Abstract
Budd -Chiari syndrome (BCS) is a hepatic vascular disorder which affects hepatic veins or inferior vena cava. Portal vein thrombosis (PVT) occurs in around 15%-25% of patients with BCS. The presence of PVT in patients with BCS makes it more difficult to intervene radiologically. We present a case of a BCS-related chronic liver disease that presented with a history of variceal upper gastrointestinal bleeding and worsening ascites. The patient had thrombosed hepatic veins (HV) and partial right portal vein thrombosis. He was started on anticoagulation, and treatment for portal hypertension was initiated. Given the inaccessibility of all the HVs for trans-jugular intrahepatic portosystemic shunts (TIPS), the patient underwent direct intrahepatic portosystemic shunts (DIPS). Next-generation sequencing identified the factor V Leiden mutation. Following DIPS, the patient's ascites disappeared, and liver function tests improved. On a nine-month follow-up, the patient was symptom-free with a patent DIPS. DIPS has been widely used in patients with BCS with thrombosed hepatic veins, but there are only a few case reports on the feasibility of DIPS in BCS patients with PVT. This is one of the very few case reports where a patient with BCS-PVT was successfully managed with DIPS.
Keywords: Budd Chiari syndrome, cirrhosis, Leiden mutation, thrombophilia, venous thrombosis
The management of Budd Chiari syndrome (BCS) includes anticoagulation and treatment for portal hypertension, followed by radiological venous recanalization or surgery.1 The ultimate treatment goal of BCS is to prevent thrombus extension by anticoagulation, recanalization of the thrombosed inferior vena cava (IVC) or hepatic veins (HV), and prevention of portal hypertension (PHTN)-related complications. Trans-jugular intrahepatic portosystemic shunts (TIPS) are shunts that are formed between the hepatic veins and portal veins, and they are the standard of care for vascular recanalization in patients with BCS. However, complete thrombosis of all HVs along with portal vein thrombosis (PVT) makes it technically challenging to perform TIPS. A direct intrahepatic portosystemic shunt (DIPS), a shunt created between PV and IVC, has been performed in such patients with high technical and clinical success rates.2,3 Additionally, PVT imposes higher technical difficulties on such patients.4 In BCS, PVT may be due to an underlying prothrombotic condition. PVT adversely affects the outcome of patients with established portal hypertension. Concomitant PVT in patients with BCS has limited therapeutic options and carries a poor prognosis.5 Here we present a case of factor V Leiden mutation-related BCS with PVT, successfully managed with DIPS.
CASE PRESENTATION
A 31-year-old male presented with ascites for five months and one episode of hematemesis two days ago. There was no history of jaundice, altered sensorium, pain in the abdomen, or decreased urine output. On upper gastrointestinal endoscopy, the patient had large esophageal varices with stigmata of recent hemorrhage, which were tackled by endoscopic variceal ligation. On investigations, he had anemia and thrombocytopenia, hypoalbuminemia, and coagulopathy [ hemoglobin 9.1 g/dL (normal 13.2–16.6 g/dL); total leukocyte count: 4400/mm3 (normal 4000–11000/mm3); platelet count: 60,000/mm3 (normal 1.5–4.5 lacs/mm3); albumin: 3.1 g/L (normal 3.4–4.5 g/L); international normalized ratio (INR): 1.54 (normal 1.1–1.3)]; bilirubin 3.2 mg/dL (normal 0.4–1.2 mg/dL); serum glutamic-oxalacetic transaminase (SGOT): 34 U/L (normal: <40 U/L); serum glutamic-pyruvic transaminase (SGPT): 27 U/L (normal: <40 U/L); alkaline phosphatase (ALP): 127 U/L (normal: <150 U/L)]. Ultrasonography of the abdomen revealed a shrunken liver with a nodular outline, non-visualized HVs, and grade III ascites. Ascites fluid albumin and protein were 1.1 g/dL, and 1.9 g/dL respectively. So, it was a high serum ascites albumin gradient (SAAG = 2) and low protein ascites suggestive of BCS-chronic liver disease (CLD)-related ascites. His models for end-stage liver disease (MELD-sodium), Child-Pugh score, and BCS-TIPS prognostic index (PI) score were 27, 12, and 6.42, respectively. In a triple-phase computed tomography (CT) scan (Figure 1A) of the abdomen, all three HVs were thrombosed, and there was a thrombus in the right PV occluding its lumen. There were no arterial-enhancing lesions in the liver. The diagnosis was consistent with chronic BCS-related decompensated cirrhosis with non-tumoral PVT. All other alternate etiologies were ruled out. After the prompt institution of anticoagulation, diuretic and beta blocker, and vascular intervention for revascularization was planned.
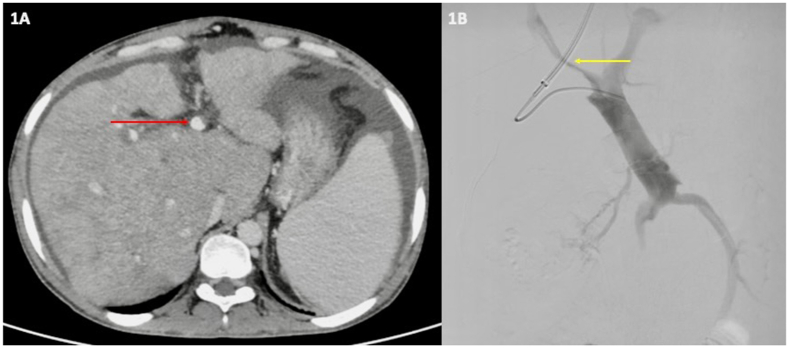
Figure 1.
A. Triple-phase computed tomography angiography scan showing volume re-distribution in the liver parenchyma with caudate lobe hypertrophy and ascites. No HV is visualized, and there is partial (<50%) thrombosis (red arrow) of the branch of the portal vein consistent with the diagnosis of BCS-PVT. B. Hepatic venogram after portal puncture showing thrombosed branches of the portal vein with luminal narrowing (yellow arrow).
TIPS was not feasible for the patient as all three HVs were thrombosed and non-accessible, an alternative therapy was considered. The patient's BCS-TIPS-PI score was less than seven, so a different radiological intervention was planned. The patient was posted for DIPS. The interventionist used the right transjugular approach to access the thrombosed portal vein and negotiated it through the trans-caval route under direct ultrasonographic guidance. A Porto-venogram confirmed the thrombosed right PV (Figure 1B). After performing balloon angioplasty of the thrombosed right PV (Figure 2A, B), 10 × 10 cm stents (FLUENCY) and 10 × 6 cm (COVERA) grafts were deployed across the parenchyma tract. 10 × 10 cm self-expanding metallic stent (SEMS) was deployed across the right portal vein thrombus into the main portal vein (Figure 2C). Post-stenting angioplasty was done using a 10 × 8 mm balloon (Boston Scientific, MUSTANG), and a satisfactory hepato-petal flow was achieved. The main PV was not targeted to avoid extrahepatic puncture. In the index case, due to volume redistribution, the left or main PV wasn't suitable for cannulation, and therefore the right portal vein was chosen. The patient's pre-DIPS portosystemic gradient (PSG) was 34 mm of Hg, which reduced significantly following the procedure (post-DIPS PSG = 14 mm Hg). There were no periprocedural complications. Warfarin and the underlying chronic liver disease (CLD) alter the serum levels of different anticoagulant and procoagulant proteins. So the patient underwent a thrombophilia-related gene panel workup using next-generation sequencing (NGS) to detect an underlying procoagulant state. It revealed a heterozygous variant of the F5 gene (mutation: p. Arg534Gln; c.1601G>A). This gene variant (factor V Leiden) is associated with activated protein C resistance (APCR) and an increased risk of venous thromboembolism.6 At discharge, the patient was on warfarin, beta-blockers, and diuretics.
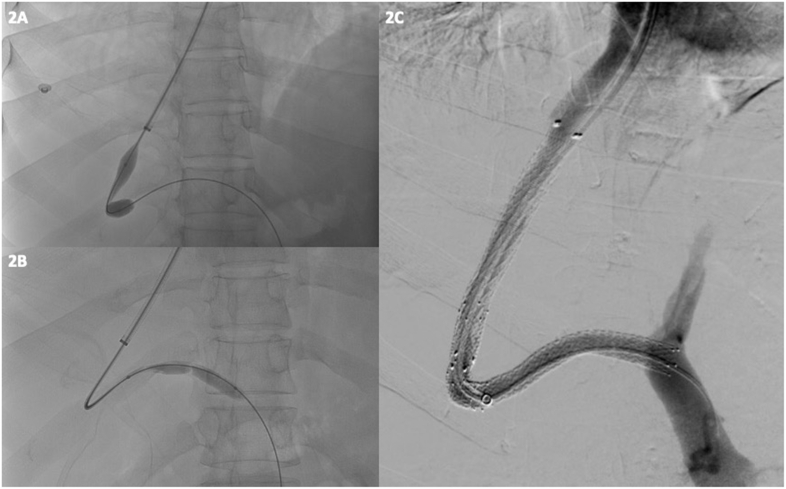
Figure 2.
A and B: Serial balloon dilation of PV done using a balloon dilator. C. Post-stenting good flow is noted across the Stent.
Portal venous (PV) pressure and inferior vena cava (IVC) pressure were measured for portosystemic gradient (PSG) before and after the procedure. Pre-DIPS: PV pressure = 44 mm Hg; IVC pressure: 10 mm Hg (PSG = 34 mm Hg). Post DIPS: PV pressure = 33 mm Hg; IVC pressure: 19 mm Hg (PSG = 14 mm Hg).
After three months of the intervention, the patient's liver stiffness measurement (LSM) was 12 kPa (pre-procedural LSM: 39 kPa). The patient's ascites also disappeared on minimal diuretics, bilirubin normalized, and body mass index (BMI) improved (BMI of 15.5 kg/m2 to 19 kg/m2). The patient did not experience further decompensation following DIPS for up to nine months of follow-up.
DISCUSSION
15–25% of patients with BCS have PVT, which is associated with a poor prognosis. Patients with PVT in the BCS group have lower albumin and higher bilirubin levels than those without PVT.7,8 Technical risks may be posed in cases where all HVs are completely thrombosed and PVT is present. In such cases, DIPS may serve as an alternative hybrid therapy that has demonstrated favorable outcomes.4 TIPS and DIPS are highly successful therapeutic options for patients with BCS. However, limited data are available on the feasibility of DIPS in the presence of PVT in BCS patients.5
A retrospective study by Murad et al. involving 282 BCS patients reported PVT in 42 patients (15%). TIPS was performed in four patients, surgical shunts in seven, and two required liver transplants in the BCS-PVT group. In contrast, 20 patients were treated conservatively, and five died during follow-up. The BCS-PVT group had a worse prognosis than the BCS group without PVT, with a five-year survival rate of 59% versus 85%.6 Mahmoud et al. found that 25% (13 of 51) of BCS patients had concomitant PVT, and they managed these cases with liver transplantation (four patients), surgical shunts (one), and balloon dilation of hepatic veins (one patient). Six patients were managed conservatively.8 In most case reports (Table 1) on BCS-PVT, patients were treated with TIPS or surgical management.9, 10, 11, 12 One case reported DIPS in a 22-year-old female patient with acute liver failure secondary to BCS and extensive thrombosis involving the PV and superior mesenteric vein. TIPS was not feasible in this case. Previous studies have validated the TIPS-BCS-PI score as the prognostic parameter for patients undergoing TIPS. Patients with a TIPS-BCS-PI score <7 had a higher liver transplant-free survival following TIPS.13 Our patient had a score of 6.42, so DIPS was performed. The patient showed drastic improvement following the intervention.
Table 1.
Management Options for Patients with Budd-Chiari Syndrome ith Portal Vein Thrombosis.
| References | Number of patients | Management |
|---|---|---|
| Murad et al.7 | 15 | Surgical shunts |
| TIPS | ||
| Liver transplant | ||
| Mahmoud et al.8 | 13 | Liver transplantation |
| Meso-atrial shunt | ||
| Balloon dilatation of hepatic veins | ||
| Artru et al.9 | 1 | TIPS |
| Souvanet et al.10 | 1 | Senning procedure |
| Watanabe et al.11 | 1 | TIPS |
| Opitz et al.12 | 1 | DIPS |
| Pedereson et al.4 | 1 | DIPS |
DIPS, direct intrahepatic portosystemic shunt; TIPS, transjugular intrahepatic portosystemic shunt; LT, liver transplant.
In our patient activated protein C resistance (APCR) was discovered to be the underlying prothrombotic condition caused by a rare factor V Leiden mutation. Factor V typically undergoes degradation by activated protein C resistance (APCR), which degrades factors Va and VIIIa using protein S as a cofactor. The mutation of factor V leads to resistance to this breakdown, allowing for longer thrombin generation and a procoagulant state.14 Previous studies have linked this rare factor V Leiden mutation to a higher risk of venous thromboembolism.7 One study from India found that 11% of BCS patients had a factor V mutation.15 Another study found that 64% of patients with TIPS-PVT had systemic hypercoagulability, 9% had a local etiology, and 24% had idiopathic thrombosis. In this study, two out of 33 patients (6%) had a factor V Leiden mutation. In patients with BCS and PVT, multifactorial etiologies were present in 53% of cases.8 Our patient was diagnosed with Factor V Leiden mutation-related thrombophilia without evidence of HCC or local pathology. TIPS were not feasible due to thrombosed HVs; therefore, he underwent DIPS, which resulted in clinical improvement and a significant decrease in liver stiffness measurement (LSM). On follow-up, his liver functions improved without further decompensation. This is one of the few case reports demonstrating the feasibility of DIPS in patients with BCS-PVT.
DIPS is a feasible treatment option for the rare subgroup of BCS patients with PVT. All these patients should be tested for underlying thrombophilia.
Credit authorship contribution statement
Sayan Malakar: Conceptualization and drafting of the case report.
Sayan Malakar, Vivek Shirol, Akash Mathur and Piyush Mishra: Management of the patients.
Ayushi Agarwal and Rajnikant R Yadav: Radiological intervention.
Uday C Ghoshal and Akash Mathur: Critical review and supervision.
Conflicts of interest
None of the other authors has any conflict of interest to declare concerning this paper.
Funding
None.
Informed consent
Consent was taken from the patient for the publication of this report and the images.
References
- 1.Shukla A., Shreshtha A., Mukund A., et al. Budd-Chiari syndrome: consensus guidance of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2021;15:531–567. doi: 10.1007/s12072-021-10189-4. PMID 34240318. [DOI] [PubMed] [Google Scholar]
- 2.Hatzidakis A., Galanakis N., Kehagias E., et al. Ultrasound-guided direct intrahepatic portosystemic shunt in patients with Budd-Chiari syndrome: short- and long-term results. Interv Med Appl Sci. 2017;9:86–93. doi: 10.1556/1646.9.2017.2.14. PMID 28932502. PMCID PMC5598128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keshava S.N., Moses V., Sharma A., et al. Technical and medium-term clinical outcomes of transjugular intrahepatic portosystemic shunt with fluoroscopy and additional trans-abdominal ultrasound guidance. Indian J Radiol Imaging. 2021;31:858–866. doi: 10.1055/s-0041-1735928. PMID 35136497. PMCID PMC8817814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen M.R., Molloy P., Wood D., Seetharam A. Direct intrahepatic portocaval shunt for treatment of portal thrombosis and Budd-Chiari syndrome. Ann Hepatol. 2016;15:127–130. doi: 10.5604/16652681.1184288. PMID 26626649. [DOI] [PubMed] [Google Scholar]
- 5.Mukhiya G., Zhou X., Han X., et al. Evaluation of outcome from endovascular therapy for Budd-Chiari syndrome: a systematic review and meta-analysis. Sci Rep. 2022;12 doi: 10.1038/s41598-022-20399-x. PMID 36171454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Athar M., Abduljaleel Z., Ghita I.S., et al. Prevalence of the factor V Leiden mutation Arg534Gln in western region of Saudi Arabia: functional alteration and association study with different populations. Clin Appl Thromb Hemost. 2021;27 doi: 10.1177/1076029620978532. PMID 33448877, PMCID PMC7812389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darwish Murad S., Valla D.C., de Groen P.C., et al. Pathogenesis and treatment of Budd-Chiari syndrome combined with portal vein thrombosis. Am J Gastroenterol. 2006;101:83–90. doi: 10.1111/j.1572-0241.2006.00353.x. PMID 16405538. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoud A.E., Helmy A.S., Billingham L., Elias E. Poor prognosis and limited therapeutic options in patients with Budd-Chiari syndrome and portal venous system thrombosis. Eur J Gastroenterol Hepatol. 1997;9:485–489. doi: 10.1097/00042737-199705000-00014. PMID 9187882. [DOI] [PubMed] [Google Scholar]
- 9.Artru F., Moschouri E., Denys A. Direct intrahepatic portocaval shunt (DIPS) or transjugular transcaval intrahepatic portosystemic shunt (TIPS) to treat complications of portal hypertension: indications, technique, and outcomes beyond Budd-Chiari syndrome. Clin Res Hepatol Gastroenterol. 2022;46 doi: 10.1016/j.clinre.2022.101858. PMID 34999250. [DOI] [PubMed] [Google Scholar]
- 10.Sauvanet A., Panis Y., Valla D., Vilgrain V., Belghiti J. Budd-Chiari syndrome with extensive portal thrombosis: treatment with Senning's procedure. Hepato-Gastroenterology. 1994;41:174–176. PMID 8056409. [PubMed] [Google Scholar]
- 11.Watanabe H., Shinzawa H., Saito T., et al. Successful emergency treatment with a transjugular intrahepatic portosystemic shunt for life-threatening Budd-Chiari syndrome with portal thrombotic obstruction. Hepato-Gastroenterology. 2000;47:839–841. PMID 10919043. [PubMed] [Google Scholar]
- 12.Opitz T., Buchwald A.B., Lorf T., Awuah D., Ramadori G., Nolte W. The transjugular intrahepatic portosystemic stent-shunt (TIPS) as rescue therapy for complete Budd-Chiari syndrome and portal vein thrombosis. Z Gastroenterol. 2003;41:413–418. doi: 10.1055/s-2003-39328. PMID 12772054. [DOI] [PubMed] [Google Scholar]
- 13.Seijo S., Plessier A., Hoekstra J., et al. Good long-term outcome of Budd-Chiari syndrome with step-wise management. Hepatology. 2013;57:1962–1968. doi: 10.1002/hep.26306. PMID 23389867. [DOI] [PubMed] [Google Scholar]
- 14.Dahlbäck B., Carlsson M., Svensson P.J., et al. The discovery of activated protein C resistance. J Thromb Haemost. 2003;1:3–9. doi: 10.1046/j.1538-7836.2003.00016.x. PMID 12871530. [DOI] [PubMed] [Google Scholar]
- 15.Amarapurkar D.N., Punamiya S.J., Patel N.D. Changing spectrum of Budd-Chiari syndrome in India with special reference to non-surgical treatment. World J Gastroenterol. 2008;14:278–285. doi: 10.3748/wjg.14.278. PMID 18186568, PMCID PMC2675127. [DOI] [PMC free article] [PubMed] [Google Scholar]