Abstract
Objectives
To compare outcomes of femtosecond-enabled deep anterior lamellar keratoplasty (FE-DALK) and standard deep anterior lamellar keratoplasty (S-DALK).
Methods
An open label, randomized controlled trial (Kensington Eye Institute, Toronto, ON, Canada) including 100 eyes of 97 participants with either keratoconus or corneal scarring, randomized to either FE-DALK (n = 48) or S-DALK (n = 49). Primary outcomes: postoperative astigmatism and surgically induced corneal astigmatism (SIA) – both at 15 months. Secondary outcomes: 6-, 12- and 15-month postoperative uncorrected- and best spectacle-corrected visual acuity, steep and flat keratometry, manifest sphere and astigmatism, rate of conversion to penetrating keratoplasty (PK), big-bubble success, central corneal thickness, endothelial cell count and complications.
Results
In intention-to-treat analysis, mean postoperative astigmatism in the FE-DALK (n = 30) and S-DALK (n = 30) groups at 15 months was 7.8 ± 4.4 D and 6.3 ± 5.0 D, respectively (p = 0.282) with an adjusted mean difference of 1.3 D (95% CI −1.08, +3.65). Mean SIA (arithmetic) was 9.2 ± 7.8 and 8.8 ± 5.4 D, respectively (p = 0.838) with a mean difference of 0.4 D (95% CI −3.13, +3.85). In an analysis of successful DALK cases only, mean postoperative astigmatism in the FE-DALK (n = 24) and S-DALK (n = 20) groups at 15 months (after excluding 4 eyes with AEs) was 7.3 ± 4.4 and 6.2 ± 4.9 D, respectively (p = 0.531) with an adjusted mean difference of 0.9 D (95% CI −1.94, +3.71). Mean SIA (arithmetic) was 9.1 ± 7.8 and 7.9 ± 4.6 D, respectively (p = 0.547) with a mean difference of 1.2 D (95% CI −2.70,+5.02). Comparison of secondary outcomes showed only weak statistical evidence.
Conclusions
In this randomized controlled trial, FE-DALK and S-DALK showed comparable functional and anatomical outcomes.
Subject terms: Outcomes research, Transplantation
Introduction
Penetrating keratoplasty (PK) has been performed for over 100 years to improve vision and comfort in patients suffering from severe corneal diseases. A full-thickness procedure such as PK can lead to several complications of which suprachoroidal haemorrhage, wound dehiscence and corneal endothelial rejection are the most devastating. For corneal pathologies that are limited to the stroma, deep anterior lamellar keratoplasty (DALK) enables replacement of diseased stroma while preserving the host endothelium. DALK is more technically challenging to perform [1], but has the advantages over PK of reduced intraoperative complications, and no risk of postoperative endothelial rejection [2, 3]. DALK has therefore become the gold standard in treatment of common corneal stromal diseases such as keratoconus and corneal scarring [4].
Similar to PK, visual recovery following DALK may be prolonged, with substantial rates of high regular and irregular postoperative astigmatism which can significantly affect outcomes. Femtosecond laser technology in corneal transplantation is becoming more common since it enables the creation of precise incisions in the host and donor tissues as well as customized shelved incision profiles. Femtosecond-assisted PK has been shown to promote faster wound healing and quicker visual recovery compared with manual PK [5, 6]. Retrospective studies comparing femtosecond-enabled DALK (FE-DALK) to standard DALK (S-DALK), report faster visual recovery following FE-DALK due to superior wound healing. However, there are no conclusive findings regarding differences in final outcomes between the two techniques [7–10].
The purpose of this study was to compare outcomes of S-DALK and FE-DALK. To the best of our knowledge, this is the first prospective randomized controlled trial comparing S-DALK to FE-DALK.
Methods
Study design
A single-centre, open label, randomized controlled trial comparing FE-DALK and S-DALK.
Study participants
Included were patients 18–70 years old who had either significant and visually limiting corneal irregularity secondary to advanced keratoconus or corneal scarring of any aetiology (including keratoconus), who needed to undergo a DALK procedure.
Exclusion criteria included previous keratoplasty, advanced glaucoma, any retinal or optic nerve pathology that could potentially affect vision, strabismus, amblyopia, significant limbal stem cell deficiency (involving more than 3 limbal clock hours), pregnancy or breastfeeding, and active autoimmune disease.
One-hundred eyes of 97 participants were recruited between 2015 and 2017, and after providing informed consent were randomized to either FE-DALK (n = 50) or S-DALK (n = 50). Only one eye from each participant was included in the analysis (FE-DALK, n = 48; S-DALK, n = 49).
The study was approved by the University of Toronto Research Ethics Board (protocol no. 30457) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants. The study was registered in the clinicaltrials.gov register (identifier NCT03732599; available at: clinicaltrials.gov).
Procedures
Eyes were randomized using random permuted blocks of size 2 and 4 to FE-DALK arm or S-DALK arm at a 1:1 ratio. Block sizes were concealed from study staff, thereby maintaining allocation concealment. Randomization was centralized, web-based, and generated by the trial statistician at the Applied Health Research Centre (AHRC) at the Li Ka Shing Knowledge Institute at St Michaels Hospital, Unity Health.
Preoperative assessment for eligibility was performed no more than 3 weeks prior to the DALK procedure.
Five corneal surgeons were involved. All surgeries and study procedures were performed at the Kensington Eye Institute (Toronto, ON, Canada). Postoperative assessments were performed at 6, 12 and 15 months. Additional standard of care follow-up was also performed at each surgeon’s office. Surgeons were permitted to use their discretion as to when to remove corneal sutures to minimize postoperative corneal astigmatism.
Surgical techniques
All donor tissue used was stored in corneal storage solution (Optisol; Bausch & Lomb, Rochester, NY, USA) and was received from the Eye Bank of Canada, Ontario Division.
Standard DALK (S-DALK)
The donor tissue was trephined using a Barron donor vacuum punch (Katena Inc, Denville, NJ) in a straight-cut configuration, and was stripped of its Descemet’s membrane. Diameter was chosen by the surgeon according to corneal diameter measurements obtained using a surgical calliper. The donor and recipient were same-sized. Removal of recipient stroma was performed using a modified version of the “big bubble” technique described initially by Anwar et al. [11]. In cases where a partial big bubble was created, Melles technique was used to complete the stromal dissection [12]. The donor button was secured using sixteen 10–0 nylon sutures.
Femtosecond-enabled DALK (FE-DALK)
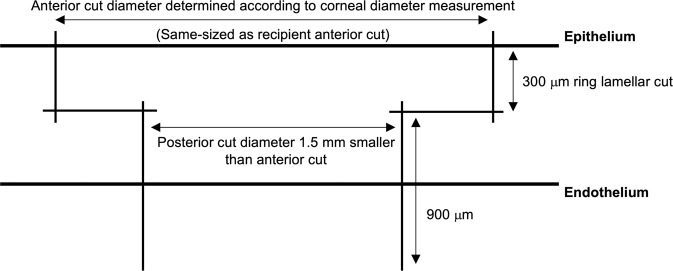
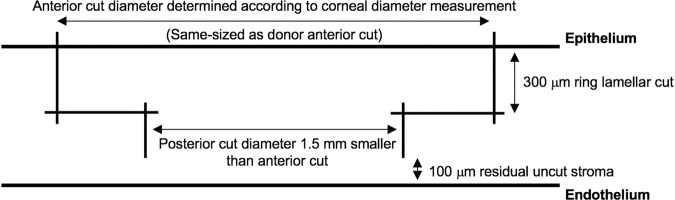
The surgical technique has been previously described by our group [9, 10, 13]. Briefly, the donor cornea, mounted on a Barron artificial anterior chamber (Katena Inc, Denville, NJ), was cut first using the 150-kHz IntraLase iFS femtosecond platform (Johnson & Johnson, New Brunswick, NJ, USA). A full-thickness mushroom-configuration incision was made in the donor cornea, along with 8 radial alignment incisions. The thickness of the anterior mushroom step (ring lamellar cut) was set to a standard depth of 300 μm. The diameter of the anterior step was chosen by the surgeon according to corneal diameter measurements (white-to-white measurements obtained using a surgical calliper). The depth of the posterior side cut (mushroom’s stalk) in the donor cornea was set at 1200 μm from the epithelium (900 μm deeper than the ring lamellar cut) to ensure a full-thickness graft incision (Fig. 1). The alignment incisions were highlighted using a sterile surgical marking pen, and the corneal button was removed from the rim and kept in storage media. Next, the recipient’s mushroom-configuration shaped non-penetrating cut was created along with 8 radial alignment incisions, ensuring that the depth of the posterior side cut (mushroom’s stalk) left at least 100 μm depth of uncut residual stromal bed (calculated based on ultrasound pachymetry measurements at 8 locations over the approximate area of the proposed incision). The thickness and diameter of the anterior step (ring lamellar cut) were similar to those of the donor. Vertical and lamellar cuts in both the donor and the recipient overlapped by 10 μm horizontally and 30 μm vertically, to eliminate the chance of an incomplete cut (Fig. 2). An angulation of 90 degrees was used for all cuts. The donor and recipient were same-sized.
Fig. 1. Femtosecond laser settings for the creation of a full-thickness mushroom configuration in the donor cornea.
Vertical cuts overlap 10 μm horizontally and 30 μm vertically with the horizontal lamellar cut at a 90° angulation to the corneal surface to ensure completely cut intersecting wound edges. The ring lamellar cut is made at a depth of 300 μm. The posterior side-cut depth is set at 1200 μm from the epithelium (or 900 μm deep from the ring lamellar cut).
Fig. 2. Femtosecond laser settings for the creation of a partial thickness mushroom configuration in the recipient cornea.
Vertical cuts overlap 10 μm horizontally and 30 μm vertically with the horizontal lamellar cut at a 90° angulation to the corneal surface to ensure completely cut intersecting wound edges. The ring lamellar cut is made at a depth of 300 μm. The posterior side-cut depth is calculated by subtracting 100 μm of residual corneal stroma from the thinnest corneal pachymetry.
After transporting the participant from the laser room to the operating room, a 4-mL sub-Tenon’s or retrobulbar block was given with a 1:1 mixture of xylocaine 2% and bupivacaine 0.5%. The femtosecond anterior side cut and lamellar plane were identified in the recipient using gentle traction with 0.12 forceps. The anterior lamella was retracted, and a Fogla DALK dissector (Bausch & Lomb, Rochester, NY, USA) was inserted into the deep stroma from the posterior edge of the posterior side cut and advanced toward the paracentral cornea. The dissector was retracted and a Tan DALK cannula (ASICO, Westmont, IL, USA), attached to a 5-mL syringe with air, was then advanced along the tract. Air was forcefully injected until a big bubble extending to the keratotomy was achieved. The anterior lamella was then dissected and removed. The big bubble was punctured and viscoelastic was injected into the bubble. Scissors were used to remove the remaining overlying stroma, leaving Descemet’s membrane intact. In cases where a partial big bubble was created, Melles technique was used to manually complete the stromal dissection [12]. The donor button was secured using sixteen 10–0 nylon sutures.
Main outcome measures
The primary outcome measure was postoperative astigmatism at 15 months. A post-hoc analysis was also performed for surgically induced astigmatism (SIA) at 15 months to account for the vector component of astigmatism. Secondary outcomes included SIA at 6 and 12 months, as well as 6-, 12- and 15-month best spectacle-corrected visual acuity (BSCVA) and uncorrected visual acuity (UCVA), steep & flat keratometry, manifest sphere & astigmatism, central corneal thickness (CCT), endothelial cell count (ECC), rates of conversion to penetrating keratoplasty (PK), big-bubble success and complications.
Measurements and data
At the preoperative assessment and at 6, 12 and 15 months postoperatively, we obtained UCVA and BSCVA using a 4 m Early Treatment Diabetic Retinopathy Study (ETDRS) chart (CSV 1000, VectorVision), manifest refraction, slit-lamp biomicroscopy, tonometry (Tonopen, Reichert Technology, Buffalo, NY, USA), corneal tomography (Pentacam; Oculus Optikgeräte GmbH, Wetzlar, Germany), ultrasound pachymetry (PachPen Pachymeter 24–5100, Accutome Inc., Malvern, PA, USA), specular microscopy and endothelial cell count (Robo KSS 300; Konan Medical, Hyogo, Japan).
Ocular history was documented at the preoperative assessment. Treatment-related unanticipated adverse events (AEs) were captured at study visits or through reports from the surgeons.
Data were recorded in REDCap (Vanderbilt University, Nashville, TN). Corneal tomography data were electronically exported directly to Microsoft Excel (Microsoft Corporation, Redmond, WA).
Statistical analysis
One eye from each participant was used in the analysis. With a sample size of 50 in each group, a conversion rate to PK of 30% and a power of 80%, this study was designed to detect a difference of 1.5D and more of postoperative astigmatism between the two groups. To calculate change in astigmatism, a post-hoc astigmatism vector analysis was performed at the corneal plane (vertex of 12 mm) using the Alpins method to produce mean values (arithmetic) of SIA and SIA single-angle vector graphs [14]. Postoperative astigmatism at 15 months was analyzed by baseline-adjusted linear regression (ANCOVA). Baseline-adjusted mean differences and 95% confidence intervals were estimated. Since the SIA calculation includes the baseline value, there is no need to adjust for baseline. Therefore, SIA values were compared between the groups using a two-sample t-test and the mean difference and 95% confidence intervals were estimated. In the secondary-outcome analysis, categorical variables were compared using chi-square test, and continuous variables were compared (at 6, 12 and 15 months) using a linear mixed effects model with treatment group, visit, treatment-by-visit interaction, and baseline value as fixed effects, except for SIA outcomes that did not have a baseline. These models permitted estimation of the treatment effects at 6, 12 and 15 months along with 95% confidence intervals. Likelihood ratio tests tested for an overall treatment effect by comparing with a model excluding treatment and the time by treatment interaction and tested for an interaction effect by comparing to a main effects model. The threshold for statistical significance was defined as a p-value < 0.05 for the primary outcome only. P-values are reported for all analyses as measures of the evidence against null treatment effects. No corrections for multiple testing are applied since all p-values are reported giving the necessary context for interpretation without resorting to multiple hypothesis tests.
Results
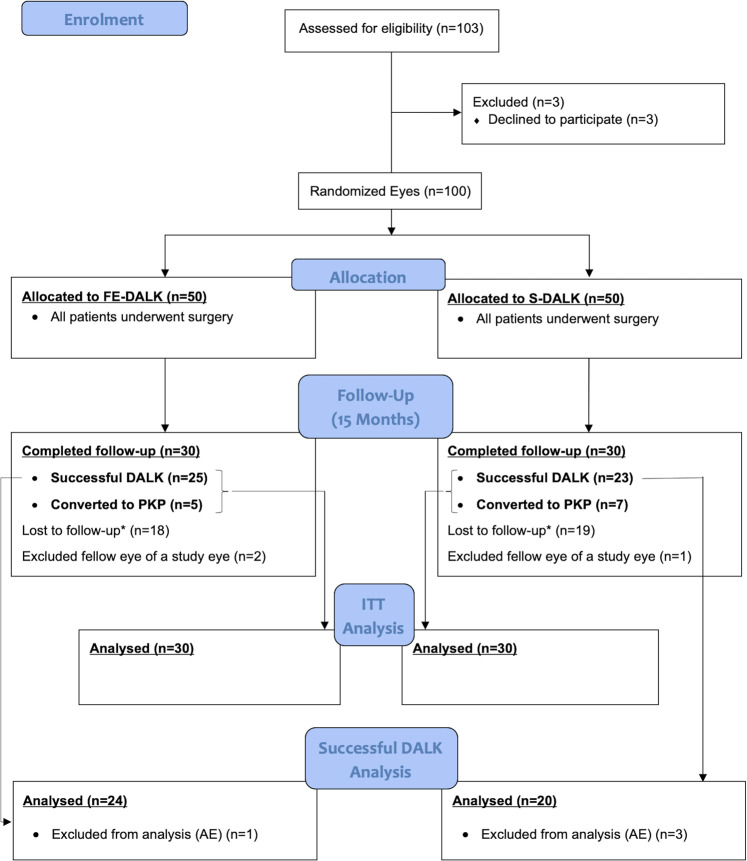
A total of 97 eyes of 97 participants were included in the analysis (FE-DALK, n = 48; S-DALK n = 49). Figure 3 illustrates the flow of participants through the study [15].
Fig. 3. CONSORT Flow Diagram.
Flow of participants through the study.
Demographics and baseline characteristics of the two groups are shown in Table 1.
Table 1.
Demographics and baseline patient characteristics of the femtosecond-enabled deep anterior lamellar keratoplasty (FE-DALK) and standard deep anterior lamellar keratoplasty (S-DALK) groups.
| FE-DALK (n = 48) | S-DALK (n = 49) | |
|---|---|---|
| Age (years) [mean ± SD] | 40.5 ± 13.6 | 37.3 ± 9.8 |
| Gender – Male [n (%)] | 29 (62%) | 27 (55%) |
| Laterality – Right [n (%)] | 23 (48%) | 21 (45%) |
| Surgery indication | ||
| Keratoconus [n (%)] | 40 (83%) | 39 (80%) |
| Scarring [n (%)] | 8 (17%) | 10 (20%) |
| Uncorrected visual acuity (logMAR) [mean ± SD] | 1.12 ± 0.37 | 1.14 ± 0.38 |
| Best spectacle-corrected visual acuity (logMAR) [mean ± SD] | 0.82 ± 0.36 | 0.79 ± 0.33 |
| Steep keratometry (D) [mean ± SD] | 64.1 ± 12.5 | 62.6 ± 12.5 |
| Flat keratometry (D) [mean ± SD] | 57.8 ± 12.1 | 56.8 ± 11.8 |
| Corneal astigmatism (D) [mean ± SD] | 6.3 ± 6.3 | 5.8 ± 3.5 |
| Manifest sphere (D) [mean ± SD] | 8.8 ± 6.4 | 8.3 ± 7.1 |
| Manifest astigmatism (D) [mean ± SD] | 3.1 ± 2.4 | 3.2 ± 2.2 |
| Intraocular pressure (mmHg) [mean ± SD] | 12.4 ± 4.6 | 11.9 ± 3.8 |
| Central corneal thickness (μm) [mean ± SD] | 467 ± 88 | 461 ± 91 |
FE-DALK femtosecond-enabled deep anterior lamellar keratoplasty, S-DALK standard deep anterior lamellar keratoplasty.
Postoperative astigmatism
Data for ITT analysis (15 months) were available for 60 eyes (FE-DALK, n = 30; S-DALK n = 30). Mean postoperative astigmatism was 7.8 ± 4.4 D in the FE-DALK group and 6.3 ± 5.0 D in the S-DALK group. The adjusted mean difference between the groups was 1.3 D (95% CI −1.08, +3.65; p = 0.282).
Data for analysis (15 months) of successful DALK cases that did not convert to PK were available for 48 eyes (FE-DALK, n = 25; S-DALK n = 23). Following exclusion of 4 eyes with AEs considered to affect primary outcome, the mean postoperative astigmatism was 7.3 ± 4.4 D in the FE-DALK group (n = 24) and 6.2 ± 4.9 D in the S-DALK group (n = 20). The adjusted mean difference between the groups was 0.9 D (95% CI −1.94, +3.71; p = 0.531).
Surgically induced astigmatism (SIA)
Data for ITT analysis (15 months) were available for 59 eyes (FE-DALK, n = 30; S-DALK n = 29). Mean SIA (arithmetic) was 9.2 ± 7.8 D in the FE-DALK group and 8.8 ± 5.4 D in the S-DALK group. The mean difference between the groups was 0.4 D (95% CI −3.13, +3.85; p = 0.838).
Data for analysis (15 months) of successful DALK cases that did not convert to PK were available for 47 eyes (FE-DALK, n = 25; S-DALK n = 22). Following exclusion of 3 eyes with AEs considered to affect the primary outcome, the mean SIA (arithmetic) was 9.1 ± 7.8 D in the FE-DALK group (n = 24) and 7.9 ± 4.6 D in the S-DALK group (n = 20). The mean difference between the groups was 1.2 D (95% CI −2.70, +5.02; p = 0.547). The SIA analysis was repeated in the FE-DALK group after removal of one outlier (an eye with preoperative astigmatism of 37.7 D, leading to SIA value of 39.2 D). Following removal of the outlier, the mean SIA (arithmetic) was 7.8 ± 4.6 D in the FE-DALK group (n = 23). Supplemental Fig. 1 (online only) depicts single-angle vector SIA analysis in the FE-DALK and S-DALK groups. The mean corneal vectors at 15 months were 1.27 D at 51 degrees in FE-DALK and 4.20 D at 114 degrees in S-DALK.
Conversion to PK and big-bubble success
Rate of conversion to PK was 15 of 48 eyes (31%) in the FE-DALK group and 18 of 49 eyes (37%) in the S-DALK group (p = 0.722).
Among eyes that converted to PK, the percentage of eyes with corneal scarring was 6 of 33 eyes (18%). Among eyes with successful DALK (not converted to PK), the percentage of eyes with corneal scarring was 12 of 64 eyes (19%). When separating successful DALK cases into FE-DALK and S-DALK groups, the percentage of eyes with corneal scarring was 6 of 33 (18%) and 6 of 31 (19%), respectively.
In eyes with successful DALK, the rate of big-bubble success was 22 of 33 eyes (67%) in the FE-DALK group and 16 of 31 eyes (52%) in the S-DALK group (p = 0.262).
Secondary outcomes
Linear mixed modelling over 6, 12 and 15 months of mean postoperative astigmatism and arithmetic SIA yielded similar results with slightly smaller estimated differences at 15 months (Table 2).
Table 2.
Postoperative secondary outcomes compared between the FE-DALK and S-DALK groups.
| FE-DALK Mean ± SD (n) |
S-DALK Mean ± SD (n) |
Adjusted difference FE-DALK - S-DALK (95% CI) |
p-Value (Overall)a |
p-Value (Interaction)b |
|
|---|---|---|---|---|---|
| Mean postoperative astigmatism (D) | |||||
| 6 months | 7.1 ± 3.8 (28) | 7.2 ± 3.6 (23) | −0.63 (−2.80, +1.55) | 0.289 | 0.187 |
| 12 months | 7.3 ± 4.0 (24) | 5.0 ± 2.2 (19) | +1.66 (−0.68, +3.99) | ||
| 15 months | 7.5 ± 4.5 (25) | 6.3 ± 4.7 (23) | +0.78 (−1.44, +3.00) | ||
| Surgically induced astigmatism (D) | |||||
| 6 months | 12.4 ± 7.0 (16) | 7.8 ± 3.5 (13) | +2.48 (−1.46, +6.42) | 0.573 | 0.495 |
| 12 months | 9.9 ± 7.7 (20) | 8.4 ± 4.0 (16) | +1.02 (−2.75, +4.80) | ||
| 15 months | 8.8 ± 8.8 (25) | 7.9 ± 4.4 (22) | +0.89 (−2.70, +4.47) | ||
| Uncorrected visual acuity (logMAR) | |||||
| 6 months | 0.90 ± 0.33 (27) | 0.79 ± 0.27 (23) | +0.076 (−0.121, +0.274) | 0.809 | 0.940 |
| 12 months | 0.88 ± 0.41 (26) | 0.79 ± 0.31 (19) | +0.047 (−0.163, +0.258) | ||
| 15 months | 0.91 ± 0.43 (25) | 0.81 ± 0.37 (20) | +0.085 (−0.120, +0.291) | ||
| Best spectacle-corrected visual acuity (logMAR) | |||||
| 6 months | 0.54 ± 0.27 (27) | 0.43 ± 0.17 (23) | +0.042 (−0.122, +0.206) | 0.172 | 0.333 |
| 12 months | 0.56 ± 0.31 (25) | 0.41 ± 0.26 (20) | +0.120 (−0.050, +0.290) | ||
| 15 months | 0.57 ± 0.36 (25) | 0.37 ± 0.29 (22) | +0.170 (+0.003, +0.337) | ||
| Steep keratometry (D) | |||||
| 6 months | 47.7 ± 3.2 (28) | 49.4 ± 3.0 (23) | −1.77 (−3.47, −0.07) | 0.057 | 0.035 |
| 12 months | 49.0 ± 3.0 (24) | 48.3 ± 2.6 (19) | +0.42 (−1.38, +2.22) | ||
| 15 months | 48.4 ± 3.2 (25) | 48.5 ± 2.8 (23) | −0.32 (−2.05, +1.40) | ||
| Flat keratometry (D) | |||||
| 6 months | 40.6 ± 3.6 (28) | 42.2 ± 2.5 (23) | −0.99 (−3.20, +1.22) | 0.786 | 1.000 |
| 12 months | 41.8 ± 3.9 (24) | 43.2 ± 3.1 (19) | −1.00 (−3.31, +1.31) | ||
| 15 months | 40.8 ± 4.2 (25) | 42.3 ± 5.3 (23) | −0.98 (−3.22, +1.25) | ||
| Manifest sphere (D) | |||||
| 6 months | −5.9 ± 5.1 (27) | −5.5 ± 5.5 (23) | +0.59 (−2.23, +3.40) | 0.885 | 0.811 |
| 12 months | −6.6 ± 5.1 (25) | −6.7 ± 5.8 (20) | +1.01 (−1.91, +3.93) | ||
| 15 months | −6.0 ± 5.6 (24) | −4.9 ± 5.6 (22) | +0.02 (−2.89, +2.92) | ||
| Manifest astigmatism (D) | |||||
| 6 months | 4.1 ± 2.9 (25) | 4.4 ± 2.4 (21) | −0.22 (−1.66, +1.22) | 0.956 | 0.854 |
| 12 months | 4.1 ± 2.7 (24) | 3.9 ± 2.1 (20) | +0.26 (−1.21, +1.74) | ||
| 15 months | 4.4 ± 2.5 (24) | 4.3 ± 2.0 (22) | −0.12 (−1.55, +1.32) | ||
| Central corneal thickness (μm) | |||||
| 6 months | 572 ± 79 (28) | 551 ± 34 (23) | +16.8 (−14.5, +48.1) | 0.220 | 0.148 |
| 12 months | 583 ± 72 (24) | 561 ± 34 (18) | +16.0 (−15.9, +48.0) | ||
| 15 months | 564 ± 31 (25) | 574 ± 40 (22) | +2.1 (−29.4, +33.6) | ||
| Endothelial cell count (cells/mm2) | |||||
| 6 months | 2482 ± 537 (22) | 2564 ± 438 (22) | −253.5 (−639.6, +132.5) | 0.419 | 0.265 |
| 12 months | 2543 ± 311 (22) | 2611 ± 311 (17) | −27.5 (−405.0, +350.0) | ||
| 15 months | 2573 ± 312 (22) | 2602 ± 300 (17) | +131.7 (−233.8, +497.2) | ||
FE-DALK femtosecond-enabled deep anterior lamellar keratoplasty, S-DALK standard deep anterior lamellar keratoplasty.
aOverall difference (treatment and time by treatment interaction combined).
bTime by treatment interaction only.
Comparison of the remaining secondary outcomes showed only weak statistical evidence over 6, 12 and 15 months of follow-up, except possibly for steep keratometry values over the follow-up period (Table 2). However, this one difference appears to be the result of a difference at the 6-month time point, but the direction of difference is inconsistent among the time-points suggesting extreme caution in interpretation of this result.
Safety/adverse events
In the S-DALK group, there was one case that converted to PK where during the “open sky” portion of the procedure the crystalline lens was expulsed due to elevated posterior pressure. The surgeon was able to complete the PK procedure, and the presence of a suprachoroidal haemorrhage was ruled out by a postoperative ultrasound scan performed shortly after the procedure. At the time of study conclusion, the eye was aphakic with a clear graft. In the S-DALK group, there was one case of a partial Descemet’s membrane detachment which resolved completely, and one case of a primary graft failure. In the FE-DALK group, there was one case of wound dehiscence which was successfully managed with corneal graft re-suturing. There were no cases of graft rejection or secondary graft failure.
Discussion
Femtosecond laser technology has been incorporated into corneal transplantation surgery to improve visual outcomes and speed visual recovery. Previous retrospective studies comparing visual outcomes of FE-DALK and S-DALK had conflicting results, with some showing faster wound healing and visual recovery, and others demonstrating no significant differences in visual outcomes between the two techniques [7–9]. To the best of our knowledge, this study is the first randomized controlled trial comparing the two. No significant differences were found between FE-DALK and S-DALK in terms of keratometric and visual outcomes, nor any other outcome measure.
Postoperative astigmatism is a significant factor affecting visual outcomes after keratoplasty. Values of postoperative astigmatism and mean arithmetic SIA did not differ significantly between FE-DALK and S-DALK at 6, 12 and 15 months after surgery, indicating similar astigmatic outcomes. However, looking at the 15-month vector analysis, it appears that the mean SIA corneal vector value was lower in FE-DALK compared with S-DALK (Supplemental Fig. 1 – online only). This indicates that the direction of surgically induced astigmatism in FE-DALK was quite evenly distributed. In S-DALK, however, the induction of astigmatism tended to occur around the 114-degree axis, as evident by the larger mean corneal vector value directed along this axis at 15 months postoperatively. The reason for this difference between the two techniques is unclear. One possible explanation could be that manual recipient trephination is less symmetric than femtosecond trephination. Manual trephination is always completed using scissors. Therefore, certain areas may be more ergonomically difficult for the surgeon to cut than others, producing a consistent deformation which could lead to induced astigmatism in a more consistent axis as was evident in the S-DALK group. Another possible explanation may be related to the fact that FE-DALK was in a mushroom configuration while S-DALK was in a straight-cut configuration. Use of a mushroom configuration, especially one that is created by laser on both the donor and recipient, allows better alignment of host and donor tissues with less chance of step formation or tissue slippage at the graft-host junction. Some areas are more prone to graft-host mismatch, such as the inferior thinner host cornea in keratoconus patients, and in such cases, a consistent vector of induced astigmatism may be more prominent in S-DALK than in FE-DALK. Therefore, the differences in vectoral values found between the groups may suggest an advantage of FE-DALK. However, this did not translate into better overall outcomes in corneal astigmatism or vision.
Li et al. retrospectively compared FE-DALK and S-DALK and found improved UCVA, BSCVA, and astigmatism following straight-cut FE-DALK [8]. In a previous study by our group, earlier visual recovery was identified with mushroom-shaped FE-DALK compared with S-DALK at 3 months. However, visual results were comparable at 6 months and 1 year [9]. This current trial showed no superior visual outcomes of FE-DALK over S-DALK at 6, 12 and 15 months after surgery. This is consistent with findings reported by Alio et al., in a retrospective study that showed no significant difference in visual outcomes between FE-DALK (n = 25) and S-DALK (n = 25) [7].
One of the rationales for using femtosecond laser in DALK is the potential to improve the accuracy with which the optimal depth that is required to form a big bubble is achieved, while minimizing the risk of perforating Descemet’s membrane. This will potentially reduce the rate of conversion to PK and increase the rate of successful big bubble formation. In this trial, no strong statistical evidence was found between FE-DALK and S-DALK neither in rates of PK conversion nor in rates of successful big bubble formation. It should be noted, however, that the big-bubble success rate was 67% in FE-DALK and only 52% in S-DALK. Since the current trial was powered to investigate the primary outcomes of astigmatism and SIA, it may not have been sufficiently powered to address the differences in big-bubble success. This should be further investigated in larger prospective studies. Modified FE-DALK techniques are now emerging whereby in addition to femtosecond-assisted trephination, the femtosecond laser is also being used to create an intrastromal channel for the insertion of an air cannula up to the precisely required big-bubble depth under the guidance of either preoperative or intraoperative optical coherence tomography (OCT). Initial reports in recent literature show big-bubble success rates of 90–100% using these modifications [16–19]. As these modified techniques continue to mature, future clinical trials comparing them to S-DALK may be warranted.
The presence of corneal scarring may affect DALK success rates. In the current study, 19% of all recruited eyes had corneal scarring. If the presence of corneal scarring had affected DALK success rates, we would have expected to see more eyes with corneal scarring among those converted to PK than among those where DALK was successful. However, the rate of corneal scarring was nearly identical between eyes that converted to PK (18%) and eyes that underwent a successful DALK (19%). Similarly, within the group of eyes with a successful DALK, 18% of the FE-DALK and 19% of the S-DALK groups had corneal scarring. These findings suggest that corneal scarring had no substantial effect on DALK success rates in the current study.
One important aspect of femtosecond laser technology is the added cost and logistics involved with its use. A previously published editorial by Yoo et al. and a recently published review by Deshmukh et al. emphasize cost and cost-effectiveness as potential disadvantages of FE-DALK when compared with S-DALK [20, 21]. The issue of cost-effectiveness should be taken into consideration and evaluated in future study designs comparing the two techniques.
This randomized controlled trial had several limitations. First, to keep the postoperative scenario as close to a real-life setting as possible and to ensure optimal patient safety and benefit, surgeons removed corneal sutures at their discretion to optimize their patients’ postoperative outcomes. This fact may have been a confounder of the comparison of postoperative astigmatism between the groups. Second, participants were undergoing both routine postoperative follow-up exams at their surgeon’s clinic and study visits at the study site. Therefore, some of the patients were not motivated to come in for an additional study visit and did not attend their 15-month follow-up.
In conclusion, in this randomized controlled trial, femtosecond DALK and standard DALK showed comparable functional and anatomical outcomes.
Summary
What was known before
Some retrospective studies have suggested that femtosecond DALK has an advantage over standard DALK in terms of faster visual recovery and reduced rates of conversion to PK.
No conclusive data exists in the literature on differences in final outcomes between the two techniques.
What this study adds
In this randomized controlled trial, femtosecond DALK and standard DALK showed comparable anatomic and functional outcomes.
Study findings suggest that further research is required to better understand the advantages of femtosecond DALK with focus on recent modifications of the femtosecond DALK technique.
Supplementary information
Acknowledgements
We would like to acknowledge Professor Noel Alpins for his assistance and guidance in the planning of vector analysis in the study, and Ms. Fei Zuo for her assistance in data analysis.
Author contributions
NSo was responsible for designing the study, drafting the protocol, analysis planning, interpreting results, and drafting the manuscript. WH was responsible for designing the study, drafting the protocol, recruiting participants, collecting data, interpreting results, and revising the manuscript. MM was responsible for analysis planning, interpreting results and revising the manuscript. HFC was responsible for recruiting participants, collecting data, and revising the manuscript. DSR was responsible for recruiting participants, collecting data, and revising the manuscript. ARS was responsible for recruiting participants, collecting data, and revising the manuscript. MCB was responsible for recruiting participants, collecting data, and revising the manuscript. CCC was responsible for recruiting participants, collecting data, and revising the manuscript. KET was responsible for analysis planning, performing the analysis, interpreting results, and revising the manuscript. MP was responsible for designing the study, drafting the protocol, and revising the manuscript. VS was responsible for recruiting participants, conducting follow-up testing, collecting data, and revising the manuscript. NSi was responsible for designing the study, analysis planning, drafting the protocol, supervising study procedures, recruiting participants, collecting data, interpreting results, and revising the manuscript.
Funding
This study is funded by Kensington Research Institute.
Data availability
The datasets generated and analyzed during the current study are not publicly available to protect individual privacy but are available from the corresponding author on reasonable request following the approval of the University of Toronto Research Ethics Board.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02387-1.
References
- 1.Shimmura S, Tsubota K. Deep anterior lamellar keratoplasty. Curr Opin Ophthalmol. 2006;17:349–55. doi: 10.1097/01.icu.0000233953.09595.91. [DOI] [PubMed] [Google Scholar]
- 2.Tan DTH, Mehta JS. Future directions in lamellar corneal transplantation. Cornea. 2007;26:S21–8. doi: 10.1097/ICO.0b013e31812f685c. [DOI] [PubMed] [Google Scholar]
- 3.Reinhart WJ, Musch DC, Jacobs DS, Lee WB, Kaufman SC, Shtein RM. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. 2011;118:209–18. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Archila EA. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1984;3:217–8. [PubMed]
- 5.Gaster RN, Dumitrascu O, Rabinowitz YS. Penetrating keratoplasty using femtosecond laser-enabled keratoplasty with zig-zag incisions versus a mechanical trephine in patients with keratoconus. Br J Ophthalmol. 2012;96:1195–9. doi: 10.1136/bjophthalmol-2012-301662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamiya K, Kobashi H, Shimizu K, Igarashi A. Clinical outcomes of penetrating keratoplasty performed with the VisuMax femtosecond laser system and comparison with conventional penetrating keratoplasty. PLoS ONE. 2014;9:1–6. doi: 10.1371/journal.pone.0105464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alio JL, Abdelghany AA, Barraquer R, Hammouda LM, Sabry AM. Femtosecond laser assisted deep anterior lamellar keratoplasty outcomes and healing patterns compared to manual technique. Biomed Res Int. 2015;2015:397891. doi: 10.1155/2015/397891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Wang T, Bian J, Wang F, Han S, Shi W. Precisely controlled side cut in femtosecond laser-assisted deep lamellar keratoplasty for advanced keratoconus. Cornea. 2016;35:1289–94. doi: 10.1097/ICO.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 9.Shehadeh-Mashor R, Chan CC, Bahar I, Lichtinger A, Yeung SN, Rootman DS. Comparison between femtosecond laser mushroom configuration and manual trephine straight-edge configuration deep anterior lamellar keratoplasty. Br J Ophthalmol. 2014;98:35–39. doi: 10.1136/bjophthalmol-2013-303737. [DOI] [PubMed] [Google Scholar]
- 10.Chan CC, Ritenour RJ, Kumar NL, Sansanayudh W, Rootman DS. Femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea. 2010;29:290–5. doi: 10.1097/ICO.0b013e3181b77873. [DOI] [PubMed] [Google Scholar]
- 11.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403. doi: 10.1016/S0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 12.Melles GR, Lander F, Rietveld FJ, Remeijer L, Beekhuis WH, Binder PS. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83:327–33. doi: 10.1136/bjo.83.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shehadeh-Mashor R, Chan C, Yeung SN, Lichtinger A, Amiran M, Rootman DS. Long-term outcomes of femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea. 2013;32:390–5. doi: 10.1097/ICO.0b013e318254a4e4. [DOI] [PubMed] [Google Scholar]
- 14.Alpins NA, Goggin M. Practical astigmatism analysis for refractive outcomes in cataract and refractive surgery. Surv Ophthalmol. 2004;49:109–22. doi: 10.1016/j.survophthal.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Schulz KF, Altman DG, Lepage L. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–62. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 16.Liu YC, Wittwer VV, Yusoff NZM, Lwin CN, Seah XY, Mehta JS, et al. Intraoperative optical coherence tomography-guided femtosecond laser-assisted deep anterior lamellar keratoplasty. Cornea. 2019;38:648–53. doi: 10.1097/ICO.0000000000001851. [DOI] [PubMed] [Google Scholar]
- 17.Guindolet D, Nguyen DT, Bergin C, Doan S, Cochereau I, Gabison EE. Double-docking technique for femtosecond laser-assisted deep anterior lamellar keratoplasty. Cornea. 2018;37:123–6. doi: 10.1097/ICO.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 18.Gogri PY, Bore MC, Rips AGT, Reddy JC, Rostov AT, Vaddavalli PK. Femtosecond laser-assisted big bubble for deep anterior lamellar keratoplasty. J Cataract Refract Surg. 2021;47:106–10. doi: 10.1097/j.jcrs.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 19.Lucisano A, Giannaccare G, Pellegrini M, Bernabei F, Yu AC, Carnevali A, et al. Preliminary results of a novel standardized technique of femtosecond laser-assisted deep anterior lamellar keratoplasty for keratoconus. J Ophthalmol. 2020;2020:5496162. doi: 10.1155/2020/5496162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo SH, Hurmeric V. Femtosecond laser-assisted keratoplasty. Am J Ophthalmol. 2011;151:189–91. doi: 10.1016/j.ajo.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Deshmukh R, Stevenson LJ, Vajpayee RB. Laser-assisted corneal transplantation surgery. Surv Ophthalmol. 2021;66:826–37. doi: 10.1016/j.survophthal.2021.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available to protect individual privacy but are available from the corresponding author on reasonable request following the approval of the University of Toronto Research Ethics Board.