Abstract
Objective
Despite its relatively high prevalence, our understanding of the natural clinical course of acute low-tone hearing loss (ALHL) without vertigo remains incomplete. The purpose of this study is to summarize the findings of studies that evaluated recovery from hearing loss (HL), recurrence and/or fluctuation of HL, and progression to Meniere’s Disease (MD) of patients presenting with ALHL without vertigo.
Methods
A scoping review of the English literature was performed. On May 14, 2020 and July 6, 2022, MEDLINE, Embase, and Scopus were searched to identify articles related to the prognosis of ALHL. To be included, articles had to present outcomes that were clearly distinguishable for patients with ALHL without vertigo. Two reviewers evaluated articles for inclusion and extracted data. Disagreements were adjudicated by a third reviewer.
Results
Forty-one studies were included. There was extensive heterogeneity between studies in regards to defining ALHL, treatment methods, and time of follow up. Most of the cohorts (39 out of 40) reported partial or complete recovery of hearing in the majority (>50%) of patients, although reports of recurrence were relatively common. Progression to MD was infrequently reported. Shorter time from onset of symptoms to treatment predicted better hearing outcomes in 6 of 8 studies.
Conclusion
The literature suggests that although the majority of patients with ALHL experience hearing improvement, recurrence and/or fluctuation are common and progression to MD occurs in a minority of patients. Additional trials utilizing standardized inclusion and outcome criteria are needed to determine the ideal treatment for ALHL.
Keywords: low-tone, hearing loss, Meniere’s disease, fluctuation, recurrence, treatment
Lay Summary:
This scoping review found that the majority of patients with acute low-tone hearing loss without vertigo experience hearing improvement. Recurrence and/or fluctuation are common and progression to Meniere’s disease occurs in a small proportion of patients.
INTRODUCTION
Acute low-tone hearing loss (ALHL) was first recognized as a distinct form of sudden sensorineural hearing loss in 19821. It is commonly defined as sudden sensorineural hearing loss (SSHL) confined to the lower frequencies without accompanying vertigo2, 3. Reports from epidemiologic studies have found that ALHL accounts for roughly 1 in 5 cases of SSHL4. Despite its relatively high prevalence, our understanding of the natural clinical course of ALHL remains incomplete.
There is a large body of literature from institutions in East Asia reporting the clinical outcomes of patients presenting with ALHL. However, these studies mostly consist of retrospective or prospective case series of patients adhering to specific treatment regimens, thereby presenting an incomplete view of the overall clinical course of ALHL. An epidemiologic study found that most patients with ALHL go on to experience complete or partial recovery of their hearing loss (HL)5.
It has also been hypothesized that ALHL may represent an early manifestation of Meniere’s disease (MD), given that a subset of patients with ALHL progress to having MD6–8. Indeed, laboratory-based studies have demonstrated an association between ALHL, endolymphatic hydrops, and MD, as characterized by positive glycerol test results8, 9, abnormal electrocochleography8, 10, 11, demonstration of cochlear/vestibular hydrops as well as saccular enlargement on magnetic resonance imaging2, 12, 13, and temporal bone studies14, 15. Furthermore, a subset of patients who recover from ALHL go on to experience fluctuating or recurrent low-tone HL6, 7, 16–18 and other auditory manifestations of MD without vestibular symptoms8. Given the limited sample size of these studies, however, it is unclear what proportion of patients with ALHL actually go on to experience full-blown MD or an intermediate disease state with recurrent ALHL.
No studies have attempted to condense all the available evidence regarding the clinical progression of ALHL. A previous meta-analysis of three retrospective chart reviews compared the odds of achieving hearing recovery in patients with ALHL treated with steroids versus diuretics19. Zhu et al found no difference in outcomes between those treated with steroids versus diuretics. Notably, this meta-analysis did not incorporate the findings of numerous retrospective and prospective studies because they lacked comparison treatment groups. The meta-analysis also did not evaluate rates of ALHL recurrence or progression to MD. As such, a comprehensive evaluation of the world literature is needed to better understand the natural history of ALHL, prognosis, and treatment outcomes.
A scoping review was therefore conducted to determine the extent of available evidence and gaps in the English literature related to ALHL. The primary goal of this review was to identify and characterize the clinical outcomes (recovery from HL, recurrence and/or fluctuation of HL, and progression to MD) of patients presenting with ALHL. The secondary goal was to summarize the clinical factors that have been reported to be associated with favorable hearing outcomes.
METHODS
This scoping review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Scoping Review (PRISMA-ScR) guidelines20.
Scoping Literature Search
The literature search was composed by a medical librarian (author S.J.K.) and utilized subject headings and keywords to represent the concepts of ALHL and prognosis. The databases MEDLINE (via PubMed), Embase (via Elsevier), and Scopus (via Elsevier) were searched from inception to May 14, 2020, with an additional search conducted on July 6, 2022. Non-human studies were removed. Reproducible search strategies are available in Supplemental Digital Content 1. Per PRISMA guidelines, the protocol described above was registered with the international prospective register of systematic reviews (PROSPERO) and is available for review21 (PROSPERO CRD42020189829).
All studies were imported into the online screening platform Covidence (Cochrane). Study selection is depicted in Figure 1 (See Supplemental Digital Content 2 for PRISMA-ScR checklist). All study abstracts were screened independently by two reviewers. Abstracts which passed initial screening then underwent full-text review by two independent reviewers. Studies were included or excluded based on the criteria below. Conflicts in both abstract screening and full-text review were adjudicated by a third reviewer.
Figure 1:
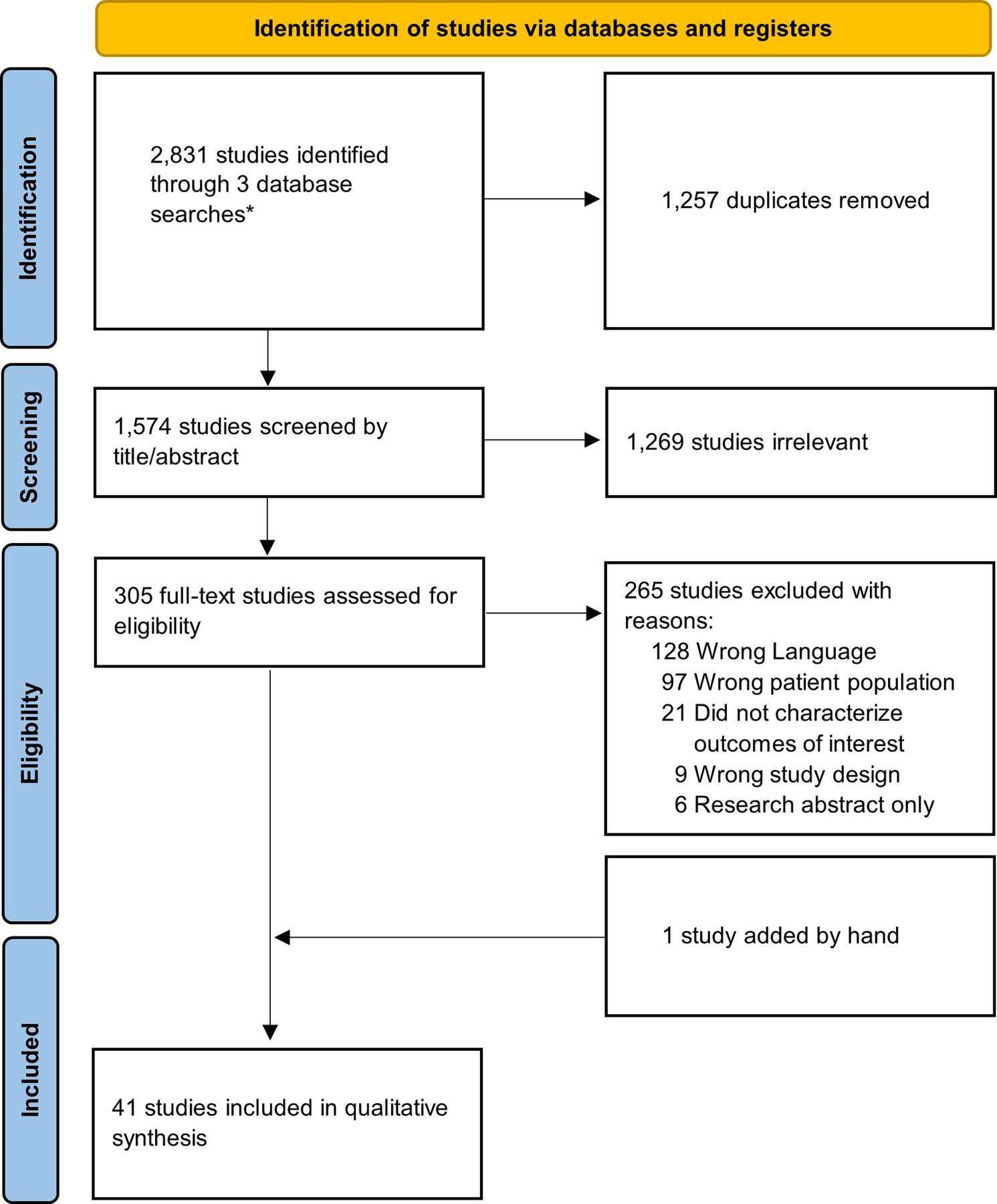
Study inclusion flowchart.
Inclusion/Exclusion Criteria
Studies were included if they met both of the following inclusion criteria:
Patients with either unilateral or bilateral ALHL, as defined by the study authors, without concurrent vertigo.
The study reported any outcomes related to recovery from ALHL, recurrence and/or fluctuation of HL, or progression to MD in patients with ALHL without vertigo. If articles included patients with other types of HL or patients with vertigo, then outcomes had to be clearly distinguishable for patients with ALHL without vertigo.
Studies were excluded if they met either of the following criteria:
The focus of the study was on pediatric presentations of ALHL.
The primary population of interest was ALHL presenting with concurrent vertigo.
Cohorts with retrocochlear disease, history of otologic surgery, acoustic trauma, barotrauma, acute or chronic otitis media, or history of MD were excluded. Non-English articles, case reports (i.e., single patient studies), editorials, letters, comments, practice guidelines, consensus development conferences, and book chapters were also excluded.
Data Extraction
The following data of interest were collected when available: study characteristics, patient characteristics, and patient outcomes. Study characteristics included study design, country of origin, study-specific audiometric definition of ALHL, and follow-up period. Patient characteristics included demographics, laterality and severity of HL, presence of tinnitus, medical comorbidities, vestibular evaluation, and interventions received. Patient outcomes were defined by the study and included recovery from HL, recurrence and/or fluctuation of HL, and progression to MD. Data were extracted independently by 2 reviewers and discrepancies were resolved via discussion. Studies that had no treatment comparison groups relative to the outcomes of interest were treated as case series during the data extraction period
Extraction and Categorization of Outcome Data
For each given outcome of interest (recovery from HL, recurrence or fluctuation of ALHL, and progression to MD), results were reported across all applicable studies. For recovery from ALHL, studies often presented outcomes in four categories (“Complete Recovery”, “Partial Recovery”, “No Change”, and “Progression”), with varying definitions for each category.
Although studies often presented outcomes in four recovery categories (as described above), other studies simply dichotomized recovery (e.g., “Any Recovery” vs. “No Recovery”). Therefore, we reported the dichotomous outcomes when needed (“Improvement” vs. “No Improvement”). Four-category outcomes were also converted to dichotomous outcomes in the following manner: Complete and Partial Recovery were considered Improvement while No Change and Progression were considered No Improvement. The findings of studies which reported quantitative rather than categorical hearing outcomes were reported separately.
Recurrence of ALHL, fluctuation of ALHL, and progression to MD were characterized dichotomously (“Yes” or “No”). The range of follow-up periods for these outcomes was also reported. Only 1 out of 12 studies13 evaluating recurrence provided audiometric criteria for defining recurrence while 7 out of 7 studies8, 17, 18, 22–25 evaluating fluctuation provided audiometric criteria for defining fluctuation. Therefore, the outcomes of recurrence and fluctuation may have reflected the same event: worsening HL after initial HL recovery. In accordance with this possibility, a separate outcome “Recurrence or Fluctuation of low-tone hearing loss” was created by aggregating outcomes of either recurrence or fluctuation across all studies.
All outcomes of interest were stratified by the following treatment modalities: Systemic Steroids alone, Diuretics alone, Steroid and Diuretic, No Steroid or Diuretic, and Treatment Unspecified or Unclear. Cohorts in which “most” patients received a specific intervention or for which treatment regimens “generally comprised” of specific interventions were categorized into the Treatment Unspecified or Unclear group. The findings of cohorts treated with intratympanic (IT) steroids for the treatment of ALHL were reported separately.
Clinical factors and their potential association with hearing outcomes were also extracted. These factors were stratified by studies which evaluated associations without adjustment for potential confounders, studies which evaluated associations with adjustment for potential confounders, and epidemiologic studies. Epidemiologic studies were tabulated separately due to their large sample sizes, and thus, greatly increased chance of detecting significant associations.
RESULTS
A total of 2,831 studies were identified by the literature searches. Among these, 1,257 duplicates were removed and 1,574 articles were screened by title and abstract. A total of 305 full-text studies were reviewed for eligibility. One additional study17 was identified within the references of one of the full-texts and manually added to the full-text review. Among all full-texts reviewed, 41 studies3–8, 10, 13, 16, 17, 22–52 were determined to meet the study inclusion and exclusion criteria and these studies subsequently underwent data extraction (Figure 1).
Overview of Included Studies
Of 41 studies, 30 evaluated outcomes without comparison of interventions, 11 compared outcomes by interventions, and 1 was an epidemiologic study. Of studies that compared interventions for ALHL, only one51 was a randomized controlled trial. All other studies were observational studies. A majority (34 out of 41 studies) originated from East Asia. Four studies originated from institutions in Europe with no studies originating from North America. Most studies (31 out of 41) were published in the 2000s or 2010s. The earliest included study was published in 198634. Published studies consistently demonstrated a female preponderance, and the age ranged from 14 to 77 years with the vast majority of studies reporting a mean age of onset in the 4th decade of life. All included studies are summarized in Table 1.
Table 1:
Characteristics of Included Studies
| Study | Design | Audiometric Definition of Low-Tone Loss | Number of Patients (M/F) | Mean Age (years) | Treatments | Outcomes Measured | Categorical Audiometric Outcome Measure (if available) | Follow-up Period |
|---|---|---|---|---|---|---|---|---|
| Alatas 2009 | Case series | Hearing threshold > 30 dB HL at 3+ of the following frequencies: 0.125, 0.25, 0.5, 1.0, 1.5 kHz. Average hearing loss at three higher frequencies (4, 6, 8 kHz) is ≤ 10 dB. | 14 (3M/11F) |
Mean (SD): 43 (15.02) | IT dexamethasone + oral prednisolone | Hearing recovery and recurrence/fluctuation | 4 categoriesa | Varied: After conclusion of therapy or after complete recovery achieved. |
| Chang et al. 2016 | Cohort study | 2011 Japanese Ministry of Health and Welfare Definitionb | 47 (11M/36F) |
Mean 43 | Oral methylprednisone vs. oral methylprednisone + HCTZ | Progression to MD | NA | 1 month for initial outcomes. Unclear when MD assessed. |
| Chen et. Al 2021 | Case seriesc | The average hearing loss at .125, .25, and .5 kHz is ≥ 30 dB and sum at 2, 4, and 8 kHz is ≤ 60 dB | 49 (13M/36F) |
Mean 41.3 | Oral prednisone + mannitol | Hearing recovery | NA | 7 days |
| Choi et al. 2011 | Case series | The average hearing loss at .125, .25, and .5 kHz is ≥ 30 dB, and the average hearing loss at 2, 4, and 8 kHz is ≤ 20 dB | 61 (18M/43F) |
Mean (SD) 43.2 (15.2) | Oral prednisolone, HCTZ, betahistine, gingko biloba, nicergoline | Hearing recovery and progression to MD | 4 categories | 3 months |
| Derinsu et al. 2006 | Case series | Upsloping Audiogram | 7 (1M/6F) |
Mean 43.7 | Steroids | Hearing recovery | ≥ 10 dB improvement (yes/no) in 4-frequency PTA (0.5, 1, 2, and 4 kHz) | Unclear |
| Fuse et al. 2002 | Cohort study | Sum of hearing thresholds at .125, .250, and .5 kHz is ≥ 100 dB and sum at 2, 4, and 8 kHz is ≤ 60 dB | 19 (NA) |
NA | Oral and IV prednisolone | Hearing recovery | 4 categories | 2 months |
| Fushiki et al. 2009 | Case seriesc | Average threshold at .125, .25, .5 kHz is at least 10 dB worse than at 2, 4, and 8 kHz. Less than 10 dB difference in hearing threshold at 1 kHz in comparison with that of adjacent frequencies | 82 (31M/51F) |
Mean (SD): 41.6 (13.8) | Most received IV corticosteroids | Recurrence/fluctuation and progression to MD | NA | Mean, range: 56, 6–196 months |
| Fushiki et al. 2010 | Case seriesc | Average hearing threshold at .125, .25, .5 kHz is at least 10 dB worse than that at 2, 4, and 8 kHz, and there is less than 10 dB difference in the hearing threshold at 1 kHz compared to adjacent frequencies (500 Hz and 2 kHz). | 35 (11M/24F) |
Mean (SD): 37.8 (11.9) | Most received IV dexamethasone | Hearing recovery, recurrence/fluctuation and progression to MD | Recovery (yes/no), not defined by the authors. | Mean, range: 48, 6–196 months Unclear when initial recovery from HL assessed |
| Hong et al. 2013 | Case seriesc | Average hearing threshold at .125, .25, and .5 kHz at least 10 dB poorer than average hearing threshold at 2, 4, 8 kHz. Pure tone threshold of 30 dB or higher at three low consecutive low frequencies. | 28 (7M/21F) |
Mean, range: 43, 18–61 | Oral prednisolone | Recurrence/fluctuation and progression to MD | Hearing Improvement (yes/no), defined as > 15 dB gain, unclear at which frequencies. | Mean, range: 510, 133–879 days |
| Huy and Sauvaget 2005 | Case seriesc | Low-tone hearing loss characterized by audiogram shape | 59 (NA) |
NA | IV steroids + mannitol | Hearing recovery | Complete Recovery (yes/no), defined as final PTA (re: 0.5, 1, 2, and 4 kHz) in the affected ear < 25 dB. | 30 days |
| Im et al. 2016 | Case series | Average hearing threshold ≥ 30 dB at .125, .25, .5 kHz and ≤ 20 dB at 2, 4, and 8 kHz. | 31 (13M/18F) |
Mean (SD): 39.4 (13) | Generally, methylprednisolone + HCTZ | Hearing recovery | Hearing Recovery (yes/no), defined as PTA gain of 10 dB or more at .125, .25, and .5 kHz. | 12 weeks |
| Imamura et al.1997 | Case series | Average hearing thresholds at .125, .25, and .5 kHz is ≥ 30 dB, and the average hearing threshold at 2,4, and 8 kHz is ≤ 20 dB. | 137 (36M/101F) |
Mean 40.6 | Vitamin B12 + ATP disodium + varying prednisone dosages | Hearing recovery, recurrence/fluctuation | 4 categories | >3 weeks |
| Inui et al. 2019 | Case series | 2011 Japanese Ministry of Health, Labor and Welfare for Acute Profound Deafness definitionb | 47 (11M/36F) |
Mean, range: 43.3, 17–71 | Hydrocortisone and/or betamethasone | Hearing recovery and recurrence/fluctuation | Cure vs. No Cure. Cure defined as recovery of thresholds to ≤ 25 dB at each of .125, .25, and .5 kHz. Individuals marked with Recurrence added to Cure group for initial HL outcome |
Mean, range: Cure group: 16.8, 3–32 weeks No Cure group: 20.8, 4–28 weeks Recurrence group: 22.5, 2–32 weeks |
| Jung et al. 2016 | Cohort study | Thresholds at .25 and .5 kHz >30 dB and ≤ 25 dB at 1, 2, 3, 4, and 8 kHz. | 50 (10M/40F) |
Mean (SD): 39.7 (12.1) | Oral prednisolone vs. IT steroids vs. Oral prednisolone and IT steroids | Hearing recovery | 4 categories | 8 weeks |
| Junicho et al. 2008a | Case seriesc | Average hearing threshold at .125, .25, and .5 kHz is worse compared to 2, 4, 8 kHz by 10 dB or more. Hearing threshold at 1 kHz within 10 dB of hearing thresholds at .5 and 2 kHz. | 177 (NA) |
NA | Unclear | Recurrence/fluctuation and progression to MD | NA | For patients without recurrence, mean, range: 67, 7–210 months |
| Junicho et al. 2008b | Case seriesc | Average hearing threshold at .125, .25, and .5 kHz is at least 10 dB worse than that at 2, 4, 8 kHz. | 82 (NA) |
NA | Most received IV steroids | Recurrence/fluctuation and progression to MD | NA | Mean, range: 67, 6–210 months |
| Kim et al. 2020 | Case series | Average hearing loss of ≥30 dB at 125, 250, and 500 Hz and ≤20 dB at 2, 4, and 8 kHz. | 58 (19M/39F) |
Mean (SD): 39.3 (12.9) | Prednisolone + HCTZ | Hearing recovery and progression to MD | Hearing Recovery (yes/no), defined as PTA gain of 10 dB or more at .125, .25, and .5 kHz. | Range 1 to 12 weeks for hearing outcomes, Mean 7.4 months for progression of MD |
| Kumagami and Osawa 1986 | Case series | Unclear | 6 (NA) |
Mean 33 | Isosorbide vs. steroid vs. CO2 inhalation | Hearing recovery | Improvement (Yes/No), criteria not defined by authors. | Range 42 days to 2.5 years |
| Lee et al. 2018 | Cohort study | Thresholds at .25, .5 kHz > 30 dB and thresholds at 1, 2, 3, 4, and 8 kHz ≤ 25 dB on the affected side. Thresholds at .25, .5, 1, 2, and 4 kHz ≤ 25 dB on the unaffected side. |
170 (52M/118F) |
Mean (SD): 44.0 (16.8) | Low dose steroids vs. high dose steroids vs. steroids + diuretics vs. IT steroids + diuretics | Hearing recovery | Recovery (yes/no), defined as either Complete or Partial Recovery | 4 weeks |
| Ma et al. 2021 | Case seriesc | Sum of hearing levels at 0.125, 0.25 and 0.5 kHz is ≥ 70 dB or the sum of hearing levels at 2, 4 and 8 kHz ≤ 60 dB | 115 (43M/72F) |
Mean (SD): 42.3 (14.33) | IT steroids | Recurrence/fluctuation and progression to MD | NA | Mean follow up time of 17.61 months |
| Morita et al. 2010 | Cohort study | 2011 Japanese Ministry of Health and Welfare Definitionb | 156 (52M/104F) |
Mean, range: 48.7, 14–77 | Oral steroids (prednisolone or betamethasone) vs. diuretics vs. oral steroids (prednisolone or betamethasone) + diuretics vs. vitamin B12 and ATP | Hearing recovery and recurrence/fluctuation | 4 categories | 8 weeks |
| Morita et al. 2016 | Cohort study | 2011 Japanese Ministry of Health and Welfare Definitionb | 90 (24M/66F) |
Median, range: 49.5, 13–61 | Salvage IT steroids vs. salvage diuretics vs. observation. All initially treated with oral steroid, diuretic, vitamin B12, and ATP | Hearing recovery, recurrence/fluctuation, progression to MD | Recovery (yes/no), defined as either Complete or Partial Recovery | Medina, range: 36, 18–108 months |
| Nakashima and Yanagita 1993 | Case series | Average threshold at 0.25 and 0.5 kHz surpasses average of 4 and 8 kHz by 30 dB or more. Contralateral thresholds have to be 20 dB or less at all frequencies 0.25 – 8kHz. | 77 (NA) |
NA | Unclear | Hearing recovery | Average thresholds of patients in six assessed frequencies | 2 months |
| Oishi et al. 2010 | Case series | Sum of thresholds at .125, .25, and .5 kHz ≥ 70 dB; hearing threshold difference between both ears < 10 dB at high frequenciesd. | 49 (19M/30F) |
Mean (SD): 46 (14) | Most treated with prednisone (oral or IV), diuretic, vasodilator | Hearing recovery and recurrence/fluctuation |
4 categories | 10–21 years |
| Okada et al. 2012 | Cohort study | Sum of thresholds at .125, .25, and .5 kHz ≥ 70 dB. Definite ALHL: sum of thresholds at 2,4, and 8 kHz ≤ 60 dB. Probable ALHL: Sum of thresholds at 2,4, and 8 kHz > 80 dB. Probable patients excluded if high-tone sum shows a difference of more than 10 dB from the contralateral ear or if low-tone sum shows less than a 10 dB difference from the contralateral ear. |
178 (56M/122F) |
Mean (SD): 47.3 | Prednisolone vs. isosorbide vs. prednisolone + isosorbide vs. Wu-Ling San vs. Steroid + Wu-Ling San vs. no treatment | Hearing recovery | 4 categories | 1 week |
| Park et al. 2018 | Case seriesc | Average threshold at .25 and .5 kHz > 30 dB and average threshold at 1, 2, 3, 4, and 8 kHz < 25 dB. | 53 (13M/40F) |
Mean, range: 39, 22–64 | Oral steroids, salvage IT steroid, diuretic | NA | NA | 8 weeks |
| Psillas et al. 2019 | Case Seriesc | The average threshold at .125, .25, .5 kHz ≥ 30 dB, and the average threshold at 2, 4, 8 kHz ≤ 20 dB. | 27 (10M/17F) |
Mean (SD): 44.1 (13.1) | IV dexamethasone | Hearing recovery, recurrence/fluctuation, and progression to MD | 4 categories | Mean follow-up of 3.3 years |
| Qin et al. 2021 | Case seriesc | Hearing loss at frequencies under 1 kHz with the least reduction by 20 dB HL at 250 and 500 Hz | 21 (12M/9F) |
Mean (SD): 54.1 (8.3) | Varied; mostly IV dexamethasone | Hearing recovery | Recovery (yes/no), defined as ≥ 30 dB improvement in 250 and 500 Hz | unclear |
| Roh et al. 2015 | Case series | Sum at .25 and .5 kHz ≥ 50 dB. Hearing threshold difference between both ears < 10 dB at 2 and 4 kHz. | 33 (13M/20F) |
Mean (SD): 41 (12.7) | Methylprednisolone +/− spironolactone +/− IT dexamethasone | Hearing recovery and recurrence/fluctuation | 4 categories | Mean, range: 22, 3–79 months, initial recovery assessed at 1 month |
| Sato et al. 2017 | Epidemiological survey | 2011 Japanese Ministry of Health and Welfare Definitionb | 642 (205M/433F/4U) |
Mean (SD): 43.8 (15.5) | Unclear | Hearing recovery | 4 categories | Unclear |
| Selivanova et al. 2005 | Case series | Air conduction hearing threshold > 30 dB at 2 or more of the following: 0.125, 0.25, 0.5, 0.75, 1, and 1.5 kHz. | 18 (9M/9F) |
Mean, range: 50, 26–62 | IT hyaluronic acid, dexamethasone | Hearing recovery and recurrence/fluctuation | Significant improvement (yes/no), defined as ≥ 10 dB improvement in at least 2 frequencies | Following end of IT injections |
| Shin et al. 2021 | Cohort study | 2011 Japanese Ministry of Health and Welfare Definitionb | 49 (13M/36F) |
Mean (SD): 42.0 (12.6) | Oral prednisolone vs. oral prednisolone + isosorbide, HCTZ, or spironolactone | Hearing recovery | Recovery (yes/no), defined as mean hearing threshold of 125, 250, and 500 Hz ≤20 dB. | 2 weeks |
| Stolzel et al. 2018 | Case seriesc | Threshold > 30 dB in the frequencies from .125 to .5 kHz or pantonal hearing loss. | 23 (NA) |
NA | Unclear | Progression to MD | NA | 5 years |
| Sugiura et al. 2003 | Case series | Sum of hearing thresholds at .125, .25, and .5 kHz ≥ 100 dB and sum of hearing thresholds at 2, 4, and 8 kHz ≤ 60 dB | 25 (9M/16F) |
Mean (SD): 39.7 (12.7) | Unclear | Hearing recovery and recurrence/fluctuation | 4 categories | Range 1.5–3.5 years |
| Suzuki et al. 2006 | Cohort study | Sum of thresholds at .125, .25, and .5 kHz is ≥ 80 dB and 40 dB poorer than the contralateral side. Thresholds at frequencies 1–8 kHz do not differ more than 10 dB between right and left ears. | 225 (99M/126F) |
NA | Oral prednisolone, IV hydrocortisone, and/or diuretics. All patients received vitamin B12 and ATP | Predictors of hearing recovery | NA | 1 month |
| Tanigawa et al. 2010 | Case series | Unclear | 5 (0M/5F) |
Mean 42 | IV Hydrocortisone vs. anti-anxiety medication | Hearing recovery and recurrence/fluctuation | 4 categories | Range 6–22 months |
| Waissbluth et al. 2021 | Case seriesc | Upsloping audiogram | 48 (NA) |
NA | Varied combinations of IT steroid, oral steroid | Hearing recovery | 4 categories | Range 14 days to 3 months |
| Wang et al. 2010 | Case series | Average hearing threshold at .125, .25, and .5 kHz > 30 dB, average hearing threshold at 2, 4, and 8 kHz < 20 dB | 21 (7M/14F) |
Mean, range: 40, 14–61 | Isosorbide | Hearing recovery | Improvement (Yes/No), defined as > 50% recovery | 3 months |
| Wu and Young 2004 | Case control | Average hearing threshold at .125, .25, .5 kHz > 30 dB, average hearing threshold at 2, 4, 8 kHz < 20 dB | 12 (5M/7F) |
Mean, range: 35, 20–61 | Isosorbide | Hearing recovery and progression to MD | Return to normal hearing (Yes/No). Normal hearing unclearly defined. | 1 year |
| Yakunina et al. 2019 | Randomized trial | 2011 Japanese Ministry of Health and Welfare Definitionb | 92 (31M/61F) |
Mean 45.5 | Concurrent oral methylprednisolone + HCTZ + isosorbide vs. sequential HCTZ + isosorbide + methylprednisolone (if indicated) | Hearing recovery | 4 categories | 28 days |
| Yamasoba et al. 1994 | Case series | Sum of thresholds at .125, .25, and .5 kHz ≥ 100 dB. Sum of thresholds at 2, 4, and 8 kHz ≤ 80 dB. | 80 (22M/58F) |
Mean 42.3 | Most treated with prednisolone + diuretic + vasodilator | Hearing recovery, recurrence/fluctuation and progression to MD | Improvement (Yes/No), no change, progression, and fluctuation | 3 months for hearing outcome, and at least 3 years for assessment of recurrence and MD progression |
Four categories as follows: Complete Recovery, Partial Recovery, No Change, Progression.
Sum of thresholds at .125, .25, and .5 kHz ≥ 70 dB. Sum of thresholds at 2, 4, and 8 kHz ≤ 60 dB.
This study was technically a cohort study. However, because all patients received the same treatment regimen, it was considered a case series for the purposes of this review.
High frequencies not specified.
Note: M, Male; F, Female; U, Unknown; NA, Not Applicable; kHz, kilohertz, SD, standard deviation, dB, decibel; PTA, pure tone average, IT=intratympanic
Definition of Low-Tone Hearing Loss
Among all included studies, 21 different audiometric criteria were used to define ALHL (see Table 1). The most commonly used criterion (7 out of 41 studies) was the 2011 Acute Profound Deafness Research Committee of the Japanese Ministry of Health, Labor, and Welfare Definition of ALHL: the sum of thresholds at 125, 250, and 500 Hz must be 70 dB or greater and the sum of thresholds at 2, 4, and 8 kHz must be 60 dB or less.
Reported Treatment Modalities
Among the included papers, 13 studies reported hearing outcomes following administration of systemic steroids alone, 4 studies following diuretics alone, 10 studies following a combination of systemic steroid and diuretics, 8 studies following intratympanic steroids, and 15 with other or unspecified treatments. Descriptions of all study cohorts in included papers are displayed by treatment type in Supplemental Digital Contents 3–8.
Outcomes
A total of 32 studies evaluated recovery from HL, with reported mean or median follow-up time ranging from one week3, 28 to 3.3 years39. Among these 32 studies, 15 studies reported outcomes in four recovery categories and 30 studies reported outcomes that could be dichotomized. Among these 30 studies, there were 40 distinct patient cohorts. A cohort was defined as a group of patients receiving the same treatment within a study. The overwhelming majority of study cohorts (39/40) reported a greater than 50% rate of hearing improvement (i.e., >50% of patients in each cohort achieved some form of hearing improvement) within a diverse range of follow up times. This was true whether or not an intervention was provided and regardless of the treatment rendered. The rate of complete hearing recovery was most frequently reported to occur in the 40–80% range for 14 out of 22 cohorts, regardless of treatment status. A summary of these studies and their outcomes can be found in Supplemental Digital Contents 3–8. In addition to categorical outcomes, recovery from HL was also reported using a variety of quantitative metrics in 13 studies (Supplemental Digital Content 9).
A total of 18 studies evaluated recurrence or fluctuation of hearing loss following ALHL, with reported mean or median follow-up time ranging from 8 weeks22, 24, 25 to 5.6 years16, 33. Though variable, the reported rates of fluctuation or recurrence were below 50% in all (21/21) cohorts. A total of 14 studies evaluated progression to MD with reported mean or median follow-up time ranging from 1 year50 to 5.6 years16, 33. The progression to MD was consistently reported to occur in fewer than 40% of patients and was 20% or less in 10 out of 14 cohorts.
Clinical Factors Associated with Hearing Outcomes
Assessment of potential factors that may be associated with better hearing outcomes was examined. Overall, very few studies reported on potential factors that may influence hearing outcomes. Demographic factors such as age and sex were examined in 6 studies, time to onset of treatment in 8 studies, severity of initial HL in 9 studies, presence of tinnitus in 2 studies, and other comorbid conditions such diabetes and hypertension in 3 studies. The epidemiological study by Sato et al (2017) found that age, gender, time to onset of treatment, and severity of initial loss were significant predictors of hearing outcome. Increased time to onset of treatment was the most consistently reported risk factor for poorer hearing outcomes across all clinical studies that examined this relationship (Table 2).
Table 2:
Reported Predictors of Better Hearing Outcomes
| No. Studies (without adjustment)a | No. Studies (with adjustment) | Epidemiological Studyb | |
|---|---|---|---|
| Prognostic Factor | |||
| Age (Younger) | 2/6d | 1/2e | Yes |
| Sex (Female) | 0/6f | -- | Yes |
| Time to Onset of Treatment (Shorter) | 6/8g | 2/2h | Yes |
| Severity of Initial Loss (Less Severe) | 4/9i | 1/2j | Yes |
| Tinnitus | 1/2k | -- | -- |
| Diabetes | 0/3l | -- | No |
| Hypertension | 0/3m | -- | No |
| ECOG (elevated SP/AP ratio) | 1/3n | -- | -- |
| ENGc | 1/2o |
Not including Sato et al. 2017
Sato et al. 2017
Evaluated for association with progression to Meniere’s Disease
Alatas, 2009; Choi et al., 2011; Jung et al., 2016; Lee et al., 2018; Psillas et al., 2019; Suzuki et al., 2006
Lee et al., 2018; Suzuki et al., 2006
Choi et al., 2011; Jung et al., 2016; Lee et al., 2018; Psillas et al., 2019; Shin et al., 2021; Suzuki et al., 2006
Alatas, 2009; Chang et al., 2016; Choi et al., 2011; Jung et al., 2016; Lee et al., 2018; Psillas et al., 2019; Shin et al., 2021; Suzuki et al., 2006
Lee et al., 2018; Suzuki et al., 2006
Alatas, 2009; Choi et al., 2011; Im et al., 2016; Inui et al., 2019; Jung et al., 2016; Lee et al., 2018; Roh et al., 2015; Suzuki et al., 2006; Wang et al., 2010
Lee et al., 2018; Suzuki et al., 2006
Choi et al., 2011; Jung et al., 2016
Jung et al., 2016; Lee et al., 2018; Shin et al., 2021
Jung et al., 2016; Lee et al., 2018; Shin et al., 2021
Choi et al., 2011; Im et al., 2016; Kim et al., 2020
Fushiki et al., 2009; Im et al., 2016
DISCUSSION
This scoping review sought to understand the available evidence that characterizes the clinical outcomes of patients presenting with ALHL in an effort to shape future research inquiry and inform clinical practice. Due to heterogeneity in the populations of interest, outcome measures, and follow-up periods, a systematic review with meta-analysis of the current literature was deemed inappropriate.
General trends in the available data demonstrated that many ALHL patients experience partial or complete recovery of hearing although reports of recurrence were relatively common. Progression to MD was reported to occur in a minority of ALHL cases. Overall, the literature consistently reported that shorter time to onset of treatment predicted better hearing outcomes in those with ALHL. Among all studies that examine treatment effects in this review, Suzuki et al.46 authored the only observational study to control for potential confounders while comparing different treatment modalities for ALHL. Their multivariate analysis showed that systemic steroid treatment was significantly associated with hearing recovery whereas diuretic treatment was not. The only randomized clinical trial51 compared sequential diuretic and steroid treatment (i.e., steroids were only administered if the patient did not achieve complete recovery within 2 weeks on diuretics alone) versus simultaneous diuretic and steroid administration. The authors reported that sequential treatment was non-inferior to simultaneous treatment. However, nearly one-third of all patients in the sequential arm eventually required systemic steroid administration. The findings of these two studies would suggest that systemic steroid administration may be slightly more effective than diuretics in achieving hearing recovery in patients with ALHL. To definitively answer this question however, additional studies and trials comparing steroid and diuretic treatment with adjustment for potential confounders are required.
Despite the general observed trends in the literature, our scoping review has revealed several remaining knowledge gaps. First, there is a dearth of literature on ALHL from research institutions outside of East Asia. As such, it remains unknown whether the overall prevalence and prognosis of ALHL is the same for other and more diverse populations. The relative contribution of genetic, environmental, and social factors on disease presentation and treatment response is unknown and warrants further investigation in more populations. Epidemiologic surveys from research institutions outside of East Asia would help improve our understanding of the overall global disease burden of ALHL, which may spur needed advances in its identification and treatment. Second, more studies are needed to assess how ALHL compares to other forms of SSHL regarding hearing recovery, recurrence of HL, and the effect of treatment on recovery and recurrence. A handful of studies found that patients with ALHL had both a higher rate of hearing recovery39 and HL recurrence16, 33, 39, 44 than patients with sudden high-tone deafness, but more studies are needed to compare outcomes against other types of SSHL (pan-tonal loss, profound deafness). Third, a greater number of longitudinal studies (with follow-up beyond 5 years) are needed to more accurately determine the rates of recurrence/fluctuation and progression to MD for patients with ALHL. Concurrently, quality-of-life studies are needed to help characterize the disease burden of ALHL and identify potential strategies to mitigate these effects.
Moving forward, consensus is needed regarding a common set of audiometric criteria for the diagnosis of ALHL as well as the evaluation of hearing outcomes. Within our review, there was significant variability in how hearing outcomes were reported and the criteria used to define various outcome categories. The provision of raw audiometric data as digital supplementary material would aid in advancing our understanding of this disease entity across multiple populations. The duplication of previously published data or patient cohorts should also be disclosed and highlighted to reduce bias. Similarly, studies must have clear criteria for distinguishing recurrence from fluctuation of HL. Having uniform methods for characterizing outcomes and high-quality studies that account for confounders, would facilitate more robust systematic reviews and meta-analyses and allow research groups to better determine optimal interventions for ALHL53–56. The selection of a global standard would be a worthwhile goal and could be addressed through collaborative action by international professional organizations of otolaryngologists and audiologists. In addition to the methodological challenges encountered in the literature, there were additional limitations. First, non-English articles were excluded in this scoping review and our results may not reflect all the available literature on ALHL. Second, authors may have published more than once on the same patient cohort, creating the possibility for duplicate data.
Calls by the National Institutes of Health and other funders to promote standardization and transparency have increased in recent years and provides us even greater impetus to move our fields forward to create accessible raw data and utilize standard ‘common data elements’ in our research. Consistent reporting on patient characteristics and treatment protocols are a basic requirement to fill knowledge gaps, elucidate prognostic factors, and inform clinical decision making and patient counseling.
CONCLUSIONS
While the literature suggests that the majority of patients with ALHL appear to experience hearing improvement, recurrence and/or fluctuation are common. Progression to MD appears to occur infrequently. Shorter time from onset of symptoms to onset of treatment may be associated with better hearing outcomes. Additional studies and randomized controlled trials utilizing standardized criteria for diagnosis, study inclusion and hearing outcome are needed to determine the natural history and ideal treatment for ALHL.
Supplementary Material
SUPPLEMENTAL DIGITAL CONTENT 1: Scoping Review Search Strategy
SUPPLEMENTAL DIGITAL CONTENT 3: Demographics and Categorical Outcomes of Patients Treated with Systemic Steroids Alone
SUPPLEMENTAL DIGITAL CONTENT 2: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist
SUPPLEMENTAL DIGITAL CONTENT 4: Demographics and Categorical Outcomes of Patients Treated with Diuretics Alone
SUPPLEMENTAL DIGITAL CONTENT 6: Demographics and Categorical Outcomes of Patients Treated with Neither Steroids nor Diuretics
SUPPLEMENTAL DIGITAL CONTENT 5: Demographics and Categorical Outcomes of Patients Treated with Systemic Steroids and Diuretics
SUPPLEMENTAL DIGITAL CONTENT 7: Demographics and Categorical Outcomes of Patients Treated with Unclear or Unspecified Interventions
SUPPLEMENTAL DIGITAL CONTENT 8: Results of Studies Evaluating Intratympanic Steroids
SUPPLEMENTAL DIGITAL CONTENT 9: Results of Studies Evaluating Non-Categorical Hearing Outcomes
Acknowledgments:
This study was not funded. Ryan J. Huang was supported by a Pfizer Foundation grant and the Duke Clinical Translational Science Institute (CTSI). Amanda Del Risco was supported by NIH NIDCD R25DC020172 for work on this project. Kristal M. Riska has grants from the National Institutes of Health and Department of Defense that are unrelated to this project. Howard W. Francis has grants from the National Institutes of Health that are unrelated to this project.
Footnotes
Conflict of Interest Statement: Howard W. Francis serves on the surgical advisory boards for Med El and Advanced Bionics. There are no other conflicts of interest.
Meeting Presentation: This work has not been presented previously.
Level of Evidence: NA
REFERENCES
- 1.Abe T Acute sensorineural hearing loss in low frequencies. Otolaryngol (Tokyo). 1982;54:385–392. [Google Scholar]
- 2.Shimono M, Teranishi M, Yoshida T, et al. Endolymphatic hydrops revealed by magnetic resonance imaging in patients with acute low-tone sensorineural hearing loss. Otology & Neurotology. 2013;34(7):1241–1246. [DOI] [PubMed] [Google Scholar]
- 3.Okada K, Ishimoto S-i, Fujimaki Y, Yamasoba T Trial of Chinese medicine Wu-Ling-San for acute low-tone hearing loss. ORL. 2012;74(3):158–163. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima T, Sato H, Gyo K, et al. Idiopathic sudden sensorineural hearing loss in Japan. Acta Otolaryngol. Nov 2014;134(11):1158–63. doi: 10.3109/00016489.2014.919406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato H, Kuwashima S, Nishio S-y, et al. Epidemiological survey of acute low-tone sensorineural hearing loss. Acta oto-laryngologica. 2017;137(sup565):S34–S37. [DOI] [PubMed] [Google Scholar]
- 6.Fushiki H, Junicho M, Aso S, Watanabe Y. Recurrence rate of idiopathic sudden low-tone sensorineural hearing loss without vertigo: a long-term follow-up study. Otol Neurotol Apr 2009;30(3):295–298. doi: 10.1097/mao.0b013e31819d3496 [DOI] [PubMed] [Google Scholar]
- 7.Fushiki H, Junicho M, Kanazawa Y, Aso S, Watanabe Y. Prognosis of sudden low-tone loss other than acute low-tone sensorineural hearing loss. Acta Otolaryngol. May 2010;130(5):559–64. doi: 10.3109/00016480903311245 [DOI] [PubMed] [Google Scholar]
- 8.Yamasoba T, Kikuchi S, Sugasawa M, Yagi M, Harada T. Acute low-tone sensorineural hearing loss without vertigo. Arch Otolaryngol Head Neck Surg. May 1994;120(5):532–5. doi: 10.1001/archotol.1994.01880290042007 [DOI] [PubMed] [Google Scholar]
- 9.Nozawa I, Imamura S-i, Honda H, Mizukoshi A, Okamoto Y. Clinical study of acute low-tone sensorineural hearing loss: survey and analysis of glycerol test and orthostatic test. Annals of Otology, Rhinology & Laryngology. 2002;111(2):160–164. [DOI] [PubMed] [Google Scholar]
- 10.Im GJ, Kim SK, Choi J, Song JJ, Chae SW, Jung HH. Analysis of audio-vestibular assessment in acute low-tone hearing loss. Acta Otolaryngol. Jul 2016;136(7):649–54. doi: 10.3109/00016489.2016.1152506 [DOI] [PubMed] [Google Scholar]
- 11.Noguchi Y, Nishida H, Kawashima Y, Tokano H, Kitamura K. Comparison of acute low-tone sensorineural hearing loss versus Meniere’s disease by electrocochleography. Annals of Otology, Rhinology & Laryngology. 2004;113(3):194–199. [DOI] [PubMed] [Google Scholar]
- 12.Attyé A, Eliezer M, Medici M, et al. In vivo imaging of saccular hydrops in humans reflects sensorineural hearing loss rather than Meniere’s disease symptoms. Eur Radiol. Jul 2018;28(7):2916–2922. doi: 10.1007/s00330-017-5260-7 [DOI] [PubMed] [Google Scholar]
- 13.Inui H, Sakamoto T, Ito T, Kitahara T. Magnetic resonance imaging of the endolymphatic space in patients with acute low-tone sensorineural hearing loss. Auris Nasus Larynx. Dec 2019;46(6):859–865. doi: 10.1016/j.anl.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Merchant SN, Adams JC, Nadol JB Jr. Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. Jan 2005;26(1):74–81. doi: 10.1097/00129492-200501000-00013 [DOI] [PubMed] [Google Scholar]
- 15.Nadol JB. Focal Endolymphatic Hydrops as Seen in the Pars Inferior of the Human Inner Ear. Otology & Neurotology. 2016;37(7):859–864. doi: 10.1097/mao.0000000000001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junicho M, Aso S, Fujisaka M, Watanabe Y. Prognosis of low-tone sudden deafness–does it inevitably progress to Meniere’s disease? Acta oto-laryngologica. 2008;128(3):304–308. [DOI] [PubMed] [Google Scholar]
- 17.Oishi N, Inoue Y, Saito H, Kanzaki S, Kanzaki J, Ogawa K. Long-term prognosis of low-frequency hearing loss and predictive factors for the 10-year outcome. Otolaryngol Head Neck Surg. Apr 2010;142(4):565–9. doi: 10.1016/j.otohns.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 18.Imamura S-i, Imamura M, Nozawa I, Murakami Y. Clinical observations on acute low-tone sensorineural hearing loss: survey and analysis of 137 patients. Annals of Otology, Rhinology & Laryngology. 1997;106(9):746–750. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Li G, Zhuang H, et al. Meta-Analysis Comparing Steroids and Diuretics in the Treatment of Acute Low-Tone Sensorineural Hearing Loss. Ear Nose Throat J. Jun 2021;100(3_suppl):281s–285s. doi: 10.1177/0145561319869610 [DOI] [PubMed] [Google Scholar]
- 20.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018/10/02 2018;169(7):467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 21.Huang R, Cooper M, Francis H. Acute Low Tone Hearing Loss: Prognostic Indicators and Treatment. . https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020189829
- 22.Fuse T, Aoyagi M, Funakubo T, Sakakibara A, Yoshida S. Short-term outcome and prognosis of acute low-tone sensorineural hearing loss by administration of steroid. ORL J Otorhinolaryngol Relat Spec. Jan-Feb 2002;64(1):6–10. doi: 10.1159/000049079 [DOI] [PubMed] [Google Scholar]
- 23.Hong SK, Nam SW, Lee H-J, et al. Clinical observation on acute low-frequency hearing loss without vertigo: the role of cochlear hydrops analysis masking procedure as initial prognostic parameter. Ear and Hearing. 2013;34(2):229–235. [DOI] [PubMed] [Google Scholar]
- 24.Jung AR, Kim MG, Kim SS, Kim SH, Yeo SG. Clinical characteristics and prognosis of low frequency sensorineural hearing loss without vertigo. Acta Otolaryngol. 2016;136(2):159–63. doi: 10.3109/00016489.2015.1094824 [DOI] [PubMed] [Google Scholar]
- 25.Morita S, Suzuki M, Iizuka K. A comparison of the short-term outcome in patients with acute low-tone sensorineural hearing loss. ORL J Otorhinolaryngol Relat Spec. 2010;72(6):295–9. doi: 10.1159/000314695 [DOI] [PubMed] [Google Scholar]
- 26.Alatas N Use of intratympanic dexamethasone for the therapy of low frequency hearing loss. Eur Arch Otorhinolaryngol. Aug 2009;266(8):1205–12. doi: 10.1007/s00405-008-0895-2 [DOI] [PubMed] [Google Scholar]
- 27.Chang J, Yum G, Im H-Y, Jung JY, Rah YC, Choi J. Short-term outcomes of acute low-tone sensorineural hearing loss according to treatment modality. Journal of Audiology & Otology. 2016;20(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen D, Wang Z, Jia G, Mao H, Ni Y. The Role of Anti-Endothelial Cell Autoantibodies and Immune Response in Acute Low-Tone Hearing Loss. Ear Nose Throat J. Jun 2021;100(3_suppl):292s–300s. doi: 10.1177/0145561320952501 [DOI] [PubMed] [Google Scholar]
- 29.Choi HG, Park KH, Seo JH, Kim DK, Yeo SW, Park SN. Clinical and Audiologic Characteristics of Acute Low-Tone Sensorineural Hearing Loss: Therapeutic Response and Prognosis. Korean J Audiol. 4 2011;15(1):8–13. [Google Scholar]
- 30.Derinsu U, Terlemez Ş, Akdaş F. Idiopathic sudden sensorineural hearing loss. Marmara Medical Journal. 01/January 2006;19 [Google Scholar]
- 31.Huy PT, Sauvaget E. Idiopathic sudden sensorineural hearing loss is not an otologic emergency. Otol Neurotol. Sep 2005;26(5):896–902. doi: 10.1097/01.mao.0000185071.35328.6d [DOI] [PubMed] [Google Scholar]
- 32.Imamura S, Nozawa I, Imamura M, Murakami Y. Clinical observations on acute low-tone sensorineural hearing loss. Survey and analysis of 137 patients. Ann Otol Rhinol Laryngol. Sep 1997;106(9):746–50. doi: 10.1177/000348949710600906 [DOI] [PubMed] [Google Scholar]
- 33.Junicho M, Fushiki H, Aso S, Watanabe Y. Prognostic value of initial electronystagmography findings in idiopathic sudden sensorineural hearing loss without vertigo. Otol Neurotol. Oct 2008;29(7):905–9. doi: 10.1097/MAO.0b013e31817fdf94 [DOI] [PubMed] [Google Scholar]
- 34.Kumagami H, Osawa H. Electrocochleographic study of low-tone hearing loss without vertigo. ORL J Otorhinolaryngol Relat Spec. 1986;48(1):16–23. doi: 10.1159/000275837 [DOI] [PubMed] [Google Scholar]
- 35.Lee CK, Lee JB, Park KH, et al. Significance of 1 kHz Pure-tone Threshold in Acute Low-frequency Sensorineural Hearing Loss. Otol Neurotol. Dec 2018;39(10):e950–e955. doi: 10.1097/mao.0000000000002023 [DOI] [PubMed] [Google Scholar]
- 36.Ma Y, Sun Q, Zhang K, Bai L, Du L. High level of IgE in acute low-tone sensorineural hearing loss: A predictor for recurrence and Meniere Disease transformation. Am J Otolaryngol. Mar-Apr 2021;42(2):102856. doi: 10.1016/j.amjoto.2020.102856 [DOI] [PubMed] [Google Scholar]
- 37.Morita S, Nakamaru Y, Fujiwara K, et al. The Short- and Long-Term Outcome of Intratympanic Steroid Therapy as a Salvage Treatment for Acute Low-Tone Sensorineural Hearing Loss without Episodes of Vertigo. Audiol Neurootol. 2016;21(3):132–40. doi: 10.1159/000444577 [DOI] [PubMed] [Google Scholar]
- 38.Park MJ, Kim SH, Kim SS, Yeo SG. Clinical Characteristics and Short-term Outcomes of Acute Low Frequency Sensorineural Hearing Loss With Vertigo. Clinical and Experimental Otorhinolaryngology. 2018;11(2):96–101. doi: 10.21053/ceo.2017.00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psillas G, Rizou A, Rachovitsas D, Tsiropoulos G, Constantinidis J. Hearing Outcome of Low-tone Compared to High-tone Sudden Sensorineural Hearing Loss. Int Arch Otorhinolaryngol. Jan 2019;23(1):65–69. doi: 10.1055/s-0038-1657789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin H, He B, Wu H, et al. Visualization of Endolymphatic Hydrops in Patients With Unilateral Idiopathic Sudden Sensorineural Hearing Loss With Four Types According to Chinese Criterion. Front Surg. 2021;8:682245. doi: 10.3389/fsurg.2021.682245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh KJ, Lee EJ, Park AY, Choi BI, Son EJ. Long-Term Outcomes of Acute Low-Tone Hearing Loss. Journal of Audiology and Otology. 2015;19(2):74–78. doi: 10.7874/jao.2015.19.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selivanova OA, Gouveris H, Victor A, Amedee RG, Mann W. Intratympanic dexamethasone and hyaluronic acid in patients with low-frequency and Meniere’s-associated sudden sensorineural hearing loss. Otology & Neurotology. 2005;26(5):890–895. [DOI] [PubMed] [Google Scholar]
- 43.Shin SH, Byun SW, Park S, Kim EH, Kim MW, Lee HY. Optimal First-Line Therapy for Acute Low-Tone Sensorineural Hearing Loss. J Audiol Otol. Oct 2021;25(4):209–216. doi: 10.7874/jao.2021.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stölzel K, Droste J, Voß LJ, Olze H, Szczepek AJ. Comorbid Symptoms Occurring During Acute Low-Tone Hearing Loss (AHLH) as Potential Predictors of Menière’s Disease. Front Neurol. 2018;9:884. doi: 10.3389/fneur.2018.00884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugiura M, Naganawa S, Nakashima T, Misawa H, Nakamura T. Magnetic resonance imaging of endolymphatic sac in acute low-tone sensorineural hearing loss without vertigo. ORL J Otorhinolaryngol Relat Spec. Sep-Oct 2003;65(5):254–60. doi: 10.1159/000075222 [DOI] [PubMed] [Google Scholar]
- 46.Suzuki M, Otake R, Kashio A. Effect of corticosteroids or diuretics in low-tone sensorineural hearing loss. ORL J Otorhinolaryngol Relat Spec. 2006;68(3):170–6. doi: 10.1159/000091343 [DOI] [PubMed] [Google Scholar]
- 47.Tanigawa T, Tanaka H, Sato T, et al. 3D-FLAIR MRI findings in patients with low-tone sudden deafness. Acta Otolaryngol. Dec 2010;130(12):1324–8. doi: 10.3109/00016489.2010.496461 [DOI] [PubMed] [Google Scholar]
- 48.Waissbluth S, Sepúlveda V, Urzúa P. Sudden sensorineural hearing loss: Recovery rates according to audiometric patterns. Acta Otorrinolaringológica Española. 2021/12/09/ 2021;doi: 10.1016/j.otorri.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 49.Wang CT, Fang KM, Young YH, Cheng PW. Vestibular-evoked myogenic potential in the prediction of recovery from acute low-tone sensorineural hearing loss. Ear Hear. Apr 2010;31(2):289–95. doi: 10.1097/AUD.0b013e3181c5b743 [DOI] [PubMed] [Google Scholar]
- 50.Wu CL, Young YH. Vestibular Evoked Myogenic Potentials in Acute Low‐Tone Sensorineural Hearing Loss. The Laryngoscope. 2004;114(12):2172–2175. [DOI] [PubMed] [Google Scholar]
- 51.Yakunina N, Lee WH, Ryu Y-J, Nam E-C. Sequential versus Combination Treatment Using Steroids and Diuretics for Acute Low-Frequency Sensorineural Hearing Loss: A Noninferiority Trial. Otology & Neurotology. 2019;40(3):305–311. [DOI] [PubMed] [Google Scholar]
- 52.Kim H, Lee H, Kim Y-C, et al. Update on Clinical Manifestation in Acute Low-Tone Hearing Loss. Korean Journal of Otorhinolaryngology-Head and Neck Surgery. 2020;63(9):403–408. doi: 10.3342/kjorl-hns.2019.00759 [DOI] [Google Scholar]
- 53.Finckh A, Tramèr MR. Primer: strengths and weaknesses of meta-analysis. Nature Clinical Practice Rheumatology. 2008/03/01 2008;4(3):146–152. doi: 10.1038/ncprheum0732 [DOI] [PubMed] [Google Scholar]
- 54.Lee YH. Strengths and Limitations of Meta-Analysis. Korean J Med. 10 2019;94(5):391–395. doi: 10.3904/kjm.2019.94.5.391 [DOI] [Google Scholar]
- 55.Sharpe D Of apples and oranges, file drawers and garbage: why validity issues in meta-analysis will not go away. Clin Psychol Rev. Dec 1997;17(8):881–901. doi: 10.1016/s0272-7358(97)00056-1 [DOI] [PubMed] [Google Scholar]
- 56.Zhou S, Shen C. Avoiding Definitive Conclusions in Meta-analysis of Heterogeneous Studies With Small Sample Sizes. JAMA Otolaryngology–Head & Neck Surgery. 2022;doi: 10.1001/jamaoto.2022.2847 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL DIGITAL CONTENT 1: Scoping Review Search Strategy
SUPPLEMENTAL DIGITAL CONTENT 3: Demographics and Categorical Outcomes of Patients Treated with Systemic Steroids Alone
SUPPLEMENTAL DIGITAL CONTENT 2: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist
SUPPLEMENTAL DIGITAL CONTENT 4: Demographics and Categorical Outcomes of Patients Treated with Diuretics Alone
SUPPLEMENTAL DIGITAL CONTENT 6: Demographics and Categorical Outcomes of Patients Treated with Neither Steroids nor Diuretics
SUPPLEMENTAL DIGITAL CONTENT 5: Demographics and Categorical Outcomes of Patients Treated with Systemic Steroids and Diuretics
SUPPLEMENTAL DIGITAL CONTENT 7: Demographics and Categorical Outcomes of Patients Treated with Unclear or Unspecified Interventions
SUPPLEMENTAL DIGITAL CONTENT 8: Results of Studies Evaluating Intratympanic Steroids
SUPPLEMENTAL DIGITAL CONTENT 9: Results of Studies Evaluating Non-Categorical Hearing Outcomes


