Abstract
Perivascular epithelioid cell neoplasm (PEComa) is one of the rare entities which is challenging to diagnose clinically. These tumors occur due to tuberous sclerosis complex gene mutations leading to upregulation and overexpression of the mammalian target of rapamycin (mTOR). Malignant PEComas are rare, and we report a peculiar case of PEComa treated with mTOR inhibitors. A 43-year-old woman presented with complaints of back pain, intermittent fever, dysuria, and cough with expectoration for one month. Abdominal computed tomography (CT) revealed heterogeneously enhancing exophytic mass of the left kidney. A positron emission tomography CT whole body showed a primary malignancy in the left kidney, sclerotic lesions in the bony skeleton, and lymphangitis carcinomatosis in both lungs. A biopsy of the left renal mass revealed PEComa, focally positive for melanocytic and muscle markers. She was commenced on treatment with intravenous temsirolimus, and there was a complete tumor regression by the end of the completion of six cycles.
Keywords: sarcomas, tuberous sclerosis, renal neoplasms, mtor inhibitors, epithelioid cell tumors
Introduction
Perivascular epithelioid cell neoplasms (PEComas) are mesenchymal tumors with perivascular clear cell and epithelioid features that co-expresses melanocytic and muscle markers [1]. PEComa encompasses a rare subgroup of sarcoma which include groups of tumors like angiomyolipoma (AML), clear-cell “sugar” tumor (CCST) of the lung and extrapulmonary sites, lymphangioleiomyomatosis (LAM), clear-cell myomelanocytic tumor of the falciform ligament/ligamentum teres and rare clear-cell tumors of other anatomical sites [2]. PEComas exhibit a peculiar morphology and are characterized by myomelanocytic tumor markers expression.
PEComas are seen to arise more in middle age and have a marked female predominance of 7:1 [2]. They have been seen to arise most commonly in the gynecologic tract, pelvis, and retroperitoneum and can be of varying malignant potential, but they can occur anywhere in the body. PEComas, particularly AMLs, and LAM, are among the typical physical manifestations of tuberous sclerosis complex (TSC) called Bourneville-Pringle disease [3].
These tumors have been seen to be associated with TSC, an autosomal dominant genetic disease due to losses of the genes TSC1 (9q34) or TSC2 (16p13.3), which have also been identified in PEComas [3,4]. These genetic alterations activate the mammalian target of rapamycin (mTOR) in adenosine monophosphate-activated protein kinase (AMPK) and mitogen-activated protein kinase pathways (MAPK), resulting in aberrantly high mTOR activity, which increases TSC-associated AML and PEComas [5-8]. Most PEComas are benign; however, some PEComas show malignant behavior as evidenced by size, lack of circumscription, nuclear atypia, and uncontrolled metastasis [6]. There is no universally effective therapy against PEComas today; however, mTOR inhibitors have shown efficacy in a few studies [8,9]. Hereby, we present a malignant metastatic PEComa of a kidney treated with mTOR inhibitor.
Case presentation
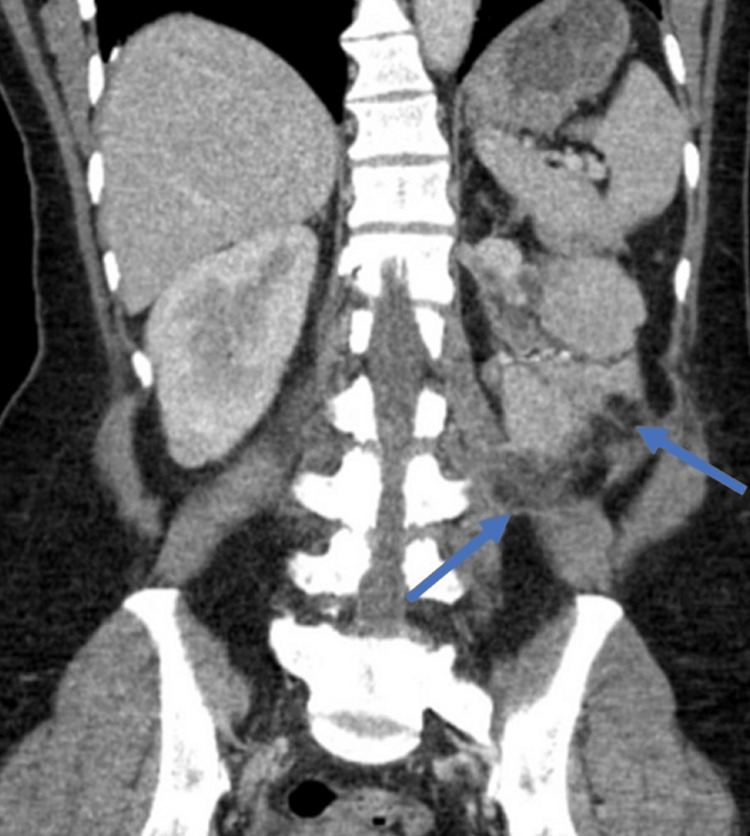
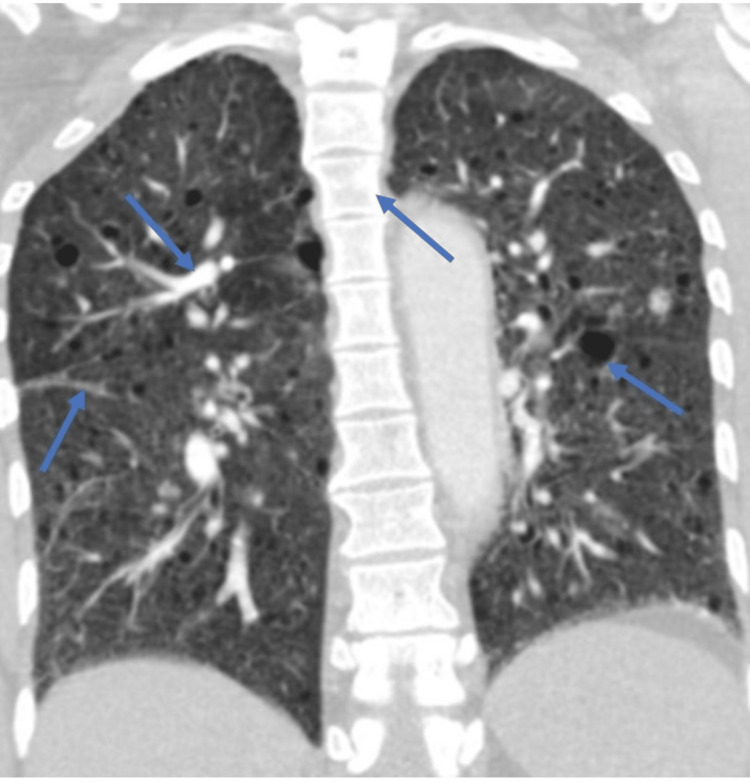
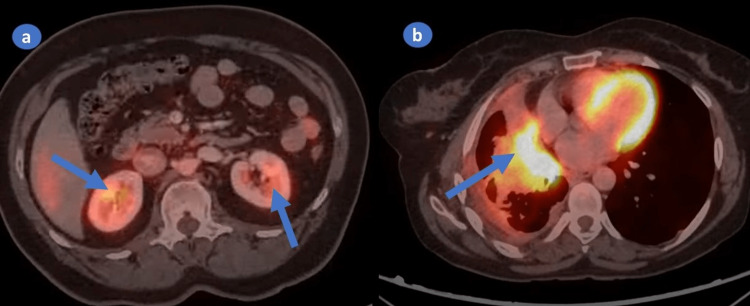
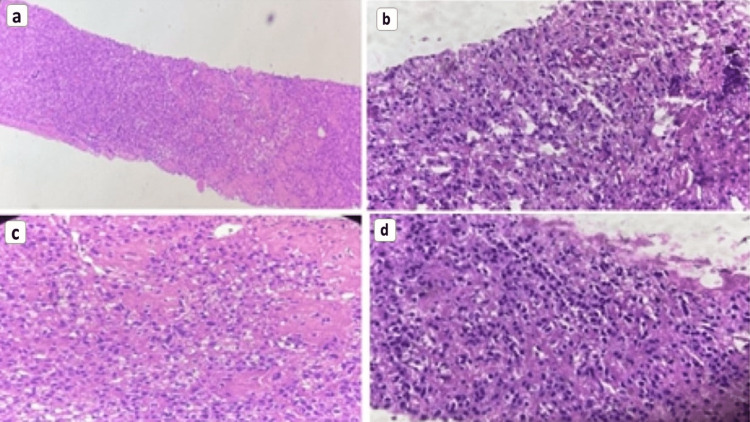
A 43-year-old woman presented to the hospital with complaints of back pain, intermittent fever, and dysuria, cough with expectoration for one month. She had no history of smoking, alcohol or substance abuse. A computed tomography (CT) abdomen was performed because of the above symptoms. It showed well-circumscribed oval to round heterogeneously enhancing exophytic mass lesions measuring approximately 76 × 35 × 68mm and 36 × 33 × 27mm with central enhancing areas involving the upper and mid pole of the left kidney, respectively (Figure 1). Multiple sclerotic lesions were noted in the dorso-lumbar spine, sacrum, pelvic bones, bilateral ribs, sternum, and slightly prominent para-aortic, external iliac, and inguinal lymph nodes. CT chest revealed multiple pulmonary nodules, ill-defined opacities, numerous thin-walled parenchymal cysts, and sclerotic lesions of the vertebral bodies (Figure 2). For further evaluation, positron emission tomography whole body (PET-WB) was done, suggesting primary malignancy in the left kidney, sclerotic lesions in the skeleton, and lymphangitic carcinomatosis in both the lungs and right kidney (Figures 3a, 3b). Biopsy from the left renal mass revealed PEComa. On biopsy, the tumor cells were focally positive for a marker of melanocytic differentiation human melanoma black 45 (HMB45), desmin, smooth muscle actin (SMA), and vimentin and negative for pan-CK (cytokeratin), paired box gene 8 (Pax8), and common acute lymphocytic leukemia antigen (CALLA/CD45); occasional lymphovascular emboli were also noted within the tumor (Figures 4a-4d).
Figure 1. CT abdomen demonstrating an enhancing lesion in the left kidney (blue arrows).
Figure 2. CT chest showing multiple pulmonary nodules, ill-defined opacities, numerous thin-walled parenchymal cysts and sclerotic lesions of the vertebral bodies (blue arrows).
CT: computed tomography.
Figure 3. PET-CT revealing enhanced tumor activity in kidneys (a) and lungs (b).
PET-CT: positron emission tomography-computed tomography.
Figure 4. Left kidney biopsy on histopathology demonstrating severe atypia, high mitotic activity, cytoplasmic pleomorphism, bundles of atypical mitotic figures (a-c), and marked coagulative necrosis (d).
Therefore, a diagnosis of malignant PEComa of the kidney with metastasis was made, and the patient was started on treatment with intravenous (IV) temsirolimus on a two-weekly schedule. Post first dose, she had a dramatic improvement in her general condition. She completed six doses of IV temsirolimus which she tolerated well. The patient responded well to the treatment administered and was completely asymptomatic after completing six cycles.
Follow-up PET-WB post-treatment showed a complete metabolic response with interval reduction in the size of lesions in the left kidney and significant reduction in the extent of lymphangitic carcinomatosis in both lungs and stable skeletal lesions, suggestive of an overall good response to the administered treatment. Following this, she was started on an oral mTOR inhibitor, tablet everolimus, 10 mg, and was kept on monthly follow-up. She developed grade-2 stomatitis, occasional headaches, hypercholesterolemia, fluctuating blood sugars, and elevated blood pressure recordings (systolic range: 110-170 mmHg) and was treated symptomatically during her follow-up.
Discussion
PEComa has been defined as “a mesenchymal tumor composed of histologically and immunohistochemically distinctive perivascular epithelioid cells.” PEComas are considered ubiquitous tumors and have been described in various organs. Criteria for classifying these tumors as “benign”, “of uncertain malignant potential”, and “malignant” have been proposed. A significant association between tumor size >5 cm, infiltrative growth pattern, high nuclear grade, necrosis, and mitotic activity >1/50 high power field with subsequent aggressive clinical behavior of PEComas has been found. PEComas are defined by the immunohistochemical (IHC) co-expression of myoid markers (SMA, desmin, caldesmon) and melanocytic markers (HMB-45, Melan-A, MiTF). Expression varies with morphology: tumors with predominant spindle cell morphology show strong expression of muscle markers and limited expression of melanocytic markers; predominantly epithelioid tumors may strongly express melanocytic markers with limited muscle marker expression. Recently, cathepsin K, a transcriptional target of the MiTF family, emerged as a sensitive marker for PEComa [10].
Malignant PEComas are rare; hence, large-volume studies have not been feasible. Consensus on the treatment of this disease has also been lacking. Prior to the availability of mTOR inhibitors, metastatic PEComas were treated with chemotherapy and vascular endothelial growth factor (VEGFR) inhibitors with varying results [11]. Acknowledgment of the usage of mTOR inhibitors first came to light by Wagner et al. 2010, who first published a report with a series of three patients diagnosed with metastatic cancer [12]. Additionally, a study done by Benson et al. on malignant PEComa from a series of 25 patients with disease types of AML and LAM treated with sirolimus for 12 months has shown promising results [13].
We have summarized the relevant studies on 79 PEComa patients treated with treatment modalities such as chemotherapy, surgery, VEGFR, and mTOR inhibitors (Table 1) [13-16]. The following studies weigh the effectiveness of various treatment options, from chemotherapy or surgery to mTOR inhibitors. So, responses from these studies need to be considered before starting treatment for rare diseases like PEComas. The median age of these patients lies between 26 and 76 years. These studies include 58 females and 21 males with the disease most commonly involving the gynecological tract, kidney, gastrointestinal tract, retroperitoneal sites, bone, trunk, and other Visceral organs. Data include 79 patients, of which 51 were treated with mTOR inhibitors, 27 with chemotherapy, 12 with VEGFR inhibitors as a second- and third-line treatment, and one underwent surgery and had been treated with sirolimus due to tumor recurrence following surgery.
Table 1. Relevant studies conducted on PEComa treatment.
NR: not reported, m: month, d: day, RECIST: response evaluation criteria in solid tumor, PR: partial response, N/A: not available, SD: stable disease, PD: progressive disease, CR: complete response, PFS: progression-free survival.
| Study | No. of patients | Treatment | Treatment duration | Response | Side effects | RECIST1.1 response | Survival rate (%) | PFS (months) | Median follow-up (months) |
| GenBenson et al. [13] | 10 | Sirolimus, temsilorimus | 7 d-45.5 m | Good (7), No response (3) | Hypertriglyceridemia, hyperglycemia, thrombocytopenia | PR (5), N/A (3), SD (1), PD (1) | 78.80% | 26 m | 21 m |
| Sanlippo et al. [14] | 53 | Chemotherapy (23), VEGFR, mTOR inhibitors (40), sirolimus (32), everolimus (5), temsirolimus (3) | 24 m | Good | NR | CR (1), PR (15), SD (14), PD (9) | 41% | 9 m | 32.5 m |
| Gennata et al. [15] | 1 | Surgery, imatinib, everolimus | 10 m | Good | Stomatitis, nasopharyngitis, acne-like skin lesions | CR | NR | >10 m | 37 m |
| Switaj et al. [16] | 15 | Sirolimus (11), chemo (4) | 25 m | Good (15) | Hypertriglyceridemia, hypercholesteremia | PR (8), SD-= (4), CR (3) | 83% (sirolimus), 65% (overall) | 42.6 m | 55.7 m |
Consequently, studies showed that patients treated with mTOR inhibitors have a good response, with survival rates ranging from 41% to 83%. The treatment duration differs from seven days to 45.5 months, with progression-free survival ranging between 4.9 months and 42.6 months, as seen in our case. The most common adverse effects seen in these studies are hypertriglyceridemia, hyperglycemia, thrombocytopenia, stomatitis, and skin lesions. So far, studies have provided firm evidence that mTOR inhibitors provide more benefits than other treatment options [13-16]. The role of surgical resection and chemo- and radiotherapy is currently poorly defined. However, locally advanced or metastatic disease portends a poor prognosis, and strategies incorporating chemotherapy, radiation, and immunotherapy have been reported. There are obvious difficulties in performing a therapeutic trial, mainly due to the rarity of the disease. Furthermore, the potential benefits of adjuvant chemotherapy have not been investigated [17]. Limited studies and other single case reports have also shown similar responses. Likewise, we treated our patient initially with IV temsirolimus followed by oral everolimus [15]. She showed an excellent response and is seen to have regressing disease on follow-up and is completely asymptomatic now.
Conclusions
PEComas are mesenchymal neoplasms with a perivascular appearance on histopathology. This case highlights a potential case of malignant PEComa treated with mTOR inhibitors. Our case also underlines the importance of early diagnosis and management to avoid significant morbidity and mortality. Although the efficacy of mTOR inhibitors has been established in treating malignant PEComa with rare side effects, further studies on a larger scale are warranted with regular follow-up.
Acknowledgments
MH drafted the manuscript, carried out the literature search, wrote the case presentation, and added a table to the case presentation with appropriate lab data. PS and NG carried out the PubMed search and extracted the relevant data and wrote the discussion with appropriate references. MS participated in writing an introduction from relevant data and drafted the abstract of the study. CT wrote down the conclusion and added the table to the discussion section with appropriate references. NA and NG added modified figures as per journal guidelines and revised the case presentation and case discussion and also participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript and collaboratively worked on the manuscript, and contributed to data curation, methodology, validation, and writing, reviewing, and editing the draft.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1."Malignant" perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Bleeker JS, Quevedo JF, Folpe AL. Sarcoma. 2012;2012:541626. doi: 10.1155/2012/541626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perivascular epithelioid cell tumor. Armah HB, Parwani AV. Arch Pathol Lab Med. 2009;133:648–654. doi: 10.5858/133.4.648. [DOI] [PubMed] [Google Scholar]
- 3.Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Int J Cancer. 2013;132:1711–1717. doi: 10.1002/ijc.27800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. Bissler JJ, McCormack FX, Young LR, et al. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Argani P, Olgac S, Tickoo SK, et al. Am J Surg Pathol. 2007;31:1149–1160. doi: 10.1097/PAS.0b013e318031ffff. [DOI] [PubMed] [Google Scholar]
- 6.A distinctive subset of PEComas harbors TFE3 gene fusions. Argani P, Aulmann S, Illei PB, et al. Am J Surg Pathol. 2010;34:1395–1406. doi: 10.1097/PAS.0b013e3181f17ac0. [DOI] [PubMed] [Google Scholar]
- 7.PEC and sugar. Bonetti F, Pea M, Martignoni G, Zamboni G. Am J Surg Pathol. 1992;16:307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Italiano A, Delcambre C, Hostein I, et al. Ann Oncol. 2010;21:1135–1137. doi: 10.1093/annonc/mdq044. [DOI] [PubMed] [Google Scholar]
- 9.Malignant PEComa. Haiges D, Kurz P, Laaff H, Meiss F, Kutzner H, Technau-Hafsi K. J Cutan Pathol. 2018;45:84–89. doi: 10.1111/cup.13061. [DOI] [PubMed] [Google Scholar]
- 10.Malignant epithelioid soft tissue tumours- a pathologist's perspective with review of literature. Dey B, Srinivas BH, Badhe B, Nachiappa Ganesh R, Gochhait D, Toi PC, Jinkala S. Cureus. 2020;12:0. doi: 10.7759/cureus.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PEComa: morphology and genetics of a complex tumor family. Thway K, Fisher C. Ann Diagn Pathol. 2015;19:359–368. doi: 10.1016/j.anndiagpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A retrospective study of patients with malignant PEComa receiving treatment with sirolimus or temsirolimus: the Royal Marsden Hospital experience. Benson C, Vitfell-Rasmussen J, Maruzzo M, et al. https://pubmed.ncbi.nlm.nih.gov/24982384/ Anticancer Res. 2014;34:3663–3668. [PubMed] [Google Scholar]
- 14.Role of chemotherapy, VEGFR Inhibitors, and mTOR Inhibitors in advanced perivascular epithelioid cell tumors (PEComas) Sanfilippo R, Jones RL, Blay JY, et al. Clin Cancer Res. 2019;25:5295–5300. doi: 10.1158/1078-0432.CCR-19-0288. [DOI] [PubMed] [Google Scholar]
- 15.Successful treatment with the mTOR inhibitor everolimus in a patient with perivascular epithelioid cell tumor. Gennatas C, Michalaki V, Kairi PV, Kondi-Paphiti A, Voros D. World J Surg Oncol. 2012;10:181. doi: 10.1186/1477-7819-10-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efficacy of sirolimus treatment in PEComa-10 years of practice perspective. Świtaj T, Sobiborowicz A, Teterycz P, et al. J Clin Med. 2021;10:3705. doi: 10.3390/jcm10163705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lack of response of a metastatic renal perivascular epithelial cell tumor (PEComa) to successive courses of DTIC based-therapy and imatinib mesylate. Rigby H, Yu W, Schmidt MH, Fernandez CV. Pediatr Blood Cancer. 2005;45:202–206. doi: 10.1002/pbc.20305. [DOI] [PubMed] [Google Scholar]