Abstract
In a long-term rodent bioassay evaluating the carcinogenicity of triethanolamine, there was equivocal evidence of carcinogenic activity in male B6C3F1 mice, based on a marginal increase in the number of hepatocellular adenomas and hepatoblastomas. Interpretation was complicated by the presence of Helicobacter hepaticus in selected silver-stained liver sections which also had histological evidence of karyomegaly and oval cell hyperplasia. An increase in numbers of liver tumors, as evidence of carcinogenic activity, was also noted in female mice. However, H. hepaticus was not considered a complicating factor, because the livers of the female mice did not have histological features compatible with H. hepaticus infection. A retrospective analysis of 51 liver tissue samples from the original carcinogenicity study was conducted to determine the incidence of H. hepaticus infection and to evaluate different diagnostic approaches for assessing the presence of H. hepaticus in livers lacking characteristic lesions. In an initial evaluation of seven mice with liver tumors, argyrophilic bacteria resembling H. hepaticus were observed in liver sections, associated with characteristic liver lesions of hepatocytic karyomegaly and oval cell hyperplasia. Frozen liver tissue was available from four of these mice; all were confirmed to be infected with H. hepaticus by culture and PCR. In a larger subsequent analysis using frozen liver tissues from 44 mice without characteristic hepatic lesions, H. hepaticus-specific DNA was amplified from the livers of 21 of 44 of the mice (47%), compared to 14 of 44 of the mice (32%) having H. hepaticus cultured from their frozen liver tumors. The results of H. hepaticus culture and H. hepaticus-specific PCR concurred (i.e., both positive and negative results) in 84% of the cases. Microscopic detection of immunofluorescence-labeled or silver-stained bacteria in liver sections was relatively insensitive compared to either culture or PCR detection. This study confirms the widespread prevalence of H. hepaticus in mice, its potential to confound experimental results, and the need to include diagnostic testing for H. hepaticus in a murine health monitoring program.
Helicobacter hepaticus, a newly described murine pathogen, is now known to cause persistent hepatitis in a variety of outbred and inbred mice. In one inbred strain, A/JCr, the infection is associated with development of hepatoma and hepatocellular carcinoma (4, 16). Chronic infection and associated hepatic lesions have also been observed in the B6C3F1 hybrid mouse strain, which is commonly used for in vivo toxicologic evaluations. The occurrence of lesions in this strain appears to have a later onset than that observed in other more susceptible strains (e.g., strain A mice). Recently H. hepaticus infection was suggested by histologic criteria in selected mice used in a toxicopathologic evaluation of triethanolamine (TEA), directed by the National Toxicology Program (NTP) (10).
TEA is widely used (in low levels) as an ingredient in industrial settings in alkalizing agents, in cosmetic products, as a chemical intermediate for anionic and nonionic surfactants, and in surface-active agents in household cleaning agents and herbicides and other products. The most widespread potential dermal human exposure to ethanolamines occurs through the use of cosmetics (1), although exposure also can result from contact with household detergents, other surfactants containing this compound, pharmaceutical ointments, cutting fluids, adhesives, and sealants. Although previous studies indicated that TEA was not carcinogenic in rodents (1, 9), questions about the oncogenic potential of TEA were raised following a report citing a slight increase in the incidence of lymphoma and total number of malignant neoplasms in female ICR-JCL mice receiving 0.03 or 0.3% TEA in their diet (8).
Because of the potential for widespread human exposure to the chemical and possible exposure of industrial workers, TEA was selected by the NTP for a 2-year chronic exposure carcinogenesis bioassay using Fischer 344 rats and B6C3F1 mice. Dermal application was chosen as the route of exposure to mimic the principal means of human exposure to TEA and because considerable systemic exposure is achieved with this route. Following completion of the study, lesions consistent with H. hepaticus infection in A/JCr male mice (i.e., those displaying karyomegaly and oval cell hyperplasia) were noted in the livers of some male B6C3F1 mice. In addition, an increase in hepatocellular carcinomas was also noted in both male and female mice and the incidence of these tumors was most elevated in the groups treated with the highest dose of TEA (10).
It is conceivable that the interpretation of hepatic lesions in chronic chemical exposure studies using H. hepaticus-infected B6C3F1 mice may be complicated by the persistent bacterial infection. The purpose of this study therefore was to (i) perform definitive tests to ascertain if H. hepaticus was present in the livers of the mice used for the TEA carcinogenicity bioassay, (ii) evaluate several alternative diagnostic methods for determining the presence of the organism, and (iii) determine if the presence of H. hepaticus could be identified in the livers of animals that lacked the characteristic nonneoplastic liver lesions, previously associated with H. hepaticus infection in male A/JCr mice (5, 16).
MATERIALS AND METHODS
The fifty-one mouse livers evaluated in this study were obtained from an NTP-sponsored study evaluating dermal exposure to TEA (10). The total incidences of primary hepatocellular neoplasms in the high-dose groups (i.e., males receiving 2,000 mg of TEA per kg of body weight and females receiving 1,000 mg of TEA per kg) were significantly greater than in sex-matched control groups (10). Overall, 42 of 50 (84%) males in the high-dose group had liver tumors, compared with 31 of 50 (62%) of the controls. A similar trend of increasing tumor incidence was also observed in female mice, in which 41 of 50 (82%) of the high-dose female group had liver tumors, compared to 23 of 50 (46%) of the controls. In both male and female test groups, the increased tumor incidences were due chiefly to increased incidences of hepatocellular adenomas in the high-dose groups (male, 54%; female, 44%). Furthermore, the numbers of mice with multiple adenomas were significantly greater in each of the high-dose groups than in the respective control groups. With regard to other primary liver tumors, hepatoblastomas were present in three male mice (6%) in the high-dose group but were not observed in the control male group and hepatocellular carcinomas were observed in five female mice in the high-dose group (10%) in comparison to only one control female (2%).
Confirmatory assays for detecting H. hepaticus were conducted on liver tissue from B6C3F1 mice used in the chronic TEA exposure study. Tissues were kindly supplied by the NTP. For the phase I evaluation, seven mice were selected from the control and treatment groups. For the phase II evaluations, 44 mice, which had hepatic neoplasms and a concurrent lack of histologic lesions suggestive of H. hepaticus infection, were selected from the high-dose treatment group. The presence of H. hepaticus in the liver specimens was assessed by microbiologic culture, PCR amplification, and microscopic evaluation of tissue.
Confirmation of H. hepaticus infection. (i) H. hepaticus culture.
In phase I, five frozen liver samples were analyzed by microaerobic culture; four liver tumors were initially selected from four male mice which had been previously diagnosed with chronic active hepatitis, hyperplasia, and karyomegaly. An additional frozen tumor from a female mouse liver was included because the mouse was considered negative for H. hepaticus, based on the absence of characteristic H. hepaticus-associated hepatitis and lack of organisms on review of silver-stained liver sections. In phase II of the study, frozen liver tumors from 19 males and 25 females were cultured for H. hepaticus and PCR was performed on the tissues.
Procedures developed in our laboratory and previously described were used to culture and identify H. hepaticus (3, 4, 13). H. hepaticus was isolated from livers by streaking liver homogenates onto trimethoprim-vancomycin-polymyxin blood agar plates (Remel Labs, Lenexa, Kans.) and incubating the plates in vented jars evacuated at a pressure of 63 cm of Hg and refilled under microanaerobic conditions with a gas mixture containing 90% N2, 5% H2, and 5% CO2. Cultures were incubated at 37°C for 5 days. H. hepaticus was characterized by typical colony morphology, Gram stain reaction, and urease, catalase, and oxidase activities as previously described (3, 4). Cultures were held for 3 weeks to verify a negative status.
(ii) PCR.
Analysis of frozen liver specimens from phase I and phase II of the study by PCR followed protocols previously described and published (13). Briefly, DNA was extracted from frozen mouse liver tissue or bacteria. Approximately 15 mg of tissue was homogenized to uniformity with a plastic, microcentrifuge-adapted pestle. Tissues or bacterial cultures were then processed by using the Rapid Prep Genomic DNA kit as outlined by the manufacturer (Pharmacia Biotech, Piscataway, N.J.). DNA pellets were dissolved in 100 μl of double-distilled H2O. Forty microliters of a 50% Chelex 100 solution (Bio-Rad, Hercules, Calif.) was added. The samples were incubated at 56°C for 30 min and subsequently heated at 94°C for 10 min. The samples were centrifuged at 12,000 × g for 5 min. The primer sequences chosen for PCR amplification recognized a region of the 16S rRNA gene specific for H. hepaticus. These two oligonucleotides, 5′ GCA TTT GAA ACT GTT ACT CTG 3′ and 5′ CTG TTT TCA AGC TCC CC 3′, produced an amplified product of 417 bp. Twenty microliters of the DNA preparation was added to a 100-μl (final volume) reaction mixture containing 1× Tth polymerase buffer (supplied by the manufacturer but supplemented with 1 M MgCl2 to a final concentration of 2.75 mM), a 0.5 μM concentration of each of the two primers, a 200 μM concentration of each deoxynucleotide, and 200 μg of bovine serum albumin per ml. Samples were heated at 94°C for 4 min, briefly centrifuged, and cooled to 61°C. Then, 3.2 U of Tth polymerase (Pharmacia) and 1.25 U of polymerase enhancer (Perfect Match; Stratagene, La Jolla, Calif.) were added, followed by an overlay of 100 μl of mineral oil. The following conditions were used for amplification: denaturation at 94°C for 1 min, annealing at 61°C for 2 min, and elongation at 72°C for 2 min. A total of 35 cycles were performed and were followed by an elongation step of 7 min at 72°C. Ten to 15 μl of the sample was then electrophoresed through a 6% Visigel separation matrix (Stratagene); this was followed by ethidium bromide staining and viewing by UV illumination.
(iii) Histology.
Formalin-fixed, paraffin-embedded liver specimens from the 51 mice analyzed in this study were used for histologic evaluation; these represented all the mice for which there were frozen specimens of liver tumors. Fixed specimens consisted of samples from each of three different hepatic lobes. Sections of formalin-fixed, paraffin-embedded liver (5 μm thick) were stained with hematoxylin and eosin (H&E) stain for histopathologic evaluation, and Warthin-Starry silver stain was used to identify the presence of H. hepaticus. The H&E-stained liver sections were evaluated for histologic lesions on a blind basis by pathologist 1. In the Warthin-Starry-stained sections, tissues identified as H. hepaticus positive were identified based on the characteristic slender, curved, argyrophilic bacterial forms within interhepatocytic canalicular sites. The presence of argyrophilic bacterial forms, consistent with H. hepaticus, was assessed independently and in a blind fashion by two pathologists. The evaluation of some sections was complicated by spurious staining of bile canaliculi. In most areas this artifactual pattern was readily discerned by the longer and wider argyrophilic deposits, relative to the pattern of staining associated with H. hepaticus. However, occasionally slender argyrophilic deposits consistent in size with H. hepaticus were observed in areas with obvious canalicular staining. Pathologist 1 evaluated liver sections excluding 13 cases which had excessive artifactual canalicular staining. Pathologist 2 evaluated all liver sections including areas with canalicular staining.
(iv) Immunofluorescence staining of mouse livers.
Livers were processed for immunofluorescence staining using polyclonal anti-H. hepaticus rabbit antisera as previously described (5). Tissue sections were deparaffinized and rehydrated through xylene and ethanol to water. The slides were incubated with 0.05% pronase (Sigma catalog no. P 5147) for 30 min at 37°C and washed with phosphate-buffered saline (PBS) for 5 min. The tissue sections were covered with either rabbit preimmune serum or postimmune serum to H. hepaticus whole-cell sonicate extract (both 1:100 in PBS) and incubated for 60 min at 37°C in a humid atmosphere. Slides were then washed twice in PBS (5 min each time), incubated for 30 min with anti-rabbit immunoglobulin G-fluorescein isothiocyanate conjugate (1:50 in PBS; Sigma catalog no. F 0511) at 37°C, and rinsed in PBS for 5 min. The slides were mounted with coverslips and sealed with buffered glycerol. Slides were examined with a Zeiss fluorescence microscope (5).
Comparison of sensitivity and specificity of H. hepaticus diagnostic tests.
In phase II, the presence of H. hepaticus in each of forty-four liver samples was evaluated by four independent techniques: culture, PCR, immunofluorescence, and histologic assessment of Warthin-Starry-stained sections. The results of the battery of tests for each specimen were used to calculate the sensitivity and specificity of each test. Because these calculated values vary based on a benchmark test used for comparison, the performance of each test was calculated by using the results of culture, PCR, and immunofluorescence as independent benchmarks. The sensitivity and specificity of each technique and the concurrence of the two techniques under comparison were then calculated.
RESULTS
Confirmation of H. hepaticus infection. (i) Culture.
All five frozen mouse livers analyzed in phase I were positive for H. hepaticus by culture. Organisms were gram negative, motile, 1.5 to 5 μm long, and 0.2 to 0.3 μm wide. The bacteria were oxidase, catalase, and strongly urease positive (Table 1). A female mouse with an adenoma in the liver was positive for H. hepaticus by culture and PCR, although karyomegaly or oval cell hyperplasia was not observed in histologic sections of liver and H. hepaticus was not observed in silver-stained sections of liver (Table 1).
TABLE 1.
Phase I diagnosis of H. hepaticus in B6C3F1 mice
| Animal no. | Groupa | Warthin-Starry staind | Liver tumor(s) | Oval cell hyperplasia/karyomegalyb | Hepatitis | Result of culture and PCR with frozen liver |
|---|---|---|---|---|---|---|
| 181 | Hm | ++++ | Adenoma | 3/3 | Chronic active | —f |
| 210 | Hm | +++ | Carcinoma, adenoma | 3/3 | Chronic active | — |
| 217 | Hm | ++++ | Multiple adenoma | 2/3 | Chronic active | Positivec |
| 121 | Mm | ++++ | Multiple carcinoma, multiple adenoma | 2/3 | Chronic active | Positive |
| 31 | Cm | +++ | Multiple carcinoma, multiple adenoma | 2/3 | Chronic active | Positive |
| 32 | Cm | +++ | Carcinoma, multiple adenoma | 2/2 | Chronic active | Positive |
| 246 | Cf | − | Adenoma | −/−e | No significant lesions | Positive |
H, high dose (2,000 mg of TEA/kg); M, medium dose (630 mg of TEA/kg); C, control; m, male; f, female.
Based on three different sections of liver tissue.
H. hepaticus positive by culture and confirmed by PCR.
Cells stained extremely strongly (++++) or strongly (+++) for H. hepaticus or failed to stain (−).
All samples were negative for oval cell hyperplasia and karyomegaly.
—, no tissue.
In phase II, H. hepaticus was cultured from some of the frozen mouse liver tumor specimens from the additional 44 mice surveyed, all of which lacked any evidence of karyomegaly or oval cell hyperplasia (10). Of the female mice, 7 of 25 (28%) had H. hepaticus recovered from their livers, and 7 of 19 (37%) of the male livers were H. hepaticus positive by culture. Higher rates of H. hepaticus identification among the male mice were also observed by using each of the other methods (Table 2). H. hepaticus organisms cultured from livers of mice in both phase I and II were confirmed by PCR using H. hepaticus-specific primers (4).
TABLE 2.
H. hepaticus in NTP high-dose TEA study using B6C3F1 micea
| Sex | No. (%) of mice positive for H. hepaticus by:
|
|||
|---|---|---|---|---|
| Warthin-Starry stainingb | FA assay | Culture | PCR | |
| Female (n = 25) | 11 (44) | 8 (32) | 7 (28) | 11 (44) |
| Male (n = 19) | 12 (63) | 9 (47) | 7 (37) | 10 (53) |
Mice without characteristic liver lesions of oval cell hyperplasia and karyomegaly.
Cumulative value based on one or both pathologists’ interpreting slides as positive.
(ii) PCR.
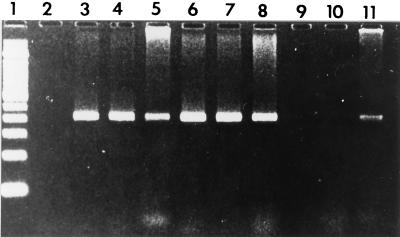
In phase I, a PCR product specific for H. hepaticus DNA was amplified from all five H. hepaticus isolates and from DNA extracted from the H. hepaticus-positive livers (Table 1). In the second phase of the study, H. hepaticus-specific PCR products were amplified from the livers of 11 of 25 (44%) female mice and from 10 of 19 (53%) male mice (Table 2; Fig. 1).
FIG. 1.
Electrophoresis of DNA amplified by PCR on a 6% Visigel separation matrix with 16S rRNA primers specific for H. hepaticus. Lane 1, 100-bp marker; lane 2, reagent control; lane 3, H. hepaticus positive control; lanes 4 to 8 and 11, DNA extracted from bacteria isolated from liver and from mouse liver samples positive for H. hepaticus; lanes 9 and 10, DNA extracted from mouse liver samples negative for H. hepaticus.
Concurrence of both positive and negative results by culture and PCR was noted in 84% (Table 3) of the cases and was observed to be independent of sex (data not shown). Comparing culture and PCR, with H. hepaticus culture results serving as the benchmark, the sensitivity was 100%, indicating all culture-positive livers were also classified as PCR positive. The calculated specificity value was 77%, affected by eight additional animals which were PCR positive but culture negative for H. hepaticus. Conversely, when PCR results were used as the benchmark for the performance of the culture technique, positive culture results were obtained for 67% of PCR-positive livers. All PCR-negative animals were confirmed as culture negative.
TABLE 3.
Comparison of sensitivity, specificity, and concurrence of H. hepaticus diagnostic tests in NTP high-dose TEA study using B6C3F1 mice
| Test or observer | No. of positive mice/total no. of mice | Comparison with the following H. hepaticus-specific benchmark test:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Culture
|
PCR
|
Immuno- fluorescence
|
||||||||
| Sa | SPb | Cc | S | SP | C | S | SP | C | ||
| Culture | 14/44 | 67 | 100 | 84 | 47 | 78 | 66 | |||
| PCR | 21/44 | 100 | 77 | 84 | 65 | 63 | 64 | |||
| Immunofluoresence | 17/44 | 57 | 70 | 66 | 52 | 74 | 64 | |||
| Pathologist 1d | 7/31 | 50 | 90 | 77 | 31 | 87 | 58 | 33 | 84 | 65 |
| Pathologist 2 | 21/44 | 57 | 57 | 57 | 52 | 57 | 55 | 53 | 56 | 55 |
Percent sensitivity compared to benchmark test.
Percent specificity compared to benchmark test.
Percent concurrence between two tests.
Values in this row are adjusted for the number of Warthin-Starry slides (13) set aside by pathologist 1 as unreadable based on confounding stain artifacts.
(iii) H. hepaticus FA assay.
The fluorescent antibody (FA) assay for demonstrating H. hepaticus had a reduced but similar correspondence with both culture and PCR (Table 3), with calculated sensitivities of 57 and 52%, respectively. Comparative specificities were slightly higher, at 70 and 74%, respectively.
(iv) Histologic evaluation.
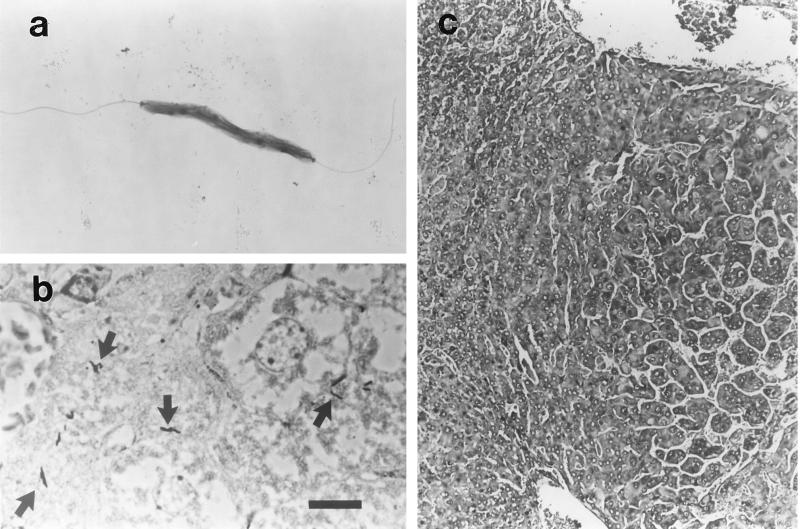
In phase I, organisms compatible with H. hepaticus were seen histologically in six of the seven mouse livers initially analyzed (Table 1). H. hepaticus organisms were identified in silver-stained liver sections by their curved to spiral shape and their location between hepatocytes (Fig. 2).
FIG. 2.
(a) Electron micrograph of Helicobacter hepaticus demonstrating bipolar flagella. (b) Warthin-Starry-stained sample. Argyrophilic, slender, curved bacterial rods characteristic of H. hepaticus are numerous and oriented along hepatocyte plasma membranes (arrows) within the bile canaliculi. The liver illustrated here contained many bacteria juxtaposed to two hepatocellular adenomas. Bar = 10 μm. (c) H&E-stained sample of hepatocellular carcinoma from an H. hepaticus-infected male mouse treated with TEA. The neoplastic focus is compressing the adjacent hepatic parenchyma at its margin. Within the tumor, atypical hepatocytes form irregular trabeculae.
Livers from 19 males and 25 females which had been previously examined histologically and had no karyomegaly or oval cell hyperplasia were examined further in phase II of the study. There was 68% concurrence between the two pathologists in the evaluation of Warthin-Starry-stained liver sections when the 31 cases evaluated by pathologist 1 were considered. Pathologist 1 classified seven livers as H. hepaticus positive, whereas pathologist 2 concurred on five of the positive cases, all of which were from male mice. Among the 13 animals excluded from evaluation by pathologist 1 because of interfering staining artifacts, pathologist 2 classified eight additional mice as positive. On a cumulative basis, Helicobacter-like organisms were seen in 23 of 44 livers. Although this number of positive classifications is similar to the number of PCR-positive cases, the association between histologic classification and PCR amplification results was weak.
Comparison of the histologic evaluations by both pathologists to the culture and PCR results did not demonstrate any strong associations, with the exception of a low percentage of false positives by pathologist 1. The specificity of histologic evaluation by pathologist 1 was 90 or 87% compared to culture or PCR results, respectively. The sensitivity of determining the presence of H. hepaticus by either pathologist was not high. Based on comparison to culture or PCR results, the sensitivity of histologic evaluation by pathologist 1 was 50 or 31%, respectively. The sensitivity of histologic evaluation by pathologist 2 was slightly higher; however, the associated specificity of this series of evaluations was diminished to 57% compared to either culture or PCR. The latter effect was associated with the inclusion of the cases with artifactual staining, which was anticipated to increase the rate of false positives.
Evaluation of karyomegaly and oval cell hyperplasia as predictors of H. hepaticus infection.
In a small group of animals analyzed, the presence of histopathologic liver lesions corresponding to H. hepaticus infection was a good indicator of H. hepaticus infection as determined by PCR and culture. However, the absence of these lesions corresponded poorly with the absence of the bacteria. In phase I of the study, all six livers evaluated that had lesions characteristic of H. hepaticus infection had H. hepaticus present in Warthin-Starry-stained liver sections. Frozen liver tumors from five of these mice (including the female mouse without characteristic H. hepaticus hepatitis) were confirmed positive for H. hepaticus by culture. In phase II, of the 44 mice analyzed without characteristic oval cell hyperplasia and karyomegaly, 21 were positive for H. hepaticus by PCR using species-specific H. hepaticus primers. Among these, 19 of the positive PCR results were supported by at least one other test method. The two animals found to be positive solely by PCR were both female mice.
DISCUSSION
An analysis of our findings indicates that several diagnostic approaches, beyond screening for characteristic hepatic lesions, should be utilized to rule out widespread infection and the potential confounding effects of H. hepaticus in long-term carcinogenesis assays. Indeed, in a retrospective analysis performed by the NTP, the presence of characteristic liver lesions was not a prerequisite for identification of H. hepaticus in B6C3F1 mice (7). Based upon limited PCR results, the NTP also concluded that both female and male mice analyzed without characteristic liver lesions had liver tumors positive for H. hepaticus (7). In our retrospective analysis, based on a combination of both culture and PCR analyses, H. hepaticus was cultured from all five frozen liver tumor samples from phase I of the study. In the second phase of the retrospective study, H. hepaticus was also identified by culture in several male and female mice and by PCR from a significant number of animals, 11 of 25 (44%) and 10 of 19 (53%) female and male B6C3F1 mice, respectively. Among these 21 PCR-positive cases, 19 of the positive PCR results were supported by at least one other test method, corroborating the validity of the PCR results. The two cases found to be positive solely by PCR were both female mice, which may have been colonized at relatively low bacterial densities. In studies performed with both A/JCr male and female mice as well as outbred germfree female mice experimentally infected with H. hepaticus, H. hepaticus status was ascertained by culture and/or PCR (5, 6). In these two studies, neither the presence of karyomegaly and oval cell hyperplasia nor visualization of spiral organisms in liver by silver staining was an accurate biomarker of H. hepaticus infection (5, 6). Our results also clearly demonstrate that even though the livers of mice from the second phase of our study did not have any karyomegaly or oval cell hyperplasia, they were infected with H. hepaticus. It is evident that the absence of these particular liver lesions corresponds poorly with the absence of infection, and therefore high rates of H. hepaticus infection in mice may be present in the absence of characteristic liver lesions. In fact, our analyses probably underestimated the rate of infection among the mice tested. For each mouse, the analyses were performed on a single sample of neoplastic tissue which was not representative of the liver as a whole. Previous histological observations indicate that tumorous tissue has fewer H. hepaticus organisms than nonneoplastic liver tissue (5, 15). Thus, further analysis of additional liver tissue, without tumors, may have increased our overall detection of H. hepaticus infection.
The presence of reticulum fibers and other argyrophilic structures within mouse livers makes the identification of H. hepaticus difficult, especially in the absence of numerous organisms. The latter was recognized as a complication in evaluating both female and male B6C3F1 mice in this study. In our phase II evaluation of the 44 mice from the TEA study, neither use of special silver stains to detect H. hepaticus nor specific histological markers were consistent predictors of infection. Detection of argyrophilic bacteria in our study was the least effective compared to the other approaches, was solely based on microscopic morphology, and cannot be regarded as specific. Specific microscopic detection was performed through the use of immunofluorescent reagents; however, sensitivity was not substantially improved compared to evaluation of Warthin-Starry-stained sections. The deficiency in sensitivity of immunofluorescence may be rectified by the adaptation of immunohistochemical detection techniques.
H. hepaticus infection in B6C3F1 mice is likely to be persistent in nature, as it is in A/JCr mice; this undoubtedly contributed to the spread of the bacteria within the mice in the NTP bioassay. In a longitudinal study of H. hepaticus infection in A/JCr mice, H. hepaticus efficiently colonized the ceca and colons of virtually 100% of both male and female mice on a persistent basis and was routinely cultured from livers of mice of both sexes throughout the study (5). Furthermore, germfree outbred female Swiss mice monocontaminated with H. hepaticus develop persistent H. hepaticus colonization in the intestine as well as chronic hepatitis (6). Thus, it is likely that a high percentage of the B6C3F1 mice were also persistently infected with H. hepaticus throughout the 2-year study.
Because of the recent findings of H. hepaticus in B6C3F1 mice in NTP studies, the NTP initiated a retrospective analysis of 76 2-year bioassay studies which commenced between October 1988 and January 1991. Nine of the 76 mouse studies analyzed were identified as having mice with liver lesions compatible with H. hepaticus-associated hepatitis (predominantly in males) (7). By using silver stains, H. hepaticus-like bacteria were observed in livers of mice in eight of these studies. H. hepaticus was confirmed in four of the nine studies (one being the TEA study) using a PCR-restriction fragment length polymorphism-based assay. Of 67 additional studies evaluated commencing before 1988 or after 1991, similar lesions have not been identified. However, the report cites three other studies which found H. hepaticus-positive animals without characteristic liver lesions; these studies were from an earlier or later time point, but detailed analyses of them have not been completed. Importantly, neoplasms of the liver (hepatocellular and hemangiosarcoma) were significantly increased (P < 0.05) in the control groups of male mice from the 9 affected studies compared to the 26 unaffected studies analyzed (7).
Due to the known carcinogenic effect of H. hepaticus in male A/JCr mice and the confounding effect of intercurrent H. hepaticus persistent infection in the TEA-treated and control mice, it is our opinion that the interpretation of carcinogenic activity of TEA in male (and perhaps female) B6C3F1 mice is not possible (10). Mechanisms to explain the carcinogenic potential of H. hepaticus are under active study (2, 5, 14). The epidemiological data linking H. pylori to gastric adenocarcinoma and gastric mucosa-associated lymphoid tumors make studies of H. hepaticus carcinogenesis particularly relevant to understanding basic mechanisms of bacterially induced tumorigenesis in humans (11, 12).
In summary, because of the demonstrated potential of H. hepaticus to confound experimental results in this study and others (7, 15) and its widespread prevalence in mouse colonies (13), we recommend the inclusion of diagnostic screening for H. hepaticus in all murine health surveillance programs. Based on the comparisons described here, culture and PCR techniques are the superior diagnostic tests for identifying H. hepaticus. Of these two techniques, it appears that PCR may offer greater sensitivity, although PCR tests have to date not been assigned “gold standard” status in most microbiology laboratories.
ACKNOWLEDGMENTS
This work was supported by grants RO1 CA67529 and P01CA26731 from the National Cancer Institute and a grant from the Chemical Manufacturing Association.
We acknowledge the critical review and constructive comments of this manuscript by Rick Haley, Dave Malarkey, and Bob Maronpot, Laboratory of Experimental Pathology, National Institute of Environmental Health Sciences.
REFERENCES
- 1.Beyer C H, Bergfeld W F, Berndt W O, Boutwell R K, Carlton W W, Hoffmann D K, Schroeter A L. Final report on the safety assessment of triethanolamine, diethanolamine, and monoethanolamine. J Am Coll Toxicol. 1983;2:183–235. [Google Scholar]
- 2.Canella K A, Diwan B A, Gorelick P L, Donovan P J, Sipowicz M A, Kasprzak K S, Weghorst C M, Snyderwine E G, Davis C D, Keefer L K, Kyrtopoulos S A, Hecht S S, Wang M, Anderson L M, Rice J M. Liver tumorigenesis by Helicobacter hepaticus: considerations of mechanism. In Vivo. 1996;10:285–292. [PubMed] [Google Scholar]
- 3.Foltz C J, Fox J G, Yan L, Shames B. Evaluation of antibiotic therapies for eradication of Helicobacter hepaticus. Antimicrob Agents Chemother. 1995;39:1292–1294. doi: 10.1128/aac.39.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox J G, Li X, Yan L, Cahill R J, Hurley R, Lewis R, Murphy J C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haley, R., J. K. Haseman, J. R. Bucher, A. E. Radovsky, D. E. Malarkey, A. Nyska, and R. P. Maronpot. Impact of Helicobacter hepaticus infection in B6C3F1 mice from 12 NTP two year carcinogenesis studies. Toxicol. Pathol., in press. [DOI] [PubMed]
- 8.Hoshino H, Tanooka H. Carcinogenicity of triethanolamine in mice and its mutagenicity after reaction with sodium nitrite in bacteria. Cancer Res. 1978;38:3918–3921. [PubMed] [Google Scholar]
- 9.Konishi Y, Denda A, Uchida K, Emi Y, Ura H, Yokose Y, Shiraiwa K, Tsutsumi M. Chronic toxicity carcinogenicity studies of triethanolamine in B6C3F1 mice. Fundam Appl Toxicol. 1992;18:25–29. doi: 10.1016/0272-0590(92)90191-j. [DOI] [PubMed] [Google Scholar]
- 10.National Toxicology Program. Draft report: toxicology and carcinogenesis studies of triethanolamine (case no. 102-71-6) in F344/N rats and B6C3F1 mice (dermal studies). U.S. Bethesda, Md: Department of Health and Human Services, Public Health Service, National Institutes of Health; 1996. [Google Scholar]
- 11.Nomura A, Stemmerman G N, Chyou P H, Kato I, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 12.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 13.Shames B, Fox J G, Dewhirst F, Yan L, Shen Z, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sipowicz M A, Chomarat P, Diwan B A, Anver M A, Awasthi Y C, Ward J M, Rice J M, Kasprzak K S, Wild C P, Anderson L M. Increased oxidative DNA damage and hepatocyte overexpression of specific cytochrome P450 isoforms in hepatitis of mice infected with Helicobacter hepaticus. Am J Pathol. 1997;151:933–941. [PMC free article] [PubMed] [Google Scholar]
- 15.Ward J M, Anver M R, Haines D C, Benveniste R E. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 16.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Gorelick P L, Nagashima K, Gonda M A, Gilden R V, Tully J G, Russell R J, Benveniste R E, Paster B J, Dewhirst F E, Donovan J C, Anderson L M, Rice J M. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]