Abstract
The discrimination of protein biological functions in different phases of the cell cycle is limited by the lack of experimental approaches that do not require pre-treatment with compounds affecting the cell cycle progression. Therefore, potential cycle-specific biological functions of a protein of interest could be biased by the effects of cell treatments. The OsTIR1/auxin-inducible degron (AID) system allows “on demand” selective and reversible protein degradation upon exposure to the phytohormone auxin. In the current format, this technology does not allow to study the effect of acute protein depletion selectively in one phase of the cell cycle, as auxin similarly affects all the treated cells irrespectively of their proliferation status. Therefore, the AID system requires coupling with cell synchronization techniques, which can alter the basal biological status of the studied cell population, as with previously available approaches. Here, we introduce a new AID system to Regulate OsTIR1 Levels based on the Cell Cycle Status (ROLECCS system), which induces proteolysis of both exogenously transfected and endogenous gene-edited targets in specific phases of the cell cycle. We validated the ROLECCS technology by down regulating the protein levels of TP53, one of the most studied tumor suppressor genes, with a widely known role in cell cycle progression. By using our novel tool, we observed that TP53 degradation is associated with increased number of micronuclei, and this phenotype is specifically achieved when TP53 is lost in S/G2/M phases of the cell cycle, but not in G1. Therefore, we propose the use of the ROLECCS system as a new improved way of studying the differential roles that target proteins may have in specific phases of the cell cycle.
Subject terms: Cell biology, Molecular biology
Introduction
The cell-division cycle, also known as cell cycle, is the fundamental, precise and complex process at the basis of life and physiological processes such as development, tissue growth, homeostasis, regeneration, and aging in multicellular organisms [1, 2]. In mitotic cells, the division into two daughter cells (cytokinesis) occurs after the parental cell undergoes the semiconservative synthesis (S phase) of a new copy of its entire genome, followed by the mitotic chromosomal segregation (M phase). Two gap phases, G0/G1 and G2, precede the S and M phases, respectively [1–3].
The mechanisms leading to and controlling DNA replication and segregation are historically among the most studied and understood processes happening throughout cell division [4]. Critical molecular players involved in cell cycle regulation and control have been identified based on the effect that their mutation, overexpression, or silencing have on genome replication, either in physiological conditions or in response to DNA damaging agents [3, 5, 6].
However, genomic DNA is not the only cellular component undergoing dramatic changes during cell cycle progression. Proteins, organelles, and cellular membranes experience profound modifications to allow the appropriate segregation of all the required materials in the daughter cells [7, 8]. One obvious paradigm is constituted by the nuclear membrane, which disassembles immediately before cells enter mitosis to be promptly re-assembled at the completion of the cell division cycle [7–9]. During this process, the nuclear content and proteins of the nuclear pore complexes are released in the open cytoplasm, and novel protein-protein interactions can take place [10–12]. Hence, it could be assumed that virtually any cellular protein might become part of alternative multi-protein complexes and perform different biological tasks, such as preserving DNA integrity [13] or cytoskeletal dynamics [14].
To date, existing technical limitations have prevented an appropriate discrimination of phase-specific protein functions, especially in physiological conditions. Insights into the cell cycle regulatory networks were initially obtained by analyzing cells synchronized in specific phases of the cell cycle. However synchronization is routinely achieved by exposing cells to stress conditions, such as serum starvation, inhibition of DNA synthesis, or by disrupting microtubule dynamics [15, 16].
To assess the cell cycle phase-specific functions of a protein of interest, one option is to take advantage of “cell cycle tags”, cell cycle-dependent protein degrons that can restrict protein expression to a specific phase of the cell cycle [17–19]. However, these approaches suffer from intrinsic limitations, as cell cycle degrons do not respond to external stimuli to regulate their functions. Therefore, previously reported cell cycle tags are constitutively functioning and not tunable.
A major advancement in the field of cell cycle tags was represented by the development of the FUCCI (Fluorescent Ubiquitination-based Cell Cycle Indicator) system [20–22]. This technology is based on the enzymatic activity of two E3 ubiquitin ligases, APCCdh1 and SCFSkp2 [22–25], involved in the control and proteasomal degradation of Geminin (targeted by APCCdh1) and Cdt1 (targeted by SCFSkp2) [26]. Cdt1 and geminin are critical regulators of the licensing of replication origins, with opposite functions and biological effects [26]. By fusing Cdt1 and Geminin with a variety of fluorescent proteins, a number of tools were engineered to accurately discriminate the cell cycle status of individual cells, either microscopically or by flow cytometry [22, 27–30], both in vitro and in vivo. Additional live-cell sensors, such as CDK2-activity fluorescent reporters [31], have also been used for in silico cell synchronization and study of protein biological functions by microscopy.
These novel approaches provide an efficient way to identify, visualize and select cells in specific phases of the cell cycle. However, some of them do not allow the isolation of large number of cells for downstream experiments, such as multi-omic or functional analyses. More importantly, they must be still combined with other technologies to perturb the levels of the protein of interest (POI) and assess its biological role throughout the cell cycle. Despite the advancements of genetic tools such as CRISPR/Cas9-based gene editing [32], gene silencing [33], or inducible gene expression approaches [34], none of these systems displays readiness of activity compatible with the kinetics of cell cycle progression. Conversely, an alternative to obtain rapid degradation of the POI is represented by targeted proteolysis using PROteolysis-Targeting Chimeras (PROTACs), or polypeptide tags (also known as degrons) [35, 36]. In one of the most commonly used degron systems, the POI is fused with an Auxin-Inducible Degron (AID) sequence, such as the 7 kDa degron termed mini-AID (mAID), in cell lines expressing the Oryza sativa TIR1 (OsTIR1) F-box protein [37–39]. When the phytohormone auxin (indole-3-acetic acid IAA) is provided, OsTIR1 binds the mAID-POI and induces its quick proteasomal degradation [37, 38]. However, despite their speed, reversibility, and fine-tuning, degron-based systems still lack cell cycle phase-specificity and require conventional cell synchronization [40].
Here, we report the engineering of the “Regulated OsTIR1 Levels of Expression based on the Cell-Cycle Status” (ROLECCS) technology, which combines the AID and the FUCCI systems. In this new tool, the OsTIR1 protein is fused to the fluorescent indicator mEmerald and the FUCCI tags Cdt1/Geminin, which are responsible for the restricted G1 and S/G2 expression, respectively. Upon auxin treatment, only the cells expressing the fusion-protein OsTIR1-mEmerald-Cdt1/Geminin, (i.e. in the desired cell cycle phase) degrade the mAID-POI. We further developed a second ROLECCS system (ROLECCSv2), which is triggered by the synthetic auxin analog 5-phenyl-indole-3-acetic acid (5-Ph-IAA), overcoming the major drawbacks of auxin treatment, such as basal level protein degradation in the absence of the phytohormone [35]. We tested our ROLECCS systems for the cell cycle phase-specific control of both exogenous overexpressed targets and endogenous CRISPR/Cas9 gene-edited proteins. Finally, we assessed the capability of ROLECCS to discriminate between some of the G1 and S/G2 specific functions of the tumor suppressor TP53 in preserving genomic stability.
Methods
Plasmids
All the plasmids used in this study were generated by Gibson Assembly using NEBuilder® HiFi DNA Assembly Master Mix (New England Biolabs, E2621L) as per manufactory’s instructions. To construct pAAVS1-ROLECCS AS, pAAVS1-ROLECCS G1, and pAAVS1-ROLECCS G2 plasmids, multiple fragments were PCR amplified from different donor plasmids and assembled as follow: pMK232 CMV-OsTIR1-PURO (Addgene #72834 [37]) was used as donor plasmid for the expression of OsTIR1 from the AAVS1 locus, the mEmerald tag was PCR amplified from mEmerald-PLK1-N-16 vector (Addgene #54234; http://n2t.net/addgene:54234; RRID:Addgene_54234), while pEN435 - pCAGGS-TagBFP-hGeminin-2A-mCherry-hCdt1-rbgpA-Frt-PGK-EM7-PuroR-bpA-Frt Tigre targeting (Addgene #92139 [28]) was used as template for both hGeminin and hCdt1 tags. The vector for the mAID-mCherry expression was generated using the pEGFP-C1 backbone (Clontech), replacing the GFP gene with the mAID-mCherry cassette derived from pMK292 mAID-mCherry2-NeoR (Addgene #72830 [37]). The bicistronic lentiviral vectors for ROLECCS AS, ROLECCS G1, ROLECCS G2, and mAID-mCherry expression were similarly obtained, although linker sequences (P2A) were synthesized (IDT) and cloned by Gibson assembly. AID version2 ROLECCS system (ROLECCSv2), bearing the OSTIR1 (F74G) mutant, was obtained from pAAVS1-ROLECCS AS, pAAVS1-ROLECCS G1, and pAAVS1-ROLECCS G2 plasmids, via site directed mutagenesis PCR.
To generate the donor plasmids for TP53 editing, the genomic region (~2000bp) encoding for the natural stop codon of TP53 was first cloned into the pUC19 vector (New England Biolabs, N3041S) by Gibson assembly. More specifically, genomic DNA from H460 cell line (TP53 wild-type) [41] was used as template to amplify the TP53 genomic region (Chromosome 17: 7,668,421-7,687,490, Transcript: TP53-201 ENST00000269305.9) of 1 kb upstream and 1 kb downstream the TP53 translation stop codon. These regions were further used as homology arms for HDR-mediated CRISPR/Cas9-mediated knock-ins. Secondly, the homology arms containing plasmid was mutated using QuickChange II XL Site-Directed Mutagenesis Kit (Agilent, #200522) to delete the single-guide RNA (sgRNA) recognition sequence, to prevent Cas9 from re-cutting after homology-directed repair-mediated insertion at the desired genetic locus. Finally the mAID-mCherry cassette containing a selection marker was amplified from pMK292 mAID-mCherry2-NeoR (Addgene #72830 [37]) or pMK293 mAID-mCherry2-Hygro (Addgene #72831 [37]) and inserted between the homology arms (about 1000 bp each), replacing the TP53 stop codon, making sure that the tags sequences were cloned in frame with the gene of interest, in order to generate a fusion protein.
To construct the CRISPR/Cas9 TP53 gene targeting vector, a single-guide RNA (sgRNA) (5’-ACTGACAGCCTCCCACCCCC-3’) was designed (http://crispr.mit.edu) to specifically target TP53 translation stop site and it was cloned into pX330-U6-Chimeric_BB-CBh-hSpCas9-hGem (1/110) (Addgene #71707) according to the protocol of Ran et al. [42]. The same protocol was followed to clone the sgRNA used for the ROLECCS transgene insertion in the AAVS1 locus [37], into the pX330-U6-Chimeric_BB-CBh-hSpCas9-hGem (1/110) [43].
All the plasmids will be deposited on Addgene or are available from the investigators upon kind request.
Treatments
To induce the degradation of mAID-fused proteins, cells were treated with 500 µM indole-3-acetic acid (auxin, IAA, dissolved 500 mM in water) (I5148, Millipore Sigma,) for ROLECCS experiments, or 1 µM 5-phenyl-indole-3-acetic acid (5-Ph-IAA) (Targetmol, T8885) for ROLECCS v2 experiments, for 1 h, unless otherwise stated. To suppress the partial degradation of TP53-mAID-mCherry in HCTT116 constitutively expressing pAAVS1-ROLECCS AS, pAAVS1-ROLECCS G1 and pAAVS1-ROLECCS G2 control cells were pretreated overnight with 200 µM auxinole (BioAcademia, Inc., Japan; #30–001, dissolved 200 mM in DMSO) and maintained in auxinole for the duration of the experiments where indicated.
To induce TP53 activation, cells were either treated with 20 µM cis-Diamineplatinum(II) dichloride (cisplatin, stock diluted in 0.9% NaCl, Millipore Sigma, #479306) for the indicated time point or exposed to ionizing radiation through a Gammacell Gamma Irradiator (5 Gy).
Statistical analysis
All the experiments are representative of at least two independent experiments (technical and/or biological replicates). The number of replicates for each experiment is specified in the relative figure legend. For statistical analysis, two-tailed t-test was performed and data were considered statistically significant for p < 0.05.
Results
Designing and engineering a cell cycle phase-specific OsTIR1
To engineer a cell cycle phase-specific degron system, we generated a variant of the mAID system where the expression of OsTIR1, necessary for the recognition and degradation of the mAID-tagged protein upon auxin exposure, was dependent on the G0/G1 or S/G2/M phase.
In our design, the OsTIR1 coding gene was fused in-frame with a mEmerald (mEM) fluorescent reporter (a brightly fluorescent monomeric variant of GFP [44]) that allows the identification of cells expressing these constructs by fluorescence microscopy and flow cytometry. Then, we added the sequences corresponding to either human Cdt1 (aa 30-120) or Geminin (aa 1-110) to restrict OsTIR1 expression to different phases of the cell cycle, like in the FUCCI system. For convenience, the hCdt1 (30-120) and hGeminin (1-110) tags are indicated hereafter as Cdt1 and GEM, respectively. We also generated a construct where no additional tag was added, to allow OsTIR1-mEmerald expression independently on the phase of the cell cycle (Fig. 1).
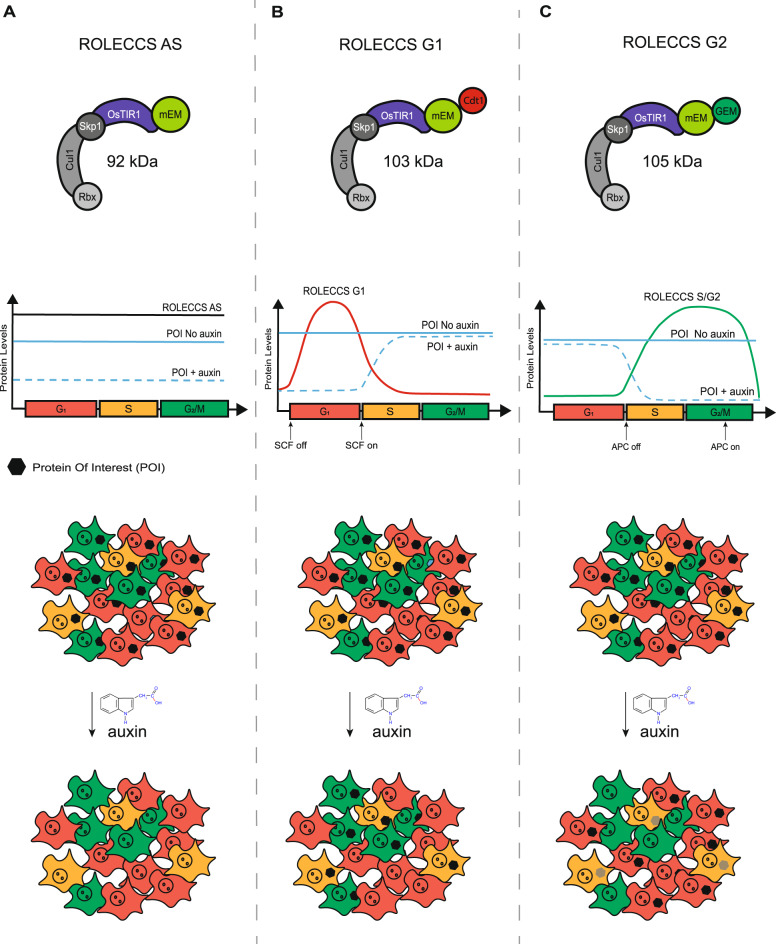
Fig. 1. Schematic representation of the design for Regulated OsTIR1 Levels of Expression based on the Cell Cycle Status (ROLECCS) variants.
A OsTIR1-mEmerald protein (asynchronous ROLECCS, ROLECCS AS, 92 KDa) is stably expressed throughout the cell cycle. Upon auxin treatment, OsTIR1 enzymatic activity elicits the degradation of the mAID-tagged protein of interest (POI) in any cell, independently of the cell cycle status. B The expression of the ROLECCS G1 variant (OsTIR1-mEmerald-Cdt1, 103 KDa) is restricted to the G1/early S phase by the presence of the Cdt1 tag, when the SCFSkp2 E3 ligase activity is off. This, in turn, leads to auxin-dependent ubiquitylation and proteasome degradation of mAID-tagged POIs. In cells transitioning during S, G2 and M phases, SCFSkp2 activity is naturally restored, leading to ROLECCS G1 degradation by ubiquitylation, and stabilization of the POI even in the presence of auxin. C The Geminin tag of the ROLECCS G2 variant (OsTIR1-mEmerald-GEM, 105 KDa) ensures its restricted expression during the late S-G2-M phase, as APCCdh1-mediated ubiquitylation and degradation is rapidly triggered during M/G1 transition. Therefore, auxin treatment induces degradation of the POI exclusively in cells going through the late S-G2-M phase of the cell cycle.
In our design, engineered variants of OsTIR1-mEmerald, OsTIR1-mEmerald-Cdt1, and OsTIR1-mEmerald-GEM genes are actively transcribed throughout the cell cycle. However, the presence of the Cdt1 and the Geminin tags determine the Regulated OsTIR1 Levels of Expression based on the Cell Cycle Status (ROLECCS system). We predicted that the OsTIR1-mEmerald protein would be stably present throughout the cell cycle. Therefore, auxin treatment would trigger OsTIR1 enzymatic activity and degradation of the mAID-tagged protein of interest in any cell, independent of the cell cycle status (from now on: asynchronous ROLECCS, ROLECCS AS) (Fig. 1A).
On the other hand, the presence of OsTIR1-mEmerald-Cdt1 (from now on: ROLECCS G1) protein would be restricted the G1/early S phase, because ubiquitylation by SCFSkp2 E3 ligase leads to its prompt degradation during S-phase transition. Thus, addition of auxin would lead to OsTIR1-mediated proteasomal degradation of the POI exclusively in those cells in G1/S phase during the treatment (Fig. 1B).
Similarly, presence of OsTIR1-mEmerald-GEM (from now on: ROLECCS G2) protein would be restricted during the late S-G2-M phase, peaking during the G2, as APCCdh1-mediated ubiquitylation and degradation is rapidly triggered during M/G1 transition. Consequently, auxin treatment would cause degradation of the POI exclusively in cells going through the late S-G2-M phase of the cell cycle during the treatment (Fig. 1C). To provide flexibility to the system and make it usable in different paradigms, the three CMV-driven ROLECCS constructs were subcloned in ad hoc vector [37] that allows either transient expression or CRISPR/Cas9-mediated integration into the AAVS1 safe harbor site of the human genome (Supplementary Fig. 1A–C).
ROLECCS G1 and ROLECCS G2 expression during cell cycle
To demonstrate that ROLECCS G1 and ROLECCS G2 expression is restricted to specific phases of the cell cycle, we first assessed their relative abundance in transiently transfected HEK-293T cells. As shown in Fig. 2A, each ROLECCS construct was abundantly expressed at 72 h from transfection.
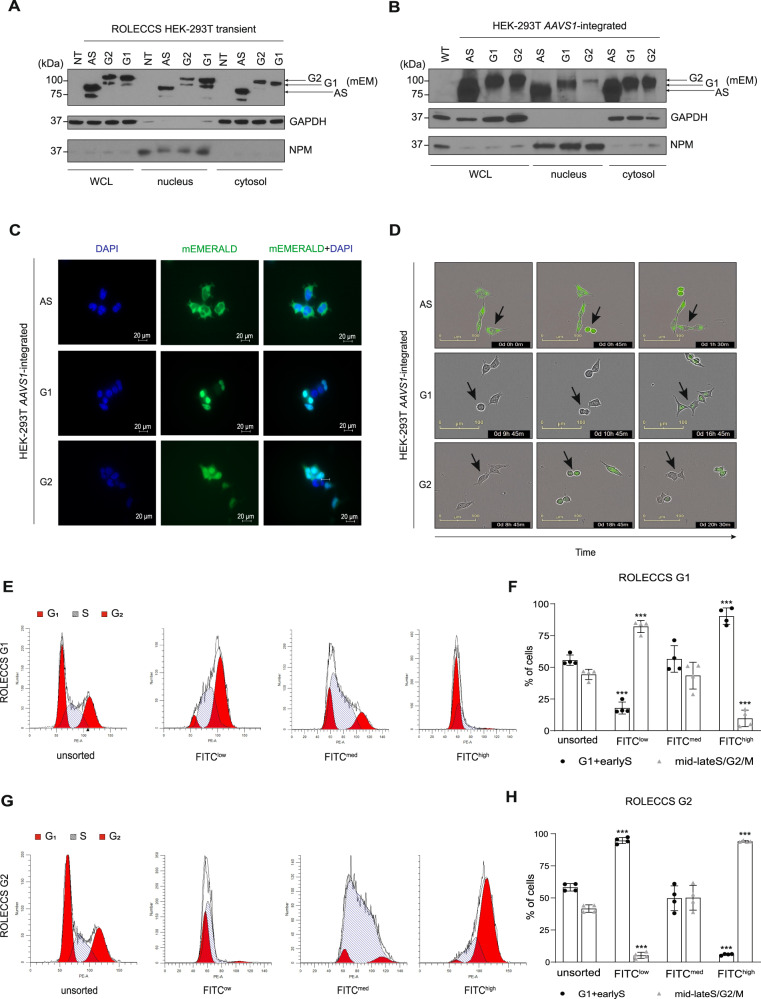
Fig. 2. Characterization of ROLECCS AS, G1, and G2 cellular distribution and during cell-cycle progression.
A, B Representative WB analysis of nuclear/cytoplasmic distribution of ROLECCS proteins upon transient (72 h) transfection (A) or AAVS1 integration (B) in HEK-293T cells. ROLECCS AS, ROLECCS G1, ROLECCS G2 (see main text) were detected using anti-GPF antibody that recognizes the mEmerald tag of the proteins (see arrows). Nucleophosmin (NPM) and GAPDH antibodies were used as loading and purity control for nuclear and cytoplasmic soluble protein fractions, respectively. Not transfected (NT) or wild-type (WT) HEK-293T were used as negative control. WCL indicates Whole Cell Lysate. C Direct fluorescence images of HEK-293T AAVS1-integrated clones. DAPI staining (blue) was used to label nuclei, mEmerald (green) signal was detected from ROLECCS variants (AS, G1, G2). D Time-frame pictures of duplicating HEK-293T AAVS1-integrated clones. Note the cell cycle-dependent changes in fluorescence of specific ROLECCS variants (AS, G1, G2) (green). Arrows indicate cells that are completing a cell cycle. E, G Cell-cycle distribution histograms of HEK-293T AAVS1-integrated clones expressing ROLECCS G1 and G2, obtained by propidium iodide staining and flow cytometry analysis. Red peaks indicate G1 and G2 phase, stripes indicate S phase. Cells were prior sorted based on FITC levels (FITClow, FITCmed, FITChigh), as described in Supplementary Fig. 2. Not sorted (unsorted) populations are reported for comparison. Data are representative of four independent experiments (n = 4). F, H Quantification of experiments reported in E and G. FITClow, FITCmed, FITChigh subpopulations were analyzed for cells composition as percentage of cells in G1+earlyS and cells in mid-lateS/G2/M, using ModFit software v5.0. Error bars indicate mean ± SD. ***p < 0.001, N.S. not significant. Statistics (two-tailed t-test) is calculated versus respective unsorted populations. Data are the average of four independent experiments (n = 4).
To obtain cell populations expressing a uniform level of the ROLECCS proteins, we generated stable HEK-293T cell lines. To this aim, the AAVS1 ROLECCS vectors were integrated into the AAVS1 safe harbor locus by CRISPR/Cas9-mediated gene knock-in. Also in this case, sustained and ubiquitous expression of the ROLECCS proteins was observed by nuclear/cytoplasmic protein fractionation (Fig. 2B), and direct fluorescence imaging (Fig. 2C and Supplementary Fig. 1D). Unlike the previously published FUCCI probes [22], all the ROLECCS proteins were present both in the nucleus and in the cytoplasm of transfected cells, although ROLECCS G1 and ROLECCS G2 (but not ROLECCS AS, which does not display any FUCCI tag) displayed a marked nuclear enrichment, possibly due to the Cdt1 and Geminin moieties of these constructs.
Next, we aimed to demonstrate that ROLECCS G1 and ROLECCS G2 protein levels oscillate reciprocally during cell cycle transition. Live cell imaging was performed on ROLECCS AS, G1 and G2 knock-in HEK-293T to monitor cell division and green fluorescence in real time. Figure 2D (top) and Supplementary Video 1 show that ROLECCS AS expression did not change during a full cell cycle. Conversely ROLECCS G1 was not visible in actively dividing cells (Fig. 2D, middle and Supplementary Video 2), becoming detectable immediately upon completion of cell division, as assessed by contrast phase imaging. Finally, ROLECCS G2 was visible only in actively dividing cells, with the fluorescence intensity peaking at G2/M transition (cells with round shape in contrast phase imaging, Fig. 2D, bottom and Supplementary Video 3).
To orthogonally validate ROLECCS G1 and ROLECCS G2 as cell cycle indicators, we sorted AAVS1-integrated ROLECCS HEK-293T based on their green fluorescence (FITC channel, to detect mEmerald) level and cellular complexity (Side Scatter, SSC) (Supplementary Fig. 2A–D), as described in the Methods section. DNA content analysis demonstrated that FITChigh-sorted ROLECCS G1 population mostly comprised cells in the G1/early S phase (90.3 ± 6.5%), in comparison with FITCmed and FITClow sorted populations (56.6 ± 10.6% and 17.9 ± 4.7% respectively) (Fig. 2E and F). Conversely, FITChigh ROLECCS G2 population showed a significant enrichment in late S/G2 phase cells (94.1 ± 0.6%), compared to FITCmed and FITClow sorted cells (50.2 ± 9.7% and 5.2 ± 2.4%, respectively) (Fig. 2G and H). Conversely, unsorted ROLECCS G1 and ROLECCS G2 populations displayed cell cycle distribution typical of unsynchronized HEK-293T cells.
Altogether, our findings indicate that engineered ROLECSS G1 and G2 protein levels are efficiently restricted to specific phases of the cell cycle, and their fluorescence intensity can be used as a good surrogate marker of cell cycle distribution.
Biological activity of ROLECCS
The addition of large tags to proteins might affect their biological activity [45]. Therefore, we wanted to assess that the enzymatic activity of OsTIR1-containing SCF complexes was not hampered by the mEmerald-Cdt1 and mEmerald-GEM tags of the ROLECCS G1 and G2, respectively. We transiently transfected AAVS1-integrated ROLECCS AS, ROLECCS G1, and ROLECCS G2 HEK-293T cells with a mAID-mCherry fluorescent reporter and measured its protein levels. As shown in Supplementary Fig. 3A, mAID-mCherry levels were appreciably reduced upon auxin treatment when performed at 8 h after reporter vector transfection, indicating that the biological activity of OsTIR1 was preserved. However, transient transfection could lead to multiple sub-populations of ROLECCS-expressing cells with different levels of mAID-mCherry due to inconsistent transduction. Moreover, at later time points, auxin-dependent degradation of mAID-mCherry was negligible (not shown). Therefore, we hypothesized that an overexpressed target could be efficiently degraded only if the molar ratio between the POI and the ROLECCS was favorable to the latter, as in the very first hours (<8 h) after transfection. This is in line with the findings of other groups, which have generated All-in-One systems to achieve equimolar levels of OsTIR1 and its targets [46].
As shown in Fig. 3A–C, we created 3 all-in-one lentiviral vectors (pLentiROLECCS AS, G1, and G2) in which the ROLECCS proteins were fused with mAID-mCherry using an autoproteolytic P2A sequence [47]. This approach allows the simultaneous and equimolar expression of both OsTIR1 and its target which, upon translation, are released as independent molecules. Figure 3D shows that transient and stable transfection of pLentiROLECCS vectors led to sustained expression of both the ROLECCS proteins and of mAID-mCherry.
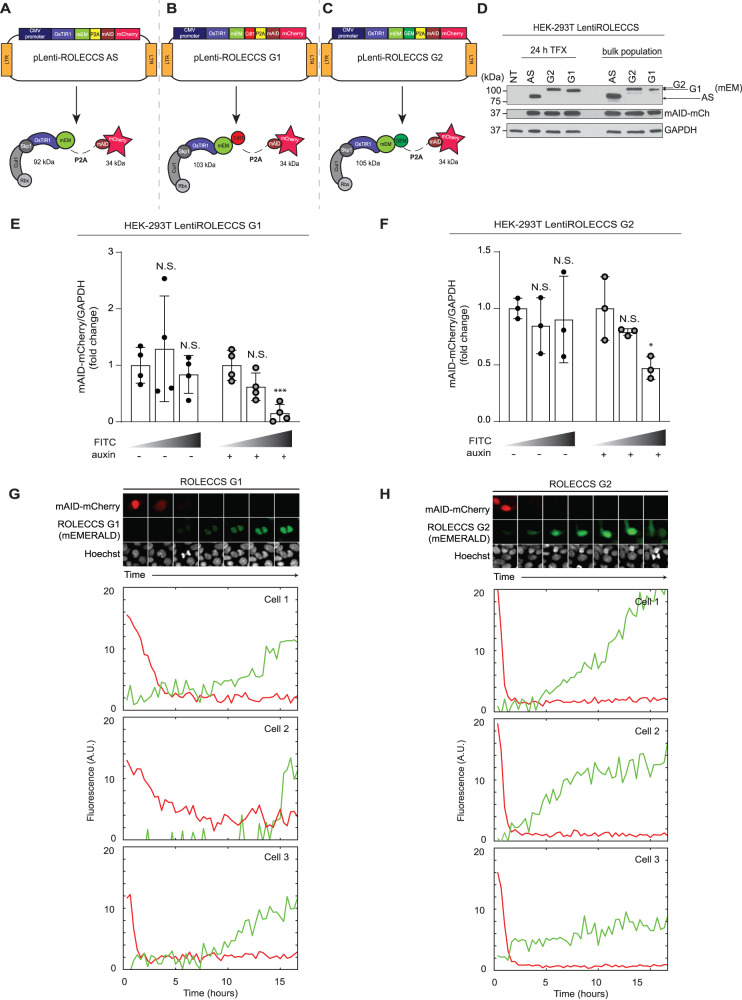
Fig. 3. Biological activity of ROLECCS proteins.
A–C Schematic representation of lentiviral vectors (pLentiROLECCS AS, G1, and G2) and their corresponding translated proteins with respective molecular weight. D WB analysis of transient (24 h) and stable transfection (bulk population) of pLentiROLECCS vectors in HEK-293T cells. Anti-GPF antibody, recognizing the mEmerald tag (mEM) of the proteins, was used to detect ROLECCS proteins (see arrows), anti-mCherry antibody was used to detect mAID-mCherry. Not transfected HEK-293T (NT) and GAPDH were used as negative and loading control respectively. E Densitometric quantification of mAID-mCherry normalized on GAPDH intensity of WB analyses of HEK-293T cells transfected with pLentiROLECCS G1, presented in Supplementary Fig. 3 B (clone 1) and C (clone 2). Relative quantification versus FITClow sorted population is reported. Error bars indicate mean ± SD. ***p < 0.001, N.S. not significant. Statistics (two-tailed t-test) is calculated versus FITClow sorted population. Data are representative of four independent experiments (n = 4). F Densitometric quantification of mAID-mCherry normalized on GAPDH intensity of WB analyses of HEK-293T cells transfected with pLentiROLECCS G2, presented in Supplementary Fig. 3 D (clone 1) and E (clone 2). Relative quantification versus FITClow sorted population is reported. Error bars indicate mean ± SD. *p < 0.05, N.S. not significant. Statistics (two-tailed t-test) is calculated versus FITClow sorted population. Data are representative of three independent experiments (n = 3). G, H Live-cell confocal microscopy imaging on MCF 10a normal breast epithelial cells, transduced with LentiROLECCS G1 (G) or LentiROLECCS G2 (H). Upon 5-Ph-IAA cells treatment, red fluorescent signal (mAID-mCherry) faded away before the green-fluorescent signal (ROLECCS G1, panel G and ROLECCS G2, panel H) could be detected. Hoechst staining (greyscale) of DNA content was performed to follow cell cycle division, confirming ROLECCS G1 expression increase after completion of cell division (G) and ROLECCS G2 detection during the progression through S and G2 phase (H). G Interval between still images is 100 min. Single cell traces (mAID-mCherry as red trace, ROLECCS G1 as green trace) of three different cells are representative and do not correspond with the images above. (H) Interval between still images is 120 min. Single cell traces (mAID-mCherry as red trace, ROLECCS G2 as green trace) are representative and do not correspond with the images above.
Next, we treated HEK-293T cells stably expressing LentiROLECCS G1 or LentiROLECCS G2 with auxin for 1 h. Cells were sorted based on their FITC fluorescence intensity and SSC and analyzed by Western Blot. Figure 3E, F show that downregulation of the mAID-mCherry protein levels was specifically achieved in FITCmed and FITChigh populations upon auxin treatment. Western blot analysis also confirmed that these sorted cell populations expressed the highest levels of ROLECCS G1 and ROLECCS G2 (Supplementary Fig. 3B–E). Notably, highest levels of ROLECCS G1 corresponded to highest expression of Cdt1 (a G1-specific marker, frequently identified as a doublet corresponding to Cdt1/phosphoCdt1 [48]) and to the lowest levels of Cyclin B1 (a late-S/G2 marker). Importantly, downregulation of the target mAID-mCherry was only observed in sorted FITChigh ROLECCS G1 cells upon auxin treatment, and not in the untreated or FITClow auxin-treated controls (Supplementary Fig. 3B, C). Similarly, ROLECCS G2 accumulation was observed in cell populations displaying highest levels of Cyclin B1 and lowest levels of Cdt1, but target downregulation was only observed upon auxin treatment (Supplementary Fig. 3D, E). These results were confirmed using 2 independent HEK-293T LentiROLECCS clones.
To confirm the cell cycle phase-specificity of ROLECCS G1 and G2 proteolytic activity, HEK-293T cells stably expressing LentiROLECCS G1 or LentiROLECCS G2 were treated with RO-3306, a potent cell cycle inhibitor, able to block the cell cycle in the G2/M phase of the cell cycle [49] (Supplementary Fig. 4A and D). For ROLECCS G1-transduced cells, we observed a significant inhibition of ROLECCS-mediated auxin-dependent proteolytic activity against mAID-mCherry, due to the reduction of the relative abundance of cells in the G1 phase upon RO-3306 treatment (Supplementary Fig. 4B, C). Conversely, RO-3306 enhanced the activity of ROLECCS G2, due to the increase of the relative abundance of the cells in the S/G2 phase.
Finally, we wanted to assess how promptly ROLECCS G1 and ROLECCS G2 could induce targeted proteolysis during cell cycle progression, in real time. To this aim, we performed live-cell confocal microscopy imaging on MCF 10a normal breast epithelial cells, transduced with LentiROLECCS G1 or LentiROLECCS G2. Figure 3G and H show that degradation of mAID-mCherry was noticeable even before the green-fluorescent signal from the ROLECCS G1 and G2 could be detected. Moreover, DNA imaging using Hoechst stain confirmed that ROLECCS G1 fluorescence was detected immediately after completion of cell division, when the cells enter the G1 phase of the cell cycle. Contrariwise, the ROLECCS G2 fluorescence increased during the progression through S and G2 phase, and promptly decreased upon cell division, in agreement with the data shown in Fig. 2.
Taken together, our data indicate that the ROLECCS system allows fast and temporally restricted degradation of mAID-tagged targets based on the cell cycle phase.
The ROLECCS and the ROLECCSv2 systems allow cell cycle phase-specific downregulation of endogenous proteins
It has been previously demonstrated that the mAID system is suitable for the downregulation of endogenous protein, when the gene of interest is modified by CRISPR/Cas9-mediated knock-in to include the mAID sequence [37]. We aimed to demonstrate that the ROLECCS system allows the same capability, but specifically in the phase of the cell cycle of interest. We decided to test whether the ROLECCS system could accomplish the cell cycle phase-specific downregulation of TP53, a well-known transcriptional factor playing a central role in the control of cell cycle progression, and genomic stability, especially in response to DNA-damaging agents [50–52]. Moreover, one of the main mechanisms of physiological negative regulation of TP53 is its MDM2-mediated ubiquitylation and proteasomal degradation [50]. Therefore, we postulated that ROLECCS-mediated synthetic degradation of TP53 could represent a valid alternative to its physiological mechanism of regulation.
First, we generated HCT116 cell lines where both wild-type TP53 alleles were modified by CRISPR/Cas9-mediated knock-in (HCT116 TP53-mAID-mCherry). For gene editing purposes, the stop codon of the endogenous TP53 gene was replaced by a mAID-mCherry fusion cassette (as described in Methods section) (Fig. 4A, Supplementary Fig. 5A). Appropriate editing by site-specific integration of the donor cassette was verified by PCR using integration-specific primer sets, as shown in Supplementary Fig. 5B–C.
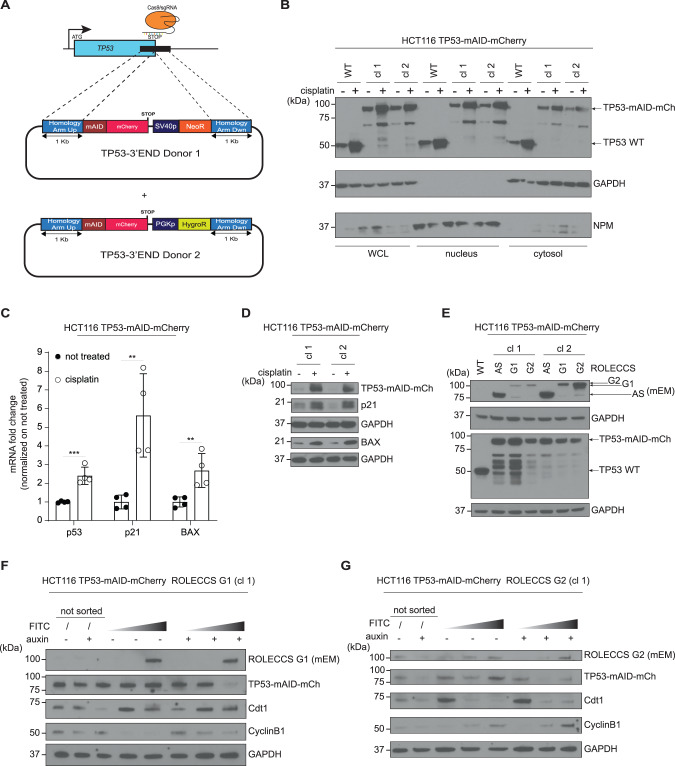
Fig. 4. ROLECCS system downregulates endogenous proteins in a cell cycle-specific fashion.
A Diagram of TP53 gene editing strategy in HCT116 via CRISPR/Cas9-mediated knock-in. The stop codon was replaced by mAID-mCherry fusion cassette, cloned between 1-kb long Homology Arms. To achieve targeting of both TP53 alleles, two donor plasmids (TP53-3’END Donor 1 and Donor 2) were used, bearing Neomycin (NeoR) or Hygromycin (HygroR) resistance genes, respectively. The antibiotic resistance genes are under the transcriptional control of independent promoters (SV40 and PGK, respectively). B WB analysis of nuclear/cytoplasmic distribution of TP53 protein (TP53-mAID-mCherry, 87 kDa) in HCT116 TP53-mAID-mCherry clone 1 (cl 1) and clone 2 (cl 2). HCT116 wild type (WT) were loaded as control for TP53 activation upon cisplatin (20 µM) treatment for 48 h. NPM and GAPDH were used as purity and loading controls for nuclear and cytoplasmic soluble protein fractions, respectively. WCL indicates Whole Cell Lysate. Images are representative of two independent experiments. C Messenger RNA fold change of TP53, p21 and BAX genes in HCT116 TP53-mAID-mCherry cells treated with cisplatin (20 µM for 24 h) quantified by Real Time PCR. GAPDH gene was used as housekeeping control and data were normalized on not treated samples. Error bars indicate mean ± SD. ***p < 0.001, **p < 0.01, N.S. not significant. Statistics (two-tailed t-test) is calculated versus not treated. Experiment was repeated twice on two independent clones (n = 4). D WB analysis of cisplatin-induced TP53-mAID-mCherry (87 kDa), p21 (21 kDa) and BAX (21 kDa) proteins increase in HCT116 TP53-mAID-mCherry. Cells were treated with 20 µM cisplatin and collected for protein extraction at 48 h. Lysates were loaded in duplicate to probe membranes with antibodies against proteins with same molecular weight, GAPDH was used as loading control. Two independent HCT116 TP53-mAID-mCherry clones (cl 1, cl 2) were analyzed. Images are representative of two independent experiments. E WB analysis of characterization of HCT116 TP53-mAID-mCherry with AAVS1-integrated ROLECCS variants (AS/G1/G2). ROLECCS AS, ROLECCS G1, and ROLECCS G2 were detected using anti-GPF antibody (see arrows), TP53 wild type (WT) and TP53-mAID-mCherry (TP53-mAID-mCh) were detected using anti-TP53 antibody. GAPDH was used as loading control. HCT116 wild type (WT) were loaded for comparison. Two independent HCT116 TP53-mAID-mCherry ROLECCS clones (cl 1, cl 2) were analyzed. Images are representative of two independent experiments. F WB analysis of HCT116 TP53-mAID-mCherry AAVS1-edited with ROLECCS G1, clone 1 (cl 1) after sorting. Cells were treated with auxin or left untreated for one hour and then sorted for FITC intensity (ascending grey gradient triangle). Membrane was probed with anti-GFP antibody (reecognizing mEmerald, mEM) for ROLECCS G1 detection and mCherry antibody for TP53-mAID-mCherry (TP53-mAID-mCh) detection. Cdt1 and CyclinB1 were used as G1 phase and G2 phase specific markers, respectively. GAPDH was used as loading control. Not sorted (unsorted) cells were loaded for comparison. G WB analysis of HCT116 TP53-mAID-mCherry AAVS1-edited with ROLECCS G2, clone 1 (cl 1) after sorting. Treatments, sortings, and antibodies are the same as shown in (F). Blots are representative of two independent experiments.
Western blot analysis showed that gene-edited TP53 had a marked molecular size increase (final predicted molecular weight ~87KDa, compared to WT TP53, 53KDa), due to the presence of the mAID and mCherry tags (Fig. 4B). Of note, when probed with a TP53-specific antibody, edited clones displayed additional lower molecular weight bands, possibly due to an unstable linker sequence, previously described at the N-terminal domain of mCherry [53]. Importantly, gene-edited TP53 was still upregulated by DNA damaging agents such as cisplatin treatment, and its nuclear and cytoplasmic localization followed the expected distribution pattern [50, 51] (Fig. 4B). The additional lower bands displayed a similar trend upon genotoxic stress. Notably, gene-edited TP53 preserved transcriptional activity on the p21 and BAX promoters, as shown by Real Time (Fig. 4C) and Western Blot (Fig. 4D) analysis, upon cisplatin treatment. Endogenous TP53 expression was also increased at the transcriptional level (Fig. 4C) upon treatment.
Next, we further edited HCT116 TP53-mAID-mCherry cells inserting the ROLECCS constructs in the AAVS1 safe harbor site, generating HCT116 TP53-mAID-mCherry ROLECCS cell lines. As shown in Fig. 4E, sustained expression of ROLECCS AS, G1, and G2 with the expected molecular weight was achieved in at least 2 independent clones. Since this analysis was performed on asynchronously growing HCT116 TP53-mAID-mCherry ROLECCS cells, the three ROLECCS constructs apparently displayed different expression levels. However, these differences are likely due to the fact that ROLECCS G1 and ROLECCS G2 are expressed only in phase-specific cell subpopulations, while ROLECCS AS is equally expressed throughout the cell cycle. We also noticed a mild reduction in the levels of edited TP53 in comparison with parental HCT116 cells, compatible with the partial leakiness observed for the AID system [54, 55]. For this reason, for all the functional studies, cells were pre-treated with auxinole (as described in the Methods section), a previously reported inhibitor of OsTIR1 [54], to neutralize the activity of the ROLECCS system in the absence of auxin.
Finally, to validate that the ROLECCS system could allow cell-cycle specific target degradation of an endogenous target, we treated HCT116 TP53-mAID-mCherry ROLECCS G1 and G2 cells with auxin. At 1 h after auxin treatment, cells were sorted (Supplementary Fig. 6A–D) based on their green fluorescence and SSC, as described in the Methods section. As shown in Fig. 4F, G, TP53 downregulation was noticeable in unsorted populations both in ROLECCS G1- and G2-expressing cells. However, upon sorting, we observed that TP53 downregulation upon auxin treatment was only achieved in FITCmed and FITChigh sorted populations, in comparison with FITClow cells for both ROLECCS constructs. Importantly, Cdt1 and Cyclin B1 levels confirmed that ROLECCS G1 FITCmed and FITChigh represented a cell population enriched in G1/early S phase of the cell cycle. On the other hand, ROLECCS G2 FITCmed and FITChigh cells were mostly representing cells in the late S/G2 phase (see also Supplementary Fig. 9). Similar results were obtained using two independent clones for each ROLECCS protein (Supplementary Fig. 7A–D).
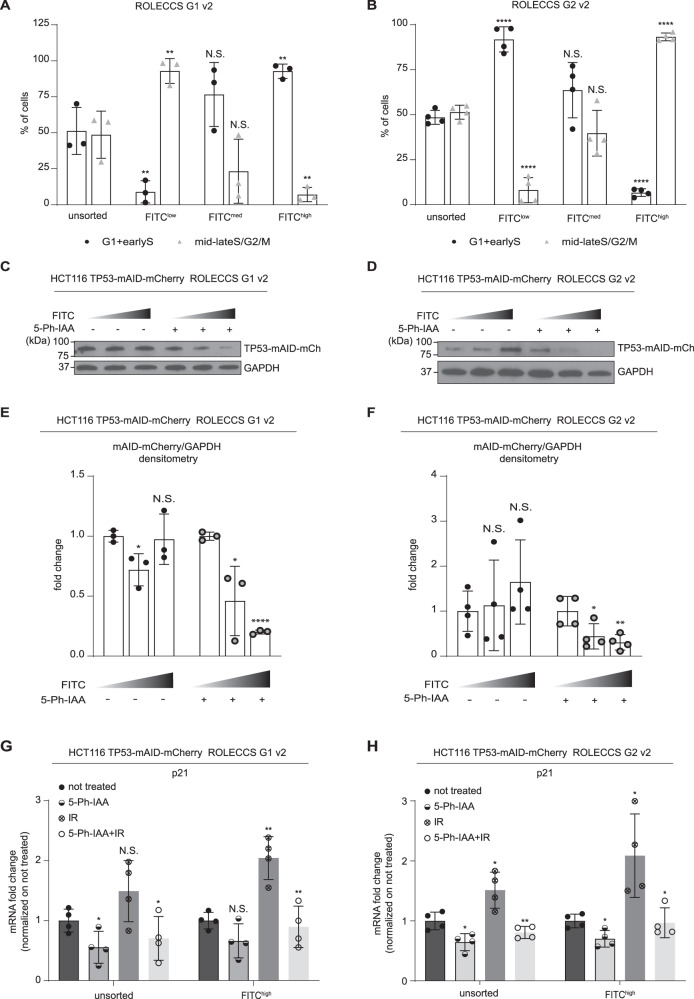
After these initial experiments, we noticed that the efficiency of the ROLECCS technology showed so far was only partially efficient, especially for ROLECCS G2. Nonetheless, these experiments required prior treatment with auxinole to prevent any leakiness shown by the OsTIR1 F-box protein, as described elsewhere [35, 55]. These drawbacks have been shown to be dramatically reduced by using an engineered variant of OsTIR1 containing a point mutation (F74G), which makes the system responsive to a synthetic variant of auxin, named 5-phenyl-indole-3-acetic acid (5-Ph-IAA) [35]. The OsTIR1(F74G) is able to induce efficient degradation of mAID-tagged proteins with no noticeable leakiness, more rapidly than the original AID system, in response to lower (more than 500 times) concentrations of the ligand, both in vitro and in vivo. Therefore, we decided to implement the same point mutant in our ROLECCS technology, generating the ROLECCS G1 v2 and the ROLECCS G2 v2 systems. HCT116 TP53-mAID-mCherry cells were edited inserting the ROLECCS v2 constructs in the AAVS1 safe harbor site, generating HCT116 TP53-mAID-mCherry ROLECCS v2 cell lines, as described above (Supplementary Fig. 8A), where TP53 expression levels could be abrogated promptly and reversibly (Supplementary Fig. 8B). Figure 5A, B show that, similarly to the previous ROLECCS constructs, ROLECCS G1 v2 and G2 v2 were specifically expressed in the G1 or lateS/G2/M phase of the cell cycle, respectively. Sorting (Supplementary Fig. 8C, D) of FITChigh ROLECCS G1 v2 resulted in a significant enrichment of cells in the G1 phase of the cell cycle, while FITClow cells were significantly enriched in cells progressing through S/G2/M, as demonstrated by propidium iodide staining after sorting (Fig. 5A). Conversely sorting of FITChigh ROLECCS G2 v2 resulted in a significant enrichment of cells in the S/G2/M phase of the cell cycle, while FITClow cells were significantly enriched in cells progressing through G1 (Fig. 5B). We then performed WB analysis on protein extracts from samples sorted as described above, and assessed the levels of TP53-mAID-mCherry (Fig. 5C–F). Notably, both ROLECCS G1 v2 and ROLECCS G2 v2 displayed a noticeable and significant downregulation of their target, specifically when ROLECCS v2 levels were highest (FITChigh, corresponding to enriched G1 or S/G2/M, respectively). Importantly, FITClow ROLECCS v2 systems did not display noticeable differences in the levels of their target, when comparing control vs 5-Ph-IAA treated samples.
Fig. 5. Cell cycle phase-specific expression and functionality of ROLECCS v2 proteins.
A, B Quantification of cell-cycle distribution experiments of HCT116 TP53-mAID-mCherry AAVS1-integrated clones expressing ROLECCS v2 G1 (A) and G2 (B). Cells were prior sorted based on FITC levels (FITClow, FITCmed, FITChigh), as described in Supplementary Fig. 8, and then stained with propidium iodide as described in Methods. FITClow, FITCmed, FITChigh subpopulations were analyzed for cells composition as percentage of cells in G1+earlyS and cells in mid-lateS/G2/M, using ModFit software v5.0. Not sorted (unsorted) populations are reported for comparison. Data are the average of three independent experiments (n = 3, Fig. 5A) and four independent experiments (n = 4, Fig. 5B). Error bars indicate mean ± SD. ****p < 0.0001, **p < 0.01, N.S. not significant. Statistics (two-tailed t-test) is calculated versus respective unsorted populations. C WB analysis of HCT116 TP53-mCherry AAVS1-edited with ROLECCS v2 G1 after sorting. Cells were treated with 1 µM 5-Ph-IAA or left untreated for 1 h and then sorted for increasing FITC intensity (ascending grey gradient triangle). Membrane was probed with anti-GFP antibody, that recognizes the mEmerald tag (mEM) of the protein, was used for ROLECCS G1 detection and mCherry antibody for TP53-mAID-mCherry (TP53-mAID-mCh) detection. GAPDH was used as loading control. D WB analysis of HCT116 TP53-mCherry AAVS1-edited with ROLECCS v2 G2 after sorting. Treatments, sortings and antibodies are the same as (C). E Densitometric quantification of TP53-mAID-mCherry normalized on GAPDH intensity of WB analyses. Relative quantification versus FITClow sorted population is reported. Densitometric analyses are the average of at least one experiment on 2 different clones (n = 3). Error bars indicate mean ± SD. ****p < 0.0001, *p < 0.05, N.S. not significant. Statistics (two-tailed t-test) is calculated versus respective FITClow sorted population. F Densitometric quantification performed as in (E). Error bars indicate mean ± SD. **p < 0.01, *p < 0.05, N.S. not significant. Statistics (two-tailed t-test) is calculated versus respective FITClow sorted population (n = 4). G, H Messenger RNA fold changes of p21 gene in HCT116 TP53-mAID-mCherry AAVS1-integrated clones expressing ROLECCS v2 G1 (G) and G2 (H) quantified by Real Time PCR. Cells were treated with 5-Ph-IAA or left untreated for one hour. Cells were then exposed to ionizing radiation or not with 5 Gy. After two hours from radiation, cells were sorted based on FITC levels (FITClow, FITCmed, FITChigh), as described in Supplementary Fig. 8, and collected for RNA extraction. OAZ1 gene was used as housekeeping control and data were normalized on not treated samples. Error bars indicate mean ± SD. **p < 0.01, *p < 0.05, N.S. not significant. Statistics (two-tailed t-test) is calculated versus not treated. Experiment was repeated twice on two independent clones (n = 4).
Finally, we assessed whether ROLECCS G1 v2 and ROLECCS G2 v2 could consistently downregulate TP53 and prevent its transcriptional activity. To this end, HCT116 TP53-mAID-mCherry ROLECCS v2 cells were pre-treated with 5-Ph-IAA for 1 h, then exposed to 5 Gy of gamma-irradiation to trigger TP53 transcriptional activity, allowed to recover for 2 h, and sorted as described above to collect total RNA. We then performed qRT-PCR to assess the levels of CDKN1A mRNA, which encodes for the tumor suppressor p21 (CIP1/WAF1) protein, a known transcriptional target of TP53 throughout the cell cycle. As shown in Fig. 5G, H, ROLECCS v2-mediated TP53 degradation significantly prevented the activation of CDKN1A expression in response to gamma-radiation. The inhibition of CDKN1A was already noticeable in unsorted cells, but cell sorting-mediated enrichment of cells expressing ROLECCS G1 v2 or ROLECCS G2 v2 further increased the significance of the observed phenotype.
Altogether, these data indicate that the ROLECCS and the ROLECCSv2 systems can be used to achieve the phase-specific downregulation of an endogenous target, appropriately gene edited to include a mAID tag.
Cell cycle phase-specific TP53 degradation has different effects on micronuclei formation
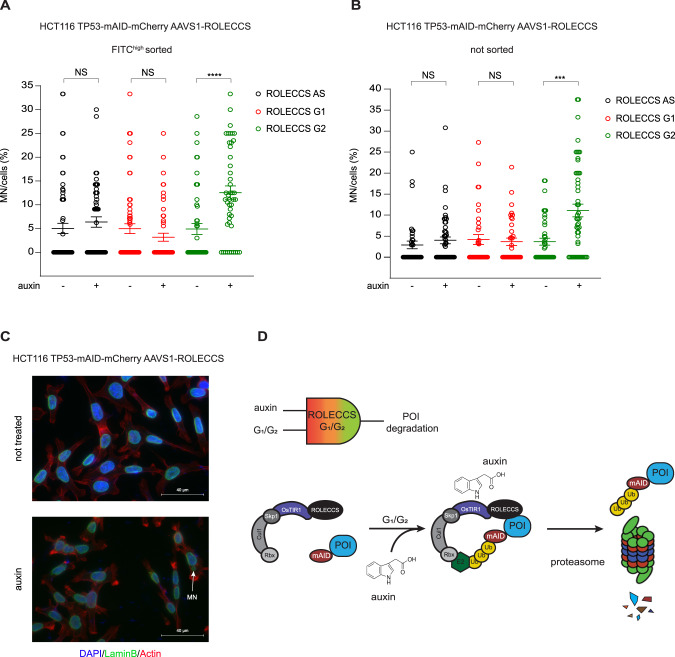
TP53 is one of the most frequently mutated genes in human cancer [50, 52, 56]. Its most well studied biological function is the regulation of expression of genes involved in cell cycle arrest, DNA repair, and apoptosis [52, 56]. Loss of TP53 results in altered DNA damage response, reduced cell death even in response to anti-neoplastic treatments, and genomic instability [52, 56]. For these reasons, TP53 is considered the main “guardian of the genome” [50, 57]. Micronuclei are the result of missegregated chromosomes that, upon mitotic exit, can recruit a lamin B-positive nuclear envelope, creating subcellular structures that are frequently identified in genetically unstable human tumors [58]. One of the still not completely understood roles of TP53 in maintaining genome stability is its capability of preventing micronuclei formation or regulating the faith of micronucleated cells [59–61]. To gain insights into novel potential cell cycle-phase specific functions of TP53, we assessed the capability of HCT116 TP53-mAID-mCherry ROLECCS AS, G1, and G2 cells to spontaneously form micronuclei upon auxin treatment. Asynchronously growing cells were treated for 1 h with auxin, harvested, sorted as described above (Supplementary Fig. 8C, D) and plated on glass coverslips in complete media containing auxin for 24 h. In parallel, cells were similarly collected and used for both WB analysis to confirm TP53-mAID-mCherry degradation upon auxin treatment (Supplementary Fig. 9A) and enrichment of cells in G1 or G2 (Supplementary Figs. 9B–D). ROLECCS AS cells were used as control for generalized (non-cell cycle phase-specific) TP53 degradation. At 24 h from the plating, cells were fixed and stained for Lamin B and DNA to visualize nuclei and micronuclei, and the relative abundance of micronucleated cells was quantified by fluorescence microscopy (Fig. 6A and C). Interestingly, neither TP53 degradation in any phase of the cell cycle (ROLECCS AS, black) nor specifically in the G1 (ROLECCS G1, red) led to an altered formation of micronuclei that could reach statistical significance. However, when TP53 was specifically degraded in S/G2/M phase (ROLECCS G2, green), we observed a significant increase in the number of micronucleated cells. To demonstrate that cell sorting is not required to perform this assay, and it is not related to the observed phenotype, we repeated the same experiment on asynchronously growing HCT116 TP53-mAID-mCherry ROLECCS AS, G1, and G2 cells, continuously treated with auxin for 24 h. As shown in Fig. 6B, ROLECCS G2, but not ROLECCS G1 and AS, displayed a significant increase in the number of micronucleated cells upon auxin treatment. These results indicate that TP53 plays different cell cycle phase-specific roles in preventing accumulation of micronucleated cells.
Fig. 6. Micronuclei accumulation upon cell cycle phase-specific TP53 abrogation.
A HCT116 TP53-mAID-mCherry ROLECCS AS, G1, and G2 were treated with auxin or left untreated for 1 h and then FITChigh population was sorted and plated on glass coverslips in the presence of auxin or medium for 24 h before fixation and IF staining for Lamin B. Dot plot graph represents percentage of micronucleated HCT116 TP53-mAID-mCherry ROLECCS cells with micronuclei per field. B Asynchronously growing HCT116 TP53-mAID-mCherry ROLECCS AS, G1, and G2 were seeded on glass coverslips for 24 h, then treated with auxin or left untreated for 24 h before fixation and IF staining. Dot plot graph representing percentage of cells with micronuclei per field. Error bars in A and B indicate mean ± SD. ****p < 0.0001, ***p < 0.001, N.S. not significant. Statistics (two-tailed t-test) is calculated versus respective not treated. Data represent four independent experiments. C Representative images of micronuclei immunofluorescence staining in HCT116 TP53-mAID-mCherry ROLECCS cells. Micronuclei (MN, white arrow) are identified as separate extra-nuclear structures with rounded shape, positive for DAPI (blue) and encased by nuclear envelope positive to laminin B1 staining (green). Phalloidin-iFluor 647 staining (red) was used to stain actin, to facilitate single cell identification. D Schematic description of the ROLECCS system for cell cycle-specific targeted proteolysis. The ROLECCS system performs a Boolean logic computation. The contemporary presence of auxin and appropriate phase of the cell cycle are both simultaneously required to lead to targeted protein degradation. ROLECCS G1 and G2 are stable only through specific phases of the cell cycle (G1/early S for ROLECCS G1, late S/G2/M for ROLECCS G2), therefore their biological activity is restricted to those phases. However, auxin is required to trigger OsTIR1-mediated protein ubiquitylation, allowing proteasomal degradation of the POI only “on demand”, and only in the appropriate phase of the cell cycle.
Discussion
The temporal discrimination of protein functions is critical to fully understand how the same factor might carry out different tasks during different phases of the cell cycle, ultimately leading to diverse biological outcomes. Therefore, “timing is everything” [62].
The development of mAID systems has allowed sharp and quick modulation of the levels of a protein of interest [36, 63, 64]. Considering the relatively short duration of cell division cycle, a rapid depletion of the protein of interest is of paramount importance. In this report, we introduced a novel tool to rapidly and reversibly regulate levels of virtually any protein in a cell cycle status-dependent manner, using the “Regulated OsTIR1 Levels of Expression based on the Cell-Cycle Status” (ROLECCS) system.
We generated two different ROLECCS proteins (ROLECCS G1 and ROLECCS G2), by fusing the Oryza sativa TIR1 (OsTIR1) F-box protein, the fluorescent indicator mEmerald and the FUCCI tags Cdt1 and Geminin, respectively. The ROLECCS system exerts its targeted proteolytic activity based on a Boolean-logic computational process. In fact, the ROLECCS proteins follow a bi-modal activation status (enzyme is active in the appropriate phase of the cell cycle/enzyme is inactive in the unwanted phase of the cell cycle). In fact, the presence of the phytohormone auxin and the appropriate cell cycle status are both simultaneously required to trigger the biological functions of ROLECCS proteins. As a result, the degradation of the mAID-tagged POI is temporally restricted to a specific cell cycle status, and only in the presence of auxin (Fig. 1 and Fig. 6D).
In our first design, the ROLECCS system is specifically integrated by CRISPR/Cas9 knock-in in the AAVS1 safe harbor genomic locus (Fig. 2). In these settings, we observed appropriate phase-specific expression of the ROLECCS proteins. Western blot, flow cytometry and live cell imaging using fluorescence and contrast-phase microscopy confirmed that ROLECCS G1 and ROLECCS G2 expression had a maximum expression peak in G1 and late S/G2, respectively (Fig. 2 and Supplementary Videos 1–3). However, we noticed that exogenous targets (e.g. transiently transfected mAID-tagged POIs) were effectively down-regulated only at very short time points after the transfection. We hypothesized that this was due to molar excess of the transfected target POI in comparison with ROLECCS proteins, especially at longer time points.
For this reason, we designed All-in-One constructs, similar to others recently reported [46], allowing the simultaneous expression of the ROLECCS proteins and their mAID-tagged targets (pLentiROLECCS), using mAID-mCherry for our tests. Our results supported the conclusion that ROLECCS proteins require to be at least in equimolar ratio to their targets to achieve consistent target degradation upon auxin treatment. Therefore, the pLentiROLECCS system is a flexible and relatively simple way to generate cell lines in which an exogenous POI can be modulated on-demand (upon auxin treatment) in specific phases of the cell cycle (Fig. 3).
Our ultimate goal was to generate a system to synthetically control endogenous protein levels based only on the cell cycle status, minimizing potential artificial factors such as the use of an exogenous promoter. Therefore, as proof-of-principle, we attempted to regulate the levels of a protein encoded by an endogenous gene, fused by CRISPR/Cas9 knock-in with the mAID tag. We chose the gene TP53 because of its well-known role in the regulation of cell cycle progression [51]. Moreover, gene editing of this gene with the mAID tag was previously reported [37]. Here, we show that cell cycle specific TP53 degradation was effectively achieved with both the ROLECCS G1 and the ROLECCS G2 systems (Fig. 4).
We also noticed that proteolysis of TP53, although significant, was not complete, especially with the ROLECCS G2 system. Therefore, the first iteration of ROLECCS G1 and G2 systems allowed us to demonstrate the appropriate restricted expression of the system in quiescent or dividing cells, respectively, but did not display sufficient biological activity for downstream uses.
Recently, a point mutant of OsTIR1 (F74G) was reported, establishing the mAID version 2 (mAID2) system, which does not respond to natural auxin but only to a synthetic ligand (5-Ph-IAA). Interestingly, this point mutant displayed no detectable leaky degradation of the target, was responsive to 670-times lower concentration of the ligand [35] and it was functional also in vivo using mouse models. For these reasons, we decided to implement this mutation in our first ROLECCS system, which resulted in the ROLECCS v2 technology. The ROLECCS v2 displayed a more potent and reliable cell cycle phase-specific targeted proteolysis of TP53.
Last, we wanted to assess whether our targeted proteolytic system could allow the identification of novel biological functions of TP53 related to cell cycle status, such as accumulation of micronucleated cells [59, 60]. First, our results confirmed that the ROLECCS system is a valuable tool to study protein functions and identify biological outcomes, which would not be noticeable or reach statistical significance when using conventional degradation or inhibition approaches. Second, our data indicate that the biological role of TP53 in preventing micronuclei accumulation is related to differential biological functions exerted in different phases of the cell cycle. In fact, our findings suggest that, at least in our model, TP53 abrogation during the S/G2, but not in the G1 phase of the cell cycle results in accumulation of micronucleated cells even in the absence of DNA-damaging agents (Fig. 6). Interestingly, when TP53 was abrogated in any phase of the cell cycle (using ROLECCS AS), a small, but not statistically significant, increase in the relative abundance of micronuclei was observed, highlighting the importance of the use of a phase-specific targeted proteolytic system. Future studies taking advantage of the ROLECCS system could allow to understand the mechanism through which S/G2/M phase-specific TP53 downregulation leads to an increase of micronucleated cells. One potential explanation of the observed phenotype is that TP53 actively regulates phase-specific genes involved in the prevention of the formation of micronuclei, especially in G2/M phase. On the other end, absence of TP53 could alter micronuclei formation similarly in G1 or S/G2/M, but with different outcomes regarding cell proliferation or activation of apoptosis. The characterization of TP53 biological functions are beyond the scope of the future study, but here we show how the implementation of the ROLECCS system could provide the appropriate technical solutions to answer biological questions with a novel temporal resolution during the cell division cycle.
The ROLECCS systems described here are the initial prototypes of multiple potential cell cycle phase-specific degron technologies. For example, the choice of different FUCCI tags, as described previously, could lead to a more accurate phase-specific protein degradation. In fact, the FUCCI tags used for the ROLECCS proteins in the present study are partially co-expressed in late-G1/early-S phase. However, it has been previously demonstrated the FUCCI systems (PIP-FUCCI, FUCCI(CA) and FUCCI(SCA)) could be used to achieve a sharp down-regulation of the ROLECCS system during cell cycle transitions [27, 65]. It is also important to note that the Cdt1 tag used in the current ROLECCS iteration acquires stability right after the anaphase, during the late stages of mitosis. Therefore, additional protein tags might be required to specifically dissect protein biological functions during different stages of mitosis.
Protein biological functions and cell cycle progression are intimately connected and reciprocally affected. Hence, the cell cycle status should be taken into account for the study of any biological phenomenon. Thanks to its phase specificity, rapidity, reversibility, and low overall perturbation of other biological processes, the ROLECCS technology represents a unique tool for the investigation of biological phenomena and their relationship with the cell cycle progression.
Supplementary information
Acknowledgements
We thank Dr. Emanuele Cocucci for the scientific discussions during the preparation of this manuscript. We also thank the countless investigators who made their plasmids and reagents available through public repositories such as Addgene.
Author contributions
MC, DP and CMC conceived and designed the project. MC, DP, AT, WOM conceived and planned the experiments. MC, DP, AT, JM, GLRV, CL, WOM executed the experiments. AED and DL performed and analyzed live microscopy experiments. MC, DP, AT, GLRV, CL, WOM and AED analyzed the data. BM provided technical support for flow cytometry experiments and cell sorting. MC and DP, wrote the manuscript. AT, JM, GLRV, CL, WOM, VC and CMC provided critical feedback and contributed to the final version of the manuscript.
Funding
This work was supported by seed funds from The Ohio State University Comprehensive Cancer Center (DP, CMC), Pelotonia (DP), NCI-NIH (NIH R35CA197706) (CMC), and ORIP-NIH (K01OD031811-01) (AED). The Gene Editing Shared Resource, the Flow Cytometry Shared Resource and the Genomics Shared Resource that contributed to this study are supported by the Cancer Center Support Grant P30CA016058.
Data availability
Original unprocessed data used for the preparation of the manuscript are available upon kind request. All data supporting the findings of this study are available within the paper and its Supplementary Information files (Source Data File).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dario Palmieri, Carlo M. Croce.
Contributor Information
Dario Palmieri, Email: dario.palmieri@osumc.edu.
Carlo M. Croce, Email: carlo.croce@osumc.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-023-01191-4.
References
- 1.Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35:461–78. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. An overview of the cell cycle. 2002.
- 3.Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annu Rev Genet. 2007;41:237–80. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda DY, Dutta A. DNA replication and progression through S phase. Oncogene. 2005;24:2827–43. doi: 10.1038/sj.onc.1208616. [DOI] [PubMed] [Google Scholar]
- 5.Lew D Cell Cycle. In: Encyclopedia of Genetics. Elsevier, 2001, pp 286–96.
- 6.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–49. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Güttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol. 2009;10:178–91. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 8.Carlton JG, Jones H, Eggert US. Membrane and organelle dynamics during cell division. Nat Rev Mol Cell Biol. 2020;21:151–66. doi: 10.1038/s41580-019-0208-1. [DOI] [PubMed] [Google Scholar]
- 9.Smoyer CJ, Jaspersen SL. Breaking down the wall: the nuclear envelope during mitosis. Curr Opin Cell Biol. 2014;26:1–9. doi: 10.1016/j.ceb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Salina D, Enarson P, Rattner JB, Burke B. Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J Cell Biol. 2003;162:991–1001. doi: 10.1083/jcb.200304080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raemaekers T, Ribbeck K, Beaudouin J, Annaert W, Van Camp M, Stockmans I, et al. NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization. J Cell Biol. 2003;162:1017–29. doi: 10.1083/jcb.200302129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeBrasseur N. When nuclear proteins go mitotic. J Cell Biol. 2003;162:958–9. [Google Scholar]
- 13.Chao HX, Poovey CE, Privette AA, Grant GD, Chao HY, Cook JG, et al. Orchestration of DNA damage checkpoint dynamics across the human cell cycle. Cell Syst. 2017;5:445–459.e5. doi: 10.1016/j.cels.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Microtubule dynamics and motor proteins during Mitosis. 2000.
- 15.Schorl C, Sedivy JM. Analysis of cell cycle phases and progression in cultured mammalian cells. Methods. 2007;41:143–50. doi: 10.1016/j.ymeth.2006.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzbekov RE. Analysis of the cell cycle and a method employing synchronized cells for study of protein expression at various stages of the cell cycle. Biochem (Mosc) 2004;69:485–96. doi: 10.1023/B:BIRY.0000029845.11184.30. [DOI] [PubMed] [Google Scholar]
- 17.Bittmann J, Grigaitis R, Galanti L, Amarell S, Wilfling F, Matos J et al. An advanced cell cycle tag toolbox reveals principles underlying temporal control of structure-selective nucleases. eLife 2020; 9. 10.7554/ELIFE.52459. [DOI] [PMC free article] [PubMed]
- 18.Karras GI, Jentsch S. The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell. 2010;141:255–67. doi: 10.1016/j.cell.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Hombauer H, Campbell CS, Smith CE, Desai A, Kolodner RD. Visualization of eukaryotic DNA mismatch repair reveals distinct recognition and repair intermediates. Cell. 2011;147:1040–53. doi: 10.1016/j.cell.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zielke N, Edgar BA. FUCCI sensors: powerful new tools for analysis of cell proliferation. Wiley Interdiscip Rev: Dev Biol. 2015;4:469–87. doi: 10.1002/wdev.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saitou T, Imamura T. Quantitative imaging with Fucci and mathematics to uncover temporal dynamics of cell cycle progression. Dev, Growth Differ. 2016;58:6–15. doi: 10.1111/dgd.12252. [DOI] [PubMed] [Google Scholar]
- 22.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–98. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Vodermaier HC APC/C and SCF: Controlling each other and the cell cycle. Curr Biol. 2004; 14. 10.1016/j.cub.2004.09.020. [DOI] [PubMed]
- 24.Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–8. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 25.Benmaamar R, Pagano M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle. 2005;4:1230–2. doi: 10.4161/cc.4.9.2048. [DOI] [PubMed] [Google Scholar]
- 26.Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–8. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- 27.Sakaue-Sawano A, Yo M, Komatsu N, Hiratsuka T, Kogure T, Hoshida T, et al. Genetically encoded tools for optical dissection of the mammalian cell cycle. Mol Cell. 2017;68:626–640.e5. doi: 10.1016/j.molcel.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Nora EP, Goloborodko A, Valton AL, Gibcus JH, Uebersohn A, Abdennur N, et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell. 2017;169:930–944.e22. doi: 10.1016/j.cell.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajar BT, Lam AJ, Badiee RK, Oh YH, Chu J, Zhou XX, et al. Fluorescent indicators for simultaneous reporting of all four cell cycle phases. Nat Methods. 2016;13:993–6. doi: 10.1038/nmeth.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe T, Sakaue-Sawano A, Kiyonari H, Shioi G, Inoue KI, Horiuchi T, et al. Visualization of cell cycle in mouse embryos with Fucci2 reporter directed by Rosa26 promoter. Dev (Camb) 2013;140:237–46. doi: 10.1242/dev.084111. [DOI] [PubMed] [Google Scholar]
- 31.Spencer SL, Cappell SD, Tsai F-C, Overton KW, Wang CL, Meyer T. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell. 2013;155:369–83. doi: 10.1016/j.cell.2013.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38:824–44. doi: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- 33.Ashfaq MA, Dinesh Kumar V, Soma Sekhar Reddy P, Anil Kumar C, Sai Kumar K, Narasimha Rao N, et al. Post-transcriptional gene silencing: basic concepts and applications. J Biosci. 2020;45:1–10. doi: 10.1007/s12038-020-00098-3. [DOI] [PubMed] [Google Scholar]
- 34.Kallunki T, Barisic M, Jäättelä M, Liu B. How to choose the right inducible gene expression system for mammalian studies? Cells. 2019; 8. 10.3390/cells8080796. [DOI] [PMC free article] [PubMed]
- 35.Yesbolatova A, Saito Y, Kitamoto N, Makino-Itou H, Ajima R, Nakano R, et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat Commun. 2020;11:1–13.. doi: 10.1038/s41467-020-19532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Röth S, Fulcher LJ, Sapkota GP. Advances in targeted degradation of endogenous proteins. Cell Mol Life Sci. 2019;76:2761–77. doi: 10.1007/s00018-019-03112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natsume T, Kiyomitsu T, Saga Y, Kanemaki MT. Rapid protein depletion in human cells by auxin-inducible degron tagging with short homology donors. Cell Rep. 2016;15:210–8. doi: 10.1016/j.celrep.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 39.Kubota T, Nishimura K, Kanemaki MT, Donaldson AD. The Elg1 replication factor c-like complex functions in PCNA unloading during DNA replication. Mol Cell. 2013;50:273–80. doi: 10.1016/j.molcel.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann S, Fachinetti D. Real-time de novo deposition of centromeric histone-associated proteins using the auxin-inducible degradation system. In: Methods Mol Biol. Humana Press Inc., 2018, pp 223-41. [DOI] [PubMed]
- 41.Lai S-L, Perng R-P, Hwang J. p53 gene status modulates the chemosensitivity of non-small cell lung cancer cells. J Biomed Sci. 2000;7:64–70. doi: 10.1007/BF02255920. [DOI] [PubMed] [Google Scholar]
- 42.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutschner T, Haemmerle M, Genovese G, Draetta GF, Chin L. Post-translational regulation of Cas9 during G1 enhances homology-directed repair. Cell Rep. 2016;14:1555–66. doi: 10.1016/j.celrep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90:1103–63. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 45.Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2003;60:523–33. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- 46.Yesbolatova A, Saito Y, Kanemaki MT. Constructing auxin-inducible degron mutants using an all-in-one vector. Pharmaceuticals 2020; 13. 10.3390/ph13050103. [DOI] [PMC free article] [PubMed]
- 47.Kim JH, Lee S-R, Li L-H, Park H-J, Park J-H, Lee KY, et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE. 2011;6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu E, Li X, Yan F, Zhao Q, Wu X. Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J Biol Chem. 2004;279:17283–8. doi: 10.1074/jbc.C300549200. [DOI] [PubMed] [Google Scholar]
- 49.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci USA. 2006;103:10660. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 51.Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062–78. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levine AJ p53: 800 million years of evolution and 40 years of discovery. 10.1038/s41568-020-0262-1. [DOI] [PubMed]
- 53.Huang L, Pike D, Sleat DE, Nanda V, Lobel P. Potential pitfalls and solutions for use of fluorescent fusion proteins to study the lysosome. PLoS ONE 2014; 9. 10.1371/journal.pone.0088893. [DOI] [PMC free article] [PubMed]
- 54.Yesbolatova A, Natsume T, Hayashi KI, Kanemaki MT. Generation of conditional auxin-inducible degron (AID) cells and tight control of degron-fused proteins using the degradation inhibitor auxinole. Methods. 2019;164–165:73–80. doi: 10.1016/j.ymeth.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Li S, Prasanna X, Salo VT, Vattulainen I, Ikonen E. An efficient auxin-inducible degron system with low basal degradation in human cells. Nat Methods. 2019;16:866–9. doi: 10.1038/s41592-019-0512-x. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani F, Collavin L, Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–6. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 58.Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salazar AM, Sordo M, Ostrosky-Wegman P. Relationship between micronuclei formation and p53 induction. Mutat Res. 2009;672:124–8. doi: 10.1016/j.mrgentox.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–81. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kakoti S, Yamauchi M, Gu W, Kato R, Yasuhara T, Hagiwara Y, et al. p53 deficiency augments nucleolar instability after ionizing irradiation. Oncol Rep. 2019;42:2293–302. doi: 10.3892/or.2019.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadonaga JT. Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors. Cell. 2004;116:247–57. doi: 10.1016/S0092-8674(03)01078-X. [DOI] [PubMed] [Google Scholar]
- 63.Lambrus BG, Moyer TC, Holland AJ. Applying the auxin-inducible degradation system for rapid protein depletion in mammalian cells. In: Methods Cell Biol. Academic Press Inc., 2018, pp 107–35. [DOI] [PubMed]
- 64.Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods. 2009;6:917–22. doi: 10.1038/nmeth.1401. [DOI] [PubMed] [Google Scholar]
- 65.Grant GD, Kedziora KM, Limas JC, Cook JG, Purvis JE. Accurate delineation of cell cycle phase transitions in living cells with PIP-FUCCI. Cell Cycle (Georget, Tex) 2018;17:2496–516. doi: 10.1080/15384101.2018.1547001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original unprocessed data used for the preparation of the manuscript are available upon kind request. All data supporting the findings of this study are available within the paper and its Supplementary Information files (Source Data File).