Abstract
Purpose of Review
The purpose of this study is to evaluate the effectiveness of eHealth interventions for weight loss and weight loss maintenance among adults with overweight or obesity through a systematic review of systematic reviews.
Recent Findings
This study included 26 systematic reviews, covering a total of 338 original studies, published between 2018 and 2023. The review indicates that eHealth interventions are more effective than control interventions or no care and comparable to face-to-face interventions. The effect sizes remain relatively small when comparing eHealth interventions to any control conditions, with mean differences of weight loss results from − 0.12 kg (95% CI − 0.64 to 0.41 kg) in a review comparing eHealth interventions to face-to-face care to − 4.32 kg (− 5.08 kg to − 3.57 kg) in a review comparing eHealth interventions to no care. The methodological quality of the included studies varies considerably. However, it can be concluded that interventions with human contact work better than those that are fully automated.
Summary
In conclusion, this systematic review of systematic reviews provides an updated understanding of the development of digital interventions in recent years and their effectiveness for weight loss and weight loss maintenance among adults with overweight or obesity. The findings suggest that eHealth interventions can be a valuable tool for delivering obesity care to more patients economically. Further research is needed to determine which specific types of eHealth interventions are most effective and how to best integrate them into clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13679-023-00515-2.
Keywords: eHealth, Digital health, Telemedicine, Obesity, Weight loss, Weight loss maintenance
Introduction
A range of health complications, including type 2 diabetes, cardiovascular disease, hypertension, sleep apnea, certain forms of cancer, and increased mortality, are associated with obesity [1]. According to the World Health Organization, more than one billion people worldwide are living with obesity, and the number is still increasing [2]. As such, reducing overweight and obesity is the key to public health.
Lifestyle changes, such as adopting a healthy diet and increasing physical activity, are known to be challenging but feasible for achieving long-term weight management or weight loss [3, 4]. Behavioral weight management interventions have been found to result in approximately 2–2.5 kg more weight loss than control conditions at the 12–18 month mark [5, 6]. However, the long-term success in maintaining weight loss is limited, with only about 20% of individuals estimated to keep the weight off for a year or more [7, 8]. Despite this, these interventions are crucial due to their additional advantages, such as preventing diabetes and premature mortality [9].
The multifactorial causes of obesity necessitate targeting different levels of contributing factors (e.g., social support, favorable environment, individual factors) in obesity treatment. However, there are several challenges in delivering these services in primary care. Weight counseling is perceived as laborious by health professionals, and there is a limited understanding of obesity care and uncertainty about how to initiate a discussion on weight and what is the appropriate terminology and language to use [10, 11]. Effective weight loss requires time, as frequent contact between healthcare professionals and patients is associated with greater weight loss outcomes [5]. Limited time and resources, including the organization of weight management groups or provision of constructive support, are frequently cited as obstacles to individual-level obesity treatment [10, 11].
Digital health, defined by the World Health Organization as the use of information and communication technologies for improving health, has been proposed as a solution to promote healthy lives and well-being for people of all ages [12]. This novel approach is also suitable for reducing obesity as it can recognize the complexity of obesity and provide patient-centered, multidisciplinary care that considers the personalized needs of individuals living with obesity. Digital health technologies also have the potential to reach a considerable number of people and improve access to obesity care as well as reduce the costs of healthcare systems as they are cost-effective [13, 14]. Furthermore, digital health tools are accessible to a wide range of people regardless of their location or physical abilities and are flexibly available in terms of time.
The COVID-19 pandemic expedited the need and development of healthcare provided in ways other than in person. The interest in eHealth grew, and new innovations emerged. This time also prompted a welcomed uprising in eHealth research. Obesity was early on recognized as a risk factor for COVID-19 complications [15]. Thus, digital weight loss interventions were of more interest than ever before.
Even prior to the pandemic, digital weight loss and weight maintenance programs were already gaining popularity. As more trials were conducted, numerous systematic reviews were published to conclude their effectiveness and components. This article aims to review the past five years of systematic reviews on eHealth weight loss and weight maintenance interventions to provide an updated understanding of the development of digital interventions in recent years. Our primary focus is to offer a comprehensive overview of the effectiveness of eHealth interventions for weight loss and weight loss maintenance among adults with overweight or obesity.
Methods
We followed guidelines for conducting systematic reviews of systematic reviews suggested by Smith et al. [16] as well as the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [17]. We followed a pre-defined, albeit not prospectively registered, protocol.
Inclusion and Exclusion Criteria
We followed the PICOS (population, intervention, control, outcome, study design) framework when formulating our inclusion and exclusion criteria (Table 1). We defined eHealth interventions as interventions delivered via websites, mobile phone applications, or other kinds of online programs with electronic components. This excluded interventions delivered solely through, e.g., phone or video calls, short messaging services (SMS), electronic chats, social media groups, or email, or the use of wearable devices without an accompanying eHealth intervention. We also excluded digital tools used outside an eHealth intervention design (e.g., meal logging applications combined with standard care) and interventions without interactivity (e.g., information-only website). We included only interventions for weight loss or weight loss maintenance rather than interventions for treating any specific underlying disease (e.g., type 2 diabetes, metabolic syndrome, hypertension, cardiovascular risk factors, or cancer). We also excluded studies focusing on lifestyle change, rather than weight loss or weight loss maintenance (such as interventions focusing solely on increasing vegetable and fruit intake, reducing sedentary behavior, or increasing physical activity).
Table 1.
Inclusion and exclusion criteria of the included reviews
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population | Adults aged 18 or older with overweight or obesity (BMI ≥ 25 kg/m2, or BMI ≥ 23 kg/m2 in people of Asian ethnicity) | Pregnant people or those in the postpartum period; people who have had or are waiting for bariatric surgery; people waiting for other types of surgery |
| Intervention | eHealth weight loss or weight loss maintenance interventions | No eHealth weight loss intervention; information-only interventions; main focus on the treatment of a disease |
| Control | Not applicable | Not applicable |
| Outcome | Weight change (kg or %) from baseline or change in BMI (kg/m2) | Weight loss not a primary outcome (main focus, e.g., methodology, theory use, acceptability, or attrition) |
| Study design | Systematic reviews, may include both RCTs and/or NRSIs | Not a systematic review |
BMI body mass index (calculated as weight in kilograms divided by height in meters squared), RCT randomized controlled trial, NRSI non-randomized study of intervention
Search Methods
We searched several databases, including PubMed, Ovid Medline, PsycNet, Scopus, Web of Science, Cochrane Library, Global Index Medicus, and the Centre for Review and Dissemination.
To make the searches as comprehensive as possible, we identified several search term synonyms for population (obesity, obese, overweight) and intervention (telemedicine, telehealth, digital health, eHealth, mHealth, web-based), utilizing and expanding Medical Subject Headings (MeSH) keywords whenever possible. We also combined these search terms with Boolean operators to account for different combinations. While our keywords were in English, we did not limit the search results to any specific language. When possible, we limited the search based on article type (reviews only) and publication date (from January 1st 2018 to February 27th 2023).
We went through the reference lists of included studies to find possible articles not found through our electronic searches. Additionally, we searched the reference lists of non-systematic reviews and umbrella reviews encountered through our primary search. We also searched through several potential databases and registries for gray literature.
A full search strategy, including all searched databases and registries, can be found in the Online Resource.
Selection Process
Two reviewers (SK and AJ) independently screened all records comparing them to the pre-existing inclusion and exclusion criteria. The selection process was carried out in two phases: first, clearly ineligible records were excluded based on their titles and abstracts. Then, full texts were retrieved and analyzed. If the reviewers did not reach consensus on any given reference, a third reviewer (LS) made the decision.
Data Extraction
We extracted basic information from the reviews, including aims, inclusion criteria, number of studies included, age, percentage of women, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), duration, country or ethnicity of participants, outcomes, behavior change technique employed, and funding sources. For reviews with meta-analysis, we extracted key values such as heterogeneity, arms compared, and effect sizes. Two independent reviewers (SK and AJ) extracted the data used in this study.
Several primary studies were included in multiple meta-analyses included in this review, making a meta-analysis of meta-analyses not viable due to issues related to statistical independence [16].
Methodological Quality Assessment
We evaluated the overall confidence of the results of each review using the AMSTAR 2 tool [18]. It is feasible for evaluating reviews consisting of not only randomized controlled trials (RCTs) but also non-randomized studies of interventions (NRSIs). It consists of 16 items, 13 of which focusing on the review itself and 3 on meta-analysis. The tool is not meant to produce a summary score. Instead, the aim is to evaluate whether the review has one or multiple critical flaws (i.e., suboptimal literature review, risk of bias not discussed adequately, poorly chosen methodology for meta-analysis) or non-critical flaws (i.e., data extraction leaving out important domains, authors not selecting studies or extracting data in duplicate, no list of excluded studies). To score high, the review needs to have no critical weaknesses, and to score moderate, only one critical weakness is accepted. Multiple non-critical weaknesses lower the overall quality of the study.
The developers of AMSTAR 2 encourage that researchers adapt the tool to their specific requirements. For the purpose of this review, we deemed that not including a reference list of excluded studies should not be considered a critical quality flaw. While only a minority of reviews included a reference list of excluded studies, most had stated the number of excluded references for each reason, suggesting that they had documented the reasons for exclusions even if not explicitly reported.
Results
Included Reviews
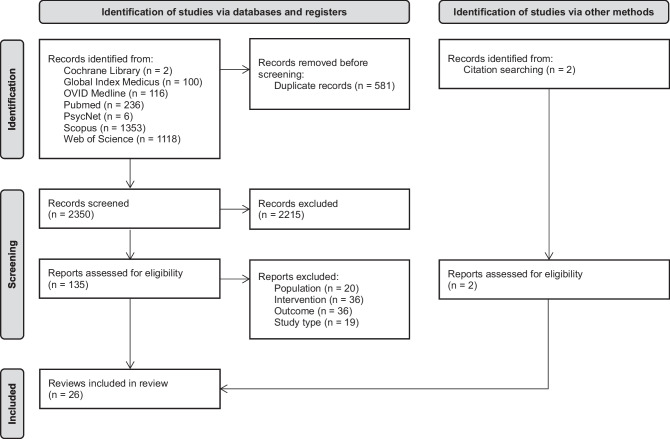
Through our searches, we found 2933 reports in total (Fig. 1). We excluded 581 duplicate reports and 2215 reports not fulfilling our inclusion criteria based on the information provided by their title and abstract. For the remaining 137 reports, we retrieved the full text for further evaluation. After this final evaluation, we included 26 systematic reviews in this review. The exclusion reasons for the other articles retrieved for full text screening can be found in the Online Resource. The 26 included reviews covered a total of 338 original studies (Online Resource).
Fig. 1.
Flowchart of the record screening process
Description of Included Reviews
Tables 2 and 3 describe the characteristics of the included reviews. Funding information can be found in the Online Resource. Fourteen studies focused on eHealth interventions in a general sense [19–32], five on interventions delivered through mobile phones [33••, 34–37], and three on interventions combining eHealth and human-delivered care [38•, 39, 40]. One compared web-based interventions to mobile phone applications [41]. One investigated a variety of behavioral and pharmacotherapy weight loss and weight loss maintenance interventions, 20 of which were technology-based [6]. Three studies focused on weight loss maintenance through eHealth interventions [34, 42, 43].
Table 2.
Characteristics of the included systematic reviews
| Author(s), publication year | Aim of the review | Inclusion criteria of studies | Included studies (n) | Duration of the intervention, mean or range | Outcomes | Theoretical/behavioral frameworks/techniques utilized |
|---|---|---|---|---|---|---|
| Ang et al. (2021) | Determine the efficacy of interventions incorporating apps for weight loss and health behavior change in the Asian population | Published in English, interventions delivered partially or fully through a mobile app, minimal study duration 2 mo, age ≥ 18, participants were of Asian ethnicity, outcome weight change | 21 | Mean 18 wk, range 8 wk to 52 wk | Weight change (kg or %), BMI, waist circumference | N/A |
| Antoun et al. (2022) | To review studies evaluating the effectiveness of smartphone apps on weight loss when combined with other interventions | RCTs, included the use of a smartphone app, weight loss as an outcome, adults only | 34 | 6 wk to 96 wk | Mean weight change (kg) from baseline to 3, 6, and 12 months | Social cognitive theory, transtheoretical model, self-efficacy theory |
| Beleigoli et al. (2019) | Investigate the effectiveness of web-based digital health interventions for weight loss and lifestyle habit changes | BMI ≥ 25, age ≥ 18, no pregnant people, RCTs examining web-based digital interventions vs. offline or in-person interventions or waitlist, overweight or obesity as a primary selection criterion | 11 | N/A | weight, BMI | Several behavioral strategies, e.g., goal setting, self-monitoring and management, social support, modeling and feedback |
| Berry and Kassavou et al. (2021) | Examine if digital self-monitoring of diet and PA is effective at supporting weight loss, increasing PA or improving eating behavior in adults with obesity or overweight, explore intervention components that might explain variations in effectiveness | BMI ≥ 25, age ≥ 18, interventions enabling digital self-monitoring of both eating behavior and PA, RCTs, comparison groups did not use digital self-monitoring | 12 | 3 mo to 12 mo | Weight loss, physical activity, eating behavior | Self-monitoring, goal setting, feedback, social support, education |
| Berry and Sala et al. (2021) | Examine the effectiveness of automated digital interventions for improving the outcomes of human coach-delivered weight loss treatment | Individuals aged ≥ 18 with overweight or obesity, peer-reviewed academic journal articles written in English, reported directly quantitative outcomes of an RCT or quasi-RCT with a prepost design, at least one arm with treatment supplemented with a fully automated digital intervention and one without, ADI did not solely track calorie intake | 13 | 12 wk to 24 mo | weight change | Behavior change theory, self-regulation theory, social cognitive theory, health belief model |
| Besson et al. (2020) | To examine the effectiveness of theoretical and operational inputs in digital interventions for healthy eating | Published in English, adult participants free from other acute illnesses or chronic disease, digital devices primary means of intervention in at least one treatment group, outcome weight loss or weight maintenance | 15 | mean 32 wk (SD 25.6) | Quantitative measures relating to weight loss or weight loss maintenance | Monitoring, feedback or social support |
| Chew et al. (2022) | Examine the effects, and the sustainability of these effects, of smartphone apps on anthropometric, metabolic, and dietary outcomes | BMI ≥ 25 for Western populations or ≥ 23 for Asian populations, weight loss as an outcome, reported outcomes beyond the baseline and after the intervention, RCTs, written in English | 16 | 12 wk to 24 mo, FU 8 wk to 24 mo | Weight loss, waist circumference, HDL, LDL, HbA1c, energy intake, blood pressure | Self-monitoring, social support, goal setting, feedback |
| Dounavi et al. (2019) | To identify existing evidence on the efficacy of mobile health technology in facilitating weight management behaviors | Peer-reviewed primary studies published between 2012 and 2017, adults of typical intellectual ability only, outcome weight loss or management | 39 (17 NRSIs, 22 RCTs) | N/A | Weight change, weight management behaviors, use of mobile technology | Feedback, peer support, goal setting, self-monitoring |
| Holmes et al. (2018) | Examine the effectiveness of interventions using digital technology for weight loss maintenance | Main outcome weight loss maintenance, digital health technologies only, RCTs, published in English between 2006 and February 2018 | 7 | 3 mo to 30 mo, mean 12 mo | BMI, BMI-SDS (BMI Standard Deviation Score), weight change | Self-monitoring and reporting, behavior reinforcement |
| Houser et al. (2019) | To identify different types of technologies used for obesity management and their outcomes | Published in English between 2010 and 2017, peer-reviewed, empirical studies, used some form of digital technology, conducted inside the USA, adults with no cognitive impairments, sample size > 10 | 23 | N/A | Weight change (kg or %), change in BMI, physical activity, dietary intake habits, and time spent in sedentary positions | N/A |
| Huang et al. (2018) | Evaluate the clinical effectiveness of telemedicine on changes in BMI for people with overweight, obesity, diabetes, or hypertension | Age ≥ 19, RCTs, one arm involved any form of telemedicine, one control group receiving usual care or standard treatment, main outcome BMI, original articles published in English or Chinese | 25 | 9 wk to 2 yr | BMI | N/A |
| Islam et al. (2020) | Assess the efficacy of mobile phone app interventions for weight loss and increasing physical activity | Written in English, mobile app interventions, study design included control group, outcomes included changes in body weight, BMI, or waist circumference | 12 | 6 wk to 9 mo | Weight loss, BMI, PA | N/A |
| Jahangiry et al. (2021) | Investigate the effectiveness of web-based interventional programs for weight loss | BMI ≥ 25, age ≥ 18, apparently healthy individuals, RCTs on web-based interventions with a non-web user control group, primary outcome percentage change in body weight | 8 | 12 to 24 wk | weight | Theory of planned behavior, social cognitive theory, behavioral change theory, cognitive behavioral theory |
| LeBlanc et al. (2018) | Investigate the benefits and harms of behavioral and pharmacotherapy weight loss and weight loss maintenance interventions in adults | Age ≥ 18, BMI ≥ 25 or other suboptimal weight-related measure, weight loss or weight maintenance as a primary outcome, population generalizable to the primary care population, controls received no or minimal intervention or were attention controls | 124 (20 utilizing eHealth) | N/A | Health outcomes (mortality, morbidity, depression, health-related quality of life, disability), intermediate outcomes (weight measurements, adiposity measures, incidence or prevalence of obesity-related conditions), adverse events (treatment-related harms) | N/A |
| Lahtio et al. (2022) | Examine the effectiveness of PA promoting web- and mobile-based distance weight loss interventions in rehabilitation settings on body composition | Age 18–65 years, PA-promoting web- and mobile-based distance weight loss interventions in rehabilitation settings, control groups did not use technology, RCTs, outcomes included BMI, waist circumference, or body fat percentage, published in English, Finnish, or Swedish | 30 | Mean 30,4 wk, range 4wk to 2yrs | BMI, waist circumference, body fat percentage | N/A |
| Lau et al. (2020) | Investigate the effectiveness and identify key features of personalized eHealth interventions for weight loss | BMI ≥ 25, age 18–64 years, tailored eHealth interventions incorporating one or more behavioral change techniques, controls received no eHealth intervention, primary outcome weight change | 15 | 12 to 48 wk | Weight | Diverse behavioral strategies, e.g., self-monitoring, goal setting, social support, motivational interviewing and/or prompts |
| Lee et al. (2022) | To review RCTs on weight loss interventions using digital health for employees with obesity | Adult employees with overweight or obesity, weight loss interventions using digital health, outcome weight or BMI, RCTs published in English or Korean | 11 | 12 wk to 12 mo | Weight change (kg) | Social cognitive theory |
| Mamalaki et al. (2022) | To examine the effects of technology-based interventions for weight loss maintenance | RCTs, published in English, adults only, at least one web- or app-based intervention arm vs. a control group of minimum intervention or in-person intervention, outcome weight change after the weight maintenance period | 12 | 3 mo to 30 mo | Weight change | Self-monitoring, goal setting, feedback |
| Mata-Gonzáles et al. (2020) | Examine the efficacy of online interventions for weight loss for adults | Open-access original articles published in English or Spanish, online weight loss interventions, quantitative empirical studies, participants aged 18–60 with overweight or obesity | 21 | 6 wk to 140 wk | Primary outcome change in weight (kg), secondary outcomes body fat, waist circumference, BMI | Self-monitoring, goal setting, social support, feedback |
| Novaes et al. (2022) | To evaluate the efficacy of digital and hybrid interventions for weight loss for people with severe mental illness | People with severe mental illness (e.g. bipolar disorder, psychotic disorders), illness not in the acute phase, remote or hybrid conducted psychoeducative interventions for weight loss and improved health behavior, age 18–65 years; outcomes related to obesity and weight loss | 16 (7 remotes, 9 hybrids) | Remote: 8 wk to 48 wk; hybrid: 10 wk to 52 wk | Change in weight (BMI), outcomes related to metabolic markers and metabolic syndrome | N/A |
| O’Boyle et al. (2022) | To evaluate the use of mobile technology versus web-based interventions and weight loss outcomes with or without individualized clinician feedback in adults with overweight or obesity | RCTs or controlled cohort studies (with n ≥ 25, dropout rate ≤ 20% or > 20% intent to treat calculated), age ≥ 18, studies not focusing on disease, participants in good health and not pregnant, published in English between January 2010 and January 2020 | 14 | 8 wk to 24 mo; eHealth 12 wk to 18 mo | Weight | Self-monitoring, feedback, goal setting |
| Podina et al. (2018) | Investigate the efficacy of multicomponent behavioral e-health interventions for weight loss | BMI ≥ 25, age ≥ 18, RCTs in which a multicomponent behavioral eHealth intervention was compared with a passive control and/or an active in-person treatment intervention | 47 | 3 mo to 24 mo | Weight loss, BMI, waist circumference, body fat percentage, waist-to-hip ratio, PA, eating behaviors | Social cognitive theory in most interventions; most used techniques were intention formation, self-monitoring, feedback, social support |
| Puigdomènech et al. (2019) | Examine the efficacy and safety of mHealth interventions (mobile phone apps) for weight control, overweight, and obesity management | assessed the efficacy and/or safety and/or effectiveness of mHealth interventions for overweight or obesity management, sample size over 10 | 28 | 3 wk to 24 mo | Efficacy (changes in PA and diet), safety | Feedback, goal setting, self-monitoring |
| Rumbo-Rodriguez et al. (2020) | Examine how different types of technologies (e.g., mobile phones, internet, social networks, virtual reality) may aid in weight loss in patients with overweight or obesity | BMI ≥ 25, age ≥ 18, published in English or Spanish, studies with at least two groups for comparison where at least one group received an intervention through technology, outcomes included weight | 47 | N/A | weight loss | Self-monitoring, feedback |
| Shi et al. (2022) | Examine the effectiveness and components of web-based interventions for overweight or obesity | BMI ≥ 25, age ≥ 18, no pregnant people, RCTs examining a web-based intervention, reported weight change as an outcome, original papers written in English or Japanese | 97 | 2 to 24 mo | Weight change, intervention components | Mostly used behavioral approaches in interventions: self-monitoring, social support, goal setting, information about health consequences |
| Varela et al. (2021) | To assess the effectiveness of Internet-based behavioral treatments for adults with overweight and obesity, includes network meta-analysis | Age 18–65 years, BMI 25–39.9 kg/m2, web-based behavioral weight loss interventions, main outcome weight change (kg), RCTs only | 15 | Median 18.3 wk, range 12 to 48 wk | Weight change (kg) | N/A |
BMI body mass index (calculated as weight in kilograms divided by height in meters squared), RCT randomized controlled trial, NRSI non-randomized study of intervention, ADI automated digital intervention, PA physical activity, SD standard deviation, FU follow up, wk week, mo month, yr year
Table 3.
Participant characteristics of the included systematic reviews
| Author(s), publication year | N | Age (years) | Women, % | BMI | Country (ethnicity) |
|---|---|---|---|---|---|
| Ang et al. (2021) | 21 173 | ≥ 18, mean 45.9 (SD 9.84, range 25.8–60.5) | 45.1 | 27.1 (SD 2.47, range 23.0–30.5) | 8 in KR (Korean), 5 in CN (Chinese), 2 in IN (Indian), the USA (Filipino American), SG (multiracial), HK (Chinese), JP (Japanese), 2 in TW (Chinese; Indonesian) |
| Antoun et al. (2022) | 16 to 449, mean 113 | ≥ 18 | N/A | ≥ 25 | 22 in the USA, 3 in AU and NZ, SE, DE, the UK, JP, SG, KR |
| Beleigoli et al. (2019) | 1525 | 18–65 | predominantly women | ≥ 25 | N/A |
| Berry and Kassavou et al. (2021) | 1190 | ≥ 18 | N/A | ≥ 25 | 7 in the USA, 3 in AU, 1 in DE, 1 in the UK |
| Berry and Sala et al. (2021) | 1471 | ≥ 18 | 12 out of 13 trials reported participants’ gender characteristics. Across these trials, women formed a weighted mean percentage of 62.76 (IQR 68.95 to 88.03) | BMI ≥ 25 | 6 studies included predominantly white people (56–77%), 1 only non-white people, 1 study reported 37.5–72.2% of the population being African American, 5 studies did not report ethnicity or race |
| Besson et al. (2020) | Mean sample size 142 (SD 110.4) | ≥ 18 | N/A | ≥ 25 | 11 in the USA, AU, KR, BE, FI |
| Chew et al. (2022) | 2870 | mean 22.7–70.1 | 0–90.7 | Mean 27.5–36.2 | 12 in the USA, UK, AU, JP, CN |
| Dounavi et al. (2019) | N/A | ≥ 18 | NRSIs: 16 included men and women, 1 only women | “Increased”; in RCTs ≥ 25 | NRSIs: 9 in the USA, 3 in the UK, AU, CA, CN, NZ, N/A; RCTs: 8 in the USA, 5 in AU, 2 in FI, CA, RU, IE, 1 in the NL, 1 in the UK, KR |
| Holmes et al. (2018) | 1939, mean 277, range 34–1032 | Mean in children 9.9, mean in adults 44.5 | Mean 63 | N/A | N/A |
| Houser et al. (2019) | from 20 to nearly 2500 | adults | N/A | N/A | US |
| Huang et al. (2018) | 6253 | ≥ 18 | N/A | N/A | N/A |
| Islam et al. (2020) | 1714 | mean 12.7–44.9 | mostly women | N/A | 5 in AU, 4 in North America, 2 in Europe, 1 in Asia |
| Jahangiry et al. (2021) | 779 | ≥ 18 | N/A | ≥ 25 | USA, AU, DE, KR |
| Lahtio et al. (2022) | 6103 | mean 40.2 | Mean 58 | N/A | N/A |
| Lau et al. (2020) | 5816 | 18–64 | N/A | ≥ 25 | USA, AU, IL |
| LeBlanc et al. (2018) | 272 526 | Mean 22.7–65.1, range ≥ 18 ≤ 80 years | N/A | N/A | 71 in the USA, 28 in Europe (excluding the UK), 16 in the UK, 5 in AU or NZ, 3 in CA, 3 in JP |
| Lee et al. (2022) | 13 392 | ≥ 18, reported means from 34.2 to 51 | N/A | N/A* | 4 in the USA, 2 in JP, 2 in DE, DK, IR, the NL |
| Mamalaki et al. (2022) | 2941 | ≥ 18 | N/A | N/A | N/A |
| Mata-Gonzáles et al. (2020) | 9897 (from 49 to 6795) | 18–60 | N/A | ≥ 25 | 10 in the USA, 7 in AU, 2 in the UK, CA, AT |
| Novaes et al. (2022) | Remote: 739 (from 15 to 333, mean 105.6); hybrid 1145 (from 13 to 412, mean 127) | Remote: means from 37 to 55.5; hybrid: between 18 and 65 | Remote: 45; hybrid: 55.19 | N/A | remote: 4 in the USA, CH, TW, ZA; hybrid: 6 in the USA, 2 in the UK, the NL |
| O’Boyle et al. (2022) | mHealth from 25 to 351, eHealth from 60 to 398 | from 18 to 68; eHealth 18 to 75 | N/A | ≥ 25 | N/A |
| Podina et al. (2018) | 15 349 | ≥ 18 | N/A | ≥ 25, reported means 29–35.7 | N/A |
| Puigdomènech et al. (2019) | Sample size from 10 to 15 310, 17 studies had a sample size of less than 100 | N/A | Majority of women | N/A | 17 in the UK, 3 in AU, 2 in KR, UK, BE, ES, the NL, CN, IL |
| Rumbo-Rodriguez et al. (2020) | 22 918 | Mean 40.9 | 51.1 | ≥ 25 | 34 in the USA, 3 in AU, 2 in ES, 3 in CN, 2 in the UK, IT, KR, IR |
| Shi et al. (2022) | N/A | Mean 20.5–69.0 | N/A | Mean 26.1–38.1 | 57 in the USA, 10 in AU, 8 in the UK, 6 in JP, 5 in the NL, 3 in ES, IR, CA, TR, FI, FR, PL, KR, HK |
| Varela et al. (2021) | 2426 | ≥ 18 | 48.4 | 29–33.9 | 8 in AU, 7 in the USA |
BMI body mass index (calculated as weight in kilograms divided by height in meters squared), RCT randomized controlled trial, NRSI non-randomized study of intervention, SD standard deviation, IQR interquartile range, USA United States of America, UK United Kingdom, AU Australia, NZ New Zealand, CA Canada, JP Japan, KR Korea (the Republic of), CN China, IN India, SG Singapore, HK Hong Kong, TW Taiwan, SE Sweden, DE Germany, BE Belgium, FI Finland, RU Russia, IE Ireland, NL Netherlands, IL Israel, DK Denmark, AT Austria, CH Switzerland, ZA South Africa, ES Spain, IT Italy, IR Iran, TR Turkey, FR France, PL Poland
Fourteen of the studies included only RCTs or quasi-RCTs. Most reviews included a majority of trials concluded in the USA, Australia, or the UK. One review focused on only Asian populations [33••]. Most reviews that included information on the gender characteristics reported that a majority of the participants were women. The duration of the interventions ranged from 3 weeks to 24 months. Social cognitive theory was the most often reported theory framework used. Common characteristics of included interventions were self-monitoring, goal setting, social support, and feedback. The methods for follow-up and outcome measures varied considerably between studies. While some studies incorporated scales utilizing wireless communication, most outcome data were self-reported.
Methodological Quality of Included Reviews
We rated two reviews as being of moderate methodological quality [6, 37], 13 as low [19, 20, 23, 25–27, 29, 31, 32, 33••, 34, 38•, 43], and 11 as critically low [21, 22, 24, 28, 30, 35, 36, 39–42]. A detailed evaluation of each AMSTAR 2 criteria can be found in the Online Resource. A considerable number of studies were rated as lower quality because the authors did not justify language restrictions in their search or included studies, did not include a list of excluded studies, or did not justify why they restricted their scope to only some study designs (notably RCTs only). If even one of these issues applicable had been addressed, both studies rated “moderate” and one rated “low” would have been rated “high,” and five of the studies rated “low” would have scored “moderate”. All studies evaluated as being of critically low quality had other substantial flaws and would not have risen in quality appraisal even if the authors addressed these aforementioned issues.
Efficacy of eHealth Interventions for Weight Loss
The effect sizes of individual meta-analyses are reported in Table 4. It also includes measures of heterogeneity, sample size, and number of studies included in the analyses. All in all, eHealth interventions were concluded to have a positive impact on weight loss results. However, across the reviews, effect sizes range from small to moderate, implying that although statistical significance was achieved, the clinical impact may not be substantial. All reviews highlighted notable heterogeneity among the results of the included studies. The results of interventions delivered via smartphone were similar to those delivered via computer.
Table 4.
Summary of meta-analyses
| Author(s), publication year | Review and comparison | No. of included studies | Outcome (units) | N | Heterogeneity | Effect size (95% CI) | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 (df) | P | T2 | I2 | |||||||
| Ang et al. (2021) | Intervention using mobile app vs. control (RCTs only) | 14 | Weight change (kg) | 68.3 | − 0.26 (− 0.41, − 0.11) SMD | < 0.01 | ||||
| Intervention using mobile app vs. control (RCTs only) | 11 | Change in BMI (kg/m2) | 69.9 | − 0.21 (− 0.42, − 0.01) SMD | 0.04 | |||||
| App combined with usual care vs. usual care (RCTs only) | 11 | Weight change (kg) | 67.6 | − 0.28 (− 0.47, − 0.09) SMD | < 0.01 | |||||
| App combined with usual care vs. usual care (RCTs only) | 7 | Change in BMI (kg/m2) | 76.2 | − 0.27 (− 0.54, 0.01) SMD | 0.05 | |||||
| Antoun et al. (2022) | Smartphone app combined with nonapp intervention vs. control without app, at 3 mo | 14 | Weight change (kg) | 1673 | 66.71 (13) | < 0.001 | 81 | − 1.99 (− 2.19, − 1.79) MD | < 0.001 | |
| Smartphone app combined with nonapp intervention vs. control without app, at 6 mo | 17 | Weight change (kg) | 2368 | 161.48 (16) | < 0.001 | 90 | − 2.80 (− 3.03, − 2.56) MD | < 0.001 | ||
| Beleigoli et al. (2019) | Web-based only vs. offline intervention | 10 | Weight change (kg) | 1497 | 141.32 (9) | < 0.001 | 4.43 | 94 | − 0.77 (− 2.16, 0.62) MD | 0.28 |
| Web-based only vs. offline intervention | 8 | Change in BMI (kg/m2) | 1244 | 52.98 (7) | < 0.001 | 0.46 | 87 | − 0.12 (− 0.64, 0.41) MD | 0.66 | |
| Web-based only vs. offline intervention for studies with < 6 months follow-up duration | 4 | Weight change (kg) | 393 | 2.96 (3) | 0.40 | 0.00 | 0 | − 2.13 (− 2.71, − 1.55) MD | < 0.001 | |
| Web-based only vs. offline intervention for studies with ≥ 6 months follow-up duration | 6 | Weight change (kg) | 1104 | 79.81 (5) | < 0.001 | 5.18 | 94 | − 0.17 (− 2.10, 1.76) MD | 0.86 | |
| Web-based only vs. active nontechnology intervention | 6 | Weight change (kg) | 1051 | 9.71 (5) | 0.08 | 0.38 | 49 | 0.82 (0.06, 1.59) MD | 0.04 | |
| Web-based only vs. wait list | 5 | Weight change (kg) | 886 | 46.85 (4) | < 0.001 | 91 | − 2.14 (− 2.65, − 1.64) MD | < 0.001 | ||
| Berry and Kassavou et al. (2021) | Digital intervention vs. control | 12 | Weight change (kg) | 1190 | 34.96 (11) | < 0.001 | 1.60 | 69 | − 2.87 (− 3.78, − 1.96) MD | < 0.001 |
| Berry and Sala et al. (2021) | Coaching combined with automated digital intervention vs. offline coaching | 13 | Weight change (kg) | 1471 | 156.18 (12) | < 0.001 | 0.50 | 92 | − 0.54 (− 0.95, − 0.13) SMD | 0.01 |
| Chew et al. (2022) | Smart phone app intervention vs. control, < 3 mo | 8 | Weight change (kg) | 1021 | < 0.01 | 4.63 | 91.3 | − 1.15 (− 3.02, 0.72) MD | 0.19 | |
| Smart phone app intervention vs. control, 3 mo | 11 | Weight change (kg) | 1406 | < 0.01 | 3.80 | 87.3 | − 2.18 (− 3.59, − 0.78) MD | < 0.01 | ||
| Smart phone app intervention vs. control, 6 mo | 13 | Weight change (kg) | 1604 | 0.01 | 2.02 | 52.4 | − 2.15 (− 3.25, − 1.05) MD | < 0.01 | ||
| Smart phone app intervention vs. control, 9–12 mo | 5 | Weight change (kg) | 899 | 0.52 | 0.03 | 0 | − 1.63 (− 2.99, − 0.26) MD | 0.03 | ||
| Huang et al. (2018) | Telehealth weight loss intervention vs. control | 5 | Change in BMI (kg/m2) | 0.17 | 37.2 | − 0.68 (− 1.07, − 0.30) MD | < 0.01 | |||
| Islam et al. (2020) | Mobile phone app intervention vs. control | 11 | Weight change (kg) | Q = 42.65 | < 0.1 | 1.40 | 76.6 | − 1.07 (− 1.92, − 0.21) MD | 0.01 | |
| Mobile phone app intervention vs. control | 10 | Change in BMI (kg/m2) | Q = 42.65 | < 0.1 | 0.17 | 78.0 | − 0.45 (− 0.78, − 0.12) MD | 0.008 | ||
| Mobile phone app intervention vs. control, ≤ 3 mo | 5 | Weight change (kg) | − 0.004 (− 0.79, 0.80) MD | 0.99 | ||||||
| Mobile phone app intervention vs. control, > 3 mo | 6 | Weight change (kg) | − 1.63 (− 2.64, − 0.61) MD | < 0.002 | ||||||
| Jahangiry et al. (2021) | Web-based intervention vs. non-web-based control | 8 | Weight change (kg) | 779 | 0.001 | 74.8 | 0.56 (− 3.47, 4.59) WMD | 0.786 | ||
| Lahtio et al. (2022) | Web- or mobile-based distance weight loss intervention vs. no technology control | 28 | Change in BMI (kg/m2) | 4060 | < 0.001 | 90 | − 0.83 (− 1.15, − 0.51) MD | < 0.001 | ||
| Lau et al. (2020) | Personalized eHealth intervention vs. control, population with obesity | 12 | Weight change (kg) | 1640 | − 3.10 (− 4.05, − 2.15) MD | < 0.001 | ||||
| Personalized eHealth intervention vs. control before sensitivity analysis | 11 | Change in BMI (kg/m2) | 5317 | 80 | − 0.77 (− 1.03, − 0.51) MD | < 0.001 | ||||
| Personalized eHealth intervention vs. control after sensitivity analysis | 9 | Change in BMI (kg/m2) | 1226 | 13 | − 0.92 (− 1.10, − 0,74) MD | < 0.001 | ||||
| Mamalaki et al. (2022) | Technology-based weight loss maintenance intervention vs. minimum intervention | 10 | Weight change (kg) | 2187 | Q = 4.63 (df 9) | 0.87 | 0.00 | 0.0 | − 0.07 (− 0.57, 0.42) MD | 0.77 |
| Technology-based weight loss maintenance intervention vs. in-person intervention | 4 | Weight change (kg) | 1105 | Q = 4.78 (df 3) | 0.19 | 0.45 | 37.2 | 1.36 (0.29, 2.43) MD | 0.01 | |
| Podina et al. (2018) | eHealth intervention vs. passive control at posttreatment | 50 | Weight outcomes | 84 | 0.34 (0.24, 0.44) g | |||||
| eHealth intervention vs. passive control at follow-up | 10 | Weight outcomes | 84 | 0.25 (0.05, 0.46) g | ||||||
| eHealth intervention vs. active control at posttreatment | 7 | Weight outcomes | < 0.01 | − 0.31 (− 0.43, − 0.20) g | ||||||
| Shi et al. (2022) | Web-based intervention vs. offline control | 44 | Weight change (kg) | 7062 | 481.60 (43) | < 0.001 | 0.29 | 91 | − 0.57 (− 0.75, − 0.40) SMD | < 0.001 |
| Varela et al. (2021) | Intensive contact websitea vs. wait list | 3 | Weight change (kg) | 320 | 0.94 (2) | 0.63 | 0.00 | 0 | − 4.32 (− 5.08, − 3.57) MD | < 0.001 |
| Minimal contact websitea vs. wait list | 3 | Weight change (kg) | 480 | 0.81 (2) | 0.67 | 0.00 | 0 | − 3.23 (− 3.80, − 2.66) MD | < 0.001 | |
| Intensive contact website vs. self-help | 5 | Weight change (kg) | 790 | 0.40 (4) | 0.98 | 0.00 | 0 | − 1.79 (− 2.33, − 1.24) MD | < 0.001 | |
BMI body mass index (calculated as weight in kilograms divided by height in meters squared), RCT randomized controlled trial, MD mean difference, SMD standardized mean difference, WMD weighted mean difference, g Hedge’s g, Q Cochran’s Q, I2 percentage of the variation across studies that can be attributed to study heterogeneity, T2 between-study variance
aIntensive contact website: personalized feedback provided by a healthcare professional at least once a week. Minimal contact website: feedback provided at least once a month; provided by a professional (personalized) or machine (not personalized)
The results of narrative reviews are described in Table 5. Overall, they concluded that eHealth interventions are associated with weight loss outcomes that are at least comparable, and in some cases superior, to those achieved through control conditions.
Table 5.
Summary of narrative reviews
| Author(s), publication year | Number of studies | Main findings |
|---|---|---|
| Besson et al. (2020) | 15 | Digital interventions can be beneficial in weight loss for adults with overweight. Nine studies out of fifteen showed a significant difference between treatments favoring digital interventions. Also, the results of the rest of the studies (6) indicated that when comparing the digital interventions to the active compare group, they could be as successful as the comparison intervention. Interventions that promoted weight loss utilized, e.g., personalized feedback and counseling, social support, and self-monitoring through web programs, Internet chats, text messages, and mobile apps. The results were mixed when digital interventions were compared to traditional face-to-face/human counseling interventions: some found that hybrid (human and digital) intervention was the most effective for weight loss, whereas some did not find the difference. Digital interventions succeeded well in the short term compared to human intervention, but human counseling was more effective in the longer term |
| Dounavi et al. (2019) | 39 | The results of both RCTs and non-randomized studies showed that digital apps were useful and easy to use for the purposes of weight management and weight loss. Including tools that enabled the effortless self-monitoring of health behaviors, interaction with peers, tailored feedback, and reminders to continue with the app, increased engagement in the process and thus supported successful weight management |
| Holmes et al. (2018) | 7 | The use of digital health technologies promoted successful weight-loss maintenance in short-term periods (3–24 months). Four RCTs out of seven reported that compared to controls with no contact or face-to-face contact; the technology significantly aided the weight management process. Self-monitoring and reporting were essential components of digital health technologies. The results of five trials suggested that digital interventions could support goal setting and social interaction. Also, personalized contacts were seen as necessary for participants |
| Houser et al. (2019) | 23 | The statistically significant association between the use of digital components and weight loss was seen in 14 studies. The review noticed that 73% of the studies that utilized mobile health devices showed a statistically significant association between weight loss and used technology, whereas 40% of the studies that used telemedicine and 50% of the studies that used eHealth reported statistically significant differences. Digital tools were utilized to provide reminders and encourage health-promoting behaviors |
| Lee et al. (2022) | 11 | All studies showed a statistically significant weight loss after the digital health intervention. Seven studies reported a significant difference between the intervention and comparison groups, and among those studies, six showed that both intervention and control groups lost weight. Still, the intervention groups’ weight reduction was greater than the control groups. The remaining four studies failed to show a significant difference between the groups. Intervention strategies included in the studies were, e.g., tailored advice, personalized goal settings and daily messages, tailored remote group meetings, and online social support |
| Mata-Gonzáles et al. (2020) | 21 | The online intervention showed significant differences in weight when compared to the control group or face-to-face intervention. Web-based programs that promoted weight management focused on, e.g., increasing physical activity and making healthier changes to diet. Successful interventions utilized self-monitoring and social support as well as goal setting |
| Novaes et al. (2022) | 16 | Both remote and hybrid (digital + face-to-face) interventions found significant outcomes favoring the interventions. However, as statistical methods and study outcomes varied, direct comparisons were difficult to make. In conclusion, digital approaches seem practical, competent, and valuable tools to reduce sedentary behavior, improve life quality and healthier lifestyle, and lose weight among patients with severe mental illnesses |
| O’Boyle et al. (2022) | 14 | The review found that both mHealth (8) and eHealth (6) interventions positively impacted weight loss and behavior change. When combining human support (regular clinician coaching through, e.g., phone calls, text messages, and e-feedback) with digital components, the participants gained the most successful outcomes regarding weight and behavior change. Self-regulation, reporting weight-related behaviors, and tailored feedback were essential factors in successful interventions |
| Puigdomènech et al. (2019) | 28 | The review aimed to find methods of how mHealth interventions had evaluated the efficacy, effectiveness, and safety of digital interventions for weight loss/management. Most of the studies (78%) assessed the reduction in weight/BMI as a primary marker for the efficacy of mHealth intervention, followed by changes in physical activity and diet. Feedback messaging, goal setting, and self-monitoring were the most used tools in apps. Peer support and gamification might be useful to increase engagement and motivation and thus improve the efficacy of the intervention. The weight loss results were controversial: some found no difference between intervention and control groups, whereas some found significant or non-significant reductions in weight-loss markers between groups. Interventions that included face-to-face elements in their program obtained the most successful outcomes |
| Rumbo-Rodriguez et al. (2020) | Different technologies such as smartphones, apps, websites, and personal digital assistants were used in weight loss interventions for patients with overweight and obesity. Almost half of the interventions (47%) reported a significant impact of the technology-based interventions for weight loss compared to the control or comparison group. The use of digital tools also seems to improve treatment adherence as they offer more straightforward and faster self-monitoring via technology. Also, some findings indicate that the adherence level is further increased when it is accompanied by immediate feedback. Additionally, the short-term weight loss results highlight the crucial role of personalized feedback in weight management, but the association has not been observed in the long term |
eHealth Interventions vs. Any Control Conditions
Seventeen reviews reported weight loss outcomes by comparing intervention effects to any control conditions, including standard care, waitlists, paper-based communication, or minimal interventions such as an educational website or a single face-to-face educational session [19, 20, 22–28, 30, 31, 34–37, 40, 42].
The results favored eHealth interventions, with mean differences of statistically significant (P < 0.05) weight loss outcomes ranging from − 1.07 (95% CI − 1.92, − 0.21) kg to − 3.10 (− 4.05, − 2.15) kg for absolute weight change and from − 0.12 (− 0.64, 0.41) kg/m2 to − 0.92 (− 1.10, − 0.74) kg/m2 for changes in BMI. Of the 15 reviews that included meta-analysis, only two did not find significant differences between weight loss outcomes [19, 24]. All 8 narrative reviews concluded that eHealth interventions reach at least similar and often even greater weight loss results than control conditions.
eHealth Interventions vs. No or Minimal Interventions
Three reviews compared eHealth interventions to minimal interventions, finding the former to be more effective. The control conditions varied, but they included no interventions (e.g., waitlist or no care) or minimal interventions (e.g., newsletter or information pamphlet). Podina et al. found effect sizes to be small but consistent across trials (Hedge’s g 0.34 (0.24, 0.44)) [29]. Beleigoli et al. found those in the eHealth intervention to achieve better weight loss results than those on a wait-list (mean difference (MD) − 2.14 (− 2.65, − 1.64) kg, P < 0.001) [19]. Moreover, Varela et al. reported that those in an eHealth intervention with professional feedback at least weekly reached the strongest outcomes compared to those on a wait-list (MD − 4.32 (− 5.08, − 3.57) kg, P < 0.001) while those in a mostly automated eHealth intervention reached slightly weaker results (MD − 3.23 (− 3.80, − 2.66) kg, P < 0.001) [32].
eHealth Interventions Combined with Professional Contact
All three reviews examining the effects of feedback found that eHealth interventions with personalized feedback provided by a health professional were significantly more effective than interventions with no or machine-generated feedback, with mean differences in weight loss outcomes as great as − 4.3 kg (95% CI − 5.08, − 3.57 kg) [25, 26, 32]. Similarly, Varela et al. concluded that web-based interventions with professional contact were more effective than self-help websites (MD − 1.79 (− 2.33, − 1.24) kg) [32].
Both in-person and e-counseling were found to increase intervention success. Puigdomènech et al. concluded that interventions including face-to-face elements obtained the most successful outcomes [37], while Shi et al. found that eHealth interventions with e-counseling had better results than those without (standardized mean difference (SMD) − 0.42 (− 0.75, − 0.08) kg, P = 0.04) [31]. This finding was supported by O’Boyle et al., who found that phone calls, text messages, and e-feedback also enhance intervention success [41]. Besson et al. stated mixed results, where some studies found hybrid interventions to be more effective than eHealth interventions only, while others did not find a difference [21].
Combining face-to-face interventions with an eHealth intervention also leads to enhanced weight loss results. Berry and Sala et al. found that combining human coaching with an automated digital intervention provided better results than coaching alone (MD − 2.18 (− 4.39 kg to − 0.03 kg) and − 2.21% (− 4.49 to 0.08%) in body weight from baseline) [39]. Similarly, both Ang et al. and Antoun et al. found that combining usual care with a mobile application was more effective than usual care alone (Hedge’s g − 0.28 (− 0.47, − 0.09); P < 0.01 and MD − 2.80 (− 3.03, -2.56) kg; P < 0.001, respectively) [33••, 38•]. Surprisingly, Berry and Sala et al. concluded that combined interventions with lower duration or frequency of coach contact (< 10 h) provided better results than interventions with more intensive coaching (> 10 h) (SMD 0.11 (− 0.24, 0.45) kg vs. 0.86 (0.20, 1.52) kg, P = 0.05).
eHealth Interventions vs. Face-to-Face Interventions
Four reviews reported weight loss outcomes in eHealth interventions compared with face-to-face interventions, such as usual care without an eHealth component. Beleigoli et al. found that stand-alone web-based interventions had poorer weight loss outcomes than in-person interventions (MD 0.82 (0.06, 1.59) kg, P = 0.04) [19]. Similarly, Podina et al. found active control groups to have more beneficial results than eHealth groups (Hedge’s g − 0.31 (− 0.43, − 0.20)). On the other hand, Besson et al. found that eHealth interventions could be as successful as active offline comparison interventions or even more effective in the short term [21].
Efficacy of eHealth Interventions for Weight Loss Maintenance
The outcomes of eHealth interventions on weight loss maintenance seem less pronounced than on initial weight loss. Mamalaki et al. found that eHealth interventions produced similar results than minimal interventions while leading to greater weight regain in comparison with in-person care [43]. In contrast, Podina et al. found eHealth interventions to be more effective in weight loss maintenance than passive control conditions, although the effect sizes remained small (Hedge’s g = 0.25 (0.05, 0.46) before and 0.15 (0.02, 0.27) after sensitivity analysis) [29].
Chew et al. found that only 6% (1 out of 16 studies) of included mobile application interventions reported significant weight loss at both 18- and 24-month follow-up [34]. On the other hand, Holmes et al. concluded that digital health technologies aided in weight loss maintenance in short time periods (3–24 months), with four out of seven RCTs reporting significant improvements compared to no contact or in-person care [42].
Discussion
This review article summarizes 26 systematic reviews, covering a total of 338 original studies, that evaluate the efficacy of web-based interventions for weight loss or weight loss maintenance. The review indicates that eHealth interventions are more effective than control interventions or no care and comparable to face-to-face interventions. The effect sizes remain relatively small when comparing eHealth interventions to any control conditions, with mean differences of weight loss results from − 0.12 kg (95% CI − 0.64 to 0.41 kg) in a review comparing eHealth interventions to face-to-face care to − 4.32 kg (− 5.08 kg to − 3.57 kg) in a review comparing eHealth interventions to no care. The methodological quality of the included studies varies considerably. However, it can be concluded that interventions with human contact work better than those that are fully automated.
Features Enhancing Intervention Effect
Features linking effective interventions together were individual feedback, tailored content, self-monitoring, and the use of multiple intervention modalities. A common finding was that interventions with human contact performed better than fully automated interventions. The contact does not have to be time-consuming: Varela et al. found that weekly tailored feedback provided by a professional resulted in more favorable outcomes than no or fully automated feedback [32]. Furthermore, Berry et al. found that when combined with a fully automated intervention, a lighter amount of additional coaching led to greater weight loss results than more intensive coaching [39]. Additionally, personal support has been shown to increase adherence to eHealth interventions [44•, 45–47].
The Importance of Cultural and Social Awareness
In cultures that value community and interpersonal relationships, close contact with service providers may lead to more favorable outcomes and greater acceptance of interventions. For instance, Ang et al. [33••] discovered that mobile applications developed for Asian populations were typically culturally adapted and commonly enabled patient-professional communication, whereas applications designed for Western populations often relied on self-directed learning. The authors also emphasized the significance of proper localization, including culturally appropriate advice, locally adapted educational content, and the use of native language. Similarly, Rosenbaum et al. noted that racial minorities were more likely to enroll in trials that used both smartphones and in-person care, suggesting that these populations were seeking social support or a sense of connection to service providers [48].
Cultural and social awareness may also improve the feasibility of eHealth interventions when treating vulnerable groups such as immigrants, aging populations, people with low health literacy, or people with low socioeconomic status [4, 44•, 49, 50••, 51]. It is critical to successfully engage these populations in eHealth interventions because these may provide certain advantages over non-eHealth interventions. For example, because eHealth interventions are multimodal, they can deliver information not only in text but also in video or audio format. Moreover, they may alleviate the burden of limited transportation or caregiving duties [48]. eHealth can also enhance healthcare accessibility for previously underserved populations [52].
From Statistical Significance to Clinical Significance
The majority of trials included in the present reviews reported absolute change in weight (kg) or BMI (kg/m2). While results based on these measures may achieve statistical significance, their clinical significance remains uncertain, particularly given inconsistent reporting of participants’ weight at baseline. We need to know what to compare the results to in order to make meaningful assumptions about the clinical significance based on absolute weight change. For example, a 2 kg weight loss in a person who weighs 70 kg at baseline may be significant, but it is not in a person who weighs 120 kg at baseline.
One way to support the evaluation of clinical significance is to report relative weight change (Δ% from baseline). Although this approach is not without limitations, it does produce more useful results. A 5% weight loss is generally considered clinically significant, and even a 3% weight loss is likely to result in positive health changes [53]. Reporting relative weight change also mitigates distortion of final results caused by participants with widely different baseline weights. It acknowledges that people with higher baseline weights require greater absolute weight loss to achieve clinically significant relative weight loss.
We must also keep in mind that reported effect sizes from meta-analyses only show differences between groups, not how patients’ weight changed from baseline. For example, a mean difference only reflects the difference in means between groups, not the difference in means within groups from baseline. Similarly, when reporting standardized mean differences, the assumption should be that the differences in standard deviations are due to different measurement scales rather than real differences and high variability within study populations. Trials may include participants with a broader range of baseline characteristics, leading to higher standard deviation. Given that the included studies in this review exhibited high heterogeneity, it is reasonable to assume that at least some of the differences in standard deviation were due to this variability. Therefore, effect sizes alone do not provide a reliable estimate of the clinical impact of the interventions.
We Need More Research in Real-Life Settings
While randomized controlled trials are often regarded as the gold standard for intervention research, including non-randomized studies on intervention effects may help us understand how these interventions work in clinical practice. They may be more feasible when addressing long-term outcomes, outcomes in different populations or settings, or alternative methods of intervention delivery. We also know that retention rates may differ between trial and real-life interventions [54]. Subsequently, efficacy information based solely on RCTs conducted in non-real-world settings may not be widely applicable or generalizable when transitioning from trials to healthcare.
Several trials excluded participants with chronic diseases or underlying diagnoses. In reality, many patients with obesity also have concomitant or comorbid diseases such as hypertension, asthma, type 2 diabetes, or heart disease. A recent real-life cohort study by Kupila et al. with nearly 1300 patients enrolled in a digital obesity management program demonstrated that weight loss success was not affected by the number of concurrent diagnoses or medications [55•]. Enrolling a wider selection of patients would likely result in larger trial populations and more clinically generalizable data without introducing significant confounding factors.
Furthermore, we require more long-term research to ascertain the effects of eHealth interventions in managing obesity. Several studies included in the reviews had brief study and follow-up periods. Although these study designs may demonstrate if an eHealth intervention initially works, obesity care and weight management demand long-term and sustainable solutions that integrate into patients’ daily lives. Short-term study durations fail to provide information on how these interventions function in the long run or if their effects last.
We Need a Change from Theory to Practice
Examining a large body of evidence invites us to consider its practical implications. Many trials employ eHealth interventions created specifically for research purposes. While this approach provides valuable information about eHealth interventions themselves, it does not facilitate the much-needed step towards digitalization, unless interventions are developed for use in clinical practice.
The question “does it work?” has been extensively researched, but there is limited knowledge on “how does it work?”. Simply observing impact or efficacy does not help us develop more effective interventions. Many reviews explore and discuss various aspects of successful interventions. While this information is welcome, we need more trials examining underlying factors that influence intervention success. Weight loss outcomes may vary based on intervention details such as theoretical frameworks used, components, amount of human contact, or modes of delivery. They may also vary due to population differences such as age, socioeconomic status, gender, or baseline health status. We need to know what works and with whom in order to not only develop interventions but also deliver them to those who will most likely benefit from them. On the other hand, we must be able to identify those who may need more face-to-face support.
There is relatively little research on intervention acceptability. Not only is an effective intervention necessary, but users must also accept it as part of their care. Reviews have highlighted that eHealth interventions may work, but only if the users adhere. Developing interventions from top to bottom, from researchers and developers to end-users, may overlook any hidden needs or wishes of patients or professionals. This raises critical unanswered questions, such as which types of interventions patients and professionals believe will benefit them the most, what types of feedback or human contact will best support lifestyle change, and what will help users commit to the intervention and empower them to take agency over their own health and care?
Risk of Bias in the Included Studies
The majority of the included reviews excluded studies published in languages other than English. Furthermore, the vast majority of studies were conducted in the USA or Europe, which limits the generalizability of their findings to other populations. Although we searched the Global Index Medicus for potential sources published in low- to middle-income countries, we found none that met our inclusion criteria. Nevertheless, it is important to note that we identified thousands of original articles published in languages other than English when we searched with the same keywords but with no restrictions on publication type.
This suggests that existing systematic reviews may overlook a significant number of available publications by restricting their search language. Consequently, this may introduce language and publication bias and skew our understanding of the impact of eHealth innovations by only considering their impact in Western populations.
Most authors provided the number of full-text articles that were excluded and their reasons for doing so. However, only a few authors provided a list of articles excluded after full-text examination. This lack of transparency reduces the credibility of research findings. Therefore, we suggest that authors include a list of excluded articles in future reviews.
We know that a disproportionate number of weight loss intervention participants drop out during the intervention [56]. We did not examine the attrition rates of included reviews as this was not the purpose of this review. However, for an intervention to be effective, its participants must adhere to it. Weight loss results from only those who completed an intervention may be reported in the absence of an intent-to-treat analysis. When applied to a real-world situation, the outcomes will likely differ. Thus, if attrition rates were reported in the original trials, it would be beneficial to report them in future reviews. This would aid the reader in reaching a deeper understanding of the interventions in question.
Although most reviews with meta-analysis observed no significant publication bias, often concluded by visually inspecting forest plots, only a few reviews in this study compared eHealth research interventions to real-life interventions. This raises the question of whether this is due to a lack of study designs that allow for such comparisons or whether it is an indication of publication bias due to potentially unfavorable results. Furthermore, many reviews limited their search language to English or did not search for gray literature (research produced by organizations outside of the traditional commercial or academic publishing), potentially increasing publication bias. While it could be argued that these restrictions would not have affected the final results in a meaningful way, we have no way of knowing what the true impact would have been in the context of any individual review.
Heterogeneity in the Included Studies
The included reviews generally exhibited a high degree of heterogeneity. The terms “eHealth” and “digital health” lack clear definitions, leading authors to interpret them in their own ways. A single review could have included a variety of intervention modalities, such as phone or video calls, websites, DVDs, rapid messaging, mobile phone applications, or social media. Furthermore, the duration and intensity of the interventions varied, and the participants ranged in age, background, and health status.
With a more focused research question, the included interventions could be narrowed down, resulting in a more uniform review synthesis. This could also be accomplished with adequate subgroup analysis. Combining various interventions in meta-analyses produces non-generalizable results that cannot be interpreted in any specific context. This makes it difficult for clinicians and policymakers to make meaningful recommendations based on available research.
The interventions examined in these reviews were based on a variety of behavior change theories (e.g., the Social Cognitive Theory, the Self-Determination Theory, the Theory of Planned Behavior). It is plausible that frameworks that are effective in real-life settings are also applicable in virtual settings. However, as with most therapeutic interventions, the therapeutic alliance may be a more significant determinant of treatment outcomes than any particular theoretical model [57, 58].
Strengths and Limitations
We only included systematic reviews in this review because they follow a robust and reproducible methodology that aims to find all relevant trials available. Additionally, we screened the reference lists of other reviews of reviews, both systematic and non-systematic, to identify any additional reviews that could be included. With the large number of systematic reviews found, and our extensive search of gray literature, we are confident that our scope is sufficiently broad and comprehensive.
To ensure the reliability of our review, we followed a predetermined protocol and conducted both reference searches and data extraction in duplicate. We also aimed to report our review process and findings as transparently as possible, with the aid of the additional material included in the Appendix.
We included reviews published in 2018 or later. Although the majority of the trials included in these reviews were published relatively recently, a few were published as early as 2001. This could have partially influenced the results because we know that newer eHealth interventions, specifically those developed in the 2010s or later, yield better results than older interventions [31]. This could be attributed to the rise of digitalization, which has resulted in improved technology literacy and advancements in device and application design.
As previously discussed, the included reviews generally had a high or moderate risk of bias, as well as high heterogeneity among the included trials and results. This must be taken into consideration when interpreting the findings of this study.
Conclusion
The review indicates that eHealth interventions are more effective than control interventions or no care and comparable to face-to-face interventions. Notably, the most significant weight loss results have been observed in eHealth interventions that combine a digital program with personal counseling or coaching from a qualified professional, delivered either remotely or in-person. Common features of effective interventions include individual feedback, personalized content, self-monitoring, and the utilization of various intervention modalities. However, studies focusing on the maintenance of weight loss are limited, leaving a gap in knowledge regarding the long-term effectiveness of eHealth interventions for weight loss and weight loss maintenance.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
All authors contributed to the study conception and design. Sakris Kupila and Anu Joki performed the literature search and data extraction with Laura Suojanen resolving potential discrepancies. Sakris Kupila formatted the tables and figures. The first draft of the manuscript was written by Sakris Kupila and Anu Joki. All authors reviewed and edited the manuscript. All authors read and approved the final manuscript. The project was administered by Sakris Kupila and supervised by Kirsi Pietiläinen.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This study was supported by Academy of Finland, grant numbers 335443, 314383, 272376, 266286; Finnish Medical Foundation, grant number 5891; Gyllenberg Foundation; Novo Nordisk Foundation, grant numbers NNF20OC0060547, NNF17OC0027232, NNF10OC1013354; Finnish Diabetes Research Foundation; Finnish Foundation for Cardiovascular Research; Government Research Funds; Helsinki University Hospital; and University of Helsinki. The funders had no role in study design, data collection and analysis, Cmdecision to publish, or manuscript preparation.
Compliance with Ethical Standards
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/14/2023
A Correction to this paper has been published: 10.1007/s13679-023-00530-3
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. [DOI] [PMC free article] [PubMed]
- 2.World Health Organization. Obesity and overweight. [cited 2023 Apr 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 3.Varkevisser RDM, van Stralen MM, Kroeze W, Ket JCF, Steenhuis IHM. Determinants of weight loss maintenance: a systematic review. Obes Rev. 2019;20:171–211. doi: 10.1111/obr.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean B, Hossain N, Donison V, Gray M, Durbano S, Haase K, et al. Providing medical information to older adults in a web-based environment: systematic review. JMIR Aging. 2021;4:e24092. doi: 10.2196/24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madigan CD, Graham HE, Sturgiss E, Kettle VE, Gokal K, Biddle G, et al. Effectiveness of weight management interventions for adults delivered in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2022;377:e069719. doi: 10.1136/bmj-2021-069719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US preventive services task force. JAMA United States. 2018;320:1172–1191. doi: 10.1001/jama.2018.7777. [DOI] [PubMed] [Google Scholar]
- 7.Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle GR. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37–46. doi: 10.2147/DMSO.S89836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn M, Stathi A. Moral discourse in general practitioners’ accounts of obesity communication. Soc Sci Med. 2019;230:166–173. doi: 10.1016/j.socscimed.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn M, Stathi A, Keogh E, Eccleston C. Raising the topic of weight in general practice: perspectives of GPs and primary care nurses. BMJ Open. 2015;5:e008546. doi: 10.1136/bmjopen-2015-008546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Digital health. [cited 2023 Apr 26]. Available from: https://www.who.int/health-topics/digital-health.
- 13.Pappa GL, Cunha TO, Bicalho PV, Ribeiro A, Couto Silva AP, Meira W, et al. Factors associated with weight change in online weight management communities: a case study in the LoseIt Reddit community. J Med Internet Res. 2017;19:e17. doi: 10.2196/jmir.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentili A, Failla G, Melnyk A, Puleo V, Tanna GLD, Ricciardi W, et al. The cost-effectiveness of digital health interventions: a systematic review of the literature. Front Public Health. 2022;10. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85137319829&doi=10.3389%2ffpubh.2022.787135&partnerID=40&md5=61a716abe696bfa8b9926cc34e289fed. [DOI] [PMC free article] [PubMed]
- 15.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. The Lancet Diabetes & Endocrinology Elsevier. 2021;9:350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beleigoli AM, Andrade AQ, Cançado AG, Paulo MN, Diniz MDFH, Ribeiro AL. Web-based digital health interventions for weight loss and lifestyle habit changes in overweight and obese adults: systematic review and meta-analysis. Canada. J Med Internet Res. 2019;21:e298. [DOI] [PMC free article] [PubMed]
- 20.Berry R, Kassavou A, Sutton S. Does self-monitoring diet and physical activity behaviors using digital technology support adults with obesity or overweight to lose weight? A systematic literature review with meta-analysis. England. Obes Rev. 2021;22:e13306. [DOI] [PubMed]
- 21.Besson M, Gurviez P, Carins J. Using digital devices to help people lose weight: a systematic review. Emerald Publishing Limited. J Soc Mark. 2020;10:289–319.
- 22.Houser S, Joseph R, Puro N, Burke D. Use of technology in the management of obesity: a literature review. Perspect Health Inf Manag. 2019;16:1c. [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J-W, Lin Y-Y, Wu N-Y. The effectiveness of telemedicine on body mass index: a systematic review and meta-analysis. JOURNAL OF TELEMEDICINE AND TELECARE. 2019;25:389–401. doi: 10.1177/1357633X18775564. [DOI] [PubMed] [Google Scholar]
- 24.Jahangiry L, Farhangi MA. Obesity paradigm and web-based weight loss programs: an updated systematic review and meta-analysis of randomized controlled trials. Bangladesh. J Health Popul Nutr. 2021;40:16. [DOI] [PMC free article] [PubMed]
- 25.Lahtio H, Rintala A, Immonen J, Sjogren T. The Effectiveness of physical activity-promoting web- and mobile-based distance weight loss interventions on body composition in rehabilitation settings: systematic review, meta-analysis, and meta-regression analysis. Canada. J Med Internet Res. 2022;24:e25906. [DOI] [PMC free article] [PubMed]
- 26.Lau Y, Chee DGH, Chow XP, Cheng LJ, Wong SN. Personalised eHealth interventions in adults with overweight and obesity: a systematic review and meta-analysis of randomised controlled trials. United States. Prev Med. 2020;132:106001. [DOI] [PubMed]
- 27.Lee Y, Lee NY, Lim HJ, Sung S. Weight reduction interventions using digital health for employees with obesity: a systematic review. Diabetes Metab Syndr Obes. 2022;15:3121–3131. doi: 10.2147/DMSO.S384450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mata-Gonzalez E, Meza-Pena C, Garcia C. Intervention programs through the Internet for weight loss in adults with overweight and obesity: a systematic review. REVISTA ESPANOLA DE NUTRICION HUMANA Y DIETETICA. 2020;24:324–335. [Google Scholar]
- 29.Podina IR, Fodor LA. Critical review and meta-analysis of multicomponent behavioral e-health interventions for weight loss. Health Psychology US: American Psychological Association. 2018;37:501–515. doi: 10.1037/hea0000623. [DOI] [PubMed] [Google Scholar]
- 30.Rumbo-Rodriguez L, Sanchez-SanSegundo M, Ruiz-Robledillo N, Albaladejo-Blazquez N, Ferrer-Cascales R, Zaragoza-Marti A. Use of technology-based interventions in the treatment of patients with overweight and obesity: a systematic review. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed]
- 31.Shi Y, Wakaba K, Kiyohara K, Hayashi F, Tsushita K, Nakata Y. Effectiveness and components of web-based interventions on weight changes in adults who were overweight and obese: a systematic review with meta-analyses. Switzerland. Nutrients. 2022;15. [DOI] [PMC free article] [PubMed]
- 32.Varela C, Oda-Montecinos C, Andrés A, Saldaña C. Effectiveness of web-based feedback interventions for people with overweight and obesity: systematic review and network meta-analysis of randomized controlled trials. J Eat Disord. 2021;9:75. doi: 10.1186/s40337-021-00432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.•• Ang SM, Chen J, Liew JH, Johal J, Dan YY, Allman-Farinelli M, et al. Efficacy of interventions that incorporate mobile apps in facilitating weight loss and health behavior change in the Asian population: systematic review and meta-analysis. J Med Internet Res. 2021;23:e28185. Multicomponent interventions that incorporate apps produce a small to moderate effect in reducing weight, BMI, and waist circumference in Asian populations. Main factors for intervention success and adherence are patient-professional communication, self-monitoring, and cultural adaptation. [DOI] [PMC free article] [PubMed]
- 34.Chew HSJ, Koh WL, Ng JSHY, Tan KK. Sustainability of weight loss through smartphone apps: systematic review and meta-analysis on anthropometric, metabolic, and dietary outcomes. Canada. J Med Internet Res. 2022;24:e40141. [DOI] [PMC free article] [PubMed]
- 35.Dounavi K, Tsoumani O. Mobile health applications in weight management: a systematic literature review. American Journal of Preventive Medicine Elsevier. 2019;56:894–903. doi: 10.1016/j.amepre.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Islam MM, Poly TN, Walther BA, Jack Li Y-C. Use of mobile phone app interventions to promote weight loss: meta-analysis. Canada. JMIR Mhealth Uhealth. 2020;8:e17039. [DOI] [PMC free article] [PubMed]
- 37.Puigdomenech Puig E, Robles N, Saigi-Rubio F, Zamora A, Moharra M, Paluzie G, et al. Assessment of the efficacy, safety, and effectiveness of weight control and obesity management mobile health interventions: systematic review. JMIR Mhealth Uhealth. 2019;7. [DOI] [PMC free article] [PubMed]
- 38.• Antoun J, Itani H, Alarab N, Elsehmawy A. The effectiveness of combining nonmobile interventions with the use of smartphone apps with various features for weight loss: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2022;10:e35479. Mobile phone applications have a role in weight loss management, especially when used in conjunction with usual care. Combining an mHealth intervention with human-delivered coaching led to most favorable weight loss results at 6 months. [DOI] [PMC free article] [PubMed]
- 39.Berry MP, Sala M, Abber SR, Forman EM. Incorporating automated digital interventions into coach-delivered weight loss treatment: a meta-analysis. Health Psychology US: American Psychological Association. 2021;40:534–545. doi: 10.1037/hea0001106. [DOI] [PubMed] [Google Scholar]
- 40.Novaes Oliveira AC, Menezes Guariente SM, Zazula R, Mesas AE, Coral Oliveira CE, Vissosi Reiche EM, et al. Hybrid and remote psychosocial interventions focused on weight and sedentary behavior management among patients with severe mental illnesses: a systematic review. Psychiatr Q. 2022;93:813–840. doi: 10.1007/s11126-022-09994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Boyle J, Davidson P. The effects of mhealth versus eHealth on weight loss in adults: a systematic review. Top Clin Nutr. 2022;37:69. doi: 10.1097/TIN.0000000000000268. [DOI] [Google Scholar]
- 42.Holmes WS, Moorhead SA, Coates VE, Bond RR, Zheng H. Impact of digital technologies for communicating messages on weight loss maintenance: a systematic literature review. Eur J Pub Health. 2019;29:320–328. doi: 10.1093/eurpub/cky171. [DOI] [PubMed] [Google Scholar]
- 43.Mamalaki E, Poulimeneas D, Tsiampalis T, Kouvari M, Karipidou M, Bathrellou E, et al. The effectiveness of technology-based interventions for weight loss maintenance: a systematic review of randomized controlled trials with meta-analysis. England. Obes Rev. 2022;23:e13483. [DOI] [PubMed]
- 44.• Arsenijevic J, Tummers L, Bosma N. Adherence to electronic health tools among vulnerable groups: systematic literature review and meta-analysis. Canada. J Med Internet Res. 2020;22:e11613. eHealth interventions with multimodal content and direct communication between patients and healthcare providers increase intervention adherence among vulnerable groups. [DOI] [PMC free article] [PubMed]
- 45.Jakob R, Harperink S, Rudolf AM, Fleisch E, Haug S, Mair JL, et al. Factors influencing adherence to mHealth apps for prevention or management of noncommunicable diseases: systematic review. J Med Internet Res. 2022;24. [DOI] [PMC free article] [PubMed]
- 46.Asbjornsen RA, Smedsrod ML, Nes LS, Wentzel J, Varsi C, Hjelmesaeth J, et al. Persuasive system design principles and behavior change techniques to stimulate motivation and adherence in electronic health interventions to support weight loss maintenance: scoping review. J Med Internet Res. 2019;21. [DOI] [PMC free article] [PubMed]
- 47.Mohr D, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to ehealth interventions. J Med Internet Res. 2011;13:e1602. doi: 10.2196/jmir.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenbaum DL, Piers AD, Schumacher LM, Kase CA, Butryn ML. Racial and ethnic minority enrollment in randomized clinical trials of behavioural weight loss utilizing technology: a systematic review. Obes Rev. 2017;18:808–817. doi: 10.1111/obr.12545. [DOI] [PubMed] [Google Scholar]
- 49.Bodie GD, Dutta MJ. Understanding health literacy for strategic health marketing: eHealth literacy, health disparities, and the digital divide. Health Mark Q. 2008;25:175–203. doi: 10.1080/07359680802126301. [DOI] [PubMed] [Google Scholar]
- 50.•• Sung M, He J, Zhou Q, Chen Y, Ji JS, Chen H, et al. Using an integrated framework to investigate the facilitators and barriers of health information technology implementation in noncommunicable disease management: systematic review. J Med Internet Res. 2022;24:e37338. eHealth interventions are convenient, improve the quality of care, and increase the accessibility of healthcare. Implementation success is facilitated by well-designed applications with easy usability as well as social support and communication with healthcare providers. [DOI] [PMC free article] [PubMed]
- 51.Coupe N, Cotterill S, Peters S. Tailoring lifestyle interventions to low socio-economic populations: a qualitative study. BMC Public Health. 2018;18:967. doi: 10.1186/s12889-018-5877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasselli JR, Juray S, Trasino SE. Success and failures of telehealth during COVID-19 should inform digital applications to combat obesity. Obes Sci Pract. 2021 doi: 10.1002/osp4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, et al. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors’ expert forum. Diabetes Care. 2015;38:1567–1582. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgess E, Hassmén P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clinical Obesity. 2017;7:123–135. doi: 10.1111/cob.12183. [DOI] [PubMed] [Google Scholar]
- 55.Kupila SKE, Venäläinen MS, Suojanen L-U, Rosengård-Bärlund M, Ahola AJ, Elo LL, et al. Weight loss trajectories in healthy weight coaching: cohort study. JMIR Form Res. 2022;6:e26374. doi: 10.2196/26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colombo O, Ferretti VVV, Ferraris C, Trentani C, Vinai P, Villani S, et al. Is drop-out from obesity treatment a predictable and preventable event? Nutr J. 2014;13:13. doi: 10.1186/1475-2891-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flückiger C, Del Re AC, Wampold BE, Horvath AO. The alliance in adult psychotherapy: a meta-analytic synthesis. Psychotherapy (Chic) 2018;55:316–340. doi: 10.1037/pst0000172. [DOI] [PubMed] [Google Scholar]
- 58.Sturgiss EA, Sargent GM, Haesler E, Rieger E, Douglas K. Therapeutic alliance and obesity management in primary care – a cross-sectional pilot using the Working Alliance Inventory. Clinical Obesity. 2016;6:376–379. doi: 10.1111/cob.12167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.