Abstract
Objective and design.
Our aim was to study if an extracellular, oxidative antimicrobial mechanism inherent to tracheal epithelial cells is capable of inactivating influenza virus.
Material or subjects.
Epithelial cells were isolated from tracheas of male Sprague-Dawley rats. Both human and rat tracheobronchial epithelial cells were differentiated in air-liquid interface cultures.
Treatment.
A/swine/Illinois/02860/09 (swH1N2) influenza A virions were added to the apical side of the airway cells for 1 hour in the presence or absence of lactoperoxidase or thiocyanate.
Methods.
Characterization of rat epithelial cells (morphology, Duox expression) occurred via western blotting, PCR, hydrogen peroxide production measurement and histology. The number of viable virions was determined by plaque assays. Statistical difference of the results was analyzed by ANOVA and Tukey’s test.
Results.
Our data show that rat tracheobronchial epithelial cells develop a differentiated, polarized monolayer with high transepithelial electrical resistance, mucin production and expression of dual oxidases. Influenza A virions are significantly inactivated by human and rat epithelial cells via a dual oxidase-, lactoperoxidase- and thiocyanate-dependent mechanism.
Conclusions.
Differentiated air-liquid interface cultures of rat tracheal epithelial cells provide a novel model to study Duox-influenza interactions. The dual oxidase/lactoperoxidase/thiocyanate extracellular oxidative system producing hypothiocyanite is a fast and potent anti-influenza mechanism inactivating H1N2 viruses prior to infection.
Keywords: Dual oxidase, hypothiocyanite, influenza, airway epithelium, hydrogen peroxide
1. Introduction
Tracheobronchial epithelial cells (TBEC) in the airways provide the first line of defense against inhaled pathogenic infectious agents [1]. The respiratory epithelium alerts the innate immune system to initiate inflammation [1]. TBECs themselves are capable of fighting pathogens by producing reactive oxygen species, mucins and antimicrobial peptides [1–4]. TBECs orchestrate an oxidative extracellular antiviral system present in the airway surface liquid consisting of the protein lactoperoxidase (LPO), the thiocyanate anion (SCN−) and hydrogen peroxide (H2O2) [5–7]. LPO and SCN− are both present in large quantities in the airway surface liquid [6, 7]. LPO oxidizes SCN− using H2O2 into hypothiocyanite (OSCN−), which has demonstrated antimicrobial effects [8–11]. This antimicrobial system was originally described in milk and saliva [8, 9, 11], and only recently in the airways [5, 6].
Cellular H2O2 is derived from two NADPH oxidases highly expressed in TBECs: dual oxidase 1 and 2 (Duox1, Duox2) [5, 12, 13]. Duox1 and Duox2 are the most likely candidates to provide H2O2 for the H2O2/LPO/SCN− antimicrobial system since these enzymes are the dominant NADPH oxidases expressed in TBECs [5, 14, 15], and are ideally localized to the apical membrane to produce extracellular H2O2 into the airway surface liquid [5, 16]. The H2O2/LPO/SCN− antimicrobial system is effective against several microbes including viruses [17, 18]. Its virucidal effects have been described for HIV and RSV but not for other viruses including influenza [17, 18].
Influenza A virus (IAV) causes yearly epidemics with high morbidity and deaths in humans [19, 20]. IAVs have several subtypes that are classified based on their hemagglutinin (HA) and neuraminidase (NA) surface proteins [21]. The IAV subtypes most commonly infecting humans are H1N1, H1N2, and H3N2 [21]. A recent study showed that enzyme-free OSCN− has antiviral activity against a pandemic influenza strain (A/H1N1/2009) [22]. Different IAV subtypes (H1N1, H3N2) were shown to elicit discrete responses (Duox up-regulation) in TBECs [23]. These data suggest that the Duox/H2O2/LPO/SCN− system in TBECs is a potent anti-influenza mechanism of the respiratory innate immune system [22, 23]. The potential role of the H2O2/LPO/SCN− antimicrobial system in inactivation of the IAV subtype H1N2 has yet to be determined. H1N2 viruses represent a serious public health problem in humans and pigs [24]. The H1N2 IAV subtype resulted from reassortment between H1N1 and H3N2 subtypes [25] and are endemic in the United States [26].
The purpose of this study was to determine if TBECs are capable of inactivating extracellular IAV virions of the H1N2 subtype by the Duox/H2O2/LPO/SCN− system. Air-liquid interface (ALI) cultures of polarized, differentiated TBECs provide the best in vitro model of the respiratory epithelium. These cultures develop transepithelial electric resistance (TEER); contain ciliated, goblet and basal cells; produce mucins, and release cytokines upon microbial stimulus [14, 15, 27–31]. We and others have also shown that TBECs express Duox at their later, differentiated stage in ALI cultures [14, 15, 23, 32]. Human TBECs are difficult to obtain, costly and exhibit large donor-to-donor variation. For this reason, we established a complementary model: ALI cultures of primary rat TBECs. These cells are easier to isolate, cost-effective, less prone to variations and are suitable for larger scale studies; therefore rat TBECs provide an excellent alternative to human TBECs in vitro [33].
In this article, we provide detailed characterization of ALI cultures of primary rat TBECs. Rat cells express both, Duox1 and Duox2, and produce extracellular H2O2 in a calcium-dependent manner. We also show that rat TBECs inactivate H1N2 IAV to a remarkable extent by the H2O2/LPO/SCN− system. These data were further confirmed using ALI cultures of normal human bronchial epithelium (NHBE). In summary, our data establishes rat TBECs as a model to study influenza-Duox interactions and show for the first time that H1N2 subtype of IAV is efficiently inactivated by the Duox/H2O2/LPO/SCN− antiviral system.
2. Materials and Methods
2.1. Animals.
Male Sprague-Dawley rats were purchased from Harlan Laboratories (South Easton, MA). The animals were between 15–20 weeks old at the time of euthanasia via CO2. All animal-related procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia (Rada, IACUC protocol numbers: A2012 11-004-Y2-A1, A2015 03-030-Y1-A0.)
2.2. Culture of primary human and rat airway epithelial cells.
Primary normal human bronchial epithelial cells (NHBE) were purchased from Lonza (Walkersville, MD) and cultured as previously described [15]. Briefly, cells were seeded onto 24-well polyester (0.4 micron pore) membrane transwells (Costar), precoated with 0.3% rat tail collagen I (Sigma), at a density of 2.0 × 104 cells/ well. B-ALI™ growth medium was used in the apical and basal chambers until the cells reached confluence. The upper chamber medium was aspirated and the lower medium was replaced with B-ALI™ Differentiation medium (Lonza, Walkersville, MD). Cells were maintained on the air-liquid interface (ALI) for 4–5 weeks by feeding every other day with ALI differentiation medium (the surface of ALI cultures was washed with sterile HBSS every other day). Antibiotics (penicillin and streptomycin, Life Technologies, Grand Island, NY) were supplemented in the media up to four days before experiments.
Rat tracheas were removed under sterile conditions from male Sprague-Dawley rats. The tracheas were incubated overnight at 4°C in Dulbecco’s modified Eagle’s medium (DMEM; Sigma) and Ham’s nutrient F-12 medium (F-12; Sigma) (1:1) with 5% protease (Sigma). Fetal bovine serum 10% (FBS; Hyclone) was then added to the incubation medium (DMEM/F-12) and TBECs were flushed out. The cells were collected by centrifugation (450g, 4°C, 10 min) and were washed twice with DMEM/F-12 containing 10% FBS. Growth medium for RTE cells consisted of DMEM/F-12 supplemented with 1% L-Glutamine, 1% Pen/Strep, 10 μg/mL insulin, 0.1 μg/mL hydrocortisone, 0.1 μg/mL cholera toxin, 5 μg/mL transferrin, 5ug/mL Transferrin, 25 ng/mL epidermal growth factor (all reagents from Sigma), 1% bovine pituitary extract (Life technologies), 3 mg/mL bovine serum albumin, 50 nM retinoic acid. Polyester permeable membranes on culture inserts (6.5-mm-diameter, 0.4-μm-pore-size; Costar) were precoated with 100 μL 0.3% rat tail collagen I (Sigma). Rat TBEcs were plated onto the apical surface of the inserts with 0.2 mL of growth medium in the upper (apical) compartments of the culture plates (6.0 × 104 cells/membrane). Cultures were grown in 95% air and 5% CO2 at 37°C. After 72 hours, media in the apical chamber was changed. The medium was changed every other day using 0.5 and 0.2 mL growth medium in the basal and apical compartments, respectively. After 7 days the cells were confluent (determined visually and by measuring transepithelial electrical resistance (TEER)). The medium was removed from the apical chamber taking the cells to ALI. The basal chamber was changed every other day. Cells were cultured between 3–4 weeks on ALI when used for experiments. 4 days prior to experiments cells were given antibiotic-free medium. To characterize their morphology, rat TBEC cultures were fixed with 4% paraformaldehyde (GE Healthcare Life Sciences, Pittsburgh, PA) and subjected to H&E and Mayer’s mucicarmine staining (detecting mucins) (UGA, Histology Laboratory, Athens, GA) (Figure 1.).
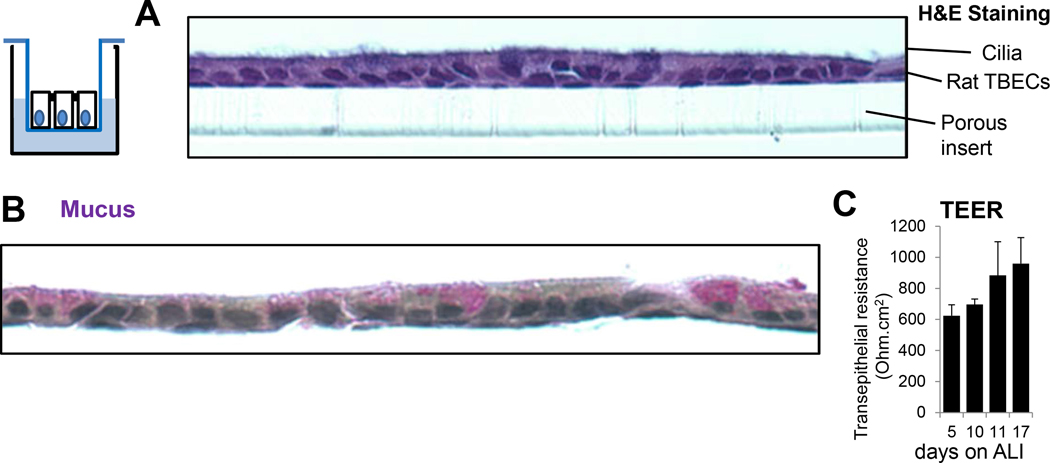
Figure 1. Air-liquid interface cultures of polarized, differentiated rat tracheal epithelial cells provide an excellent in vitro model of the bronchial epithelium.
(A) H&E staining shows formation of a ciliated monolayer of polarized cells after 17 days of culture on ALI. One representative results, n=3. (B) Mayer’s mucicarmine staining detects mucins. These large glycoproteins were identified by positive staining (purple) originating from mucin-producing Goblet cells. One representative result, n=3. (C) Transepithelial resistance (Ohm.cm2) was measured on days 5, 10, 11, 17 post ALI using a voltohmmeter. Mean+/−S.E.M., n=7. TEER, transepithelial electrical resistance; TBEC, tracheobronchial epithelial cell; ALI, air-liquid interface.
2.3. Influenza A virus.
Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing high glucose (HyClone) supplemented with 5% heat-inactivated fetal bovine serum (FBS) and maintained at 37°C with 5% CO2. A/swine/Illinois/02860/09 (swH1N2) IAV viral stocks were cultured in MDCK cells using infection medium (DMEM containing high glucose supplemented with 1 mM L-glutamine with 1-μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone [TPCK]-treated trypsin).
2.4. Western blotting
NHBE cells were lysed by Nonidet P-40 lysis buffer (Boston Bioproducts, Ashland, MA) containing 150 μM PMSF (Fluka Biochemika) and protease inhibitor cocktail (Sigma-Aldrich) [15]. Protein levels were determined using the bicinchoninic acid assay (Pierce, Grand Island, NY). Lysates were electrophoresed on SDS-polyacrylamide gels (8%; Tris-glycine gel, Invitrogen). Gels were blotted on nitrocellulose membrane (Invitrogen) using the iBlot dry blotting system (Life Technologies, Carlsbad, CA). Blots were blocked overnight in TTBS (TBS buffer containing 5% milk powder and 0.05% Tween 20). Blots were incubated with primary anti-Duox antibody (rabbit, polyclonal; 1/2000) [34] followed by incubation with secondary HRP-linked anti-rabbit IgG antibody GE Healthcare; 1/1000). Blots were developed by chemiluminescence using the Lumigen DS detection kit (GE Healthcare).
2.5. RNA isolation and RT-PCR
RNA was isolated from rat TBECs by RNAzol (Sigma-Aldrich, St. Louis, MO)/chloroform extraction followed by isopropanol precipitation as described [35]. RNA Concentrations and purities were determined using a Nanodrop spectrophotometer. cDNA synthesis was carried out with the Thermoscript cDNA synthesis kit (Life Technologies) using 1 μg total RNA, oligo dT primers and RNaseH treatment. To detect duox1, duox2 and actin gene expressions, the following gene-specific primers were used in the PCR reaction (PCR Thermocycler, Eppendorf): rat duox1 (F: CTGGAGCTCT CCGGGTTT, R: GGCACTGAGG AGGCTGACTA, product: 767 bp); rat duox2 (F: GGTGGAGATC AGTGTGGTGA, R: GCTAGGAAGC CCCTCTGC, product: 665 bp); rat actin (F: GGAAATGCAC TCCCTTGTGT, R: TGTTAGCTTT GGGGTTCAGG, product: 453 bp). PCR program: 94 C (0.5 min), 62 C (0.5 min), 72 C (1.5 min), 37 cycles.
2.6. Measurement of H2O2 production.
H2O2 production was measured through horseradish peroxidase (HRP)-mediated oxidation of homovanillic acid (HVA) as previously described [14]. Flourescence was measured using Varioskan Flash fluorescence microplate reader (ThermoScientific, excitation wavelength 320nm, emission wavelength 405 nm) for one hour taking readings every minute. H2O2 production was then calculated and expressed as nmol H2O2/hr/106 cells.
2.7. Viral inactivation assay.
After human or rat TBECs had been on ALI for the respective time, 4 days prior to use cells were switched to antibiotic-free medium and the apical chamber was washed with HBSS (Mediatech, Manassas, VA)once. Each component of the system was tested at the following concentrations 100 μM ATP, 6.5 μg/ml LPO, 400 μM SCN− (in HBSS as assay medium). The reaction volume was set to 40 μL with the appropriate concentration of each component. Virus was diluted to an MOI of 0.1 (NHBE cells ~5000 viral particles, rat TBECs ~8000 viral particles). Catalase (15,000 U/mL, Sigma-Aldrich, St. Louis, MO) was also used in one of the reactions to inhibit the system. Once the components were assembled, the 40 μL was pipetted to the apical chamber of the transwell and placed in a 37 °C 5% CO2 incubator for one hour. After one hour supernatants from each respective well were collected and stored at −80c. Plaque assays were performed to determine viral concentrations as previously described [24].
2.9. Statistics.
Data for the viral inactivation assay were log-transformed and significance was calculated using a one-way ANOVA and a Tukey post-hoc test performed using Minitab17. *, p<0.05; **, p<0.01; ***, p<0.001.
3. Results
3.1. Characterization of ALI cultures of differentiated rat TBECs.
Recently published results suggest that the Duox/H2O2/LPO/SCN− system responds to H1N1 and H3N2 subtypes of IAV [22, 23]. Whether the third IAV subtype commonly infecting humans, H1N2, can be inactivated by TBECs is unknown. To study this, we used ALI cultures of primary, differentiated rat TBECs as an in vitro model to study epithelial-influenza interactions. Rat TBECs were cultured on ALI (Fig. 1. scheme) in 24-well transwells for 3 weeks and subjected to different assays to characterize them. Hematoxylin and eosin staining revealed that TBECs formed the characteristic monolayer containing polarized epithelial cells with apical cilia (Fig. 1A). Basal cells near the transwell membrane support can be visualized (Fig. 1A). Mayer’s mucicarmine staining detecting mucins as large glycoproteins identified positively stained (purple), mucin-producing Goblet cells (Fig. 1B). Unstained polarized cells are ciliated epithelial cells (Fig. 1B). Differentiated TBECs develop high transepithelial electric resistance (TEER) suggesting formation of tight junctions [36]. Figure 1C shows the time-dependent development of high TEER values in rat TBECs. These data confirm that rat TBECs form polarized, mucus-producing, ciliated monolayers in our hands providing an excellent in vitro model of the respiratory epithelium.
3.2. Rat TBECs express functional Duox1 and Duox2.
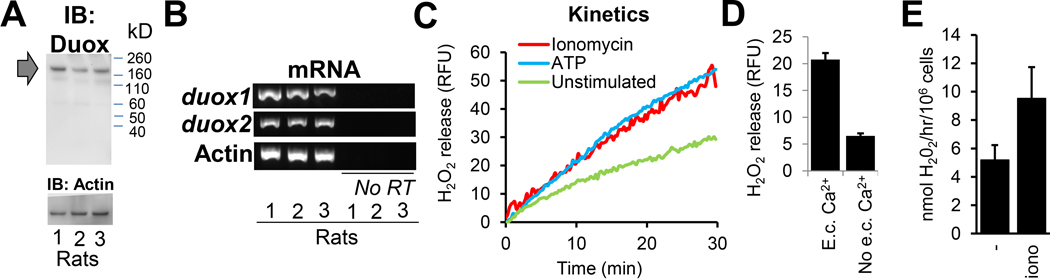
In humans, dual oxidases are expressed in TBECs in vivo [5] or in vitro in ALI cultures [14, 15, 32, 37]. Duox proteins were also detected in in vitro cultures of rat and cow TBECs although detailed description of their culture conditions were not provided [32]. Here we provide detailed characterization of Duox expression and function in rat TBECs. As shown in Figure 2A, polarized rat TBECs express Duox protein (~180 kDa molecular weight) to similar extent in all three animals studied. Although the antibody favors detection of Duox1, it is not isoform-specific [38]. Therefore we detected gene expression levels of each isoform, Duox1 and Duox2, by reverse transcriptase PCR. Figure 2B shows that both isoforms are expressed in rat TBECs. Reverse transciptase was omitted in control samples to show specificity for amplification of DUOX1 and DUOX2 and lack of contaminating host DNA. (Fig. 2B). To show that rat TBECs contain functional Duox enzymes, we measured extracellular H2O2 release by horse radish peroxidase (HRP)-mediated oxidation of homovanillic acid (HVA) – as previously described [14, 16]. Rat TBECs spontaneously produced extracellular H2O2 (referred to as basal level) that could be still enhanced by known activators of Duox: ATP and ionomycin (Fig. 2C) [15, 28, 39]. In the current study we used ATP to stimulate Duox activity (see later Figs. 4. and 5.). Duox requires an increase in cytosolic calcium to be activated [37, 40]. Scavenging extracellular calcium by EGTA largely reduces basal H2O2 output of rat TBECs suggesting that Duox enzymes are the main H2O2 producers in rat TBECs, and that extracellular calcium is required for their activation (Fig. 2D). In summary, rat TBECs express both Duox isoforms that are the suggested source of H2O2 production.
Figure 2. Rat tracheal epithelial cells express Duox and produce extracellular H2O2 in a calcium-dependent manner.
Air-liquid interface cultures of primary rat tracheal cells were cultured for 17 days and subjected to the following analyses: (A) Duox protein was detected in cell lysates by western blotting. Samples of three animals shown. (B) Gene expression levels of rat duox1, duox2 and actin genes were detected by reverse transcriptase PCR. Samples of three animals shown. (C) Kinetics (left) and endpoint (right) measurements of extracellular hydrogen peroxide production detected by homovanillic acid oxidation assay. Kinetics: one representative result, n=3. ATP (100 μM), ionomycin (1 μM). Endpoint: mean+/−S.E.M., n=3. Extracellular calcium was chelated by addition of 1mM EGTA. IB, immunoblot; RT, reverse transcriptase; RFU, relative fluorescence unit.
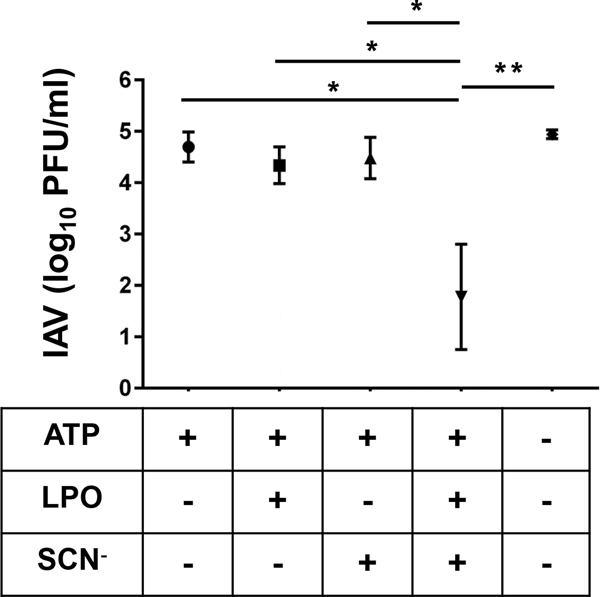
Figure 4. Rat TBECs inactivate influenza virions in a Duox/H2O2/LPO/SCN−-dependent manner.
ALI cultures of differentiated primary Sprague-Dawley rat TBECs were exposed to 8000 PFU of H1N2 IAV (swH1N2) in presence or absence of ATP (Duox activator, 100 μM), LPO (6.5 μg/ml) or SCN− (400 μM) in the indicated combinations. After 1 hr incubation supernatants were collected and concentration of viable virus particles was determined by PFU assay using MDCK cells. Mean+/−S.E.M., n=4. ANOVA, Brown-Forsythe test, Tukey’s multiple comparisons. *, p<0.05; **, p<0.01. IAV, influenza A virus; PFU, plaque forming unit.
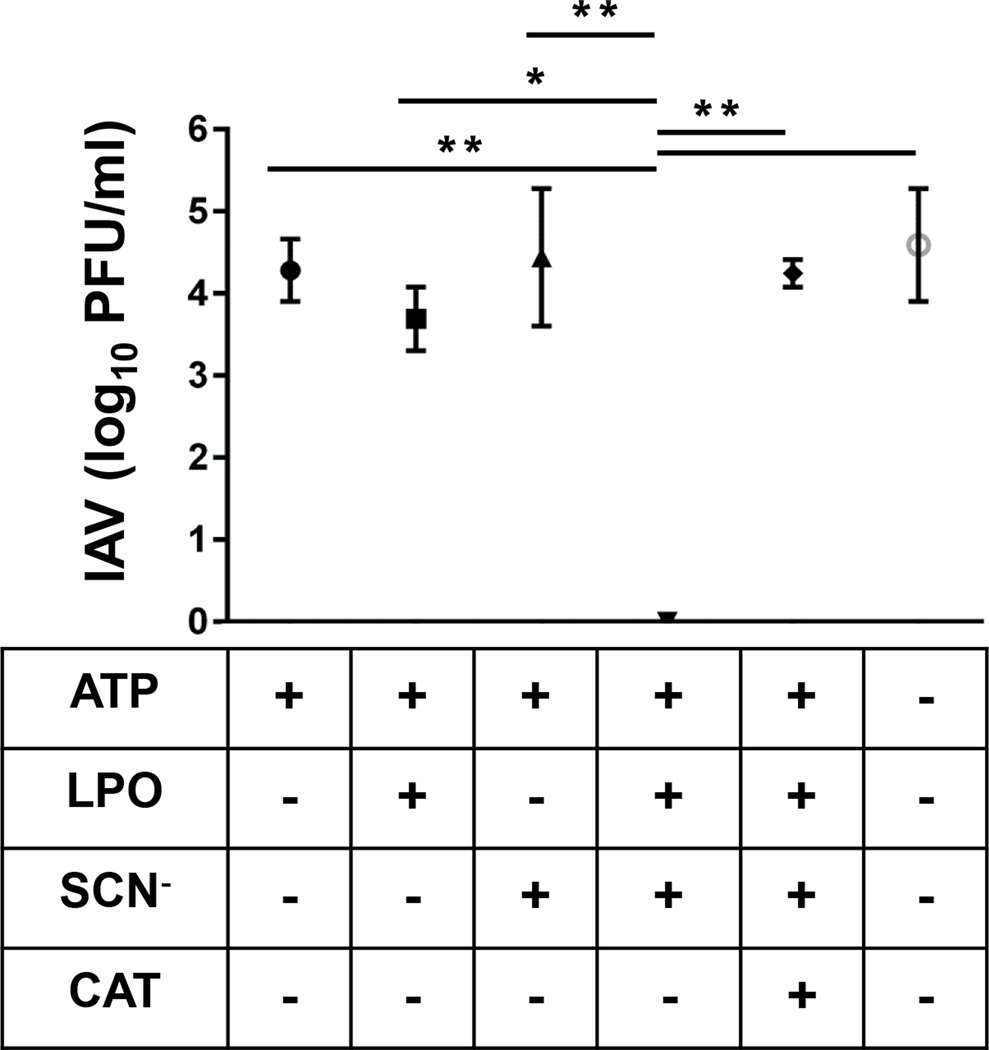
Figure 5. Human bronchial epithelial cells inactivate influenza virions in a Duox/H2O2/LPO/SCN− -dependent manner.
ALI cultures of differentiated human NHBE cells were exposed to 5000 PFU of H1N2 IAV (swH1N2) in presence or absence of ATP (Duox activator, 100 μM), LPO (1 μM), SCN− (400 μM) or catalase (CAT, 15.000 U/ml) in the indicated combinations. After 1 hr incubation supernatants were collected and concentration of viable virus particles was determined by PFU assay using MDCK cells. Mean+/−S.E.M., n=2. ANOVA, Brown-Forsythe test, Tukey’s multiple comparisons. *, p<0.05; **, p<0.01; ***, p<0.001. IAV, influenza A virus; PFU, plaque forming unit.
3.3. Rat TBECs inactivate H1N2 influenza A virus in a H2O2/LPO/SCN−-dependent manner.
Although enzyme-free OSCN− has virucidal effect against the pandemic A/H1N1/2009 influenza virus [22], and NHBE cells upregulate Duox in response to H1N1 and H3N2 IAV strains [23], there are no data to show direct IAV inactivation by the Duox/H2O2/LPO/SCN− system and response of TBECs to the H1N2 subtype. To address this knowledge gap, we exposed TBEC cultures to the H1N2 IAV strain A/Swine/Illinois/02860/09 (swH1N2) at a multiplicity of infection (MOI) of 0.1 for 1.5 hour (see explanatory scheme in Figure 3). swH1N2 is an endemic swine strain originally isolated in the state of Illinois in 2009 [24, 41]. Supernatants were collected from infected TBEC cultures to prepare 10-fold serial dilutions (Fig. 3.). Diluted viral suspensions were added to Madin-Darby Canine Kidney Epithelial Cells (MDCK) (Fig. 3.). Plaques were counted after three days of incubation, and changes in viable virion concentrations were calculated (Fig. 3.). Although Duox enzymes show high spontaneous activity in rat TBECs, ATP was added to enhance their H2O2 output (Fig. 2.). LPO and SCN− were added as indicated at levels found in human airways (SCN−: 400 μM, LPO 6.5 μg/ml) (Fig. 4.) [32, 37]. Figure 4 shows that a significant 2–3 log reduction in the number of viable H1N2 IAV virions occurred when the complete H2O2/LPO/SCN− system was reconstituted on top of rat TBEC cultures. Omitting LPO, SCN− or both resulted in complete loss of virion inactivation (Fig. 4.), indicating that the full system needs to be present and OSCN− is responsible for viral inactivation.
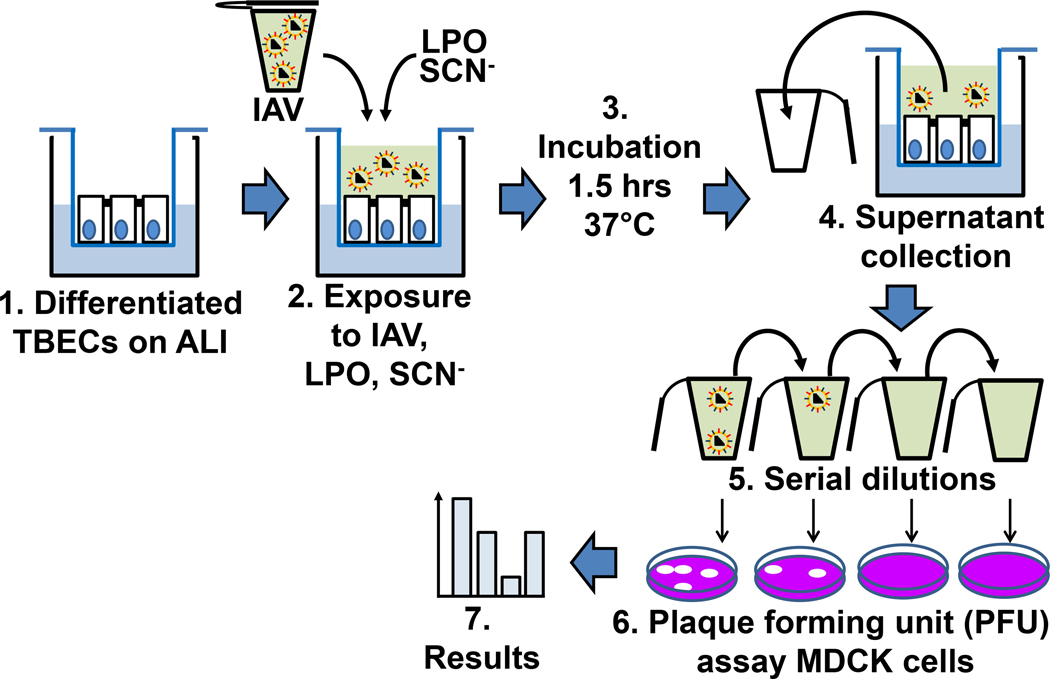
Figure 3. Steps of measuring inactivation of extracellular influenza virions on tracheobronchial epithelial cells.
ALI cultures of differentiated TBECs (1) are exposed to IAV in combination with exogenous LPO and SCN− (2) for 1 hour at 37oC (3). Supernatants were then collected (4), 10-fold serial dilutions were performed (5) and plaque forming unit assays on MDCK cells (6) were used to determine the PFU/mL of virus remaining (7). MDCK, Madin-Darby canine kidney cells; PFU. Plaque forming unit; LPO, lactoperoxidase; SCN-, thiocyanate; IAV, influenza A virus; ALI, air-liquid interface; TBEC, tracheobronchial epithelial cell.
3.4. H1N2 IAV inactivation by the H2O2/LPO/SCN− system of NHBE.
To determine if primary cultures of human bronchial epithelial cells behave similarly to rat TBECs, we exposed human NHBE cells to H1N2 IAV under the same conditions as the rat cells (Fig. 4) and followed influenza virion inactivation as described. Similarly to the results shown in Figure 4., NHBE caused H1N2 inactivation when the full H2O2/LPO/SCN− system was assembled. If LPO, SCN− or both were left out, influenza inactivation was entirely inhibited (Fig. 5.). This highlights again the crucial antiviral role of the final product, OSCN− against influenza, and proves that NHBE cells can produce sufficient H2O2 by Duox to fuel this antiviral system. In addition, we also show that the H2O2 scavenger catalase entirely blocks the virion-inactivating effect of the H2O2/LPO/SCN− system (Fig. 5, see “CAT”). This further suggests that Duox-derived H2O2 is required and sufficient for influenza inactivation. Overall, the Duox/H2O2/LPO/SCN− system of both rat and human cultures of primary airway epithelial cells have strong antiviral activity against the IAV H1N2 strain. This further confirms that rat TBECs provide an excellent model to study IAV-Duox interactions in the respiratory epithelium.
Discussion
TBECs are the earliest responders to influenza challenge in the airways [1, 42]. Their reaction to the first invading virions is essential in determining the later fate of the inflammatory response. One of the inflammatory mechanisms by which TBECs fight pathogens is the production of reactive oxygen species [1, 5, 23]. TBECs release hydrogen peroxide into the airway surface liquid that is used by LPO to oxidize its most abundant substrate, SCN− to produce antimicrobial OSCN− [6, 7, 43, 44]. LPO is produced primarily in serous acini of submucosal glands in the airways, not in the epithelium [5, 45]. Its main substrate, SCN− is present extracellularly in submillimolar concentrations in human airways [43]. SCN− is transported from the blood trough the epithelium via several suggested transport proteins [46–48]. Both, LPO and SCN− are abundantly present in airway secretion and H2O2 production represents the rate-limiting factor in the activity of the system. In intact airways, H2O2 is primarily provided by two NADPH oxidases, Duox1 and Duox2 [5]. Both Duox enzymes and their maturation factors (Duox activators) localize to the apical plasma membrane of bronchial epithelial cells ideally suited to produce extracellular H2O2 [5, 16]. In vitro ALI cultures of differentiated and polarized human respiratory epithelium express high amounts of Duox, produce apical H2O2 and have been shown to kill several microorganisms in an H2O2-dependent manner [15, 23, 32, 43]. However, working with cultures of primary human cells has significant limitations: 4–5 weeks of culturing time, cost and most importantly large donor-to-donor variations. We used ALI cultures of rat TBECs to complement our data obtained with human cells. Obtaining primary rat cells is cost effective, the cells are faster to culture, show little variation among donors and allow larger scale studies. Our rat TBECs reconstitute all the features of human cultures and express both dual oxidases (Figs. 1–2.). This is in accordance with previous studies that also used rat TBEC ALI cultures [32, 49–51]. Our data showing that rat TBECs inactivate IAV similarly to human cultures indicates that rat TBECs provide an excellent, alternative model to study influenza-human epithelial cell interactions (Figs. 4–5.).
TBECs are capable of producing sufficient H2O2 to supply the anti-influenza effect of LPO (Figs. 4–5.). All three components (Duox/H2O2, LPO, SCN−) are necessary to inactivate IAV. Omitting one, two or all three components results in no significant inactivation of IAV. Thus, not only enzyme-free [22] but also enzyme-derived OSCN− efficiently inactivates IAV. Detailed responses of human epithelial cells to H1N1 and H3N2 subtypes of IAV have been shown but their direct extracellular inactivation by the Duox-based system has not been documented [23]. Our studies are the first to show that extracellular influenza viruses can be inactivated efficiently by epithelial-derived OSCN−. This adds influenza to the growing list of pathogenic infectious agents that the H2O2/LPO/SCN− system is capable of inactivating/killing [15, 18, 32, 43]. We also show that Duox and LPO are efficient against H1N2 subtype of IAV.
There are two isoforms of Duox, both of which are highly expressed in TBECs [2, 5, 15, 23, 52]. Which isoform is more important to produce H2O2 in TBECs is unclear at this point. It is well-accepted that in human TBECs Duox1 is the main Duox isoform expressed [5, 14]. Basal Duox2 expression is lower but Duox2 can be induced to a larger extent by microbial stimuli [53]. While the in vivo role of Duox1 is still unknown, the main physiological function of Duox2 is clearly to provide H2O2 for thyroid hormone biosynthesis. This is evident since both Duox2-deficient mice and human patients with Duox2 mutations develop hypothyroidism [54, 55]. Our data indicate that rat TBECs express both Duox1 and Duox2 at similar levels. Although Duox2 has been proposed to be the main isoform in rat TBECs [32], this conclusion was drawn from an siRNA transfection experiment which showed minimal effect on apical H2O2 output without showing isoform specificity of the siRNAs. The situation is likely more complicated and both isoforms could be activated by different stimuli or could overtake each other’s function to provide redundancy. Therefore, further data are needed to clearly identify the main Duox isoform responsible for the antimicrobial and inflammatory effect of TBECs.
It is important to emphasize the very fast action of the Duox/H2O2/LPO/SCN− system to inactivate IAV. Under our experimental conditions, IAV was only added for 1 hour on the top of TBECs. During this time there is a significant decrease in the concentration of viable extracellular virions if the whole system is assembled (Figs. 4–5). We assume that this rapid and robust inactivation of IAV by the Duox-based system largely reduces their potential to infect TBECs and to cause inflammation. To our current knowledge very few, if any, airway immune responses act so quickly [1, 56]. Antimicrobial peptides are already present in the airway surface liquid but we estimate that the OSCN−-generating mechanism has a larger virucidal capacity and is more manipulative (it can be turned on or off fast) [57, 58]. These features make the Duox/H2O2/LPO/SCN− antiviral system ideal for pharmaceutical intervention. By enhancing its anti-influenza effect, we could eliminate extracellular virions still on the airway surface before establishing infections in TBECs and triggering inflammation. All the downstream effects of IAV infection could be theoretically prevented at the earliest intervention time point possible.
Acknowledgements.
This work was supported by the 1) startup fund of Dr. Rada provided by the Office of Vice President for Research and 2) the funds of Dr. Tripp provided by the Georgia Research Alliance. We thank to Dr. Thomas Leto (NIAID, NIH) for the critical reading of the manuscript.
Abbreviations
- ALI
air-liquid interface
- Duox
dual oxidase
- HA
hemagglutinin
- LPO
lactoperoxidase
- MDCK
Madin-Darby canine kidney cells
- MOI
multiplicity of infection
- NA
neuraminidase
- NHBE
normal human bronchial epithelium
- OSCN−
hypothiocyanite
- SCN−
thiocyanate
- TBEC
tracheobronchial epithelial cell
- TEER
transepithelial electrical resistance
References
- 1.Vareille M, et al. , The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev, 2011. 24(1): p. 210–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rada B and Leto TL, Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol, 2008. 15: p. 164–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose MC and Voynow JA, Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev, 2006. 86(1): p. 245–78. [DOI] [PubMed] [Google Scholar]
- 4.Voynow JA, Gendler SJ, and Rose MC, Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol, 2006. 34(6): p. 661–5. [DOI] [PubMed] [Google Scholar]
- 5.Geiszt M, et al. , Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J, 2003. 17(11): p. 1502–4. [DOI] [PubMed] [Google Scholar]
- 6.Conner GE, Salathe M, and Forteza R, Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med, 2002. 166(12 Pt 2): p. S57–61. [DOI] [PubMed] [Google Scholar]
- 7.Conner GE, et al. , The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett, 2007. 581(2): p. 271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebanoff SJ, Clem WH, and Luebke RG, The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta, 1966. 117(1): p. 63–72. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff SJ and Luebke RG, The Antilactobacillus System of Saliva. Role of Salivary Peroxidase. Proc Soc Exp Biol Med, 1965. 118: p. 483–6. [DOI] [PubMed] [Google Scholar]
- 10.Zeldow BJ, Studies on the antibacterial action of human saliva. III. Cofactor requirements of Lactobacillus bactericidin. J Immunol, 1963. 90: p. 12–6. [PubMed] [Google Scholar]
- 11.Bjorck L, et al. , Antibacterial activity of the lactoperoxidase system in milk against pseudomonads and other gram-negative bacteria. Appl Microbiol, 1975. 30(2): p. 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Deken X, et al. , Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem, 2000. 275(30): p. 23227–33. [DOI] [PubMed] [Google Scholar]
- 13.Dupuy C, et al. , Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem, 1999. 274(52): p. 37265–9. [DOI] [PubMed] [Google Scholar]
- 14.Rada B, et al. , Histamine stimulates hydrogen peroxide production by bronchial epithelial cells via histamine H1 receptor and dual oxidase. Am J Respir Cell Mol Biol, 2014. 50(1): p. 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rada B, et al. , The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol, 2008. 181(7): p. 4883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morand S, et al. , Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J, 2009. 23(4): p. 1205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikola H, Waris M, and Tenovuo J, Inhibition of herpes simplex virus type 1, respiratory syncytial virus and echovirus type 11 by peroxidase-generated hypothiocyanite. Antiviral Res, 1995. 26(2): p. 161–71. [DOI] [PubMed] [Google Scholar]
- 18.Pourtois M, et al. , Inhibition of HIV infectivity by lactoperoxidase-produced hypothiocyanite. J Biol Buccale, 1990. 18(4): p. 251–3. [PubMed] [Google Scholar]
- 19.Poland GA, Jacobson RM, and Targonski PV, Avian and pandemic influenza: an overview. Vaccine, 2007. 25(16): p. 3057–61. [DOI] [PubMed] [Google Scholar]
- 20.Thompson WW, et al. , Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA, 2003. 289(2): p. 179–86. [DOI] [PubMed] [Google Scholar]
- 21.Steel J and Lowen AC, Influenza A virus reassortment. Curr Top Microbiol Immunol, 2014. 385: p. 377–401. [DOI] [PubMed] [Google Scholar]
- 22.Cegolon L, et al. , In vitro antiviral activity of hypothiocyanite against A/H1N1/2009 pandemic influenza virus. Int J Hyg Environ Health, 2014. 217(1): p. 17–22. [DOI] [PubMed] [Google Scholar]
- 23.Strengert M, et al. , Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection. Antioxid Redox Signal, 2014. 20(17): p. 2695–709. [DOI] [PubMed] [Google Scholar]
- 24.Dlugolenski D, et al. , Swine Influenza Virus PA and Neuraminidase Gene Reassortment into Human H1N1 Influenza Virus Is Associated with an Altered Pathogenic Phenotype Linked to Increased MIP-2 Expression. J Virol, 2015. 89(10): p. 5651–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karasin AI, Olsen CW, and Anderson GA, Genetic characterization of an H1N2 influenza virus isolated from a pig in Indiana. J Clin Microbiol, 2000. 38(6): p. 2453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karasin AI, et al. , Genetic characterization of H1N2 influenza A viruses isolated from pigs throughout the United States. J Clin Microbiol, 2002. 40(3): p. 1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rada B and Leto TL, Redox warfare between airway epithelial cells and Pseudomonas: dual oxidase versus pyocyanin. Immunol Res, 2009. 43(1–3): p. 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rada B and Leto TL, Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett, 2010. 584(5): p. 917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauser MJ, et al. , Antiviral responses by Swine primary bronchoepithelial cells are limited compared to human bronchoepithelial cells following influenza virus infection. PLoS One, 2013. 8(7): p. e70251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshansky CM, et al. , Respiratory syncytial virus F and G proteins induce interleukin 1alpha, CC, and CXC chemokine responses by normal human bronchoepithelial cells. J Infect Dis, 2010. 201(8): p. 1201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshansky CM, et al. , Avian influenza viruses infect primary human bronchial epithelial cells unconstrained by sialic acid alpha2,3 residues. PLoS One, 2011. 6(6): p. e21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskwa P, et al. , A novel host defense system of airways is defective in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine, 2007. 175(2): p. 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura S, et al. , The effect of sevoflurane on ciliary motility in rat cultured tracheal epithelial cells: a comparison with isoflurane and halothane. Anesth Analg, 2006. 102(6): p. 1703–8. [DOI] [PubMed] [Google Scholar]
- 34.El Hassani RA, et al. , Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol, 2005. 288(5): p. G933–42. [DOI] [PubMed] [Google Scholar]
- 35.Rada B, et al. , Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol, 2011. 4(2): p. 158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascual JM, et al. , Epoxygenase metabolites of arachidonic acid affect electrophysiologic properties of rat tracheal epithelial cells1. J Pharmacol Exp Ther, 1998. 286(2): p. 772–9. [PubMed] [Google Scholar]
- 37.Forteza R, et al. , Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol, 2005. 32(5): p. 462–9. [DOI] [PubMed] [Google Scholar]
- 38.Pachucki J, et al. , Structural and functional characterization of the two human ThOX/Duox genes and their 5’-flanking regions. Mol Cell Endocrinol, 2004. 214(1–2): p. 53–62. [DOI] [PubMed] [Google Scholar]
- 39.Boots AW, et al. , ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem, 2009. 284(26): p. 17858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ameziane-El-Hassani R, et al. , Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem, 2005. 280(34): p. 30046–54. [DOI] [PubMed] [Google Scholar]
- 41.Dlugolenski D, et al. , Bat cells from Pteropus alecto are susceptible to influenza A virus infection and reassortment. Influenza Other Respir Viruses, 2013. 7(6): p. 900–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholls JM, The battle between influenza and the innate immune response in the human respiratory tract. Infect Chemother, 2013. 45(1): p. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wijkstrom-Frei C, et al. , Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol, 2003. 29(2): p. 206–12. [DOI] [PubMed] [Google Scholar]
- 44.Gerson C, et al. , The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol, 2000. 22(6): p. 665–71. [DOI] [PubMed] [Google Scholar]
- 45.Salathe M, et al. , Isolation and characterization of a peroxidase from the airway. Am J Respir Cell Mol Biol, 1997. 17(1): p. 97–105. [DOI] [PubMed] [Google Scholar]
- 46.Fragoso MA, et al. , Transcellular thiocyanate transport by human airway epithelia. J Physiol, 2004. 561(Pt 1): p. 183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedemonte N, et al. , Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol, 2007. 178(8): p. 5144–53. [DOI] [PubMed] [Google Scholar]
- 48.Lorentzen D, et al. , Concentration of the antibacterial precursor thiocyanate in cystic fibrosis airway secretions. Free Radic Biol Med, 2011. 50(9): p. 1144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang M, et al. , Recombinant rat CC16 protein inhibits LPS-induced MMP-9 expression via NF-kappaB pathway in rat tracheal epithelial cells. Exp Biol Med (Maywood), 2015. [DOI] [PMC free article] [PubMed]
- 50.Poussin C, et al. , The species translation challenge-a systems biology perspective on human and rat bronchial epithelial cells. Sci Data, 2014. 1: p. 140009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.St-Laurent J, Boulet LP, and Bissonnette E, Cigarette smoke differently alters normal and ovalbumin-sensitized bronchial epithelial cells from rat. J Asthma, 2009. 46(6): p. 577–81. [DOI] [PubMed] [Google Scholar]
- 52.Harper RW, et al. , Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett, 2005. 579(21): p. 4911–7. [DOI] [PubMed] [Google Scholar]
- 53.Gattas MV, et al. , Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic Biol Med, 2009. 47(10): p. 1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno JC, et al. , Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med, 2002. 347(2): p. 95–102. [DOI] [PubMed] [Google Scholar]
- 55.Grasberger H, Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol, 2010. 322(1–2): p. 99–106. [DOI] [PubMed] [Google Scholar]
- 56.Tamura S and Kurata T, Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis, 2004. 57(6): p. 236–47. [PubMed] [Google Scholar]
- 57.Ganz T, Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest, 2002. 109(6): p. 693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hertz CJ, et al. , Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol, 2003. 171(12): p. 6820–6. [DOI] [PubMed] [Google Scholar]