Abstract
Although outcomes of children and adolescents with newly diagnosed acute myeloid leukemia (AML) have improved significantly over the past two decades, more than one-third of patients continue to relapse and experience suboptimal long-term outcomes. Given the small numbers of patients with relapsed AML and historical logistical barriers to international collaboration including poor trial funding and drug availability, the management of AML relapse has varied among pediatric oncology cooperative groups with several salvage regimens utilized and a lack of universally defined response criteria. The landscape of relapsed pediatric AML treatment is changing rapidly, however, as the international AML community harnesses collective knowledge and resources to characterize the genetic and immunophenotypic heterogeneity of relapsed disease, identify biological targets of interest within specific AML subtypes, develop new precision medicine approaches for collaborative investigation in early-phase clinical trials, and tackle challenges of universal drug access across the globe. This review provides a comprehensive overview of progress achieved to date in the treatment of pediatric patients with relapsed AML and highlights modern, state-of-the-art therapeutic approaches under active and emerging clinical investigation that have been facilitated by international collaboration among academic pediatric oncologists, laboratory scientists, regulatory agencies, pharmaceutical partners, cancer research sponsors, and patient advocates.
Introduction
Outcomes of children and adolescents/young adults with newly-diagnosed acute myeloid leukemia (AML) have improved over the past 20 years with overall survival (OS) rates now approaching 65-70%.1-4 These survival gains have been attributed largely to advances in biological and genetic characterization of heterogeneous pediatric AML subtypes via next-generation sequencing with clinical outcome correlation, and to enhanced supportive care measures focused on reducing toxicities from intensive multi-agent chemotherapy regimens required for cure. Recent advances in flow cytometric and molecular measurable residual disease detection have further enhanced modern risk-stratified approaches to chemotherapy and allocation to allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR1) when indicated.
While these measures have improved event-free survival (EFS) for children and adolescents/young adults with de novo AML, 30-40% of patients ultimately relapse. Management of patients in first relapse has varied among pediatric oncology consortia with no universally agreed-upon standard of care at this time. Accordingly, a wide range of second complete remission (CR2) rates from 23% to 81%5,6 and 5-year OS rates from 21% to 42%7-1 1 has been reported across the spectrum of AML salvage regimens. A standardized approach to relapse has been difficult to achieve for several reasons. The Berlin-Frankfurt-Münster (BFM) group in Europe has historically used relapsed disease as an opportunity to conduct large randomized trials. The Children’s Oncology Group (COG) in North America, Ireland, New Zealand, and Australia and other cooperative groups have viewed relapse as an opportunity to test novel therapeutic agents efficiently in smaller cohorts of patients via early-phase clinical trials. However, access to new drugs of biological interest in pediatric AML is not equal among countries and continents, which has further affected the ability to investigate promising approaches and to standardize treatment more globally in the relapsed setting. Response criteria for pediatric patients with relapsed AML have also not yet been standardized across study groups, although efforts to do this are currently underway. Finally, patients with a first relapse of AML (without or with prior HSCT) clearly represent a different disease population from patients with primary chemorefractory disease or from those in second or greater relapse who collectively experience highly different outcomes. These populations are frequently grouped together in relapse trials given the relatively small numbers of pediatric patients with AML, which further contributes to heterogeneity of CR achievement and EFS and OS response metrics described above.
Despite these challenges, significant achievements have been made in understanding and treating relapsed AML in pediatric patients during the past decade. This review highlights recent advances in prognostic factors and reinduction regimens for children and adolescents/young adults with relapsed AML and discusses emerging treatment approaches under current and near-future clinical investigation.
Predictors of response in children with relapsed acute myeloid leukemia
Despite heterogeneity of salvage regimens and response assessment metrics for relapsed AML, some consistent predictors of the achievement of second remission have been identified. During the past two decades, several consortia have demonstrated improved survival of children with first relapse, often without introduction of new agents. This metric has been attributed to improved supportive care over time and increased utilization of HSCT with a greater pool of stem cell sources, including haploidentical donors.11 Among COG cohorts, the 5-year OS was 29% for children with relapsed AML between 2007 and 2009 and 40% between 2013 and 2017. BFM studies also reported an improvement in 5-year OS from 39% in 2009-2013 to 49% in 2013-2017 in children with relapsed AML treated with optimized salvage chemotherapy and HSCT.11
Risk stratification at initial AML diagnosis has been associated with outcomes at relapse. Children treated on the COG AAML1031 phase III trial who were initially classified as high risk by leukemia-associated genetics or end-induction MRD and subsequently relapsed had a 5-year OS of 15% versus 44% for initially low-risk patients who relapsed (P<0.001).11 Variables that have prognostic significance in childhood acute lymphoblastic leukemia, such as age and white blood cell count, have not proven predictive of clinical outcomes in children with AML.7 In adult studies, AML patients with full hematologic recovery (complete remission [CR]) have better outcomes compared to those with complete remission with incomplete platelet count recovery (CRp) or incomplete blood count recovery (CRi).12-14 The negative prognostic outcome of a CRp/CRi has not been demonstrated in pediatric studies, as children with relapsed AML who achieve CRi/CRp do as well as those in CR.5,11 In certain contexts, count recovery could be a surrogate for residual disease, rather than toxicity to normal progenitors.13 The difference in prognostic outcomes for those achieving CRp/CRi between adults and pediatric patients may also be related to fundamental biological differences in AML biology with dys-plastic marrow being more predominant in the former. The planned intercalation of MRD-based remission criteria into response assessment may further refine (or complicate) these measures.
Response to therapy at initial AML diagnosis is also predictive of survival in patients with relapse.15 In the BFM cohort, those classified as non-responders (≥10% marrow involvement after first or ≥5% after second induction) had a 5-year OS of 0% compared to 45% for those who responded to initial therapy (P=0.031). In the COG cohort, outcomes after relapse were also dependent upon detection of MRD after initial induction therapy with 5-year OS of 24% and 41% (P<0.001) for those with and without MRD, respectively. Finally, resistance to salvage chemotherapy after relapse also expectedly contributes to differential outcomes. Within a prior Therapeutic Advances in Childhood Leukaemia (TACL) consortium cohort in North America and Australia, 56% of patients with residual disease after reinduction obtained CR after a second treatment attempt, 25% after a third attempt, and 17% after any subsequent attempts.7
Well-known predictors of response are the duration of the initial AML remission and time to relapse.7,10,16-18 Relapse within 1 year of CR1 is consistently associated with poor long-term survival. In BFM studies, 5-year OS was 29% for patients relapsing within 12 months of initial AML diagnosis (early relapse) versus 55% when relapse occurred after more than 12 months (late relapse; P<0.0001).11 COG studies have similarly reported 25% and 51% 5-year OS (P<0.001) in patients with early and late relapse, respect-ively.11 Those relapsing in less than 6 months after the initial AML diagnosis had comparably poor outcomes to those relapsing at 6 to 12 months after the initial diagnosis (37% vs. 27%, P=0.55). However, the ability to achieve CR2 after reinduction even in patients with early relapse has contributed to superior outcomes, as evidenced by a 4-year OS of 41% for responders versus 8% in non-responders in recent BFM studies.15
In comparison to survival of children after a first relapse of AML, survival following a second relapse has not improved over time with a stable 5-year OS of approximately 15%.19 Intensive reinduction regimens have generally not improved outcomes,19 highlighting the need to study novel targeted agents that may better attack the ‘Achilles’s heels’ of the AML cells for these patients. Encouragingly, if third complete remission (CR3) after second relapse can be achieved, use of HSCT has improved survival. One study demonstrated 5-year OS of 40% for patients receiving chemotherapy and HSCT as third-line therapy.20 Time to relapse also remains prognostic in second relapse with a 5-year OS of 2% and 33% for those who relapsed before and after 1 year, respectively.19 Leukemia-associated high-risk genetic alterations, particularly FLT3 internal tandem duplication (ITD; either alone or with WT1 co-mutations), have also been associated with worse outcomes for patients at second relapse, as described below. Other variables, including age, receiving prior HSCT, white cell count at initial AML diagnosis, and poor treatment response at initial diagnosis, have not proven to be prognostic at second relapse.19,20
Current treatment approaches for children with relapsed acute myeloid leukemia
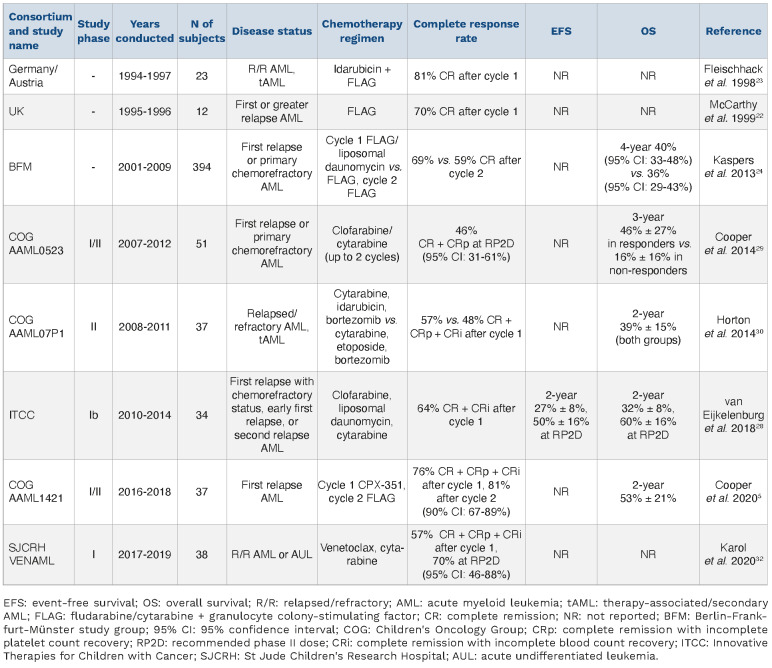
Demonstrated improvements in survival of pediatric patients with first relapse of AML warrant an aggressive reinduction attempt with HSCT consolidation in most cases. A number of reinduction regimens have demonstrated efficacy. Factors to consider when choosing a reinduction regimen include time to relapse, initial response to induction therapy, cumulative anthracycline chemotherapy dose, availability of chemotherapeutic agents, and presence of specific mutations that may be amenable to targeted therapies. Recent data have demonstrated that haploidentical transplantation outcomes using post-transplant cyclophosphamide are similar to those obtained with matched sibling donors.21 While direct comparisons between cooperative group trials have not been possible due to differing response criteria, inclusion criteria, and study designs, careful consideration of the data for various evidence-based salvage regimens remains important (Table 1).
The fludarabine and cytarabine with granulocyte colony-stimulating factor (g-csf, filgrastim) support (FLAG) regimen is frequently used for children with first relapse of AML in an attempt to provide effective reinduction therapy while reducing infectious and cardiac morbidity, particularly in patients with prior cumulative anthracycline exposure ≥450 mg/m2. CR2 rates after FLAG reinduction as high as 70% in patients with relapsed/refractory AML or ALL have been reported with many patients able to proceed to HSCT after a second cycle of FLAG to consolidate deep remission.22 Anthracycline addition to a FLAG backbone can be considered in patients who have not received maximal prior cumulative dosing or in those with high-risk disease (e.g., early relapse). In one study, a combination of FLAG with idarubicin resulted in CR rates of 81% after one cycle in heavily pre-treated patients with relapsed AML.23 However, two courses of FLAG with idarubicin have been associated with excessive toxicity,23 so a second induction cycle of FLAG without idarubicin is generally recommended instead.
To decrease treatment-related morbidity associated with cumulative anthracycline usage, the BFM group previously investigated the use of FLAG reinduction combined with a liposomal preparation of daunomycin (DaunoXome) versus FLAG in the largest pediatric first relapse AML randomized study reported to date (n=394 patients).24 All patients received FLAG for cycle 2. DaunoXome has potential benefits of decreased toxicity,25 increased half-life,26 and decreased drug resistance.27 The CR2 rate after two cycles was 69% with FLAG/DaunoXome versus 59% with FLAG (P=0.07), although OS was similar (40% vs. 36%, P=0.54). Interestingly, FLAG/DaunoXome was particularly beneficial for patients with core-binding factor AML (RUNX1::RUNX1T1 or CBFB::MYH11 fusions) with 5-year OS being 82% with FLAG/DaunoXome and 58% with FLAG (P=0.04). While results of this study were very promising, DaunoXome was never made available in the USA and is no longer manufactured.
The COG recently reported its analogous experience using CPX-351, a liposomal preparation of cytarabine and dauno-rubicin in a fixed 5:1 molar ratio, in the non-randomized AAML1421 phase I/II study in pediatric patients with first relapse of AML.5 Administration of CPX-351 in cycle 1 and FLAG in cycle 2 resulted in an overall response rate (comprising CR, CRp, and CRi) of 81% among 37 treated patients. All 14 patients with AML in late relapse (CR1 ≥12 months) achieved CR2, while 67% of patients with early relapse (CR1 <12 months) achieved CR2. Among the 30 responding patients, 29 (96.7%) were able to proceed to allogeneic HSCT. Two-year OS remained encouraging at 52.7%, demonstrating long-term benefit of this salvage approach.5 Unfortunately, CPX-351 is not available or is difficult to procure in many countries, including Canada and in Europe.
Additional reinduction methods for patients with relapsed disease have included the investigation of alternate purine analogs or proteasome inhibitors. An Innovative Therapies for Children with Cancer (ITCC) Consortium/BFM trial investigated whether replacing fludarabine with clofarabine in conjunction with cytarabine and liposomal daunorubicin would improve outcomes in patients with early first relapse, chemotherapy-refractory first relapse, or second relapse of AML.28 Among 31 evaluable patients, 64% achieved CR or CRi. The 2-year EFS and OS in this high-risk group were 27% and 32%, respectively. At the recommended phase II dosing of this regimen, the 2-year EFS was 50% and OS 60%.28 In the COG AAML0523 phase II study, clofarabine and cytarabine administered without an anthracycline resulted in a CR + CRp rate of 46%.29 In the COG AALL07P1 phase II/pilot trial, addition of bortezomib to reinduction with either high-dose cytarabine and etoposide or low-dose cytarabine and idarubicin was also deemed safe with excellent composite complete remission rates (CR + Cri + CRp) of 57% in the idarubicin-containing arm.30 The inclusion of the BCL-2 inhibitor venetoclax in intensive induction regimens has proven effective in adults with relapsed AML,31 and various other venetoclax-based therapy regimens have been investigated in both relapse and de novo settings. In the pediatric domain, the VENAML phase I/II study from St Jude Children’s Research Hospital performed dose-finding and assessed preliminary efficacy of a cytarabine and venetoclax reinduction regimen in 38 pediatric patients with relapsed/refractory AML.32 Four patients had primary chemorefractory AML, 14 of 21 patients in first relapse had received previous salvage therapy, 11 enrolled after second relapse, and two patients enrolled after third relapse. A CR rate of 57% (CR, CRp, and CRi) was achieved after cycle 1. At the recommended phase II dosing of venetoclax, 70% of patients achieved composite CR with 71% of the CR also being MRD-negative,32 which was very encouraging in a highly treatment-resistant patient population. Of note, the VENAML regimen may not be equally suitable for all AML subtypes. In this study, no patient with FLT3-ITD or FLT3-point mutations responded to therapy,32 possibly due to the lack of FLT3 inhibitor use. Children with high allelic ratio FLT3-ITD AML have an increased risk of relapse.33,34 Survival benefit has been clearly demonstrated with addition of targeted FLT3 kinase inhibitors at relapse and, more recently, to front-line chemotherapy.35,36 Initial trials studied first-generation multi-tyrosine kinase inhibitors with anti-FLT3 properties, such as midostaurin and sorafenib.37,38 Subsequent trials have investigated more selective second-generation inhibitors, including quizartinib, crenolanib, and gilteritinib, after demonstration of promising results in adult patients.39,40 A current phase I/II study is examining the safety and efficacy of quizartinib with fludarabine, cytarabine, and etoposide in pediatric patients with relapsed/refractory AML (NCT03793478). Another phase I/II study is investigating the safety and efficacy of gilteritinib with fludarabine and cytarabine in children with relapsed/refractory AML (NCT04240002). Based upon successful data in adult patients with FLT3-ITD AML that led to its approval by the US Food and Drug Administration (FDA), gilteritinib in combination with multi-agent chemotherapy is also under investigation in the COG AAML1831 phase III trial in children, adolescents, and young adults with newly-diagnosed FLT3-ITD or FLT3-mutant AML (NCT04293562). Recent studies have also demonstrated benefit of post-HSCT FLT3 inhibitor maintenance therapy, although desired anti-AML activity must be carefully balanced with risk of toxicity.37, 41 The above studies highlight current evidence-based rein-duction options for treatment of children with relapsed AML. Ideally, all patients should be enrolled on a clinical trial, and several early-phase studies of precision medicine therapeutics for children with relapsed/refractory AML are now available or will soon open (Table 2). However, if such options are not possible or clinically relevant, a pragmatic approach to salvage therapy is recommended in Figure 1. For patients with high-risk relapse, incorporation of anthracyclines where possible may offer the best chance of obtaining CR2, but should be carefully weighed against the risk of long-term cumulative cardiotoxicity. While liposomal daunomycin formulations have clearly demonstrated efficacy in AML salvage regimens (and with CPX-351 now under front-line investigation via the COG AAML1831 phase III trial), it is not yet known whether or not this agent is associated with less cardiotoxicity than co-usage of the cardioprotectant dexrazoxane with conventional anthracycline drugs, as was recently shown to be beneficial in children treated on the COG AAML1031 phase III trial.42 For patients with low-risk cytomolecular alterations and late relapse of AML or who have received maximal cumulative dosing of anthracycline chemotherapy, FLAG is a safe and generally very effective reinduction option. In recent years, addition of the CD33-targeting antibody-drug conjugate gemtuzumab ozogamicin (GO) to FLAG cycle 1 for patients with CD33+ AML has been anecdotally used with a goal of improving CR rates while maintaining tolerable side effects. More formal evaluation of FLAG with GO with or without venetoclax for children with relapsed AML is now occurring in clinical trials, such as the international Leukaemia & Lymphoma Society PedAL/EUpAL consortium APAL2020D phase III study (NCT05183035, EudraCT 2021-003212-11), and may shed additional light. If curative treatment is intended, consolidative HSCT when in CR2 or later remission should be pursued as clinically appropriate. In patients who remain refractory to reinduction attempts, there is surprisingly some evidence to support a role for HSCT even in the absence of CR. In a BFM study cohort, children with AML who had no response after relapse (≥5% residual AML after second reinduction therapy) had a poor, but not zero, OS rate of 27% at 5 years.11-
Table 1.
Recently completed clinical trials for children with relapsed acute myeloid leukemia.
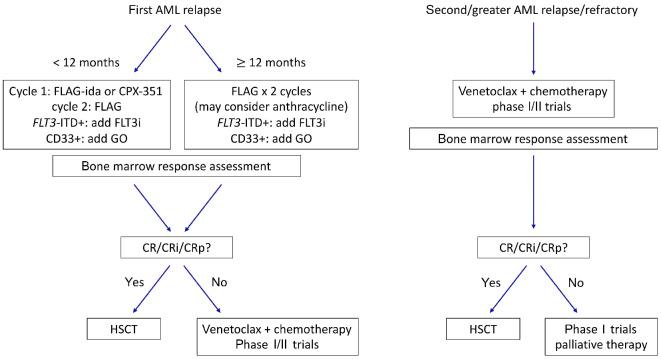
Figure 1.
Proposed approach to therapeutic decision-making for children with relapsed acute myeloid leukemia. Additional targeted therapies may be considered depending upon underlying cytomolecular genetic alterations or immunophenotypic characteristics. AML: acute myeloid leukemia; FLAG: fludarabine/cytarabine + granulocyte colony-stimulating factor; ida: idarubicin; FLT3i: FLT3 inhibitor; GO: gemtuzumab ozogamicin; CR: complete remission; CRi: complete remission with incomplete blood count recovery; CRp: complete remission with incomplete platelet count recovery; HSCT: hematopoietic stem cell transplantation.
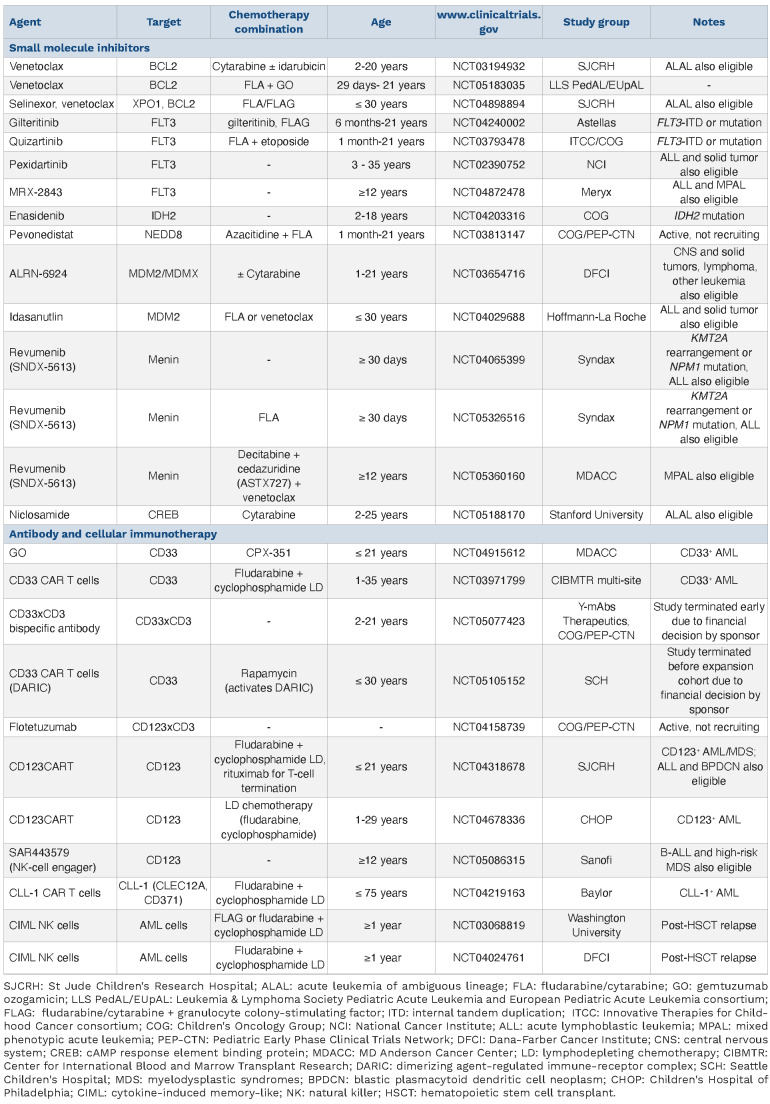
Table 2.
Current and soon-to-open clinical trials for children with relapsed/refractory acute myeloid leukemia.
Special considerations for children with relapsed myeloid leukemia of Down syndrome
Children with trisomy 21-associated AML who relapse represent another high-risk subgroup who require special attention. While outcomes for most young children with myeloid leukemia of Down syndrome (ML-DS) are excel-lent,43 the subset of patients with relapsed/refractory disease have very poor outcomes with 3-year OS of 17-26%,44-46 even with use of consolidative HSCT. Initial treatment failure in children with ML-DS is frequently secondary to disease progression, rather than due to excess toxicity.45,47 In the recent COG AAML1531 phase III trial, patients were stratified as standard- or high-risk based upon negative or positive end-induction 1 MRD, respectively, with attempted therapy de-escalation via anthracycline reduction to decrease cardiotoxicity for children with standard-risk ML-DS. However, an interim study analysis demonstrated the futility of decreased anthracycline dosing in this population with higher relapse rates than in the prior COG AAML0431 trial. Importantly, very poor salvage of children with initially standardrisk ML-DS who subsequently relapsed was achieved with a 1-year OS of 16.7%, demonstrating the importance of appropriately intensive up-front therapy for these patients to prevent relapse.46 Successful intercalation of more targeted, less toxic agents for children with ML-DS remains an important therapeutic goal.
Promising new agents for children with relapsed/refractory acute myeloid leukemia
Historically, AML in children has been treated similarly to AML in adults, and novel agents that have demonstrated activity in relapsed adult AML have been applied to relapsed pediatric disease. Although there have been some successes with this approach, many agents do not translate well into the pediatric context given fundamental biological differences in AML across the age spectrum. For example, RAS pathway mutations occur frequently in children with AML, but are uncommon in adults. Similarly, mutations in the epigenetic modifier genes DNMT3A, IDH1, and IDH2 are common in adult AML, but rare in pediatrics.48 Specific mutations may also be present at a subclonal level, but targeting these mutations may not fully eradicate disease if they are not major oncogenic drivers.
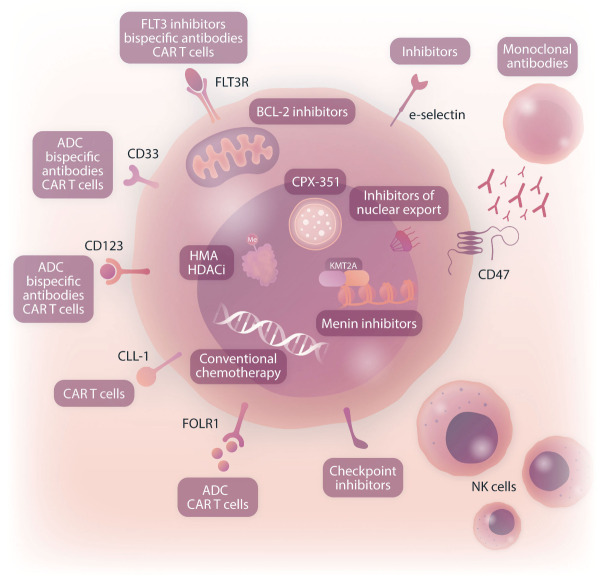
Given the poor clinical outcomes of children with relapsed/refractory AML, early-phase clinical trials of new agents may be considered for patients with persistent residual disease after a reinduction attempt, particularly if an anthracycline agent was included in reinduction. Alternative strategies include venetoclax-based regimens as described above and immunotherapeutic approaches, such as antibody-drug conjugates, bispecific antibodies, and cell ther-apies.49 Patients with particularly high-risk AML-associated genetics, including CBFA2T3::GLIS2 fusion, NUP98 rearrangements, and some KMT2A rearrangements, may also be considered for experimental therapy at the time of first relapse if available given their known very poor salvage rates. Some of these ‘boutique’ subtypes of high-risk AML occur exclusively in younger children and may be amenable to novel targeted approaches, including menin inhibition for KMT2A-rearranged and NUP98-rearranged AML and anti-CD56 and FOL1R immunotherapies for CBFA2T3::GLIS2 acute megakaryoblastic leukemia discussed in more detail below (Figure 2).
Epigenetic modifiers
Hypomethylating agents, such as azacytidine and decitabine, have demonstrated activity in adult AML, initially in elderly patients not fit for intensive chemotherapy.50 The TACL consortium recently investigated reinduction with azacytidine and fludarabine/cytarabine in 12 children with relapsed/refractory leukemia in a phase I study; 58% (7 of 12) achieved CR/CRi after one cycle with four of these responses being MRD-negative.51 The use of hypomethylating chemotherapy for ‘epigenetic priming’ prior to induction chemotherapy is now under evaluation in children and adolescents/young adults with newly-diagnosed AML in a USA-based multi-site randomized phase II trial (NCT03164057). A subsequent TACL2016-002 phase I trial also studied combining decitabine with the checkpoint inhibitor nivolumab (NCT03825367), but the study closed early due to lack of activity and poor accrual (A Verma, personal communication, 2022).
Another class of epigenetic drugs are histone deacetylase (HDAC) inhibitors, such as pabinostat and vorinostat, which remodel chromatin and alter gene expression which can lead to AML cell differentiation and apoptosis.52 A recent St Jude Children’s Research Hospital phase I trial examined the use of panobinostat before and in combination with fludarabine/cytarabine in 17 pediatric patients with relapsed/refractory AML (NCT02676323), which demonstrated the safety of the combination therapy and CR in five of six (83.3%) patients treated at dose level 3, but closed early due to poor accrual.53 The TACL consortium also studied decitabine and vorinostat with fludarabine/cytarabine in a phase I trial in pediatric and adolescent/young adult patients with relapsed/refractory AML or ML-DS (NCT02412475, NCT03263936) and recently reported 19 of 35 (54%) treated patients had achieved CR or CRi.54
Small molecule inhibitors
In addition to the aforementioned FLT3 and BCL-2 inhibitors, several new targets and associated small molecule inhibitors are of particular interest in childhood AML.
KMT2A rearrangements with a variety of fusion partners occur in 15-20% of pediatric AML cases, many of which are associated with high relapse risk and poor outcomes. The intracellular cofactor menin interacts with the KMT2A protein complex to activate HOXA-cluster genes and MEIS1, which drive leukemia progression.55 Targeted menin inhibitors have demonstrated remarkable activity in preclinical models of KMT2A-rearranged AML and ALL,56 as well as in NPM1-mutant and NUP98-rearranged AML.57,58 Revumenib (SNDX-5613) and ziftomenib (KO-539) have demonstrated safety, tolerability, and preliminary activity in adults with relapsed/refractory KMT2A-rearranged or NPM1-mutant leukemias (NCT04065399, NCT04067336).59,60 Pediatricspecific investigation of menin inhibitors is occurring via industry-supported trials and soon-to-open LLS PedAL/EUpAL phase I trials (SK Tasian, personal communication, 2022).
Selective inhibitors of nuclear export, particularly XPO1 inhibitors, include selinexor and eltanexor, and have demonstrated clinical efficacy in adults and children with relapsed/refractory AML.61,62 A pediatric phase I study of selinexor with fludarabine and cytarabine reported a 47% CR/CRi rate in children with multiply relapsed AML. Singleagent activity of selinexor has also been observed in a small number of pediatric patients with relapsed AML harboring nucleoporin genes (e.g., NUP214, NUP98) and other fusions.61 A phase I/expansion cohort study is now examining the activity of selinexor and venetoclax with chemotherapy in children and adolescents/young adults with relapsed/refractory AML (NCT04898894).
Figure 2.
Therapeutic targets in acute myeloid leukemia under current or planned pediatric-specific clinical investigation. CAR T cells: chimeric antigen receptor T-cell immunotherapy; ADC: antibody-drug conjugates; HMA: hypomethylating agents; HDACi: histone deacetylase inhibitors; NK: natural killer. Created with BioRender.com.
Another pediatric phase I trial is studying the safety and tolerability of ALRN-6294, a targeted inhibitor of MDM2 and MDMX proteins, in children with relapsed/refractory TP53 wild-type solid tumors, lymphoma, or AML (NCT03654716) based upon promising preclinical and early clinical data in adults with AML.63,64
Antibody and cellular immunotherapies
Successful development of immunotherapy targeting cell surface antigens is of particular interest in relapsed/refractory pediatric AML. Analysis of AML specimens from the COG AAML0531 phase III clinical trial demonstrated inferior EFS and OS of children with the highest CD33 protein expression.65 Importantly, the AAML0531 study also demonstrated the safety of combining the CD33-targeting antibody-drug conjugate GO with multi-agent chemotherapy and improved disease-free survival in specific high-risk subgroups.66-68 GO is now approved by the FDA and European Medicines Agency (EMA) for pediatric patients with relapsed or de novo AML based upon favorable clinical trial data from AAML0531 and other European studies. A first-in-child, multi-site phase I trial in the USA is investigating the safety and preliminary activity of CD33 chim-eric antigen receptor T cells (CD33CART) in children with multiply relapsed/refractory AML (NCT03971799) based upon promising preclinical data.69 The recently opened international first-in-child COG ADVL2111 phase I trial is also studying the safety and tolerability of a CD33xCD3 bispecific antibody in children with second or greater relapsed/refractory AML (NCT05077423).
CD123 (interleukin receptor-3 alpha chain) is another cell surface antigen of particular interest in pediatric AML given a recent similar demonstration of inferior EFS and OS and increased relapse risk in patients with highest expression.70 Several CD123-directed immunotherapies are under pediatric-specific investigation in phase I clinical trials. The COG PEPN1812 study investigated the safety and tolerability of the CD123xCD3 bispecific antibody flotetuzumab in 15 children with second or greater relapsed AML, identifying a recommended phase II dose and detecting a 20% overall response rate (NCT04158739) that was concordant with data from an adult study.71-73 Phase I trials of CD123 CAR T-cell immunotherapies in children with relapsed/refractory AML are ongoing at the Children’s Hospital of Philadelphia (NCT04678336) and St Jude Children’s Research Hospital (NCT04318678) based upon promising preclinical data and early clinical experience of similar products in adults with relapsed/refractory AML.74 -7 7 Other antigen targets of interest in pediatric AML include mesothelin, CLEC12A (CLL-1, CD371), FLT3, FOL1R, and eselectin ligand.78-84 Early-phase pediatric studies of anti-body-based or cellular immunotherapies against these targets are planned.
Finally, remarkable anti-leukemia activity has been reported with cytokine-induced memory-like natural killer (NK) cells in adults and children with relapsed/refractory AML with additional trials underway (NCT03068819, NCT04024761).85-87 Additional potential for CAR-modified T cells against NKG2D/NKG2D ligands and CAR-NK cells in patients with relapsed/refractory AML is being explored.88 Accrual is also ongoing in studies of HA-1 T-cell receptor T cells in children with relapsed/refractory AML (NCT03326921).89
Future approaches to relapse in pediatric acute myeloid leukemia
Next-generation sequencing approaches in acute myeloid leukemia
While the genomic landscape of newly diagnosed pediatric AML has recently been relatively well-described,48 the cytomolecular characteristics of relapsed AML and their potential evolution from diagnosis are less well understood. More widespread use of RNA- and DNA-based next-generation sequencing will continue to increase our understanding of relapsed pediatric AML and enable further fine-tuning of risk stratification and therapeutic decision-making. A recent study comprehensively sequenced the genome and transcriptome of 136 relapsed AML cases and identified over-representation of WT1, KMT2A, and NUP98 alterations at relapse compared to other subtypes also detected at diagnosis.90 Interestingly, tandem duplications in upstream binding transcription factor (UBTF) were identified as a previously unknown recurrent alteration in 9% of relapsed pediatric AML cases compared to a frequency of 0.9% in relapsed adult AML. These duplications occurred in AML with normal karyotype or trisomy 8 and frequently in the setting of FLT3-ITD and WT1 mutations. This alteration was also noted to be common in young adolescent patients and was associated with higher rates of end-induction MRD positivity and poor long-term survival,90 but remains incompletely understood. Recent integrated genetic and transcriptomic analyses have been posited to be superior in prediction of biological subtypes and outcomes in pediatric AML than conventional immunophenotyping and genetic mutation analyses.91 Future studies that prospectively integrate gene expression profiling, including stemness scores,92,93 into risk stratification are likely to refine further pediatric AML risk stratification and therapeutic selection.
Drug sensitivity profiling and functional precision medicine in acute myeloid leukemia
While genomic profiling provides prognostic information regarding disease heterogeneity and clonal evolution, the identification of novel therapeutic targets to match specific genetic mutations occurs in only a fraction of cases. Because the molecular complexity of AML can be influenced by metabolic and epigenetic perturbations, targeting genomic perturbations does not always translate into meaningful clinical responses. Ex vivo drug testing can provide informative targetable results that complement genomic approaches. The Beat AML (NCT03013998) and other screening studies (NCT02551718) have demonstrated the feasibility of combining functional precision medicine and ex vivo high-throughput drug sensitivity profiling with genomic and transcriptional data,94,95 although prospective data remain lacking. The recent Paediatric LEAP consortium Matched Targeted Therapy study intercalated detailed DNA-based next-generation sequencing and similar drug sensitivity profiling of a subset of relapsed or de novo high-risk pediatric leukemia specimens using the Beat AML platform and identified a high percentage of patients with potential targeted therapy recommendations.96
The EXALT study (NCT03096821) used an image-based single-cell functional precision medicine approach to evaluate the effects of 139 drugs on leukemia specimens from adults with multiply relapsed/refractory hematologic malignancies,97 including 14 patients with AML. Each patient’s progression-free survival was compared with progression-free survival from their prior therapy regimens. Progression-free survival was significantly increased with a single-cell functional precision medicine approach, and OS was also increased with this approach compared to the survival of a cohort treated with physicians’ choice of therapy. Fifty-four percent of patients had progression-free survival of at least 1.3 times the duration of that from prior therapy, and 21% had an exceptional response (defined as tripled progression-free survival duration compared with expected response duration of the respective disease entity).97 In comparison to classical sequencing approaches, results on this trial were available within a median of 5 days of sample procurement, making it a feasible, relatively real-time approach in the relapsed context. The EXALT 2 study (NCT04470947) is now randomizing patients with relapsed/refractory leukemias to therapy directed by either comprehensive genomic profiling, next generation drug screening, or physicians’ choice.
In another study, Malani and colleagues tested the utility of a functional precision medicine tumor board, integrating functional data with clinical and molecular data to guide treatment decisions.98 Ex vivo drug sensitivity and resistance testing of relapsed AML specimens were performed, and an actionable drug target was identified in 97% of patients with a median timeframe of 4 days. Thirty-seven patients with relapsed/refractory AML were treated according to the result of drug sensitivity and resistance testing with 59% demonstrating a response, including 13 CR. Importantly, achievement of these precision medicine-induced CR was transplant-enabling in five patients, who achieved long-term survival.98 While difficult to implement for every patient given access, cost, and logistic feasibility, such approaches may offer effective tools for relatively real-time clinical decision making in the relapsed setting, including in childhood AML.
Conclusions
At this time, fewer than half of pediatric patients survive relapsed AML. While outcomes have increased modestly over time, such advances have largely been attributed to improved supportive care rather than to development of more effective treatment approaches. Historically, fewer than 20% of children with relapsed AML have been treated on clinical trials,99 which has limited identification of optimal salvage regimens. Despite significant regulatory burdens, coordinated international efforts and joint relapse trials of new agents are now finally underway to rectify this major knowledge gap.
The outlook for novel therapeutics in pediatric AML has improved with the development of the LLS PedAL/EUpAL consortium.99 The concept for this joint North American/European initiative is the development of a master screening protocol with a common genetic and immunophenotypic screening platform and a robust data dictionary that identifies critical biological characteristics within relapsed acute leukemia specimens and helps to match patients with specific early-phase precision medicine clinical trials. This innovative international cooperative infrastructure has successfully engaged regulatory agencies, academic pediatric oncologists, and pharmaceutical companies to: (i) standardize relapse definitions, response criteria, and outcomes reporting, (ii) hasten pediatric-specific drug development and investigation of novel agents, and (iii) increase enrollment efficiency of less common ‘boutique’ subtypes of childhood acute leukemias within specific trials.99
Via recent advances in sophisticated genomic and extended immunophenotypic characterization of childhood AML and correlation with clinical outcomes via comprehensive clinical trial databases, the pediatric oncology community is harnessing its collective power to design clinical trials that increase global patient numbers in rare biological/genetic subgroups predicted to be amenable to specific targeted therapies and to incite collaboration from industry and regulatory partners for timely pediatric-specific investigation of novel agents. Additional efforts at genetic and biological characterization remain necessary to delineate further the complex and heterogeneous landscape of AML in children and adolescents/young adults, as well as to elucidate clinical outcomes of newly identified high-risk subgroups. As the pediatric AML community investigates new therapies in the relapsed/refractory domain, further work will also be needed to identify predictive biomarkers of treatment response versus failure and to determine which drugs should be prioritized for frontline investigation in patients with de novo disease in the future. Despite many existing challenges, the future of pediatric AML therapy looks promising, and the next decade will undoubtedly bring exciting discoveries that improve the outlook for children and adolescents/young adults with relapsed/refractory AML.
Acknowledgments
Our thanks to Luk Cox for his excellent collaboration in producing Figure 2.
Funding Statement
Funding: GE is supported by an Ontario Institute of Cancer Research (OICR) Investigator Award. SKT is supported by the National Institutes of Health/National Cancer Institute 1U01CA232486 and 1U01CA243072, Department of Defense Translational Team Science Award CA180683P1, and the V Foundation for Cancer Research. SKT is a Scholar of the Leukemia and Lymphoma Society and holds the Joshua Kahan Endowed Chair in Pediatric Leukemia Research at the Children’s Hospital of Philadelphia.
References
- 1.Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29(3):310-315. [DOI] [PubMed] [Google Scholar]
- 2.Pession A, Masetti R, Rizzari C, et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. 2013;122(2):170-178. [DOI] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aplenc R, Meshinchi S, Sung L, et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: a report from the Children's Oncology Group. Haematologica. 2020;105(7):1879-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper TM, Absalon MJ, Alonzo TA, et al. Phase I/II study of CPX-351 followed by fludarabine, cytarabine, and granulocyte-colony stimulating factor for children with relapsed acute myeloid leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2020;38(19):2170-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper TM, Sison EAR, Baker SD, et al. A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: a Pediatric Oncology Experimental Therapeutics Investigators' Consortium study (POE 10-03). Pediatr Blood Cancer. 2017;64(8):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorman MF, Ji L, Ko RH, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010;55(3):421-429. [DOI] [PubMed] [Google Scholar]
- 8.Rubnitz JE, Razzouk BI, Lensing S, Pounds S, Pui CH, Ribeiro RC. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007;109(1):157-163. [DOI] [PubMed] [Google Scholar]
- 9.Stahnke K, Boos J, Bender-Götze C, Ritter J, Zimmermann M, Creutzig U. Duration of first remission predicts remission rates and long-term survival in children with relapsed acute myelogenous leukemia. Leukemia. 1998;12(10):1534-1538. [DOI] [PubMed] [Google Scholar]
- 10.Aladjidi N, Auvrignon A, Leblanc T, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. J Clin Oncol. 2003;21(23):4377-4385. [DOI] [PubMed] [Google Scholar]
- 11.Rasche M, Zimmermann M, Steidel E, et al. Survival following relapse in children with acute myeloid leukemia: a report from AML-BFM and COG. Cancers (Basel). 2021;13(10):2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol. 2010;28(10):1766-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Xie H, Wood BL, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol. 2015;33(11):1258-1264. [DOI] [PubMed] [Google Scholar]
- 14.Norsworthy KJ, Gao X, Ko CW, et al. Response rate, event-free survival, and overall survival in newly diagnosed acute myeloid leukemia: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2022;40(8):847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creutzig U, Zimmermann M, Dworzak MN, et al. The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: results of the international study Relapsed AML 2001/01. Haematologica. 2014;99(9):1472-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells RJ, Adams MT, Alonzo TA, et al. Mitoxantrone and cytarabine induction, high-dose cytarabine, and etoposide intensification for pediatric patients with relapsed or refractory acute myeloid leukemia: Children’s Cancer Group study 2951. J Clin Oncol. 2003;21(15):2940-2947. [DOI] [PubMed] [Google Scholar]
- 17.Webb DK, Wheatley K, Harrison G, Stevens RF, Hann IM. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial. MRC Childhood Leukaemia Working Party. Leukemia. 1999;13(1):25-31. [DOI] [PubMed] [Google Scholar]
- 18.Moritake H, Tanaka S, Miyamura T, et al. The outcomes of relapsed acute myeloid leukemia in children: results from the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05R study. Pediatr Blood Cancer. 2021;68(1):e28736. [DOI] [PubMed] [Google Scholar]
- 19.Rasche M, Steidel E, Zimmermann M, et al. Second relapse of pediatric patients with acute myeloid leukemia: a report on current treatment strategies and outcome of the AML-BFM study group. Cancers (Basel). 2021;13(4):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White T, Kaspers G, Abrahamsson J, et al. Clinical outcomes of second relapsed and refractory first relapsed paediatric AML: a retrospective study within the NOPHO-DB SHIP consortium. Br J Haematol. 2022;197(6):755-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy AJ, Pitcher LA, Hann IM, Oakhill A. FLAG (fludarabine, high-dose cytarabine, and G-CSF) for refractory and high-risk relapsed acute leukemia in children. Med Pediatr Oncol. 1999;32(6):411-415. [DOI] [PubMed] [Google Scholar]
- 23.Fleischhack G, Hasan C, Graf N, Mann G, Bode U. IDA-FLAG (idarubicin, fludarabine, cytarabine, G-CSF), an effective remission-induction therapy for poor-prognosis AML of childhood prior to allogeneic or autologous bone marrow transplantation: experiences of a phase II trial. Br J Haematol. 1998;102(3):647-655. [DOI] [PubMed] [Google Scholar]
- 24.Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM study group. J Clin Oncol. 2013;31(5):599-607. [DOI] [PubMed] [Google Scholar]
- 25.Ewer MS, Martin FJ, Henderson C, Shapiro CL, Benjamin RS, Gabizon AA. Cardiac safety of liposomal anthracyclines. Semin Oncol. 2004;31(6 Suppl 13):161-181. [DOI] [PubMed] [Google Scholar]
- 26.Gabizon A, Goren D, Cohen R, Barenholz Y. Development of liposomal anthracyclines: from basics to clinical applications. J Control Release. 1998;53(1-3):275-279. [DOI] [PubMed] [Google Scholar]
- 27.Rahman A, Husain SR, Siddiqui J, et al. Liposome-mediated modulation of multidrug resistance in human HL-60 leukemia cells. J Natl Cancer Inst. 1992;84(24):1909-1915. [DOI] [PubMed] [Google Scholar]
- 28.van Eijkelenburg NKA, Rasche M, Ghazaly E, et al. Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study. Haematologica. 2018;103(9):1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper TM, Alonzo TA, Gerbing RB, et al. AAML0523: a report from the Children's Oncology Group on the efficacy of clofarabine in combination with cytarabine in pediatric patients with recurrent acute myeloid leukemia. Cancer. 2014;120(16):2482-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton TM, Perentesis JP, Gamis AS, et al. A phase 2 study of bortezomib combined with either idarubicin/cytarabine or cytarabine/etoposide in children with relapsed, refractory or secondary acute myeloid leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2014;61(10):1754-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNardo CD, Lachowiez CA, Takahashi K, et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol. 2021;39(25):2768-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karol SE, Alexander TB, Budhraja A, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol. 2020;21(4):551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108(12):3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cucchi DGJ, Denys B, Kaspers GJL, et al. RNA-based FLT3-ITD allelic ratio is associated with outcome and ex vivo response to FLT3 inhibitors in pediatric AML. Blood. 2018;131(22):2485-2489. [DOI] [PubMed] [Google Scholar]
- 35.Sexauer AN, Tasian SK. Targeting FLT3 signaling in childhood acute myeloid leukemia. Front Pediatr. 2017;5:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight TE, Edwards H, Meshinchi S, Taub JW, Ge Y. "FLipping" the story: FLT3-mutated acute myeloid leukemia and the evolving role of FLT3 inhibitors. Cancers (Basel). 2022;14(14):3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollard JA, Alonzo TA, Gerbing R, et al. Sorafenib in combination with standard chemotherapy for children with high allelic ratio FLT3/ITD+ acute myeloid leukemia: a report from the Children's Oncology Group protocol AAML1031. J Clin Oncol. 2022;40(18):2023-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwaan CM, Söderhäll S, Brethon B, et al. A phase 1/2, open-label, dose-escalation study of midostaurin in children with relapsed or refractory acute leukaemia. Br J Haematol. 2019;185(3):623-627. [DOI] [PubMed] [Google Scholar]
- 39.Inaba H, van Oosterwijk JG, Panetta JC, et al. Preclinical and pilot study of type I FLT3 tyrosine kinase inhibitor, crenolanib, with sorafenib in acute myeloid leukemia and FLT3-internal tandem duplication. Clin Cancer Res. 2022;28(12):2536-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper TM, Cassar J, Eckroth E, et al. A phase I study of quizartinib combined with chemotherapy in relapsed childhood leukemia: a Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) study. Clin Cancer Res. 2016;22(16):4014-4022. [DOI] [PubMed] [Google Scholar]
- 41.Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133(8):840-851. [DOI] [PubMed] [Google Scholar]
- 42.Getz KD, Sung L, Alonzo TA, et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2020;38(21):2398-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravindranath Y, Abella E, Krischer JP, et al. Acute myeloid leukemia (AML) in Down's syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML study 8498. Blood. 1992;80(9):2210-2214. [PubMed] [Google Scholar]
- 44.Taga T, Saito AM, Kudo K, et al. Clinical characteristics and outcome of refractory/relapsed myeloid leukemia in children with Down syndrome. Blood. 2012;120(9):1810-1815. [DOI] [PubMed] [Google Scholar]
- 45.Hitzler JK, He W, Doyle J, et al. Outcome of transplantation for acute myelogenous leukemia in children with Down syndrome. Biol Blood Marrow Transplant. 2013;19(6):893-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hitzler J, Alonzo T, Gerbing R, et al. High-dose AraC is essential for the treatment of ML-DS independent of postinduction MRD: results of the COG AAML1531 trial. Blood. 2021;138(23):2337-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meissner B, Borkhardt A, Dilloo D, et al. Relapse, not regimen-related toxicity, was the major cause of treatment failure in 11 children with Down syndrome undergoing haematopoietic stem cell transplantation for acute leukaemia. Bone Marrow Transplant. 2007;40(10):945-949. [DOI] [PubMed] [Google Scholar]
- 48.Bolouri H, Farrar JE, Triche T Jr, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. 2018;24(1):103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamble AJ, Tasian SK. Opportunities for immunotherapy in childhood acute myeloid leukemia. Blood Adv. 2019;3(22):3750-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santini V, Ossenkoppele GJ. Hypomethylating agents in the treatment of acute myeloid leukemia: a guide to optimal use. Crit Rev Oncol Hematol. 2019;140:1-7. [DOI] [PubMed] [Google Scholar]
- 51.Sun W, Triche T Jr, Malvar J, et al. A phase 1 study of azacitidine combined with chemotherapy in childhood leukemia: a report from the TACL consortium. Blood. 2018;131(10):1145-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.San José-Enériz E, Gimenez-Camino N, Agirre X, Prosper F. HDAC inhibitors in acute myeloid leukemia. Cancers (Basel). 2019;11(11):1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karol SE, Cooper TM, Mead PE, et al. Safety, pharmacokinetics, and pharmacodynamics of panobinostat in children, adolescents, and young adults with relapsed acute myeloid leukemia. Cancer. 2020;126(21):4800-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pommert L, Schafer ES, Malvar J, et al. Decitabine and vorinostat with FLAG chemotherapy in pediatric relapsed/refractory AML: report from the therapeutic advances in childhood leukemia and lymphoma (TACL) consortium. Am J Hematol. 2022;97(5):613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123(2):207-218. [DOI] [PubMed] [Google Scholar]
- 56.Borkin D, He S, Miao H, et al. Pharmacologic inhibition of the menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell. 2015;27(4):589-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kühn MW, Song E, Feng Z, et al. Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov. 2016;6(10):1166-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heikamp EB, Henrich JA, Perner F, et al. The menin-MLL1 interaction is a molecular dependency in NUP98-rearranged AML. Blood. 2022;139(6):894-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erba HP, Fathi AT, Issa GC, et al. Update on a phase 1/2 first-inhuman study of the menin-KMT2A (MLL) inhibitor ziftomenib (KO-539) in patients with relapsed or refractory acute myeloid leukemia. Blood. 2022;140(Suppl 1):153-156. [Google Scholar]
- 60.Issa GC, Aldoss I, DiPersio JF, et al. The menin inhibitor SNDX-5613 (revumenib) leads to durable responses in patients (Pts) with KMT2A-rearranged or NPM1 mutant AML: updated results of a phase (Ph) 1 study. Blood. 2022;140(Suppl 1):150-152. [Google Scholar]
- 61.Alexander TB, Lacayo NJ, Choi JK, Ribeiro RC, Pui CH, Rubnitz JE. Phase I study of selinexor, a selective inhibitor of nuclear export, in combination with fludarabine and cytarabine, in pediatric relapsed or refractory acute leukemia. J Clin Oncol. 2016;34(34):4094-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Etchin J, Berezovskaya A, Conway AS, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia. 2017;31(1):143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carvajal LA, Neriah DB, Senecal A, et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci Transl Med. 2018;10(436):eaao3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sallman DA, Borate U, Cull EH, et al. Phase 1/1b study of the stapled peptide ALRN-6924, a dual inhibitor of MDMX and MDM2, as monotherapy or in combination with cytarabine for the treatment of relapsed/refractory AML and advanced MDS with TP53 wild-type. Blood. 2018;132(Suppl 1):4066-4066. [Google Scholar]
- 65.Pollard JA, Alonzo TA, Loken M, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119(16):3705-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollard JA, Loken M, Gerbing RB, et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2016;34(7):747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollard JA, Guest E, Alonzo TA, et al. Gemtuzumab ozogamicin improves event-free survival and reduces relapse in pediatric KMT2A-rearranged AML: results from the phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2021;39(28):3149-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin H, Yang L, Chukinas JA, et al. Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design. J Immunother Cancer. 2021;9(9):e003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamble AJ, Eidenschink Brodersen L, Alonzo TA, et al. CD123 expression is associated with high-risk disease characteristics in childhood acute myeloid leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2022;40(3):252-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamble AJ, Liu X, Minard C, et al. Safety and activity of flotetuzumab in pediatric and young adult patients with relapsed/refractory acute myeloid leukemia: results from the COG PEPN1812 phase 1 trial. Blood. 2022;140(Suppl 1):6209-6210. [Google Scholar]
- 72.Uy GL, Aldoss I, Foster MC, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood. 2021;137(6):751-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vadakekolathu J, Lai C, Reeder S, et al. TP53 abnormalities correlate with immune infiltration and associate with response to flotetuzumab immunotherapy in AML. Blood Adv. 2020;4(20):5011-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gill S, Tasian SK, Ruella M, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tasian SK, Kenderian SS, Shen F, et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood. 2017;129(17):2395-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonifant CL, Szoor A, Torres D, et al. CD123-engager T cells as a novel immunotherapeutic for acute myeloid leukemia. Mol Ther. 2016;24(9):1615-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaidya A, Doherty E, Wu X, et al. Improving the anti-acute myeloid leukemia activity of CD123-specific engager T cells by MyD88 and CD40 costimulation. Haematologica. 2023,108(4):1039-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonti AR, Pardo L, Alonzo TA, et al. Transcriptome profiling of glycosylation genes defines correlation with E-selectin ligand expression and clinical outcome in AML. Blood. 2019;134(Suppl1):3772. [Google Scholar]
- 79.Le Q, Castro S, Tang T, et al. Therapeutic targeting of mesothelin with chimeric antigen receptor T cells in acute myeloid leukemia. Clin Cancer Res. 2021;27(20):5718-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Q, Hadland B, Smith JL, et al. CBFA2T3-GLIS2 model of pediatric acute megakaryoblastic leukemia identifies FOLR1 as a CAR T cell target. J Clin Invest. 2022;132(22):e157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaeding AJ, Barwe SP, Gopalakrishnapillai A, et al. Mesothelin is a novel cell surface disease marker and potential therapeutic target in acute myeloid leukemia. Blood Adv. 2021;5(9):2350-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krawczyk E, Zolov SN, Huang K, Bonifant CL. T-cell activity against AML improved by dual-targeted T cells stimulated through T-cell and IL7 receptors. Cancer Immunol Res. 2019;7(4):683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willier S, Rothämel P, Hastreiter M, et al. CLEC12A and CD33 coexpression as a preferential target for pediatric AML combinatorial immunotherapy. Blood. 2021;137(8):1037-1049. [DOI] [PubMed] [Google Scholar]
- 84.Niswander LM, Graff ZT, Chien CD, et al. Potent preclinical activity of FLT3-directed chimeric antigen receptor T cell immunotherapy against FLT3-mutant acute myeloid leukemia and KMT2A-rearranged acute lymphoblastic leukemia. Haematologica. 2023;108(2):457-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berrien-Elliott MM, Cashen AF, Cubitt CC, et al. Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 2020;10(12):1854-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berrien-Elliott MM, Foltz JA, Russler-Germain DA, et al. Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia. Sci Transl Med. 2022;14(633):eabm1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bednarski JJ, Zimmerman C, Berrien-Elliott MM, et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood. 2022;139(11):1670-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25(8):1769-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krakow EF, Summers C, Dahlberg A, et al. Phase I study of adoptive immunotherapy with HA-1-specific CD8+ and CD4+ memory T cells for children and adults with relapsed acute leukemia after allogeneic hematopoietic stem cell transplantation (HCT): trial in progress. Blood. 2020;136(Suppl 1):45-46. [Google Scholar]
- 90.Umeda M, Ma J, Huang BJ, et al. Integrated genomic analysis identifies UBTF tandem duplications as a recurrent lesion in pediatric acute myeloid leukemia. Blood Cancer Discov. 2022;3(3):194-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fornerod M, Ma J, Noort S, et al. Integrative genomic analysis of pediatric myeloid-related acute leukemias identifies novel subtypes and prognostic indicators. Blood Cancer Discov. 2021;2(6):586-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elsayed AH, Rafiee R, Cao X, et al. A six-gene leukemic stem cell score identifies high risk pediatric acute myeloid leukemia. Leukemia. 2020;34(3):735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang BJ, Smith JL, Farrar JE, et al. Integrated stem cell signature and cytomolecular risk determination in pediatric acute myeloid leukemia. Nat Commun. 2022;13(1):5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyner JW, Tognon CE, Bottomly D, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562(7728):526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kongtim P, Percival M-EM, Martins TJ, et al. Long-term outcomes of a clinical trial of molecular and functional drug screening for individualized therapy in relapsed and refractory (R/R) acute leukemia (AL). Blood. 2022;140(Suppl 1):1200-1202.35767897 [Google Scholar]
- 96.Pikman Y, Tasian SK, Sulis ML, et al. Matched targeted therapy for pediatric patients with relapsed, refractory, or high-risk leukemias: a report from the LEAP consortium. Cancer Discov. 2021;11(6):1424-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kornauth C, Pemovska T, Vladimer GI, et al. Functional precision medicine provides clinical benefit in advanced aggressive hematologic cancers and identifies exceptional responders. Cancer Discov. 2022;12(2):372-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malani D, Kumar A, Brück O, et al. Implementing a functional precision medicine tumor board for acute myeloid leukemia. Cancer Discov. 2022;12(2):388-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pearson ADJ, Zwaan CM, Kolb EA, et al. Paediatric Strategy Forum for medicinal product development for acute myeloid leukaemia in children and adolescents: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration. Eur J Cancer. 2020;136:116-129. [DOI] [PMC free article] [PubMed] [Google Scholar]