Abstract
Respiratory symptoms and hypoxemia can complicate chronic liver disease and portal hypertension. Various pulmonary disorders affecting the pleura, lung parenchyma, and pulmonary vasculature are seen in end-stage liver disease, complicating liver transplantation (LT). Approximately 8% of cirrhotic patients in an intensive care unit develop severe pulmonary problems. These disorders affect waiting list mortality and posttransplant outcomes. A thorough history, physical examination, and appropriate laboratory tests help diagnose and assess the severity to risk stratify pulmonary diseases before LT. Hepatopulmonary syndrome (HPS), portopulmonary hypertension (POPH), and hepatic hydrothorax (HH) are respiratory consequences specific to cirrhosis and portal hypertension. HPS is seen in 5–30% of cirrhosis cases and is characterized by impaired oxygenation due to intrapulmonary vascular dilatations and arteriovenous shunts. Severe HPS is an indication of LT. The majority of patients with HPS resolve their hypoxemia after LT. When pulmonary arterial hypertension occurs in patients with portal hypertension, it is called POPH. All other causes of pulmonary arterial hypertension should be ruled out before labeling as POPH. Since severe POPH (mean pulmonary artery pressure [mPAP] >50 mm Hg) is a relative contraindication for LT, it is crucial to screen for POPH before LT. Those with moderate POPH (mPAP >35 mm Hg), who improve with medical therapy, will benefit from LT. A transudative pleural effusion called hepatic hydrothorax (HH) is seen in 5–10% of people with cirrhosis. Refractory cases of HH benefit from LT. In recent years, increasing clinical expertise and advances in the medical field have resulted in better outcomes in patients with moderate to severe pulmonary disorders, who undergo LT.
Keywords: hepatopulmonary syndrome, portopulmonary hypertension, hepatic hydrothorax, pulmonary artery hypertension, liver transplantation
Liver transplantation (LT) is the treatment of choice for end-stage liver disease, a major cause of death in adults and children. Various pulmonary disorders affecting the pleura, lung parenchyma, and pulmonary vasculature are seen in end-stage liver disease, complicating LT. Severe pulmonary complications occur in about 8% of cirrhotic patients in an intensive care unit (ICU) who require an LT.1,2 Pulmonary manifestation in cirrhosis can be (1) pulmonary diseases specific to chronic liver diseases and portal hypertension,3, 4, 5, 6 (2) coincidental pulmonary diseases,2,7, 8, 9, 10 or (3) those diseases that can involve both liver and lung (Table 1).9,10 A thorough pretransplant pulmonary assessment is required to identify these problems, determine prognosis, and decide on transplant candidacy.
Table 1.
Pulmonary Manifestation in Chronic Liver Disease.
| Pulmonary diseases specific to chronic liver diseases and portal hypertension |
| Hepatopulmonary syndrome (HPS) |
| Portopulmonary hypertension (POPH) |
| Hepatic hydrothorax, |
| Coincidental pulmonary diseases |
| Pneumonia (viral, bacterial, Fungal) |
| Pulmonary alveolar hemorrhage |
| Adult respiratory distress syndrome (ARDS) |
| Transfusion-related acute lung injury (TRALI) |
| Transfusion-related circulatory overload (TACO) |
| Interstitial lung diseases and obstructive airway diseases, |
| Diseases involving both the liver and lungs |
| Alpha-1 antitrypsin deficiency |
| Cystic fibrosis |
| Autoimmune disorders |
| Adverse drug reactions |
| Sarcoidosis |
| Hereditary haemorrhagic telangiectasia |
Hypoxemia is relatively common in end-stage liver disease, which is multifactorial and often asymptomatic.11 Anemia of chronic liver disease, tense ascites with the elevated diaphragm, bilateral basilar lung atelectasis, and chest wall edema can interfere with ventilation in cirrhotic patients.11,12 Sarcopenia and frailty are common in chronic liver disease and contribute to hypoxemia.11 Hepatopulmonary syndrome (HPS), portopulmonary hypertension (POPH), and hepatic hydrothorax (HH) occur in cirrhosis and portal hypertension and have a significant impact on quality of life and posttransplant survival. In high endemic areas, pulmonary tuberculosis can complicate cirrhosis.13 Also, preexisting lung diseases such as chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), bronchiectasis, and bronchial asthma can coexist in patients with chronic liver disease.8, 9, 10 Unlike HPS and POPH, no guidelines define the severity of coexisting pulmonary diseases that would rule out an LT.9 Most centers would not accept patients with moderate to severe coexisting pulmonary disease with poor functional status and high short-term mortality. Respiratory failure in acute on chronic liver failure (ACLF) often occurs secondary to inflammatory sequelae of ACLF, lung infections, or aspiration.2,11 In ACLF, respiratory failure is defined by the chronic liver failure consortium–organ failure (CLIF-OF) scoring system as a partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (P/F ratio) of less than 200 mmHg.2,11 Respiratory failure requiring mechanical ventilation is a poor prognostic sign in patients with ACLF, with 1-year mortality as high as 89%.2,11 Diagnosing and treating pulmonary disorders pretransplant is essential to ensure optimal functional status, quality of life, tissue oxygenation, and good outcomes posttransplant. In this paper, we aim to review the preoperative pulmonary assessment of LT candidates (Figure 1).
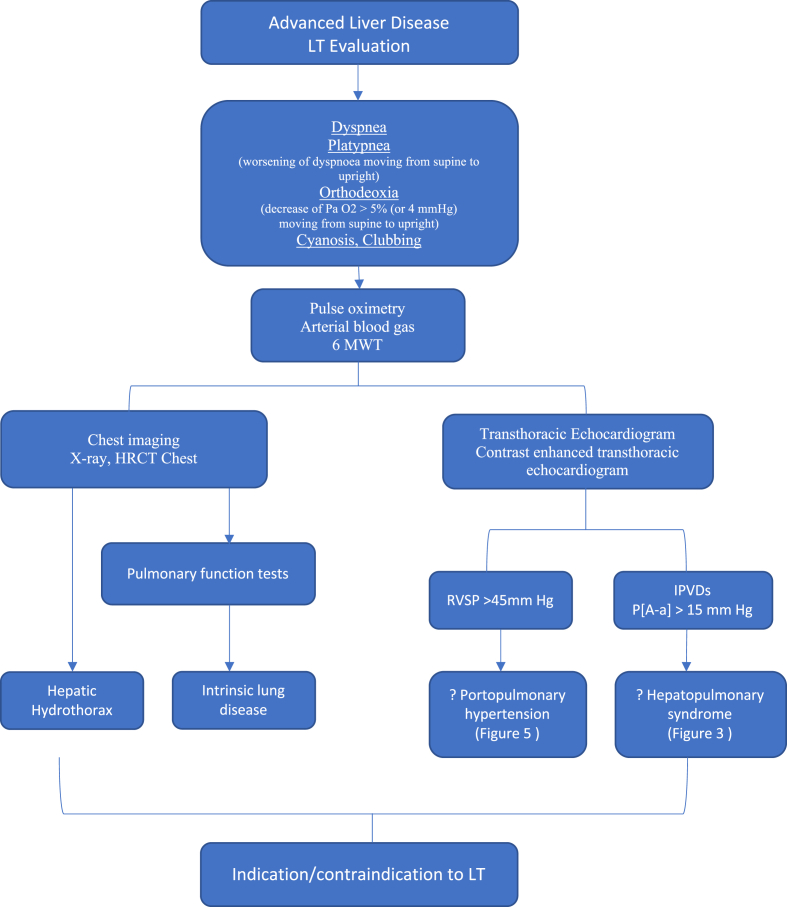
Figure 1.
Pulmonary evaluation in liver transplantation (LT): Pulmonary evaluation of a liver transplant recipient starts with thorough history and physical examination. Pulse oximetry is a cost-effective method to screen for hypoxemia. All patients undergo CXR, ECG, ABG, 6-MWT, TTE, CE TTE, and HRCT as part of pretransplant evaluation. RHC, 99Tc MAA scan and pulmonary angiography are reserved for select cases. LT, liver transplantation; CXR, chest X-ray; ECG, electrocardiogram; ABG, arterial blood gas; 6-MWT, 6 -min walk test; P [A–a], pulmonary alveolar arterial pressure gradient; RVSP, right ventricular systolic pressure; IPVDs, intrapulmonary vascular dilatations; TTE, transthoracic echocardiogram; CE TTE, contrast-enhanced transthoracic echocardiogram; HRCT, high-resolution computed tomography; RHC, right heart catheterization; 99Tc MAA, scan technetium 99 macroaggregated albumin scintigraphy.
Pulmonary Evaluation of a Liver Transplant Candidate
To diagnose asymptomatic hypoxemia, a thorough history and physical examination are required. Look for risk factors such as smoking, contact with tuberculosis, allergen exposure, and occupational history. Past history and family history aid in the diagnosis of concomitant pulmonary illnesses.3,14,15 The simplest screening test for hypoxemia is pulse oximetry (SpO2) while breathing ambient room air in a sitting posture. An arterial blood gas (ABG) further characterizes hypoxemia in patients with a SpO2 below 96% in room air.14,15 In our unit, all patients get a chest X-ray, electrocardiogram (ECG), transthoracic echocardiography (TTE), contrast-enhanced transthoracic echocardiogram (CE TTE), pulmonary function test (PFT) and high-resolution computed tomography of the chest (HRCT) as part of the pretransplant evaluation. A 6-min walk test is done to assess exercise capacity. A reduced 6-min walk distance of less than 250 m is associated with a poor post-LT outcome.16 TTE assesses for structural heart disease, the right and left ventricular functions, and pulmonary artery hypertension (PAH).14,17,18 We do a right heart catheterization (RHC) for any patient with right ventricular systolic pressure (RVSP) of >45 mm Hg and/or RV dysfunction.14,17,18 RHC helps to confirm the diagnosis and characterize PAH. A CE TTE is done to look for intrapulmonary shunts.19 The appearance of microbubbles in the left atrium at three to six cardiac cycles after appearance in the right atrium indicates intrapulmonary shunts.18,19 The contrast-enhanced transesophageal echocardiography (CE TEE) is significantly better for diagnosing intrapulmonary vascular dilatations (IPVDs) as it can differentiate intracardiac from intrapulmonary shunts and has a higher sensitivity.18 But it is invasive and is not readily available for screening. Our unit uses TEE for intraoperative monitoring of the patient. In patients with HPS and a coexisting pulmonary parenchymal disease, technetium-labeled macroaggregated albumin (99mTc MAA) scan helps determine the contribution of HPS to hypoxemia. A shunt fraction of more than 6% incriminates HPS as the cause of hypoxemia.18, 19, 20 Pulmonary angiography is reserved for patients suspected to have discrete pulmonary arteriovenous communications that can be occluded.18, 19, 20 All patients evaluated for LT undergo pulmonary rehabilitation to improve functional status, tissue oxygenation, and prognosis on the waiting list and after LT.21
Hepatopulmonary Syndrome (HPS) and Portopulmonary Hypertension (POPH)
Pathogenic processes within the liver and portal venous system impact pulmonary circulation in patients with chronic liver disease and portal hypertension.1,3,4 Pulmonary vasculature abnormalities result from increased production or failure to clear the circulating inflammatory, vasoactive, proliferative, or angiogenic mediators.1,3,4 The prolonged stimulation by these mediators leads to the remodeling the pulmonary vascular bed with diffuse or localized vasodilatation in HPS or hyperplastic lesions in POPH (Table 2).4 The occurrence of these seemingly unrelated illnesses in the same patient population raises the possibility that disease modifiers have a part in dictating a patient's pulmonary vascular phenotype. These pulmonary vascular abnormalities have distinctive pathogenesis, diagnostic methods, therapeutic modalities, and implications for LT.
Table 2.
Hepatopulmonary Syndrome and Portopulmonary Hypertension.
| HPS | POPH | |
|---|---|---|
| Symptomatology | Mild cases asymptomatic | Progressive dyspnea |
| Progressive dyspnoea | ||
| Platypnea | ||
| Orthodeoxia | ||
| Clinical examination | Cyanosis | Chest pain syncope No cyanosis or clubbing RV heave Pronounced P2 component |
| Finger clubbing | ||
| Spider angiomas | ||
| Pathophysiology | IPVDs | Pulmonary vasoconstriction. Concentric intimal fibrosis, smooth muscle hyperplasia and hypertrophy. Increased ET-1, 5HT and Thromboxane. Decreased NO and prostacycline |
| A-V shunts | ||
| Angiogenesis | ||
| Increased NO, CO, VEGF | ||
| ECG findings | None | RBBB Rightward axis RV hypertrophy |
| Arterial blood gas | Moderate to severe hypoxemia | No/mild hypoxemia |
| Chest radiography | Normal | Cardiomegaly Hilar enlargement |
| TTE | Normal | RVSP increased May show impaired right ventricular function |
| CE TTE | Always positive; bubbles appear in left atrium >3–6 cardiac cycles after right atrial opacification |
Usually negative |
| 99mTcMAA shunting | >6% | <6% |
| Pulmonary hemodynamics | Normal mPAP, Normal/low PVR | mPAP >25 mm Hg, PVR >240 dyn/s/cm−5 |
| Pulmonary angiography | Normal/"spongy" appearance (type I) Discrete AV communications (type II) |
Large main pulmonary arteries Distal arterial pruning |
| OLT | Always indicated in severe HPS (PaO2 <60 mm Hg) | Indicated only if initial mPAP is <35 mm Hg or mPAP is <35 mm Hg and PVR <400. Dyn/sec/cm−5 with treatment or mPAP >35 mmHg and <45 mmHg and PVR <240 dyn/s/cm5 (<3 Wood units (WU)). |
HPS, hepatopulmonary syndrome; POPH, portopulmonary hypertension; IPVDs, intrapulmonary vascular dilatations; A_V shunts, arteriovenous shunts; NO, nitric oxide; CO, carbon monoxide; VEGF, vascular endothelial growth factor; ET-1, endothelin 1; RBBB, right bundle branch block; TTE, transthoracic echocardiogram; RVSP, right ventricular systolic pressure; CE TTE, contrast-enhanced transthoracic echocardiogram; mPAP, mean pulmonary artery pressure; PVR, pulmonary artery resistance; OLT, orthotopic liver transplantation.
Hepatopulmonary Syndrome (HPS)
About 5–30% of cirrhosis patients have HPS. It presents as impaired oxygenation due to the formation of arteriovenous fistulas and IPVDs in the pulmonary vascular bed.5,22 It is a significant cause of mortality and morbidity on the waiting list and peritransplant period.5,22 The HPS is defined by the presence of (1) congenital portosystemic shunts, chronic liver disease, or portal hypertension; (2) abnormal arterial oxygenation; and (3) IPVDs (Table 3).18 A ABG sampling is done to confirm HPS as pulse oximetry saturation is not sufficiently sensitive or specific.18,22 Occasionally, PaO2 criterion is not met in cases of HPS, and the alveolar-arterial (A–a) gradient is used to diagnose aberrant gas exchange.22
Table 3.
Diagnostic Criteria and Severity of Hepatopulmonary Syndrome, Portopulmonary Hypertension and Hepatic Hydrothorax.
 |
Pathophysiology and Pathogenesis
Three processes mostly explain abnormal gas exchange in HPS. (1) In HPS, the pulmonary capillary network has decreased tone and is abnormally dilated (>15 μm), called IPVDs.23, 24, 25, 26 The extra alveolar-capillary diameter and the reduced transit time due to high cardiac output (CO) hinder red blood cell oxygenation23, 24, 25, 26 (Figure 2). (2) IPVDs cause a ventilation-perfusion (V/Q) mismatch, which is made worse by inadequate hypoxia-mediated pulmonary vasoconstriction.24,25 (3) The precapillary arteriovenous fistulae allow the mixing of pulmonary arterial and venous blood resulting in a true shunt.24, 25, 26 The hypoxemia brought on by IPVDs and V/Q mismatch can be partially improved with the help of additional oxygen therapy. True shunt-related hypoxemia is typically resistant to supplemental oxygen.27, 28, 29 Human studies in patients with HPS have documented high levels of NO, ET-1, VEGF, intrapulmonary monocytes, and increased expression of endothelin-B receptors in the pulmonary circulation30, 31, 32, 33, 34, 35 (Figure 2). Additionally, HPS is linked to single-nucleotide polymorphisms that control angiogenesis, implicating pulmonary angiogenesis as a contributor to HPS.36 The fact that two distinct phenotypes (HPS and POPH) occur in the same disease indicates yet-to-be-identified external modifiers that influence the phenotypic presentation.
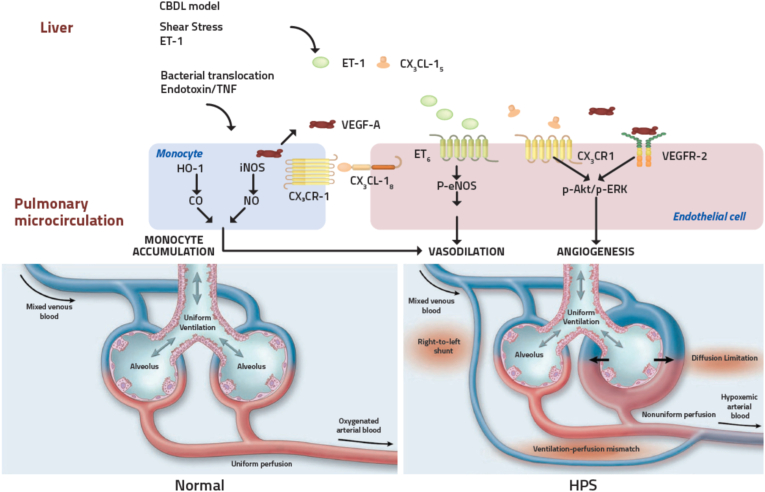
Figure 2.
Pathophysiology of hepatopulmonary syndrome: Portal hypertension leads to an increased hyperdynamic circulatory state and shear stress which, along with chronic inflammation and oxidative stress, leads to dysregulation of key regulators of pulmonary vascular tone resulting in pulmonary vasodilatation and intrapulmonary shunts. Excess Vascular endothelial growth factor leads to angiogenesis which contributes to arteriovenous shunts and impaired oxygenation. Chronic inflammation also leads to increased intrapulmonary monocytes, which increase excess NO and CO, further contributing to vasodilatation. Adapted with permission from Machicao VI et al.3 andRodriguez-Roisin R et al.5 ET-1, endothelin-1; TNF, tumor necrosis factor; HO-1, heme oxygenase-1; CO, carbon monoxide; NO, nitric oxide; VEGF-A, vascular endothelial growth factor-A; iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase; ETB, endothelin B receptor; VEGFR2, vascular endothelial growth factor receptor 2.
Clinical presentation and evaluation
Most cases of HPS are asymptomatic.5,19,22 Patients with moderate to severe HPS will have dyspnea, platypnea (worsening of dyspnea moving from supine to upright), and orthodeoxia (decrease of Pa O2 > 5% or 4 mmHg moving from supine to upright).19 A detailed history and physical examination are needed to rule out all other potential causes of hypoxemia.19,22 As the severity of hypoxemia increases, cyanosis and clubbing occur. Chest examination is usually noncontributory. Even though a prospective multicentric study revealed that a SpO2 <96% has low sensitivity for detecting significant hypoxemia, patients are initially screened with pulse oximetry (SpO2) as it is cost-effective.37 A room air SpO2 of less than 96% would trigger further evaluation with ABG and CE TTE.14,18 ABGs in room air and 100% oxygen are done to confirm diagnosis and document response to supplemental oxygen (Figure 3).14,18 Primary lung pathologies are ruled out with chest X-ray and computed tomography of the chest.22,38 Typically, PFTs reveal a diminished CO diffusion capacity (DLCO) proportionate to the degree of hypoxemia and a mild restrictive pattern.5,19,22
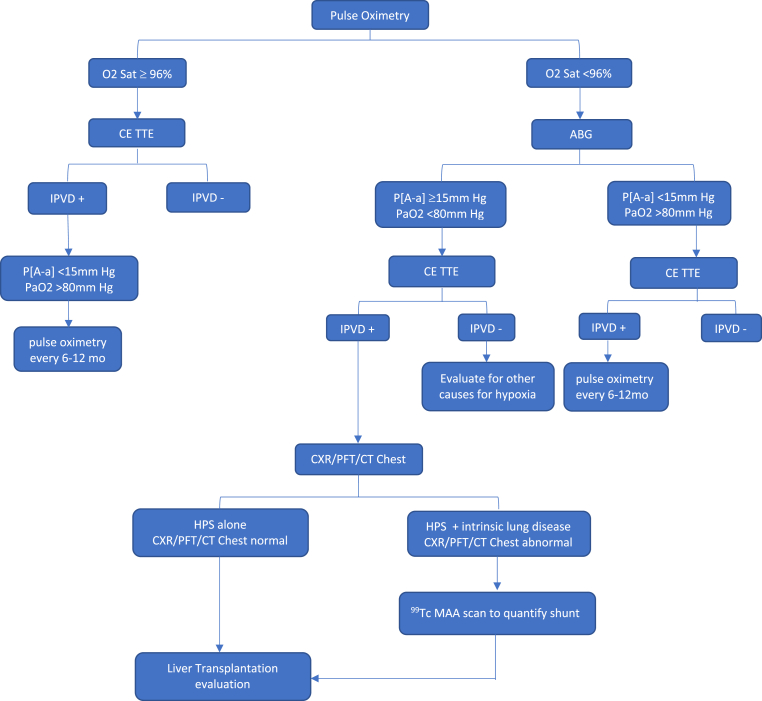
Figure 3.
Approach to hepatopulmonary syndrome (HPS): Pulse oximetry is the simplest and cost-effective method to screen for HPS. All patients undergo CE TTE as part of pretransplant evaluation. When there are competing etiologies for hypoxemia, 99TC MAA scan is done to quantify the shunt fraction and to a confirm HPS as the cause for hypoxemia. Pulmonary angiography is reserved for patients identified to have discrete shunts on CT chest that can be embolized. CE TTE, contrast-enhanced transthoracic echocardiography; ABG, arterial blood gas; IPVD, intrapulmonary vascular dilatations; P [A–a], pulmonary alveolar arterial oxygen gradient; CXR, chest X-ray; PFT, pulmonary function test; CT, computed tomography.
A CE TTE is a sensitive technique to identify IPVDs and rule out structural cardiac anomalies, intracardiac shunts, and coexisting pulmonary hypertension.5,19,22 CE TTE (bubble contrast study) is done by injecting agitated saline intravenously. The normal pulmonary capillaries (diameter of 8–15 um) trap the saline bubbles and do not permit entry to the left heart.5,19,22 IPVDs (with a diameter >15 um) permit unrestricted entry of these bubbles into the left heart, allowing for the diagnosis of intrapulmonary shunts.5,19,22 When an intracardiac shunt is present, bubbles often appear in the left heart 1–2 cycles after it appears in the right atrium. In intrapulmonary shunting, bubbles will appear in the left heart in 3–6 cardiac cycles after it appears in the right atrium.5,19,22 The CE TEE is significantly better for diagnosing IPVDs as it can differentiate intracardiac from intrapulmonary shunts and has a higher sensitivity.18 But it is invasive and is not readily available for screening. Our unit uses TEE for intraoperative monitoring of the patient. The 99mTc MAA lung perfusion scan is reserved for patients with HPS and coexisting lung parenchymal disease.39 A 99mTc MAA lung perfusion scan with a high brain shunt index fraction (>6%) would argue for HPS being the predominant cause of hypoxemia than lung parenchymal disease.14,18 Also, in patients with severe HPS (PaO2 <60 mm Hg), a brain shunt index fraction >20% indicates a poor prognosis.39 Patients with severe hypoxemia (PaO2 <60 mm Hg) whose high-resolution chest computed tomography is suspicious for discrete pulmonary arteriovenous malformations or those in whom PaO2 does not rise to >200 mm Hg with 100% inhaled oxygen undergo a pulmonary angiography to detect discrete arteriovenous malformations that can be embolized.14,18
Management of HPS
In the absence of LT, patients with HPS have twice the risk of dying compared to those with cirrhosis of comparable severity without HPS. HPS also worsens the quality of life of patients with cirrhosis.40,41 The risk of dying from HPS with or without LT is highest in people with very severe hypoxemia (PaO2 <50 mm Hg).40, 41, 42 Posttransplant morbidity and mortality are also higher in patients with severe HPS whose hypoxemia does not improve with 100% inhaled oxygen.40, 41, 42 There is no evidence that medical treatment for HPS increases survival, and LT remains the standard of care for patients with severe HPS (PaO2 <60 mm Hg).40, 41, 42 Lowering portal pressure with the transjugular intrahepatic portosystemic shunt (TIPS) has not shown benefit in HPS.18 HPS has been successfully treated in children by stenting spontaneous inferior vena cava to portal vein shunts and ligating/coiling congenital portosystemic shunts in the Abernethy malformation.43,44 Rarely, discrete pulmonary arteriovenous anomalies may be detected on high-resolution chest computed tomography or pulmonary angiography, and coil embolization may improve hypoxemia.18
Medical therapies in hepatopulmonary syndrome
Medical treatment of symptomatic HPS is supportive with oxygen supplementation to maintain a SpO2 >88%.14,18 No medication has been demonstrated to be helpful or to offer a long-lasting response in HPS.14,18 Patients with HPS should be referred for LT before progressing to severe or very severe categories.14,18 Numerous medications, including methylene blue, cyclooxygenase inhibitors, N(G)-nitro-l-arginine methyl ester (l-NAME), somatostatin, propranolol, inhaled prostacyclin derivatives, almitrine, and withdrawal of chronic methadone, have been tried in uncontrolled studies; none have been proven to be beneficial in HPS (Table 4).45 Sorafenib, a tyrosine kinase inhibitor that prevents angiogenesis or norfloxacin used for gut decontamination, did not show benefit in the pilot research.46,47 Garlic extracts have shown some benefits in HPS.48,49 In a randomized placebo-controlled trial, patients with HPS who received high-dose garlic (1–2 mg/m2) had a more significant increase in PaO2 (24.66% vs. 7.37%; P < 00.1).49 Recently, better oxygenation in experimental HPS has been attributed to pentoxifylline, a phosphodiesterase inhibitor with known modest TNF-α and NO inhibition. Small uncontrolled studies using pentoxifylline on human HPS have produced conflicting outcomes.50,51 Overall, no intervention other than LT has proven beneficial in HPS. These medical therapies should only be resorted to in critically ill patients with hypoxemia who have no LT options.
Table 4.
Drugs Used in HPS.
| Drugs | Mechanism of action |
|---|---|
| Methylene blue | Causes vasoconstriction by inhibiting NO and also decrease angiogenesis. |
| NG-nitro-l-arginine methyl ester | Inhibit nitric oxide synthase |
| Pentoxifylline | Tumor necrosis factor-alpha inhibitor with vasodilator and anti-angiogenesis actions |
| Norfloxacin | Decreases bacterial translocation |
| Garlic | Contains allicin which is a potent vasodilator and anti-angiogenesis. |
| Mycophenolate mofetil | An inhibitor of angiogenesis and nitric oxide production |
| Sorafenib | Tyrosine kinase inhibitor that can reduce angiogenesis. |
| Letrozole | Nonsteroidal inhibitor of aromatase which effectively blocks estrogen synthesis. |
| Almitrine bismesylate | Pulmonary vasoconstrictor |
| Bosentan | Dual ETA and ETB receptor subtypes antagonist. |
Liver transplantation (LT)
Only LT can enhance oxygenation and chances of survival in patients with HPS.18 Large case series have shown that individuals with HPS who underwent LT completely recovered from IPVDs and hypoxemia.52, 53, 54, 55 The standard model for end-stage liver disease (MELD) exception scores are provided to people with severe hypoxemia due to HPS (PaO2 of <60 mm Hg).56 Studies from the MELD exception era have shown excellent outcomes in these patients.56, 57, 58 A review of the UNOS database recently revealed that patients with HPS had an 8% waiting list mortality rate.59 The same study also showed more significant posttransplant mortality if the pretransplant room air PaO2 was less than 44 mm Hg.59 Recent studies indicate individuals with very severe hypoxemia (PaO2 <50 mm Hg) who undergo LT also do well.60,61 The decision to accept candidates with very severe HPS for LT is center specific. No specific PaO2 threshold has been agreed upon as an absolute contraindication to LT.
Intraoperative management of HPS is supportive. Most HPS patients can obtain sufficient oxygen saturation with 100% inspired oxygen.62,63 There are no defined PaO2 cut-offs for canceling a case. Continuous monitoring of mixed venous oxygen saturation (SvO2) during surgery is recommended.62 Oxygen saturation frequently worsens after the transplant due to volume overload, atelectasis, hypoventilation, sedation, and/or aspiration.62,63 The goal should be to extubate as soon as possible to reduce infectious problems. In patients with HPS, severe posttransplant hypoxemia—defined as the need for 100% inspired oxygen to maintain an oxygen saturation level of 85%—develops in 6–21% of cases and is linked to longer ICU stays and a death rate of 45%.64 Trendelenburg positioning, 100% inspired high-flow oxygen, inhaled vasodilators such as epoprostenol or nitric oxide (by increasing the transit time of erythrocytes it enables sufficient oxygen binding to take place and improves V-Q mismatch), and intravenous methylene blue (with or without inhaled vasodilators) are all options for treating severe posttransplant hypoxemia.62,63 Intravenous methylene blue is a vasoconstrictor that improves V-Q matching.62,63 The oxygenation of patients with extremely severe HPS has also been shown to improve with venovenous (VV)-extracorporeal membrane oxygenation (ECMO), both before and after LT, and to help with the transition to LT or recovery after LT.62,63
Nearly, all cases of HPS-related hypoxemia improve after LT. However, the degree of pretransplant hypoxemia may determine how long it takes to recover.55 Following surgery, patients are routinely monitored using pulse oximetry, and when room air oxygen saturation is above 88%, stopping the use of supplemental oxygen is considered.63 A 5-year post-LT survival of 76% has been observed in patients with HPS, comparable to that in patients with cirrhosis without HPS.55
Portopulmonary hypertension
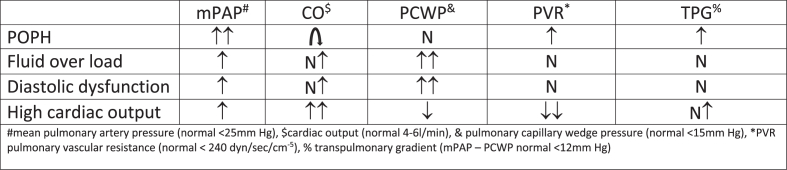
POPH is defined as pulmonary arterial hypertension (PAH) resulting from portal hypertension with or without cirrhosis.14,18 Nearly, 20% of cirrhotic individuals who undergo echocardiography have PAH although only 1 in 4 has true POPH.65,66 The remaining patients either have a fluid overload or hyperdynamic circulation due to high CO, which affects pulmonary hemodynamics.65,66 The diagnosis of POPH requires a RHC showing an increased mean pulmonary artery pressure (mPAP >25 mm Hg) due to increased pulmonary vascular resistance (PVR > three wood units or 240 dyn/s per cm−5) in the setting of a normal pulmonary capillary wedge pressure (PCWP <15 mm Hg) (Table 3).18 In 2019, the 6th World Symposium on Pulmonary Hypertension (WSPH) redefined pulmonary artery hypertension as mPAP > 20 mm Hg and PVR ≥3 wood units.67 This new definition has not been incorporated in hepatology literature. Before diagnosing POPH, other causes of pulmonary artery hypertension (PAH), such as high flow state, fluid overload, diastolic dysfunction, obstructive/restrictive lung disease, and obstructive sleep apnea, should be ruled out.14,17,18 Rarely, pulmonary vascular resistance (PVR) and pulmonary capillary wedge pressure (PCWP) increase POPH.68,69 In this situation, an elevated transpulmonary gradient (mPAP-PCWP of >12 mm Hg) indicates the presence of true precapillary pulmonary hypertension (POPH).68,69
Pathophysiology and pathogenesis
The pathologic alterations in POPH, such as the muscularization of the arterioles, smooth muscle hypertrophy, intimal thickening, in situ thrombosis, and plexiform lesions, are comparable to those in other types of PAH.70,71 Portal hypertension leads to a hyperdynamic circulatory state, and the resulting shear stress triggers the pulmonary vascular proliferative process, which results in POPH (figure 4).71 Excess circulating growth hormones, mediators of smooth muscle proliferation, and the imbalance between vasoconstrictors and vasodilators contribute to the POPH phenotype. In clinical studies, people with POPH have elevated endothelin-1, low prostacyclin, and low NO in their pulmonary circulation.71, 72, 73 Genetics may also be involved because not all people with portal hypertension go on to develop POPH.74 Like other forms of PAH, oxidative stress, cGMP metabolism, and estrogen signaling play a crucial role in POPH.75
Clinical features and evaluation
POPH may be asymptomatic and diagnosed during liver transplant evaluation. Depending on the severity, some patients may have dyspnea on exertion, chest pain, syncope, or evidence of right heart failure.76 Typically, the lung examination is unremarkable. As POPH's duration and severity increase, the chest X-ray shows enlarged pulmonary arteries and cardiomegaly. When POPH is severe, the electrocardiogram could exhibit a right-axis deviation, a right bundle branch block pattern, and T wave inversion in the precordial V1–V4 leads. PFTs are usually nonspecific in POPH.76
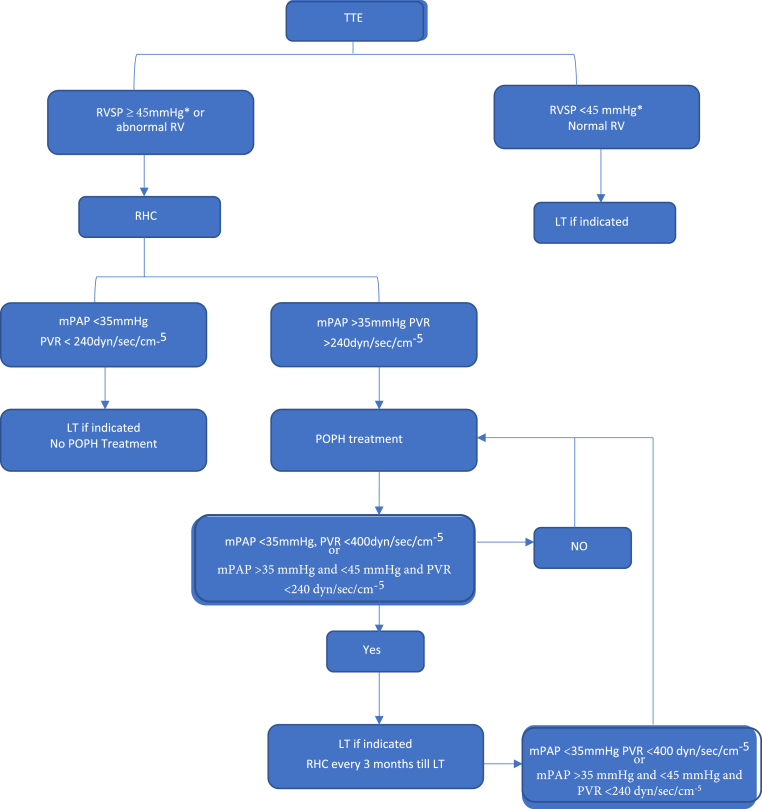
The best screening procedure for POPH is transthoracic echocardiography (TTE).18 TTE has a 97% sensitivity and a 77% specificity for detecting moderate to severe PAH.77 In patients with portal hypertension, the RVSP is calculated using tricuspid regurgitant peak velocity, the modified Bernoulli equation, and an estimation of the right atrial pressure (RVSP = 4(V)2 + RAP).77, 78, 79 It is an indirect measure of pulmonary artery systolic pressure (PASP) (Figure 4). With the aid of this screening method, it is possible to choose the patients who should have RHC (Figure 5). In most institutions, the cutoff value for proceeding with a RHC is an RVSP >50 mm Hg even though AASLD recommends RHC if RVSP is >45 mm Hg.14,17,18 In our unit, any patient with RVSP >45 mm Hg or evidence of RV strain/dysfunction undergoes an RHC. POPH is classified as mild (mPAP 25–35 mm Hg), moderate (mPAP 35–45 mm Hg), and severe (mPAP >45 mm Hg) based on RHC findings.18,76 Other indirect measures of PAH include tricuspid regurgitant velocity >2.8 m/s l, RV/LV basal diameter/area ratio >1.0, flattening of the interventricular septum (LVEI >1.1 in systole and/or diastole), RVOT acceleration time <105 ms, TAPSE/PASP ratio <0.55 mm/mmHg, and early diastolic pulmonary regurgitation velocity >2.2 m/s.77, 78, 79 Echocardiography also help to assess the right ventricular function. The features of RV dysfunction on echocardiography include the tricuspid annular plane systolic excursion (TAPSE) <18 mm, RV fractional area change (RV-FAC) <35%, RV free-wall strain, tricuspid annulus velocity (S′ wave) <9.5 cm/s, and RV ejection fraction (RVEF) <45%.79
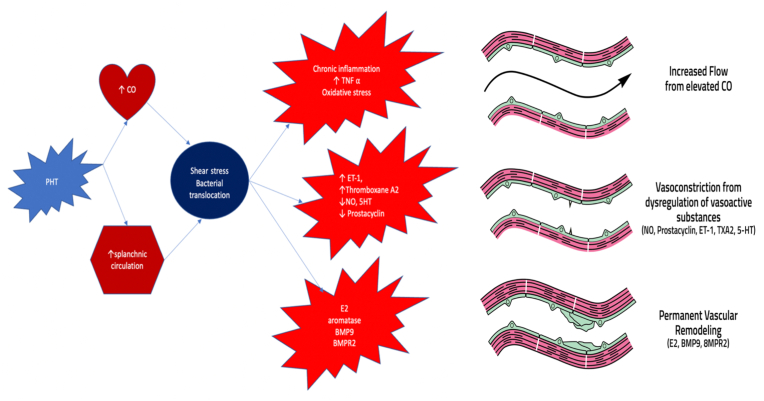
Figure 4.
Pathogenesis of portopulmonary hypertension: Portal hypertension leads to an increased hyperdynamic circulatory state and shear stress along with chronic inflammation and oxidative stress leading to dysregulation of key regulators of pulmonary vascular tone resulting in pulmonary vasoconstriction. At the same time, damage to the pulmonary endothelium and the underlying smooth muscle along with genetic factors, results in permanent vascular remodeling, ultimately leading to the development of pulmonary hypertension. Adapted with permission from Thomas C et al.95 CO, cardiac output; NO, nitric oxide; ET-1, endothelin-1; TXA2, thromboxane A2; 5-HT, serotonin; E2, estrogen; BMP9, bone morphogenic protein 9; BMPR2, bone morphogenic receptor 2.
Figure 5.
Approach to portopulmonary hypertension: TTE, transthoracic echocardiogram; POPH, portopulmomary hypertension; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; RHC, right heart catheterization. ∗AASLD guidelines recommend RHC if mPAP >45 mmHg while ILTS recommends RHC if mPAP >50 mmHg.
The diagnosis of POPH requires a RHC showing an increased mean PA pressure (mPAP >25 mm Hg) with an increased pulmonary vascular resistance (PVR > three wood units or 240 dyn/s per cm−5) in the setting of a normal pulmonary capillary wedge pressure (PCWP <15 mm Hg).14,17,18,80 Some patients with cirrhosis and elevated RVSP have changes indicative of hyperdynamic circulation or volume overload with normal PVR (Figure 6). These patients don't seem to be at risk for adverse results with LT.18,80 Transpulmonary pressure gradient (TPG) calculation (mPAP - PCWP) aids in determining which patients have POPH (TPG >12 mmHg).18,80
Figure 6.
Hemodynamic patterns on right heart catheterization in cirrhosis patients with elevated RVSP on echocardiogram.
Management of portopulmonary hypertension
Patients with POPH not on pharmacological therapy or receiving LT have an estimated 5-year survival rate of only 14%.81 Right-sided heart failure and decompensated cirrhosis are responsible for mortality in POPH. Patients with POPH with an mPAP of more than 35 mm Hg are at risk for poor results with LT and require PAH-specific medicines.82 In a study of 43 patients with PoPH (confirmed by RHC) not on medical treatment and underwent LT, 100% of the patients with severe (mPAP > 50 mm Hg) and 50% of the patients with moderate POPH (mPAP 35–50 mm Hg) died due to cardiopulmonary events in the peritransplant period.83 No mortality was reported among the patients with mild POPH (mPAP <35 mmHg).83
Patients with POPH are treated with general measures that target portal hypertension and the complications of PAH. Diuretics are used as the fluid overload is frequently present in patients with POPH from right heart failure and liver dysfunction.84, 85, 86, 87 To minimize pulmonary vasoconstriction, supplementary oxygen should be utilized to maintain oxygen saturation >89%. Anticoagulants are not recommended in POPH.84, 85, 86, 87 Calcium channel blockers should be avoided in POPH as they can worsen splanchnic vasodilatation.84, 85, 86, 87 Any coexisting conditions that worsen pulmonary hypertension, like primary cardiac or pulmonary disease, are treated.84 Beta-blockers may compromise the right ventricular function and be avoided in moderate to severe POPH.88 TIPS can momentarily raise mPAP, CO, and PVR and is not advised in patients with severe POPH (mPAP >45 mm Hg).84, 85, 86, 87
Medications with vasodilator, antiplatelet, and antiproliferative characteristics help to improve pulmonary hemodynamics and restore normal right ventricular function.84,85 Prostacyclin analogs such as epoprostenol, treprostinil, iloprost, and prostacyclin receptor agonist selexipag target the prostacyclin pathway.89, 90, 91, 92, 93, 94 Endothelin receptor antagonists, bosentan, macitentan, and ambrisentan, act by inhibiting the endothelin-1 pathway.95, 96, 97, 98 Phosphodiesterase-5 inhibitors, such as tadalafil and sildenafil, and soluble guanylate cyclase stimulators, such as riociguat, target the nitric oxide pathway.99, 100, 101, 102, 103, 104 (Table 5) These agents have been used to manage POPH, based mainly on clinical experience in idiopathic pulmonary artery hypertension (IPAH). A prospective cohort study from the French Pulmonary Hypertension Registry (FPHR) examined data on 637 patients with POPH. It showed that PAH-specific therapies improved WHO functional class, 6-min walk distance, and hemodynamic parameters.89
Table 5.
Drugs Used in POPH.
| Class | Drug | Starting dose | Target dose | Mechanism of action |
|---|---|---|---|---|
| Endothelin receptor antagonists | Bosentan | 62.5 mg b.i.d | 125 mg b.i.d | Dual ETA and ETB receptor subtypes antagonist. Specifically, inhibition of ET-1 receptors. |
| Ambrisentan | 5 mg o.d | 10 mg o.d | Highly selective ETA receptor inhibition. | |
| Macitentan | 10 mg o.d | 10 mg o.d | High affinity ETA than ETB antagonist. | |
| Phosphodiesterase-5 inhibitors | Sildenafil | 20 mg t.i.d | 20 mg t.i.d | High selectivity for PD5 vs PD2, 3 and 4. |
| Tadalafil | 20 mg o.d | 40 mg o.d | High selectivity for PD5 compared with PD1, 4, 7 and 10. | |
| Vardenafil | 10 mg o.d | 20 mg o.d | 20 times more potent than sildenafil for inhibiting PD5 | |
| Prostanoids | Epoprostenol | 2 ng/kg/mt | 16–30 ng/kg/mt | Synthetic prostacyclin with potent effects of vasodilatation and platelet aggregator inhibitor. |
| Treprostinil | 1.25 ng/kg/mt | 25–60 ng/kg/mt | Long acting tricyclic benzindene synthetic analog of prostacyclin. Vasodilator and inhibits platelet inhibition. | |
| Inhaled Iloprost | 2.5mcg 6-9times/d | 5mcg 6–9 times/d | Orally, intravenously, or as an inhaler. | |
| Selexipag | 200mcg b.i.d | 1600mcg b.i.d | Oral prostacyclin receptor agonist with vasodilatory, antiproliferative and antiplatelet action. | |
| Beraprost | 20mcg t.i.d | 40mcg t.i.d | Oral prostacyclin analog binding to prostacyclin membrane receptors inhibit the release of Ca2+ leading to relaxation of the smooth muscle cells and vasodilation | |
| Soluble guanylate cyclase stimulator | Riociguat | 2.5mcg 6-9times/d | 5mcg 6-9times/d | Riociguat has a dual mode of action, acting in synergy with endogenous nitric oxide and also directly stimulating soluble guanylate cyclase, independent of nitric oxide availability |
Patients with mild POPH do not require pharmacologic therapy but are clinically monitored for disease progression.14,18 In POPH, no evidence suggests that one agent is better than another. Patients with moderate to severe POPH are started on an oral PDE5i or an ERA.84,105 In patients with a poor initial response to monotherapy, combination therapy with PDE5i, and ERA or riociguat with ERA may be used. Inhaled or s/c prostacyclin analogs may be added in nonresponders.84,105 Only patients who require hastened LT or have severe signs of POPH like syncope, right heart failure, or dyspnea at rest should receive intravenous prostacyclin therapy.84,105 Bosentan necessitates continuous monitoring of liver function tests because it is linked to liver damage and liver failure.84, 85, 86 Ambrisentan and macitentan do not require regular monitoring of liver function tests. Still, it is recommended that patients have baseline liver function tests, and generally, ERAs are not recommended for individuals with moderate to severe liver function impairment and transaminases that are more than three times the upper limit of normal.18 Current data demonstrate that medications targeted at PAH can enhance hemodynamics and functional outcomes in POPH patients.84, 85, 86 They aid in qualifying for LT and improve survival.
Liver transplantation
POPH is not considered an indication of LT in the absence of decompensated liver disease. Patients with properly managed POPH and good RV function can safely undergo LT. POPH improves after the transplant in about 29–64% of patients.18 As per retrospective studies, moderate-to-severe POPH (mPAP >35 mmHg) is associated with a higher mortality rate after LT and LT are contraindicated in patients with an mPAP >50 mmHg as perioperative mortality reaches 100%.18 Recent case series using current PAH-specific therapy have shown favorable short-term post-LT outcomes in individuals with moderate POPH who respond to treatment by achieving an mPAP <35 mm Hg and a PVR <400 dyn/s per cm−5 (<5 WU) before surgery or mPAP greater than or equal to 35 mmHg and less than 45 mmHg and PVR less than 240 dyn/s/cm5 (or less than 3 Wood units [WU]).14,18,106, 107, 108, 109 Following LT, a small percentage of these patients could stop their POPH medications.107,108 At present, UNOS policy allows for a MELD exception for POPH, awarding a MELD score of 22 to POPH patients with baseline mPAP values > 35 mmHg, provided that an RHC documented mPAP values < 35 mmHg, PVR values < 400 dyn/s per cm−5 (<5 WU) or mPAP greater than or equal to 35 mmHg and less than 45 mmHg and PVR less than 240 dyn/s/cm5 (or less than 3 WU) and normal right ventricular function following medical therapy.14,18,107,109,110
In patients with moderate POPH, continuous mPAP monitoring is recommended in the peritransplant period.18 Deferment of LT is advised if mPAP is > 45–50 mmHg before abdominal incision.18 We use intraoperative TEE to monitor RV function. During LT, wide fluctuations in pulmonary pressure occur due to changes in CO. PAH-targeted therapy should be continued throughout the perioperative and immediate posttransplant period.84,85 In patients on parenteral prostacyclin therapy, abruptly stopping PAH treatment can result in severe right heart failure and mortality.84,85 Other therapeutic options during and after LT include inhaled vasodilators, such as nitric oxide or epoprostenol, intravenous prostacyclin analogs, and continuing a patient's preoperative PAH-targeted therapy.111,112 Rarely, venoarterial (VA)–extracorporeal membrane oxygenation (ECMO) has been used as a rescue option to support cardiovascular function throughout the transplant surgery and the posttransplant recovery period.113 During VA-ECMO, blood is extracted from the right atrium and returned to the arterial system, bypassing the heart and lungs and providing respiratory and hemodynamic support. This process provides indirect support to the RV by reducing preload, reducing RV wall tension, and delivering oxygenated blood to the coronary circulation.113
Invasive hemodynamic monitoring is continued in the ICU to help manage pulmonary hypertension. ICU care for POPH entails avoiding hypoxia, hypercapnia, hypovolemia, and hypotension.84,85 Inotropes, such as dobutamine or milrinone, may increase contractility in individuals who develop right ventricular failure, and vasopressors may be required to prevent hypotension.84,85,112,. Following the transplant, PAH medication should be continued, and weaning should be considered based on symptoms, the results of echocardiography, and pulmonary hemodynamics.114 Major alterations are typically not recommended during the initial posttransplant period. Weaning or stopping therapy after a transplant may take up to 3 months or longer.114 Case reports and series suggest that 29–64% of patients with moderate to severe POPH under long-term follow-up posttransplant have been able to discontinue therapy over time.114,115 POPH patients have worse 1-year mortality or graft failure than patients without POPH.
Hepatic hydrothorax
HH is a transudative pleural effusion typically higher than 500 mL in a patient with portal hypertension with no other underlying major cardiac or pulmonary etiology.7,116 HH represents 2–3% of all cases of pleural effusions. Approximately 5–10% of patients with cirrhosis develop HH.7,116 HH occurs on the right side in 85% of the cases, left in 13%, and bilateral in 2%.7,116 Diaphragmatic defects are the primary factor causing ascitic fluid to leak into the pleura.117 Additionally, it is believed that negative intrathoracic pressure promotes ascitic fluid's unidirectional flow out of the abdominal cavity.118 Azygous vein hypertension with plasma leakage, peritoneal fluid leakage through the lymphatics, lymphatic leakage from the thoracic duct, and hypoalbuminemia that reduces oncotic pressure are few more reasons of HH.118
The presence of pleural effusion is confirmed by chest radiography, and thoracentesis is often necessary for the initial diagnosis of HH.118,119 It is recommended to perform a thoracentesis to determine the etiology of pleural effusion, rule out infection in the fluid, and alleviate the symptoms.118,119 In uncomplicated HH, the polymorphonuclear cell count (PMN) is 250 cells/mm3, the pleural fluid total protein is 2.5 g/dL, and the serum-to-pleural fluid albumin gradient (SPAG) is > 1.1 (Table 3).118,119 The evaluation of the pleural fluid should include cell count and differential, Gram stain, culture, cytology, protein concentrations, albumin concentrations, and lactate dehydrogenase concentrations.118, 119, 120 Depending on the clinical circumstances, further tests to rule out chylothorax, pancreatitis, malignancy, tuberculosis, heart or renal failure, and other etiology of pleural effusion are done.118, 119, 120
Infection of HH in the absence of underlying pneumonia is called spontaneous bacterial pleuritis (SBPL).121, 122, 123 SBPL occurs in 10–16% of patients with HH.121 The following conditions are necessary for the diagnosis of SBPL (Table 3): (1) a positive pleural fluid culture and a neutrophil cell count of more than 250 cells/mm3; (2) a negative pleural fluid culture and a neutrophil cell count of more than 500 cells/mm3; and (3) the absence of pneumonia on chest imaging.121 Enterobacteriaceae species, Enterococcus species, Streptococcus species, and Pseudomonas aeruginosa are the most typical bacteria that cause SBPL.121, 122, 123 SBEM is treated with the timely beginning of a broad-spectrum antibiotic for 7–10 days.122,123 Albumin therapy to prevent hepatorenal syndrome is not evaluated in SBPL patients.121 Antibiotic prophylaxis following an SBPL has also not been studied.123 A bout of SBPL is associated with a >20% mortality rate.123
Treatment of HH
The medical management of HH includes sodium restriction in the diet and diuresis, which is quite similar to the medical management of ascites. Patients are instructed to limit their daily salt intake to 88 mEq (2000 mg).124 Aldosterone receptor antagonists are typically started in conjunction with loop diuretics, and depending on the response and symptomatology, dose titration is done.124 The HH is deemed refractory if the patient's response to medical treatment is insufficient or experiences complications from the diuresis. It occurs in 20–25% of cases and is an indication for LT.124,125 Therapeutic thoracentesis is often helpful in alleviating respiratory symptoms. Paracentesis is typically performed before considering thoracentesis in individuals with concurrent large-volume ascites because it may alleviate respiratory problems.124,125 Limiting pleural fluid drainage to 1–2 L is advisable to reduce the possibility of reexpansion of pulmonary edema.126 There are no studies demonstrating the benefit of albumin administration during thoracentesis.124 TIPS is an option in patients with refractory hydrothorax with contraindications for LT or as a bridge to LT.127, 128, 129, 130 The response rate to TIPS in carefully selected patients is between 70% and 80%.130 Presence of advanced liver disease, recurrent encephalopathy, RVSP >45 mm Hg, and left ventricular ejection fraction <60% are contraindications to TIPS.130 Because of worries about protein loss, electrolyte imbalance, and infection, inserting an indwelling catheter and chest tube for HH is considered a relative contraindication.131 The pleural infection and catheter-related sepsis incidence are high when indwelling catheters are placed for refractory HH.132,133 We use indwelling catheters only for the short term as a bridge to TIPS or living donor liver transplantation (LDLT).18 For refractory HH, LT is the definitive treatment.14,18,134 Patients receiving LT for HH have similar outcomes compared to other indications for LT.134,135 For patients with refractory HH who are not candidates for LT or TIPS, indwelling pleural catheters, mechanical or chemical pleurodesis, and surgical repair of diaphragmatic defects are palliative options.136,137
Summary
Respiratory symptoms and hypoxemia can complicate chronic liver disease and portal hypertension. Approximately 8% of cirrhotic patients in an intensive care unit develop severe pulmonary problems. HPS, POPH, and HH are respiratory consequences specific to cirrhosis and portal hypertension. These disorders affect waiting list mortality and posttransplant outcomes. A thorough history, physical examination and appropriate laboratory tests help to make a diagnosis and assess the severity to risk stratify pulmonary disorders before LT. All patients undergo a chest X-ray, ECG, TTE, CE TTE, HRCT chest, PFT, and ABG as part of their pretransplant evaluation. CE TEE, 99mTc MAA scan, pulmonary angiography, and RHC are reserved for select patients identified on the initial screening.
Severe HPS (PaO2 <60 mm Hg) is an indication for LT, and hypoxemia secondary to HPS almost universally improves following LT. Even though POPH per se is not an indication for LT, patients with moderate to severe HPS whose mPAP and PVR can be brought down to less than 35 mm Hg and 400 dyn/s per cm−5, respectively, benefit from LT. In POPH, the posttransplant course is unpredictable, and case reports and series suggest that 29–64% of patients with moderate to severe POPH under long-term follow-up posttransplant have been able to discontinue therapy over time. The definitive treatment for refractory HH is LT. With increasing clinical expertise and advances in the medical field, better posttransplant outcomes have recently been seen in severe HPS and POPH.
Credit authorship contribution statement
Charles Panackel: Conceptualization, writing original draft, visualization. Joe Francis Mathew: Editing and reviewing the article, visualization. Mohamed Fawas N: Editing and reviewing the article, visualization. Mathew Jacob: Editing and reviewing the article, supervising.
Conflicts of interest
The authors have none to declare.
Funding
The authors have received no funding for the article.
References
- 1.Bauer M., Fuhrmann V., Wendon J. Pulmonary complications in liver disease. Intensive Care Med. 2019;45:1433–1435. doi: 10.1007/s00134-019-05721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang P., Formanek P., Scaglione S., Afshar M. Risk factors and outcomes of acute respiratory distress syndrome in critically ill patients with cirrhosis. Hepatol Res. 2019;49:335–343. doi: 10.1111/hepr.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machicao V.I., Balakrishnan M., Fallon M.B. Pulmonary complications in chronic liver disease. Hepatology. 2014;59:1627–1637. doi: 10.1002/hep.26745. [DOI] [PubMed] [Google Scholar]
- 4.Fallon M.B., Abrams G.A. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859–865. doi: 10.1053/jhep.2000.7519. [DOI] [PubMed] [Google Scholar]
- 5.Krowka M.J., Cortese D.A. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528–1537. doi: 10.1378/chest.105.5.1528. [DOI] [PubMed] [Google Scholar]
- 6.Montani D., Günther S., Dorfmüller P., et al. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013;8:97. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardenas A., Kelleher T., Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20:271–279. doi: 10.1111/j.1365-2036.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 8.Kwo P.Y. Shortness of breath in the patient with chronic liver disease. Clin Liver Dis. 2012;16:321–329. doi: 10.1016/j.cld.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Krowka M.J., Wiesner R.H., Heimbach J.K. Pulmonary contraindications, indications and MELD exceptions for liver transplantation: a contemporary view and look forward. J Hepatol. 2013;59:367–374. doi: 10.1016/j.jhep.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Spagnolo P., Zeuzem S., Richeldi L., du Bois R.M. The complex interrelationships between chronic lung and liver disease: a review. J Viral Hepat. 2010;17:381–390. doi: 10.1111/j.1365-2893.2010.01307.x. [DOI] [PubMed] [Google Scholar]
- 11.Karcz M., Bankey B., Schwaiberger D., Lachmann B., Papadakos P.J. Acute respiratory failure complicating advanced liver disease. Semin Respir Crit Care Med. 2012;33:96–110. doi: 10.1055/s-0032-1301738. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A., Fletcher A., Lipscomb G.R. Pulmonary oedema after therapeutic ascitic paracentesis: a case report and literature review of the cardiac complications of cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:241–245. doi: 10.1097/MEG.0b013e32833110f7. [DOI] [PubMed] [Google Scholar]
- 13.Dhiman R.K., Saraswat V.A., Rajekar H., Reddy C., Chawla Y.K. A guide to the management of tuberculosis in patients with chronic liver disease. J Clin Exp Hepatol. 2012 Sep;2:260–270. doi: 10.1016/j.jceh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh Shweta A., Shrivastava Piyush, Agarwal Anil, et al. LTSI consensus guidelines: preoperative pulmonary evaluation in adult liver transplant recipients. J Clinic Experiment Hepatol. 2022 doi: 10.1016/j.jceh.2022.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raevens S., Geerts A., Van Steenkiste C., Verhelst X., Van Vlierberghe H., Colle I. Hepatopulmonary syndrome and portopulmonary hypertension: recent knowledge in pathogenesis and overview of clinical assessment. Liver Int. 2015;35:1646–1660. doi: 10.1111/liv.12791. [DOI] [PubMed] [Google Scholar]
- 16.Cox-Flaherty K., Moutchia J., Krowka M.J., et al. Six-Minute walk distance predicts outcomes in liver transplant candidates [published online ahead of print, 2023 Jan 25] Liver Transplant. 2023 doi: 10.1097/LVT.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin P., DiMartini A., Feng S., Brown R., Jr., Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American association for the study of liver diseases and the American society of transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 18.Krowka M.J., Fallon M.B., Kawut S.M., et al. International Liver Transplant Society Practice Guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. 2016;100:1440–1452. doi: 10.1097/TP.0000000000001229. [DOI] [PubMed] [Google Scholar]
- 19.Koch D.G., Fallon M.B. Hepatopulmonary syndrome. Clin Liver Dis. 2014;18:407–420. doi: 10.1016/j.cld.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Krowka M.J., Wiseman G.A., Burnett O.L., et al. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000;118:615–624. doi: 10.1378/chest.118.3.615. [DOI] [PubMed] [Google Scholar]
- 21.Lin F.P., Visina J.M., Bloomer P.M., et al. Prehabilitation-driven changes in frailty metrics predict mortality in patients with advanced liver disease. Am J Gastroenterol. 2021;116:2105–2117. doi: 10.14309/ajg.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Roisin R., Krowka M.J. Hepatopulmonary syndrome–a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. doi: 10.1056/NEJMra0707185. [DOI] [PubMed] [Google Scholar]
- 23.Agusti A.G., Roca J., Rodriguez-Roisin R. Mechanisms of gas exchange impairment in patients with liver cirrhosis. Clin Chest Med. 1996;17:49–66. doi: 10.1016/s0272-5231(05)70298-7. [DOI] [PubMed] [Google Scholar]
- 24.Schraufnagel D.E., Kay J.M. Structural and pathologic changes in the lung vasculature in chronic liver disease. Clin Chest Med. 1996;17:1–15. doi: 10.1016/s0272-5231(05)70295-1. [DOI] [PubMed] [Google Scholar]
- 25.Keal E.E., Harington M. Cirrhosis and hypoxia. Proc Roy Soc Med. 1970;63:621–622. doi: 10.1177/003591577006300628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartin-Ceba R., Krowka M.J. Pulmonary complications of portal hypertension. Clin Liver Dis. 2019;23:683–711. doi: 10.1016/j.cld.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Del Valle K., DuBrock H.M. Hepatopulmonary syndrome and portopulmonary hypertension: pulmonary vascular complications of liver disease. Compr Physiol. 2021;11:3281–3302. doi: 10.1002/cphy.c210009. [DOI] [PubMed] [Google Scholar]
- 28.Porres-Aguilar M., Gallegos-Orozco J.F., Garcia H., Aguirre J., Macias-Rodriguez R.U., Torre-Delgadillo A. Pulmonary vascular complications in portal hypertension and liver disease: a concise review. Rev Gastroenterol México. 2013;78:35–44. doi: 10.1016/j.rgmx.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Surani S.R., Mendez Y., Anjum H., Varon J. Pulmonary complications of hepatic diseases. World J Gastroenterol. 2016;22:6008–6015. doi: 10.3748/wjg.v22.i26.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thenappan T., Goel A., Marsboom G., et al. A central role for CD68(1) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med. 2011;183:1080–1091. doi: 10.1164/rccm.201008-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Ling Y., Luo B., et al. Analysis of pulmonary heme oxygenase-1 and nitric oxide synthase alterations in experimental hepatopulmonary syndrome. Gastroenterology. 2003;125:1441–1451. doi: 10.1016/j.gastro.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Mejias M., Garcia-Pras E., Tiani C., et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 33.Cremona G., Higenbottam T.W., Mayoral V., et al. Elevated exhaled nitric oxide in patients with hepatopulmonary syndrome. Eur Respir J. 1995;8:1883–1885. doi: 10.1183/09031936.95.08111883. [DOI] [PubMed] [Google Scholar]
- 34.Koch D.G., Bogatkevich G., Ramshesh V., et al. Elevated levels of endothelin-1 in hepatic venous blood are associated with intrapulmonary vasodilatation in humans. Dig Dis Sci. 2012;57:516–523. doi: 10.1007/s10620-011-1905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrivet P., Cadranel J., Housset B., et al. Mechanisms of impaired arterial oxygenation in patients with liver cirrhosis and severe respiratory insufficiency. Effects of indomethacin. Chest. 1993;103:500–507. doi: 10.1378/chest.103.2.500. [DOI] [PubMed] [Google Scholar]
- 36.Roberts K.E., Kawut S.M., Krowka M.J., et al. Genetic risk factors for hepatopulmonary syndrome in patients with advanced liver disease. Gastroenterology. 2010;139 doi: 10.1053/j.gastro.2010.03.044. 130–9.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arguedas M.R., Singh H., Faulk D.K., et al. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol. 2007;5:749–754. doi: 10.1016/j.cgh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Luo B.W., Du Z.Y. Advances in diagnostic imaging of hepatopulmonary syndrome. Front Med. 2022;8 doi: 10.3389/fmed.2021.817758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abrams G.A., Nanda N.C., Dubovsky E.V., et al. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114:305–310. doi: 10.1016/s0016-5085(98)70481-0. [DOI] [PubMed] [Google Scholar]
- 40.Poterucha J.J., Krowka M.J., Dickson E.R., et al. Failure of hepatopulmonary syndrome to resolve after liver transplantation and successful treatment with embolotherapy. Hepatology. 1995;21:96–100. [PubMed] [Google Scholar]
- 41.Swanson K.L., Wiesner R.H., Krowka M.J. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology. 2005;41:1122–1129. doi: 10.1002/hep.20658. [DOI] [PubMed] [Google Scholar]
- 42.Fritz J.S., Fallon M.B., Kawut S.M. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med. 2013 Jan 15;187:133–143. doi: 10.1164/rccm.201209-1583CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Leary J.G., Rees C.R., Klintmalm G.B., Davis G.L. Inferior vena cava stent resolves hepatopulmonary syndrome in an adult with a spontaneous inferior vena cava-portal vein shunt. Liver Transplant. 2009;15:1897–1900. doi: 10.1002/lt.21884. [DOI] [PubMed] [Google Scholar]
- 44.Sahu M.K., Bisoi A.K., Chander N.C., Agarwala S., Chauhan S. Abernethy syndrome, a rare cause of hypoxemia: a case report. Ann Pediatr Cardiol. 2015;8:64–66. doi: 10.4103/0974-2069.149526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raevens S., Fallon M.B. Potential clinical targets in hepatopulmonary syndrome: lessons from experimental models. Hepatology. 2018;68:2016–2028. doi: 10.1002/hep.30079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawut S.M., Ellenberg S.S., Krowka M.J., et al. Sorafenib in hepatopulmonary syndrome: a randomized, double-blind, placebo-controlled trial. Liver Transplant. 2019;25:1155–1164. doi: 10.1002/lt.25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta S., Faughnan M.E., Lilly L., et al. Norfloxacin therapy for hepatopulmonary syndrome: a pilot randomized controlled trial. Clin Gastroenterol Hepatol. 2010;8:1095–1098. doi: 10.1016/j.cgh.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Abrams G.A., Fallon M.B. Treatment of hepatopulmonary syndrome with Allium sativum L. (garlic): a pilot trial. J Clin Gastroenterol. 1998;27:232–235. doi: 10.1097/00004836-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 49.De B.K., Dutta D., Pal S.K., et al. The role of garlic in hepatopulmonary syndrome: a randomized controlled trial. Can J Gastroenterol. 2010;24:183–188. doi: 10.1155/2010/349076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanikella R., Philips G.M., Faulk D.K., Kawut S.M., Fallon M.B. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transplant. 2008;14:1199–1203. doi: 10.1002/lt.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta L.B., Kumar A., Jaiswal A.K., et al. Pentoxifylline therapy for hepatopulmonary syndrome: a pilot study. Arch Intern Med. 2008;168:1820–1823. doi: 10.1001/archinte.168.16.1820. [DOI] [PubMed] [Google Scholar]
- 52.Stoller J.K., Moodie D., Schiavone W.A., et al. Reduction of intrapulmonary shunt and resolution of digital clubbing associated with primary biliary cirrhosis after liver transplantation. Hepatology. 1990;11:54–58. doi: 10.1002/hep.1840110111. [DOI] [PubMed] [Google Scholar]
- 53.Arguedas M.R., Abrams G.A., Krowka M.J., et al. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192–197. doi: 10.1053/jhep.2003.50023. [DOI] [PubMed] [Google Scholar]
- 54.Lange P.A., Stoller J.K. The hepatopulmonary syndrome. Effect of liver transplantation. Clin Chest Med. 1996;17:115–123. doi: 10.1016/s0272-5231(05)70302-6. [DOI] [PubMed] [Google Scholar]
- 55.Taille C., Cadranel J., Bellocq A., et al. Liver transplantation for hepatopulmonary syndrome: a ten-year experience in Paris, France. Transplantation. 2003;75 doi: 10.1097/01.TP.0000061612.78954.6C. 1482–9 [discussion: 46–7] [DOI] [PubMed] [Google Scholar]
- 56.Fallon M.B., Mulligan D.C., Gish R.G., et al. Model for end-stage liver disease (MELD) exception for hepatopulmonary syndrome. Liver Transplant. 2006;12(suppl 3):S105–S107. doi: 10.1002/lt.20971. [DOI] [PubMed] [Google Scholar]
- 57.Iyer V.N., Swanson K.L., Cartin-Ceba R., et al. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology. 2013;57:2427–2435. doi: 10.1002/hep.26070. [DOI] [PubMed] [Google Scholar]
- 58.Raevens S., Rogiers X., Geerts A., et al. Outcome of liver transplantation for hepatopulmonary syndrome: a Eurotransplant experience. Eur Respir J. 2019;53 doi: 10.1183/13993003.01096-2018. [pii:1801096] [DOI] [PubMed] [Google Scholar]
- 59.Goldberg D.S., Krok K., Batra S., et al. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology. 2014;146:1256–12565.e1. doi: 10.1053/j.gastro.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta S., Castel H., Rao R.V., et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354–363. doi: 10.1111/j.1600-6143.2009.02822.x. [DOI] [PubMed] [Google Scholar]
- 61.Iyer V.N., Swanson K.L., Krowka M.J. Survival benefits of liver transplant in severe hepatopulmonary syndrome. Am J Respir Crit Care Med. 2013;188:514. doi: 10.1164/rccm.201302-0245LE. [DOI] [PubMed] [Google Scholar]
- 62.Fauconnet P., Klopfenstein C.E., Schiffer E. Hepatopulmonary syndrome: the anaesthetic considerations. Eur J Anaesthesiol. 2013;30:721–730. doi: 10.1097/EJA.0b013e328365bb6f. [DOI] [PubMed] [Google Scholar]
- 63.Nayyar D., Man H.S., Granton J., Lilly L.B., Gupta S. Proposed management algorithm for severe hypoxemia after liver transplantation in the hepatopulmonary syndrome. Am J Transplant. 2015;15:903–913. doi: 10.1111/ajt.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nayyar D., Man H.S., Granton J., Gupta S. Defining and characterizing severe hypoxemia after liver transplantation in hepatopulmonary syndrome. Liver Transplant. 2014;20:182–190. doi: 10.1002/lt.23776. [DOI] [PubMed] [Google Scholar]
- 65.Krowka M.J. Evolving dilemmas and management of portopulmonary hypertension. Semin Liver Dis. 2006;26:265–272. doi: 10.1055/s-2006-947294. [DOI] [PubMed] [Google Scholar]
- 66.Krowka M.J. Pulmonary hypertension: diagnostics and therapeutics. Mayo Clin Proc. 2000;75:625–630. doi: 10.4065/75.6.625. [DOI] [PubMed] [Google Scholar]
- 67.Galiè N., McLaughlin V.V., Rubin L.J., Simonneau G. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J. 2019;53 doi: 10.1183/13993003.02148-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krowka M.J., Miller D.P., Barst R.J., et al. Portopulmonary hypertension: a report from the US-based REVEAL registry. Chest. 2012;141:906–915. doi: 10.1378/chest.11-0160. [DOI] [PubMed] [Google Scholar]
- 69.Edwards B.S., Weir E.K., Edwards W.D., et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol. 1987;10:1233–1238. doi: 10.1016/s0735-1097(87)80123-7. [DOI] [PubMed] [Google Scholar]
- 70.Yeager M.E., Frid M.G., Stenmark K.R. Progenitor cells in pulmonary vascular remodeling. Pulm Circ. 2011;1:3–16. doi: 10.4103/2045-8932.78095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuder R.M., Cool C.D., Geraci M.W., et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 72.Kamath P.S., Carpenter H.A., Lloyd R.V., et al. Hepatic localization of endothelin-1 in patients with idiopathic portal hypertension and cirrhosis of the liver. Liver Transplant. 2000;6:596–602. doi: 10.1053/jlts.2000.9735. [DOI] [PubMed] [Google Scholar]
- 73.Pellicelli A.M., Barbaro G., Puoti C., et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology. 2010;61:802–806. doi: 10.1177/0003319710369101. [DOI] [PubMed] [Google Scholar]
- 74.Roberts K.E., Fallon M.B., Krowka M.J., et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835–842. doi: 10.1164/rccm.200809-1472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arnal J.F., Fontaine C., Billon-Gales A., et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 76.Krowka M.J. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17–25. doi: 10.1055/s-0032-1301731. [DOI] [PubMed] [Google Scholar]
- 77.Kim W.R., Krowka M.J., Plevak D.J., et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant. 2000;6:453–458. doi: 10.1053/jlts.2000.7573. [DOI] [PubMed] [Google Scholar]
- 78.Lau E.M., Tamura Y., McGoon M.D., Sitbon O. The 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: a practical chronicle of progress. Eur Respir J. 2015;46:879–882. doi: 10.1183/13993003.01177-2015. [DOI] [PubMed] [Google Scholar]
- 79.Kossaify A. Echocardiographic assessment of the right ventricle, from the conventional approach to speckle tracking and three-dimensional imaging, and insights into the "right way" to explore the forgotten chamber. Clin Med Insights Cardiol. 2015 Jul 5;9:65–75. doi: 10.4137/CMC.S27462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53 doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swanson K.L., Wiesner R.H., Nyberg S.L., et al. Survival in portopulmonary hypertension: mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445–2453. doi: 10.1111/j.1600-6143.2008.02384.x. [DOI] [PubMed] [Google Scholar]
- 82.Ashfaq M., Chinnakotla S., Rogers L., et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7:1258–1264. doi: 10.1111/j.1600-6143.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 83.Krowka M.J., Plevak D.J., Findlay J.Y., Rosen C.B., Wiesner R.H., Krom R.A. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant. 2000;6:443–450. doi: 10.1053/jlts.2000.6356. [DOI] [PubMed] [Google Scholar]
- 84.Jose Arun, Kay Dana, Jean M., Elwing Treatment of portopulmonary hypertension (PoPH): a review. J Liver Transplant. 2022 [Google Scholar]
- 85.Jose A., Jones C.R., Elwing J.M. Struggling between liver transplantation and portopulmonary hypertension. Heart Fail Clin. 2023;19:55–65. doi: 10.1016/j.hfc.2022.08.017. [DOI] [PubMed] [Google Scholar]
- 86.Savale L., Guimas M., Ebstein N., et al. Portopulmonary hypertension in the current era of pulmonary hypertension management. J Hepatol. 2020;73:130–139. doi: 10.1016/j.jhep.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 87.Thomas C., Glinskii V., de Jesus Perez V., Sahay S. Portopulmonary hypertension: from bench to bedside. Front Med. 2020;7 doi: 10.3389/fmed.2020.569413. Published 2020 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Provencher S., Herve P., Jais X., et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology. 2006;130:120–126. doi: 10.1053/j.gastro.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 89.Faisal M., Siddiqi F., Alkaddour A., Bajwa A.A., Shujaat A. Effect of PAH specific therapy on pulmonary hemodynamics and six-minute walk distance in portopulmonary hypertension: a systematic review and meta-analysis. Pulm Med. 2014;2014 doi: 10.1155/2014/528783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krowka M.J., Frantz R.P., McGoon M.D., et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): a study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30:641–648. doi: 10.1002/hep.510300307. [DOI] [PubMed] [Google Scholar]
- 91.Kuo P.C., Johnson L.B., Plotkin J.S., et al. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation. 1997;63:604–606. doi: 10.1097/00007890-199702270-00020. [DOI] [PubMed] [Google Scholar]
- 92.Hoeper M.M., Seyfarth H.J., Hoeffken G., et al. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096–1102. doi: 10.1183/09031936.00032407. [DOI] [PubMed] [Google Scholar]
- 93.Melgosa M.T., Ricci G.L., Garcia-Pagan J.C., et al. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transplant. 2010;16:348–356. doi: 10.1002/lt.21997. [DOI] [PubMed] [Google Scholar]
- 94.Holthaus N., Prins K., Rose L., Prisco S., Pritzker M., Thenappan T. EXPRESS: transition from parental prostacyclin to selexipag: a case series of five pulmonary arterial hypertension patients. Pulm Circ. 2019;9 doi: 10.1177/2045894019862167. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Savale L., Magnier R., Le Pavec J., et al. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur Respir J. 2013;41:96–103. doi: 10.1183/09031936.00117511. [DOI] [PubMed] [Google Scholar]
- 96.Cartin-Ceba R., Swanson K., Iyer V., Wiesner R.H., Krowka M.J. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139:109–114. doi: 10.1378/chest.10-0574. [DOI] [PubMed] [Google Scholar]
- 97.Cartin-Ceba R., Swanson K., Iyer V., et al. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139:109–114. doi: 10.1378/chest.10-0574. [DOI] [PubMed] [Google Scholar]
- 98.Sitbon O., Bosch J., Cottreel E., et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med. 2019;7:594–604. doi: 10.1016/S2213-2600(19)30091-8. [DOI] [PubMed] [Google Scholar]
- 99.Fisher J.H., Johnson S.R., Chau C., Kron A.T., Granton J.T. Effectiveness of phosphodiesterase-5 inhibitor therapy for portopulmonary hypertension. Cancer Res J. 2015;22:42–46. doi: 10.1155/2015/810376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hemnes A.R., Robbins I.M. Sildenafil monotherapy in portopulmonary hypertension can facilitate liver transplantation. Liver Transplant. 2009;15:15–19. doi: 10.1002/lt.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jevnikar M., Nathan E., Jais X., Boucly A., Montani D., Humbert M., et al. Efficacy and safety of tadalafil in portopulmonary hypertension. Eur Respir J. 2018;52:PA3050. [Google Scholar]
- 102.Ghofrani H.A., Galie N., Grimminger F., et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 103.Rubin L.J., Galie N., Grimminger F., et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2) Eur Respir J. 2015;45:1303–1313. doi: 10.1183/09031936.00090614. [DOI] [PubMed] [Google Scholar]
- 104.Cartin-Ceba R., Halank M., Ghofrani H.A., et al. Riociguat treatment for portopulmonary hypertension: a subgroup analysis from the PATENT-1/-2 studies. Pulm Circ. 2018;8 doi: 10.1177/2045894018769305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 106.Weinfurtner K., Forde K. Hepatopulmonary syndrome and portopulmonary hypertension: current status and implications for liver transplantation. Current Hepatol Report. 2020;19:174–185. doi: 10.1007/s11901-020-00532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.AbuHalimeh B., Krowka M.J., Tonelli A.R. Treatment barriers in portopulmonary hypertension. Hepatology (Baltimore, Md. 2019;69:431–443. doi: 10.1002/hep.30197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benz F., Mohr R., Tacke F., Roderburg C. Pulmonary complications in patients with liver cirrhosis. J Transl Int Med. 2020;8:150–158. doi: 10.2478/jtim-2020-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.DuBrock H.M., Del Valle K.T., Krowka M.J. Mending the model for end-stage liver disease: an in-depth review of the past, present, and future portopulmonary hypertension model for end-stage liver disease exception. Liver Transplant. 2022;28:1224–1230. doi: 10.1002/lt.26422. [DOI] [PubMed] [Google Scholar]
- 110.Goldberg D.S., Batra S., Sahay S., Kawut S.M., Fallon M.B. MELD exceptions for portopulmonary hypertension: current policy and future implementation. Am J Transplant. 2014;14:2081–2087. doi: 10.1111/ajt.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Safdar Z., Bartolome S., Sussman N. Portopulmonary hypertension: an update. Liver Transplant. 2012;18:881–891. doi: 10.1002/lt.23485. [DOI] [PubMed] [Google Scholar]
- 112.Ramsay M.A., Spikes C., East C.A., et al. The perioperative management of portopulmonary hypertension with nitric oxide and epoprostenol. Anesthesiology. 1999;90:299–301. doi: 10.1097/00000542-199901000-00036. [DOI] [PubMed] [Google Scholar]
- 113.Barbas A.S., Schroder J.N., Borle D.P., et al. Planned initiation of venoarterial extracorporeal membrane oxygenation prior to liver transplantation in a patient with severe portopulmonary hypertension. Liver Transplant. 2021;27:760–762. doi: 10.1002/lt.25871. [DOI] [PubMed] [Google Scholar]
- 114.Tokushige K., Kogiso T., Egawa H. Current therapy and liver transplantation for portopulmonary hypertension in Japan. J Clin Med. 2023 Jan 10;12:562. doi: 10.3390/jcm12020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Savale L., Sattler C., Coilly A., et al. Long-term outcome in liver transplantation candidates with portopulmonary hypertension. Hepatology. 2017;65:1683–1692. doi: 10.1002/hep.28990. [DOI] [PubMed] [Google Scholar]
- 116.Lv Y., Han G., Fan D. Hepatic hydrothorax. Ann Hepatol. 2018;17:33–46. doi: 10.5604/01.3001.0010.7533. [DOI] [PubMed] [Google Scholar]
- 117.Huang P.M., Chang Y.L., Yang C.Y., Lee Y.C. The morphology of diaphragmatic defects in hepatic hydrothorax: thoracoscopic finding. J Thorac Cardiovasc Surg. 2005;130:141–145. doi: 10.1016/j.jtcvs.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 118.Norvell J.P., Spivey J.R. Hepatic hydrothorax. Clin Liver Dis. 2014;18:439–449. doi: 10.1016/j.cld.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 119.Al-Zoubi R.K., Abu Ghanimeh M., Gohar A., Salzman G.A., Yousef O. Hepatic hydrothorax: clinical review and update on consensus guidelines. Hosp Pract. 2016;44:213–223. doi: 10.1080/21548331.2016.1227685. [DOI] [PubMed] [Google Scholar]
- 120.Alonso J.C. Pleural effusion in liver disease. Semin Respir Crit Care Med. 2010;31:698–705. doi: 10.1055/s-0030-1269829. [DOI] [PubMed] [Google Scholar]
- 121.Reiche W., Deliwala S., Chandan S., et al. Spontaneous bacterial empyema in cirrhosis: a systematic review and meta-analysis. World J Hepatol. 2022;14:1258–1268. doi: 10.4254/wjh.v14.i6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tu C.Y., Chen C.H. Spontaneous bacterial empyema. Curr Opin Pulm Med. 2012;18:355–358. doi: 10.1097/MCP.0b013e328352b50f. [DOI] [PubMed] [Google Scholar]
- 123.Chen C.H., Shih C.M., Chou J.W., et al. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int. 2011;31:417–424. doi: 10.1111/j.1478-3231.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 124.Krok K.L., Cardenas A. Hepatic hydrothorax. Semin Respir Crit Care Med. 2012;33:3–10. doi: 10.1055/s-0032-1301729. [DOI] [PubMed] [Google Scholar]
- 125.Baikati K., Le D.L., Jabbour, et al. Hepatic hydrothorax. Am J Therapeut. 2014;21:43–51. doi: 10.1097/MJT.0b013e318228319e. [DOI] [PubMed] [Google Scholar]
- 126.Feller-Kopman D., Berkowitz D., Boiselle P., Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656–1661. doi: 10.1016/j.athoracsur.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 127.Dhanasekaran R., West J.K., Gonzales P.C., et al. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105:635–641. doi: 10.1038/ajg.2009.634. [DOI] [PubMed] [Google Scholar]
- 128.Gordon F.D., Anastopoulos H.T., Crenshaw W., et al. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366–1369. doi: 10.1002/hep.510250611. [DOI] [PubMed] [Google Scholar]
- 129.Jeffries M.A., Kazanjian S., Wilson M., et al. Transjugular intrahepatic portosystemic shunts and liver transplantation in patients with refractory hepatic hydrothorax. Liver Transplant Surg. 1998;4:416–423. doi: 10.1002/lt.500040506. [DOI] [PubMed] [Google Scholar]
- 130.Boyer T.D., Haskal Z.J. American association for the study of liver diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2010;51:306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 131.Orman E.S., Lok A.S. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3:582–586. doi: 10.1007/s12072-009-9136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Runyon B.A., Greenblatt M., Ming R.H. Hepatic hydrothorax is a relative contraindication to chest tube insertion. Am J Gastroenterol. 1986;81:566–567. [PubMed] [Google Scholar]
- 133.Ridha A., Al-Abboodi Y., Fasullo M. The outcome of thoracentesis versus chest tube placement for hepatic hydrothorax in patients with cirrhosis: a nationwide analysis of the national inpatient sample. Gastroenterol Res Pract. 2017;2017 doi: 10.1155/2017/5872068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiol X., Tremosa G., Castellote J., et al. Liver transplantation in patients with hepatic hydrothorax. Transpl Int. 2005;18:672–675. doi: 10.1111/j.1432-2277.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- 135.Shirali A.S., Grotts J., Elashoff D., et al. Vol 31. 2019. Predictors of outcomes after thoracic surgery in orthotopic liver transplant recipients with pleural disease; pp. 604–611. (Seminars in Thoracic and Cardiovascular Surgery). Autumn. [DOI] [PubMed] [Google Scholar]
- 136.Hou F., Qi X., Guo X. Effectiveness and safety of pleurodesis for hepatic hydrothorax: a systematic review and meta-analysis. Dig Dis Sci. 2016;61:3321–3334. doi: 10.1007/s10620-016-4260-9. [DOI] [PubMed] [Google Scholar]
- 137.Huang P.M., Kuo S.W., Chen J.S., et al. Thoracoscopic mesh repair of diaphragmatic defects in hepatic hydrothorax: a 10-year experience. Ann Thorac Surg. 2016;101:1921–1927. doi: 10.1016/j.athoracsur.2015.11.023. [DOI] [PubMed] [Google Scholar]