Abstract
Outcomes for patients with acute myeloid leukemia (AML) remain poor due to the inability of current therapeutic regimens to fully eradicate disease-initiating leukemia stem cells (LSC). Previous studies have demonstrated that oxidative phosphorylation (OXPHOS) is an essential process that is targetable in LSC. Sirtuin 3 (SIRT3), a mitochondrial deacetylase with a multi-faceted role in metabolic regulation, has been shown to regulate OXPHOS in cancer models; however, it has not yet been studied in the context of LSC. Thus, we sought to identify if SIRT3 is important for LSC function. Using RNAi and a SIRT3 inhibitor (YC8-02), we demonstrate that SIRT3 is a critical target for the survival of primary human LSC but is not essential for normal human hematopoietic stem and progenitor cell function. In order to elucidate the molecular mechanisms by which SIRT3 is essential in LSC we combined transcriptomic, proteomic, and lipidomic approaches, showing that SIRT3 is important for LSC function through the regulation of fatty acid oxidation (FAO) which is required to support OXPHOS and ATP production in human LSC. Further, we discovered two approaches to further sensitize LSC to SIRT3 inhibition. First, we found that LSC tolerate the toxic effects of fatty acid accumulation induced by SIRT3 inhibition by up-regulating cholesterol esterification. Disruption of cholesterol homeostasis sensitizes LSC to YC8-02 and potentiates LSC death. Second, SIRT3 inhibition sensitizes LSC to the BCL-2 inhibitor venetoclax. Together, these findings establish SIRT3 as a regulator of lipid metabolism and potential therapeutic target in primitive AML cells.
Introduction
Acute myeloid leukemia (AML) is a devastating disease with a high rate of relapse and poor survival outcomes.1 In many patients, disease relapse is caused by the persistence of the disease-initiating leukemic stem cells (LSC),2 necessitating the development of LSC-directed therapies. LSC uniquely rely on oxidative phosphorylation (OXPHOS) for ATP production and ultimately their survival.3 One family of proteins which have been shown to regulate energy metabolism in cancer are sirtuins (SIRT). The SIRT family of proteins are responsible for removing multiple post-translational modifications including acetylation, ribosylation, succinylation, and malonylation. Although SIRT are highly studied in the context of aging and cancer,4 less is known about the role of SIRT in cancer stem cells (CSC). Sirtuins 1,5-7 28 and 69 have been associated with CSC in solid tumors. In AML, sirtuins 1,10,11 2,12,13 3,14,15 5,16 6,17 and 717 have been shown to play important roles in leukemic survival, however only SIRT1 has been reported to regulate LSC function.10,11
SIRT3 is one of three SIRT which localize to the mitochondria where it plays a key role in orchestrating several critical metabolic pathways through deacetylation of mitochondrial proteins on lysine residues.18 In cancer cells, SIRT3 has been shown to suppress reactive oxygen species (ROS) levels, promote glutamine metabolism, regulate fatty acid synthesis, inhibit and promote glycoly-sis, regulate iron metabolism, decrease HIF1α activity, and increase the activity of the citric acid cycle.15,19-21 However, the function of SIRT3 in LSC has not been elucidated. In this study we show that SIRT3 is critical for LSC function in part by promoting fatty acid oxidation (FAO). Inhibition of SIRT3 results in the loss of energy production and fatty acid accumulation in LSC. Further, we demonstrate that LSC are uniquely protected from fatty acid-induced cell death by upregulating protective cholesterol metabolism pathways, which can be targeted to further sensitize LSC to SIRT3 inhibition. Finally, we demonstrate that SIRT3 inhibition sensitizes LSC to the BCL-2 inhibitor venetoclax which is commonly used to treat chemotherapy ineligible AML patients.
Methods
Primary acute myeloid leukemia and cell culture
Primary cells obtained were obtained from donors who gave informed consent for sample procurement under the Princess Margaret Leukemia Tissue Bank protocol and analyzed with University Health Network Research Ethics Board approval (20-5031). Cells were cultured in X-Vivo10 media (Lonza; 04-380Q), supplemented with 20% BIT (StemCell; 09500) and cytokines (IL-3, IL-6, SCF, FLT3 ligand). Patient details are available in the Online Supplementary Table S1. Additional culture information is available in the Online Supplementary Appendix.
BODIPY staining
BODIPY 581/591 C11 was prepared in media at 2-4 mM. Cells were incubated for 20-45 minutes at 37°C.
Inhibitors/chemicals
YC8-02 was synthesized as previously described.19 Dipyridamole was obtained from Selleck Chemicals (S1895-10 mM/1 mL). Dimethyl 2-oxogluterate (349631-5G) was obtained from Sigma-Aldrich. Linoleic acid (L8134), palmitic acid 13C16 (605573), and palmitic acid 16-13C (605646) were obtained from Sigma-Aldrich. Venetoclax was obtained from Cedarlane (HY-15531-500 mg).
CD34 enrichment
Cord blood (CB) samples were CD34+ enriched using the Miltenyi Biotec’s CD34 microbead magnetic separation kit (130-046-702) following product specifications.
Animal studies
Animal studies were performed in accordance with UHN’s Animal Resource Center, under animal use protocol 6366 as previously described.22 Detailed methods are available in the Online Supplementary Appendix.
Flow cytometry
Primary AML samples were sorted for enriched LSC and blast populations as previously described.23 Additional information is available in the Online Supplementary Appendix.
RNA sequencing
Primary samples enriched for LSC, were treated with YC8-02 inhibitor at 10 mM and 25 mM for 4 hours. RNA was isolated using Qiagen’s RNeasy micro kit (74004). Sample quality was assessed using Agilent Bioanalyzer prior to library preparation and sequencing. RNA sequencing was performed using Illumina Novaseq 6000 using a 100-cycle paired-read protocol with multiplexing resulting in ~40 million reads/sample. Detailed data analysis available in the Online Supplementary Appendix. SIRT3 network analysis is also available in the Online Supplementary Appendix.
Proximity-dependent biotinylation
BioID was performed as previously described.24 Detailed methods are available in the Online Supplementary Appendix.
Immunobloting
Cell lysates were loaded on 4-15% precast gels (Bio-Rad) and transferred to PVDF membrane (Bio-RAD). Blots were probed with primary anti-FLAG antibody (Sigma-Aldrich, 8146S), GAPDH antibody (SantaCruz; sc-32233), or SIRT3 antibody (Cell Signaling; 5490S) overnight at 4°C, followed by 1-hour incubation with secondary antibody (LI-COR Bio-sciences, IRDye® 680RD). Blots were imaged using Odyssey® DLx system (LI-COR Biosciences).
Quantitative real-time polymerase chain reaction
RNA was isolated as described above, cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad; 1708891) and quantitative real-time polymerase chain reaction (RT-qPCR) was performed using Itaq Universal SYBR (BioRad; 1725122).
Enzyme activity and ATP quantification assays
ATP levels were quantified using a kit Roche (11699709001) following the manufacture’s protocol.
Other methods
Colony-forming unit (CFU) assays,22 small interfering RNA (siRNA) transfection,25 seahorse analysis,22 and metabolic analysis26 were performed as previously described. Additional information is available in the Online Supplementary Appendix.
Results
SIRT3 is essential for acute myeloid leukemia survival
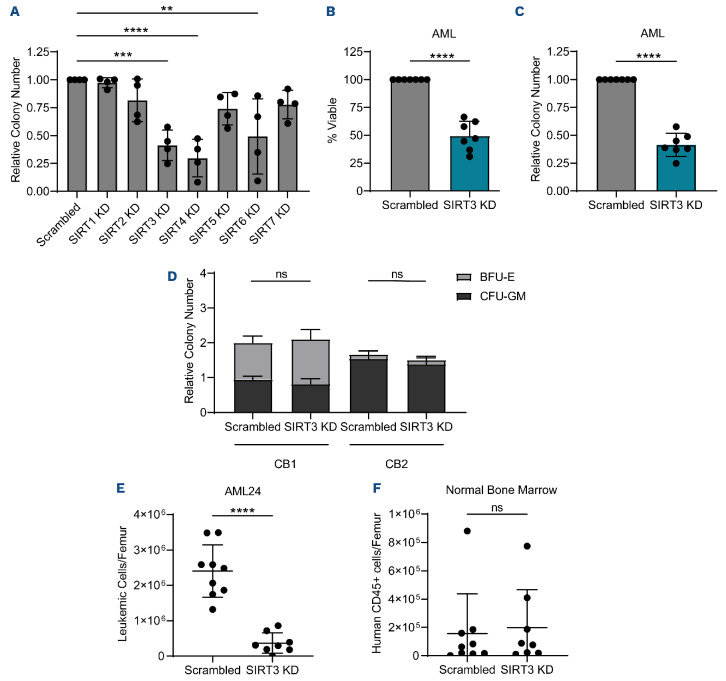
In order to establish whether SIRT were essential in LSC, we used siRNA to knockdown each sirtuin in four primary AML specimens (Online Supplementary Figure S1A), then measured cell viability (Online Supplementary Figure S1B) and colony-forming potential (Figure 1A). These data revealed that knockdown of SIRT3 and SIRT4 consistently decreased the viability and colony-forming potential of AML specimens. We chose to focus our subsequent analysis on SIRT3 because of its well characterized role in mitochondrial energy metabolism, an Achilles heel of LSC.3 Knockdown of SIRT3 in three additional primary AML specimens (Online Supplementary Figure S1C) and the Molm13 AML cell line confirmed that SIRT3 knockdown decreased AML viability and colony-forming potential (Figure 1B, C; Online Supplementary Figure S1D, E). Importantly, knockdown of SIRT3 in CD34+ enriched human CB, did not alter colony-forming ability (Figure 1D; Online Supplementary Figure S1F). In order to understand the impact of SIRT3 on stemness, we evaluated the colony-forming ability of primary AML specimens and normal bone marrow upon secondary replating of colonies. In four primary AML specimens, colony-forming potential was significantly decreased upon replating of SIRT3 knockdown cells compared to the non-targeting control (Online Supplementary Figure S1G, H). In contrast, there was no effect on the serial colony-forming ability of normal bone marrow samples upon knockdown (Online Supplementary Figure S1I, J) demonstrating a potential therapeutic window to target SIRT3 in AML while minimally affecting normal hematopoietic stem and progenitor cells (HSPC).
In order to assess the impact of SIRT3 on LSC specifically, we knocked down SIRT3 in enriched LSC and AML blast populations from primary AML patient specimens. LSC and AML blasts were enriched using relative reactive oxygen species (ROS) level as previously described.23 LSC enrichment was validated by measuring colony-forming potential (Online Supplementary Figure S1K) which revealed that cells with relatively low levels of ROS (enriched LSC) had increased colony-forming potential compared to cells with high levels of ROS (enriched AML blasts). We also confirmed that ROS low cells are enriched for CD34+, a well-established marker of LSC,27 (Online Supplementary Figure S1L) as previously shown.22 When SIRT3 was knocked down in enriched LSC populations, colony-forming potential was significantly decreased (Online Supplementary Figure S1M, N).
Next, we used the gold-standard assay for assessing LSC and HSC function, engraftment into immune deficient mice28 upon SIRT3 knockdown. SIRT3 was knocked down by siRNA in a primary human AML and a normal bone marrow sample, then transplanted into NSG-SGM3 mice (Online Supplementary Figure S1O). SIRT3 knockdown (Online Supplementary Figure S1P) resulted in a significant decrease in AML engraftment (Figure 1E) but did not impair the engraftment or lineage output of normal bone marrow (Figure 1F; Online Supplementary Figure S1Q, R).
Perturbation of SIRT3 targets leukemia stem cells
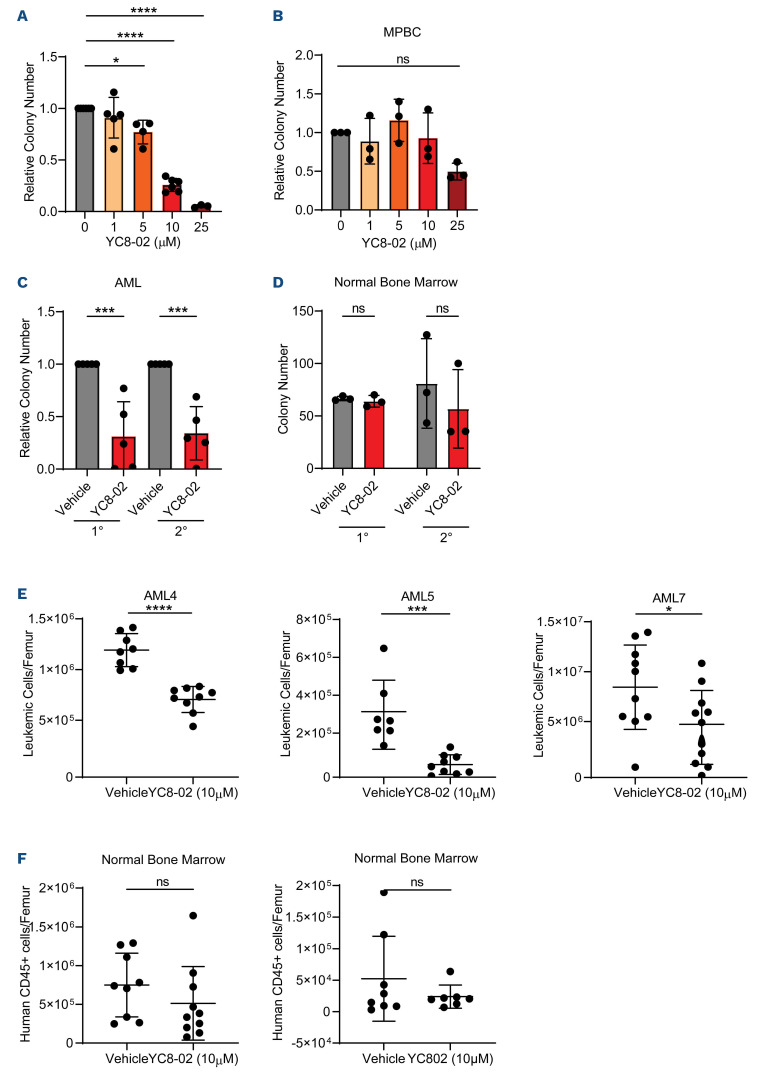
In order to further evaluate SIRT3 in AML, we assessed viability and colony-forming potential upon treatment with a SIRT3 inhibitor, YC8-0219,29 which has previously been shown to target mitochondrial SIRT3 with minor effects on other class one sirtuin proteins, SIRT1 and SIRT2. Importantly, knockdown of SIRT1 and SIRT2 did not affect AML viability or colony-forming potential (Figure 1A; Online Supplementary Figure S1B) suggesting that any effects of YC8-02 in AML would be SIRT3-mediated. In line with our SIRT3-knockdown experiments, cell viability and colony-forming potential of AML cell lines was significantly decreased upon YC8-02 treatment (Online Supplementary Figure S2A, B), which correlated with a dose-dependent increase in apoptosis (Online Supplementary Figure S2C). We then determined the effect of YC8-02 on LSC and blasts enriched from primary AML. Enriched populations were cultured for 48 hours with or without YC8-02 before assessing viability and colony-forming ability. YC8-02 treatment resulted in a significant decrease in LSC and AML blast viability (Online Supplementary Figure S2D) and a decrease in colony-forming potential of LSC compared to vehicle treatment (Figure 2A). SIRT3 inhibition upon YC8-02 treatment did not decrease the CD34+ cell frequency or colony-forming potential of HSPC isolated from MPBC (Online Supplementary Figure S2B, E). In order to evaluate the effect of YC8-02 on stemness, secondary colony-forming potential was assessed in AML and normal bone marrow. Upon secondary replating of colonies, the colony-forming potential was significantly decreased in AML and unaffected in normal bone marrow when treated with YC8-02 (Figure 2C, D).
Finally, to assess the effect of SIRT3 inhibition on LSC and HSPC function, we used the gold-standard assay, engraftment into immune deficient mice. Three primary human AML samples and two normal bone marrow specimens were treated with YC8-02 for 24 hours, then transplanted into NSG-SGM3 mice. YC8-02 treatment resulted in a significant decrease in AML engraftment for each AML specimen (Figure 2E) but did not significantly impair the engraftment or lineage output of normal bone marrow (Figure 2F; Online Supplementary Figure S2F). These findings demonstrate that inhibiting SIRT3 significantly impairs LSC function and demonstrate a potential therapeutic window in which LSC can be targeted with minimal effects on normal bone marrow.
Figure 1.
SIRT3 knockdown targets acute myeloid leukemia but not hematopoietic stem and progenitor cells. (A) Colony-forming ability of four primary acute myeloid leukemia (AML) specimens (AML1-4) post scrambled and sirturin (SIRT) targeting small interfering RNA (siRNA) transfection. Colony-forming unit (CFU) assay was prepared immediately after electroporation. Statistical significance was determined by one-way ANOVA analysis. Each dot represents a primary AML specimen. (B) Viability of bulk AML 48 hours post scrambled or SIRT3 targeting siRNA transfection in 7 primary AML specimens (AML 1-5, 14 and 15). Statistical significance was determined using a paired t-test. Each dot represents a primary AML specimen. (C) Colony-forming potential of bulk AML post scrambled or SIRT3 targeting siRNA transfection in 7 primary AML specimens (AML. 1-5, 14, and 15). CFU assay was prepared immediately after electroporation. Statistical significance was determined using a paired t-test. Each dot represents a primary AML specimen. (D) Colony-forming potential of 2 CD34-enriched cord blood samples post scrambled or SIRT3 targeting siRNA transfection. CFU assay was prepared immediately after electroporation. Statistical significance was determined using a paired t-test. (E) Engraftment of AML24 post scrambled or SIRT3 targeting siRNA transfection. Each point represents a single mouse. Statistical significance was determined using an unpaired t-test. (F) Engraftment of normal bone marrow post scrambled or SIRT3 targeting siRNA transfection. Each point represents a single mouse. Statistical significance was determined using an unpaired t-test. All error bars represent standard deviation. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant.
SIRT3 regulates mitochondrial energy metabolism
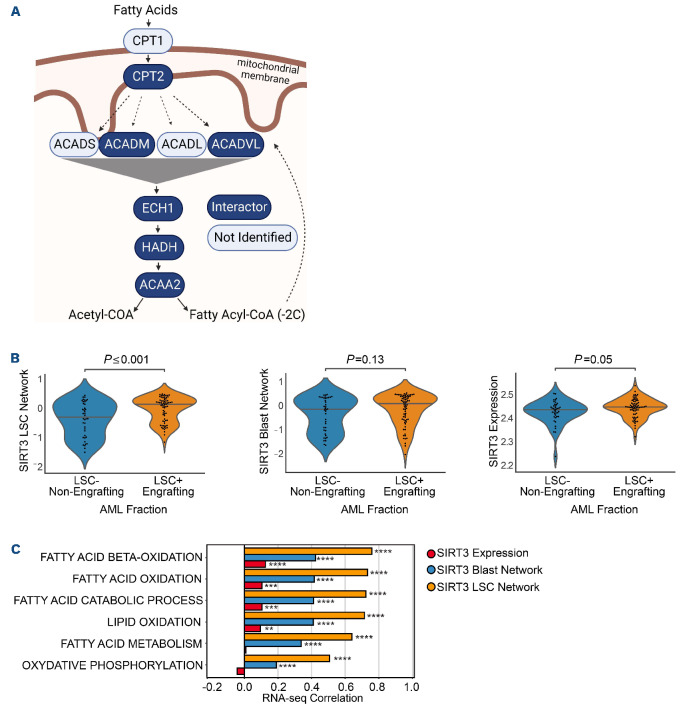
In order to determine the molecular mechanisms by which SIRT3 is important in LSC and AML, we performed proximity-dependent biotin labeling (BioID) to map the SIRT3 interactome. SIRT3 was expressed in 293 Flp-In cells (Online Supplementary Figure S3A), with an in-frame C-terminal BirA*-Flag tag, and BioID was conducted as previously described.30 SIRT3 interactors identified by mass spectrometry were compared to those interacting with the Flag-BirA* tag alone, and with the interactomes of two other mitochondrial matrix proteins, NLN31 and ClpP.32 After subtraction of interactors detected with all three mitochondrial proteins, 316 high confidence SIRT3-specific proximity interactors were identified (Online Supplementary Table S2). Three-hundred and one of these hits (95%) are annotated as mitochondrial proteins (Online Supplementary Table S2), linked to lipid, amino acid, and carbohydrate metabolism as well as tricarboxylic acid (TCA) cycle, and respiratory electron transport functions (Online Supplementary Figure S3B). We next compared our BioID analysis to two previously published SIRT3 proteomic analyses: one which interrogated SIRT3 interactors by SIRT3 immunoprecipitation followed by mass spectrometry (IP-MS) and another identified differentially acetylated proteins differentially acetylated upon SIRT3 knockout. SIRT3 IP-MS studies identified 84 interactors, 44 of which were also identified in our BioID analysis (Online Supplementary Figure S3B, red outlines33). We also identified 54 previously reported SIRT3 de-acetylation substrates (Online Supplementary Figure S3B in rectangular nodes34). Overall, these data suggest that SIRT3 may have an important function in regulating mitochondrial energy metabolism, but which metabolic pathway(s) are essential for LSC function were unclear.
Figure 2.
SIRT3 inhibition perturbs leukemia stem cell function and spares normal hematopoietic stem and progenitor cells. (A) Colony-forming ability of reactive-oxygen species (ROS) low leukemia stem cells (LSC) was assessed from 6 primary acute myeloid leukemia (AML) (AML 4, 5, 7, 8, 9, 14) and treated with YC8-02 for 24 hours at increasing doses, when possible, prior to performing the colony-forming unit (CFU) assay. Each dot represents a unique AML. Statistical significance was determined using ordinary one-way ANOVA. (B) Colony-forming ability of representative mobilized peripheral blood cells (MPBC) following treatment with YC8-02 for 24 hours at increasing doses prior to performing the CFU assay. Statistical significance was determined using two-way ANOVA. (C) Serial colony-forming ability of primary AML 7, 9, 10, and 24 treated with 10 µM YC8-02 for 24 hours. Each dot represents a unique AML. Statistical significance was determined using an unpaired t-test. (D) Serial colony-forming ability of normal bone marrow treated with 10 µM YC8-02 for 24 hours. Statistical significance was determined using an unpaired t-test. (E) Engraftment of 3 primary AML treated with YC8-02. Each point represents a single mouse. Statistical significance was determined using an unpaired t-test. (F) Engraftment of 2 normal bone marrow specimens in NSG-SGM3 mice following treatment with YC8-02. Each point represents a single mouse. Statistical significance was determined using an unpaired t-test. All error bars represent standard deviation. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant.
While BioID has been previously used to interrogate the functions of proteins in AML,31,32 one limitation of these studies is that it is not currently feasible to perform this analysis in rare cell populations like LSC. In order to interrogate SIRT3 biology specifically in enriched LSC and AML blasts, we performed a global transcriptomic analysis on LSC and blasts upon YC8-02 treatment. LSC and AML blasts from three primary AML specimens were treated with vehicle or YC8-02 for 4 hours. Eight hundred and sixty-nine and 57 genes were significantly altered in LSC and AML blasts upon SIRT3 inhibition, respectively. Gene set enrichment analysis demonstrated significant alterations in several biological functions including pathways pertaining to mitochondrial metabolism (Online Supplementary Figure S3C). Intriguingly, OXPHOS and FAO were amongst the most highly enriched gene sets in LSC (Online Supplementary Figure S3D, E). Notably, proteins involved in each step of FAO were identified as SIRT3 proximity interactors (Figure 3A). Next, we integrated the BioID and gene expression data with the goal of identifying genes most likely to be regulated by SIRT3 in LSC. Specifically, we identified transcripts that were significantly altered in LSC or AML blasts upon YC8-02 treatment, and which encode SIRT3 proximity interactors, to generate SIRT3 LSC and SIRT3 AML blast networks comprising 45 and nine genes, respectively (Online Supplementary Figure S3F; Online Supplementary Table S3). Utilizing published datasets (details available in the Online Supplementary Appendix), we observed that the SIRT3 LSC network but not the SIRT3 AML blast network was enriched in functional LSC compared to non-LSC. It is also notable that SIRT3 itself was also enriched in LSC populations but to a lesser extent (Figure 3B). Finally, to interrogate the biological significance of these SIRT3 networks, we correlated the GSVA enrichment scores of each SIRT3 network against enrichment scores of biological pathways across 812 primary AML samples. This analysis revealed correlations with several fatty acid metabolism and OX-PHOS processes that were strongest with the SIRT3 LSC network compared to the SIRT3 blast network or SIRT3 itself (Figure 3C). Together, the integration of our proteomic and transcriptomic data suggests a role of SIRT3 in the regulation of OXPHOS and fatty acid metabolism in LSC.
SIRT3 regulates OXPHOS
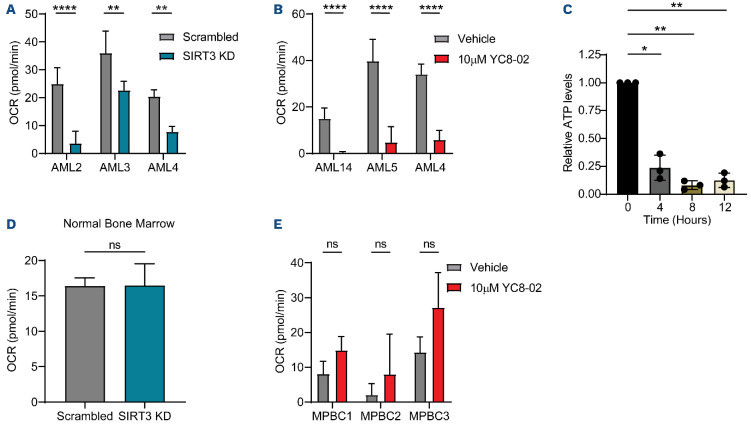
In order to functionally demonstrate a link between SIRT3 and OXPHOS in LSC we measured OXPHOS in AML cell lines, primary AML specimens and LSC upon SIRT3 perturbation using a seahorse assay. OXPHOS was decreased in Molm13, MV4;11, and TEX cells, after 24 hours of SIRT3 knockdown (Online Supplementary Figure S4A, B) and upon 4, 8, and 24 hours of YC8-02 treatment at increasing doses (Online Supplementary Figure S4C-E). These changes were evident as early as 4 hours post treatment, and the effects were further increased at later time points (8 and 24 hours) in a dose-dependent manner. Next, we examined the effect of SIRT3 knockdown (Figure 4A) and YC8-02 treatment (Figure 4B) in primary AML specimens. SIRT3 perturbation significantly decreased basal respiration in AML specimens (Figure 4A) and primary human LSC (Figure 4B). Importantly, decreased OXPHOS corresponded with a decrease in cellular ATP in LSC (Figure 4C) indicating that SIRT3 perturbation disrupted mitochondrial energy metabolism. In contrast, SIRT3 knockdown and YC8-02 treatment of normal bone marrow and MPBC respectively did not result in decreased OXPHOS (Figure 4D, E). We observed no compensatory effect of glycolysis in the cell lines or the primary AML specimens (Online Supplementary Figure S4F-I), consistent with findings that show LSC are uniquely reliant on OXPHOS and cannot compensate with upregulation of glycolysis.35 In normal HSPC, we observed a minimal increase in glycolysis upon SIRT3 knockdown (Online Supplementary Figure S4J) and no change in glycolysis upon YC8-02 treatment (Online Supplemental Figure S4K). Overall, these data demonstrate that SIRT3 perturbation results in decreased OXPHOS in both primary LSC and cell lines, resulting in reduced cellular ATP, the likely cause of SIRT3 mediated cell death.
Figure 3.
SIRT3 regulates mitochondrial energy metabolism in acute myeloid leukemia. (A) Fatty acid oxidation proteins identified as proximity interactors of SIRT3 (dark blue) or not identified as interactors (light blue). (B) Expression of SIRT3 leukemia stem cells (LSC) network, SIRT3 blast network and SIRT3 individually in functional LSC and non-LSC based using GSVA on quantile normalized microarray data (for the networks) or quantile normalized microarray (for SIRT3 alone). Data derived from 220 sorted fractions.33 (C) Correlation of metabolic pathways with SIRT3 LSC network, SIRT3 blast network and SIRT3 individually. Bars represent the Pearson correlation across 812 diagnostic acute myeloid leukemia (AML) patients from TCGA, Beat-AML and Leucegene databases. Network data was generated by using GSVA on transcript per million (TPM) normalized RNA sequencing (RNA-seq) data and the SIRT3 data was generated using variance stabilizing transformation (VST) normalized RNA-seq data and COMBAT batch corrected. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant.
SIRT3 inhibition results in faty acid accumulation in leukemia stem cells
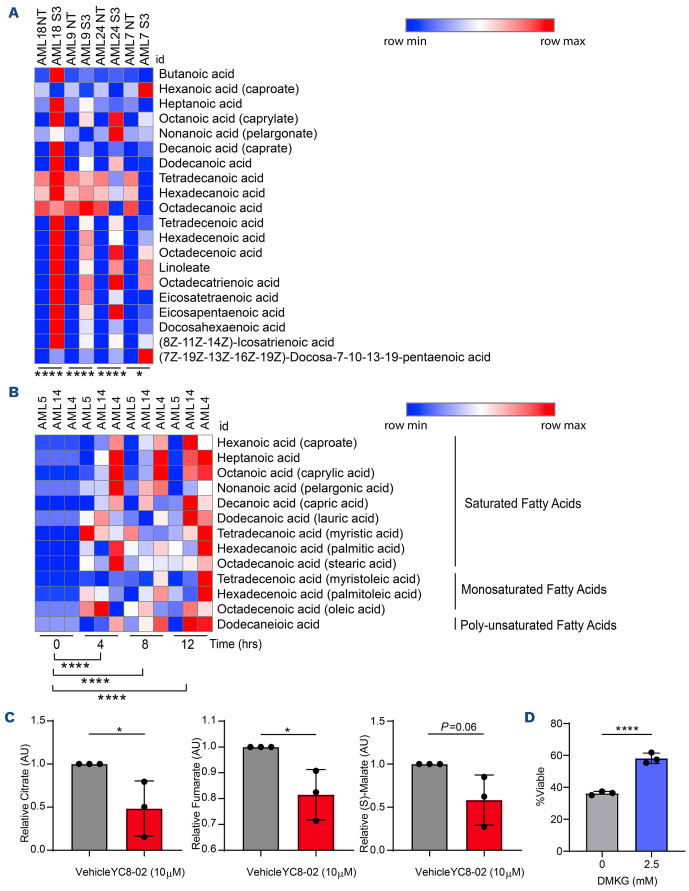
In order to determine if SIRT3 decreases OXPHOS by perturbing fatty acid metabolism in LSC, we performed mass spectrometry-mediated metabolomic analyses. In AML cell lines treated with YC8-02 for 4 hours, there was a significant elevation of fatty acid levels (Online Supplementary Figure S5A). We next examined fatty acid levels in four primary AML specimens upon SIRT3 knockdown for 24 hours, as well as LSC enriched from three primary AML specimens treated with YC8-02 for 0, 4, 8, or 12 hours. Upon SIRT3 perturbation, fatty acids levels were significantly increased in the AML samples (Figure 5A) and LSC (Figure 5B) as early as 4 hours post YC8-02 treatment. No other metabolites previously associated with SIRT3 function such as glutamate19 or succinate36 were significantly altered at 4 hours post YC8-02 treatment (data not shown).
SIRT3 inhibition decreases faty acid oxidation in leukemia stem cells
Based on our data above, we hypothesized that fatty acid levels are increased upon SIRT3 perturbation due to a reduction in FAO. In order to test this, we performed a stable isotope labeled (SIL) tracing analysis using 13C16-palmitate (Online Supplementary Figure S5B) and examined the incorporation of 13C carbons into TCA cycle intermediates. First, 13C16-palmitate tracing analysis was performed on Molm13 cells 24 hours after electroporation with control or SIRT3 targeting siRNA (Online Supplementary Figure S5C). SIRT3 knockdown resulted in a loss of TCA cycle intermediates including citrate, succinate, and fumarate derived from 13C16-palmitate, demonstrating that SIRT3 perturbation in AML decreases FAO. We then treated the Molm13 cell line with 10 mM YC8-02 for 4 hours followed by a 4- or 16-hour treatment with SIL palmitic acid (Online Supplementary Figure S5D). SIRT3 inhibition also resulted in a significant decrease of 13C enrichment in TCA cycle intermediates.
Figure 4.
SIRT3 regulates oxidative phosphorylation. (A) Basal respiration of 3 bulk primary acute myeloid leukemia (AML) transfected with small interfering RNA (siRNA) targeting SIRT3 or a non-targeting scrambled siRNA. Measurements taken 24 hours post electroporation. Statistical significance was determined using an unpaired t-test. (B) Basal respiration of leukemia stem cells (LSC) enriched from 3 primary AML treated with YC8-02 for 12 hours prior to read out. Statistical significance was determined using an unpaired t-test. (C) Total cellular ATP quantified from bulk AML (AML 4, 5, and 14) treated with 10 µM of YC8-02 for 4, 8, or 12 hours. ATP quantities are normalized to the baseline measurement. Each dot represents an AML specimen. Statistical significance was determined using RM one-way ANOVA. (D) Basal respiration of normal bone marrow samples transfected with siRNA targeting SIRT3 or a non-targeting scrambled siRNA. Measurements taken 24 hours post electroporation. Statistical significance was determined using an unpaired t-test. (E) Basal respiration of three mobilized peripheral blood cell (MPBC) samples treated with YC8-02 for 12 hours prior to readout. Statistical significance was determined using an unpaired t-test. All error bars represent standard deviation. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant. OCR: oxygen consumption rate; KD: knockout.
Next, we confirmed our findings in primary AML specimens. We performed SIL tracing using three primary AML cells treated for 4 hours with 10 mM YC8-02 followed by introduction of SIL palmitic acid for an additional 16 hours. Compared to control, treatment with YC8-02 resulted in a decrease of TCA cycle intermediates in bulk primary AML specimens (Online Supplementary Figure S5E), enriched LSC (Figure 5C), and to a lesser extent, in enriched AML blasts (Online Supplementary Figure S5F). In order to demonstrate that decreased FAO is essential to the mechanism by which SIRT3 mediated LSC death, we performed a rescue experiment by adding back the TCA cycle intermediate α-ketoglutarate analog dimethyl-2-oxogluterate (DMKG). The addition of DMKG partially rescued loss of viability upon YC8-02 treatment in primary AML but not AML cell lines (Figure 5D; Online Supplementary Figure S5G). It is important to note that recent studies highlight that DMKG affects metabolic processes outside of TCA metabolism.37 Overall, these data indicate that decreased FAO is one essential part of the mechanism by which SIRT3 inhibition targets LSC.
Elevated cholesterol metabolism protects leukemia stem cells from lipid accumulation
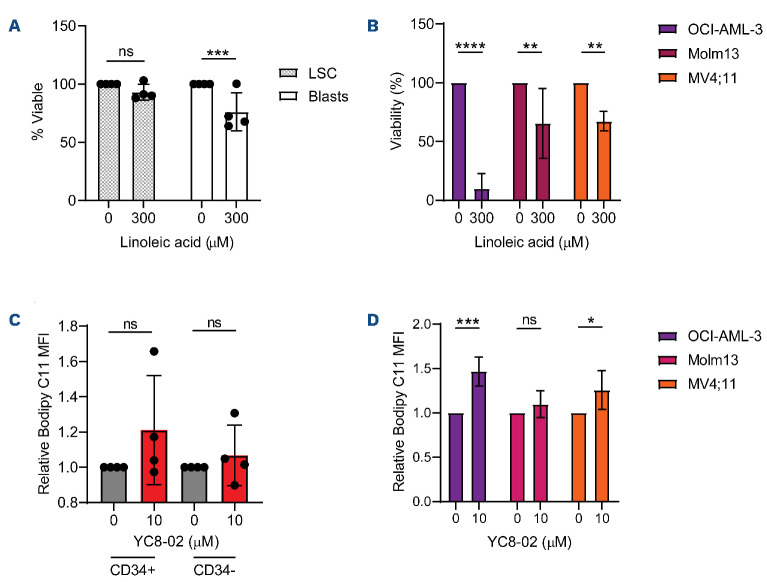
We hypothesized that lipid accumulation may also be resulting in AML cell death through lipotoxicity38 which is why we observed a partial and lack of rescue by DMKG treatment in LSC and AML cell lines respectively. Further, we postulated that LSC may be protected from lipid-mediated death due to metabolic compensatory pathways. In order to test this hypothesis, we phenocopied conditions for lipotoxicity by treating cell lines, primary human LSC, and AML blasts with linoleic acid.39,40 Strikingly different responses were observed between the primary cells (Figure 6A) and the cell lines (Figure 6B). While the cell lines, and to a lesser extent, AML blasts, have reduced viability when exposed to linoleic acid, LSC displayed no reduction in cell viability, indicating that LSC have intrinsic mechanisms to protect them from lipotoxicity. Further, primary AML specimens, both CD34+ and CD34- cells, did not display a significant increase in lipid ROS upon SIRT3 inhibition (Figure 6C). In contrast, cell lines treated with YC8-02 showed a significant accumulation lipid ROS staining (Figure 6D) in a manner corresponding to the sensitivity of each cell line to lipotoxic cell death (Figure 6B). These results show that lipid peroxidation likely plays a role in cell line-mediated cell death upon YC8-02 treatment in AML cell lines. In addition, these data suggest that the loss of LSC viability upon SIRT3 inhibition, could be further enhanced by targeting protective mechanisms to lipotoxicity.
Figure 5.
SIRT3 inhibition results in faty acid accumulation in leukemia stem cells. (A) Fatty acids measured by steady state mass spectrometry lipidomics on 4 bulk acute myeloid leukemia (AML) transfected with small interfering RNA (siRNA) targeting SIRT3 or a non-targeting scrambled siRNA. Samples were collected 24 hours post transfection. Significance was determined using a paired t-test. (B) Fatty acids in leukemia stem cells (LSC) enriched from three primary AML and treated with 10 µM YC8-02 for 4, 8 or 12 hours. Significance was determined using a paired t-test. (C) Stable isotope tracing analysis of LSC enriched from 3 primary AML specimens (AML 8, 10 and 12) treated with vehicle or 10 µM YC8-02 prior to introduction of 13C16-palmitate. Three TCA intermediates were detected in these analyses. Each point represents a unique AML specimen. Statistical significance was determined using an unpaired t-test. (D) Result of tricarboxylic acid cycle (TCA) cycle rescue on viability using bulk AML (AML 11). Cells were treated with dimethyl-2-oxoglutarate (DMKG) at 0 mM or 2.5 mM for 1 hour prior to introduction of 25 µM of YC8-02. Cells were incubated for 48 hours prior to analysis. Statistical significance was determined using ordinary one-way ANOVA. All error bars represent standard deviation. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant.
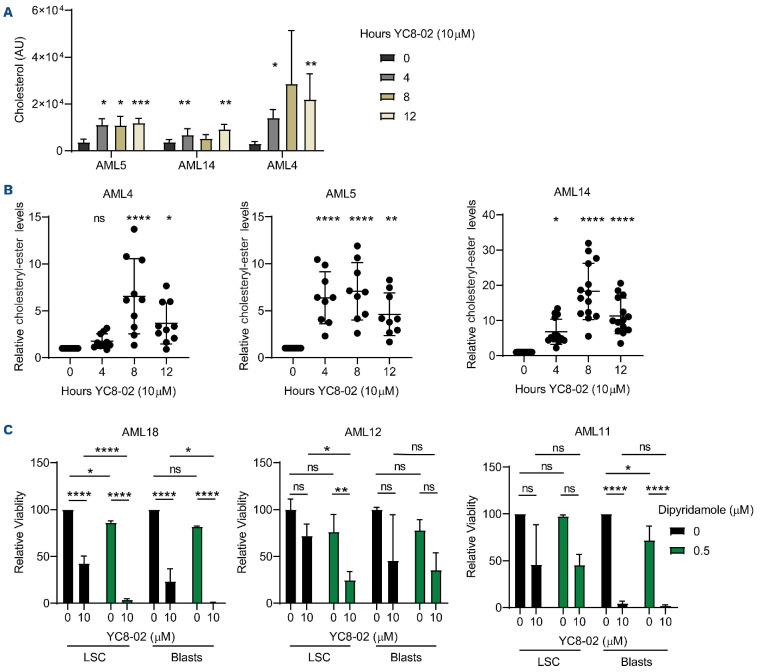
Cholesterol homeostasis is a potential mechanism that protects cells from lipotoxicity through cholesteryl-ester formation.41 Specifically, the carboxylate group of fatty acids can form an ester bond with the hydroxyl group of cholesterols to form cholesteryl-esters which are less toxic to cells. In order to determine if LSC were protected from fatty acid accumulation through altered cholesterol metabolism, we measured both cholesterols and cholesteryl-esters accumulated in the primary LSC following 4, 8, and 12 hours of YC8-02 treatment (Figure 7A, B). Cholesterol and cholesteryl-ester levels were significantly elevated as early as 4 hours post drug treatment, consistent with the time point we observed an increase in fatty acid levels. In contrast, the AML cells lines, Molm13, OCI-AML-3, and MV4;11 did not display any changes in cholesterol or cholesteryl-ester levels in response to YC8-02 treatment (Online Supplementary Figure S6A, B). We hypothesized that primary AML cells were able to protect themselves against lipid accumulation by inducing cholesterol esterification. In order to test this hypothesis, we targeted the master regulators of cholesterol homeostasis sterol regulatory-element binding protein 1 and 2 (SREBP1 and SREBP2) which have been proposed to protect cells from lipotoxicity.38,42-44 We used the SREBP1 and 2 inhibitor dipyridamole42,45,46 alone or in combination with YC8-02 and measured LSC and blast viability. The effect of YC8-02 was potentiated by dipyridamole treatment in LSC isolated from two of the three AML specimens examined (Figure 7C). Further, the combination of SIRT3 knockdown with dipyridamole treatment resulted in significantly more cell death than single treatments (Online Supplementary Figure S6C, D). In contrast, YC8-02 and dipyridamole treatment did not affect normal CD34+ cells isolated from cord blood (Online Supplementary Figure S6E).
Figure 6.
Leukemia stem cells are resistant to cell death induced by lipid accumulation. (A) Viability of leukemia stem cells (LSC) and acute myeloid leukemia (AML) blasts enriched from three primary AML (AML 6, 9, and 10) treated with linoleic acid for 48 hours. Statistical significance was determined using two-way ANOVA. (B) Viability of 3 AML cell lines (OCI-AML-3, Molm13, and MV4;11) treated with linoleic acid for 48 hours. Statistical significance was determined using ordinary one-way ANOVA. (C) BODIPY C11 mean florescence intensity (MFI) of primary AML 20, AML 21, AML 22, and AML 23 treated with 10 µM of YC8-02 for 8 hours. Statistical significance was determined using two-way ANOVA. (D) BODIPY C11 MFI of primary AML cell lines treated with 10 µM of YC8-02 for 8 hours. Statistical significance was determined using an unpaired t-test. All error bars represent standard deviation. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant.
Figure 7.
Combined inhibition of SIRT3 and cholesterol metabolism increases cell death. (A) Cholesterol levels detected by steady state mass-spectrometry lipidomic analysis in leukemia stem cells (LSC) enriched from 3 primary acute myeloid leukemia (AML) specimens and treated with 10 µM YC8-02 for 4, 8 or 12 hours. Statistical significance was determined using ordinary one-way ANOVA. (B) Cholesteryl-ester levels detected by steady state mass-spectrometry lipidomic analysis in LSC enriched from 3 primary AML specimens and treated with 10 µM YC8-02 for 4, 8 or 12 hours. Quantities are normalized to baseline control. Statistical significance was determined using ordinary one-way ANOVA. (C) Viability of LSC and AML blasts enriched from 3 primary AML specimens and treated with 10 µM YC8-02 alone or in combination with 0.5 µM dipyridamole. Statistical significance was determined using two-way ANOVA. All error bars represent standard deviation. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant.
YC8-02 treatment sensitizes acute myeloid leukemia cells to venetoclax
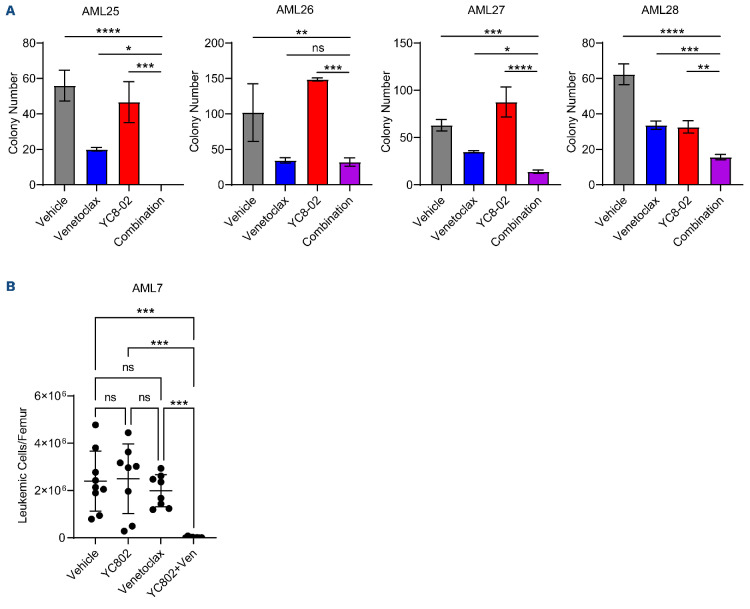
It is highly likely that effective AML therapies will be delivered using combination treatment approaches. Therefore, we sought to determine if SIRT3 inhibition could sensitize AML cells to approved AML therapies including cytarabine, anthracyclines, and venetoclax. YC8-02 treatment sensitized AML cell lines to venetoclax treatment but not cytarabine or doxorubicin (data not shown). Recent research has identified fatty acid metabolism as a mode of resistance for AML cells to venetoclax.22,25,47 Since SIRT3 targets LSC in part through decreasing FAO it is unsurprising that YC8-02 sensitized AML cells to venetoclax. Next, we treated primary AML samples with venetoclax, YC8-02, or a combination of the two and assessed colony-forming ability (Figure 8A). Specimens treated with the combination of YC8-02 and venetoclax had a significantly decreased colony-forming ability compared to the individual treatments alone. We then used the gold-standard engraftment assay on AML samples treated with venetoclax, YC8-02, or a combination to assess their effect on functional LSC (Figure 8B). Strikingly, in a patient sample with no response to YC8-02 or venetoclax, the combination treatment resulted in almost undetectable levels of engraftment. These data suggest that targeting SIRT3 sensitizes LSC to venetoclax.
Discussion
Disease relapse, which originates from residual LSC, remains a major hurdle in AML.2 Thus, it is essential to understand and target the unique properties of LSC to develop new therapies and improve patient outcomes. LSC-directed therapies such as venetoclax paired with hypomethylating agents, have shown tremendous progress in treating a subset of AML patients.48 Here, our results reveal SIRT3 as a novel target to eradicate LSC.
Figure 8.
SIRT3 inhibition improves response to venetoclax. (A) Colony-forming ability of 4 primary acute myeloid leukemia (AML) specimens treated with 10 µM YC8-02, 100 nM of venetoclax or a combination of the two, treated for 72 hours prior to performing colony-forming unit (CFU) assay. Statistical significance was determined using an ordinary one-way ANOVA. (B) Engraftment of AML 7 treated with with 10 µM YC8-02, 100 nM of venetoclax, or a combination of the two for 24 hours prior to engraftment. Each point represents a single mouse. Statistical significance was determined using ordinary one-way ANOVA. All error bars represent standard deviation. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001; ns: not significant.
In this study, we found that knockdown and pharmacologic inhibition of SIRT3 had a significant impact on LSC colony-forming potential and cell viability without significantly impacting normal HSPC function. These results highlight SIRT3 as a promising target for treating AML and demonstrate, for the first time, SIRT3’s roles in CSC. SIRT3 has been found to promote disease progression in a number of cancers including B-cell lymphoma.18,19 However, SIRT3’s role in cancer is not fully elucidated as SIRT3 is considered both a tumor suppressor and oncogene.49 The loss of LSC function upon SIRT3 inhibition was due to decreased OXPHOS, a well-established LSC vulnerability.3,35 Importantly, we demonstrate that reduced OXPHOS levels upon SIRT3 perturbation result in a decrease in ATP indicating that we are indeed decreasing overall energy production. Our findings represent a novel method to target a key metabolic pathway in LSC. Our data demonstrate that SIRT3 contributes to energy metabolism through regulation of fatty acid metabolism, which has been established as critical for AML function and response to therapy.3 As SIRT3 is a known regulator of many metabolic pathways, we sought to determine which pathways were significantly impacted by SIRT3 inhibition using a multi-omics approach, combining transcriptomic, metabolomic, and proteomic analyses. By combining these analyses and assessing several time points, we were able to focus our mechanistic studies on the earliest metabolic changes observed upon SIRT3 perturbation, which revealed FAO as a key metabolic pathway regulated by SIRT3 in LSC. FAO has previously been shown to be a metabolic vulnerability of LSC and represents a mechanism by which LSC become resistant to the BCL-2 inhibitor venetoclax.22,25,47 However, FAO had not been shown to be regulated by SIRT3 in CSC. Our work highlights the importance of examining the role of metabolic regulating proteins in various cancer types and cell types, as the essential metabolic pathways regulated by enzymes like SIRT3 vary depending on cellular context.
Lipid homeostasis and toxicity is an area of much interest in cancer biology and has been implicated in cancer development.38 When we phenocopied lipid accumulation in primary LSC we did not observe any changes in cell viability, unlike AML cell lines. Interestingly, the increase in fatty acids levels correlated with elevated cholesterol and cholesteryl-esters in LSC, but not cell lines. Cholesterol and cholesterol esterification have been proposed to be responsible for preventing lipotoxicity.50 Therefore, to obtain the full benefit of inhibiting fatty acid metabolism, we used dipyridamole to block the compensatory effect of cholesterol homeostasis and thereby enhance the effects of YC8-02 treatment. This finding was unique to LSC, demonstrating that interrogating LSC-specific biology is essential to elucidate novel targetable pathways and resistance mechanisms in AML.
SIRT3 has previously been shown to mediate treatment resistance15 through regulation of OXPHOS. In order to understand how SIRT3 inhibition may be beneficial as part of current patient therapy, we tested our SIRT3 inhibitor in combination with chemotherapy and venetoclax. We show that SIRT3 inhibition sensitizes AML cells to venetoclax. These findings are consistent with previous studies that identified that venetoclax resistance is in part driven by elevation of fatty acid metabolism, and that strategies designed to target fatty acid metabolism can sensitize AML cells to venetoclax.22,25,47 In our study, the combination of SIRT3 inhibition and venetoclax outperformed single agent treatments in primary AML specimens. Overall, we demonstrate that SIRT3 is a key regulator of FAO in LSC. Loss of FAO upon SIRT3 inhibition results in reduced OXPHOS and ATP levels ultimately leading to LSC cell death. We highlight the flexibility of primary AML in revealing the compensatory mechanism to evade fatty acid accumulation by cholesterol biosynthesis and esterification. Further, we demonstrate that SIRT3 inhibition improves response to venetoclax by inhibiting fatty acid metabolism, a mode of venetoclax resistance.
Supplementary Material
Acknowledgments
The authors thank the Leukemia Tissue Bank (Princess Margaret Cancer Center) for providing the primary AML samples. We thank Drs. Aaron Schimmer and Kristin Hope, for thoughtful suggestions regarding this manuscript and Jill Flewelling (Princess Margaret Cancer Center) for administrative assistance.
Funding Statement
Funding: This work was supported by Medical Biophysics Excellence OSOTF, Sona Noran Pancha Graduate Award, David Rae Graduate Student Scholarship (to COB), Medical Biophysics Excellence OSOTF (to TL), NIH 1F31CA250361-01 (to RCH), the Princess Margaret Cancer Center, the Princess Margaret Cancer Foundation, and the Ontario Ministry of Health (to AT, MM, AA, BR, JED and CLJ), the Princess Margaret Cancer Center Foundation, Ontario Institute for Cancer Research through funding provided by the Government of Ontario, Canadian Institutes for Health Research (RN380110-409786), Canadian Cancer Society (grant #703212 [end date 2019], #706662 [end date 2025]), Terry Fox New Frontiers Program Project Grant (project# 1106), a Canada Research Chair (to JED), the University of Colorado Cancer Center Support Grant (P30CA046934) (to AD), the Leukemia and Lymphoma Society, American Society of Hematology, the Canadian Institutes of Health Research, University of Toronto Medicine by Design Initiative which receives funding from the Canada First Research Excellence Fund, and the Ontario Institute of Cancer Research (to CLJ).
References
- 1.Pollyea DA, Jordan CT. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017;129(12):1627-1635. [DOI] [PubMed] [Google Scholar]
- 2.Shlush LI, Mitchell A, Heisler L, et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547(7661):104-108. [DOI] [PubMed] [Google Scholar]
- 3.Jones CL, Inguva A, Jordan CT. Targeting energy metabolism in cancer stem cells: progress and challenges in leukemia and solid tumors. Cell Stem Cell. 2021;28(3):378-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Sun K, Jiao S, et al. High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients. Sci Rep. 2014;4:7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JS, Park JR, Kwon OS, et al. SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of "cancer cells with neural stemness" in a p53-dependent manner. Neuro Oncol. 2015;17(1):95-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An Y, Wang B, Wang X, Dong G, Jia J, Yang Q. SIRT1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop. Cell Death Dis. 2020;11(2):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao D, Mo Y, Li MT, et al. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J Clin Invest. 2014;124(12):5453-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioris RM, Galié M, Ramadori G, et al. SIRT6 suppresses cancer stem-like capacity in tumors with PI3K activation independently of its deacetylase activity. Cell Rep. 2017;18(8):1858-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham A, Qiu S, Chacko BK, et al. SIRT1 regulates metabolism and leukemogenic potential in CML stem cells. J Clin Invest. 2019;129(7):2685-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Osdal T, Ho Y, et al. SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells. Cell Stem Cell. 2014;15(4):431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan L, Klimenkova O, Klimiankou M, et al. The role of sirtuin 2 activation by nicotinamide phosphoribosyltransferase in the aberrant proliferation and survival of myeloid leukemia cells. Haematologica. 2012;97(4):551-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu SN, Wang TS, Li X, Wang YP. SIRT2 activates G6PD to enhance NADPH production and promote leukaemia cell proliferation. Sci Rep. 2016;6:32734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell S, Zhang P, Cannon M, et al. Anti-tumor NAMPT inhibitor, KPT-9274, mediates gender-dependent murine anemia and nephrotoxicity by regulating SIRT3-mediated SOD deacetylation. J Hematol Oncol. 2021;14(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Liu B, Yu D, et al. SIRT3 deacetylase activity confers chemoresistance in AML via regulation of mitochondrial oxidative phosphorylation. Br J Haematol. 2019;187(1):49-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan D, Franzini A, Pomicter AD, et al. SIRT5 is a druggable metabolic vulnerabilty in acute myeloid leukemia. Blood Cancer Discov. 2021;2(3):266-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi X, Jiang Y, Kitano A, et al. Nuclear NAD(+) homeostasis governed by NMNAT1 prevents apoptosis of acute myeloid leukemia stem cells. Sci Adv. 2021;7(30):eabf3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaiswal A, Xudong Z, Zhenyu J, Saretzki G. Mitochondrial sirtuins in stem cells and cancer. Febs J. 2022;289(12):3393-3415. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Chiang YL, Lyssiotis CA, et al. Non-oncogene addiction to SIRT3 plays a critical role in lymphomagenesis. Cancer Cell. 2019;35(6):916-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu LX, Hao LJ, Ma JQ, Liu JK, Hasim A. SIRT3 promotes the invasion and metastasis of cervical cancer cells by regulating fatty acid synthase. Mol Cell Biochem. 2020;464(1-2):11-20. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Q, Zhou J, Li F, et al. The role and therapeutic perspectives of Sirtuin 3 in cancer metabolism reprogramming, metastasis, and chemoresistance. Front Oncol. 2022;12:910963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones CL, Stevens BM, D'Alessandro A, et al. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell. 2018;34(5):724-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens BM, O'Brien C, Jordan CT, Jones CL. Enriching for human acute myeloid leukemia stem cells using reactive oxygen species-based cell sorting. STAR Protoc. 2021;2(1):100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coyaud E, Mis M, Laurent EM, et al. BioID-based identification of Skp Cullin F-box (SCF)β-TrCP1/2 E3 ligase substrates. Mol Cell Proteomics. 2015;14(7):1781-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones CL, Stevens BM, Pollyea DA, et al. Nicotinamide metabolism mediates resistance to venetoclax in relapsed acute myeloid leukemia stem cells. Cell Stem Cell. 2020;27(5):748-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisz JA, Zheng C, D'Alessandro A, Nemkov T. Untargeted and semi-targeted lipid analysis of biological samples using mass spectrometry-based metabolomics. methods Mol Biol. 2019;1978:121-135. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730-737. [DOI] [PubMed] [Google Scholar]
- 28.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645-648. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Teater MR, Hong JY, et al. Translational activation of ATF4 through mitochondrial anaplerotic metabolic pathways is required for DLBCL growth and survival. Blood Cancer Discov. 2022;3(1):50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta GD, Coyaud É, Gonçalves J, et al. A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell. 2015;163(6):1484-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirali S, Botham A, Voisin V, et al. The mitochondrial peptidase, neurolysin, regulates respiratory chain supercomplex formation and is necessary for AML viability. Sci Transl Med. 2020;12(538):eaaz8264. [DOI] [PubMed] [Google Scholar]
- 32.Ishizawa J, Zarabi SF, Davis RE, et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35(5):721-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W, Nagasawa K, Münch C, et al. Mitochondrial sirtuin network reveals dynamic SIRT3-dependent deacetylation in response to membrane depolarization. Cell. 2016;167(4):985-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rardin MJ, Newman JC, Held JM, et al. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci U S A. 2013;110(16):6601-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finley LW, Haas W, Desquiret-Dumas V, et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One. 2011;6(8):e23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker SJ, Encarnación-Rosado J, Hollinshead KER, et al. Spontaneous hydrolysis and spurious metabolic properties of α-ketoglutarate esters. Nat Commun. 2021;12(1):4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broadfield LA, Pane AA, Talebi A, Swinnen JV, Fendt SM. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev Cell. 2021;56(10):1363-1393. [DOI] [PubMed] [Google Scholar]
- 39.Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016;57(8):1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miglietta A, Bozzo F, Bocca C, et al. Conjugated linoleic acid induces apoptosis in MDA-MB-231 breast cancer cells through ERK/MAPK signalling and mitochondrial pathway. Cancer Lett. 2006;234(2):149-157. [DOI] [PubMed] [Google Scholar]
- 41.Williams KJ, Argus JP, Zhu Y, et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res. 2013;73(9):2850-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longo J, Mullen PJ, Yu R, et al. An actionable sterol-regulated feedback loop modulates statin sensitivity in prostate cancer. Mol Metab. 2019;25:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat Rev Endocrinol. 2017;13(12):710-730. [DOI] [PubMed] [Google Scholar]
- 44.Navarro-Imaz H, Chico Y, Rueda Y, Fresnedo O. Channeling of newly synthesized fatty acids to cholesterol esterification limits triglyceride synthesis in SND1-overexpressing hepatoma cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(2):137-146. [DOI] [PubMed] [Google Scholar]
- 45.Pandyra A, Mullen PJ, Kalkat M, et al. Immediate utility of two approved agents to target both the metabolic mevalonate pathway and its restorative feedback loop. Cancer Res. 2014;74(17):4772-4782. [DOI] [PubMed] [Google Scholar]
- 46.Kim HH, Liao JK. Translational therapeutics of dipyridamole. Arterioscler Thromb Vasc Biol. 2008;28(3):s39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens BM, Jones CL, Pollyea DA, et al. Fatty acid metabolism underlies venetoclax resistance in acute myeloid leukemia stem cells. Nat Cancer. 2020;1(12):1176-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollyea DA, Stevens BM, Jones CL, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24(12):1859-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torrens-Mas M, Oliver J, Roca P, Sastre-Serra J. SIRT3: oncogene and tumor suppressor in cancer. Cancers (Basel). 2017;9(7):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2(2):132-141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.