Abstract
Circumstantial evidence suggests that “Helicobacter heilmannii” infection is an example of zoonosis. The presence of “H. heilmannii” strains in a human subject with acute gastric erosions, in his two cats, and in two unrelated cats was analyzed, and the genetic relatedness of the human and feline strains was assessed. A 580-bp, PCR-amplified sequence of “H. heilmannii” urease B gene (ureB) obtained from biopsies from the human subject and his two cats was restricted with AluI and cloned for sequencing. Analysis of the restriction fragment length polymorphism of the ureB-amplified product suggested the presence of different individual “H. heilmannii” strains in the cats and of three distinct strains in the human subject. One of the “H. heilmannii” ureB sequences amplified from the human subject’s biopsies was identical to that derived from one of his cats. The degree of similarity between the other “H. heilmannii” human and feline nucleotide sequences was higher than 97%. Most of the base substitutions were conservative. We conclude that human and animal “H. heilmannii” strains are closely related and that humans can be infected by more than one “H. heilmannii” strain, as has been observed for Helicobacter pylori.
Helicobacter pylori, a stomach-colonizing bacterium that causes gastritis and peptic ulcer disease, is a risk factor for gastric adenocarcinoma (26) and malignant mucosa-associated lymphoid tissue lymphoma (35). Virtually every infected person harbors a different H. pylori strain (33). Nevertheless, infection with the same strain can occur, especially among members of the same family, as can simultaneous infection with several isolates (2, 17, 28). The high level of genetic diversity noted for H. pylori is not common to all the members of the genus Helicobacter, since Helicobacter mustelae, for example, is known to be much more conserved (32). “H. heilmannii”, previously known as Gastrospirillum hominis, is able to infect humans to a much lesser extent than H. pylori. Frequency of infection ranges from 0.25% (14) to 1.2 to 1.7% (19, 36) in symptomatic as well as nonsymptomatic persons (22), with most of those infected suffering from chronic active gastritis (14). Sporadic cases of gastric erosion (1, 3, 10) and gastric cancer (1, 23, 36) have also been reported.
Unlike H. pylori, “H. heilmannii” is not restricted to humans, since it can naturally infect a broad range of animals, such as cats, dogs, pigs, and primates, leading to mild to moderate gastritis (6, 27). The frequency of infection in cats, dogs, and pigs ranges from 80 to 100%, and “H. heilmannii” infection in humans has been postulated to be an example of zoonosis (20, 31, 34). Studies performed by Stolte et al. demonstrated that most infected persons have close contact with animals (31). While morphological criteria (3, 14, 25) allow the identification of Gastrospirillum-like organisms (GLO) in humans and animals, they do not allow differentiation between strains.
Our aim in this study was to determine the presence of “H. heilmannii” in a human subject with acute gastric erosions and in his two pet cats by PCR and to assess the genetic relatedness of the strains by restriction fragment length polymorphism (RFLP) and sequence analysis.
MATERIALS AND METHODS
Subjects and endoscopy.
A 38-year-old dentist presented with a 4-year history of recurrent dyspepsia. Endoscopy showed multiple antral ulcers (10 superficial ulcers up to 6 mm in diameter and 2 mm deep). A rapid urease test on antral biopsies indicated the presence of urease-positive organisms, and histology revealed long, spiral GLO. Endoscopy was performed a second time, and gastric biopsies were done on the antrum and the corpus. The human subject’s two cats (cats 1 and 2) and two cats belonging to different owners (cats 3 and 4) were anesthetized and examined endoscopically as previously described (24).
DNA extraction.
DNA extraction was performed by adding 200 μl (to a frozen biopsy) or 400 μl (to a paraffin-embedded biopsy) of K buffer (10 mM Tris-HCl [pH 7.4]–100 mM NaCl–25 mM EDTA–0.5% sodium dodecyl sulfate) containing 100 or 200 mg of proteinase K per ml. After the tissue was completely dissolved, three phenol-chloroform extractions were performed and DNA was precipitated, dried, and resuspended into 100 μl of sterile distilled water.
Amplification of a ureB 580-bp DNA fragment.
The forward primer (5′-GGGCGATAAAGTGCGCTTG-3′ [19-mer]) and the reverse primer (5′-CTGGTCAATGAGAGCAGG-3′ [18-mer]) were derived from the published “H. heilmannii” urease B sequence EMBL L25079 (29) and used to amplify a 580-bp segment of “H. heilmannii” urease B subunit gene as already described (24). The amplification reaction consisted of 1- to 2-μl DNA samples in a final volume of 50 μl containing 1× PCR buffer (Pharmacia Biotech, Dübendorf, Switzerland), 200 μM (each) deoxynucleoside triphosphate (Pharmacia), 100 pmol of primers (Microsynth GmbH, Balgach, Switzerland), and 2.5 U of Taq DNA polymerase (Pharmacia). Negative reagent control reactions in which the target DNA was replaced by sterile distilled water were performed with every set of amplifications.
The temperature and time schedule was as follows: 1 cycle of denaturation at 94°C for 3 min, annealing at 57°C for 2 min, and extension at 72°C for 3 min followed by 30 cycles at 94°C for 30 s, 57°C for 30 s, and 72°C for 1 min. Following completion of the 30 cycles, the tubes were incubated at 94°C for 20 s, at 57°C for 20 s, and at 72°C for an additional 5 min. PCR products were resolved by electrophoresis in a 1% (wt/vol) agarose gel containing 0.5 μg of ethidium bromide per ml.
RFLP analysis.
The PCR products (5 to 15 μl) showing a single band of the expected size were subjected to restriction analysis with the AluI enzyme in the supplied buffer according to the manufacturer’s (Pharmacia) protocol. Restriction products were separated on a 2% metaphore agarose (FMC BioProducts, Rockland, Maine) gel in 0.045 M Tris-borate–0.001 M EDTA. The gel was stained with ethidium bromide and examined on a UV transilluminator.
Sequence and sequence analysis of the PCR products.
The amplified 580-bp “H. heilmannii” ureB fragments were cloned into the pGEM-T vector (Promega, Wallisellen, Switzerland), allowing direct cloning of the PCR products. Recombinant plasmids were purified with Qiagen-Tips 100 (Qiagen AG, Basel, Switzerland) according to the manufacturer’s instructions. Each plasmid (10 μg) was sequenced on an automatic ALF sequencer with fluoresceinated universal and reverse primers combined with dideoxynucleotides as recommended by the manufacturer (Pharmacia LKB).
The DNA and inferred amino acid sequences were analyzed with version 8.1-UNIX software (Genetics Computer Group, University of Wisconsin, Madison); homologies were estimated with the BESTFIT program.
RESULTS
PCR amplification and AluI restriction fragment length patterns of a 580-bp DNA fragment of Helicobacter ureB in human and cat biopsies.
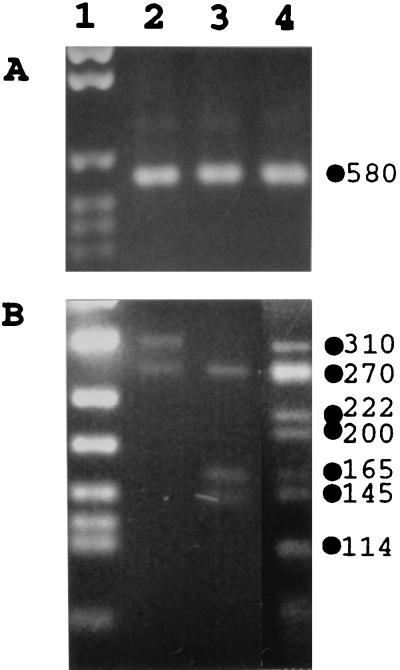
To confirm the morphological evidence suggesting that a 38-year-old human subject was infected by “H. heilmannii”, amplification reactions were performed on DNA extracted from the subject’s gastric antral biopsies with primers shown to be specific for a fragment of the “H. heilmannii” urease B gene (24). The expected 580-bp amplification product was obtained, unambiguously demonstrating the presence of “H. heilmannii” in the human subject’s gastric mucosa (Fig. 1A, lane 4); no PCR product could be detected when PCRs were run with primers specific for either H. pylori or Helicobacter felis ureB (data not shown).
FIG. 1.
(A) PCR amplification of a “H. heilmannii” ureB fragment. Agarose gel (1%) electrophoresis of products from PCR was performed with the forward 19-mer and the reverse 18-mer primers described in Materials and Methods. DNA molecular weight standard VI (Boehringer) (lane 1) and DNA template for amplification extracted from gastric biopsies of cat 1 (lane 2), cat 2 (lane 3), and the human subject (lane 4) are shown. (B) AluI restriction fragments of the “H. heilmannii” ureB 580-bp PCR products. Metaphore agarose gel (2%) electrophoresis of RFLP products was performed. DNA molecular weight standard VIII (Boehringer) (lane 1) and digested PCR product from cat 1 (lane 2), cat 2 (lane 3), and the human subject (lane 4) are shown.
Since “H. heilmannii” infection in humans is postulated to be transmitted by pets, we performed endoscopy on the human subject’s two cats and established by breath test, rapid urease test, and histology on gastric biopsies that the cats were both infected with GLO, i.e., with urease-positive organisms that resemble “H. heilmannii” (data not shown). PCRs on DNA extracted from the cats’ gastric biopsies with “H. heilmannii” ureB-specific primers resulted in a 580-bp product (Fig. 1A, lanes 2 and 3). No amplification was obtained with H. pylori- or H. felis-ureB-derived primers (data not shown).
The human and feline “H. heilmannii” PCR fragments were then subjected to RFLP analysis with the enzyme AluI (Fig. 1B). Two bands of approximately 310 and 270 bp were obtained from the “H. heilmannii” PCR fragment from cat 1 (lane 2), three bands of 270, 165, and 145 bp were obtained from the “H. heilmannii” PCR fragment from cat 2 (lane 3), and a complex pattern was obtained for the human subject’s “H. heilmannii” fragment (lane 4). The total size of the human subject’s restriction fragments was three times the size of the undigested PCR product, suggesting that he was infected by at least three strains.
Molecular analysis of the PCR fragments.
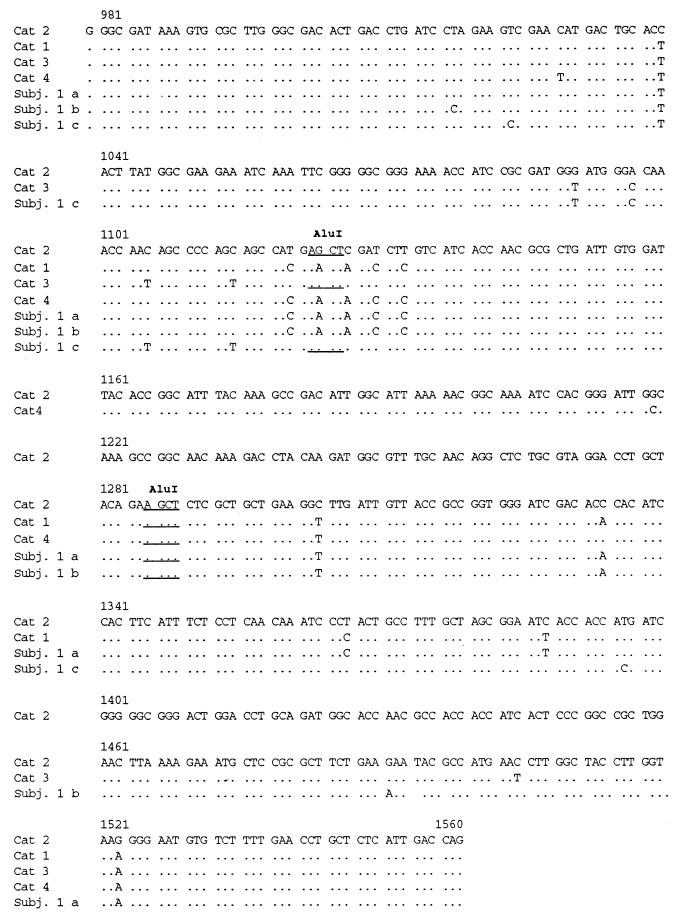
To determine the genetic relatedness of the human and cat strains, the 580-bp “H. heilmannii” ureB PCR products were cloned into the pGEMT vector. Several recombinant clones were obtained, and the nucleotide sequences were determined (Fig. 2). Three different sequences were obtained for the strains from the subject. These sequences were aligned and compared with those for the strains from the human subject’s cats (cats 1 and 2) and with those for the strains of two unrelated cats belonging to different, unrelated owners (cats 3 and 4).
FIG. 2.
Nucleotide sequences of the 580-bp ureB PCR product obtained from human and feline strains. Since the sequence of the “H. heilmannii” ureB fragment from cat 2 was found to be identical to the EMBL 25079 sequence, the nucleotide position refers to the EMBL sequence. Dots denote nucleotide identity. AluI cutting sites used in the RFLP analysis are underlined.
The AluI restriction fragments deduced from the nucleotide sequence data of “H. heilmannii” strains present in the human subject’s two cats corresponded perfectly to those resolved by RFLP. In contrast, analysis of the sequences of the human subject’s “H. heilmannii” strains could not explain all the bands visualized by RFLP, suggesting that the 580-bp product amplified from the man’s gastric biopsies contained more sequences than those that were cloned and sequenced.
Genetic relatedness of human and cat “H. heilmannii” sequences.
Comparison of the “H. heilmannii” ureB sequences derived from cats 1 to 4 showed that the sequences were not identical but were nevertheless highly homologous (97.6 to 99.1%). Interestingly, while identity between feline strains was not observed, one of the three sequences derived from the human subject’s biopsy was found to be 100% identical to the sequence from one of his cats (cat 1) (Table 1). Furthermore, the “H. heilmannii” ureB sequence derived from cat 2 perfectly matched the human sequence found in data banks (EMBL L25079).
TABLE 1.
Sequence similarity between the “H. heilmannii” ureB 580-bp fragments from feline and human strains
| Cat no. | % DNA homology for human subject sequencea
|
||
|---|---|---|---|
| 1a | 1b | 1c | |
| 1 | 100 | 99.1 | 97.2 |
| 2 | 98.1 | 98.3 | 98.8 |
| 3 | 97.6 | 97.4 | 99.3 |
| 4 | 99.1 | 99 | 97.4 |
The three sequences (1a, 1b, and 1c) from the human subject were aligned with the four feline sequences (from cats 1 to 4) with the BESTFIT program (Genetics Computer Group).
When all the sequences were compared, point mutations were observed at 22 different positions along the 580-bp sequence; 16 (73%) occurred in the third base position of the codon, 4 (18%) occurred at the second base position, and 2 (9%) occurred at the first base position. Most of these base substitutions were found to be conservative; i.e., they either did not change the encoded amino acid or substituted a homologous amino acid; indeed, of a total of 6 amino acid substitutions, only two, one in a strain from the human subject (sequence 1b) (E→K188) and one in a strain from cat 4 (H→Y34), were not conservative (Table 2).
TABLE 2.
Analysis of the nucleotide and inferred amino acid substitutions in the “H. heilmannii” ureB 580-bp fragment
| Source of “H. heilmannii” ureB sequence | No. of substitutions
|
|
|---|---|---|
| Nucleotides | Amino acidsa | |
| Human subject | ||
| 1a | 11 | 0 |
| 1b | 10 | 2 (L→P30, E→K188) |
| 1c | 7 | 2 (V→A32, M→T156) |
| Cat | ||
| 1 | 11 | 0 |
| 2 | 0 | 0 |
| 3 | 7 | 0 |
| 4 | 10 | 2 (H→Y34, G→A97) |
The positions of the amino acids refer to the urease B amino acid sequence derived from sequence EMBL 25079 (ATG→M1).
These data demonstrate that, despite a significant degree of heterogeneity in the “H. heilmannii” ureB DNA sequence, amino acid sequences are well conserved and some feline strains are indistinguishable from human strains.
DISCUSSION
Colonization by GLO of the gastric mucosa in various pets, especially cats and dogs, has often been described. Thus, in contrast to H. pylori, “H. heilmannii” has a natural nonhuman reservoir, and transmission from animal to human hosts could occur. The transmission of this bacterium does not seem to be very effective, however, since the prevalence of “H. heilmannii” is very high in pets (15) but, fortunately, very low in humans (16). In cats and dogs, “H. heilmannii” colonization is associated with mild to moderate gastritis (6, 24); in humans, it causes mild gastritis (16), but people infected by “H. heilmannii” have also developed gastric ulcers (16, 27, 30, 37) and even cancer (23, 36).
Similarity between the spirilla found in humans and animal bacteria was illustrated by Lee et al. (21). However, despite morphological similarities, it is not clear whether the human “H. heilmannii” strains are identical to those observed in animals. While “H. heilmannii” strains can be maintained in vivo by feeding rodents homogenized gastric biopsies from colonized patients or animals (5, 21), attempts to culture the human and cat “H. heilmannii” in vitro have so far been largely unsuccessful (4). GLO resembling “H. heilmannii” have been described in dogs, but these organisms, named Helicobacter bizzozeronii and Helicobacter salomonis, are culturable, in contrast to “H. heilmannii” (12, 13, 18).
We used PCR to amplify, clone, and sequence a fragment of the “H. heilmannii” urease B gene directly from human and cat gastric biopsies. The structural gene ureB from “H. heilmannii” is highly homologous to ureB from H. pylori and H. felis (29), but we have shown previously that the primers used in our PCRs do not cross-hybridize with DNA from related species and are therefore highly specific (24). The DNA and predicted amino acid sequences reported here are subject to the fidelity constraints of Taq polymerase. The reported measured error rate of Taq polymerase ranges from 2 × 10−4 to less than 1 × 10−5 errors per nucleotide per cycle (7). For the 580 bp of the ureB fragment, we expect <1 to 1 error per molecule. Therefore, the 7 to 11 base mutations we observed in the 580-bp fragment can truly be attributed to strain heterogeneity and not to Taq polymerase infidelity.
The heterogeneity observed in the “H. heilmannii” ureB sequences (with a DNA sequence similarity ranging between 97.2 and 100%) appears to be comparable to that reported for a partial DNA sequence of ureC from 15 strains of H. pylori (sequence similarity, 95.3 to 99.2%) (9). Together, these data confirm that Helicobacter ureases are encoded by well-conserved genes; other genes such as H. pylori adhesin HpA present a much larger heterogeneity between isolates (8).
While sequence comparisons showed that all the urease fragments from the “H. heilmannii” cat strains tested were different, they twice revealed 100% homology between urease fragments of “H. heilmannii” strains of human and cat origin. The “H. heilmannii” ureB sequence of cat 2 matched perfectly that of an Australian patient described in the gene banks, and the “H. heilmannii” ureB sequence of cat 1 was found to be identical to that of its owner.
In contrast to the former case, which is unlikely to be a case of zoonosis because of geographical constraints, the latter case might be an example of direct animal-to-human transmission, as the human subject had been in contact with pets since his childhood. We do not know whether the man’s strains are clonal variants or whether they were acquired from his previous pets, nor do we know whether the heterogeneity that we observed in the ureB fragment reflects the situation in the whole genome. However, we demonstrate for the first time that, despite the reported heterogeneity between “H. heilmannii” strains, some human and cat strains harbor identical ureB sequences.
Although the cats’ owner probably acquired the “H. heilmannii” strain(s) directly from his pet(s), the possibility cannot be excluded that he infected them or that the cats and their owner became infected from the same sources, as other studies have shown that “H. heilmannii” can be found in dogs and pigs (6, 11, 27).
We tried to culture the “H. heilmannii” organisms on artificial media but failed. We also tried to maintain and propagate the organisms in vivo by inoculating the human and cat gastric tissues into the stomachs of pathogen-free mice (5). We succeeded with the feline strains but not with the human strains. Propagation of “H. heilmannii” would have allowed us to extend our study and compare the 16S rRNA gene sequences which are already known. We do not know, however, whether this method would have allowed the maintenance of multiple strains, as passage through a different host might lead to the preferential selection of one of the clones.
We report here for the first time that humans can be infected simultaneously with several “H. heilmannii” strains. This type of multiple infection was previously known to be possible in humans only in the case of H. pylori (17, 28). Three different sequences were obtained from the human subject in this study, but the restriction profiles obtained were nevertheless unaccounted for. Thus, our data demonstrate (i) that some human and feline “H. heilmannii” strains are very similar, if not identical, suggesting the possibility of transmission between household pets and their owners and (ii) that humans can be infected by more than one “H. heilmannii” strain, as has been observed for H. pylori.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Foundation (grants no. 31.46858.96 to I.C.-T. and no. 31.43240.95 to A.B.). I. Corthésy-Theulaz received a Swiss Confederation Grant for Academical Scientists (Bourse de Relève).
We thank S. Hopkins for critical reading of the manuscript and are indebted to M. Roulet, H. Bouzourène, E. Saraga, and G. Neiger for their clinical support and to D. Schorderet for access to his sequencing facilities.
REFERENCES
- 1.Akin O Y, Tsou V M, Werner A L. Gastrospirillum hominis-associated chronic active gastritis. Pediatr Pathol Lab Med. 1995;15:429–435. doi: 10.3109/15513819509026978. [DOI] [PubMed] [Google Scholar]
- 2.Akopyanz N, Bukanov N O, Westblom T U, Berg D E. PCR-based RFLP analysis of DNA sequence diversity in the gastric pathogen Helicobacter pylori. Nucleic Acids Res. 1992;20:6221–6225. doi: 10.1093/nar/20.23.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Himyary A J, Zabaneh R I, Zabeneh S S, Barnett S. Gastrospirillum hominis in acute gastric erosion. South Med J. 1994;87:1147–1150. doi: 10.1097/00007611-199411000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Andersen L P, Norgaard A, Holck S, Blom J, Elsborg L. Isolation of a Helicobacter heilmannii-like organism from the human stomach. Eur J Clin Microbiol Infect Dis. 1996;15:95–96. doi: 10.1007/BF01586196. [DOI] [PubMed] [Google Scholar]
- 5.Dick E, Lee A, Watson G, O’Rourke J. Use of the mouse for the isolation and investigation of stomach-associated, spiral-helical shaped bacteria from man and other animals. J Med Microbiol. 1989;29:55–62. doi: 10.1099/00222615-29-1-55. [DOI] [PubMed] [Google Scholar]
- 6.Eaton K A, Dewhirst F E, Paster B J, Tzellas N, Coleman B E, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert K A, Kunkel T A. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1991;1:17–24. doi: 10.1101/gr.1.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Evans D G, Evans D J, Jr, Lampert H C, Graham D Y. Restriction fragment length polymorphism in the adhesin gene hpaA of Helicobacter pylori. Am J Gastroenterol. 1995;90:1282–1288. [PubMed] [Google Scholar]
- 9.Ferrero R L, Labigne A. Organization and expression of the Helicobacter pylori urease gene cluster. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 171–190. [Google Scholar]
- 10.Goddard A F, Logan R P H, Atherton J C, Jenkins D J, Spiller R C. Healing of duodenal ulcer following eradication of Helicobacter heilmannii. Lancet. 1997;349:1815–1816. doi: 10.1016/S0140-6736(05)61696-0. [DOI] [PubMed] [Google Scholar]
- 11.Grasso G M, Ripabelli G, Sammarco M L, Ruberto A, Iannitto G. Prevalence of Helicobacter-like organisms in porcine gastric mucosa: a study of swine slaughtered in Italy. Comp Immunol Microbiol Infect Dis. 1996;19:213–217. doi: 10.1016/0147-9571(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 12.Hanninen M L, Happonen I, Saari S, Jalava K. Culture and characteristics of Helicobacter bizzozeronii, a new canine gastric Helicobacter sp. Int J Syst Bacteriol. 1996;46:160–166. doi: 10.1099/00207713-46-1-160. . (Erratum, 46:839.) [DOI] [PubMed] [Google Scholar]
- 13.Hanninen M L, Jalava K, Saari S, Happonen J, Westermarck E. Culture of “Gastrospirillum” from gastric biopsies of dogs. Eur J Clin Microbiol Infect Dis. 1995;14:145–146. doi: 10.1007/BF02111876. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann K L, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 16.Hilzenrat N, Lamoureux E, Weintrub I, Alpert E, Lichter M, Alpert L. Helicobacter heilmannii-like spiral bacteria in gastric mucosal biopsies. Prevalence and clinical significance. Arch Pathol Lab Med. 1995;119:1149–1153. [PubMed] [Google Scholar]
- 17.Hirschl A M, Richter M, Makristathis A, Pruckl P M, Willinger B, Schutze K, Rotter M L. Single and multiple strain colonization in patients with Helicobacter pylori-associated gastritis: detection by macrorestriction DNA analysis. J Infect Dis. 1994;170:473–475. doi: 10.1093/infdis/170.2.473. [DOI] [PubMed] [Google Scholar]
- 18.Jalava K, Kaartinen M, Utriainen M, Happonen I, Hanninen M L. Helicobacter salmonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int J Syst Bacteriol. 1997;47:975–982. doi: 10.1099/00207713-47-4-975. [DOI] [PubMed] [Google Scholar]
- 19.Kubonova K, Trupi J, Jancula L, Polak E, Vrablik V. Presence of spiral bacteria (“Gastrospirillum hominis”) in the gastric mucosa. Eur J Clin Microbiol Infect Dis. 1991;10:459–460. doi: 10.1007/BF01968031. [DOI] [PubMed] [Google Scholar]
- 20.Lavelle J P, Landas S, Mitros F A, Conklin J L. Acute gastritis associated with spiral organisms from cats. Dig Dis Sci. 1994;39:744–750. doi: 10.1007/BF02087417. [DOI] [PubMed] [Google Scholar]
- 21.Lee A, Hazell S L, O’Rourke J, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzucchelli L, Wilder-Smith C H, Ruchti C, Meyer-Wyss B, Merki H S. Gastrospirillum hominis in asymptomatic, healthy individuals. Dig Dis Sci. 1993;38:2087–2089. doi: 10.1007/BF01297089. [DOI] [PubMed] [Google Scholar]
- 23.Morgner A, Bayerdorffer E, Meining A, Stolte M, Kroher G. Helicobacter heilmannii and gastric cancer. Lancet. 1995;346:511–512. doi: 10.1016/s0140-6736(95)91364-5. [DOI] [PubMed] [Google Scholar]
- 24.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthésy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634–637. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliva M M, Lazenby A J, Perman J A. Gastritis associated with Gastrospirillum hominis in children. Comparison with Helicobacter pylori and review of the literature. Mod Pathol. 1993;6:513–515. [PubMed] [Google Scholar]
- 26.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 27.Queiroz D M, Rocha G A, Mendes E N, De Moura S B, De Oliveira A M, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996;111:19–27. doi: 10.1053/gast.1996.v111.pm8698198. [DOI] [PubMed] [Google Scholar]
- 28.Shortridge V D, Stone G G, Flamm R K, Beyer J, Versalovic J, Graham D W, Tanaka S K. Molecular typing of Helicobacter pylori isolates from a multicenter U.S. clinical trial by ureC restriction fragment length polymorphism. J Clin Microbiol. 1997;35:471–473. doi: 10.1128/jcm.35.2.471-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solnick J V, O’Rourke J, Lee A, Tompkins L S. Molecular analysis of urease genes from a newly identified uncultured species of Helicobacter. Infect Immun. 1994;62:1631–1638. doi: 10.1128/iai.62.5.1631-1638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolte M, Kroher G, Meining A, Morgner A, Bayerdorffer E, Bethke B. A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 1997;32:28–33. doi: 10.3109/00365529709025059. [DOI] [PubMed] [Google Scholar]
- 31.Stolte M, Wellens E, Bethke B, Ritter M, Eidt H. Helicobacter heilmannii (formerly Gastrospirillum hominis) gastritis: an infection transmitted by animals? Scand J Gastroenterol. 1994;29:1061–1064. doi: 10.3109/00365529409094888. [DOI] [PubMed] [Google Scholar]
- 32.Taylor D E, Chang N, Taylor N S, Fox J G. Genome conservation in Helicobacter mustelae as determined by pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1994;118:31–36. doi: 10.1111/j.1574-6968.1994.tb06799.x. [DOI] [PubMed] [Google Scholar]
- 33.Taylor D E, Eaton M, Chang N, Salama S M. Construction of a Helicobacter pylori genome map and demonstration of diversity at the genome level. J Bacteriol. 1992;174:6800–6806. doi: 10.1128/jb.174.21.6800-6806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wegmann W, Aschwanden M, Schaub N, Aenishanslin W, Gyr K. Gastritis associated with Gastrospirillum hominis—a zoonosis? Schweiz Med Wochenschr. 1991;121:245–254. . (In German.) [PubMed] [Google Scholar]
- 35.Wotherspoon A C, Ortiz-Hidalgo C, Falzon M R, Isaacson P G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Li X, Xu Z, Zhou D. “Helicobacter heilmannii” infection in a patient with gastric cancer. Dig Dis Sci. 1995;40:1013–1014. doi: 10.1007/BF02064190. [DOI] [PubMed] [Google Scholar]
- 37.Yeomans N D, Kolt S D. Helicobacter heilmannii (formerly Gastrospirillum): association with pig and human gastric pathology. Gastroenterology. 1996;111:244–247. doi: 10.1053/gast.1996.v111.agast961110244. [DOI] [PubMed] [Google Scholar]