Subretinal administration of voretigene neparvovec-rzyl (VN) was shown to improve light sensitivity, visual fields, and navigational ability under dim lighting conditions in patients with RPE65-associated retinal degeneration. Real-world evidence is mostly consistent with clinical trial results [1]. Nevertheless, some cases of considerable loss of best-corrected visual acuity (BCVA) [1], intraocular inflammation [2] and development of new or increased areas of chorioretinal atrophy [3] have been reported.
We present the case of a 45-year-old male with parental consanguinity and clinical diagnoses of early-onset rod-cone degeneration and end-stage renal disease. Whole exome sequencing (including copy number variation analysis) revealed probable homozygosity for the c.560G>A p.(Gly187Glu) likely pathogenic variant in RPE65 (NM_000329.2) and homozygosity for a pathogenic deletion of NPHP1 and partial loss of MALL: seq[GRCh37] del(2)(q13) chr2:g.110855123_110962791del. After multidisciplinary discussion, the patient was proposed for VN treatment. Bilateral, sequential (i.e., one week apart) 25G pars plana vitrectomy followed by subretinal injection of 0.3 mL of VN were performed by two senior vitreoretinal surgeons.
The patient underwent a comprehensive ophthalmic examination at three timepoints: baseline (i.e., pre-treatment), efficacy (3 months after surgery), and durability (6 months after surgery). Efficacy was defined as a change < −0.3 log units (cd.s/m2) in combined white full-field stimulus threshold (FST, DiagnosysFST®) in dark-adapted conditions at 3 months.
Results from clinical trials showed average sensitivity changes <−2 log units (cd.s/m2) (i.e., around 100-fold improvement in sensitivity) one year after treatment [4]. The clinically meaningful effect, which is nearly maximal by 30 days, is maintained for at least four years [4]. Our patient’s average change at 3 months was 0.861 log units (cd.s/sq2), denoting an almost 10-fold reduction in retinal sensitivity. Additionally, the patient lost 31 and 19 ETDRS letters OD and OS, respectively. Bilateral functional worsening persisted at 6 months. On spectral-domain optical coherence tomography (SD-OCT), both central retinal thickness and ellipsoid zone layer width decreased at 3 and 6 months (Fig. 1). The functional and structural worsening witnessed in our patient was not paralleled by new and/or increased areas of chorioretinal atrophy, nor the advent of postoperative intraocular inflammation. Subjectively, the patient reported decreased visual acuity and worsened dark adaptation immediately after treatment and persisting 6 months postoperatively.
Fig. 1. Multimodal retinal imaging at baseline and six months after Voretigene Neparvovec-rzyl treatment.
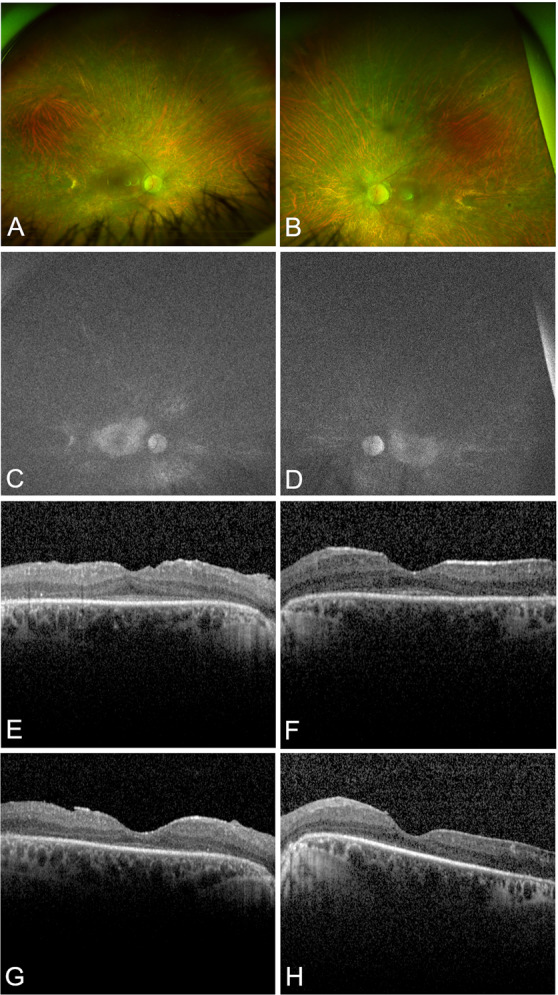
Baseline ultra-widefield color fundus photography (UWF-CFP) and fundus autofluorescence (UWF-FAF) imaging of the right (A, C) and left eyes (B, D). CFP shows bilateral diffuse outer retinal degeneration, vessel attenuation, optic disc pallor and bone spicule hyperpigmentation. FAF shows an overall reduction in normal autofluorescence usually observed in RPE65-associated retinal degeneration. Six months after treatment, there were no significant changes in either imaging modality (not shown). Spectral-domain optical coherence tomography (SD-OCT) macular B-scans of the right (E, G) and left (F, H) eyes at baseline (top row) and 6 months (bottom row) after treatment. Six months after treatment, there is evidence of bilateral foveal thinning and disruption of the ellipsoid zone (EZ) layer.
Animal studies showed that in the absence of nephrocystin-1 (the product of NPHP1 gene), the outer segment faces an accumulation of proteins normally located in the inner segment and that this disruption of protein homeostasis leads to retinal degeneration [5]. It is conceivable to hypothesize that the disruption of protein homeostasis could render the outer segments more susceptible to mechanical trauma caused by subretinal VN administration. Other than the potential genetic modifying effect of the NPHP1 deletion, vitrectomy and bleb formation could have acted as catalysts of an otherwise inevitable outcome in a 45-year-old with advanced retinal degeneration.
In summary, we present a case of manifest worsening in retinal function after VN gene therapy. While this study represents only one case, our findings might help clinicians in the decision whether to pursue RPE65 augmentation therapy in the setting of concurrent ciliopathies.
Author contributions
ERN was responsible for the study design, data collection, and drafting of the manuscript. JPM was responsible for study design, data collection, revision, and final approval of the manuscript. ALC was responsible for genetic testing, drafting of the manuscript, revision, and final approval of the manuscript. JF, JM, MA, CP, and TM were responsible for revision of the paper and its final approval. All authors are accountable for all aspects of this work and ensure the integrity of what it contains.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
ERN, ALC: No relevant conflicts of interest. JPM: Consultancy fees (Novartis); PERCEIVE study Principal Investigator in CHUC. JF: Consultancy fees (Novartis); PERCEIVE study Subinvestigator in CHUC. JM: Consultancy fees (Novartis). MA, CP, TM: PERCEIVE study Subinvestigators in CHUC.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sengillo JD, Gregori NZ, Sisk RA, Weng CY, Berrocal AM, Davis JL, et al. Visual acuity, retinal morphology, and patients’ perceptions after voretigene neparovec-rzyl therapy for RPE65-associated retinal disease. Ophthalmol Retina. 2022;6:273–83. doi: 10.1016/j.oret.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Kessel L, Christensen UC, Klemp K. Inflammation after voretigene neparvovec administration in patients with RPE65-related retinal dystrophy. Ophthalmology. 2022;129:1287–93. doi: 10.1016/j.ophtha.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Gange WS, Sisk RA, Besirli CG, Lee TC, Havunjian M, Schwartz H, et al. Perifoveal chorioretinal atrophy after subretinal voretigene neparvovec-rzyl for RPE65-mediated leber congenital amaurosis. Ophthalmol Retina. 2022;6:58–64. doi: 10.1016/j.oret.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maguire AM, Russell S, Chung DC, Yu Z-F, Tillman A, Drack AV, et al. Durability of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease: phase 3 results at 3 and 4 years. Ophthalmology. 2021;128:1460–8. doi: 10.1016/j.ophtha.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Datta P, Cribbs JT, Seo S. Differential requirement of NPHP1 for compartmentalized protein localization during photoreceptor outer segment development and maintenance. PLoS ONE. 2021;16:e0246358. doi: 10.1371/journal.pone.0246358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


