv.fr/fr/ Available at.
- 67.Ngiam N.H.W., Yee W.Q., Teo N., et al. Building digital literacy in older adults of low socioeconomic status in Singapore (Project Wire Up): nonrandomized controlled trial. J Med Internet Res. 2022;24 doi: 10.2196/40341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Offodile A.C., Seitz A.J., Peterson S.K. Digital health navigation: an enabling infrastructure for optimizing and integrating virtual care into oncology practice. JCO Clin Cancer Inform. 2021;5:1151–1154. doi: 10.1200/CCI.21.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franzoi M.A., Degousée L., Martin E., et al. Implementing a PROACTive care pathway to empower and support survivors of breast cancer. JCO Oncol Pract. 2023;19:353–361. doi: 10.1200/OP.23.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howell D., Mayer D.K., Fielding R., et al. Management of cancer and health after the clinic visit: a call to action for self-management in cancer care. J Natl Cancer Inst. 2021;113:523–531. doi: 10.1093/jnci/djaa083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aapro M., Bossi P., Dasari A., et al. Digital health for optimal supportive care in oncology: benefits, limits, and future perspectives. Support Care Cancer. 2020;28:4589–4612. doi: 10.1007/s00520-020-05539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodday S.M., Karlin E., Brooks A., et al. Better Understanding of the Metamorphosis of Pregnancy (BUMP): protocol for a digital feasibility study in women from preconception to postpartum. NPJ Digit Med. 2022;5:40. doi: 10.1038/s41746-022-00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Education That Empowers - EUPATI. https://eupati.eu/ Available at.
- 74.Abernethy A., Adams L., Barrett M., et al. The promise of digital health: then, now, and the future. NAM Perspect. 2022 doi: 10.31478/202206e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Enhanced Patient Matching Is Critical to Achieving Full Promise of Digital Health Records. The Pew Charitable Trusts. https://www.pewtrusts.org/en/research-and-analysis/reports/2018/10/02/enhanced-patient-matching-critical-to-achieving-full-promise-of-digital-health-records Available at.
- 76.National Institute of Standards and Technology Framework for Improving Critical Infrastructure Cybersecurity, Version 1.1. NIST CSWP 04162018. 2018. http://nvlpubs.nist.gov/nistpubs/CSWP/NIST.CSWP.04162018.pdf Available at.