β-thalassemia (β-thal) is a genetic red cell disorder characterized by chronic hemolytic anemia due to ineffective erythropoiesis and reduced red cell survival.1-3 Chronic transfusion and intensive iron chelation are standard treatments for β-thalassemic syndromes,1 but new therapeutic options are being developed, including gene therapy4 and novel pharmacologic approaches. We have shown that mitapivat, a pyruvate kinase activator, improves anemia and ineffective erythropoiesis in Hbbth3/+ mice, a widely used model for β-thal.5 The effects of mitapivat are not limited to the erythroid compartment: mitapivat also modulates DMT1 expression, controlling iron absorption in the duodenum in Hbbth3/+ mice, with an increase of hepcidin related to the improvement in ineffective β-thalassemic erythropoiesis.5 Results from a phase II trial of mitapivat in non–transfusion-dependent β-thal patients previously demonstrated a sustained long-term increase in hemoglobin (Hb ≥1 g/dL) with improvement of hemolysis and ineffective erythropoiesis.6 Here, we asked whether mitapivat might be a potential therapeutic option also for β-thal patients under chronic transfusion regimen. In order to address this question, we exposed female Hbbth3/+ mice (3-4 months of age) to chronic transfusion with or without mitapivat (50 mg/kg twice daily [BID]). Hbbth3/+ mice were treated by oral gavage with mitapivat (50 mg/kg BID) or vehicle for 10 days, and then transfused with 400 µL washed red blood cells at 40-45% hematocrit (Hct)7 (Figure 1A). We defined Hb values ≤10.5 g/dL as the transfusion threshold, corresponding to the reduction of ~50% of post-transfusion Hb values. Normality of data was assessed with the Shapiro-Wilk test. Two-tailed unpaired Student t-test or two-way analysis of variance with Tukey’s multiple comparisons were used for data analyses. Data show values from individual mice and are presented with mean ± standard error of the mean (differences with P<0.05 were considered significant).
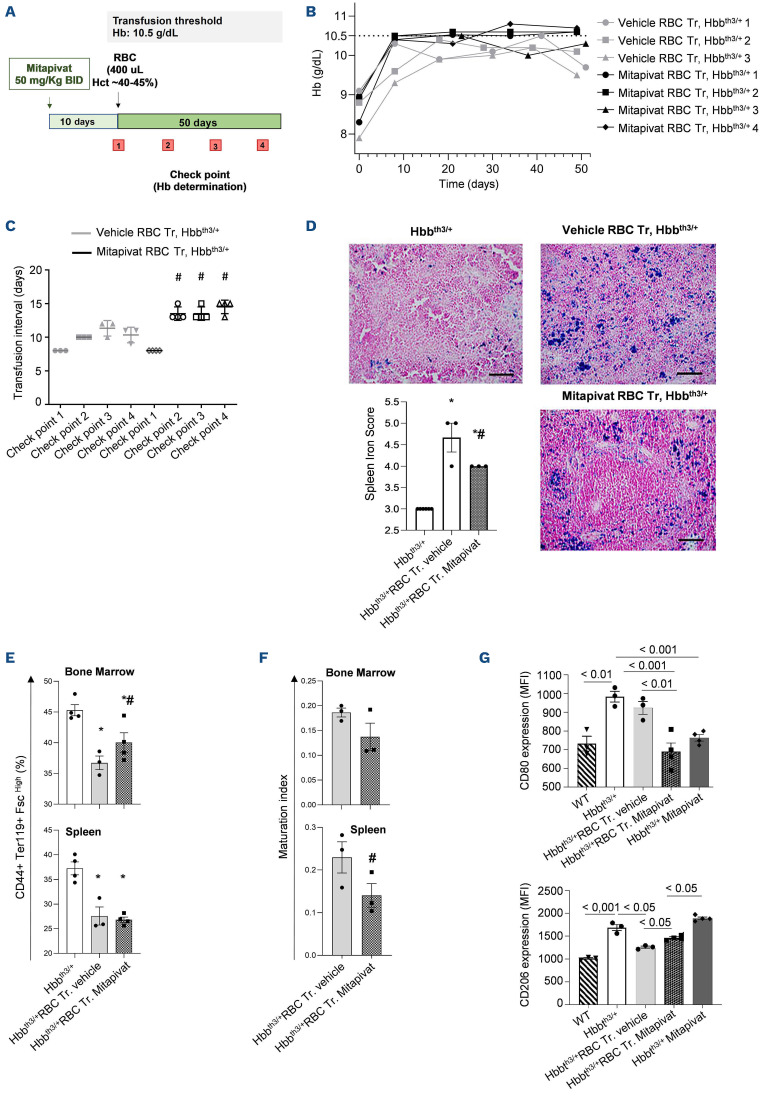
As shown in Figure 1B, mitapivat-treated β-thal mice exposed to chronic transfusion displayed a greater sustained rise in Hb from baseline compared to vehicle-treated transfused β-thal mice. This resulted in a longer interval between transfusions (13.8±1.0 days in mitapivat-treated β-thal mice vs. 10.5±1.0 days in vehicle-treated β-thal mice; Figure 1C). Chronic transfusion resulted in a significant reduction of splenomegaly in both mitapivat- and vehicle-treated β-thal mice (Online Supplementary Figure 1SA) compared to untreated β-thal mice, but spleen iron accumulation was significantly lower in mitapivat-treated β-thal mice when compared to vehicle-treated β-thal mice (Figure 1D). A significant reduction of both bone marrow and spleen ineffective erythropoiesis was observed in all transfused β-thal mice (Figure 1E; Online Supplementary Figure S1B). Of note, mitapivat-treated transfused β-thal mice showed a slight increase of bone marrow erythropoiesis with a trend towards an improvement of maturation index compared to vehicle-treated transfused β-thal mice evaluated at the end of the study.5 This is most likely related to a protective effect of mitapivat on residual bone marrow and spleen erythropoiesis (Figure 1F). Indeed, plasma erythropoietin was lower in mitapivat-treated transfused β-thal mice than in vehicle-treated transfused β-thal mice (Online Supplementary Figure S1C). Since splenic macrophages contribute to both erythrophagocytosis and iron recycling, we evaluated the functional profile of spleen macrophages in the different mouse groups.8 As shown in Figure 1G, flow cytometric analysis of the surface expression of the M1 marker CD80 and the M2 marker CD206 on spleen macrophages (MΦ) revealed that mitapivat promoted a proresolving profile of splenic macrophages in transfused β-thal mice when compared to vehicle-treated transfused β-thal mice (Online Supplementary Figure S2A). This effect was still observed in non-transfused mitapivat-treated mice compared to vehicle-treated β-thal mice (Figure 1G; Online Supplementary Figure S2A). Collectively, these data support the role of mitapivat in reprograming macrophages from proinflammatory to proresolving and repairing the phenotype in β-thal mice with or without chronic transfusion.9
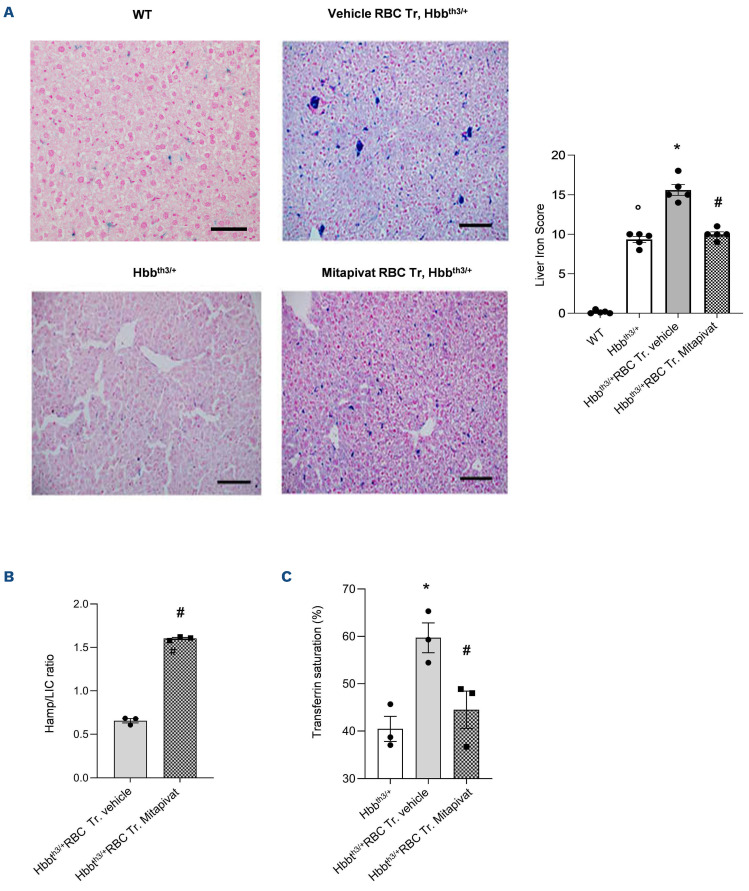
We then evaluated the impact of mitapivat on iron metabolism in transfused β-thal mice. Mitapivat-treated transfused β-thal mice showed lower liver iron accumulation when compared to vehicle-treated transfused βthal mice (Figure 2A). This might be due in part to the reduction of the transfusion burden but also to the multimodal action of mitapivat, which we previously showed to modulate hepcidin indirectly by the reduction of ineffective erythropoiesis and downregulation of DMT1 expression in the duodenum.5 Indeed, in mitapivat-treated transfused β-thal, we found a significant increase in liver hepcidin/LIC ratio (Figure 2B) and a marked reduction in the percentage of serum transferrin saturation when compared to vehicle-treated transfused β-thal mice (Figure 2C). The reduced transfusion burden observed in mitapivat treated β-thal mice might favorably contribute to the general reduction of iron-overload in β-thal mice exposed to chronic transfusion. Our preclinical results in combination with clinical data from non-transfusion-dependent β-thal patients treated with mitapivat6 suggest that the increase in the length of time between transfusions with mitapivat treatment may be associated with improvement in the quality of life in patients as well as a decrease in iron-overload-related organ damage.
Figure 1.
Mitapivat reduces transfusion burden in β-thalassemia mice exposed to chronic transfusion with associated repro-gramming of splenic macrophage phenotype. (A) Experimental study design to assess the effects of mitapivat on hematologic phenotype of β-thalassemia (β-thal) mice exposed to chronic transfusion. (B) Hemoglobin (Hb) changes over time in transfused (Tr.) β-thal (Hbbth3/+) mice treated with either vehicle or mitapivat (50 mg/kg twice daily [BID]) shown as single animals (n=3 vehicle-treated mice; n=4 mitapivat-treated mice). Grey dotted line shows the transfusion threshold (10.5 g/dL). (C) Transfusion time intervals in β-thal (Hbbth3/+) mice treated with either vehicle or mitapivat (50 mg/kg BID). Data are presented as means ± standard error of the mean (SEM) (n=3 vehicle-treated mice; n=4 mitapivat-treated mice); #P<0.05 compared to vehicle-treated transfused β-thal mice. (D) Iron staining (Perl’s Prussian blue is a semi-quantitative method to assess organ iron accumulation) in spleen from Hbbth3/+ mice treated with either vehicle or transfusion plus vehicle or transfusion plus mitapivat. One representative image from 3 with similar results. Left panel: quantification of iron staining in spleen. Data are mean ± SEM (n=3). *P<0.05 compared with vehicle Hbbth3/+ mice and #P<0.05 compared with vehicle-treated transfused Hbbth3/+ mice. (E) Flow cytometric analysis (CD44+Ter119+ and cell size markers, see also the Online Supplementary Figure S2) of bone marrow and spleen from Hbbth3/+ mice exposed to either vehicle or to chronic transfusion with and without mitapivat treatment (see also Matte et al.5). Data are mean ± SEM (n=3-4). *P<0.05 compared with vehicle Hbbth3/+ mice and #P<0.05 compared with vehicle-treated transfused Hbbth3/+ mice. (F) Maturation index as ratio between pop II (Baso E.) and pop IV (Ortho E.) in bone marrow and spleen from Hbbth3/+ mice treated with either vehicle or exposed to chronic transfusion with or without mitapivat, analyzed by flow cytometry. Data are mean ± SEM (n=3-4). (G) Flow cytometric quantification of M1 (CD80) and M2 (CD206) expression on spleen macrophage cell surface from wild-type (WT) or Hbbth3/+ mice exposed to either vehicle or mitapivat or to chronic transfusion with and without mitapivat treatment. Spleen macrophages (MΦ) were isolated with the GentleMACS cell dissociator (Miltenyi Biotech, Germany). MΦ were identified and gated as CD45+/F4/80+ cells. Anti-CD45 PE-Cy5.5, F4/80 PE, CD206 PerCP-Cy5.5 and CD80 were from BioLegend, USA. Data are mean ± SEM (n=3-4). MFI: mean fluorescence intensity; RBC: red blood cells.
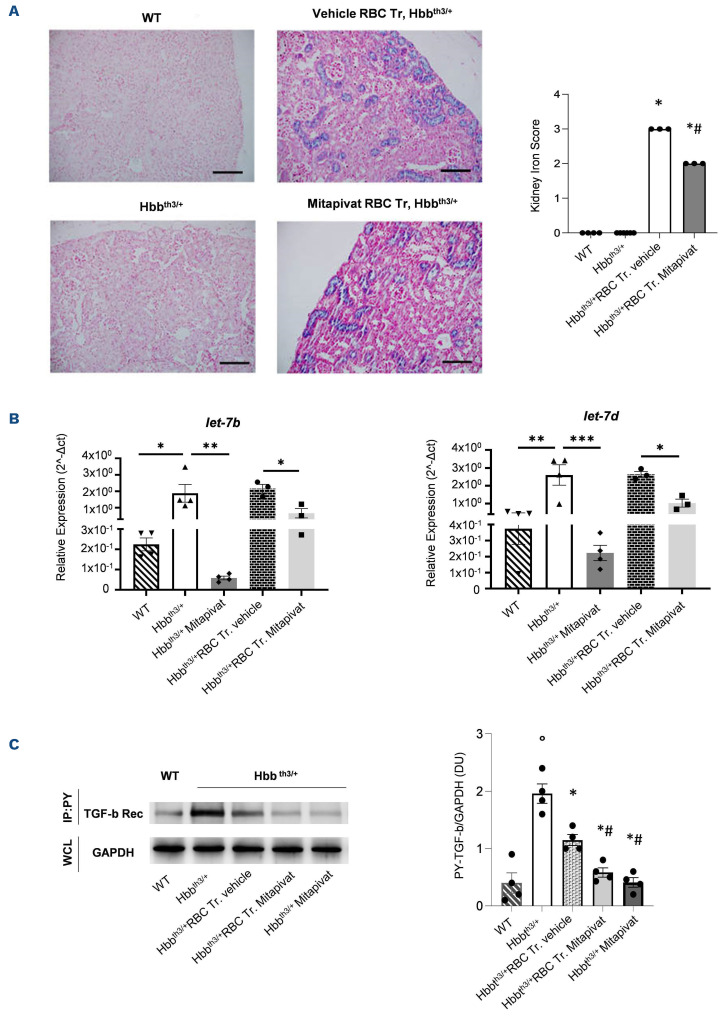
Recent reports in transfusion-dependent β-thal patients have highlighted a correlation between ferritin levels and kidney iron accumulation assessed by magnetic resonance T2* imaging, or in sample analysis from kidney biopsies or autopsy series.10 Kidney iron overload mainly involved the tubular compartment which has been related to chronic anemia and might be reversed by iron chelation.10 In vehicle-treated transfused β-thal mice, we found tubular accumulation of iron, which was significantly reduced in mitapivat-treated transfused animals (Figure 3A). No major difference in creatinine was observed in both β-thal mouse groups exposed to chronic transfusion (Online Supplementary Figure S2B). Previous studies suggest that kidney iron accumulation promotes local oxidative stress, contributing to profibrotic signaling in addition to hypoxia.10,11 MicroRNA (miRNA) let-7b, -c, and -d have been shown to be linked to renal fibrosis throughout the transforming growth factor-β cascade (TGF-β).12 In this study, miRNA let-7b and -d were upregulated in vehicle-treated β-thal mice with or without chronic transfusion (Figure 3B; Online Supplementary Figure S2C), while mitapivat downregulated miRNA let-7b and -d in β-thal mice with or without chronic transfusion (Figure 3B; Online Supplementary Figure S2C).
miRNA let-7 have been reported to reduce ATP production by deactivating pyruvate dehydrogenase kinase (PDK).13 Here, we found normalization of the amount of the active form of the TGF-β receptor in β-thal mice treated with mitapivat when compared to vehicle-treated β-thal mice with or without transfusion (Figure 3C). Previously, in β-thal mice, the activation of TGF-β receptor was reduced by chronic transfusion, hypoxia being a trigger of activation of TGF-β receptor.14 Taken together, our data indicate that mitapivat might play a pivotal role in kidney protection by reducing the transfusion burden and iron overload as well as by preserving energy cell metabolism. This might represent an added value of mitapivat as a therapeutic option for patients with β-thal taking iron chelators who develop renal toxicity or chronic kidney disease.
Finally, we explored the effects of the co-administration of mitapivat and deferiprone (DFP) on β-thal mice, since iron chelation is part of the gold standard treatment of β-thal patients.1 DFP was administered to Hbbth3/+ mice treated with mitapivat in drinking water at the dosage of either 1.25 or 0.75 mg/mL15 (Online Supplementary Figure S3A). Previously, Casu et al. reported that DFP alone has no effect on hematologic parameters and red cell features in murine β-thal.15 The beneficial effects of mitapivat on murine β-thal anemia was maintained when mitapivat was co-administered with DFP at both dosages, as supported by the stable and sustained increase in Hb and the reduction in circulating erythroblasts compared to baseline values (Online Supplementary Figure S3B, C). In agreement with Matte et al.,5 we found a significant reduction in α-globin membrane precipitates in red blood cells from mitapivat DFP-treated Hbbth3/+ mice compared with vehicle-treated animals (Online Supplementary Figure S3D). Of note, DFP iron chelation efficacy represented by a change in LIC was preserved in β-thal mice treated with both DFP and mitapivat (Online Supplementary Figure S3E).
In conclusion, our study shows for the first time that mitapivat improves the transfusion burden and reduces organ iron overload in β-thal mice exposed to a chronic transfusion regimen. We also observed that mitapivat might protect the kidney against profibrotic stimuli related to local iron accumulation by two different mechanisms: the reduction in transfusion requirement and the local modulation of miRNA involved in profibrotic signaling. Finally, the observed reprograming of spleen macrophages toward a proresolving phenotype might represent an added value to the known improvement of ineffective erythropoiesis induced by mitapivat in β-thal mice.5 Thus, the beneficial effects of mitapivat in β-thal mice exposed to chronic transfusion support its use as a potential new therapeutic tool in clinical management of thalassemic patients under chronic transfusion regimen.
Figure 2.
Mitapivat-treated transfused β-thalassemia mice show reduced liver iron accumulation and improved iron homeostasis. (A) Left and central panels: iron staining (Perl’s Prussian blue is a semi-quantitative method to assess organ iron accumulation) in liver from wild-type (WT) and Hbbth3/+ mice treated with either vehicle or transfusion (Tr.) or transfusion plus mitapivat. One representative image from 5 with similar results. Right panel: quantification of iron staining in liver. Data are mean ± standard error of the mean (SEM) (n=5). °P<0.05 compared to WT, *P<0.05 compared with vehicle Hbbth3/+ mice and #P<0.05 compared with vehicle-treated transfused (Tr.) Hbbth3/+ mice. (B) Liver mRNA expression normalized over liver iron concentration (LIC) as determined using the bathophenanthroline method. Data are presented as means ± SEM (n=3). #P<0.05 compared with vehicle-treated transfused Hbbth3/+ mice. (C) Transferrin saturation in Hbbth3/+ mice treated with either vehicle or transfusion or transfusion plus mitapivat. Transferrin saturation was calculated as the ratio between serum iron and total iron binding capacity, using the Total Iron Binding Capacity Kit (Randox Laboratories, UK) and 50 mL of serum, according to the manufacturer’s instructions. Data are presented as means ± SEM (n=3). *P<0.05 compared with vehicle Hbbth3/+ mice and #P<0.05 compared with vehicle-treated transfused Hbbth3/+ mice. RBC: red blood cells.
Figure 3.
In transfused ^-thalassemia mice, mitapivat reduces kidney iron accumulation and downregulates profibrotic kidney miRNA let-7 expression. (A) Upper panels: iron staining (Perl’s Prussian blue is a semi-quantitative method to assess organ iron accumulation) in kidney from wild-type (WT) and Hbbth3/+ mice treated with either vehicle or transfusion (Tr.) or transfusion plus mitapivat. One representative image from 3-6 with similar results. Lower panels: quantification of iron staining in kidney. Data are mean ± standard error of the mean (SEM) (n=3-6). *P<0.05 compared with vehicle Hbbth3/+ mice and #P<0.05 compared with vehicle-treated transfused (Tr.) Hbbth3/+ mice. (B) Relative expression of miRNA let-7b and -7d in kidneys from WT or Hbbth3/+ mice exposed to either vehicle or mitapivat or to chronic transfusion with and without mitapivat treatment. Small RNA was isolated from frozen kidneys using a silica spin column-based Quick-RNA kit (Zymo Research), quantified with a UV NanoPhotometer (Implen), and reverse transcribed with the qScript microRNA cDNA Synthesis for RT-PCR (QuantaBio). For real time polymerase chain reaction (RT-PCR) analysis of let-7b and let-7d miRNA, 3 ng of cDNA were used as a template in reaction mixtures (10 mL final volume) including a PowerUp SYBR Green Master Mix (5 mL, Applied Biosystems), miRNA-specific forward and universal reverse primers (1 mL each, miRCURY assays, Qiagen), and PCR-grade water. The expression of the indicated mRNA was quantitated by the comparative ΔCt method. RNU6-1 was used as control for normalization. Data are mean ± SEM (n=3-4). *P<0.05. **P<0.01. ***P<0.001. (C ) Phospho-tyrosine immunoprecipitation of kidneys from WT or Hbbth3/+ mice exposed to either vehicle or mitapivat or to chronic transfusion with and without mitapivat treatment, using anti-phospho-tyrosine specific antibodies (IP: PY, clone PY99 from SCBT, Santa Cruz, CA and clone 4G10 from Merck KGaA, Darmstadt, Germany), revealed with anti-TGF-β receptor (Rec) specific antibody. GAPDH in whole-cell lysate (WCL) is used as loading control. One representative gel from 4 others with similar results is presented. Blots were developed using the Luminata Forte Chemiluminescent HRP Substrate from Merck Millipore (Armstadt, Germany), and images were acquired with the Alliance Q9 Advanced imaging system (Uvitec, UK). Densitometric analysis of immunoblots is shown on the right. Data are mean ± SEM (n=4). °P<0.05 compared to WT; *P<0.05 compared with vehicle Hbbth3/+ mice, #P<0.05 compared with vehicle-treated transfused Hbbth3/+ mice. RBC: red blood cells.
Supplementary Material
Funding Statement
Funding: This study was supported by an Agios Pharmaceuticals, Inc. research collaborative grant to LDF. Editorial assistance was provided by Avant Healthcare, LLC and Excel Medical Affairs, Horsham, UK, supported by Agios.
References
- 1.Taher AT, Musallam KM, Cappellini MD. Beta-Thalassemias. N Engl J Med. 2021;384(8):727-743. [DOI] [PubMed] [Google Scholar]
- 2.De Franceschi L, Bertoldi M, Matte A, et al. Oxidative stress and β-thalassemic erythroid cells behind the molecular defect. Oxid Med Cell Longev. 2013;2013:985210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivella S. β-thalassemias: paradigmatic diseases for scientific discoveries and development of innovative therapies. Haematologica. 2015;100(4):418-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locatelli F, Thompson AA, Kwiatkowski JL, et al. Betibeglogene autotemcel gene therapy for non-beta(0)/beta(0) genotype beta-Thalassemia. N Engl J Med. 2022;386(5):415-427. [DOI] [PubMed] [Google Scholar]
- 5.Matte A, Federti E, Kung C, et al. The pyruvate kinase activator mitapivat reduces hemolysis and improves anemia in a beta-thalassemia mouse model. J Clin Invest. 2021;131(10):e144206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo KHM, Layton DM, Lal A, et al. Safety and efficacy of mitapivat, an oral pyruvate kinase activator, in adults with non-transfusion dependent alpha-thalassaemia or beta-thalassaemia: an open-label, multicentre, phase 2 study. Lancet. 2022;400(10351):493-501. [DOI] [PubMed] [Google Scholar]
- 7.Park SY, Matte A, Jung Y, et al. Pathologic angiogenesis in the bone marrow of humanized sickle cell mice is reversed by blood transfusion. Blood. 2020;135(23):2071-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos P, Casu C, Gardenghi S, et al. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med. 2013;19(4):437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvan-Pena S, O'Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demosthenous C, Vlachaki E, Apostolou C, et al. Beta-thalassemia: renal complications and mechanisms: a narrative review. Hematology. 2019;24(1):426-438. [DOI] [PubMed] [Google Scholar]
- 11.Musallam KM, Taher AT. Mechanisms of renal disease in beta-thalassemia. J Am Soc Nephrol. 2012;23(8):1299-1302. [DOI] [PubMed] [Google Scholar]
- 12.Hong S, Lu Y. Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: targeting novel lipid mediator pathways in mitigation of acute kidney injury. Front Immunol. 2013;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma X, Li C, Sun L, et al. Lin28/let-7 axis regulates aerobic glycolysis and cancer progression via PDK1. Nat Commun. 2014;5:5212. [DOI] [PubMed] [Google Scholar]
- 14.Chou YH, Pan SY, Shao YH, et al. Methylation in pericytes after acute injury promotes chronic kidney disease. J Clin Invest. 2020;130(9):4845-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casu C, Aghajan M, Oikonomidou PR, et al. Combination of Tmprss6- ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica. 2016;101(1):e8-e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.