Abstract
Postoperative radiotherapy is the standard method for inhibition of breast cancer recurrence and metastasis, whereas radiation resistant and ineluctable skin radiation injury are still key problems encountered in the prognosis of breast cancer. Herein, we design an internally implantable biodegradable hydrogel and extracutaneously applicable antioxidant bioadhesive to concurrently prevent postoperative tumor recurrence and radioactive skin injury after adjuvant radiotherapy. The biodegradable silk fibroin/perfluorocarbon hydrogel loading doxorubicin (DOX) formed by consecutive ultrasonication-induced β-sheets-crosslinked amphiphilic silk fibroin/perfluorocarbon/DOX nanoemulsion, exhibits continuous release of oxygen in physiological environment to improve hypoxia and sensitivity of radiotherapy, as well as simultaneous release of DOX to finally achieve effective anti-cancer effect. A stretchable bioadhesive is fabricated by copolymerization of α-thioctic acid and N, N-diacryloyl-l-lysine, and gold nanorods and gallic acid are loaded into the bioadhesive to afford gentle photothermal therapy and antioxidant functions. The near-infrared light-induced controlled release of gallic acid and mild photothermal therapy can efficiently eliminate excess free radicals generated by radiotherapy and promote radioactive wound healing. Ultimately, in vivo animal studies substantiate the efficacy of our methodology, wherein the post-tumor resection administration of hydrogel and concomitant application of an antioxidant bioadhesive patch effectively inhibit tumor recurrence and attenuate the progression of skin radiation damage.
Keywords: Breast cancer, Biodegradable hydrogel, Bioadhesive, Adjuvant radiotherapy, Radiation skin injury
Graphical abstract
Highlights
-
•
A biodegradable hydrogel that releases O2 and DOX, and an antioxidant bioadhesive are developed.
-
•
The stretchable bioadhesive exhibits antioxidant and photothermal properties.
-
•
Combination of the hydrogel and bioadhesive concurrently prevents tumor recurrence and attenuates skin radiation damage.
1. Introduction
Surgical resection is the major form for patients who are diagnosed with breast cancer [1]. Clinically, more than 90% of breast cancer patients in early stage need to receive surgery [2]. Although five-year survival of patients with breast cancers has been improved, the high risk of postoperative recurrence and distant metastases is still a fatal problem, which has become the leading causes of death for breast cancer [3]. In clinic, adjuvant radiotherapy (RT) after breast-conserving surgery that is the standard treatment for surgical patients can remarkably reduce the local recurrence rate and mortality [4]. However, the severe side effect of RT is impossible to be ignored, while beyond 90% patients after RT endure the skin radiation damage [5]. The grievous unbearable skin radiation injury is commonly unable to self-cure and vulnerable to be infected, not to mention the bleeding immunocompromised surgical wound. Unfortunately, there are still no effective ways to solve the contradictory conflict between tumor recurrence control and radiation skin injury in postoperative RT.
Radiosensitization is an efficient method that remarkably improves the local therapeutic effects of RT and effectively alleviates damage to normal tissue [[6], [7], [8]]. Hypoxic microenvironment in tumors and insufficient O2 supply caused by massive consumption of oxygen during radiotherapy are the main obstacles for radiotherapy tolerance [9,10]. Under this condition, it is difficult to ensure the production of enough ROS to eliminate the residual tumor [11]. Thus, the enhancement of local O2 contents in tumor surgical site is the key to enhancing adjuvant radiotherapeutic effect for prevention of tumor reoccurrence. An X-ray triggered catalysis system based on gas-radiotherapy for ROS enhancement is a promising strategy for cancer synergistic radiosensitization [12]. Hydrogels are biocompatible soft wet materials containing abundant water that can be loaded with massive O2 and maintain oxygen-enriched environment by continuous release of O2, which is expected to be an ideal radiosensitization agent for postoperative RT [13]. A nano-oxygenated hydrogel loaded with lyophilized oxygen-encapsulated nanoparticles for chronic wound healing was recently reported [14]. The hydrogel was able to deliver dissolved oxygen to relieve local and permeable hypoxia. The radiotherapy capacity could be dramatically enhanced by improving tumor hypoxia microenvironment through self-supplied O2 strategy; nonetheless, the skin radiation injury may also become more serious due to overproduction of ROS. It is a vicious circle that the more irradiation of RT, the more severe the radiation damage to normal tissue. Therefore, merely relying on improving the effect of adjuvant RT cannot fundamentally solve the contradiction between tumor recurrence control and radiation skin injury.
The radiation-induced skin injury, including dyspigmentation, ulceration, erythema, is more complicated and intractable compared with normal wounds [15]. Even worse, the aggravation condition of radiation damage may occur due to potential infection and uncontrollable bleeding in surgical wound. The interaction between radiation and water generates continuous reactive oxygen species, causing DNA damage and inducing apoptosis. Eventually, radiation damage becomes more difficult to heal than normal skin damage, seriously affecting the quality of life of patients [16]. Thus, the wound closure and ROS clearance after surgery and adjuvant RT are indispensable procedures for treatment of radioactive surgical trauma. Notably, the skin injury often occurs several weeks or more after RT, but the postoperative treatment of tumor recurrence is mainly performed in the short term after surgery. This difference motivates us to develop a novel strategy for two-step postoperative treatment with on-demand radiosensitization and ROS elimination property.
To achieve this goal, we designed an internally implantable biodegradable hydrogel and extracutaneous applicable antioxidant bioadhesive to concurrently prevent postoperative tumor recurrence and radioactive skin injury after adjuvant RT. The biodegradable hydrogel was constructed by ultrasonication of silk fibroin/perfluorocarbon (SF/PF) emulsion loading doxorubicin (DOX). In clinic, the perfluorinated chemicals (e.g., perfluorodecalin) are widely applied in vitreoretinal surgery to deliver oxygen as an oxygen-carrying agent [[17], [18], [19], [20], [21], [22], [23]]. Consecutive ultrasonication induced transition of β-sheets of SF to trigger the formation of hydrogel, in which PF was well-dispersed. The biodegradable hydrogel was capable of continually oxygenating and releasing DOX to alleviate local hypoxia and enhance the antitumor efficacy via radiosensitization and combined chemo-radiotherapy around tumor site. Meanwhile, the antioxidant bioadhesive was fabricated by copolymerization of α-thioctic acid (TA) and N, N-diacryloyl-l-lysine that could stabilize Poly-TA (PTA) and provide additional adhesive groups, with addition of gold nanorods (AuNRs) and gallic acid (GA) (Scheme 1). The resultant PTA-based patches served as wound dressing after postoperative RT. As a natural disulfide-containing compound, α-thioctic acid can be transformed into a PTA patch with adhesive, self-healing and stretchable properties, which could rapidly stop bleeding, seal wounds and inhibit bacterial infections for surgical wound [[24], [25], [26]]. The combination of positively charged AuNRs and negatively charged Poly-TA network through electrostatic interactions endowed the patches with a photothermal function that allowed for controlled drug release and provided a mild-temperature photothermal therapy to promote skin wound healing [[27], [28], [29], [30]]. Furthermore, the antioxidant activity could be triggered by responsive release of loaded GA via photothermal property of AuNRs after near-infrared (NIR) irradiation [[31], [32], [33]].
Scheme 1.
Schematic illustration of the fabrication of SF/PF@DOX hydrogel and PTA@Au-GA patch and their application in treatment of breast cancer recurrence and radiation skin injury.
It is imagined that the residual tumor after surgery could be thoroughly eliminated through radiosensitization and combination of RT and chemotherapy by self-supplied O2 of biodegradable hydrogel, ensuring the prevention of postoperative tumor recurrence. After adjuvant RT, the excessive ROS could be scavenged by NIR-controlled release of gallic acid from the bioadhesive patches at skin surface to alleviate radiation skin damage. Moreover, the bioadhesive patches could conform to irregular wounds to protect wounds from infection, and promote wound healing by mild photothermal therapy. Thus, the combination of biodegradable hydrogel and antioxidant bioadhesive are indispensable in synergistic therapy of breast cancer recurrence and skin radiation injury. This strategy points to a new inspiration to unify tumor recurrence and radiation skin injury through on-demand ROS enhancement and elimination.
2. Materials and methods
2.1. Materials
l-lysine hydrochloride (99%), gallic acid (GA, 99%) and thioctic acid (TA, 99%) were purchased from Macklin (Shanghai, China). Acryloyl chloride (98%, Sigma-Aldrich, Shanghai, China), doxorubicin hydrochloride (DOX, 95%, TCI), perfluorotributylamine (99%, Heowns Biochem Technologies LLC, Tianjin, China), DCFH-DA (sigma-aldrich, D6883), Anti-gamma H2AX antibody(abcam, ab22551), and Rabbit Anti-Mouse IgG H&L (Alexa Fluor® 488) preadsorbed (abcam, ab150125) were used as received. Gold nanorods (AuNRs, CTAB modified, 0.1 mg/mL, 40 × 10 nm) were purchased from Nanjing XFNANO Tech. Co., Ltd. B. mori cocoons were purchased from sericulture factory in Shaanxi. All the other reagents are of analytical grade and used without further purification.
2.2. Procedure of preparation
First, N, N-diacryloyl-l-lysine as crosslinkers was synthesized with minor modification to the previously described method [34]. 18.26 g of l-lysine hydrochloride, aqueous potassium carbonate (80 mL of water, 15 g of potassium carbonate) and 60 mL of ether were added into a 250 mL flask under vigorous stirring. The ether solution of acryloyl chloride was added drop by drop to the flask under stirring at 0 °C for 1 h. After 3–4 h, adjust the pH of the aqueous phase to 12 and wash the solution with ether. Subsequently, adjust the pH of the solution to 2 and extract with ethyl acetate, add anhydrous magnesium sulphate to remove excess water. Finally, the solvent was removed by rotary evaporation to obtain the resultant crosslinker.
PTA was prepared by a one-pot heating method. TA and N, N-diacryloyl-l-lysine were mixed with varied ratios and then heated to 130 °C. The polymerization was carried out for 4 h. The yellow transparent solid copolymer was obtained after cooling down to room temperature. In order to remove unreacted crosslinker and TA monomer from the PTA, the resulting solid is dissolved in ethanol and dialyzed using a large amount of ethanol. The dialyzed PTA ethanol solution were dried in an oven at 70 °C to obtain a PTA patch with adhesive properties. Similarly, 1 mL of AuNRs solution and 1 mg of gallic acid were added into 10 mL of PTA solution and were dried to obtain the PTA-GA@Au patch.
Silk fibroin was prepared as previously described [35]. SF/PF hydrogels were formed according to protocols described below. Different concentrations of aqueous silk fibroin solutions were mixed with perfluorotributylamine (PF) with various ratios. An ultrasonic crusher was used to perform an ultrasonic process to the mixed solution. The probe of the ultrasonic crusher was inserted below the liquid level and sonicated four times (5 s each time at 5 s intervals) at a power of 200 W/min to 400 W/min. After sonication, a white emulsion was formed in the test tube. Subsequently, the SF/PF nanoemulsion spontaneously formed a white SF/PF hydrogel after 3–4 h. SF/PF@DOX hydrogels were prepared by the same method after dissolving DOX in SF solution.
2.3. Characterizations
The microscopic morphology of the hydrogels was characterized by a scanning electron microscope (SEM) after gold spraying. Fourier transform infrared (FTIR) spectrometry (PerkinElmer spectrum 100, USA) was used to characterize the transformation of the second structure of silk fibroin. For the tensile test, all samples were cut into rectangular pieces with a dimension of 30 mm × 8 mm × 2 mm and the extension rate were set at 100 mm min−1. Adhesion test was performed at room temperature by lap shear on a WDW-T05 (Tianchen, Co, Ltd, China), equipped with a liquid nitrogen refrigeration unit. The adhesive strengths were calculated as F/S (F is the maximum load, and S is the joint area). For underwater adhesion tests under different solvents, the method was similar to the previous test method.
2.4. Photothermal conversion capacity and in vitro drug release
100 mg of PTA@Au-GA was placed in 10 mL of phosphate buffered saline (PBS) and shaken at 37 °C at 100 rpm using a shaker. 1 mL of the solution was removed at different time points and PBS was added to keep the total volume of the solution constant. The absorbance of the sample at 259 nm was tested and the absorbance curve of the standard solution of gallic acid was used to calculate the concentration of the released sample. The releasing rate of the drug under NIR light was also examined. The changes in temperature and drug release rate were investigated by alternating NIR light irradiation over 30 min with 5 min intervals.
1 mL of SF/PF hydrogels loaded DOX were incubated in 10 mL of PBS (pH 5.5, 6.5, or 7.4) at 37 °C with shaking at 100 rpm. Cumulative drug release was monitored by removing and replacing the buffer at the indicated time points and measuring the absorbance at 520 nm. A dissolved oxygen meter was used to detect the O2 release from SF/PF hydrogels in vitro. Briefly, 5 mL of deoxygenated phosphate buffer solution was added to a vial and covered with fluid wax for to separate it from air. Then, 1 mL of SF/PF@O2 hydrogel was added in the water and the oxygen concentration was monitored in real time using the dissolved oxygen meter, whose probe is inserted below the liquid level of the vial. Moreover, the oxygen release property of SF@O2 hydrogel was also measured as controls.
2.5. DPPH free radical assay
The DPPH free radical assay was used to evaluate the antioxidant properties of PTA-GA@Au. DPPH was dissolved in ethanol and diluted to a final concentration of 0.1 mM. Different samples were added to the prepared 0.1 mM DPPH solution and incubated at 4 °C for 1 h. After completion of the reaction, the absorbance was tested at 517 nm using a UV spectrophotometer.
2.6. Intracellular ROS scavenging
The intracellular ROS scavenging abilities of SF/PF@DOX hydrogel were measured by DCFH-DA. L929 cells were grown in DMEM (dulbecco's modified eagle medium) culture medium, supplemented with 10% FBS (Life Technologies), 100 units·mL−1 of penicillin, and 100 μg mL−1 of streptomycin. The cells were maintained at 37 °C in a humidified, 5% (v/v) CO2 atmosphere. L929 cells were seeded in a 48 well plate at the density of 10000 cells/well and incubated overnight. Then, cells were treated with 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (500 μL, 1 μL/mL) in serum-free medium and incubated for 30 min (37 °C and 5% CO2). After that, cells were exposed to X-rays, with doses of 6 Gy. 50 mg PTA, PTA@Au, PTA-GA and PTA@Au-GA were added into the well and were co-cultured with L929 cells for 24 h. Meanwhile, all groups were treated by NIR light irradiation for 10 min with the intensity of 1.0 W cm−2. For comparison, the cells were inoculated in normal culture medium with and without X-ray irradiation as control groups (PBS group and PBS + RT group). The fluorescence intensity indicating the intracellular ROS level was recorded by a fluorescence microscope.
2.7. Rheology test
Rheological characteristics of SF/PF hydrogels with varying concentrations of silk fibroin was carried out by Anton Paar MCR302(Austria). The time sweep test was carried out with T = 25 °C, strain = 0.1%, and f = 1 Hz. For frequency sweep test, the frequency range was set from 1 to 100 Hz with strain = 1%, T = 25 °C. The linear regime was measured with a frequency of 1 Hz and T = 25 °C.
2.8. In vitro degradation of SF/PF hydrogel
The degradation behaviors of SF/PF hydrogels with varying concentrations of silk fibroin were determined by aa enzymatic degradation process. Collagenase IV was dissolved in PBS (pH 7.4, 0.1 M) at 1.0 U/mL. The SF/PF hydrogel was cut into squares and weighted. Then, the samples were incubated in 5 mL of enzyme solution for degradation tracing at 37 °C under slow shaking. The degradation remains were collected and weighted every 5 days, and the degradation solution was replaced with a fresh enzyme solution every 5 days. Quantitative changes were expressed as the percentage of weight retained relative to the initial weight.
2.9. Cytotoxicity assay and hemolysis test
The cytotoxicity of PTA@Au-GA patch and SF/PF@DOX hydrogel were determined as previously described using L929 mouse fibroblast cells [36]. The L929 cells were seeded in wells of 96-well plates at a density of 5000 cells per well. After incubated for 24 h, the L929 cells are incubated with PTA and PTA-GA@Au for another 24 h. Next, cell viabilities were determined by MTT assay. To evaluate the cytotoxicity of SF/PF hydrogels loaded with DOX, cells were seeded in 24-well plates at a density of 1 × 105 per well. After 24 h of incubation, the old medium was aspirated, 1 mL of new DMEM high sugar medium was added to each well. 200 μL of SF/PF@DOX hydrogel loaded with different concentrations of SF (SF concentrations of 3%, 4%, 5% and 6%) were added into the upper inserts and cultured with the cells. Pure SF hydrogel, DOX-free SF/PF hydrogel and free DOX solution were used as controls. Cell viabilities were determined by MTT assay after 24 h and 48 h of incubation. Similarly, the biocompatibility of SF/PF@DOX hydrogels was further confirmed by co-culture with NIH/3T3 cells.
The mouse breast cancer cells (4T1 cells) were used to access the anticancer activity of the drug-and oxygen-loaded SF/PF hydrogels. Cultures were maintained in RPMI 1640 medium supplemented with 10% FBS and 10% P/S. The same method as above was used to assess the killing effect of the hydrogel on 4T1 cells under normoxic conditions. Similarly, the cellular activity of 4T1 cells was tested by MTT assay after 24 h and 48 h of incubation. For the hypoxia groups, 4T1 cells were cultured in a cell incubator with 5% (v/v) CO2 and 1%(v/v) O2 atmosphere. As previously described, cells were seeded in 24-well plates at a density of 1 × 105 per well. After 24 h of incubation, the cells were exposed to X-rays at a dose of 6 Gy. After that, PBS, SF/PF hydrogel and SF/PF@DOX hydrogel were added into wells and co-cultured with 4T1 cells for 24 h. PBS, SF/PF and SF/PF@DOX hydrogels were also added into cells without radiation treatment as a comparison. Cell viabilities were determined by MTT assay after 24 h of incubation.
Hemolysis test was performed according to the following steps. 2 mL PBS, 20 μl erythrocytes and 200 mg PTA or PTA@Au-GA were added into vials as experimental groups. 20 μl of erythrocytes was added into 2 mL deionized water as a positive control group (PC) and 20 μl of erythrocytes was added to 2 mL of saline as a negative control group (NC). All groups were incubated at 37 °C for 2 h. At the end of the incubation, the solutions were centrifuged at 2000 rpm for 10 min and photographed and recorded. The supernatant was aspirated and its absorbance at 545 nm was read to calculate the hemolysis rate of the material according to the following formula, where As is the absorbance at 545 nm for the sample group, Anc is the absorbance at 545 nm for the negative control group and Apc is the absorbance at 545 nm for the positive control group.
| Hemolysis% = [(As - Anc)/ (Apc - Anc)] × 100% |
The hemolysis test of SF/PF hydrogels were carried out as mentioned above.
2.10. γ-H2AX detect
4T1 cells were seeded in a 12-well plate with 104 cells per well. Then cells were treated with PBS, PBS + RT (6 Gy), 100 mg SF hydrogel + RT, 100 mg SF/PF hydrogel + RT, 100 mg SF/PF@DOX + RT, respectively. After incubation for 24 h, 4T1 cells were washed with pre-cooled PBS, fixed with 4% PFA. Then the cells were permeabilized the membrane with 0.25% Triton X-100, and blocked with 3% BSA for 1 h. After that, the cells were further incubated with γ-H2AX antibody overnight at 4 °C. After washing with PBS, cells were incubated with the secondary antibody at room temperature for 2 h. Cells were stained by 4’,6-diamidino-2-phenylindole (DAPI) after being washed with PBS. The γ-H2AX within cells were finally recorded by confocal microscope and analyzed by ImageJ software.
2.11. In vivo inhibition of postoperative breast cancer recurrence
All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Peking Union Medical College and experiments were approved by the Animal Experiments and Ethics Review Committee of the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (IRM-DWLL-2023025). Healthy male Balb/c mice were randomly divided into 5 groups (5 mice per group): blank control (PBS), SF/PF, SF/PF@DOX, SF/PF + RT, and SF/PF @DOX + RT. Then, 4T1 cells (106 cells in 100 μL PBS) suspended in PBS were subcutaneously injected into the hind leg of each mouse to build the tumor model. The tumors were allowed to grow for about two weeks and were subjected to tumor resection. Before the surgery, the mice were treated with anesthesia, and then the formed tumors were excised. Different groups of materials were implanted at the resection site. The mice were observed every other day for weight, wound recovery, and tumor recurrence. The mice were executed when the tumors reached 14 mm or when the mice were moribund.
2.12. In vivo treatment of radiation-induced mice skin injury
Healthy male Balb/c mice were randomly divided into 5 groups (5 mice per group): blank control (without treatment), PTA, PTA@Au, PTA-GA, and PTA@Au-GA. First, all mice were treated with depilation of leg. The mice were anesthetized and exposed to 40 Gy of X-rays at 4 Gy/min for 10 min on the skin of their hind limbs. The irradiated wound changes of skin in each mouse are monitored daily. When the skin began to redden and ulcerate, the irradiated site was covered with the prepared material. After that, the wound sites of all groups of mice were irradiated daily with NIR light at an intensity of 1 W cm−2 for 10 min. The dressing was changed every two days.
The γ-H2AX staining with radioactive wound was performed after 5 days of X-ray irradiation (40 Gy). After 3 days of treating with hydrogels for the damage skin, the mice were sacrificed and the frozen sections of treated skin tissues were prepared. Frozen tissues were dried, fixed in 4% paraformaldehyde for 30 min at room temperature, permeabilized with ice methanol at −20 °C for 15 min and blocked with 1% BSA at room temperature for 1 h. Lymphocyte preparations were performed using the primary γ-H2AX antibody. After washing with PBS, the sections were incubated with the secondary antibody at room temperature for 1 h. Nuclei were stained with DAPI. Laser scanning confocal microscopy was performed with a confocal microscope and analyzed by ImageJ software.
2.13. Statistical analysis
Statistical analysis was performed using two-tailed Student's t-test for two groups and one-way ANOVA analysis of variance for multiple groups. P values: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (unpaired, two-way t tests). And p-value <0.05 is regard as statistically significant. All analysis were performed using GraphPad Prism version 9.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com.
3. Results and discussion
3.1. Characterization of PTA @Au-GA patches
TA is a natural small molecule that can undergo spontaneously thermal-initiated ring-opening polymerization due to the dynamic disulfide bond exchange and form supramolecular polymer networks in a hierarchical self-assembly fashion [37]. Based on the rich adhesive carboxyl groups in the polymer chain of PTA, it has been widely used in the preparation of adhesives [38]. However, the as-prepared PTA is metastable due to the inverse ring closing depolymerization initiated by terminal reactive sulfur radicals, and PTA will depolymerize into oligomers and lose adhesion when cooled to room temperature. Although PTA-based supramolecular polymers can be stabilized by introducing multiple double-bond crosslinkers and metal ions, most of these crosslinkers are non-adhesive, and the excessive complexation of metal ions to carboxyl groups will significantly reduce the adhesion of PTA-based materials [39]. In this work, we designed and synthesized a carboxyl crosslinker, N, N-diacryloyl-l-lysine, which could not only stabilize PTA, but also further enhance the adhesion of PTA-based material by using its own carboxyl groups. To our knowledge, the additional adhesion ability introduced by a crosslinker has never been reported before. The chemical structure of N, N-diacryloyl-l-lysine was confirmed by the 1H NMR spectrum (Fig. S1). The schematic synthetic route of PTA is demonstrated in Fig. 1a. Therefore, a stable, transparent and yellowish PTA-based supramolecular polymer adhesive can be obtained by simple one-pot heating of TA and N, N-diacryloyl-l-lysine (Fig. 1b). The PTA polymer was slowly dissolved in DMSO and then dialyzed with ethanol to remove unreacted small molecules and obtain a PTA ethanol solution, in which GA and Au nanorods (AuNRs) were added, to form a uniform dispersion. Finally, the PTA@Au-GA patches were prepared by solvent volatilization. The 1H NMR spectra of TA and PTA are exhibited in Fig. S2. The appearance of the resonance peaks at 2.8 ppm compared with that for TA proved the successful synthesis of PTA.
Fig. 1.
(a) Diagram showing the synthetic route of PTA. (b) Photographs showing the fabrication of PTA. (c) Adhesion strength of the patches to pig skin with different content of crosslinker (in comparison with PEGDA crosslinker). * indicates significance, ****p < 0.0001. (d) Adhesion strength of the patches-bonded pigskin immersed in PBS for 24 h. “ns” indicates not significant (p > 0.05). (e) Adhesion strength of the PTA@Au-GA patches to pig skin. * indicates significance, (***p < 0.001). (f) Tensile stress–strain curves of the PTA patches with different crosslinkers. (g) Tensile stress-strain curves of the PTA patch and the PTA@Au-GA patch with 10 wt% crosslinker. (h) Photos showing the ability of patch to adhere to the knuckle of index fingers and withstand different angle deformation (from 0° to 45°, 90° and >90°).
As observed in UV–vis spectrum (Fig. S3a), the typical peaks at 520 nm and 810 nm confirmed the successful synthesis of AuNRs. Then, the morphology and size of AuNRs were analyzed by transmission electron microscopy (TEM). The average particle diameter of AuNRs was 34.1 ± 2.2 nm (Fig. S3b), which was in the same range of the hydrodynamic diameter of 36.9 ± 1.2 nm determined by dynamic light scattering (DLS, Fig. S3c). The positive charged AuNRs was loaded in PTA networks through electrostatic interaction and the successful incorporation of AuNRs in the patches was confirmed by scanning electron microscope (SEM, Fig. S4). The FTIR spectra of PTA and PTA@Au-GA are shown in Fig. S5. The peak at 1700 cm−1 could be assigned to the stretching vibration of C O in carboxyl groups. The peaks at 2860 cm−1 and 2930 cm−1 to the stretching vibration of O–H in carboxyl groups while those at 1414 cm−1, 933 cm−1 were attributed to the bending vibration of O–H. After adding GA and AuNRs, some new peaks were observed at 500-2000 cm−1, which are similar to the characteristic GA peaks in the literature [40]. Broad vibrational bands located at 3377 cm−1 and 3491 cm−1 were observed, which were attributed to O–H stretching. The above results indicated the successful loading of GA in the patch. In addition, the O–H stretching vibration at 2860 cm−1 and 2930 cm−1 in PTA changed to 2898 cm−1 and 2972 cm−1 in PTA@Au-GA, which could be attributed to the hydrogen bonding formation between the carboxyl groups the PTA and the hydroxyl groups in GA.
3.2. Adhesive properties of PTA @Au-GA patches
The ability of carboxyls to form hydrogen bonds with amines affords the PTA polymer with robust adhesion to soft tissues [41]. The adhesion strength of PTA@Au-GA patches with different crosslinker contents were then measured by lap shear test. These patches were adhered to fresh pork skin and immersed in PBS for various time periods. As shown in Fig. 1c, the adhesion strengths of PTA patches were enhanced with the decrease of crosslinker content. When the mass fraction of the crosslinker was 10 wt%, the adhesion strength could reach 76.8 ± 5.3 kPa. To demonstrate that our elaborately designed carboxyl-containing crosslinker could enhance the adhesion of PTA, Poly(ethylene glycol diacrylate) (PEDGA) with approximate molecular weight and added amount was used to replace N,N-diacryloyl-l-lysine to copolymerize with TA. As shown in Fig. 1c, the adhesion strengths were 33.0 ± 3.8 kPa, 34.8 ± 6.5 kPa and 12.7 kPa ±5.5 kPa when the mass fractions of PEGDA were 10 wt%, 15 wt% and 20 wt%, respectively. These adhesion forces were much lower than those of N,N-diacryloyl-l-lysine-crosslinked PTA, confirming that the carboxyl groups the crosslinker could further increase the adhesive strength of PTA-based materials. There was almost no decrease in the adhesion strength for bonded pigskin after immersing in PBS for 24 h (Fig. 1d). This might be attributed to the hydrophobic property of PTA, which could prevent water molecules from penetrating into the adherent interface. After the addition of GA and AuNRs, the adhesion strengths of the PTA@Au and PTA @Au-GA was measured to be 71.8 ± 4.8 kPa and 60.3 ± 3.8 kPa, respectively, which was slightly decreased compared with PTA (Fig. 1e). A probable reason is that AuNRs and GA might interfere with the interaction of carboxyl groups with adherent surfaces and the phenolic hydroxyl groups on GA might form hydrogen bonds with part of the carboxyl groups on PTA.
Next, the tensile stress–strain curves of PTA with different crosslinker contents were measured. As shown in Fig. 1f, the tensile strength and modulus decreased after enhancement of crosslinker contents. The tensile strength of the PTA containing 10 wt% crosslinker was 80 kPa, nearly fourfold and twofold as that of PTA containing 15 wt% and 20 wt% crosslinker. Moreover, the PTA patch could be stretched in 10, 17 and over 20 times of its original length without breakage and partially recovered after release. As measured by the low-strain region (<20% strain) of the stress-strain curve, the Young's modulus was calculated as 105.3 ± 6.2 kPa, 105.9 ± 10.3 kPa and 33.77 ± 8.3 kPa when the crosslinker contents were 10 wt%, 15 wt% and 20 wt%, respectively. Also, PEGDA with approximate molecular weight and added amount was used to replace N, N-diacryloyl-l-lysine to copolymerize with TA. The tensile strength decreased with an increment of crosslinker contents, which was similar to the results with N, N-diacryloyl-l-lysine crosslinker. The tensile strength and elongation of PTA-PEGDA at break were lower than those of PTA-N, N-diacryloyl-l-lysine, further proving the superiority of the carboxyl crosslinker in enhancing the mechanical and adhesive performance. The addition of GA and AuNRs to the PTA resulted in little change in the tensile strength of PTA. The elongation at break was reduced but still adequate (Fig. 1g). Notably, the PTA@Au-GA patch exhibited an excellent mechanical strength and outstanding stretchability, and could fix securely to the fingers and be bent to different angles (Fig. 1h). On account of its high adhesive strength and appropriate mechanical property, the PTA@Au-GA with a crosslinker content of 10 wt% was chosen in the following study.
3.3. Photothermal properties of PTA@Au-GA patches and in vitro drug release
To investigate the photothermal performance of PTA@Au-GA patches, temperature changes of the patches under NIR light irradiation (808 nm; 0.5, 1.0, and 1.5 W cm−2, 450 s) were recorded. The surface temperature of PTA@Au-GA patches rapidly increased and reached a plateau of approximately 40.0 °C (0.5 W cm−2), 49.9 °C (1.0 W cm−2) and 65.7 (1.5 W cm−2) after 4 min, while the temperature of the pristine PTA only increased to 36.6 °C (Fig. 2a and b). Considering the demand of the mild photothermal therapy and the prevention of scalding, we chose NIR light irradiation with the intensity of 1.0 W cm−2 for further studies. As observed in Fig. 2c, the temperature of PTA@Au increased to 49.5 °C after NIR light irradiation for 10 min through thermal imaging camera, whereas the temperature of PTA only reached 30.1 °C. It is apparent that the laser itself caused only a slight temperature increase, while the AuNRs-loaded adhesive displayed an excellent photothermal activity. Notably, the photothermal effect of PTA@Au-GA remained unchanged after five cycles of light switching, indicating that the AuNRs could remain stable and maintain a good photothermal conversion in the adhesive patches (Fig. 2d).
Fig. 2.
Photothermal conversion curves of PTA and PTA@Au: (a) under the light irradiation of NIR with an intensity of 1 W cm−2 and (b) under different intensities of NIR light irradiation. (c) Infrared images of PTA and PTA@Au after NIR light irradiation with an intensity of 1 W cm−2 for 10 min. (d) Photothermal conversion cycle curve under intermittent NIR light irradiation with an intensity of 1 W cm−2. Gallic acid release from PTA@Au-GA (e) with or without NIR treating and (f) under intermittent NIR light irradiation. (g), (h) Antioxidative properties of PTA-GA with different content of GA. (i), (j), (k), (l) DCFH-DA staining of cells after different treatments.
Based on the excellent photothermal conversion properties of the PTA@Au-GA patch, the controlled drug release from the patch under NIR light irradiation was further explored. Gallic acid in the patch was released rapidly within 6 h, and then the release rate slowed down until a plateau was reached. The final release of gallic acid from the patch under NIR light irradiation was 87.0%, which was 1.5 times higher than that of the unirradiated group under the same conditions (Fig. 2e). To further understand the process of NIR-induced drug release, the drug release under NIR light irradiation was selected for alternating irradiation of 30 min. The PTA@Au-GA patch immersed in PBS was irradiated with NIR light for 5 min every 5 min, and the drug release rate and temperature changes were monitored over time. As observed in Fig. 2f, the temperature increased by 15 °C after NIR light irradiation and the drug release rate increased significantly. It is not difficult to understand that the local elevated temperature arising from the photothermal efficacy of PTA@Au-GA led to the rapid release of GA. In contrast, the release rate of GA decreased rapidly after the withdrawal of NIR light and remained a low level, which was comparable to the case without NIR light irradiation. Therefore, the controlled drug release and local photothermal treatment of PTA@Au-GA patches could be achieved by NIR light conditioning.
3.4. Antioxidation property of PTA@Au-GA patches
To evaluate the antioxidant activity of the PTA@Au-GA patches under NIR light irradiation, a DPPH radical scavenging assay was performed. As illustrated in Fig. 2g, the scavenge efficacies of DPPH free radicals of the PTA, PTA@Au-GA without NIR light irradiation and PTA@Au-GA with NIR light irradiation were 0%, 14.1%, and 23.5% at 60 min, respectively. Furthermore, the PTA@Au-GA under NIR light irradiation could scavenge 74.07% DPPH free radicals after 5 h, which was nearly twice higher than that of PTA@Au-GA without NIR light irradiation. As shown in Fig. 2h, the DPPH decoloration for PTA was obviously 0%, indicating that the PTA did not have any antioxidant ability. The lack of scavenging effect of PTA on DPPH revealed that the antioxidant properties of the material originate from the controlled release of GA under NIR light irradiation. These results indicated that the antioxidant property of the PTA@Au-GA patches could be tuned by adjusting NIR light irradiation.
Then, we explored the antioxidant activity of PTA@Au-GA patches in scavenging intracellular ROS. The DCFH-DA probe, an oxidative stress indicator, could evaluate the level of intracellular ROS with strong fluorescent signals [42]. As shown in Fig. 2i and j, the PTA and PTA@Au-incubated cells displayed high fluorescence signal after X-ray irradiation, indicating large production of ROS. On the contrary, the cells treated with PTA-GA and PTA@Au-GA with NIR light irradiation exhibited a small quantity of green fluorescence, which indicated the good antioxidant effect for elimination of intracellular ROS (Fig. 2k and l).
3.5. Preparation and characterizations of SF/PF hydrogel
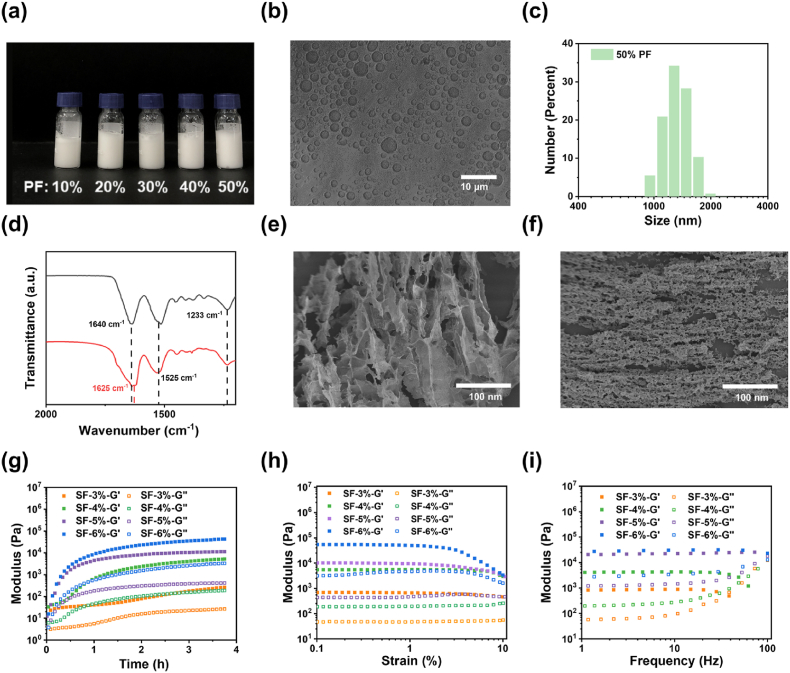
As shown in Fig. S6 and Fig. 3a, a mixture of aqueous silk fibroin solution and perfluorotributylamine (PF) with various ratios was treated using an ultrasonic processor to obtain opaque SF/PF emulsions. The amphiphilic nature and surface activity of silk fibroin allow them to adsorb to the interface between perfluorocarbon liquid and water, reducing the interfacial tension and forming a protective layer on the surface of perfluorocarbon droplets [43,44]. Microscopy images (taken 7 days after emulsion preparation) of the emulsions after dilution stabilized by silk fibroin are displayed in Fig. 3b. The droplet size of all these emulsions ranged from 1 μm to 5 μm and did not correlate well with the concentration of SF and the ratio of PF (Fig. 3c and Fig. S7). Table S1 shows the zeta potential of the droplets in the emulsions prepared with different silk fibroin concentrations and PF ratios. It was discernible that the zeta potential of the droplets varied from −23 to −49 mV, and the concentration of SF exerted a greater effect on the zeta potential than the volume fraction of PF. In general, the silk fibroin is negatively charged at pH > 4 [45]. The high zeta potential provides a strong electrostatic repulsion between droplets, which could effectively prevent the agglomeration of droplets. The SF/PF emulsion did not separate into aqueous solution and PF over an extended period of time. The above results demonstrate the excellent stability of the SF/PF emulsion. Oxygen was slowly pumped into the SF/PF emulsion to reach oxygen saturation and SF/PF emulsion gradually turned into a SF/PF hydrogel with the extension of time through the formation of β-sheets. The conformation of silk fibroin in SF/PF hydrogel was determined by ATR-FTIR (Fig. 3d). The peaks of the β-sheet conformation appear at 1616-1637 cm−1 (amide I), 1513-1525 cm−1 (amide II) and 1265 cm−1 (amide III); while the typical peaks of the random coil/α-helix are located at 1638-1660 cm−1 (amide I), 1536-1545 cm−1 (amide II) and 1235 cm−1 (amide III) [43]. Consequently, the amide I peak of raw SF appeared at 1640 cm−1, but the amide I peak of SF/PF hydrogel appeared at 1625 cm−1. This result reflected the conformational transition from random coil/α-helix to β-sheet.
Fig. 3.
(a) Digital photo of SF/PF hydrogels with different contents of PF. (b) Microscope image of the water/PF emulsions stabilized by silk fibroin. (c) Size of emulsions with different content of PF. (d) ATR-FTIR spectra of freeze-dried pure SF and SF/PF hydrogel. (e), (f) SEM images of freeze-dried SF and SF/PF hydrogels. (g) Time sweep curve, (h) strain sweep curve and (i) frequency sweep curves of storage and loss moduli of SF/PF hydrogels.
The porous morphology of pure SF hydrogels and SF/PF hydrogels was examined by SEM (Fig. 3e and f and Fig. S8). The microscopic morphology of SF/PF hydrogels was significantly different from that of pure SF hydrogels, with the former showing smaller pores. In addition, the pore size of SF/PF hydrogel was also closely related to the concentration of SF. The higher the concentration, the smaller the pore size. The pore sizes were ∼3 μm when the SF concentration was 4%, and the pores were surrounded by laminar silk layers that present a certain degree of orientation. The ultrasonication-induced self-assembly of silk fibroin replaced the use of the thermal or chemical crosslinking traditionally used to stabilize the aqueous protein phase in emulsions.
To characterize the rheological properties of the SF/PF hydrogels, an oscillatory rheological test was used. For rheology studies, SF/PF hydrogels at various silk concentrations (3, 4, 5, and 6%) were examined. The results of time sweeps indicated that the modulus of the SF/PF hydrogel gradually increased over time due to the gradual formation of the β-sheet structure of SF (Fig. 3g). 3–4 h was required for SF/PF hydrogel to reach the plateau of storage modulus. The final storage moduli averaged from three kinetic experiments were ∼3, 5, 11 and 54 kPa for the hydrogels with 3, 4, 5 and 6% SF concentrations, respectively. Dynamic strain sweeps were conducted to determine the linear viscoelastic region of the SF/PF hydrogels, and a strain amplitude of 0.1%, which was in the linear viscoelasticity region for all samples, was selected for frequency sweep tests. The strain sweeps obtained following in situ gelation indicated that failure of the hydrogels, which was influenced by silk concentrations, occurred at ∼3% strain for hydrogel with 3% SF concentration and ∼7% for hydrogels with 4, 5 and 6% SF concentrations (Fig. 3h). In the frequency sweep measurement, the hydrogels all showed constant modulus in the frequency interval of 1 Hz–10 Hz (Fig. 3i).
3.6. Self-supplied oxygen behavior, in vitro degradation of SF/PF hydrogel and DOX release
PF possesses an excellent oxygen-dissolving ability owing to the strong van der Waals interaction between PF and oxygen [46]. It is expected that our SF/PF hydrogels could also deliver oxygen when they were implanted in the tumor surgical site. The oxygen release ability of oxygen-saturated SF/PF hydrogels under hypoxic conditions was investigated by measuring the dissolved oxygen concentration in aqueous solutions in real time using an oxygen meter. PBS, oxygen-saturated SF hydrogel, and oxygen-saturated SF/PF hydrogel were added to the deoxygenated PBS. According to Fig. 4a, the dissolved oxygen concentration of SF/PF hydrogel in water rapidly increased to 7 mg/L and saturated within the first 100 s. Then, the oxygen concentration of SF/PF hydrogel slowly decreased to 5 mg/L and remained stable after incubation of 10 min. The final oxygen concentration of SF/PF hydrogel was beyond twice and 5-fold higher than that of PBS and SF, which indicated the excellent oxygenating ability of SF/PF hydrogel (Fig. 4b). The above results demonstrated that the SF/PF hydrogel has an excellent oxygen loading ability and maintain oxygen-enriched environment via continual release of loaded O2.
Fig. 4.
(a) The oxygen concentration changes of PBS solution after adding PBS@O2, SF@O2 hydrogel, and SF/PF@O2 hydrogel, respectively. (b) The oxygen concentration of PBS solution after 20 min of adding PBS@O2 solution, SF@O2 hydrogel, and SF/PF@O2 hydrogel. * indicates significance, (****p < 0.0001). (c) Wet weight remaining ratio of SF/PF hydrogels with different concentrations of SF treated with protease XIV. DOX release from SF/PF@DOX hydrogels (d) with different concentrations of SF in protease XIV solution (e) under different pH environment. (f) Relative viability of L929 cells after incubation with hydrogels for 24 h and 48 h. (g) Hemolysis rates of SF/PF@DOX hydrogels; PBS: NC, negative control group, deionized water: PC, positive control group; Inset: Digital photo showing hemolysis phenomenon. (h) Relative viability of 4T1 cells after incubation with hydrogels for 24 h and 48 h in normoxic environment. (i) Relative viability of 4T1 cells after incubation with hydrogel in hypoxic environment when combined with radiotherapy. “ns” indicates not significant (p > 0.05) and * indicates significance (*p < 0.05 and ***p < 0.001). (j) Immunofluorescent imaging and (k) quantitative analysis of γ-H2AX foci within 4T1 cells after treated with PBS, SF hydrogel, SF/PF hydrogel and SF/PF@DOX hydrogel under RT. Scale bar: 20 μm * indicates significance (*p < 0.05 and ***p < 0.001).
The degradation behavior of biomaterials is crucial for the application of controlled drug release and biosafety in vivo. SF can be degraded by proteases in vitro and in vivo, and its degradation products are soluble peptides and free amino acids that are readily metabolized and absorbed by the body [47]. PFTBA is a non-toxic, stable perfluorocarbon liquid that is also commonly used as an oxygen carrier in living organisms. The degradation behavior of SF/PF hydrogels was measured in protease XIV solution to mimic the physiological environment. As shown in Fig. 4c, the weight loss of SF/PF hydrogel decreased with the increase of SF contents. It is possible that the higher concentration of SF contents resulted in a stronger crosslink density due to formation of denser β-sheets. At day 14, the difference of various SF/PF hydrogels in weight loss was evident. After one month, the weight losses of the SF/PF hydrogels with SF concentration of 3% and 4% were respectively 68% and 47%, while the weight loss of the hydrogels with SF concentration of 6% was 20%. Thus, the degradation rate of SF/PF hydrogels could be adjusted by the concentration of SF.
To screen out the appropriate degradation rate of hydrogels for drug release, the drug release rates of SF/PF hydrogels with different SF contents loaded with DOX in buffers of pH 5.5, 6.5, and 7.4 were also examined (Fig. 4d). The content of SF in the hydrogels also had a significant influence on drug release behavior. The drug release rate of SF/PF hydrogels was accelerated with the decrease of SF contents due to the enhancement of degradation rate of hydrogels. The drug release rate was faster in the first 5 days and then gradually decreased after 5 days. Drug release from the 6 wt% SF/PF hydrogel was the slowest, resulting in a cumulative release of 26.9% DOX at pH 7.4 after 30 days. The cumulative amount released from the SF/PF hydrogels with 3, 4 and 5 wt% SF contents increased to 45.9%, 55.8% and 86.0% at pH 7.4. Based on the above analyses, at a higher SF content, highly crosslinked SF/SF hydrogel was formed, which led to a slower degradation rate. As a result, the SF/PF hydrogel exhibited a lower drug release. For all the DOX-loaded SF/PF hydrogels, drug release was rapid during the first 11 days and decreased during the rest of the time period. The results suggested that a tunable release of DOX can be achieved by adjusting the SF content in the hydrogels. However, although the SF/PF hydrogel with 3 wt% SF content possessed the fastest drug release rate, it lost its structural integrity and fragmented on the second day. Therefore, DOX-loaded SF/PF hydrogel at 4 wt% SF content was selected for in vivo studies.
In acid environment (such as tumor site), the DOX release rate SF/PF hydrogels was enhanced compared to that in PBS (pH 7.4) due to the increased solubility of DOX in an acid solution. The cumulative release of DOX in pH 5.5 and 6.5 was 73.6% and 63.9%, respectively (Fig. 4e). This result demonstrated that the SF/PF hydrogels could serve as a slow releasing platform for controlled drug delivery in tumor therapy.
3.7. In vitro biocompatibility and anticancer efficiency of SF/PF@O2 hydrogel
The biocompatibility was examined via cell viability and hemolytic activity in vitro. The cytotoxicity of the PTA@Au-GA and the SF/PF@DOX hydrogel were assayed by co-culturing fibroblast cells in media with the hydrogels. Both PTA@Au-GA patches and SF/PF hydrogels displayed negligible toxicity to L929 mouse fibroblast cells, and the cell viability was maintained to over 95% (Fig. S9a and Fig. 4f). The result suggested that the PTA@Au-GA patches and SF/PF hydrogels have no obvious cytotoxicity. The SF/PF@DOX hydrogels were slightly toxic to L929 cells, but cell viability could still reach more than 80% after 24 h and 48 h of culture. Meanwhile, the cytotoxicity of the SF/PF@DOX hydrogel on NIH/3T3 cell was also tested and the cell viability were all above 80% in different concentrations of SF after incubation of 24 h and 48 h, whereas the viability of free DOX was 28.9% and 10.5% after incubation of 24 h and 48 h, respectively (Fig. S10). These results demonstrate that the DOX-encapsulated hydrogel could reduce cytotoxicity of free DOX against normal cells. Hemolysis test is also often used to inspect the hemocompatibility of materials. As illustrated in Fig. S9b and Fig. 4g, the hemolysis rates of both PTA@Au-GA patches and SF/PF@DOX hydrogels were below the safety standard of 5%, indicating that the patches and hydrogels have good hemocompatibility. Therefore, the PTA@Au-GA patches and SF/PF@DOX hydrogels are promising to be biocompatible wound dressing and implantable materials in postoperative treatment of breast cancer for prevention of tumor recurrence and radiation skin injury.
To verify the radiosensitization potency based on self-supplied O2 strategy, the MTT assay was used to evaluate the cytotoxic effects of SF/PF@DOX hydrogels on breast cancer cells 4T1 under normoxic and hypoxic conditions. As shown in Fig. 4h, SF hydrogel, SF/PF hydrogel and SF/PF@DOX hydrogel with different concentrations of SF were co-cultured with 4T1 cells under normoxic conditions for 24 h and 48 h. It was observed that SF hydrogel and SF/PF hydrogel had no homicidal effect on tumor cells, and the activity of 4T1 cells treated with SF/PF hydrogel even exceeded that of the control group at 24 h. It was speculated that the oxygen released from the hydrogels promoted the growth and proliferation of tumor cells. In contrast, the hydrogel loaded with DOX had a significant killing effect on tumor cells, and the cell activity decreased to about 60% after 24 h of co-culture and to 10%–20% after 48 h, indicating that the slowly release of DOX from hydrogel could continually kill tumor cells to achieve a satisfied antitumor effect. Notably, the DOX release rate could be adjusted by SF contents due to difference of degradation rate of hydrogel.
Similarly, the hydrogels were co-cultured with 4T1 cells under hypoxic conditions supplemented with appropriate radiation, to study the anti-tumor effect of hydrogels as well as the effect of improving the sensitivity to radiotherapy. The specific groupings were as follows: (i) control; (ii) RT; (iii) SF/PF; (iv) SF/PF@DOX; (v) SF/PF + RT; (vi) SF/PF@DOX + RT. As shown in Fig. 4i, the activity of 4T1 cells in both the RT and SF/PF groups was the same as the control group, indicating that radiation alone in the hypoxic conditions could not generate enough ROS to kill tumor cells. The 4T1 cell activity in the SF/PF@DOX and SF/PF + RT groups decreased to 58.0% and 68.5%, respectively, demonstrating that the traditional chemotherapy and radiotherapy could have a certain degree of tumor cell killing effect. The SF/PF@ DOX + RT group had the lowest cellular activity of 16.0%, which indicated that the SF/PF@DOX hydrogel demonstrated a potent anti-tumor effect by releasing DOX along with effective oxygen release and improving the sensitivity of radiotherapy. These results corroborated the combined chemo-radiotherapy through controlled drug release and self-supplied O2 radiosensitization could quickly improve hypoxic tumor environment and efficiently eliminate tumor cells.
Moreover, the radiosensitization effects of the SF/PF@DOX hydrogel were further assessed through detection and quantification of γ-H2AX, a biomarker indicative of cellular response to DNA damage. As shown in Fig. 4j, DNA double strands breakage were observed in both SF hydrogel, SF/PF hydrogel and SF/PF@DOX hydrogel groups after X-ray irradiation. Notably, the SF/PF@DOX hydrogel group exhibited the most aggravated DNA breakage compared with other groups (Fig. 4k), suggesting that the sustainable release of O2 and DOX from SF/PF@DOX hydrogel could generate massive ROS and increase the radiosensitivity of cancer cells.
3.8. In vivo locoregional inhibition of breast cancer recurrence
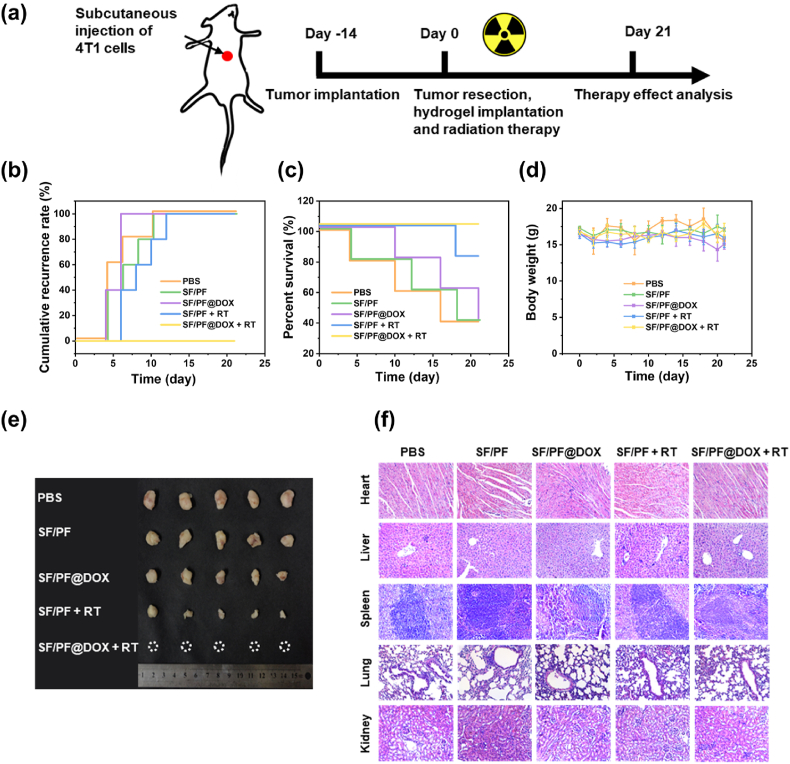
In sight of the appropriate biodegradation and anti-tumor property in vitro, the SF/PF@DOX hydrogel are promising to be developed for prevention of breast cancer recurrence after tumor resection in combination with irradiation therapy. In this experiment, a breast cancer model of Balb/c mice was established to evaluate the in vivo treatment effects (Fig. 5a–d). First, the tumors in the thorax of the mice were excised, and then PBS, SF/PF hydrogel, SF/PF@DOX hydrogel were implanted into the surgically excised cavities of the mice with or without irradiation therapy. The therapeutic effects of the hydrogel were evaluated by examining the cancer recurrence rates and percent survival after 21 days. By evaluating the above treatment groups, it was found that for the mice injected with PBS and implanted with SF/PF hydrogel and SF/PF@DOX hydrogel, cancer recurrence occurred in 3, 2, and 2 mice, respectively, by the third day (Fig. 5b). Mice in the SF/PF + RT group also showed tumor recurrence on the sixth day, and finally all of them suffered from tumor recurrence by the 12th day. However, mice in the SF/PF@DOX + RT group had no recurrence at all throughout the experiment, demonstrating that the combined radiotherapy and chemotherapy of SF/PF@DOX hydrogel could effectively eliminate residual tumor and then inhibit tumor recurrence. It is evident that the release of DOX in the SF/PF@DOX group was insufficient to kill the cancer cells. In addition, the mice in the SF/PF@DOX + RT group had the highest survival percentage (Fig. 5c). As shown in Fig. 5e, the recurrent tumors in treatment groups were removed after 21 days. The tumor size of SF/PF@DOX group and SF/PF + RT group was found to be significantly smaller than that of PBS and SF/PF groups. Although these two groups did not successfully inhibit the tumor recurrence, the chemotherapy and radiotherapy could slow down the growth of tumor tissue.
Fig. 5.
In vivo locoregional inhibition of breast cancer recurrence. (a) Schematic illustration of the establishment and treatment strategy of a in vivo 4T1 breast cancer recurrence model. (b) Cumulative recurrence rate of the mice in different treatment groups. (c) Survival curves of the mice in different treatment groups in the 4T1 tumor recurrence model. (d) Body weight of 4T1 tumor-bearing mice. (e) The images of representative tumors after different treatments on day 21. (f) H&E-stained images of the heart, liver, spleen, lung and kidney treated with PBS, SF/PF hydrogel, SF/PF@DOX hydrogel, SF/PF hydrogel + RT and SF/PF@DOX hydrogel + RT.
DOX has been shown to have a high antitumor efficacy in the treatment of various cancers, but its side effects, especially toxicity to major organs in the systemic circulation, could not be ignored [48]. According to our previous study [36], in both cases of intravenously and locally injected DOX, the mice experienced significant weight loss due to significant systemic toxicity of free DOX. In contrast, in this experiment, the body weight of mice in the treatment and control groups remained at normal levels (Fig. 5d). In addition, no toxicity was found in major organs including heart, liver, spleen, lung and kidney in the SF/PF@DOX group based on histological observations (Fig. 5f). No significant difference was observed in blood biochemistry parameters of mice before and after SF/PF@DOX treatment (Fig. S10). This suggested that the local release of hydrogel can greatly reduce the toxicity of DOX and mitigate the side effects of DOX.
3.9. In vivo wound healing of radiation-induced skin injury
It is well accepted that radiation-induced skin injury has two major characteristics of difficult healing and long-term susceptibility to carcinogenesis. Clinically, it manifested as recurrent ulcerative necrosis [49]. Therefore, it is especially important to establish effective prevention and treatment measures. To evaluate the skin radioprotective effect after RT, we tested the therapeutic potency of PTA@Au-GA in vivo by visually monitoring the irradiated skin wound changes on Balb/c mice and their corresponding histopathological phenomenon (Fig. 6a). The patches served as external-use dressing to treat radiation skin injury 2 weeks after 40 Gy X-ray irradiation. The dressings were replaced every 2 d and the tissues were retrieved after 0, 7, and 21 d treatment, and the irradiated wound sites of all groups were irradiated daily with NIR light at an intensity of 1 W cm−2 for 10 min. The BALB/c mice are divided into 5 group: (i) control; (ii) PTA; (iii) PTA@Au; (iv) PTA-GA; (v) PTA@Au-GA. The control group was the mice that receive no treatment after X-ray irradiation. Fig. 6b visually showed the skin wound changes of mice with different treatments. All the groups exhibited typical symptoms of radioactive skin injury to varying degrees after 14 days of irradiation: skin erythema, erythema and alopecia. The wounds in the PTA and control groups deteriorated further over time, with skin edema, red ulcers, and clear exudates. On day 3, all the groups showed significant edema and skin reddening. Then, by day 7, the control and PTA groups showed severe injury with the largest ulcers, while the PTA@Au and PTA-GA groups showed moderate injury with moderate ulcers. In contrast, the PTA@Au-GA showed minimal damage with a slight ulcer. The wounds of mice treated with PTA@Au-GA dressings gradually healed over the following two weeks, indicating that the release of GA could quickly eliminate ROS and delay the development of skin radiation damage. Notably, the PTA@Au-GA group showed a mild symptoms of radiation damage and clear trend of recovery of radioactive wound. This indicated that the mild photothermal therapy could promote wound healing and enhanced the antioxidant efficacy via trigger release of GA. Besides, fur regeneration occurred in the PTA-GA and PTA@Au-GA groups. Quantitative assessments were performed using Radiation Therapy Oncology Group (RTOG) scoring system, a recognized scale for scoring and documenting radiation-induced damage [50]. Fig. 6c reveals that the PTA@Au-GA group exhibited significant improvement in skin damage, and ultimately achieved an RTOG score of 0 on day 21, suggesting its effectiveness in delaying the development of skin radiation damage and repairing radioactive injury. Moreover, the use of PTA@Au and PTA-GA patches also delayed the development of radiation-induced skin damage, with final RTOG scores of 2,1, respectively. These results indicating that the NIR-induced GA release and mild photothermal therapy could alleviate the skin radiation damage and promote radioactive wound healing.
Fig. 6.
In vivo wound healing of radiation-induced skin injury. (a) Schematic illustration of the establishment and treatment strategy of a radiation-induced mice skin injury model. All groups were irradiated daily with NIR light at an intensity of 1 W cm−2 for 10 min after X-ray radiation. (b) Photographs of the skin changes of mice after different treatments at five-time points. (c) Radiation Therapy Oncology Group (RTOG) scores of different groups in treatment of skin radiation injury. (d) Representative H&E staining of skin tissue sections at 14 days after different treatments. (e) Immunofluorescent imaging and (f) quantitative analysis of γ-H2AX foci after treated with different patches under RT in vivo. * indicates significance (*p < 0.05). Hematological index of the mice after different treatments: (g) WBCs, (h) HGBs (i) RBCs and (j) PLTs cell numbers.
In addition, we collected treated skin tissues from the radiation sites and performed histopathological analysis by using H&E staining. As seen in Fig. 6d, H&E staining showed that the skin of the PTA@Au-GA group was in good condition and the inflammation subsided, while the other groups displayed varying degrees of interstitial edema, hyperkeratosis, dermal exudation and inflammatory infiltration, with the control group being the most severe. The above outcomes reveal that the PTA@Au-GA group can repair radiation-induced dermatitis well, which stems from its good free radical scavenging activity. Additionally, in vivo detection of γ-H2AX foci was performed to monitor DNA damage and repair. As depicted in Fig. 6e and f, DNA double strands breakage of radioactive tissues were observed in both groups after high dosage of X-ray irradiation, which suggested occurrence of radiation-induced skin injury. However, the DNA damage of PTA-GA and PTA@Au-GA was minor compared with PBS group, indicating excellent antioxidant properties arise from NIR-induced GA release.
To confirm the in vivo biosafety of PTA@Au-GA dressings, the blood indicators of mice after different treatment were measured. No significant difference was observed in all hematological indices of mice in different groups (Fig. 6g–j). These results demonstrated that the PTA@Au-GA dressings have good biosafety with an appealing ability to promote wound healing of radioactive skin injury.
4. Conclusions
In summary, we developed a biodegradable SF/PF@DOX hydrogel based on consecutive ultrasonication-induced β-sheets-crosslinked amphiphilic silk fibroin/perfluorocarbon/DOX nanoemulsion. A PTA@Au-GA bioadhesive patch was fabricated by copolymerization of TA and N, N-diacryloyl-l-lysine that could stabilize PTA and provided additional adhesive groups, with addition of AuNRs and GA. The SF/PF@DOX hydrogel exhibited an adjustable degradability and mechanical property with sustained release of oxygen and DOX around tumor site, thus improving tumor hypoxia and radiotherapeutic sensitivity. Due to self-supplied O2 strategy, the generation of ROS during radiotherapy was enhanced after implantation of hydrogel, and the combined chemotherapy ensured the elimination of residual tumor and prevention of tumor recurrence accordingly. The PTA@Au-GA bioadhesive could fit irregular shape of skin wound, and fix firmly to the tissue surface, showing a salient ability to scavenge free radicals by controlled release of GA and perform gentle photothermal therapy to promote angiogenesis and tissue repair for radioactive skin damage.
During 3-week of implantation of SF/PF@DOX hydrogel around surgical site after tumor resection in mice model, the enhanced radiosensitization combined with chemotherapy based on sustainable DOX release from biodegradable polymer networks prevented the breast cancer recurrence. Meanwhile, the PTA@Au-GA bioadhesive was glued to mice's skin surface of surgical wound after 2 weeks of X-ray irradiation. These adhesive patches displayed excellent antioxidant activity imparted by controllable release of GA under NIR light irradiation, thus effectively delaying the development of skin radiation damage and repairing radioactive injury, even promoting fur regeneration. In a word, this synergistic strategy may offer a more judicious alternative for preventing recurrence of breast cancer tumors after lumpectomy and improving the prognosis of individuals afflicted with breast cancer in consideration of the therapeutic dilemma of breast cancer recurrence and radioactive skin damage.
Ethics approval and consent to participate
All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Peking Union Medical College and experiments were approved by the Animal Experiments and Ethics Review Committee of the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (IRM-DWLL-2023025).
CRediT authorship contribution statement
Zhuodan Zhang: contributed equally to this work, Investigation, Data curation, Methodology, Formal analysis, Writing – original draft. Qiannan Cao: contributed equally to this work, Investigation, Data curation, Formal analysis. Yi Xia: contributed equally to this work, Investigation, Data curation, Formal analysis. Chunyan Cui: Investigation, Data curation. Ying Qi: Investigation, Data curation. Qian Zhang: Software, Data curation. Yuanhao Wu: Conceptualization, Formal analysis, Writing. Jianfeng Liu: Conceptualization, Formal analysis. Wenguang Liu: Conceptualization, Formal analysis, Writing – review & editing.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the support for this work from the National Natural Science Foundation of China (Grant No. 52233008, 51733006, 32301120), the CAMS Innovation Fund for Medical Sciences (2021-I2M-1–042, 2021-I2M-1–060), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2022-RC350-06).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2023.08.021.
Contributor Information
Yuanhao Wu, Email: wuyuanhao@irm-cams.ac.cn.
Jianfeng Liu, Email: liujianfeng@irm-cams.ac.cn.
Wenguang Liu, Email: wgliu@tju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023, CA cancer. J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Giaquinto A.N., Sung H., Miller K.D., Kramer J.L., Newman L.A., Minihan A., Jemal A., Siegel R.L. Breast cancer statistics, 2022. CA Cancer J. Clin. 2022;72:524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis C.E., Ma J.M., Gaudet M.M., Newman L.A., Miller K.D., Sauer A.G., Jemal A., Siegel R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 4.Sjostrom M., Chang S.L., Fishbane N., Davicioni E., Zhao S.G., Hartman L., Holmberg E., Feng F.Y., Speers C.W., Pierce L.J., Malmstrom P., Ferno M., Karlsson P. Clinicogenomic radiotherapy classifier predicting the need for intensified locoregional treatment after breast-conserving surgery for early-stage breast cancer. J. Clin. Oncol. 2019;37:3340–3349. doi: 10.1200/JCO.19.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.M., Zhu Y.N., Zhang Y.M., Lin W.J., Ke J., Liu J.F., Zhang L., Liu J.J. A balanced charged hydrogel with anti-biofouling and antioxidant properties for treatment of irradiation-induced skin injury. Mat Sci Eng C-Mater. 2021;131 doi: 10.1016/j.msec.2021.112538. [DOI] [PubMed] [Google Scholar]
- 6.Xie J.N., Gong L.J., Zhu S., Yong Y., Gu Z.J., Zhao Y.L. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv. Mater. 2019;31 doi: 10.1002/adma.201802244. [DOI] [PubMed] [Google Scholar]
- 7.Chan L., Chen X.D., Gao P., Xie J., Zhang Z.Y., Zhao J.F., Chen T.F. Coordination-driven enhancement of radiosensitization by black phosphorus via regulating tumor metabolism. ACS Nano. 2021;15:3047–3060. doi: 10.1021/acsnano.0c09454. [DOI] [PubMed] [Google Scholar]
- 8.Pan S.Y., Huang G.N., Sun Z.W., Chen X., Xiang X.L., Jiang W.X., Xu Y.C., Chen T.F., Zhu X.Q. X-Ray-Responsive zeolitic imidazolate framework-capped nanotherapeutics for cervical cancer-targeting radiosensitization. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 9.Chao Y., Xu L.G., Liang C., Feng L.Z., Xu J., Dong Z.L., Tian L.L., Yi X., Yang K., Liu Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018;2:611–621. doi: 10.1038/s41551-018-0262-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y.M., Feng Z.J., Liu J.J., Li H., Su Q., Zhang J.M., Huang P.S., Wang W.W., Liu J.F. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact. Mater. 2022;16:359–371. doi: 10.1016/j.bioactmat.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L., Wang D., Jia H., Yang C., Zhang Y., Li H., Liu J., Liu J. Tumor-specific peroxynitrite overproduction disrupts metabolic homeostasis for sensitizing melanoma immunotherapy. Adv. Mater. 2023 doi: 10.1002/adma.202301455. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y., Su L.C., Yuan M., Chen T., Ye J.M., Jiang Y.F., Song J.B., Yang H.H. In vivo X-ray triggered catalysis of H-2 generation for cancer synergistic gas radiotherapy. Angew. Chem., Int. Ed. 2021;60:12868–12875. doi: 10.1002/anie.202100002. [DOI] [PubMed] [Google Scholar]
- 13.Fang Z.Q., Lv Y.C., Zhang H.R., Zhang Y.X., Gao H.Q., Chen C.X., Wang D.Z., Chen P.H., Tang S.J., Li J.J., Qiu Z.H., Shi X.A., Chen L.W., Yang J.M., Chen X.S. A multifunctional hydrogel loaded with two nanoagents improves the pathological microenvironment associated with radiation combined with skin wounds. Acta Biomater. 2023;159:111–127. doi: 10.1016/j.actbio.2023.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z.Y., Chen H.H., Yang P.Z., Shen X.F., Hu Y.Q., Cheng Y.H., Yao H.W., Zhang Z.T. Nano-oxygenated hydrogels for locally and permeably hypoxia relieving to heal chronic wounds. Biomaterials. 2022;282 doi: 10.1016/j.biomaterials.2022.121401. [DOI] [PubMed] [Google Scholar]
- 15.Burke G., Faithfull S., Probst H. Radiation induced skin reactions during and following radiotherapy: a systematic review of interventions. Radiography. 2022;28:232–239. doi: 10.1016/j.radi.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Yee C., Wang K., Asthana R., Drost L., Lam H., Lee J., Vesprini D., Leung E., DeAngelis C., Chow E. Radiation-induced skin toxicity in breast cancer patients: a systematic review of randomized trials. Clin. Breast Cancer. 2018;18:E825–E840. doi: 10.1016/j.clbc.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Huang D.Q., Zhao C., Wen B.J., Fu X., Shang L.R., Kong W.T., Zhao Y.J. Oxygen-carrying microfluidic microcapsules for enhancing chemo-sonodynamic therapy on patient-derived tumor organoid models. Chem. Eng. J. 2022;435 [Google Scholar]
- 18.Liang X.L., Chen M., Bhattarai P., Hameed S., Dai Z.F. Perfluorocarbon@Porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS Nano. 2020;14:13569–13583. doi: 10.1021/acsnano.0c05617. [DOI] [PubMed] [Google Scholar]
- 19.Wang W.G., Cheng Y.H., Yu P., Wang H.R., Zhang Y., Xu H.H., Ye Q.S., Yuan A.H., Hu Y.Q., Wu J.H. Perfluorocarbon regulates the intratumoural environment to enhance hypoxia-based agent efficacy. Nat. Commun. 2019;10:1580. doi: 10.1038/s41467-019-09389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y.F., Zhang C.Q., Wu B.D., Li C.Y., Lin J., Huang P. Thermoresponsive ozone-enriched spray gel for postsurgical treatment of hepatocellular carcinoma. ACS Nano. 2023;17:3518–3527. doi: 10.1021/acsnano.2c09893. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y.X., Zhao D.Y., Wang G., Wang Y., Cao L.L., Sun J., Jiang Q.K., He Z.G. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: opportunities, challenges, and future development. Acta Pharm. Sin. B. 2020;10:1382–1396. doi: 10.1016/j.apsb.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu D.R., Zhong L., Wang M.Y., Li H.H., Qu Y., Liu Q.Y., Han R.X., Yuan L.P., Shi K., Peng J.R., Qian Z.Y. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 23.Ma T., Hao Y.M., Li S.Y., Xia B., Gao X., Zheng Y., Mei L.W., Wei Y.T., Yang C.B., Lu L., Luo Z.J., Huang J.H. Sequential oxygen supply system promotes peripheral nerve regeneration by enhancing Schwann cells survival and angiogenesis. Biomaterials. 2022;289 doi: 10.1016/j.biomaterials.2022.121755. [DOI] [PubMed] [Google Scholar]
- 24.Shao X.H., Yang X., Zhou Y., Xia Q.C., Lu Y.P., Yan X., Chen C., Zheng T.T., Zhang L.L., Ma Y.N., Ma Y.X., Gao S.Z. Antibacterial, wearable, transparent tannic acid-thioctic acid-phytic acid hydrogel for adhesive bandages. Soft Matter. 2022;18:2814–2828. doi: 10.1039/d2sm00058j. [DOI] [PubMed] [Google Scholar]
- 25.Chen C., Yang X., Li S.J., Zhang C., Ma Y.N., Ma Y.X., Gao P., Gao S.Z., Huang X.J. Tannic acid-thioctic acid hydrogel: a novel injectable supramolecular adhesive gel for wound healing. Green Chem. 2021;23:1794–1804. [Google Scholar]
- 26.Zhang Q., Shi C.Y., Qu D.H., Long Y.T., Feringa B., Tian H. Exploring a naturally tailored small molecule for stretchable, self-healing, and adhesive supramolecular polymers. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aat8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y.J., Bhattarai P., Dai Z.F., Chen X.Y. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019;48:2053–2108. doi: 10.1039/c8cs00618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng J.P., Cheng X.Z., Zhang H., Bai X.P., Ai R.Q., Shao L., Wang J.F. Gold nanorods: the most versatile plasmonic nanoparticles. Chem. Rev. 2021;121:13342–13453. doi: 10.1021/acs.chemrev.1c00422. [DOI] [PubMed] [Google Scholar]
- 29.Qin M.M., Guo Y.Q., Su F.F., Huang X.P., Qian Q.P., Zhou Y.L., Pan J.Y. High-strength, fatigue-resistant, and fast self-healing antibacterial nanocomposite hydrogels for wound healing. Chem. Eng. J. 2023;455 [Google Scholar]
- 30.Dong X.W., Ye J., Chen Y., Tanziela T., Jiang H., Wang X.M. Intelligent peptide-nanorods against drug-resistant bacterial infection and promote wound healing by mild-temperature photothermal therapy. Chem. Eng. J. 2022;432 [Google Scholar]
- 31.Kahkeshani N., Farzaei F., Fotouhi M., Alavi S.S., Bahramsoltani R., Naseri R., Momtaz S., Abbasabadi Z., Rahimi R., Farzaei M.H., Bishayee A. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225–237. doi: 10.22038/ijbms.2019.32806.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D.J., Moh S.H., Son D.H., You S., Kinyua A.W., Ko C.M., Song M., Yeo J., Choi Y.H., Kim K.W. Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules. 2016;21:899. doi: 10.3390/molecules21070899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Zhang Z., Pan Z., Liu Y. Advanced bioactive nanomaterials for biomedical applications. Explorations. 2021;1 doi: 10.1002/EXP.20210089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao F., Zhang Y.Y., Li Y.M., Xu B., Cao Z.Q., Liu W.G. Sea cucumber-inspired autolytic hydrogels exhibiting tunable high mechanical performances, repairability, and reusability. Acs Appl Mater Inter. 2016;8:8956–8966. doi: 10.1021/acsami.6b00912. [DOI] [PubMed] [Google Scholar]
- 35.Bian X.Y., Cui C.Y., Qi Y., Sun Y.G., Zhang Z.D., Liu W.G. Amino acid surfactant-induced superfast gelation of silk fibroin for treating noncompressible hemorrhage. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 36.Wu Y.H., Wang H.B., Gao F., Xu Z.Y., Dai F.Y., Liu W.G. An injectable supramolecular polymer nanocomposite hydrogel for prevention of breast cancer recurrence with theranostic and mammoplastic functions. Adv. Funct. Mater. 2018;28 [Google Scholar]
- 37.Cui C.Y., Liu B., Wu T.L., Liu Y., Fan C.C., Xu Z.Y., Yao Y., Liu W.G. A hyperbranched polymer elastomer-based pressure sensitive adhesive. J. Mater. Chem. A. 2022;10:1257–1269. [Google Scholar]
- 38.Chen J.Y., Yuan T., Liu Z.Z. Supramolecular medical antibacterial tissue adhesive prepared based on natural small molecules. Biomater Sci-Uk. 2020;8:6235–6245. doi: 10.1039/d0bm01101k. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y.J., Sun S.T., Wu P.Y. Adaptive ionogel paint from room-temperature autonomous polymerization of alpha-thioctic acid for stretchable and healable electronics. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 40.Mohammed-Ziegler I., Billes F. Vibrational spectroscopic calculations on pyrogallol and gallic acid. J Mol Struc-Theochem. 2002;618:259–265. [Google Scholar]
- 41.Zhang Z.Q., Tong P.D., Wang L., Qiu Z.H., Li J.A., Li H., Guan S.K., Lin C.G., Wang H.Y. One-step fabrication of self-healing poly(thioctic acid) coatings on ZE21B Mg alloys for enhancing corrosion resistance, anti-bacterial/oxidation, hemocompatibility and promoting re-endothelialization. Chem. Eng. J. 2023;451 [Google Scholar]
- 42.Hu B., Gao M.Z., Boakye-Yiadom K.O., Ho W., Yu W., Xu X.Y., Zhang X.Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021;6:4592–4606. doi: 10.1016/j.bioactmat.2021.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S., Choi D.W., Kim H.N., Park C.G., Lee W., Park H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12:1706. doi: 10.3390/pharmaceutics12070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haferkamp S., Arnolds O., Moog D., Wrobeln A., Nocke F., Cantore M., Pu S.F.N., Hartwig A., Franzkoch R., Psathaki O.E., Jastrow H., Schauerte C., Stoll R., Kirsch M., Jaegers J., Ferenz A.D.K.B. Deciphering the emulsification process to create an albumin-perfluorocarbon-nanoemulsion with high shelf life and bioresistivity. Langmuir. 2022;38:10351–10361. doi: 10.1021/acs.langmuir.1c03388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wongpinyochit T., Johnston B.F., Seib F.P. Degradation behavior of silk nanoparticles-enzyme responsiveness. ACS Biomater. Sci. Eng. 2018;4:942–951. doi: 10.1021/acsbiomaterials.7b01021. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Z.G., Zhang B.L., Wang S.S., Zai W.J., Yuan A., Hu Y.Q., Wu J.H. Perfluorocarbon nanoparticles mediated platelet blocking disrupt vascular barriers to improve the efficacy of oxygen-sensitive antitumor drugs. Small. 2018;14 doi: 10.1002/smll.201801694. [DOI] [PubMed] [Google Scholar]
- 47.Li C.M., Guo C.C., Fitzpatrick V., Ibrahim A., Zwierstra M.J., Hanna P., Lechtig A., Nazarian A., Lin S.J., Kaplan D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020;5:61–81. [Google Scholar]
- 48.Li Q.W., Wen J.R., Liu C.L., Jia Y.P., Wu Y.Z., Shan Y., Qian Z.Y., Liao J.F. Graphene-nanoparticle-based self-healing hydrogel in preventing postoperative recurrence of breast cancer. ACS Biomater. Sci. Eng. 2019;5:768–779. doi: 10.1021/acsbiomaterials.8b01475. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal A., Israilevich R., Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J. Am. Acad. Dermatol. 2019;81:558–567. doi: 10.1016/j.jaad.2019.02.047. [DOI] [PubMed] [Google Scholar]
- 50.Wengström Y., Forsberg C., Näslund I., Bergh J. Quantitative assessment of skin erythema due to radiotherapy—evaluation of different measurements. Radiother. Oncol. 2004;72:191–197. doi: 10.1016/j.radonc.2004.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.