Therapeutic monoclonal antibodies (mAbs) have great therapeutic potential and have changed clinical medicine. However, repeated administrations of mAbs could induce an immune response with the production of anti-drug antibodies (ADAs) [1].
The first mAb produced for patients with hemophilia A (HA), as the first non-replacement therapeutic option, was emicizumab (Hemlibra, Roche). Emicizumab mimics the cofactor activity of activated factor VIII (FVIII) by co-binding to activated factor IX and factor X zymogen, placing them in appropriate positions on vascular sites promoting thrombin formation [2]. Weekly subcutaneous administrations of emicizumab markedly reduce the annualized bleeding rate while improving clinical management and the quality of life [3]. In the frame of the phase 3 clinical trials, ADAs have been developed in 5.1% of enrolled patients, of which 0.6% were persistently detectable and associated with lower emicizumab concentration [4]. One patient in the frame of the phase 3 clinical trials had to be withdrawn from the study due to loss of emicizumab therapeutic efficacy, and the ADA was further biochemically characterized [5]. Few additional cases with partially neutralizing ADAs and/or reduction of emicizumab concentration have been found in our center [6]. In the presence of complete neutralizing ADAs and failure of treatment, emicizumab must be stopped, returning to bypassing agents or FVIII products with intravenous administration and sometimes incomplete protection, particularly in patients with FVIII inhibitors [7].
Recently, a new FVIII-mimetic mAb, mim8, (Novo Nordisk A/S) has been developed with the goal to improve activated factor IX and factor X assembling on the procoagulant membrane surface minimally interacting with the circulating targets factors. In a HA mouse model and in vitro experiments, mim8 showed an enhanced activity over emicizumab in thrombin generation and clot formation [8].
The aim of this study was to evaluate the in vitro cross-reactivity of mim8 with the antiemicizumab antibodies in order to verify whether mim8 could be a potential alternative therapeutic option for patients who can no longer be treated with emicizumab. For these in vitro purposes, emicizumab and mim8 were kindly provided by Hoffman-La Roche and Novo Nordisk A/S, respectively.
Four patients with HA followed at the Angelo Bianchi Bonomi Hemophilia and Thrombosis Centre in Milan, who developed anti-emicizumab antibodies, were analyzed [6]. Written informed consent was obtained from patients, and the Ethics Committee of Milan Area 2 approved the data analyses and publication.
Patient 1 at the time of his fifth emicizumab dose in the frame of the phase 3 clinical trials developed fully neutralizing ADAs associated with undetectable plasma drug concentration and the occurrence of spontaneous bleeding episodes. Treatment was discontinued, and bypassing agents resumed [5].
Patient 2 at week 5 of emicizumab exposure experienced spontaneous joint bleeding concomitantly with ADA detection and a decrease in emicizumab level from 49.1 μg/mL to 31.7 μg/mL. ADAs spontaneously disappeared at week 8, and emicizumab concentration gradually increased to 50.6 μg/mL at week 25. Nevertheless, this patient experienced 5 bleeding episodes and has been switched to an FVIII product.
Patient 3 at week 10 of emicizumab exposure developed ADAs with a decrease in emicizumab concentration from 39.1 μg/mL to 28.3 μg/mL. Emicizumab plasma level reached the lowest value of 17 μg/mL at week 20 and then slowly increased up to 31 μg/mL at week 52. No bleeding occurred, and he is still on emicizumab prophylaxis.
Patient 4 developed ADAs at week 9 of exposure with a reduction in plasma emicizumab concentration from 32.3 μg/mL at week 5 to 24 μg/mL at week 18 without bleeding episodes. He is still on emicizumab therapy.
Plasma samples were collected from all patients at ADAs appearance. For patient 1, an additional sample was obtained 2 years after emicizumab discontinuation.
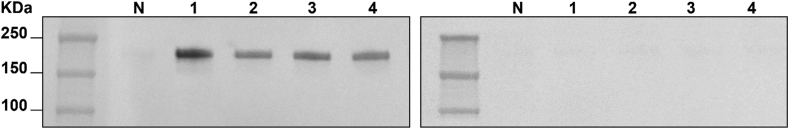
ADAs were detected in the total immunoglobulin G (IgG) fraction using the Western blot method [5]. For each sample, a fixed amount of purified material, corresponding to 2 μg of IgG, was analyzed. Two identical membranes were run; the first one was incubated and detected with biotinylated-emicizumab (bt-emi), and the second one with biotinylated-mim8 (bt-mim8), both at 1 μg/mL. All patients showed a positive result after incubation with bt-emi and a negative result after incubation with bt-mim8 (Figure 1).
Figure 1.
Western blot analysis. Purified total immunoglobulin G, 2 μg for each sample, was analyzed with Western blot. Two identical membranes were run and incubated with biotinylated-emicizumab at 1 μg/mL (left panel) and biotinylated-mim8 at 1 μg/mL (right panel). All patients bound to biotinylated-emicizumab; no binding was found to biotinylated-mim8. N represents the healthy subject; 1, 2, 3, and 4 represent the in-study patients with hemophilia A.
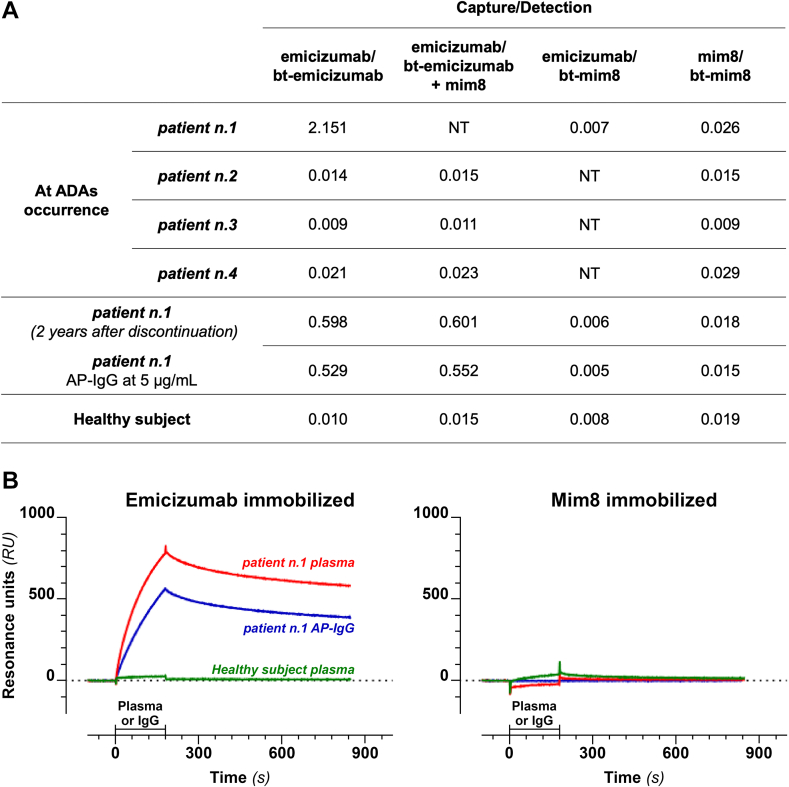
To assess the in vitro binding between ADAs and the 2 drugs, all samples were tested with an in-house enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR)–based assay. The in-house ELISA was performed using different combinations of emicizumab and mim8 in capture and detection (Figure 2A). By using emicizumab in capture and bt-emi in detection (both at 1.5 μg/mL), only samples from patient 1 and the affinity-purified IgG (AP-IgG) from the same patient had absorbance levels above that of the healthy subject. The addition of mim8 at 40 μg/mL to the bt-emi solution did not reduce the signal indicating no competition between mim8 and emicizumab for ADA binding. All samples showed absorbances as that of the healthy subject with both of the following combinations: 1) capture with emicizumab and detection with bt-mim8 and 2) capture with mim8 and detection with bt-mim8.
Figure 2.
Cross-reactivity of anti-emicizumab antibodies. (A) Enzyme-linked immunosorbent assay optical density (OD) results. By using emicizumab as capture and biotinylated (bt)-emicizumab as detection (both at 1.5 μg/mL), high absorbances were found with the 2 plasma samples from patient 1 and with the affinity-purified (AP) immunoglobulin G (IgG) (at 5 μg/mL) from the same patient but not with patients 2, 3, and 4 at anti-drug antibody (ADAs) occurrence. The addition of mim8 at 40 μg/mL to the bt-emicizumab solution did not change the binding signal. By replacing bt-emicizumab with bt-mim8, no positive sample was found. By using mim8 as capture and bt-mim8 as detection, all samples had values similar to that of the healthy subject. The 2 plasma samples from patient 1 were tested at 1:20 dilution; plasma from patients 2, 3, and 4 and from the healthy subject were tested at 1:10 dilution. (B) Surface plasmon resonance (SPR) analysis. The AP-IgG were tested at 2.7 μg/mL, plasma sample from patient 1 at ADA occurrence, and from a healthy subject at 1:40 dilution. Samples flowed from time 0 to 180 seconds over immobilized emicizumab (left panel) or mim8 (right panel). Sensorgrams show that plasma from patient 1 and the AP-IgG bind emicizumab but not mim8. The specific SPR signals were obtained after subtraction of the SPR signals measured in a parallel surface of the same chip coated with control IgG. NT, not tested.
The SPR analysis was performed using the ProteOn XPR36 Protein Interaction Array System (Bio-Rad Laboratories) apparatus. Emicizumab, mim8, and normal IgG were covalently immobilized on 3 parallel strips on the same sensor chip (GLC, Bio-Rad) at 6000 resonance units where 1000 resonance units = 1 ng/mm2 [9]. Before injection, the AP-IgG was diluted at 2.7 μg/mL, and the plasma of patient 1 was collected at ADA occurrence at a final dilution of 40-fold after acidic pretreatment. Samples flowed over the sensor chip for 3 minutes at a flow rate of 30 μL/min. All SPR assays were run at 25 °C at a rate of 30 μL/min. The time course of the SPR signal (sensorgrams) showed that the AP-IgG and the plasma of patient 1 bound to the immobilized emicizumab but not to the immobilized mim8 (Figure 2B). Patients 2, 3, and 4 showed overlapping sensorgrams with the normal control (data not shown).
The anti-emicizumab antibodies were not detected in plasma samples collected at ADA appearance from patients 2, 3, and 4 with both the ELISA and the SPR method. In our opinion, detection could depend on the low drug tolerance of the assays and the relative amounts of drugs and ADAs circulating.
It is plausible that when ADA levels do not overcome the drug levels, all the antibodies are likely engaged in immune complexes and are poorly accessible even after the acid dissociation. When the immune response is strong, as for patient 1, ADA levels overcome the drug level leaving enough free antibodies to be easily detected in plasma.
Although our study is limited by the low number of patients, we found that the mim8 molecule could escape the anti-emicizumab antibodies. This suggests that patients who failed the treatment using emicizumab for ADA occurrence and need to resume the conventional therapies might benefit from mim8 maintaining a similar therapeutic approach without returning to intravenous infusion.
Further investigation will clarify whether mim8 could be a good candidate for patients with HA who develop ADAs associated with low drug efficacy. Moreover, only real-life experience will help determine which minimum concentration of emicizumab leads to increased risk of bleeding in the event of ADAs, justifying switching to other products.
Acknowledgments
The authors gratefully acknowledge Prof Pier Mannuccio Mannucci for the critical revision of the manuscript and Dr Luigi Flaminio Ghilardini for his help in preparing the figures.
Funding
This work was partially supported by the Italian Ministry of Health, Bando Ricerca Corrente 2023. The Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico is a member of the European Reference Network EuroBloodNet.
Author contributions
R.G., C.V., and F.P. conceived the study and wrote the manuscript. R.G., S.A., A.C., and S.M.S. managed the patients. C.V., L.S., M.B., and M.G. performed the analysis and the data collection. All authors reviewed the data and revised the manuscript.
Relationship Disclosure
F.P. is a member of the advisory boards of Sanofi, Sobi, Roche, BioMarin, and CSL Behring; reports consulting fees from CSL Behring, BioMarin, Roche, Sanofi, and Sobi; and reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Takeda/Spark. R.G. is a member of the advisory boards of Roche, Takeda, Bayer, Pfizer, and Novo Nordisk. A.C. is a consultant for Bayer, reports Bayer Hemophilia Awards Fellowship, reports support for attending meetings and/or travel from Bayer and Sobi, is a member of the advisory board of Bayer. C.V., S.A., S.M.S., L.S., M.B., and M.G. have no competing interests to disclose.
Footnotes
Handling Editor: Dr Suely Rezende
References
- 1.Vaisman-Mentesh A., Gutierrez-Gonzalez M., DeKosky B.J., Wine Y. The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front Immunol. 2020;11:1951. doi: 10.3389/fimmu.2020.01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shima M., Hanabusa H., Taki M., Matsushita T., Sato T., Fukutake K., et al. Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374:2044–2053. doi: 10.1056/NEJMoa1511769. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan M.U., Negrier C., Paz-Priel I., Chang T., Chebon S., Lehle M., et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies. Blood. 2021;137:2231–2242. doi: 10.1182/blood.2020009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt C., Emrich T., Chebon S., Fernandez E., Petry C., Yoneyama K., et al. Low immunogenicity of emicizumab in persons with haemophilia A. Haemophilia. 2021;27:984–992. doi: 10.1111/hae.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valsecchi C., Gobbi M., Beeg M., Adams T., Castaman G., Schiavone L., et al. Characterization of the neutralizing anti-emicizumab antibody in a patient with hemophilia A and inhibitor. J Thromb Haemost. 2021;19:711–718. doi: 10.1111/jth.15226. [DOI] [PubMed] [Google Scholar]
- 6.Arcudi S., Gualtierotti R., Marino S., Nicolò G., Biguzzi E., Ciavarella A., et al. Real-world data on emicizumab prophylaxis in the Milan cohort. Haemophilia. 2022;28:e141–e144. doi: 10.1111/hae.14630. [DOI] [PubMed] [Google Scholar]
- 7.Mannucci P.M. Hemophilia therapy: the future has begun. Haematologica. 2020;105:545–553. doi: 10.3324/haematol.2019.232132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Østergaard H., Lund J., Greisen P.J., Kjellev S., Henriksen A., Lorenzen N., et al. A factor VIIIa-mimetic bispecific antibody, Mim8, ameliorates bleeding upon severe vascular challenge in hemophilia A mice. Blood. 2021;138:1258–1268. doi: 10.1182/blood.2020010331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beeg M., Nobili A., Orsini B., Rogai F., Gilardi D., Fiorino G., et al. A surface plasmon resonance-based assay to measure serum concentrations of therapeutic antibodies and anti-drug antibodies. Sci Rep. 2019;9:2064. doi: 10.1038/s41598-018-37950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]