Abstract
Interleukin (IL)-21 is essential for type 1 diabetes (T1D) development in the NOD mouse model. IL-21-expressing CD4 T cells are present in pancreatic islets where they contribute to T1D progression. However, little is known about their phenotype and differentiation states. To fill this gap, we generated a novel IL-21 reporter NOD strain to further characterize IL-21+ CD4 T cells in T1D. IL-21+ CD4 T cells accumulate in pancreatic islets and recognize β-cell antigens. Single-cell RNA sequencing revealed that CD4 T effector cells in islets actively express IL-21 and they are highly diabetogenic despite expressing multiple inhibitory molecules, including PD-1 and LAG3. Islet IL-21+ CD4 T cells segregate into four phenotypically and transcriptionally distinct differentiation states, less differentiated early effectors, Tfh-like cells, and two Th1 subsets. Trajectory analysis predicts that early effectors differentiate into both Tfh-like and terminal Th1 cells. We further demonstrated that intrinsic IL-27 signaling controls the differentiation of islet IL-21+ CD4 T cells, contributing to their helper function. Collectively, our study reveals the heterogeneity of islet-infiltrating IL-21+ CD4 T cells and indicates that both Tfh-like and Th1 subsets produce IL-21 throughout their differentiation process, highlighting the important sources of IL-21 in T1D pathogenesis.
Introduction
Interleukin (IL)-21 is a type I cytokine that binds to the IL-21 receptor (IL-21R) and common γ chain (γc) receptor complex and signals through the Janus kinase (JAK)-signal transducer and activator of transcription (STAT), mitogen-activated protein kinase (MAPK), and phosphoinositide 3 kinase (PI3K) pathways (1). IL-21 is mainly produced by CD4 T cells and it exerts its pleiotropic functions in diverse immune cells, including T cells, B cells, NK cells, macrophages, and dendritic cells (DCs) (1). IL-21 regulates T cell differentiation in a context dependent manner (2). In CD4 T cells, IL-21 promotes the differentiation of T follicular helper (Tfh) and Th17 cells but negatively impacts regulatory T cells (Tregs) (3–7). In addition, IL-21 has been shown to enhance Th2 responses and suppress Th1 differentiation (8, 9). In CD8 T cells, IL-21 has been reported to support memory T cell formation during acute viral infection (10). Additionally, IL-21 limits CD8 T cell exhaustion and enhances their effector function during chronic viral infection (11–14). Many of the effects exerted by IL-21 depend on STAT3 although IL-21 also activates STAT1. Accordingly, the balance between these two STAT proteins fine-tunes the outcome of IL-21 stimulation (2).
Studies in many autoimmune diseases, including type 1 diabetes (T1D), have implicated a pathogenic role of IL-21 (15). Genetic ablation of IL-21 or IL-21R completely blocks T1D development in NOD mice (16–19). IL-21 deficiency in CD4 T cells or lack of IL-21R in CD8 T cells inhibits T1D development, indicating that CD4 T cell-derived IL-21 plays an important role in promoting the pathogenic activity of CD8 T cells (20). In addition, IL-21 has been shown to promote T1D in NOD mice by enhancing the maturation and migration of DCs for CD4 T cell priming in pancreatic lymph nodes (PLNs) (21). Evidence supporting that IL-21 plays a role in human T1D includes that significantly higher levels of circulating IL-21+ CD4 T cells and IL-21 protein were found in subjects with β-cell autoimmunity compared to healthy controls (22–25). Furthermore, combined treatment of anti-IL-21 and liraglutide preserved β-cell function in recent onset T1D patients (26). Together, these reports indicate that IL-21+ CD4 T cells play a central role in the pathogenesis of both mouse and human T1D.
Th17, Tfh, and invariant natural killer T (iNKT) cells are the main sources of IL-21 (27). Previous attempt to isolate islet IL-21+ CD4 T cells in NOD mice used CCR9 as a surrogate marker (20). Transcriptional analysis of CCR9+ CD4 T cells in islets of NOD mice revealed that they are not Th17 cells (20). Instead, they are phenotypically similar to, but distinct from, Tfh cells (20). These results suggest that islet-infiltrating IL-21+ CD4 T cells follow a differentiation program that is distinct from previously described CD4 T effector lineages. However, the limitation of this study is that CCR9+ CD4 T cells did not capture the entire IL-21+ population and could contain IL-21− cells. In addition, transcriptional analysis of bulk CCR9+ CD4 T cells did not have sufficient resolution to reveal their differentiation states at a single-cell level. Understanding the differentiation states of islet IL-21+ CD4 T cells and factors that control them will enhance the ability to target these cells for therapeutic purposes. Here, we used a newly generated IL-21 reporter strain and single-cell RNA sequencing (scRNA-seq) to understand the heterogeneity and disease function of islet-infiltrating IL-21+ CD4 T cells in the NOD mouse model of T1D.
Materials and Methods
Mice
NOD/ShiLtJ (NOD) and NOD.B6-Ptprcb/6908MrkTacJ (NOD.CD45.2) mice were purchased from The Jackson Laboratory and maintained at the Medical College of Wisconsin (MCW). IL-21 reporter NOD mice were generated by JAX® Mice Model Generation Service using the CRISPR/Cas9 system to insert an internal ribosome entry site (IRES) followed by the coding sequence for the fluorescent protein Venus immediately downstream of the stop codon in exon 5 of the Il21 gene directly in NOD mouse embryos. A founder was backcrossed to NOD mice for 3 generations. The heterozygous mice (NOD.Il21V/+) used for all experiments were maintained by intercrossing Il21V/+ and Il21+/+ littermates. NOD.Rag1-/−.NY8.3 mice have been described (28). NOD.Il27ra−/− mice have been previously described (29). NOD.Il21V/+ and NOD.CD45.2 strains were crossed and then backcrossed to NOD.CD45.2 to generate NOD.CD45.2.Il21V/+ mice. NOD.Il27ra−/− mice were crossed to the NOD.Il21V/+ strain and then backcrossed to NOD.Il27ra−/− to generate NOD.Il27ra-/−.Il21V/+ mice. All mice were used in accordance with and approved by Institutional Animal Care and Use Committee guidelines at the MCW.
T1D incidence study
Mice were tested weekly for glycosuria (Bayer Diastix®) and considered diabetic after two consecutive readings of >250 mg/dL.
Generation of mixed bone marrow chimeras
For the generation of mixed bone marrow (BM) chimeras, BM cells were collected from 6–9-week-old NOD.Il27ra-/−.Il21V/+ and NOD.CD45.2.Il21V/+ females. T cells were depleted using anti-CD3e microbeads (Miltenyi Biotec). T cell-depleted NOD.Il27ra-/−.Il21V/+ and NOD.CD45.2.Il21V/+ BM cells were mixed at a 1:1 ratio (2.5×106 cells each) and infused into lethally irradiated (1100 rads) 6–8-week-old (NOD x NOD.CD45.2)F1 females.
Flow cytometry
Single cell suspension was prepared from blood, lymph nodes and spleens. Red blood cells were lysed with the ACK lysis buffer then washed cells were suspended in FACS buffer (PBS + sodium azide and 2% FBS). Islet single cell suspension was prepared as previously described (30). Cells were stained at 4°C for 30 minutes for surface markers CD45.1 (A20), CD3e (145–2C11), CD4 (RM4–5 or GK1.5), CD8 (53–6.7), CD11b (M1/70), CD11c (N418), CD19 (1D3), CD44 (IM7), CD127 (A7R34), SLAMF6 (Ly108) (330-AJ), CXCR6 (SA051D1), ICOS (15F9), PD-1 (J43), or LAG3 (eBIOC9B7W). Cells were washed once with FACS buffer and analyzed on the LSRII or LSRFortessa X20 cytometer (BD Biosciences). For MHC class II tetramer staining of islet-infiltrating IL-21+ CD4 T cells, cells were stained at 37°C for one hour with I-Ag7 tetramers loaded with the insulin B:9–23 mimotopes (1:1 mixture of p8G [HLVERLYLVCGGEG] and p8E [HLVERLYLVCGEEG]) or the 2.5-hybrid insulin peptide (HIP) (LQTLALWSRMD). The control tetramers were loaded with PVSKMRMATPLLMQA. All antibodies were from BD Biosciences, Biolegend, or eBioscience. MHC class II tetramers were obtained from the National Institutes of Health Tetramer Core Facility. Dead cells were discriminated using 7-aminoactinomycin (Sigma). For detection of IL-21, islet single cell suspensions were incubated at 37°C for 4 hours in the presence of PMA, ionomycin, and GolgiPlug (BD Biosciences). After labelling with fixable viability dye and surface markers, cells were permeabilized using the Cytofix/Cytoperm fixation and permeabilization kit (BD Biosciences) and incubated with IL-21R/Human Fc fusion protein (R&D Systems) and anti-GFP antibody (Biolegend) followed by anti-human Fc (Biolegend) at 4°C for 30 minutes. For intracellular staining of transcription factors, cells were fixed using the Cytofix/Cytoperm fixation buffer (BD Biosciences) for 30 minutes, washed with BD Perm/Wash buffer (BD Bioscience), and then incubated with ice-cold methanol for 30 minutes. Fixed cells were stained with antibody cocktail containing anti-Bcl6 (clone BCL-DWN), anti-T-bet (clone eBio4B10), or anti-FOXP3 (clone FJK-16s) for 30 minutes at room temperature. Results were analyzed with the FlowJo software (BD Biosciences).
Cell isolation
Islet single cell suspension was prepared as previously described (30). For scRNA-seq, islet cells were stained with antibodies for CD45.1, CD4, CD8, CD11c, and CD19, and sorted into Venus+ and Venus− CD4 T cells on a FACSAria III (BD Biosciences). For analysis of Il21 expression, splenic CD4 T cells were negatively selected using the CD4+ T cell isolation kit (Miltenyi Biotec). Enriched splenic CD4 T cells were stained with anti-CD4 and sorted into Venus+ and Venus− CD4 T cells on a FACSAria II (BD Biosciences). Non-CD4 T cells were plunged off the magnetic column. For adoptive transfer of islet IL-21+ CD4 T cells, islet cells were stained with antibodies for CD45.1, CD4, CD8, CD11c, and CD19, and Venus+ and Venus− CD4+ cells were sorted on a FACSAria III (BD Biosciences). Isolated islet CD4 T cells (5,000 or 25,000 cells/recipient) were injected into NOD.Rag1-/−.NY8.3 mice for testing their diabetogenic activity.
Quantitative polymerase chain reaction (qPCR)
Non-CD4 T cells and sorted Venus+ and Venus− CD4 T cells were lysed in buffer RLT (Qiagen). Total RNA was extracted using the RNeasy Mini kit (Qiagen). First-strand cDNA was synthesized using High-Capacity cDNA Reverse Transcription kit with RNase inhibitor (Applied Biosystems). qPCR was performed on a 7900HT Sequence Detection System (Applied Biosystems) using TaqMan® gene expression assays for Hprt (Mm00440502-M1) and Il21 (Mm00517640_m1). Relative expression of Il21 was determined using Hprt as the internal control using the comparative Ct method.
10x Genomics library preparation and sequencing
Sorted islet Venus+ and Venus− CD4 T cells were used to prepare scRNA-seq libraries using the Chromium Single Cell 3’ v2 Reagent Kit (10x Genomics) as previously described (30). Libraries were sequenced on an Illumina NextSeq using the NextSeq 500/550 High Output Kit v2 (150 cycles) (Illumina). Reads were demultiplexed and converted to gene-barcode matrices with mm10 as the reference transcriptome using the Cell Ranger (version 1.3.1) mkfastq and count functions respectively. The sequencing data are accessible in the GEO database (accession number GSE212009, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE212009).
Single-cell RNA sequencing data analysis
Data analysis was performed in R (version 3.6.2) using the package Seurat (version 3.1.5) (31). Venus+ and Venus− CD4 T cell samples were merged and cells with unique feature counts <200 or >5000 and percent of counts from mitochondrial genes >20% were removed. The unique molecular identifier (UMI) count data was normalized using SCTransform method (32) to adjust for different sequencing depth between samples. Variation from total counts of RNA and percentage of mitochondrial genes are regressed out in a second non-regularized linear regression. Principal component (PC) analysis was performed on the normalized dataset using the top 3000 variably expressed genes and the first 10 PCs were used as input features for following steps. All cells were initially clustered with Shared Nearest Neighbor (SNN) based Louvain clustering algorithm and visualized using Uniform Manifold Approximation and Projection (UMAP). Canonical markers were used to classify cell types. Heatmaps, violin plots, and UMAP plots were generated using the functions DoHeatmap, VlnPlot, and DimPlot respectively. Gene module scores were calculated using the AddModuleScore function. The volcano plot was generated using the R package EnhancedVolcano (version 1.4.0). Cell states prediction was done by projecting our dataset onto the reference atlas of tumor-infiltrating lymphocytes using the ProjecTILs R package (version 2.0.0) (33). For this analysis, we combined cells from clusters 1, 2, and 7 as naïve cells and combined cells from clusters 0, 3, 5, and 8 as IL-21+ cells. For each indicated cluster, the frequency of cells that have the predicted cell state from the reference were calculated. Regulon analysis was performed using the R package SCENIC (34). Raw UMI count matrix was used as the input. Motif database for mouse were downloaded following default settings of SCENIC. The regulons can be divided into two categories: main regulons are identified by using high confidence annotations only (inferred by orthology) and “extended” regulons are identified by using lower confidence annotations (inferred by motif similarity). Differential expression analysis was performed by two-sample t-tests to identify cluster-specific marker regulons (cluster identities from Seurat analysis). Trajectory analysis was performed using the R package Monocle (version 2) (35), where corrected UMI count matrix from Seurat was used as input. Top 100 cluster marker genes from each of cluster 0, 3, 5 and 8 were used as feature genes to define cells’ progress/order and to construct the trajectory. Dimension reduction method DDRTree was used to build a two-dimension tree-like trajectory plot.
Statistical analysis
Statistical tests were performed in R (version 3.6.2) for scRNA-seq related analyses and in Graphpad Prism for Log-rank test and unpaired and paired t test.
Results
Generation and characterization of a new IL-21 reporter NOD mouse strain
To study live IL-21 expressing CD4 T cells in T1D, we generated a novel reporter NOD strain. The CRISPR/Cas9 system was used to insert an internal ribosome entry site (IRES) followed by the coding sequence for the fluorescent protein Venus immediately downstream of the stop codon in exon 5 of the Il21 gene in NOD embryos (Figure 1A). A successfully targeted founder was identified and backcrossed to standard NOD mice for 3 generations to eliminate potential off-target mutations. The Il21 reporter knockin allele was heterozygously maintained for all subsequent experiments (designated NOD.Il21V/+). NOD.Il21V/+ mice had a similar T1D incidence to their wildtype littermate controls and the standard NOD mice in our colony (Figure 1B). Venus and IL-21 proteins were co-expressed in CD4 T cells (Figure 1C). Some cells expressed a lower level of Venus and were IL-21−, likely due to lower sensitivity of intracellular IL-21 staining. Detection of the Il21 transcript was limited to Venus+ CD4 T cells (Figure 1D). Venus (IL-21) protein expression in NOD.Il21V/+ mice was largely restricted to CD4 T cells (Figure 1F). Venus (IL-21)+ CD4 T cells were present in all examined lymphoid organs and pancreatic islets with the highest frequency found in the later site (Figure 1, E and F). β-cell autoreactive CD4 T cells in islets were CD44high and present in both Venus (IL-21)+ and Venus (IL-21)− populations (Figure 1G). Interestingly, the majority of the 2.5-hybrid insulin peptide (HIP)-reactive CD4 T cells expressed Venus (IL-21) whereas a significant proportion of insulin B:9–23 reactive CD4 T cells did not express Venus (IL-21) and were FOXP3+ (Figure 1G). Next, we tested the diabetogenic function of Venus (IL-21)+ CD4 T cells using NOD.Rag1-/−.NY8.3 mice as recipients. β-cell autoreactive CD8 T cells of the NY8.3 clonotype require help from CD4 T cells to fully exert their diabetogenic activity (36). Thus, the diabetogenic function of CD4 T cells can be tested by transferring them into NOD.Rag1-/−.NY8.3 recipients (37). Venus (IL-21)+ CD4 T cells sorted from pancreatic islets were highly diabetogenic when adoptively transferred into NOD.Rag1-/−.NY8.3 recipients, consistent with their pathogenic function at the site of autoimmune destruction (Figure 1H).
Figure 1.

Characterization of IL-21 reporter NOD mice. (A) The illustration of the Il21 Venus knockin reporter allele. Closed boxes depict exons. (B) T1D incidence study of NOD.Il21V/+ and NOD.Il21+/+ female littermates and standard NOD females. No significant difference was found between groups (Log-rank test). (C) Co-expression of Venus fluorescent protein and IL-21 in NOD.Il21V/+ islet-infiltrating CD4 T cells. Islet-infiltrating cells were stained for IL-21 with IL-21R-HuFc fusion protein followed by PE conjugated anti-HuFc. The negative control was only stained with anti-HuFc. Venus protein was stained by anti-GFP. Total viable CD4 T cells are shown. Similar results were seen in another two mice. (D) Expression of Il21 mRNA in Venus+ and Venus− splenic CD4 T cells and all other non-CD4 T cells isolated from NOD.Il21V/+ mice. Each symbol represents cells pooled from 4–6 male mice. (E and F) The frequency of Venus (IL-21)+ CD4 T cells in blood, spleen, pancreatic lymph nodes (PLN), mesentery lymph nodes (MLN), and pancreatic islets of 11–12-week-old NOD.Il21V/+ females. Summarized results from two experiments are shown in (E). Each symbol represents one mouse. The representative flow cytometry profiles are shown in (F). Total viable CD4 negative (CD4neg) and CD4 T cells are shown. (G) I-Ag7 MHC class II tetramer staining of islet-infiltrating IL-21+ CD4 T cells. Islet-infiltrating cells pooled from two 6–9-week-old NOD.Il21V/+ females were stained with tetramers loaded with the insulin B:9–23 mimotopes (1:1 mixture of p8G [HLVERLYLVCGGEG] and p8E [HLVERLYLVCGEEG]) or the 2.5-hybrid insulin peptide (HIP) (LQTLALWSRMD). The control tetramers were loaded with PVSKMRMATPLLMQA. A similar result was seen in another two pools of cells. Two independent experiments were performed. (H) Islet IL-21+ CD4 T cells are highly diabetogenic. Venus+ and Venus− CD4 T cells were sorted from islets of 8–13-week-old NOD.Il21V/+ females and transferred into 7–8-week-old NOD.Rag1-/−.NY8.3 female mice. A group of age matched NOD.Rag1-/−.NY8.3 females not receiving CD4 T cells were included for comparison. Results are combined from two independent transfer experiments. *p < 0.05, **p < 0.01 by Log-rank test.
Single-cell RNA seq analysis of islet-infiltrating CD4 T cells
Previous studies of islet infiltrating bulk IL-21+ CD4 T cells in NOD mice indicate that they are distinct from Tfh found in lymphoid tissues (20). A single-cell targeted RNA profiling study with a limited number of cells and transcripts identified an IL-21/IFNγ co-expressing insulin reactive CD4 T cell population in the islets of NOD mice, suggesting that IL-21 is co-expressed in some Th1 cells (38). Nevertheless, the transcriptional heterogeneity and differentiation states of IL-21+ CD4 T cell subsets in the islets of prediabetic NOD mice remain unclear. Therefore, we performed a scRNA-seq analysis on Venus+ cells sorted from the islets of 9–12-week-old prediabetic NOD.Il21V/+ females using the 10X Genomics platform (Figure 2A). For comparison, Venus− islet CD4 T cells were sorted from the same mice and processed in parallel. The final dataset passing quality control (see Materials and Methods) had 5691 cells. We integrated Venus+ and Venus− CD4 T cells to determine the transcriptional difference between these two populations. After removing two minor clusters of contaminated non-CD4 T cells, 13 clusters were identified (Figure 2B). Venus+ and Venus− CD4 T cells have minimum overlap in the UMAP plot, indicating that their gene expression patterns are very distinct (Figure 2C). Il21 was expressed in Venus+ but not Venus− cells, confirming the accuracy of our cell sorting (Figure 2D). Clusters 11 and 12 were cycling cells respectively in S and G2/M phases (Figure 2, E and F) and contained mostly Venus+ cells (Figure 2, C and E). Clusters 1, 2, and 7 displayed a transcriptional profile consistent with naïve T cells, including high levels of Lef1, Dapl1, Sell, Ccr7, and Il7r (Figure 2F). Cluster 4 had a gene expression pattern indicative of memory T cells, including elevated expression of Cd44, Itgb1, and Il7r (Figure 2F). Cluster 6 contained both Venus+ and Venus− cells that expressed an interferon (IFN)-stimulated gene signature, suggesting that they were being exposed to IFN in the islet environment at the time of isolation (Figure 2F). Clusters 9 and 10 represented two subsets of regulatory T cells (Tregs) as indicted by high Foxp3 expression and other Treg associated genes (e.g., Ikzf2, Tnfrsf4, Tnfrsf18, Ctla4, and Il2ra) (Figure 2F). Direct comparison between clusters 9 and 10 revealed that cells in cluster 10 expressed higher levels of genes associated with activated Tregs (e.g., Icos, Lag3, Ctla4, Itgae, Tnfrsf4, Tnfrsf18, Cd5, Klrg1, Maf, and Id2), suggesting that they were in a differentiation state with higher suppressive activity (Figure 2G). The majority of Venus+ cells were found in clusters 0, 3, 5, and 8 (Figures 2, B and C). Overall, these cells upregulated genes associated with activation and effector functions (Figure 2F), including Icos, Pdcd1, Lag3, Ifng, Cxcr3, Cxcr6, Id2, Batf, and Prdm1 (encoding BLIMP1) as well as Maf, a transcription factor important for Il21 expression (39–41).
Figure 2.
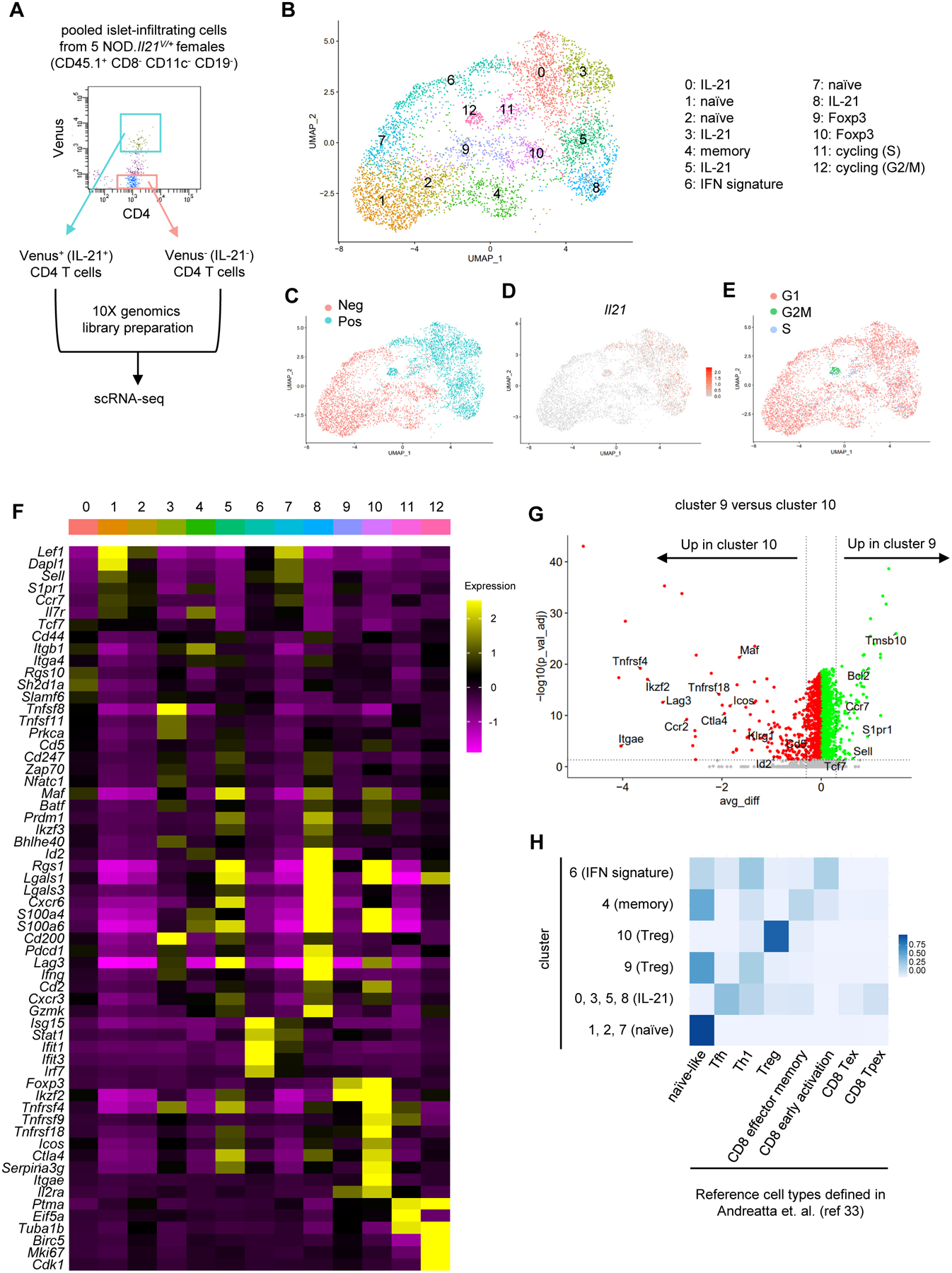
Single-cell RNA sequencing of islet-infiltrating CD4 T cells. (A) Diagram depicting the experimental design. Islet-infiltrating cells were pooled from five 9–12-week-old NOD.Il21V/+ females and sorted into Venus+ and Venus− CD4 T cells for library generation and sequencing. (B) UMAP plot of thirteen distinct clusters of islet CD4 T cells. (C) UMAP plot depicting the distribution of Venus+ and Venus− cells. (D) Feature plot of Il21 expression. (E) UMAP plot depicting G1, S, and G2M phases of the cell cycle assigned using the CellCycleScoring feature in Seurat. (F) Heatmap of the key cluster maker genes. The color scale represents the z-score distribution of the expression level. (G) Volcano plot of differentially expressed genes between Treg clusters 9 and 10. The horizontal dotted line indicates adjusted p value of 0.05. The vertical dotted lines indicate average difference (avg_diff) of −0.3 and 0.3 respectively. (H) Cell type prediction by ProjecTILs. The color scale depicts the proportion of cells within the indicated clusters predicted to be the corresponding cell types in the reference.
To further evaluate the cell differentiation states, we performed ProjecTILs analysis to predict cell types using a built-in reference constructed by publicly available scRNA-seq data from CD4 and CD8 T cells in 21 murine tumors and four tumor draining lymph node samples (33). Consistent with our definition, clusters 0, 1, and 7 are predicted to be naïve-like and cluster 10 consisted of Tregs (Figure 2H). Cells in cluster 9, identified as a Treg subset in our definition, were identified as more “naïve-like”, consistent with their resting phenotype (Figure 2H). Cells in cluster 6 appeared to have diverse phenotypes including naïve, early activation, and Th1, confirming that they did not represent a specific differentiation state and were grouped together due to high levels of IFN response genes. Clusters 0, 3, 5, and 8 showed similarity to both Tfh and Th1 cells (Figure 2H). Of note, some cells in these clusters were predicted to be similar to memory, terminally exhausted (Tex), and precursor-exhausted (Tpex) cells in the reference (Figure 2H), suggesting that clusters 0, 3, 5, and 8 contained cells of diverse differentiation states ranging from early to terminally differentiated effectors.
Flow cytometry analysis confirmed that the majority of islet Venus (IL-21)+ CD4 T cells express a CD44highCD127low phenotype and they expressed higher levels of ICOS, LAG3, and PD-1 than Venus (IL-21)− CD4 T cells (Figure 3, A–D). Additional analysis showed that significant proportions of islet CD44highCD127low CD4 T cells were Venus (IL-21)+ and FOXP3+ (Figure 3E). Conversely, the majority of islet CD44highCD127high and CD44lowCD127high CD4 T cells were negative for Venus (IL-21) and FOXP3 (Figure 3E).
Figure 3.
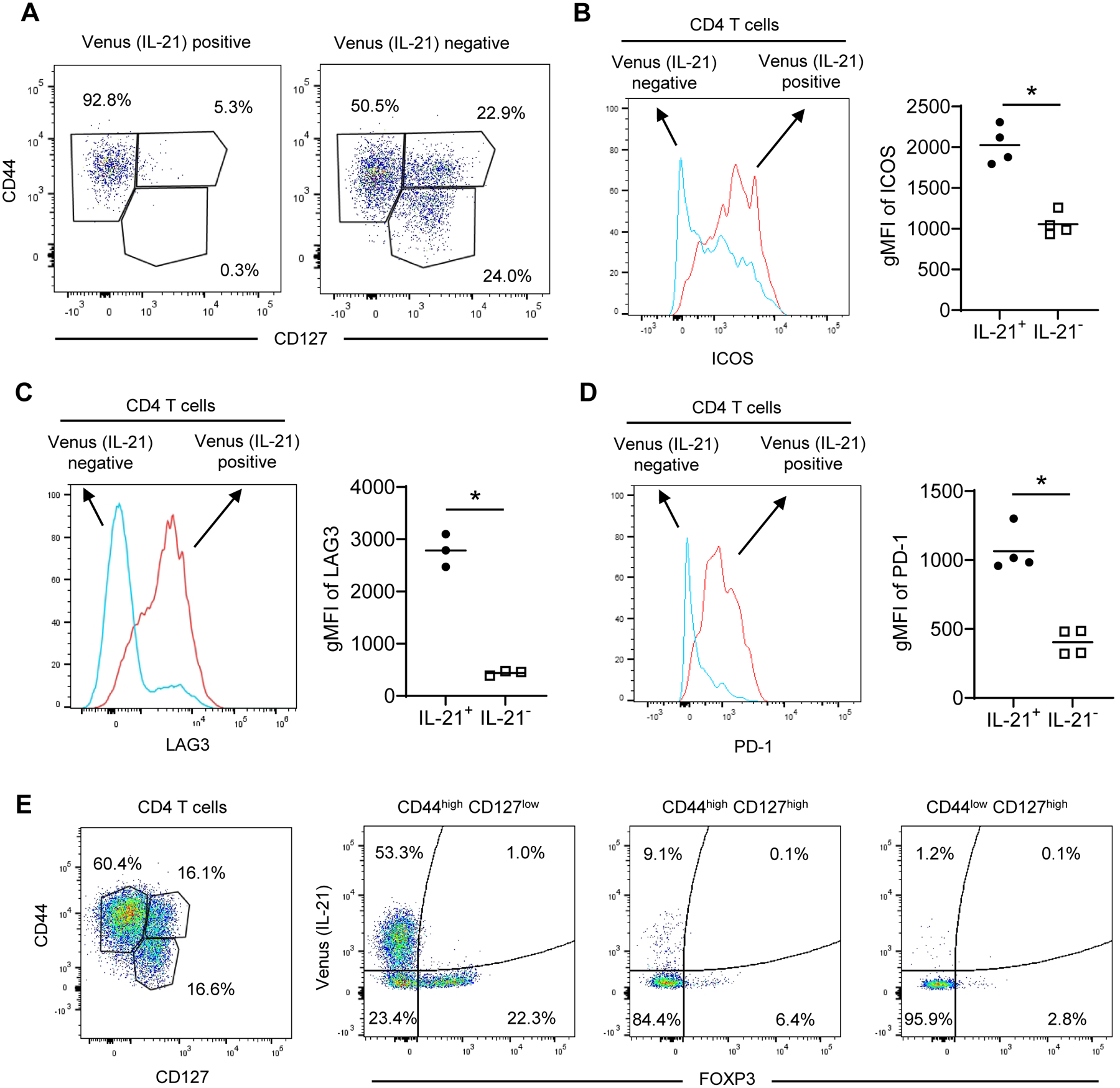
Islet IL-21+ effector CD4 T cells express high levels of LAG3, ICOS, and PD-1. (A) Islet Venus (IL-21)+ CD4 T cells of NOD.Il21V/+ mice are CD44high and CD127low effectors. Shown are single viable CD45.1+CD3+CD4+ Venus+ or Venus− cells. One of four mice with similar results is shown. At least two independent experiments were performed. (B-D) Islet Venus (IL-21)+ CD4 T cells of NOD.Il21V/+ mice express higher levels of ICOS (B), LAG3 (C), and PD-1 (D) than Venus (IL-21)− CD4 T cells. Representative histograms are on the left and the summarized results are on the right. Shown are single viable CD45.1+CD3+CD4+ Venus+ or Venus− cells. Each symbol represents one mouse, and the horizontal bars are the means. gMFI: geometric mean fluorescent intensity. *p < 0.05 by unpaired t test. (E) The heterogeneity of CD44highCD127low islet CD4 T cells. Shown are single CD45.1+CD3+CD4+ T cells and the expression of Venus (IL-21) and FOXP3 in CD44highCD127low, CD44highCD127high, and CD44lowCD127high subpopulations. One of three 11–12-week-old NOD.Il21V/+ mice with similar results is shown. Similar results were obtained in an independent experiment.
Phenotypic and transcriptional network analyses of islet IL-21+ CD4 T cell subsets
We then focused on characterizing and determining the differentiation states of the four Venus (IL-21)+ clusters. We examined clusters 0, 3, 5, and 8 for lineage-defining transcriptional signatures and cell states (Figure 4A and Supplementary Table 1). Cells in cluster 0 express elevated levels of genes typically associated with Tfh cells (Figure 4B), including Bcl6, Tcf7, Id3, Cxcr5, Sh2d1a, Slamf6, and Rgs10 (42–44). However, the levels of Bcl6 and Cxcr5 were not as high as expected for typical Tfh cells. On the other hand, cells in clusters 5 and 8 display a gene expression pattern associated with Th1 effectors (Figure 4B), including Id2, Prdm1, Bhlhe40, Ifng, Cxcr3, Cxcr6, Selplg, and Rgs1 (42, 44–46). To further distinguish these two Th1 subsets, we directly compared their gene expression. Cluster 5 cells had higher expression of Izumo1r, Ikzf2, and Tnfsf8 while cluster 8 cells were enriched for Ccl3, Ccl4, Ccl5, Cxcr6, S100a4, S100a6, Id2, Ifng, and Pdcd1 (Figure 4C). These gene expression patterns suggest that cluster 8 consists of terminally differentiated Th1 effector cells. Flow cytometry analysis confirmed that islet Venus (IL-21)+ CD4 T cells separated into CXCR6+ and CXCR6− populations with the former expressing a lower level of SLAMF6 than the latter (Figure 4D). In addition, Venus (IL-21)+ CD4 T cells expressing a high level of Bcl6 were enriched in SLAMF6high cells and high T-bet expression was associated with CXCR6+ cells (Figure 4E). Cells in cluster 3 upregulated Cd200 and Tnfsf8 (Figure 4B). However, the overall expression pattern of this cluster was less distinctive than the other clusters since it included genes associated with both Tfh or Th1 cells (e.g., Tcf7, Bhlhe40, Slamf6, and Ifng). Consistent with these results, a gene module analysis using genes previously associated with Th1 or Tfh effector cells (GSE43863, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE43863) showed that cluster 0 had the highest Tfh gene module score while cluster 8 had the highest Th1 gene module score (Figure 4F). The overall gene expression pattern in cluster 3 correlated with both clusters 0 and 5, while cluster 0 and cluster 8 were quite distinct (Figure 4G). Collectively, these data indicate that both Th1 and Tfh-like IL-21+ CD4 T cells accumulate in the insulitic lesions of NOD mice.
Figure 4.

Heterogeneity of islet-infiltrating IL-21+ CD4 T cells. (A) UMAP plot showing the four IL-21+ cell clusters (From Figure 2B). (B) Dot plots showing the key cluster marker genes. Dot size indicates the proportion of cells in a cluster expressing a particular gene. The color scale denotes the average expression of the gene. (C) Differentially expressed genes between Th1 clusters 5 and 8. The horizontal dotted line indicates adjusted p value of 0.05. The vertical dotted lines indicate average difference (avg_diff) of −0.3 and 0.3 respectively. (D) SLAMF6 and CXCR6 expression in islet Venus (IL-21)+ CD4 T cells of NOD.Il21V/+ females. Shown are single viable CD45.1+CD3+CD4+ cells. One of more than six 9–13-week-old mice in at least two experiments with similar results is shown. (E) Bcl6 and T-bet expression in islet Venus (IL-21)+ CD4 T cells of NOD.Il21V/+ females. One of four 11–13-week-old females from two experiments with similar results is shown. (F) Violin plots showing the Th1 and Tfh signature gene module scores defining the differentiation states of the four IL-21+ CD4 T cell clusters. *p < 0.05, ****p < 5×10−6 by adjusted t test. (G) Correlation of gene expression among the four IL-21+ CD4 T cell clusters. Top 3000 most variable genes were used to calculate Pearson correlation. (H) Heatmap showing the activity of the key regulons that are significantly different in at least one cluster (adjusted p value < 0.05).
To further understand the gene regulatory networks underlying autoreactive IL-21+ CD4 T cell differentiation, we performed SCENIC analysis that identifies a transcription factor and its direct binding target genes in each cell by co-expression and cis-regulatory motif analysis (34). The resulting transcription factor and its targets define a “regulon” whose activity can be determined within individual cells. This analysis revealed that the activities of several regulons were selectively increased in specific subsets of Venus (IL-21)+ CD4 T cells. Consistent with the Tfh-like phenotype, the regulon activities of Tcf7 and Lef1, transcription factors known to be important for Tfh development (43), were upregulated in cluster 0 cells (Figure 4H). The regulon activities of Prdm1, Runx2, Runx3, Tbx21 (encoding T-bet), Irf4, and Bhlhe40 were enhanced in cluster 5 and/or cluster 8 cells (Figure 4H); many of these have been linked to the differentiation and function of Th1 cells (46–48). Cluster 3 cells showed elevated regulon activities of Nfkb2, Irf4, Nfatc1, Relb, Egr1, Bhlhe40, Rel, Egr2, Nfkb1, Stat5a, and Stat4 (Figure 4H). A recent study of chronic viral infection identified a memory-like CD4 T cell subset having chromatin accessible regions enriched for NFAT, Egr1, Egr2, and NF-κB binding motifs and an ability to differentiate into both Th1 and Tfh cells (49). Although cluster 3 did not show a clear memory phenotype, the partial overlap of transcriptional networks with the virus-induced memory-like CD4 T cells during chronic infection raises the possibility that cluster 3 cells may function as a precursor of Tfh-like and/or Th1 cells.
Differentiation trajectory of islet IL-21+ CD4 T cell subsets
Next, Venus (IL-21)+ CD4 T cells were ordered along a differentiation trajectory using Monocle (35). Most cells fell along a single linear differentiation trajectory with some cluster 0 cells branching out and this analysis placed the differentiation of cell clusters in the order of 3 → 0 → 5 → 8 over pseudotime (Figure 5, A and B). Cluster 3 cells were found at the beginning and cluster 0 cells were localized to the middle of the pseudotime, followed by cluster 5 cells. Cluster 8 cells were found at the end of the trajectory, indicating that they were terminally differentiated. Two small branches, mainly occupied by cluster 0 cells, separated from the main trajectory at the beginning of the pseudotime, suggesting that some cells retain the Tfh-like phenotype as the terminal state. Next, we evaluated the transcriptional activity of Tfh and Th1 associated transcription factors along the differentiation trajectory by superimposing their regulon activities (determined by SCENIC analysis) on the pseudotime (Figure 5C). Consistent with the Tfh and Th1 gene expression patterns, the regulon activities of Tfh transcription factors Tcf7 and Lef1 were upregulated in cells of the Tfh-like branches and in those in the middle of the main trajectory before cells displayed the Th1 phenotype. Conversely, the regulon activities of Tbx21 and Prdm1 gradually increased and reached the highest levels in the terminally differentiated Th1 cells. Some early effectors also displayed elevated Tbx21 regulon activity. Interestingly, the regulon activity of Bhlhe40 was high throughout the main trajectory but was downregulated in the branches of Tfh-like cells. This activity pattern raises the possibility that reduced Bhlhe40 transcriptional activity is required for retaining the Tfh-like phenotype during the differentiation of islet-infiltrating IL-21+ CD4 T cells. Maf regulon activity was relatively stable and reached the highest level at the end of the pseudotime, consistent with its role in driving IL-21 production in T1D (41). Collectively, our scRNA-seq analysis indicates that IL-21+ CD4 T cells in islets are heterogeneous and suggests a continuum of cell differentiation from a state of less committed early effector, Tfh-like, to terminal Th1 cells with some diverging cells having a terminal Tfh-like state.
Figure 5.

Differentiation trajectory of islet-infiltrating IL-21+ CD4 T cells. (A) Monocle analysis reveals cell differentiation trajectory over pseudotime. (B) Distribution of cells within Seurat clusters 0, 3, 5, and 8 (Tfh-like, early effector, Th1, and terminal Th1, respectively) over pseudotime. (C) Regulon activities of selected transcription factors over pseudotime. The color scale indicates the regulon activity determined by SCENIC.
IL-27 regulates the differentiation of islet IL-21+ CD4 T cells
IL-27 signaling in NOD CD4 T cells is important for their pathogenic activity (29), but the underlying mechanism has not been completely defined. IL-27 has been shown to promote IL-21+ Tfh cells in response to immunization and viral infection (50, 51). This suggests a possibility that IL-27 signaling may control the differentiation of islet IL-21+ CD4 T cells. To address this question, we crossed NOD.Il21V/+ mice to our previously described NOD.Il27ra−/− mice (29). In comparison to the wildtype, the frequency of Venus (IL-21)+ CD4 T cells was not altered in the spleen but was significantly reduced in the pancreatic islets of Il27ra−/− mice (Figure 6, A and B). Next, we generated mixed BM chimeras to determine if the effect of IL-27 on islet Venus (IL-21)+ CD4 T cells is cell intrinsic. We used BM cells from congenically marked wildtype (CD45.2+) and Il27ra−/− (CD45.1+) NOD.Il21V/+ mice (mixed at a 1:1 ratio) to reconstitute lethally irradiated (NOD x NOD.CD45.2)F1 recipients (Figure 6C). Ten to eleven weeks after BM reconstitution, spleens and pancreatic islets were analyzed for Venus (IL-21)+ CD4 T cells. The frequency of Il27ra−/− BM-derived Venus (IL-21)+ CD4 T cells was significantly reduced in spleens and islets when compared to the wildtype counterparts (Figure 6D). Using CXCR6 as a marker to distinguish IL-21+ early effector/Tfh-like and Th1 CD4 T cells in islets, we found that deficiency of IL-27 signaling reduced the frequency of both CXCR6− and CXCR6+ subsets (Figure 6, E and F). While reduced in the mixed BM chimeras, significant numbers of Il27ra−/− Venus (IL-21)+ CD4 T cells were still present, suggesting that cell-extrinsic effect of IL-27 signaling and/or IL-27Ra-independent factors also contribute to the differentiation of islet IL-21+ CD4 T cells.
Figure 6.

IL-27 controls the differentiation of IL-21+ CD4 T cells. (A and B) The frequency of Venus (IL-21)+ CD4 T cells in the spleen (A) and pancreatic islets (B) of wildtype (WT) and IL-27ra-deficient (Il27ra−/−) NOD.Il21V/+ mice. Representative flow cytometry profiles are shown on the left and the summarized results from two experiments are on the right. Shown are single viable CD3+CD4+ cells. Each symbol represents one mouse, and the horizontal bars are means. ***p < 0.005 by unpaired t test. (C) The design of the mixed BM chimera experiment. (D) The frequency of wildtype (WT) and IL-27ra-deficient (Il27ra−/−) Venus (IL-21)+ CD4 T cells in the mixed BM chimeras. Representative flow cytometry profiles from the spleen are shown on the left and the summarized results are on the right. Each symbol represents cells pooled from two mice. Combined results from two independent experiments are shown. *p < 0.05; ***p < 0.005 by paired t test. (E and F) The frequency of wildtype (WT) and IL-27ra-deficient (Il27ra−/−) CXCR6− and CXCR6+ Venus (IL-21)+ CD4 T cells in the islets of the mixed BM chimeras. (E) Representative flow cytometry profiles. (F) Calculated frequencies of CXCR6− and CXCR6+ Venus (IL-21)+ subsets among total islet CD4 T cells. Summarized results are from two independent experiments. *p < 0.05 by paired t test.
Discussion
IL-21 is essential for sustaining immune responses during chronic antigen stimulation (11–13). Studies using the LCMV Clone 13 chronic infection model demonstrate that IL-21 is critical for the differentiation of CX3CR1+ cytotoxic CD8 T cells for viral control (14). IL-21 is also important for tumor control and supporting the effector function of tumor-infiltrating CD8 T cells (52, 53). Mouse models of infection and cancer have shown that Tfh cells are a critical source of IL-21 (49, 52). IL-21 is required for T1D development in NOD mice where CD4 T cell produced IL-21 acts directly on CD8 T cells to promote T1D (20). This is consistent with the chronic nature of T1D where T cells are continuously exposed to autoantigen. Existing evidence suggests that β-cell autoreactive CD8 T cells encounter IL-21 in pancreatic islets where they are reactivated (54). While the importance of this cytokine for T1D pathogenesis has been known for more than 10 years, the phenotype and function of the IL-21-producing CD4 T cells have not been comprehensively studied, in part due to lack of surface markers to identify and isolate live IL-21+ CD4 T cells. In this study, we used a newly generated IL-21 reporter NOD mouse to perform single-cell transcriptomic analysis of islet-infiltrating IL-21+ CD4 T cells to address this knowledge gap and demonstrate the presence of heterogenous subpopulations of IL-21+ CD4 T cells in pancreatic islets of NOD mice.
Previous work that characterized IL-21+ CD4 T cells in mouse models of T1D includes a study in NOD mice where CCR9 was used as a marker to isolate islet IL-21+ CD4 T cells for gene expression analysis. This study showed that islet IL-21+ CD4 T cells expressed similar levels of Maf and Bcl6 as observed in Tfh cells but expressed lower levels of Sh2d1a (encoding SAP) than Tfh cells (20). Another study that transcriptionally analyzed islet autoreactive CD4 T cells utilized the DO11 RIP-mOVA mouse model of T1D (23). In this model, expression of OVA as a self-antigen in the pancreatic β cells is driven by the rat insulin promoter and expression of the DO11.10 transgenic T cell receptors allows the development of OVA-specific “autoreactive” DO11 T cells (55). In the DO11 RIP-mOVA model, transcriptome analysis of β-cell autoreactive DO11 CD4 T cells in PLNs showed increased expression of Tfh associated genes, including Cxcr5, Pdcd1, Il21, and Bcl6, relative to those from inguinal lymph nodes (ILNs) (23). In the same study, pancreatic CD4 T cells also showed elevated levels of Cxcr5, Pdcd1, and Icos, relative to those from ILNs, suggesting the presence of Tfh cells in islets. While these bulk sequencing studies are informative, they cannot resolve the cellular heterogeneity that the current work describes. Our scRNA-seq analysis of both IL-21+ and IL-21− populations provides a comprehensive transcriptional landscape of the differentiation states of islet-infiltrating CD4 T cells, including naïve and memory cells, Tregs, and those expressing IL-21. Despite expressing high levels of inhibitory molecules PD-1 and LAG3, islet IL-21+ CD4 T cells are not exhausted and are highly pathogenic as evidenced by their ability to adoptively transfer rapid onset of T1D into NOD.Rag1-/−.NY8.3 recipients. Importantly, we provide evidence to support the co-existence of IL-21+ Tfh-like and Th1 cells in pancreatic islets during the progression of T1D. Thus, the ability to produce IL-21 is an important feature of β-cell autoreactive effector CD4 T cells disregarding their differentiation states.
We previously reported that IL-27 signaling is essential for diabetes development in NOD mice as both IL-27 and IL-27Ra deficient NOD mice were completely resistant to T1D (29). Using a CD4 and CD8 T cell co-transfer system, we reported that IL27Ra-deficient CD4 T cells were less capable of supporting CD8 T cells for T1D development in NOD.Rag1−/− recipients than the wildtype counterpart (29). In the current study, we found that IL-27 signaling was important for the generation of islet IL-21+ CD4 T cells likely through both cell intrinsic and extrinsic mechanisms. It appears that IL-27Ra deficiency in CD4 T cells has a greater impact on islet CXCR6+ IL-21+ CD4 T cells than the CXCR6− counterpart. We have previously shown that IL-27 signaling is important for the differentiation of T-bet+ CD4 T cells in islets (29). As CXCR6+ IL-21+ CD4 T cells express T-bet, these results support that IL-27 signaling is important for the differentiation of islet IL-21+ Th1 cells. Collectively, the results from our previous and current studies indicate that the IL-27-IL-21 axis functionally links β-cell autoreactive CD4 and CD8 T cells in T1D.
A distinct CD4 T helper population termed peripheral helper T (Tph) cells was recently identified in human inflamed joint tissue (56). Like Tfh cells, Tph cells express high levels of IL-21, PD-1, ICOS, MAF, and CXCL13, and are capable of providing B-cell help. However, they differ from Tfh cells in several respects including that they lack CXCR5 and express a lower level of BCL6 and higher levels of CXCR6 and BLIMP1 compared to Tfh cells (56). A mouse counterpart to human Tph cells has yet to be identified. However, the Th1 subsets of islet IL-21+ CD4 T cells described in the current work phenotypically resemble Tph cells in that they express high levels of Maf, Pdcd1 (encoding PD-1), Icos, Cxcr6, and Prdm1 (encoding BLIMP1) and lack expression of Bcl6 and Cxcr5. However, we did not detect Cxcl13 in islet IL-21+ CD4 T cells. Elevated frequency of circulating Tph cells has been associated with T1D progression in humans, supporting their role in disease pathogenicity (57). Thus, it will be important to determine if Tph cells are present in inflamed islets during the progression of human T1D and whether the islet IL-21-expressing Th1 cells identified here represent the mouse counterpart of human Tph cells.
In conclusion, we reveal that a significant proportion of islet effector CD4 T cells produce IL-21 and provide insight into the heterogeneity of their differentiation states. We also demonstrate a positive role of IL-27 in the differentiation of islet IL-21+ CD4 T cells. In addition, the novel reporter strain described here will significantly improve the analysis of IL-21+ CD4 T cells in future studies that evaluate preclinical therapeutic efficacy for T1D using the NOD mouse model.
Supplementary Material
Key points:
Differentiation states of islet IL-21+ CD4 T cells are heterogeneous in NOD mice
IL-21+ Tfh-like and Th1 CD4 T effector cells are found in islets of NOD mice
IL-27 regulates the differentiation of islet IL-21+ CD4 T cells in NOD mice
Acknowledgements
We are grateful to Caren Crumer for excellent mouse colony management. We thank the Children’s Research Institute Flow Cytometry Core for technical assistance. We thank the NIH Tetramer Core Facility for providing MHC class II tetramers. We thank The Jackson Laboratory Genetic Engineering Technologies group for technical support on this project.
This work is supported by National Institutes of Health (NIH) grants DK107541, AI144360, and DK121747 (to Y.-G.C), DK097605 (to A.M.G. and Y.-G.C), and DK118786 (to A.E.C.).
References
- 1.Leonard WJ, and Wan CK. 2016. IL-21 Signaling in Immunity. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian Y, and Zajac AJ. 2016. IL-21 and T Cell Differentiation: Consider the Context. Trends Immunol 37: 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, and Kuchroo VK. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, and Dong C. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448: 480–483. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, and Littman DR. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8: 967–974. [DOI] [PubMed] [Google Scholar]
- 6.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, and Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, and King C. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29: 127–137. [DOI] [PubMed] [Google Scholar]
- 8.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, and Grusby MJ. 2002. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med 196: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, Boon L, Karlsson Hedestam GB, Nutt SL, Hammad H, and Lambrecht BN. 2015. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity 43: 318–330. [DOI] [PubMed] [Google Scholar]
- 10.Cui W, Liu Y, Weinstein JS, Craft J, and Kaech SM. 2011. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35: 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsaesser H, Sauer K, and Brooks DG. 2009. IL-21 is required to control chronic viral infection. Science 324: 1569–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, and Kopf M. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324: 1576–1580. [DOI] [PubMed] [Google Scholar]
- 13.Yi JS, Du M, and Zajac AJ. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324: 1572–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zander R, Schauder D, Xin G, Nguyen C, Wu X, Zajac A, and Cui W. 2019. CD4(+) T Cell Help Is Required for the Formation of a Cytolytic CD8(+) T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 51: 1028–1042 e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren HM, Lukacher AE, Rahman ZSM, and Olsen NJ. 2021. New developments implicating IL-21 in autoimmune disease. J Autoimmun 122: 102689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spolski R, Kashyap M, Robinson C, Yu Z, and Leonard WJ. 2008. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A 105: 14028–14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland AP, Van Belle T, Wurster AL, Suto A, Michaud M, Zhang D, Grusby MJ, and von Herrath M. 2009. Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 58: 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire HM, Walters S, Vogelzang A, Lee CM, Webster KE, Sprent J, Christ D, Grey S, and King C. 2011. Interleukin-21 is critically required in autoimmune and allogeneic responses to islet tissue in murine models. Diabetes 60: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allred MG, Chimenti MS, Ciecko AE, Chen YG, and Lieberman SM. 2021. Characterization of Type I Interferon-Associated Chemokines and Cytokines in Lacrimal Glands of Nonobese Diabetic Mice. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, Christ D, Fulcher D, Falcone M, and King C. 2011. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity 34: 602–615. [DOI] [PubMed] [Google Scholar]
- 21.Van Belle TL, Nierkens S, Arens R, and von Herrath MG. 2012. Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity 36: 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira RC, Simons HZ, Thompson WS, Cutler AJ, Dopico XC, Smyth DJ, Mashar M, Schuilenburg H, Walker NM, Dunger DB, Wallace C, Todd JA, Wicker LS, and Pekalski ML. 2015. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia 58: 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, Heuts F, Kogimtzis A, Patel S, Rosenthal M, Ono M, Sansom DM, Narendran P, and Walker LS. 2015. Follicular helper T cell signature in type 1 diabetes. The Journal of clinical investigation 125: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viisanen T, Ihantola EL, Nanto-Salonen K, Hyoty H, Nurminen N, Selvenius J, Juutilainen A, Moilanen L, Pihlajamaki J, Veijola R, Toppari J, Knip M, Ilonen J, and Kinnunen T. 2017. Circulating CXCR5+PD-1+ICOS+ Follicular T Helper Cells Are Increased Close to the Diagnosis of Type 1 Diabetes in Children With Multiple Autoantibodies. Diabetes 66: 437–447. [DOI] [PubMed] [Google Scholar]
- 25.Baharlou R, Ahmadi-Vasmehjani A, Davami MH, Faraji F, Atashzar MR, Karimipour F, Sadeghi A, Asadi MA, and Khoubyari M. 2016. Elevated Levels of T-helper 17-associated Cytokines in Diabetes Type I Patients: Indicators for Following the Course of Disease. Immunol Invest 45: 641–651. [DOI] [PubMed] [Google Scholar]
- 26.von Herrath M, Bain SC, Bode B, Clausen JO, Coppieters K, Gaysina L, Gumprecht J, Hansen TK, Mathieu C, Morales C, Mosenzon O, Segel S, Tsoukas G, Pieber TR, I. L. l. S. G. i. Anti, and contributors. 2021. Anti-interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 9: 212–224. [DOI] [PubMed] [Google Scholar]
- 27.Spolski R, and Leonard WJ. 2014. Interleukin-21: a double-edged sword with therapeutic potential. Nature reviews. Drug discovery 13: 379–395. [DOI] [PubMed] [Google Scholar]
- 28.Forsberg MH, Ciecko AE, Bednar KJ, Itoh A, Kachapati K, Ridgway WM, and Chen YG. 2017. CD137 Plays Both Pathogenic and Protective Roles in Type 1 Diabetes Development in NOD Mice. Journal of immunology 198: 3857–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciecko AE, Foda B, Barr JY, Ramanathan S, Atkinson MA, Serreze DV, Geurts AM, Lieberman SM, and Chen YG. 2019. Interleukin-27 Is Essential for Type 1 Diabetes Development and Sjogren Syndrome-like Inflammation. Cell Rep 29: 3073–3086 e3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciecko AE, Schauder DM, Foda B, Petrova G, Kasmani MY, Burns R, Lin CW, Drobyski WR, Cui W, and Chen YG. 2021. Self-Renewing Islet TCF1(+) CD8 T Cells Undergo IL-27-Controlled Differentiation to Become TCF1(−) Terminal Effectors during the Progression of Type 1 Diabetes. J Immunol 207: 1990–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, and Satija R. 2019. Comprehensive Integration of Single-Cell Data. Cell 177: 1888–1902 e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafemeister C, and Satija R. 2019. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 20: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreatta M, Corria-Osorio J, Muller S, Cubas R, Coukos G, and Carmona SJ. 2021. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat Commun 12: 2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aibar S, Gonzalez-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine JC, Geurts P, Aerts J, van den Oord J, Atak ZK, Wouters J, and Aerts S. 2017. SCENIC: single-cell regulatory network inference and clustering. Nat Methods 14: 1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, and Trapnell C. 2017. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 14: 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, and Santamaria P. 1997. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med 186: 1663–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, and Santamaria P. 2003. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity 19: 877–889. [DOI] [PubMed] [Google Scholar]
- 38.Gioia L, Holt M, Costanzo A, Sharma S, Abe B, Kain L, Nakayama M, Wan X, Su A, Mathews C, Chen YG, Unanue E, and Teyton L. 2019. Position beta57 of I-A(g7) controls early anti-insulin responses in NOD mice, linking an MHC susceptibility allele to type 1 diabetes onset. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, and Kuchroo VK. 2009. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology 10: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, and Kuchroo VK. 2009. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. Journal of immunology 183: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu CY, Yeh LT, Fu SH, Chien MW, Liu YW, Miaw SC, Chang DM, and Sytwu HK. 2018. SUMO-defective c-Maf preferentially transactivates Il21 to exacerbate autoimmune diabetes. J Clin Invest 128: 3779–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, and Ahmed R. 2013. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 38: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue HH, and Crotty S. 2015. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol 16: 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lonnberg T, Svensson V, James KR, Fernandez-Ruiz D, Sebina I, Montandon R, Soon MS, Fogg LG, Nair AS, Liligeto U, Stubbington MJ, Ly LH, Bagger FO, Zwiessele M, Lawrence ND, Souza-Fonseca-Guimaraes F, Bunn PT, Engwerda CR, Heath WR, Billker O, Stegle O, Haque A, and Teichmann SA. 2017. Single-cell RNA-seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw LA, Belanger S, Omilusik KD, Cho S, Scott-Browne JP, Nance JP, Goulding J, Lasorella A, Lu LF, Crotty S, and Goldrath AW. 2016. Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nat Immunol 17: 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu F, Sharma S, Jankovic D, Gurram RK, Su P, Hu G, Li R, Rieder S, Zhao K, Sun B, and Zhu J. 2018. The transcription factor Bhlhe40 is a switch of inflammatory versus antiinflammatory Th1 cell fate determination. J Exp Med 215: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, and Ansel KM. 2007. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol 8: 145–153. [DOI] [PubMed] [Google Scholar]
- 48.Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi MF, Ahlers J, Janke M, Rudolph C, Mockel-Tenbrinck N, Kuhl AA, Heimesaat MM, Esser C, Im SH, Radbruch A, Rutz S, and Scheffold A. 2014. Role of Blimp-1 in programing Th effector cells into IL-10 producers. J Exp Med 211: 1807–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zander R, Kasmani MY, Chen Y, Topchyan P, Shen J, Zheng S, Burns R, Ingram J, Cui C, Joshi N, Craft J, Zajac A, and Cui W. 2022. Tfh-cell-derived interleukin 21 sustains effector CD8(+) T cell responses during chronic viral infection. Immunity 55: 475–493 e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, Cook MC, King C, Tangye SG, de Sauvage FJ, and Ghilardi N. 2010. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. The Journal of experimental medicine 207: 2895–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harker JA, Dolgoter A, and Zuniga EI. 2013. Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4(+) T cell responses and viral control during chronic infection. Immunity 39: 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M, Cheung JF, Mao T, Askari AS, Chen S, Fitzgerald B, Foster GG, Eisenbarth SC, Zhao H, Craft J, and Joshi NS. 2021. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell 184: 6101–6118 e6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Topchyan P, Xin G, Chen Y, Zheng S, Burns R, Shen J, Kasmani MY, Kudek M, Yang N, and Cui W. 2021. Harnessing the IL-21-BATF Pathway in the CD8(+) T Cell Anti-Tumor Response. Cancers (Basel) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutherland APR, Graham KL, Papadimitriou M, Jhala G, Trivedi P, Catterall T, Fynch S, Kay TWH, and Thomas HE. 2019. IL-21 regulates SOCS1 expression in autoreactive CD8(+) T cells but is not required for acquisition of CTL activity in the islets of non-obese diabetic mice. Sci Rep 9: 15302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clough LE, Wang CJ, Schmidt EM, Booth G, Hou TZ, Ryan GA, and Walker LS. 2008. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol 180: 5393–5401. [DOI] [PubMed] [Google Scholar]
- 56.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, Donlin LT, Henderson LA, Wei K, Mizoguchi F, Teslovich NC, Weinblatt ME, Massarotti EM, Coblyn JS, Helfgott SM, Lee YC, Todd DJ, Bykerk VP, Goodman SM, Pernis AB, Ivashkiv LB, Karlson EW, Nigrovic PA, Filer A, Buckley CD, Lederer JA, Raychaudhuri S, and Brenner MB. 2017. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekman I, Ihantola EL, Viisanen T, Rao DA, Nanto-Salonen K, Knip M, Veijola R, Toppari J, Ilonen J, and Kinnunen T. 2019. Circulating CXCR5(−)PD-1(hi) peripheral T helper cells are associated with progression to type 1 diabetes. Diabetologia 62: 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


