Key Points
Question
What is the evidence for use of antidepressants to treat or prevent comorbid depression in patients with medical diseases?
Findings
This umbrella systematic review identified 176 individual systematic reviews of randomized clinical trials in 43 medical diseases and quantitatively summarized and meta-analyzed the results of 52 meta-analyses of antidepressant effects in 27 medical diseases; results indicated sufficient quality of the individual meta-analyses but rather low quality of the meta-analyzed clinical trials. Compared with placebo, antidepressants showed better efficacy and worse tolerability and acceptability and were more likely to prevent depression.
Meaning
Antidepressants are effective and safe for the treatment and prevention of depression in patients with medical diseases, but too few large, high-quality trials exist.
This systematic review and meta-analysis assesses all available evidence for use of antidepressants in depression comorbid with medical diseases.
Abstract
Importance
Every third to sixth patient with medical diseases receives antidepressants, but regulatory trials typically exclude comorbid medical diseases. Meta-analyses of antidepressants have shown small to medium effect sizes, but generalizability to clinical settings is unclear, where medical comorbidity is highly prevalent.
Objective
To perform an umbrella systematic review of the meta-analytic evidence and meta-analysis of the efficacy and safety of antidepressant use in populations with medical diseases and comorbid depression.
Data Sources
PubMed and EMBASE were searched from inception until March 31, 2023, for systematic reviews with or without meta-analyses of randomized clinical trials (RCTs) examining the efficacy and safety of antidepressants for treatment or prevention of comorbid depression in any medical disease.
Study Selection
Meta-analyses of placebo- or active-controlled RCTs studying antidepressants for depression in individuals with medical diseases.
Data Extraction and Synthesis
Data extraction and quality assessment using A Measurement Tool for the Assessment of Multiple Systematic Reviews (AMSTAR-2 and AMSTAR-Content) were performed by pairs of independent reviewers following PRISMA guidelines. When several meta-analyses studied the same medical disease, the largest meta-analysis was included. Random-effects meta-analyses pooled data on the primary outcome (efficacy), key secondary outcomes (acceptability and tolerability), and additional secondary outcomes (response and remission).
Main Outcomes and Measures
Antidepressant efficacy presented as standardized mean differences (SMDs) and tolerability (discontinuation for adverse effects) and acceptability (all-cause discontinuation) presented as risk ratios (RRs).
Results
Of 6587 references, 176 systematic reviews were identified in 43 medical diseases. Altogether, 52 meta-analyses in 27 medical diseases were included in the evidence synthesis (mean [SD] AMSTAR-2 quality score, 9.3 [3.1], with a maximum possible of 16; mean [SD] AMSTAR-Content score, 2.4 [1.9], with a maximum possible of 9). Across medical diseases (23 meta-analyses), antidepressants improved depression vs placebo (SMD, 0.42 [95% CI, 0.30-0.54]; I2 = 76.5%), with the largest SMDs for myocardial infarction (SMD, 1.38 [95% CI, 0.82-1.93]), functional chest pain (SMD, 0.87 [95% CI, 0.08-1.67]), and coronary artery disease (SMD, 0.83 [95% CI, 0.32-1.33]) and the smallest for low back pain (SMD, 0.06 [95% CI, 0.17-0.39]) and traumatic brain injury (SMD, 0.08 [95% CI, −0.28 to 0.45]). Antidepressants showed worse acceptability (24 meta-analyses; RR, 1.17 [95% CI, 1.02-1.32]) and tolerability (18 meta-analyses; RR, 1.39 [95% CI, 1.13-1.64]) compared with placebo. Antidepressants led to higher rates of response (8 meta-analyses; RR, 1.54 [95% CI, 1.14-1.94]) and remission (6 meta-analyses; RR, 1.43 [95% CI, 1.25-1.61]) than placebo. Antidepressants more likely prevented depression than placebo (7 meta-analyses; RR, 0.43 [95% CI, 0.33-0.53]).
Conclusions and Relevance
The results of this umbrella systematic review of meta-analyses found that antidepressants are effective and safe in treating and preventing depression in patients with comorbid medical disease. However, few large, high-quality RCTs exist in most medical diseases.
Introduction
Major depressive disorder (MDD) has a point prevalence of approximately 5% in the general population.1,2 Among individuals with medical diseases, MDD represents one of the most common comorbidities, with point prevalence often exceeding 10% or even 20%.3,4 Furthermore, many more patients exhibit subclinical depressive symptoms.3
Treatment of depression comorbid with medical diseases is important because depression in these populations is associated with decreased quality of life and poorer prognosis.3 Antidepressants represent a first-line treatment, with the most recent meta-analysis of randomized clinical trials (RCTs)5 showing small to medium effect sizes for specific antidepressants compared with placebo for MDD in general. However, meta-analyses of antidepressant efficacy5 are primarily based on RCTs that excluded individuals with medical diseases, potentially limiting the generalizability. Given that every third to sixth patient with a medical disease is treated with antidepressants,6,7,8 it is important to establish the efficacy and safety in these patient populations.
The trials that have examined antidepressant treatment for depression comorbid with medical disease tend to be more heterogeneous than pivotal trials for regulatory purposes, ranging from small academic trials to larger consortia in a wide range of different medical diseases in different clinical or geographical settings. This situation results in a complex evidence landscape for the treatment of comorbid depression that requires careful examination.
Currently, to our knowledge, there is no direct quantitative comparison of the evidence across all individual pharmacological strategies in comorbid depression. Moreover, the quality of the meta-analyses and the included RCTs has not been evaluated, which is an indispensable step before treatment recommendations can confidently be made.
To address this gap, we conducted an umbrella systematic review of all available evidence for use of antidepressants in depression comorbid with medical diseases. We hypothesized that antidepressants would be significantly superior to placebo in treating patients with depression and medical diseases and reasonably acceptable, with potential differences across distinct medical diseases.
Methods
Design
We performed an umbrella review covering systematic reviews with or without meta-analyses of RCTs for treatment of comorbid depression in medical diseases that reported the efficacy and tolerability of antidepressants compared with placebo or each other. The protocol was prospectively registered with PROSPERO (CRD42022297478) on December 10, 2021. The protocol defined a priori the review questions, search strategy, inclusion and exclusion criteria, risk of bias assessment, and data synthesis plan in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol statement (eTable 1 in Supplement 1). The present results are reported in accordance with PRISMA checklists (eTables 2 and 3 in Supplement 1).
Participants/Population
We included all systematic reviews on human participants, irrespective of age, sex, and setting (inpatient, outpatient, or community setting), participating in placebo- or active-controlled RCTs studying antidepressants for depression in medical diseases. Hence, the participants had to have been diagnosed with a medical disease and randomized to receive antidepressants for comorbid depression. Systematic reviews of patients with comorbid depressive symptoms (ie, but not diagnosed MDD) were also included.
Types of Included Studies
We applied the following predefined inclusion criteria: studies defined as a systematic review of placebo-controlled or active-controlled RCTs of antidepressants in humans with a medical disease (defined as all conditions except mental disorders, ie, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes within the F chapter) and comorbid depression that examined the antidepressant efficacy and tolerability of antidepressants (defined as all drugs listed under the World Health Organization Anatomical Therapeutic Chemical codes N06A9) vs placebo or each other. Exclusion criteria consisted of the following: observational studies and case reports; studies in which a medical disease and comorbid depression were not the exposure of interest and in which efficacy and tolerability of antidepressants were not the outcome of interest; studies or study arms in which the active drug was compared with any control other than placebo (eg, psychotherapy); depression comorbid with a mental disorder (ie, diagnosed within the ICD-10 F chapter, which includes dementia); and animal, in vivo, and in vitro experiments.
Search Methods
We conducted the literature search from database inception until March 31, 2023, in PubMed and EMBASE (for search terms used, see the eMethods in Supplement 1). We applied no restrictions regarding year or language.
Screening Procedure
Pairs of independent reviewers (V.S. and J.B., V.S. and K.Z., W.R.C. and O.H.J., K.Z. and S.G., F.W. and M.R.S., V.S. and F.W., and V.S. and S.L.), blinded to each other’s results, screened all identified articles during each step of the screening procedure. First, titles and abstracts were screened based on the eligibility criteria outlined above. Second, full texts were examined and screened for applicability based on the above-mentioned inclusion and exclusion criteria. There was agreement between reviewers in 88.6% of studies and disagreement in 11.4%, so a senior author (O.K.-F., S.M.G., or C.O.) not involved in the initial assessment made the final decision. The Cohen κ, a measure of interrater agreement, resulted in κ = 0.74 (which is considered substantial agreement).
Selection of Systematic Reviews
In case several systematic reviews of the same medical disease were identified, we chose the review that conducted the (network) meta-analysis with the largest number of RCTs. If 2 (network) meta-analyses on the same medical disease included the same number of RCTs, we chose the one with the largest number of participants. (Network) meta-analyses meeting the inclusion criteria were removed if there was another (network) meta-analysis with more RCTs for that same medical disease and antidepressant treatment as long as more than 50% of the meta-analyzed trials overlapped and the pooled sample was larger, as done previously.10 To show representative findings, we included only systematic reviews with broad and generalizable inclusion criteria, meaning that we did not consider systematic reviews that were restricted only to specific subpopulations (eg, defined based on a specific geographical area or age group).
Data Extraction Process
For the initial data extraction performed on all systematic reviews that passed full-text screening, pairs of reviewers (V.S. and J.B., V.S. and K.Z., W.R.C. and O.H.J., K.Z. and S.G., F.W. and M.R.S., V.S. and F.W., and V.S. and S.L.) independently extracted descriptive data (listed in the eMethods in Supplement 1), representing the basis for choosing those systematic reviews to be included for detailed data extraction (as described in the Selection of Systematic Reviews subsection). Furthermore, for the largest systematic reviews (as defined in the Selection of Systematic Reviews subsection), paired independent reviewers (V.S. and J.B., V.S. and K.Z., W.R.C. and O.H.J., K.Z. and S.G., F.W. and M.R.S., V.S. and F.W., and V.S. and S.L.) performed a detailed data extraction (listed in the eMethods in Supplement 1). Any inconsistencies in the data extraction were resolved by a third author (O.K.-F., S.M.G., or C.O.). We used predesigned forms that were piloted initially on a small number of included studies. We contacted authors for missing outcome data or in case of unclear information.
Study Outcomes
Our primary aim was to examine the efficacy and safety of antidepressants in the treatment of comorbid depression. The primary outcome was antidepressant efficacy, defined as improvement on a depression rating scale during the trial (eg, the Hamilton Depression Rating Scale [HDRS]). Key secondary outcomes were (1) antidepressant acceptability, defined as all-cause discontinuation, and (2) antidepressant tolerability, defined as discontinuation for adverse effects. Additional secondary outcomes included (1) study-defined antidepressant response (eg, as ≥50% improvement on the HDRS); (2) study-defined depression remission (eg, ≤7 points on the 17-item HDRS); (3) study discontinuation for intolerability, inefficacy, and nonadherence; and (4) at least 1 adverse event as well as specific adverse events, including suicidal behavior, suicide, and death. Our secondary aim was to examine the efficacy of antidepressants in preventing comorbid depression. The primary outcome was the occurrence of comorbid depression as defined in the specific (network) meta-analyses.
Critical Appraisal of Methodological Quality
For systematic reviews included in the detailed data extraction, we applied A Measurement Tool for the Assessment of Multiple Systematic Reviews (AMSTAR)–2 (scores range from 0-16, with higher scores indicating better quality of systematic reviews), which allows measurement of the quality of the methodology of systematic reviews,11 and AMSTAR-Content (scores range from 0-9, with higher scores indicating the quality of RCTs in meta-analyses), which allows measurement of the quality of the included RCTs in systematic reviews.10 We present both AMSTAR-2 quality and AMSTAR-Content ratings of each included systematic review. The rating was performed independently by pairs of reviewers (V.S. and J.B., V.S. and K.Z., W.R.C. and O.H.J., K.Z. and S.G., F.W. and M.R.S., V.S. and F.W., and V.S. and S.L.), and discrepant scores were resolved by a third reviewer (O.K.-F., S.M.G., or C.O.). We calculated the mean scores across the included systematic review for the AMSTAR-2 quality and AMSTAR-Content to investigate whether there were limitations in the quality of the conducted systematic reviews and/or the included RCTs as done previously.10
Methods for Evidence Synthesis
We summarized the evidence from several (network) meta-analyses in different medical diseases using random-effects meta-analyses to present the standardized mean difference (SMD) for continuous measures (ie, efficacy) and risk ratios (RRs) for binary measures (eg, response or remission) from the newest or largest (network) meta-analysis within each medical disease. We converted all odds ratios (ORs) to RRs using a standard formula.12 To pool the meta-analysis estimates from (network) meta-analyses (eg, the overall SMD from all [network] meta-analyses or an SMD for specific disease groups, such as cardiology or pain), we used the DerSimonian-Laird estimator. From (network) meta-analyses, we extracted effect sizes from each specific comparison of an antidepressant vs placebo. We used the I2 test to quantify the degree of heterogeneity. Stata, version 14.0 (StataCorp LLC) was used for all analyses. Additional analyses and deviations from the protocol are described in the eMethods in Supplement 1. Two-sided P < .05 indicated statistical significance.
Results
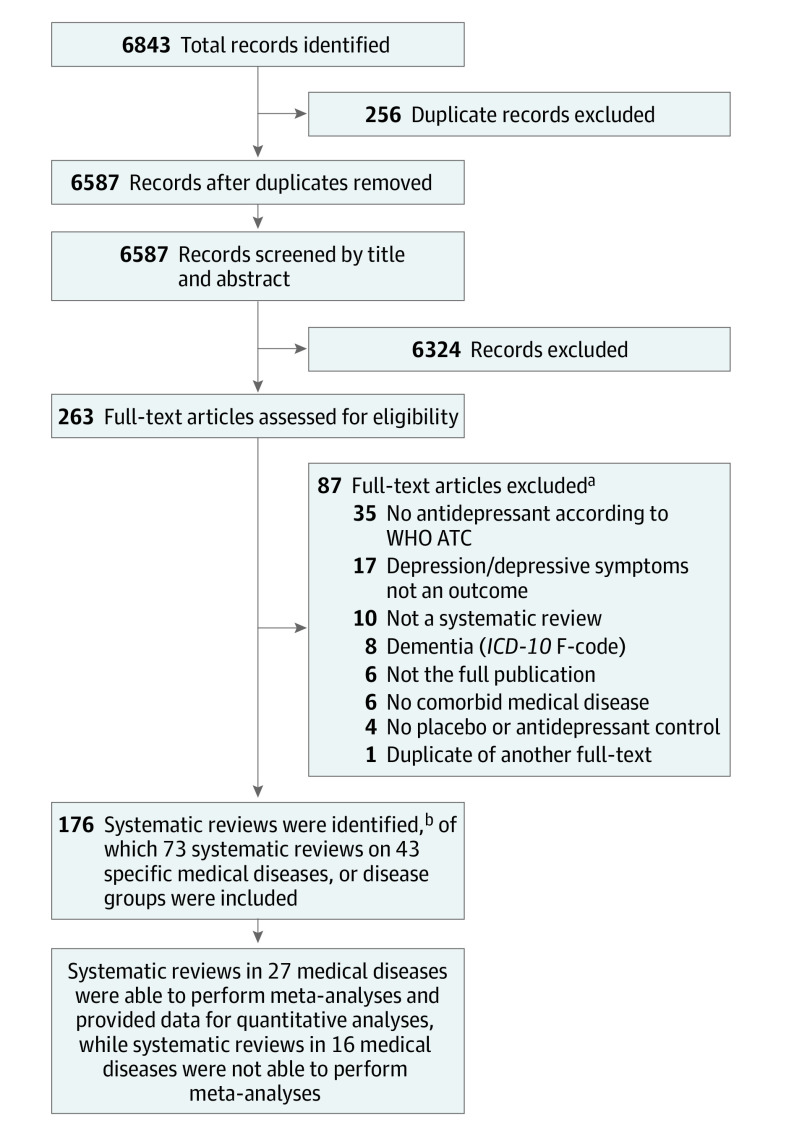
We identified 6587 references, of which 263 publications underwent full-text screening. Of those, 176 systematic reviews (including 45 Cochrane reviews) covering 43 medical diseases (Table) were eligible for initial data extraction (ie, a mean of 4.1 [range, 1-40] systematic reviews per medical disease). Figure 1 presents the study flowchart; eTable 4 in Supplement 2, the specific exclusion criteria for the 87 excluded studies; and eTable 5 in Supplement 2, the detailed bibliographic information for the included 176 systematic reviews. Most of the systematic reviews were performed in neurological (n = 79), cardiological (n = 18), and oncological (n = 20) diseases (Table). Descriptive information for all 176 systematic reviews is presented in eTable 6 in Supplement 2. Based on these descriptive data, we identified the largest systematic reviews within each medical disease. Data on primary and secondary outcome measures for these 73 systematic reviews13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83 are presented in eTable 7 in Supplement 2 for treatment of depression and in eTable 8 in Supplement 2 for prevention of depression.
Table. Number of Systematic Reviews on Antidepressant Treatment of Comorbid Depression in Medical Diseases.
| Medical disease | No. of systematic reviews | No. of Cochrane reviews | Meta-analysis performeda |
|---|---|---|---|
| All | 176 | 45 | NA |
| Neurology | 79b | 14 | Yes |
| ALS | 1 | 0 | No |
| Multiple sclerosis | 2 | 1 | Yes |
| Parkinson disease | 15 | 1 | Yes |
| Stroke | 40 | 10 | Yes |
| Traumatic brain injury | 15 | 0 | Yes |
| Epilepsy | 3 | 2 | No |
| Huntington disease | 2 | 0 | No |
| Cancer | 20b | 4 | Yes |
| Lung | 1 | 0 | No |
| Breast | 2 | 0 | Yes |
| Brain | 3 | 2 | No |
| Cardiology | 18 | 2 | |
| Acute coronary syndrome | 4 | 0 | No |
| Coronary artery disease | 5 | 2 | Yes |
| Coronary heart disease | 2 | 0 | Yes |
| Heart failure | 4 | 0 | Yes |
| Ischemic heart disease | 1 | 0 | Yes |
| Myocardial infarction | 1 | 0 | Yes |
| Cardiac rehabilitation | 1 | 0 | Yes |
| Fibromyalgia | 8 | 2 | Yes |
| Rheumatoid arthritis | 2 | 1 | Yes |
| Pain | 7 | 2 | |
| Phantom limb pain | 1 | 1 | No |
| Noncardiac chest pain | 1 | 0 | Yes |
| Neuropathic pain | 1 | 0 | Yes |
| Low back pain, chronic | 1 | 0 | Yes |
| Low back pain, nonspecific | 2 | 1 | Yes |
| Functional chest pain | 1 | 0 | Yes |
| Diabetes | 7 | 1 | Yes |
| Hepatitis C virus and interferon alfa | 7 | 0 | No |
| HIV | 4 | 1 | Yes |
| Tinnitus | 4 | 3 | No |
| Palliative | 2 | 0 | No |
| Chronic physical illness | 4 | 2 | Yes |
| Axis III disorder | 1 | 0 | Yes |
| Pulmonary diseases | 3 | 1 | |
| Asthma | 1 | 0 | Yes |
| COPD | 1 | 1 | Yes |
| Tuberculosis | 1 | 0 | No |
| IBD | 3 | 1 | Yes |
| PCOS | 1 | 1 | No |
| PMS | 1 | 0 | No |
| Overweight | 2 | 1 | No |
| Dialysis | 1 | 1 | No |
| Kidney disease | 2 | 1 | No |
Abbreviations: ALS, amyotrophic lateral sclerosis; COPD, chronic obstructive pulmonary disease; IBD, inflammatory bowel disease; NA, not applicable; PCOS, polycystic ovary syndrome; PMS, premenstrual syndrome.
Whether the newest or largest systematic review identified a sufficient number of randomized controlled trials (RCTs) to perform meta-analyses. The number of RCTs and patients represent those RCTs that studied the effect of antidepressant drugs in medical diseases. All meta-analyses within the specific medical diseases were based on fewer RCTs and patients, as many RCTs did not report necessary data.
Most systematic reviews on cancer included all patients with cancer (ie, independent of the cancer site), while 6 systematic reviews investigated specific cancer types (ie, breast, brain, and lung). In neurology, 1 systematic review included all neurological diseases, which is why the number of the systematic reviews in the specific neurological diseases adds up to 78 and not 79.
Figure 1. Flowchart of the Literature Search and Study Selection.
ICD-10 indicates International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; WHO ATC, World Health Organization Anatomical Therapeutic Chemical Code.
aeTable 4 in Supplement 2 presents the specific exclusion criteria for each of the 87 systematic reviews that were excluded during full-text screening.
bOf the 165 systematic reviews, several were on the same medical disease. Therefore, we included only the largest systematic review for quantitative synthesis.
AMSTAR-2 quality and AMSTAR-Content ratings were conducted for those 73 systematic reviews included in the detailed data extraction (the specific item scores and total scores are presented in eTable 9 in Supplement 2), of which 52 performed meta-analyses. The mean (SD) AMSTAR-2 quality rating score across these 52 meta-analyses was 9.3 (3.1 [range, 1.5-15.0]) of a maximum of 16, indicating that the meta-analyses were of moderate to good quality. In contrast, the mean (SD) AMSTAR-Content score was 2.4 (1.9 [range, 0-8.0]) of a maximum of 9, indicating that the included original RCTs were of low quality.
Systematic reviews in 16 of the 43 medical diseases contained insufficient RCTs to perform (network) meta-analyses (Table). Of the 27 medical diseases in which quantitative measures were presented, 23 meta-analyses presented SMDs on the antidepressant effect vs placebo (ie, our primary outcome measure). Those 23 (network) meta-analyses were published between 2008 and 2022 and included 1 to 26 RCTs and 30 to 1986 patients. The overall SMD from these 23 meta-analyses was 0.42 (95% CI, 0.30-0.54; I2 = 76.5%), favoring antidepressants over placebo in reducing depressive symptoms but with large heterogeneity between the disease groups (Figure 2A) (SMD range, 0.11 [95% CI, −0.02 to 0.25] to 0.79 [95% CI, 0.57-1.01]) and within specific medical diseases (Figure 2B) (SMDs, 0.06 [95% CI, 0.17-0.39] for lower back pain; 0.08 [95% CI, −0.28 to 0.45] for traumatic brain injury; 0.83 [95% CI, 0.32-1.33] for coronary artery disease; 0.87 [95% CI, 0.08-1.67] for functional chest pain; and 1.38 [95% CI, 0.82-1.93] for myocardial infarction). Compared with placebo, antidepressants were associated with better rates of response (8 meta-analyses; overall RR, 1.54 [95% CI, 1.14-1.94]; I2 = 69.3%) (eFigure 1 in Supplement 1) and remission (6 meta-analyses; RR, 1.43 [95% CI, 1.25-1.61]; I2 = 0%) (eFigure 2 in Supplement 1).
Figure 2. Standardized Mean Differences (SMDs) for Antidepressant Efficacy Compared With Placebo for Comorbid Depression.

Weights (size of diamond markers) are determined by random-effects analyses. AMSTAR indicates A Measurement Tool for the Assessment of Multiple Systematic Reviews; CAD, coronary artery disease; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; IBD, inflammatory bowel disease; IHD, ischemic heart disease; MI, myocardial infarction; MS, multiple sclerosis; NR, not reported; RCTs (as defined in Figure 3), randomized controlled trials; and TBI, traumatic brain injury. The overall SMD of 0.42 in part A is based on all specific meta-analyses presented in part B, with part A furthermore presenting the pooled SMDs within each disease category (eg, the pooled SMD for pain in part B is based on the 4 meta-analyses with pain as the disease group from part B).
Regarding key secondary outcomes, acceptability-related (24 meta-analyses; RR, 1.17 [95% CI, 1.02-1.32]; I2 = 59.2%) and tolerability-related (18 meta-analyses; RR, 1.39 [95% CI, 1.13-1.64]; I2 = 49.8%) discontinuation risks were higher with antidepressants compared with placebo (Figure 3). Occurrence of any adverse effect was also significantly more likely with antidepressants (10 meta-analyses; RR, 1.22 [95% CI, 1.06-1.38]; I2 = 29.7%) (eFigure 3 in Supplement 1). Regarding specific adverse events, antidepressants had increased risks for sexual dysfunction (RR, 2.33 [95% CI, 1.04-3.62]; I2 = 0%), dry mouth (RR, 2.12 [95% CI, 1.41-2.83]; I2 = 0%), and nausea (RR, 1.70 [95% CI, 1.07-2.33]; I2 = 0%) compared with placebo (eFigure 4 in Supplement 1).
Figure 3. Risk Ratios (RRs) for Antidepressant Acceptability and Tolerability.

Acceptability is measured by all-cause discontinuation of study medication; tolerability is measured by discontinuation of study medication due to adverse effects. Weights (size of markers) are determined by random-effects analyses. AMSTAR indicates A Measurement Tool for the Assessment of Multiple Systematic Reviews; IBD, inflammatory bowel disease; IHD, ischemic heart disease; NR, not reported; RCTs, randomized controlled trials; SSRI, selective serotonin reuptake inhibitor; and TCA, tricyclic antidepressant. If no specific antidepressant drug is mentioned (eg, SSRI), then the analyses were not restricted to specific antidepressants but could cover all antidepressants.
In additional analyses, the primary efficacy estimates were not significantly associated with the Cochrane vs non-Cochrane systematic reviews (eFigure 5 in Supplement 1) nor with the number of RCTs, publication year, or AMSTAR-Quality scores (eFigure 6 in Supplement 1). However, there were statistically significant associations between a larger sample size and higher AMSTAR Content scores with lower SMDs (eFigure 6 in Supplement 1). Few (network) meta-analyses identified data for direct comparisons between antidepressant groups and specific antidepressants, with all analyses indicating no differences between antidepressants except for paroxetine outperforming fluoxetine in patients with coronary artery disease and amitriptyline outperforming duloxetin in fibromyalgia (eFigure 7 in Supplement 1). Estimates of the specific antidepressants and antidepressant groups are presented in eFigure 8 in Supplement 1.
Of 10 systematic reviews studying antidepressants in the prevention of depression (eTable 8 in Supplement 2), 7 meta-analyses assessed antidepressant efficacy compared with placebo (Figure 4). These reviews were published between 2014 and 2021 and ranged from 2 to 13 RCTs and 147 to 1352 patients. Across medical diseases, antidepressant treatment was associated with an overall 57% reduced risk of developing depression (RR, 0.43 [95% CI, 0.33-0.53]; I2 = 20.9%) compared with placebo.
Figure 4. Forest Plot Showing Risk Ratios (RRs) for Antidepressant Drugs Compared With Placebo in Preventing the Development of Depression Among Individuals With Medical Diseases.

Weights (size of markers) are determined by random-effects analyses. AD indicates antidepressant; HCV, hepatitis C virus; IFN-α, Interferon alfa; RCTs, randomized controlled trials; SSRIs, selective serotonin reuptake inhibitors; and TBI, traumatic brain injury.
Discussion
This umbrella systematic review and meta-analysis provides 5 main findings. First, antidepressants were more efficacious compared with placebo (with a small to medium effect size) and led to higher response and remission rates. Second, antidepressants were associated with higher acceptability-related and tolerability-related discontinuation rates and a higher risk for any adverse event. Third, effect sizes for antidepressant efficacy varied considerably across specific comorbid medical diseases. Fourth, in patients with medical diseases, antidepressants prevented depression with an overall risk reduction of 57%. Finally, our quality appraisal indicated that the systematic reviews were of moderate quality, but the included RCTs often were of low quality.
The effect sizes observed were in a range similar to those reported by an earlier meta-analyses for antidepressants in patients with MDD but without medical comorbidity.5 However, the evidence base for comorbid depression generally rests on smaller and more heterogeneous trials. In MDD without medical comorbidity, more and larger RCTs have been performed (the efficacy SMDs for the specific antidepressants ranged from 0.17 to 0.48 in the most recent meta-analysis5). Accordingly, our overall SMD of 0.42 (range, 0.11-0.66) indicates comparable efficacy of antidepressants vs placebo in patients with medical diseases and comorbid depression.
Additionally, Cipriani et al5 reported ORs for acceptability ranging from 0.84 to 1.30 for specific antidepressants. This fits well with the RR of 1.17 (95% CI, 1.02-1.32) for acceptability of antidepressants vs placebo in our current analysis. Also, antidepressants were less tolerable than placebo in the meta-analysis,5 with ORs ranging from 1.21 to 4.44 for specific antidepressants. Again, this is compatible with our findings of worse tolerability of antidepressants compared to placebo with an RR of 1.39 (95% CI, 1.13-1.64). The adverse effects that occurred more often with antidepressants compared with placebo in patients with depression and medical diseases encompassed dry mouth, nausea, and sexual dysfunction, all of which are well-known adverse effects of antidepressants.84 Therefore, as a broad conclusion, clinicians can expect that antidepressants are about equally efficacious and safe in patients with depression with and without medical diseases.
Importantly, effect sizes for antidepressant efficacy varied considerably across specific comorbid medical diseases, with SMDs ranging from 0.06 in chronic low back pain70 to 1.38 after myocardial infarction72 (Figure 2B). We did not find any association between the effect size and the number of RCTs, publication year, or AMSTAR-2 quality score of the included meta-analyses (eFigure 6 in Supplement 1). This would argue for disease-specific rather than methodological reasons for differences in the effect sizes of antidepressants. For example, it is possible that the effects of antidepressants are smaller in more chronic conditions (such as chronic low back pain) compared with diseases with an acute onset (such as myocardial infarction). However, the quality of the RCTs that informed the meta-analyses was mostly low, as indicated by a mean AMSTAR-Content score of 2.4 on a scale ranging from 0 to 9. Furthermore, some meta-analyses were based on very limited RCTs with few participants. For example, the meta-analysis in chronic low back pain with the smallest effect size is based on 2 RCTs and 132 patients, whereas the meta-analysis in myocardial infarction with the largest effect size consists of 10 RCTs with 280 participants. Therefore, we would caution not to overinterpret differences across medical diseases at this stage of the data. As is so often the case, large confirmatory controlled trials in different medical diseases are needed to answer the question whether antidepressants systematically differ in their efficacy and safety across medical diseases.
In several medical diseases, we identified many systematic reviews. For example, we identified 79 systematic reviews in neurology covering a maximum of 25 RCTs, and 40 systematic reviews were published specifically in stroke covering a maximum of 10 RCTs. Hence, more systematic reviews than RCTs have been published in some medical diseases. This result emphasizes the need for large, well-conducted RCTs studying antidepressants for comorbid depression in medical diseases. Importantly, the prescription rate of antidepressants ranges from about 15% in patients with acute coronary syndrome6 or cancer7 to 35% in patients with stroke8 and 42% in patients with colon cancer.85 In other words, millions of people with a medical disease are being treated with antidepressants worldwide. Clearly, these treatment approaches need to be informed by larger and better trials.
There was an overall 57% reduced risk of developing depression in patients with a medical disease when they were treated preventively with antidepressants. However, preventive effects of antidepressants were tested in only a few medical diseases. Thus, more research is needed for such preventive approaches in individual patients with a high risk of developing a depressive episode (eg, those with a history of depressive episodes).86 However, the potential preventive efficacy should be weighed against potential adverse effects associated with antidepressants. For example, a Cochrane review found that selective serotonin reuptake inhibitors prevented depression within 12 months after stroke but were associated with an increased risk of bone fractures and seizures.42
Strengths and Limitations
Strengths of our analysis include the comprehensive and in-depth approach and formally assessing the quality of the systematic reviews and of the included RCTs. Limitations include those of the included systematic reviews and RCTs, that is, primarily the small number of RCTs and the absence of large RCTs to yield robust efficacy estimates. Second, most of the meta-analyses pooled all antidepressants with only a few analyses of specific antidepressants or antidepressant groups. Hence, it is currently not possible to make recommendations for specific antidepressants, pointing to the need for future RCTs comparing specific antidepressants head-to-head. Additionally, many systematic reviews did not report several important aspects (eg, sex, age, antidepressant dose or duration, depression scores, absolute numbers, and risk reduction rates). Accordingly, we were unable to study potential moderators of efficacy, to examine whether the absolute decrease in depression scores differed between comorbid depression and MDD without medical comorbidity,5 or to calculate the number needed to treat or number needed to harm. Third, several systematic reviews did not report tolerability, acceptability, and adverse effects, limiting detailed analyses on these important aspects. Fourth, we only considered evidence from (network) meta-analyses, meaning that we did not include evidence from RCTs that have not yet been meta-analyzed. Fifth, 2 network meta-analyses included 3-armed RCTs, meaning that the placebo was not independent because the placebo group was used repeatedly.
Conclusions
Despite the paucity of large and well-conducted RCTs studying antidepressants for comorbid depression in medical diseases, this umbrella systematic review and meta-analysis demonstrates that antidepressants are efficacious and safe for comorbid depression in medical diseases, with effect sizes that are similar to those reported for antidepressants in MDD without medical comorbidity. Furthermore, antidepressants can prevent the development of depression in some medical diseases, but this finding should be weighed against potential adverse effects. It is important to screen for and manage comorbid depression in patients with medical diseases, and clinicians should choose treatments based on patient preferences and the antidepressant’s risk-benefit ratio. Future large, high-quality RCTs should include head-to-head comparisons between antidepressants to expand the knowledge on potential differences in efficacy and safety between antidepressants for depression comorbid with medical diseases and to allow more specific treatment recommendations for distinct medical diseases.
eMethods. Search Strategy, Data Extraction, Additional Analyses, and Protocol Deviations
eTable 1. PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) 2015 Checklist: Recommended Items to Address in a Systematic Review Protocol
eTable 2. PRISMA 2020 (Preferred Reporting Items for Systematic Review and Meta-Analysis) Checklist
eTable 3. PRISMA 2020 (Preferred Reporting Items for Systematic Review and Meta-Analysis) for Abstract Checklist
eFigure 1. Risk Ratios for Antidepressant Response, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 2. Risk Ratios for Antidepressant Remission, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 3. Risk Ratios for at Least 1 Adverse Event During Antidepressant Treatment, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 4. Risk Ratios for Specific Adverse Events During Antidepressant Treatment, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 5. Standardized Mean Differences (SMDs) of Antidepressant Efficacy for Comorbid Depression in Patients With Medical Diseases, Divided Into Whether the SRs Were Cochrane or Non-Cochrane Systematic Reviews (SRs)
eFigure 6. Standardized Mean Differences (SMDs) of Antidepressant Efficacy for Comorbid Depression in Patients With Medical Diseases, Sorted by (1) No. of RCTs, (2) No. of Patients, (3) Publication Year, (4) AMSTAR-2 Quality Score, and (5) AMSTAR-Content Score
eFigure 7. Standardized Mean Differences (SMDs) and Risk Ratios (RRs) of Direct Comparisons Between Antidepressant Drugs in Comorbid Depression in Patients With Medical Diseases
eFigure 8. Standardized Mean Differences (SMDs) for Specific Antidepressant Drugs and Antidepressant Groups in Comorbid Depression in Patients With Medical Diseases
eTable 4. Reasons for Exclusion for All the 87 Systematic Reviews That Were Excluded During the Full-Text Screening Phase
eTable 5. Bibliographic Data for All 176 Identified Systematic Reviews That Were Identified During the Full-Text Screening Phase
eTable 6. Descriptive Information From All 176 Identified Systematic Reviews That Were Identified During the Full-Text Screening Phase
eTable 7. Detailed Information on Primary and Secondary Outcome Measures From the 73 Systematic Reviews Representing the Newest or Largest Cochrane and Non-Cochrane Systematic Reviews on the Treatment of Depression in Each Medical Disease
eTable 8. Detailed Information on Primary and Secondary Outcome Measures From the 73 Systematic Reviews Representing the Newest or Largest Cochrane and Non-Cochrane Systematic Reviews on the Prevention of Depression in Each Medical Disease
eTable 9. Detailed Information on AMSTAR-2 Quality and AMSTAR-Content Ratings From the 73 Systematic Reviews Representing the Newest or Largest Cochrane and Non-Cochrane Systematic Reviews on the Treatment or Prevention in Each Medical Disease
Data Sharing Statement
References
- 1.Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold SM, Köhler-Forsberg O, Moss-Morris R, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers. 2020;6(1):69. doi: 10.1038/s41572-020-0200-2 [DOI] [PubMed] [Google Scholar]
- 4.Momen NC, Plana-Ripoll O, Agerbo E, et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. 2020;382(18):1721-1731. doi: 10.1056/NEJMoa1915784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-1366. doi: 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czarny MJ, Arthurs E, Coffie DF, et al. Prevalence of antidepressant prescription or use in patients with acute coronary syndrome: a systematic review. PLoS One. 2011;6(11):e27671. doi: 10.1371/journal.pone.0027671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanjida S, Janda M, Kissane D, et al. A systematic review and meta-analysis of prescribing practices of antidepressants in cancer patients. Psychooncology. 2016;25(9):1002-1016. doi: 10.1002/pon.4048 [DOI] [PubMed] [Google Scholar]
- 8.Mortensen JK, Johnsen SP, Andersen G. Prescription and predictors of post-stroke antidepressant treatment: a population-based study. Acta Neurol Scand. 2018;138(3):235-244. doi: 10.1111/ane.12947 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Collaborating Centre for Drug Statistics Methodology . ATC/DDD index 2023. Updated January 23, 2023. Accessed May 11, 2023. https://www.whocc.no/atc_ddd_index/
- 10.Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675-684. doi: 10.1001/jamapsychiatry.2017.0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Yu KF. What’s the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. doi: 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 13.Allida S, Cox KL, Hsieh CF, House A, Hackett ML. Pharmacological, psychological and non-invasive brain stimulation interventions for preventing depression after stroke. Cochrane Database Syst Rev. 2020;5(5):CD003689. doi: 10.1002/14651858.CD003689.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allida S, Cox KL, Hsieh CF, Lang H, House A, Hackett ML. Pharmacological, psychological, and non-invasive brain stimulation interventions for treating depression after stroke. Cochrane Database Syst Rev. 2020;1(1):CD003437. doi: 10.1002/14651858.CD003437.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2011;(12):CD006380. doi: 10.1002/14651858.CD006380.pub2 [DOI] [PubMed] [Google Scholar]
- 16.Atluri DK, Chandar AK, Fass R, Falck-Ytter Y. Systematic review with meta-analysis: selective serotonin reuptake inhibitors for noncardiac chest pain. Aliment Pharmacol Ther. 2015;41(2):167-176. doi: 10.1111/apt.13015 [DOI] [PubMed] [Google Scholar]
- 17.Baldo P, Doree C, Molin P, McFerran D, Cecco S. Antidepressants for patients with tinnitus. Cochrane Database Syst Rev. 2012;2012(9):CD003853. doi: 10.1002/14651858.CD003853.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baraldi S, Hepgul N, Mondelli V, Pariante CM. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531-543. doi: 10.1097/JCP.0b013e31825d9982 [DOI] [PubMed] [Google Scholar]
- 19.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev. 2012;12:CD008381. doi: 10.1002/14651858.CD008381.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beedham W, Belli A, Ingaralingam S, Haque S, Upthegrove R. The management of depression following traumatic brain injury: a systematic review with meta-analysis. Brain Inj. 2020;34(10):1287-1304. doi: 10.1080/02699052.2020.1797169 [DOI] [PubMed] [Google Scholar]
- 21.Beevers Z, Hussain S, Boele FW, Rooney AG. Pharmacological treatment of depression in people with a primary brain tumour. Cochrane Database Syst Rev. 2020;7(7):CD006932. doi: 10.1002/14651858.CD006932.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caruso R, Ostuzzi G, Turrini G, et al. Beyond pain: can antidepressants improve depressive symptoms and quality of life in patients with neuropathic pain? a systematic review and meta-analysis. Pain. 2019;160(10):2186-2198. doi: 10.1097/j.pain.0000000000001622 [DOI] [PubMed] [Google Scholar]
- 23.Carvalho AF, Hyphantis T, Sales PM, et al. Major depressive disorder in breast cancer: a critical systematic review of pharmacological and psychotherapeutic clinical trials. Cancer Treat Rev. 2014;40(3):349-355. doi: 10.1016/j.ctrv.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Christiansen OG, Madsen MT, Simonsen E, Gögenur I. Prophylactic antidepressant treatment following acute coronary syndrome: a systematic review of randomized controlled trials. J Psychiatr Res. 2017;94:186-193. doi: 10.1016/j.jpsychires.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 25.Das A, Roy B, Schwarzer G, et al. Comparison of treatment options for depression in heart failure: a network meta-analysis. J Psychiatr Res. 2019;108:7-23. doi: 10.1016/j.jpsychires.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 26.Deng L, Qiu S, Yang Y, et al. Efficacy and tolerability of pharmacotherapy for post-stroke depression: a network meta-analysis. Oncotarget. 2018;9(34):23718-23728. doi: 10.18632/oncotarget.23891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimmock PW, Wyatt KM, Jones PW, O’Brien PMS. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356(9236):1131-1136. doi: 10.1016/S0140-6736(00)02754-9 [DOI] [PubMed] [Google Scholar]
- 28.Doyle F, Freedland KE, Carney RM, et al. Hybrid systematic review and network meta-analysis of randomized controlled trials of interventions for depressive symptoms in patients with coronary artery disease. Psychosom Med. 2021;83(5):423-431. doi: 10.1097/PSY.0000000000000944 [DOI] [PubMed] [Google Scholar]
- 29.Eshun-Wilson I, Siegfried N, Akena DH, Stein DJ, Obuku EA, Joska JA. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev. 2018;1(1):CD008525. doi: 10.1002/14651858.CD008525.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farooq S, Tunmore J, Comber R. Pharmacological or non-pharmacological interventions for treatment of common mental disorders associated with tuberculosis: a systematic review. Chron Respir Dis. 2021;18:14799731211003937. doi: 10.1177/14799731211003937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiest KM, Walker JR, Bernstein CN, et al. ; CIHR Team Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease . Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult Scler Relat Disord. 2016;5:12-26. doi: 10.1016/j.msard.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 32.Fiest KM, Hitchon CA, Bernstein CN, et al. ; CIHR Team “Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease.” . Systematic review and meta-analysis of interventions for depression and anxiety in persons with rheumatoid arthritis. J Clin Rheumatol. 2017;23(8):425-434. doi: 10.1097/RHU.0000000000000489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao C, Fu Q, Chen P, Liu Z, Zhou Q. The influence of sertraline on depressive disorder after traumatic brain injury: a meta-analysis of randomized controlled studies. Am J Emerg Med. 2019;37(9):1778-1783. doi: 10.1016/j.ajem.2019.06.050 [DOI] [PubMed] [Google Scholar]
- 34.Gnanapragasam S, Hopkins CW, Moulton CD. Can pharmacotherapy improve depressive symptoms in patients with amyotrophic lateral sclerosis? a systematic review of the literature. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(3-4):289-291. doi: 10.3109/21678421.2015.1111385 [DOI] [PubMed] [Google Scholar]
- 35.Grigolon RB, Trevizol AP, Gerchman F, et al. Is obesity a determinant of success with pharmacological treatment for depression? a systematic review, meta-analysis and meta-regression. J Affect Disord. 2021;287:54-68. doi: 10.1016/j.jad.2021.03.032 [DOI] [PubMed] [Google Scholar]
- 36.Gu J, Huang H, Chen K, Huang G, Huang Y, Xu H. Are they necessary? preventive therapies for post-stroke depression: a meta-analysis of RCTs. Psychiatry Res. 2020;284:112670. doi: 10.1016/j.psychres.2019.112670 [DOI] [PubMed] [Google Scholar]
- 37.Häuser W, Wolfe F, Tölle T, Uçeyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26(4):297-307. doi: 10.2165/11598970-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 38.Iovieno N, Tedeschini E, Ameral VE, Rigatelli M, Papakostas GI. Antidepressants for major depressive disorder in patients with a co-morbid axis-III disorder: a meta-analysis of patient characteristics and placebo response rates in randomized controlled trials. Int Clin Psychopharmacol. 2011;26(2):69-74. doi: 10.1097/YIC.0b013e328340775e [DOI] [PubMed] [Google Scholar]
- 39.Jiang HY, Deng M, Zhang YH, Chen HZ, Chen Q, Ruan B. Specific serotonin reuptake inhibitors prevent interferon-α–induced depression in patients with hepatitis C: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12(9):1452-1460.e3. doi: 10.1016/j.cgh.2013.04.035 [DOI] [PubMed] [Google Scholar]
- 40.Kampling H, Baumeister H, Bengel J, Mittag O. Prevention of depression in adults with long-term physical conditions. Cochrane Database Syst Rev. 2021;3(3):CD011246. doi: 10.1002/14651858.CD011246.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch MW, Glazenborg A, Uyttenboogaart M, Mostert J, De Keyser J. Pharmacologic treatment of depression in multiple sclerosis. Cochrane Database Syst Rev. 2011;(2):CD007295. doi: 10.1002/14651858.CD007295.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legg LA, Rudberg AS, Hua X, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. 2021;11(11):CD009286. doi: 10.1002/14651858.CD009286.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li M, Kennedy EB, Byrne N, et al. Systematic review and meta-analysis of collaborative care interventions for depression in patients with cancer. Psychooncology. 2017;26(5):573-587. doi: 10.1002/pon.4286 [DOI] [PubMed] [Google Scholar]
- 44.Maguire MJ, Marson AG, Nevitt SJ. Antidepressants for people with epilepsy and depression. Cochrane Database Syst Rev. 2021;4(4):CD010682. doi: 10.1002/14651858.CD010682.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikocka-Walus A, Prady SL, Pollok J, et al. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst Rev. 2019;4(4):CD012680. doi: 10.1002/14651858.CD012680.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moulton CD, Hopkins CW, Bevan-Jones WR. Systematic review of pharmacological treatments for depressive symptoms in Huntington’s disease. Mov Disord. 2014;29(12):1556-1561. doi: 10.1002/mds.25980 [DOI] [PubMed] [Google Scholar]
- 47.Ostuzzi G, Benda L, Costa E, Barbui C. Efficacy and acceptability of antidepressants on the continuum of depressive experiences in patients with cancer: systematic review and meta-analysis. Cancer Treat Rev. 2015;41(8):714-724. doi: 10.1016/j.ctrv.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 48.Ostuzzi G, Matcham F, Dauchy S, Barbui C, Hotopf M. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2018;4(4):CD011006. doi: 10.1002/14651858.CD011006.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostuzzi G, Turrini G, Gastaldon C, et al. Efficacy and acceptability of antidepressants in patients with ischemic heart disease: systematic review and meta-analysis. Int Clin Psychopharmacol. 2019;34(2):65-75. doi: 10.1097/YIC.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 50.Palmer SC, Natale P, Ruospo M, et al. Antidepressants for treating depression in adults with end-stage kidney disease treated with dialysis. Cochrane Database Syst Rev. 2016;2016(5):CD004541. doi: 10.1002/14651858.CD004541.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollok J, van Agteren JE, Carson-Chahhoud KV. Pharmacological interventions for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;12(12):CD012346. doi: 10.1002/14651858.CD012346.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price A, Rayner L, Okon-Rocha E, et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2011;82(8):914-923. doi: 10.1136/jnnp.2010.230862 [DOI] [PubMed] [Google Scholar]
- 53.Rabindranath KS, Butler JA, Macleod AM, Roderick P, Wallace SA, Daly C. Physical measures for treating depression in dialysis patients. Cochrane Database Syst Rev. 2005;(2):CD004541. doi: 10.1002/14651858.CD004541.pub2 [DOI] [PubMed] [Google Scholar]
- 54.Rayner L, Price A, Evans A, Valsraj K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev. 2010;(3):CD007503. doi: 10.1002/14651858.CD007503.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2011;(11):CD008920. doi: 10.1002/14651858.CD008920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarkar S, Schaefer M. Antidepressant pretreatment for the prevention of interferon alfa-associated depression: a systematic review and meta-analysis. Psychosomatics. 2014;55(3):221-234. doi: 10.1016/j.psym.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 57.Serralde-Zúñiga AE, Gonzalez Garay AG, Rodríguez-Carmona Y, Melendez G. Fluoxetine for adults who are overweight or obese. Cochrane Database Syst Rev. 2019;10(10):CD011688. doi: 10.1002/14651858.CD011688.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghazi-Noori S, Chung TH, Deane K, Rickards HE, Clarke CE. Therapies for depression in Parkinson’s disease. Cochrane Database Syst Rev. 2003;(3):CD003465. doi: 10.1002/14651858.CD003465 [DOI] [PubMed] [Google Scholar]
- 59.Shi CC, Tian G, Lou J, Wu J. Efficacy and safety of selective serotonin reuptake inhibitors for Parkinson’s disease patients with depression: a systematic review. Chin J Evidence-Based Med. 2015;15(4):439-444. doi: 10.7507/1672-2531.20150073 [DOI] [Google Scholar]
- 60.Su D, Zhang Y, Wang A, et al. Efficacy and tolerability of selective serotonin reuptake inhibitors on promoting motor recovery after stroke: meta-analysis of randomized controlled trials. Expert Rev Neurother. 2021;21(10):1179-1189. doi: 10.1080/14737175.2021.1982696 [DOI] [PubMed] [Google Scholar]
- 61.Taylor D, Meader N, Bird V, Pilling S, Creed F, Goldberg D; Pharmacology Subgroup of the National Institute for Health and Clinical Excellence Guideline Development Group for Depression in Chronic Physical Health Problems . Pharmacological interventions for people with depression and chronic physical health problems: systematic review and meta-analyses of safety and efficacy. Br J Psychiatry. 2011;198(3):179-188. doi: 10.1192/bjp.bp.110.077610 [DOI] [PubMed] [Google Scholar]
- 62.Tran L, Sharrad K, Kopsaftis Z, et al. Pharmacological interventions for the treatment of psychological distress in patients with asthma: a systematic review and meta-analysis. J Asthma. 2021;58(6):759-769. doi: 10.1080/02770903.2020.1731826 [DOI] [PubMed] [Google Scholar]
- 63.Tully PJ, Ang SY, Lee EJ, et al. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 2021;12(12):CD008012. doi: 10.1002/14651858.CD008012.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ujeyl M, Müller-Oerlinghausen B. Antidepressants for treatment of depression in palliative patients: a systematic literature review. Article in German. Schmerz. 2012;26(5):523-536. doi: 10.1007/s00482-012-1221-x [DOI] [PubMed] [Google Scholar]
- 65.Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific low back pain. Cochrane Database Syst Rev. 2008;2008(1):CD001703. doi: 10.1002/14651858.CD001703.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;2015(6):CD011735. doi: 10.1002/14651858.CD011735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker J, Sawhney A, Hansen CH, et al. Treatment of depression in people with lung cancer: a systematic review. Lung Cancer. 2013;79(1):46-53. doi: 10.1016/j.lungcan.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142. [PubMed] [Google Scholar]
- 69.Welsch P, Üçeyler N, Klose P, Walitt B, Häuser W. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;2(2):CD010292. doi: 10.1002/14651858.CD010292.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White AP, Arnold PM, Norvell DC, Ecker E, Fehlings MG. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36(21)(suppl):S131-S143. doi: 10.1097/BRS.0b013e31822f178f [DOI] [PubMed] [Google Scholar]
- 71.Zahid JA, Grummedal O, Madsen MT, Gögenur I. Prevention of depression in patients with cancer: a systematic review and meta-analysis of randomized .controlled trials. J Psychiatr Res. 2020;120:113-123. doi: 10.1016/j.jpsychires.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 72.Zhou W, Zhang Y, Meng H, Deng G, Zhou X. Efficacy and safety of newer-generation antidepressants for patients with myocardial infarction and depression: a meta-analysis. Chin J Evidence-Based Med. 2018;18(7):715-720. doi: 10.7507/1672-2531.201801101 [DOI] [Google Scholar]
- 73.Zhuang J, Wang X, Xu L, Wu T, Kang D. Antidepressants for polycystic ovary syndrome. Cochrane Database Syst Rev. 2013;2013(5):CD008575. doi: 10.1002/14651858.CD008575.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Feltz-Cornelis C, Allen SF, Holt RIG, Roberts R, Nouwen A, Sartorius N. Treatment for comorbid depressive disorder or subthreshold depression in diabetes mellitus: systematic review and meta-analysis. Brain Behav. 2021;11(2):e01981. doi: 10.1002/brb3.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srisurapanont M, Suttajit S, Kosachunhanun N, Likhitsathian S, Suradom C, Maneeton B. Antidepressants for depressed patients with type 2 diabetes mellitus: a systematic review and network meta-analysis of short-term randomized controlled trials. Neurosci Biobehav Rev. 2022;139:104731. doi: 10.1016/j.neubiorev.2022.104731 [DOI] [PubMed] [Google Scholar]
- 76.Wang XL, Feng ST, Wang YT, et al. Comparative efficacy and acceptability of drug treatments for Parkinson’s disease with depression: a systematic review with network meta-analysis. Eur J Pharmacol. 2022;927:175070. doi: 10.1016/j.ejphar.2022.175070 [DOI] [PubMed] [Google Scholar]
- 77.Lim GEH, Tang A, Chin YH, et al. A network meta-analysis of 12,116 individuals from randomized controlled trials in the treatment of depression after acute coronary syndrome. PLoS One. 2022;17(11):e0278326. doi: 10.1371/journal.pone.0278326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan L, Ai Y, Xing Y, et al. Citalopram in the treatment of elderly chronic heart failure combined with depression: a systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:1107672. doi: 10.3389/fcvm.2023.1107672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of amitriptyline and US Food and Drug Administration–approved treatments for fibromyalgia: a systematic review and network meta-analysis. JAMA Netw Open. 2022;5(5):e2212939. doi: 10.1001/jamanetworkopen.2022.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Liang C, Chen P, Cao Y, Zhang Y. Effect of antidepressants on psychological comorbidities, disease activity, and quality of life in inflammatory bowel disease: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2023;16:17562848231155022. doi: 10.1177/17562848231155022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elsnhory A, Hasan MT, Hagrass AI, et al. Recovery in stroke patients treated with fluoxetine versus placebo: a pooled analysis of 7,165 patients. Neurologist. 2023;28(2):104-116. doi: 10.1097/NRL.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 82.Kalbouneh HM, Toubasi AA, Albustanji FH, Obaid YY, Al-Harasis LM. Safety and efficacy of SSRIs in improving poststroke recovery: a systematic review and meta-analysis. J Am Heart Assoc. 2022;11(13):e025868. doi: 10.1161/JAHA.122.025868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hughes JW, Kuhn TA, Ede D, Gathright EC, Josephson RA. Meta-analysis of antidepressant pharmacotherapy in patients eligible for cardiac rehabilitation: ANTIDEPRESSANT AMBIVALENCE. J Cardiopulm Rehabil Prev. 2022;42(6):434-441. doi: 10.1097/HCR.0000000000000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sinyor M, Cheung CP, Abraha HY, et al. Antidepressant-placebo differences for specific adverse events in major depressive disorder: a systematic review. J Affect Disord. 2020;267:185-190. doi: 10.1016/j.jad.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 85.Pocobelli G, Yu O, Ziebell RA, et al. Use of antidepressants after colon cancer diagnosis and risk of recurrence. Psychooncology. 2019;28(4):750-758. doi: 10.1002/pon.5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi Y, Yang D, Zeng Y, Wu W. Risk factors for post-stroke depression: a meta-analysis. Front Aging Neurosci. 2017;9:218. doi: 10.3389/fnagi.2017.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy, Data Extraction, Additional Analyses, and Protocol Deviations
eTable 1. PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) 2015 Checklist: Recommended Items to Address in a Systematic Review Protocol
eTable 2. PRISMA 2020 (Preferred Reporting Items for Systematic Review and Meta-Analysis) Checklist
eTable 3. PRISMA 2020 (Preferred Reporting Items for Systematic Review and Meta-Analysis) for Abstract Checklist
eFigure 1. Risk Ratios for Antidepressant Response, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 2. Risk Ratios for Antidepressant Remission, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 3. Risk Ratios for at Least 1 Adverse Event During Antidepressant Treatment, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 4. Risk Ratios for Specific Adverse Events During Antidepressant Treatment, Compared With Placebo, for Comorbid Depression in Medical Diseases
eFigure 5. Standardized Mean Differences (SMDs) of Antidepressant Efficacy for Comorbid Depression in Patients With Medical Diseases, Divided Into Whether the SRs Were Cochrane or Non-Cochrane Systematic Reviews (SRs)
eFigure 6. Standardized Mean Differences (SMDs) of Antidepressant Efficacy for Comorbid Depression in Patients With Medical Diseases, Sorted by (1) No. of RCTs, (2) No. of Patients, (3) Publication Year, (4) AMSTAR-2 Quality Score, and (5) AMSTAR-Content Score
eFigure 7. Standardized Mean Differences (SMDs) and Risk Ratios (RRs) of Direct Comparisons Between Antidepressant Drugs in Comorbid Depression in Patients With Medical Diseases
eFigure 8. Standardized Mean Differences (SMDs) for Specific Antidepressant Drugs and Antidepressant Groups in Comorbid Depression in Patients With Medical Diseases
eTable 4. Reasons for Exclusion for All the 87 Systematic Reviews That Were Excluded During the Full-Text Screening Phase
eTable 5. Bibliographic Data for All 176 Identified Systematic Reviews That Were Identified During the Full-Text Screening Phase
eTable 6. Descriptive Information From All 176 Identified Systematic Reviews That Were Identified During the Full-Text Screening Phase
eTable 7. Detailed Information on Primary and Secondary Outcome Measures From the 73 Systematic Reviews Representing the Newest or Largest Cochrane and Non-Cochrane Systematic Reviews on the Treatment of Depression in Each Medical Disease
eTable 8. Detailed Information on Primary and Secondary Outcome Measures From the 73 Systematic Reviews Representing the Newest or Largest Cochrane and Non-Cochrane Systematic Reviews on the Prevention of Depression in Each Medical Disease
eTable 9. Detailed Information on AMSTAR-2 Quality and AMSTAR-Content Ratings From the 73 Systematic Reviews Representing the Newest or Largest Cochrane and Non-Cochrane Systematic Reviews on the Treatment or Prevention in Each Medical Disease
Data Sharing Statement