Abstract
Non-alcoholic fatty liver disease (NAFLD) has emerged as one of the common causes of cirrhosis and hepatocellular carcinoma (HCC) and is a leading indication for liver transplantation (LT). Patients with NAFLD-related cirrhosis and HCC are at high risk for the development of recurrent NAFLD after LT. NAFLD can also develop de novo post-transplantation in patients subjected to LT for other indications. Besides the pretransplant presence of various components of metabolic syndrome (MS) use of immunosuppressive agents in the post-LT setting forms one of the major drivers for the development of post-LT NAFLD. Individual components of conventional immunosuppressive regimens (corticosteroids, calcineurin inhibitors, and m-TOR inhibitors) are all implicated in the development of post-LT metabolic derangement and follow unique mechanisms of action and degree of disturbances. The development of cardiovascular risk is associated with post-LT NAFLD, although graft outcomes do not seem to be influenced only by the presence of post-LT NAFLD. Measures in consonance with the management of NAFLD, in general, including lifestyle modifications and control of metabolic risk factors, hold true for post-LT NAFLD. Tailoring immunosuppression strategies with early corticosteroid withdrawal and calcineurin inhibitor minimization balancing against the risk of graft rejection constitutes important nuances in the individualized management of post-LT NAFLD.
Keywords: liver transplantation, NASH, cryptogenic cirrhosis, metabolic syndrome, rational immunosuppression
Non-alcoholic fatty liver disease (NAFLD) has emerged as one of the most common causes of chronic liver disease worldwide with an estimated global prevalence of about 30%.1 The prevalence of NAFLD in the general population in India varies from 9 to 53% with a pooled prevalence of 39%.2,3 In consonance with the exponential rise, it has also established itself as one of the commonest indications of liver transplantation (LT).1,3 A seemingly paradoxical, but anticipated, challenge of NAFLD in the post-transplant setting has emerged synchronously with the increasing use of transplantation as well as its changing epidemiology.
NAFLD in the post-transplant setting can be classified into two subtypes, a more common recurrent NAFLD and a comparatively lesser common de novo NAFLD. Recurrent NAFLD is the re-occurrence of NAFLD in patients who were originally transplanted for NAFLD-related cirrhosis or hepatocellular carcinoma (HCC).4 On the other hand, de novo NAFLD is defined as the onset of liver steatosis or non-alcoholic steatohepatitis (NASH) after at least six months of transplantation who were originally transplanted for non-NAFLD indications.5 While there are multiple risk factors that predispose to the development of NAFLD post-LT, in the following sections, we delve into the impact of immunosuppression on the development of NAFLD and NASH in the post-LT setting.
Epidemiology of post-LT NAFLD
Recurrent NAFLD post-LT which reflects a re-occurrence of the primary disease has been reported to be extremely common with one study reporting a prevalence of more than 90% of which 25% had advanced fibrosis.6 Ten-year follow-up data from a single center showed the development of NAFLD in two-thirds, with one-fourth having NASH, and 18% having significant fibrosis.7 Pooled meta-analysis data show an incidence of post-LT recurrent NAFLD of 59%, 57%, and 82% at 1, 3, and 5 years following LT, respectively. Similarly, the incidence of post-LT recurrent NASH has been estimated to be 53%, 57.4%, and 38% at 1, 3, and 5 years following LT, respectively.8 On the other hand, a recent meta-analysis of 12 studies involving 2166 patients shows that de novo NAFLD has a variable prevalence of 14.7%–52% post-LT which is less common than recurrent NAFLD.5 Furthermore, the same meta-analysis also showed a variable prevalence of 0.96%–32% of biopsy-proven NASH involving eight studies in those having de novo NAFLD.5
Risk Factors for Post-transplant NAFLD
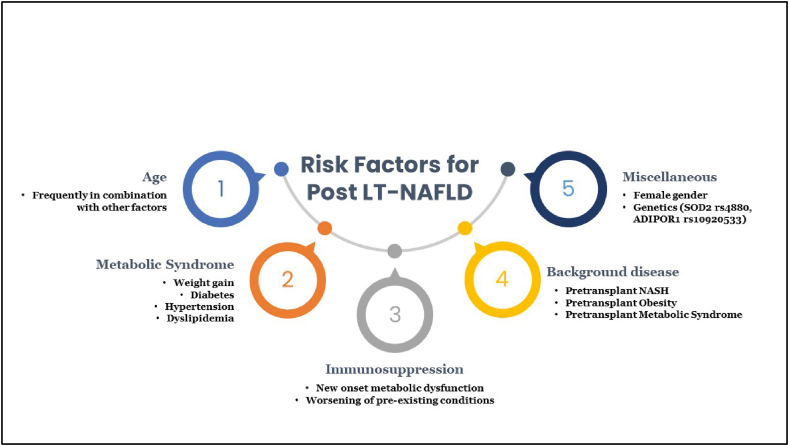
The traditional risk factors for NAFLD, including obesity, post-LT weight gain, diabetes mellitus, hypertension, and hyperlipidemia, holds true for the development of post-LT NAFLD with diabetes having a stronger association with recurrent NAFLD.9,10 Other risk factors that have been implicated in the development of post-LT NAFLD include age (in conjunction with components of metabolic syndrome (MS)), female gender, and genetic factors including PNPLA3 gene polymorphisms.11, 12, 13 Besides, these risk factors side effects mediated by immunosuppressants have been associated with the development of post-LT NAFLD. The culminating point of all the associated risk factors is the development of post-transplant metabolic syndrome (PTMS) which, in turn, has a proportional relationship with post-LT NAFLD. In addition to the aforementioned risk factors, rapid weight gain post-LT and lower exercise intensity accelerate PTMS and post-LT NAFLD. Figure 1 provides an overview of the risk factors of post-LT NAFLD.
Figure 1.
An overview of the risk factors of post-LT NAFLD.
Implication of individual immunosuppressant drugs in post-LT NAFLD
Corticosteroids
Corticosteroids form an essential component of the immunosuppressive strategy in the immediate postoperative setting to prevent graft rejection. The use of corticosteroids both in the short term and long term is associated with metabolic complications and frequently leads to hyperglycemia, hypertension, hyperlipidemia, and post-transplant obesity.14 However, current day immunosuppression protocols have progressively been moving toward rapid steroid weaning protocols and steroid-free regimens which have limited the adverse effects.15 Data from protocols using steroid-free immunosuppression have shown a significantly reduced rate of new-onset diabetes post-LT.16
Calcineurin Inhibitors
The calcineurin inhibitors (CNI) [cyclosporine A (CsA) and tacrolimus (TAC)] have transformed immunosuppression practices with TAC being the current backbone of modern immunosuppression regimens. The diabetogenic mechanisms of CNIs involve multiple axes which include induction of insulin resistance, inhibition of transcription factors for beta-cell growth, downregulation of adiponectin transcription, and CNI-mediated hypomagnesemia (renal wasting) which leads to impaired insulin signaling.17 On a comparative basis, the literature from renal transplant recipients indicate toward a higher diabetogenic potential of TAC than CsA which, however, does not translate into any differences in short-term outcomes.18
Dyslipidemias are associated with both CsA and TAC with the former having a worse profile.19 The mechanisms that have been proposed for dyslipidemia include inhibition of sterol 27-hydroxylase resulting in increased 3-hydroxy-2-methylglutaryl coenzyme A activity and a subsequent increase in cholesterol levels, decrease in bile acid synthesis from cholesterol resulting in increased cholesterol levels, and reduction in triglyceride degradation via inhibition of lipoprotein lipase.20
The development of hypertension post-transplant is common and is reported in more than 50% of the cases in some studies, and exposure to CNI has been proposed as a predominant reason for the same.21,22 Multiple mechanisms have been proposed including rennin and angiotensin II upregulation, decreased glomerular filtration, increased renal tubular reabsorption of sodium, sympathetic overactivity, and impairment of arterial vasodilation due to reduced levels of prostacyclin and nitric oxide.23 Although decreased filtration and increased sodium reabsorption with renal vasoconstriction appears to be one of the primary mechanisms, the activation of rennin-angiotensin system is not implicated as a major pathway.22
mTOR Inhibitors (mTORi)
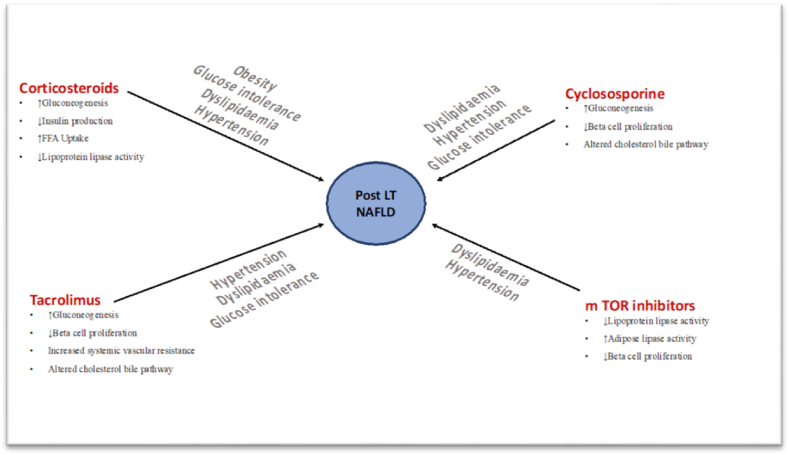
mTORi the newest class in the immunosuppressive armamentarium is commonly associated with the development of hyperglycemia and dyslipidemia. These tend to increase high-density lipoproteins (HDL), low-density lipoproteins (LDL), cholesterol, and triglycerides in approximately 40–75% of patients receiving therapy.24 Although multiple mechanisms possibly lead to mTORi-induced dyslipidemia, the primary reasons include the inhibition of lipid transport into adipocytes and increased basal lipolysis.25 mTORi-induced dyslipidemia is dose-dependent as studies from renal transplant recipients have shown that with both sirolimus and everolimus lower fixed dose leads to less profound changes than higher doses, even in the presence of standard-exposure CNI therapy.26,27 Current day practices using low dose mTORi either in combination with CNI or in a CNI free regimen have become less intensive and with concomitant statin therapy concerns about dyslipidemia have been relatively controlled.25 mTORi are also associated with hyperglycemia possibly acting through the inhibition of PI3K/AKT axis and impairment of insulin-related gene transcription.24 It can be seen in up to 50% of patients with the occurrence being more frequent in those with baseline hyperglycemia.28 A summary of the potential mechanisms of metabolic alterations due to various immunosuppressant drugs and individual derangements is shown in Table 1 and Figure 2.
Table 1.
Metabolic Derangements due to Individual Immunosuppressants.
| Metabolic derangement | Corticosteroids | Tacrolimus | Cyclosporine | mTOR inhibitors |
|---|---|---|---|---|
| Mechanisms | ↑Gluconeogenesis ↓Insulin production ↑FFA uptake ↓Lipoprotein lipase activity |
↑Gluconeogenesis ↓Cholesterol transport into bile ↓Beta cell proliferation and survival ↓Vasodilators and ↑SVR |
↑Gluconeogenesis ↓Cholesterol transport into bile ↓Beta cell proliferation and survival |
↓Lipoprotein lipase activity ↑Adipose lipase activity ↓Beta cell proliferation |
| Obesity | ++ | − | − | − |
| Impaired glucose tolerance | +++ | ++ | + | − |
| Dyslipidemia | ++ | + | ++ | +++ |
| Hypertension | + | + | ++ | + |
FFA, free fatty acid; SVR, systemic vascular resistance.
Figure 2.
A summary of the potential mechanisms of metabolic alterations due to various immunosuppressant drugs and individual derangements.
Immunosuppression – Its Implication in Post-transplant NAFLD
As elaborated in the previous sections, the adverse metabolic impact of various classes of immunosuppressive therapies remains one of the principal concerns in the post-LT setting. Corticosteroids and CNIs are especially associated with adverse effects on the metabolic profile which become more pronounced in a background of NASH in the pretransplant setting.6 While this aspect of worse metabolic profile is well established, the studies linking the effects of immunosuppressants, specifically on allograft steatosis, however, need further validation. Both tacrolimus, as well as cumulative steroid dose, have been implicated in the development of recurrent allograft NAFLD in one study.29 However, another study found no association between the use of tacrolimus and post-transplant steatosis. The use of cyclosporine was found to be a predictor of post-transplant steatosis on univariate analysis in one study but did not attain statistical significance on multivariate assessment.30 Therefore, although the risks of worsening metabolic profile are common with the use of immunosuppressive regimens in the post-transplant period, their impact on graft steatosis needs further substantiation.
Post-transplant NAFLD and implications on graft survival
In spite of post-LT NAFLD being an extremely common complication, its impact on graft survival has been variably reported. Literature from multiple studies conclude that despite a high incidence of recurrent NAFLD graft survival post-LT does not seem to be influenced by disease recurrence.6,7,31 Similarly, in another recent study with 275 patients with NASH who underwent LT, the authors found no difference in graft survival for patients with recurrent NASH or NAFL.32 However, it is important to emphasize that even if the rates for long-term survival are not different, these patients remain at higher risk for cardiovascular events due to underlying metabolic disease.30
Management of post-LT NAFLD: implications of immunosuppressive strategies and pharmacotherapy
The tenets of management of post-LT NAFLD are essentially the same as those with conventional NAFLD with stress on lifestyle modification, prevention of weight gain, dietary restriction, and achieving weight loss. The additional component involves tailoring of immunosuppression strategies to reduce the metabolic risks. The International Liver Transplantation Consensus recommends minimization of immunosuppression with an attempt for early steroid withdrawal. Additional, strategies include switching from tacrolimus to cyclosporine in cases of uncontrolled hyperglycemia or from cyclosporine to tacrolimus in cases of dyslipidemia.33 In similar lines, for patients with recurrent NASH, the Indian National Society for the study of the liver recommends early steroid taper and advocates mycophenolate mofetil as the drug with the least metabolic complications in such settings.34 However, the most important consideration that remains to be kept in the backdrop is the optimization of graft and patient survival. An approach to control metabolic derangements and rational immunosuppression is shown in Table 2. Literature regarding specific NAFLD-directed therapy in the post-LT setting is limited. None of the drugs which have been used in the pre-LT setting have robust data in post-LT NAFLD and hence are not recommended as of now. Saroglitazar, a dual peroxisome proliferator-activated receptor-α/γ agonist which has shown promising results in the pre-LT setting with regulatory approval in India, is currently being evaluated for patients with post-LT NAFLD (NCT03639623).35
Table 2.
Approaches to Control of Metabolic Derangements and Rational Immunosuppression.
| Metabolic parameters | Management options | Rational immunosuppression |
|---|---|---|
| Low-density lipoprotein >100 mg/dL Higher triglycerides |
|
|
| Diabetes mellitus |
|
|
| Hypertension |
|
Steroid and CNI minimization |
CNI, calcineurin inhibitors.
NAFLD post-LT is common and is influenced by baseline metabolic profile, post-transplant weight gain, poor metabolic control, and adverse effects of immunosuppressants. The impact of immunosuppression on individual metabolic derangements has been clearly delineated; however, its composite impact on post-LT NAFLD remains to be determined. Although overall graft and patient survival may not be affected with the development of post-LT NAFLD per se, cardiovascular outcomes are worse on account of poor metabolic profile. Strict regulation of metabolic risk factors, lifestyle interventions, and targeted pharmacotherapy toward medical management of metabolic complications and rational immunosuppression form the core strategies for the control and management of post-LT NAFLD.
Credit authorship contribution statement
Ajay Duseja: conceptualization, methodology, software Akash Roy: data curation, writing-original draft preparation. Sunil Taneja: visualization, investigation. Sunil Taneja: supervision. Akash Roy: software, validation. Ajay Duseja/Sunil Taneja: writing-reviewing and editing.
Conflicts of interest
The authors have none to declare.
Funding
Nil.
References
- 1.Younossi Z., Golabi P., Paik J., Henry A., Van Dongen C., Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023:10–97. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elhence A., Bansal B., Gupta H., Anand A., Singh T.P., Goel A. Prevalence of non-alcoholic fatty liver disease in India: a systematic review and meta-analysis. J Clin Exp Hepatol. 2021 doi: 10.1016/j.jceh.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De A., Duseja A. Nonalcoholic fatty liver disease: Indian perspective. Clin Liver Dis. 2021:158–163. doi: 10.1002/cld.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil D.T., Yerian L.M. Evolution of nonalcoholic fatty liver disease recurrence after liver transplantation. Liver Transpl. 2012;18:1147–1153. doi: 10.1002/lt.23499. [DOI] [PubMed] [Google Scholar]
- 5.Losurdo G., Castellaneta A., RendinaM, et al. Systematic review with meta-analysis: de novo non-alcoholic fatty liver disease in liver-transplanted patients. Aliment Pharmacol Ther. 2018;47:704–714. doi: 10.1111/apt.14521. [DOI] [PubMed] [Google Scholar]
- 6.Bhati C., Idowu M.O., Sanyal A.J., et al. Long-term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis-related cirrhosis. Transplantation. 2017;101:1867–1874. doi: 10.1097/TP.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 7.Malik S.M., deVera M.E., Fontes P., et al. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15:1843–1851. doi: 10.1002/lt.21943. [DOI] [PubMed] [Google Scholar]
- 8.Saeed N., Glass L., Sharma P., Shannon C., Sonnenday C.J., Tincopa M.A. Incidence and risks for nonalcoholic fatty liver disease and steatohepatitis post-liver transplant: systematic review and meta-analysis. Transplantation. 2019 Nov 1;103:e345–e354. doi: 10.1097/TP.0000000000002916. [DOI] [PubMed] [Google Scholar]
- 9.Burke A., Lucey M.R. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686–693. doi: 10.1111/j.1600-6143.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 10.Vallin M., Guillaud O., Boillot O., et al. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl. 2014;20:1064–1071. doi: 10.1002/lt.23936. [DOI] [PubMed] [Google Scholar]
- 11.Collins B.H., Pirsch J.D., Becker Y.T., et al. Long-term results of liver transplantation in older patients 60 years of age and older. Transplantation. 2000;70:780–783. doi: 10.1097/00007890-200009150-00012. [DOI] [PubMed] [Google Scholar]
- 12.Kappus M., Abdelmalek M. De novo and recurrence of nonalcoholic steatohepatitis after liver transplantation. Clin Liver Dis. 2017;21:321–335. doi: 10.1016/j.cld.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Finkenstedt A., Auer C., Glodny B., et al. Patatin-like phospholipase domain-containing protein 3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin Gastroenterol Hepatol. 2013;11:1667–1672. doi: 10.1016/j.cgh.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Lane J.T., Dagogo-Jack S. Approach to the patient with new-onset diabetes after transplant (NODAT) J Clin Endocrinol Metab. 2011 Nov 1;96:3289–3297. doi: 10.1210/jc.2011-0657. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson A., Davidson J., Dotta F., et al. Guidelines for the treatment and management of new-onset diabetes after transplantation 1. Clin Transplant. 2005 Jun;19:291–298. doi: 10.1111/j.1399-0012.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 16.Castedal M., Skoglund C., Axelson C., Bennet W. Steroid-free immunosuppression with low-dose tacrolimus is safe and significantly reduces the incidence of new-onset diabetes mellitus following liver transplantation. Scand J Gastroenterol. 2018 Jun 3;53:741–747. doi: 10.1080/00365521.2018.1463390. [DOI] [PubMed] [Google Scholar]
- 17.Van Laecke S., Desideri F., Geerts A., et al. Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transpl. 2010 Nov;16:1278–1287. doi: 10.1002/lt.22146. [DOI] [PubMed] [Google Scholar]
- 18.Vincenti F., Friman S., Scheuermann E., et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007 Jun;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 19.Sarumathy S., George P., Kumar B., Mukundan V.A., Shanmugarajan T.S., Maheshwari P. Clinical comparison of serum lipids between cyclosporine and tacrolimus treated renal transplant recipients. Res J Pharm Technol. 2016;9:694–698. [Google Scholar]
- 20.Tory R., Sachs-Barrable K., Goshko C.B., Hill J.S., Wasan K.M. Tacrolimus-induced elevation in plasma triglyceride concentrations after administration to renal transplant patients is partially due to a decrease in lipoprotein lipase activity and plasma concentrations. Transplantation. 2009 Jul 15;88:62–68. doi: 10.1097/TP.0b013e3181aa7d04. [DOI] [PubMed] [Google Scholar]
- 21.Canzanello V.J., Schwartz L., Taler S.J., et al. Evolution of cardiovascular risk after liver transplantation: a comparison of cyclosporine A and tacrolimus (FK506) Liver Transpl Surg. 1997 Jan;3:1–9. doi: 10.1002/lt.500030101. [DOI] [PubMed] [Google Scholar]
- 22.Hryniewiecka E., Żegarska J., Paczek L. Transplantation proceedings. 2011. Arterial hypertension in liver transplant recipients. [DOI] [PubMed] [Google Scholar]
- 23.Canzanello V.J., Textor S.C., Taler S.J., et al. Renal sodium handling with cyclosporin A and FK506 after orthotopic liver transplantation. J Am Soc Nephrol. 1995 May 1;5:1910–1917. doi: 10.1681/ASN.V5111910. [DOI] [PubMed] [Google Scholar]
- 24.Pallet N., Legendre C. Adverse events associated with mTOR inhibitors. Expet Opin Drug Saf. 2013 Mar 1;12:177–186. doi: 10.1517/14740338.2013.752814. [DOI] [PubMed] [Google Scholar]
- 25.Holdaas H., Potena L., Saliba F. mTOR inhibitors and dyslipidemia in transplant recipients: a cause for concern? Transplant Rev. 2015 Apr 1;29:93–102. doi: 10.1016/j.trre.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Vitko S., Wlodarczyk Z., Kyllönen L., et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. Am J Transplant. 2006 Mar;6:531–538. doi: 10.1111/j.1600-6143.2005.01193.x. [DOI] [PubMed] [Google Scholar]
- 27.Vitko S., Tedesco H., Eris J., et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6-month safety and efficacy results of two randomized studies. Am J Transplant. 2004 Apr;4:626–635. doi: 10.1111/j.1600-6143.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnston O., Rose C.L., Webster A.C., Gill J.S. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol. 2008 Jul 1;19:1411–1418. doi: 10.1681/ASN.2007111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contos M.J., Cales W., Sterling R.K., et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 30.Yalamanchili K., Saadeh S., Klintmalm G.B., et al. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 31.Narayanan P., Mara K., Izzy M., et al. Recurrent or de novo allograft steatosis and long-term outcomes after liver transplantation. Transplantation. 2019 Jan 1;103:e14–e21. doi: 10.1097/TP.0000000000002317. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka L., Chotai P.N., Slaughter J., et al. A single-center study of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis recurrence in recipients of liver transplant for treatment of nonalcoholic steatohepatitis cirrhosis. Exp Clin Transplant. 2022 Feb doi: 10.6002/ect.2021.0343. [DOI] [PubMed] [Google Scholar]
- 33.Charlton M., Levitsky J., Aqel B., et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018 May 1;102:727–743. doi: 10.1097/TP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 34.Duseja A., Singh S.P., De A., et al. Indian National Association for Study of the Liver (INASL) guidance paper on nomenclature, diagnosis and treatment of non-alcoholic fatty liver disease (NAFLD) J Clin Exp Hepatol. 2022 doi: 10.1016/j.jceh.2022.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gawrieh S., Noureddin M., Loo N., et al. Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: a randomized controlled double-blind phase 2 trial. Hepatology. 2021;74:1809–1824. doi: 10.1002/hep.31843. [DOI] [PubMed] [Google Scholar]