Abstract
Background
Use of illegal stimulants is associated with an increased risk of psychotic disorder. However, the impact of stimulant use on odds of first-episode psychosis (FEP) remains unclear. Here, we aimed to describe the patterns of stimulant use and examine their impact on odds of FEP.
Methods
We included patients with FEP aged 18–64 years who attended psychiatric services at 17 sites across 5 European countries and Brazil, and recruited controls representative of each local population (FEP = 1130; controls = 1497). Patterns of stimulant use were described. We computed fully adjusted logistic regression models (controlling for age, sex, ethnicity, cannabis use, and education level) to estimate their association with odds of FEP. Assuming causality, we calculated the population-attributable fractions for stimulant use associated with the odds for FEP.
Findings
Prevalence of lifetime and recent stimulant use in the FEP sample were 14.50% and 7.88% and in controls 10.80% and 3.8%, respectively. Recent and lifetime stimulant use was associated with increased odds of FEP compared with abstainers [fully adjusted odds ratio 1.74,95% confidence interval (CI) 1.20–2.54, P = .004 and 1.62, 95% CI 1.25–2.09, P < .001, respectively]. According to PAFs, a substantial number of FEP cases (3.35% [95% CI 1.31–4.78] for recent use and 7.61% [95% CI 3.68–10.54] for lifetime use) could have been prevented if stimulants were no longer available and the odds of FEP and PAFs for lifetime and recent stimulant use varied across countries.
Interpretation
Illegal stimulant use has a significant and clinically relevant influence on FEP incidence, with varying impacts across countries.
Keywords: first episode psychosis, stimulant use, amphetamines, methamphetamine, population attributable fractions
Introduction
Illegal amphetamine-type stimulants (hereafter illicit stimulants) are the second most widely used illegal drugs in the world after cannabis.1,2 According to the last European Drug Report 2021 from the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), the prevalence of last year stimulant consumption in adults (15–64 years) is around 1.7 million (0.5% of the total population) with 4.5% of European adults have used stimulants in their lifetime.3 Patients with first-episode psychosis (FEP) have higher rates of cooccurring substance use disorders compared to the general population,4 with alcohol, tobacco, cannabis, and stimulants the most frequently used substances.5,6 Substance use disorders in patients are associated with male sex and with an earlier age at onset of psychosis.4–6 The prevalence of stimulant use has been reported to be 8.9% in psychosis7 and 6.9% in FEP.8 The relationship between stimulant use and both psychotic symptoms and the presence of a diagnosis of psychotic disorder has been previously studied.9 Stimulants enhance dopamine neurotransmission in the brain10 and the use of illicit stimulants is associated with higher odds of developing psychotic symptoms in recreational drug users,11,12 and people with psychotic disorders.13 A prospective longitudinal study of chronic amphetamine users, found a 5-fold dose-dependent increase in the likelihood of developing psychotic symptoms.14 In addition, illicit stimulant use has been associated with roughly 2 to 3-fold increases in the odds of schizophrenia and other psychotic disorders.9,15 However, it is still uncertain whether the prevalence of illicit stimulant consumption varies across geographical regions and to what extent the incidence of psychosis can be attributed to illicit stimulant use, a concept known as population-attributable fraction (PAF).
Using data from the European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI) study16 we sought to: (1) describe patterns of illicit stimulant use in a large and representative sample of FEP and controls, (2) examine the impact of illicit stimulant use on the odds of FEP in the whole sample and across countries, (3) compute the PAF for illicit stimulant use in the whole sample and across countries, (4) investigate the relationship between stimulant prevalence use and psychosis incidence rates across countries, and (5) investigate these issues in a subsample of patients with a less heterogeneous psychotic condition, such as first-episode schizophrenia (FES).
Methods
EU-GEI Study Design and Participants
The EU-GEI project set out to estimate the incidence of psychosis and investigate risk factors for psychotic disorders by recruiting a sample of FEP cases and controls.16 The study was conducted between May 1, 2010 and April 1, 2015 across 17 catchment areas in 6 countries including urban and non-urban populations: United Kingdom, The Netherlands, Italy, France, Spain, and Brazil.
Patients were eligible if they met the following criteria during the recruitment period: ages between 18 and 64 years; first presentation with a diagnosis of psychosis per the International Statistical Classification of Diseases, Tenth Revision (codes F20–33); and residing within the catchment area. Trained researchers identified cases and clinical teams invited patients to participate. Using the Operational Criteria Checklist algorithm, all patients interviewed received a research-based diagnosis.17 Exclusion criteria included: Prior contact with psychiatric services for psychosis; any evidence of an organic cause of psychotic symptoms; transient psychological symptoms resulting from acute intoxication (International Statistical Classification of Diseases, Tenth Revision: F1x.0); and, for the case-control study only, insufficient fluency in the primary language. The incidence study included 2774 individuals by identifying all individuals with FEP seen in mental health services in each catchment area.18 Of those, 1519 were approached when considered appropriate by clinical staff, and 1130 (41% of the total incidence sample) consented to be assessed. The main reasons for non-inclusion in the case-control study were refusal to participate, language barriers, or not meeting the age inclusion criterion. A sample of 1497 controls was also recruited. The groups of patients included and not included did not differ with respect to the proportion of minority ethnic groups, but the proportion of men and younger individuals were greater among patients included vs not included. Further details can be found elsewhere.16 All sites contributed to the recruitment of control populations except for Maison Blanche. The recruitment of controls followed a mixture of random and quota sampling strategies. Local demographic data were used to set quotas for controls to ensure the best possible representativeness in age, sex, ethnicity, and catchment areas. The identification of controls was based on locally available sampling frames, including lists of postal addresses, and general practice lists from randomly selected surgeries. Additionally, we used internet and newspaper advertisements, leaflets at local stations, and job centers. All participants who agreed to participate provided written informed consent. Local research ethics committees gave ethical approval to each site. More details are provided in previous studies.17,19
Measures
The network obtained sociodemographic data using a modified version of the Sociodemographic Program of the Medical Research Council.20 Ethnicity was self-reported and classified by researchers as: Asian, Black, Mixed, North African, White, and Other. Data related to recreational use of stimulants, cannabis, and cocaine use were obtained through the updated version of the modified cannabis use experience questionnaire for EU-GEI.19 This questionnaire includes the registration of a detailed history of use of several substances in both FEP patients and controls. Researchers asked participants whether they had used illicit stimulants in their lifetime and in the year preceding the study (hereafter, recent use) and participants spontaneously provided information about the type. We defined illicit stimulant use as the use of amphetamine-type stimulants or amphetamines not prescribed by a health professional. In EU-GEI study participants reported the use of khat or amphetamines, including methamphetamine, and MDMA (Ecstasy/Molly). In affirmative cases, participants were requested to provide additional details on their pattern of use including the age at first use and the age at stop for lifetime use and the number of weeks and frequency of use (daily, weekly, or occasional use [less than once a week]) for participants with recent stimulant use. Cannabis use was recorded as (1) current and lifetime history of cannabis, (2) frequency of cannabis use, ie, the frequency that characterized the participant’s most consistent pattern of use, and (3) type of cannabis used, ie, the type preferentially used by the participant. The frequency variable was grouped as “low frequency” when subjects did not use cannabis, or used on weekends or less frequently, and “high frequency” if participants used cannabis every day. Type of cannabis was classified as “low potency” if participants used “hash-type” and “high potency” when they reported the use of skunk type.19 All the researchers undertook training on the assessment instruments before and throughout the study. To ensure the comparability of procedures and methods across sites, inter-rater reliability was assessed annually, with acceptable scores for all the scales (ie, κ > 0.7 in OPCRIT).
Statistical Analysis
All analyses were performed using R version 4.0.3.
We used adjusted logistic regression models to estimate the effect of each of the 9 measures of stimulant use on the odds of a diagnosis of FEP for the whole sample. We computed clustered standard errors to account for the fact that cases and controls are nested within sites using the command “summ” from the R package “jtools”. In addition, we computed adjusted logistic regression models of recent or lifetime use for each country and site separately using never used or not used and either lifetime or recent use as the reference group in each statistical model.
A previous study found age, sex, ethnicity, cannabis use, and education level to be significant contributors to psychosis incidence in this sample,19 and cannabis use often precedes or accompanies stimulant use.21 Therefore, we adjusted raw models for age, sex, and ethnicity, and fully adjusted models additionally controlled for current use or lifetime use of cannabis and education level. Three sets of sensitivity analyses were run: Controlling for (1) potency (high vs low) of cannabis, (2) frequency (daily vs other) of cannabis use, instead of current use or lifetime use of cannabis, and (3) adding first-degree family history of mental illness covariate to the fully adjusted models. Propensity score matching to estimate the effect of stimulant use on FEP. First, we attempted 1:1 nearest neighbor propensity score matching with a propensity score estimated using logistic regression of the diagnosis on age and sex. This matching specification yielded poor balance, so coarsened exact matching on the propensity score was used. After matching, all standardized mean differences for the covariates were below 0.001, indicating good balance. All missing values were removed for these analyses. To estimate the effect of stimulant use and its confidence intervals (CI), we fit a generalized linear regression model with diagnosis as the outcome and stimulant use and covariates, and included the coarsened exact matching, matching weights in the estimation using the MatchIt package.22
Based on the prevalence of recent and lifetime stimulant use in FEP and controls and the corresponding fully adjusted odds ratio (OR)s’ upper and lower CIs, we estimated the PAFs with 95% (CI) for recent and lifetime stimulant use, for the whole sample and for each country. PAF measures were calculated using Miettinen’s equation23 with fully adjusted OR: PAF = prevalence of exposure in cases *[(fully adjusted OR – 1)/fully adjusted OR]. The PAF measures the population effect of exposure by providing an estimate of the proportion of new FEP cases that would be prevented if the exposure were removed, assuming causality.
Cocaine shares some mechanisms of action with illicit stimulants at both behavioral and cellular levels,24 so we investigated its prevalence by calculating ORs and PAF to estimate the effect of the use of cocaine and/or stimulants on FEP (considering stimulants users if the subjects used cocaine, stimulants, or both).
We used Pearson’s correlation to test for an association between the incidence rates for psychotic disorder in each country and recent and lifetime use in controls (as representing the general population for each country).
Finally, as FEP is a heterogeneous group, including different syndromes and disorders with indeterminate neurobiological mechanisms,25 we repeated all analyses in the subsample of participants with FES, which represents a less heterogeneous group.
Results
Sample Description
This analysis included 1130 people with FEP (mean age = 31.25 years old, standard deviation[SD] = 10.61; 38.3% female, 61.7% male) and 1497 controls (mean age = 36.06 years old, SD = 12.90; 52.8% female, 47.2% male). Compared with controls, the FEP group was younger and included a higher percentage of males and ethnic minorities. Controls were likelier to have pursued higher education. Controls reported lower rates of recent and lifetime cannabis use. Demographic data are shown in table 1.
Table 1.
Demographic Variables and Cannabis Use Across All Included FEP Cases and Controls
| Variables | Controls (n = 1497) | FEP cases (n = 1130) | χ² P value |
|---|---|---|---|
| Age, years (mean, SD) | 36.06 (12.90) | 31.25 (10.61) | <.001 |
| Sex, n (%) | <.001 | ||
| Female | 791 (52.8%) | 433 (38.3%) | |
| Male | 706 (47.2%) | 697 (61.7%) | |
| Self-reported ethnicity, n (%) | <.001 | ||
| Asian | 33 (2.2%) | 35 (3.1%) | |
| Black | 121 (8.1%) | 183 (16.2%) | |
| Mixed | 116 (7.7%) | 110 (9.7%) | |
| North African | 24 (1.6%) | 52 (4.6%) | |
| White | 1178 (78.7%) | 715 (63.3%) | |
| Other | 24 (1.6%) | 35 (3.1%) | |
| Missing data | 1 (0.1%) | 0 (0%) | |
| Education, n (%) | <.001 | ||
| School, no qualifications | 72 (4.8%) | 185 (16.4%) | |
| School, with qualifications | 197 (13.2%) | 291 (25.7%) | |
| Tertiary | 431 (28.8%) | 260 (23%) | |
| Vocational | 238 (15.9%) | 198 (17.5%) | |
| Higher (undergraduate) | 343 (22.9%) | 128 (11.3%) | |
| Higher (postgraduate) | 209 (13.7%) | 56 (5%) | |
| Missing data | 7 (0.5%) | 12 (1.1%) | |
| Cannabis use, n (%) | |||
| Current | 160 (10.7%) | 242 (21.4%) | <.001 |
| Lifetime | 703 (46.9%) | 702 (62.1%) | <.001 |
| Country, n (%) | |||
| United Kingdom | 336 (22.4) | 246 (21.8) | .015 |
| The Netherlands | 210 (14.0) | 196 (17.3) | |
| Italy | 280 (18.7) | 187 (16.5) | |
| France | 147 (9.8) | 105 (9.3) | |
| Spain | 222 (14.8) | 204 (18.1) | |
| Brazil | 302 (20.2) | 192 (17.0) | |
Note: FEP, first-episode psychosis; SD, standard deviation.
Patterns of Illicit Stimulant Use and the Impact of Stimulant Use on Odds of FEP
More FEP subjects than controls reported having ever used illicit stimulants both recently (7.9% (n = 89) in FEP and 3.8% (n = 57) in controls, P < .001) and lifetime (14.5% (n = 217) in FEP and 10.8% (n = 162) in controls, P < .001). We found no significant differences between FEP and controls at the age of first illicit stimulant use and age at stopping. Among FEP and controls, the most common pattern of illicit stimulant use was less than weekly. There were no significant differences in the odds of FEP based on the frequency of illicit stimulant use (see table 2).
Table 2.
Measures of Stimulant Use and ORs for FEP
| Variables | Controls (n = 1497) | FEP cases (n = 1130) | χ² P value* | Raw OR (95% CI)† | Raw OR P value | Fully adjusted OR (95% CI)† | Fully adjusted OR P value |
|---|---|---|---|---|---|---|---|
| Recent use (last year) | |||||||
| Ever used, yes n (%) | 57 (3.81) | 89 (7.88) | <.001 | 1.84 (1.29–2.62) | <.001 | 1.74 (1.20–2.54) | .004 |
| Number of weeks, mean (SD) | 4.33 (8.56) | 7.81 (12.83) | .056 | 1.05 (1.01–1.09) | .010 | 1.04 (1.00–1.08) | .034 |
| Frequency, daily/weekly/less, n | 1/11/44 | 5/20/60 | <.001 | 7.68 (0.87–67.59) 1.95 (0.92–4.16) 1.63 (1.08–2.46) |
.066 .082 .019 |
3.44 (0.39–30.60) 1.92 (0.83–4.22) 1.58 (1.02–2.45) |
.269 .105 .040 |
| Cocaine and/or stimulant use, n (%)* | 101 (6.74) | 187 (16.55) | <.001 | 2.30 (1.76–3.00) | <.001 | 2.04 (1.53–2.72) | <.001 |
| Lifetime use** | |||||||
| Ever used, yes n (%) | 162 (10.82) | 217 (14.50) | <.001 | 1.87 (1.49–2.36) | <.001 | 1.62 (1.25–2.09) | <.001 |
| Age at first use, mean (SD) | 20.77 (5.25) | 19.83 (4.58) | .825 | 1.00 (0.95–1.05) | .964 | 1.02 (0.97–1.08) | .414 |
| Age at stop, mean (SD) | 25.71 (8.5) | 24.12 (7.8) | .931 | 1.02 (0.98–1.06) | .490 | 1.04 (0.99–1.08) | .086 |
| Frequency, daily/weekly/less, n | 4/25/91 | 16/49/112 | <.001 | 6.46 (2.12–19.74) 2.73 (1.65–4.50) 1.74 (1.29–2.35) |
.001 <.001 <.001 |
3.19 (0.99–10.29) 2.29 (1.34–3.89) 1.62 (1.17–2.24) |
.052 .002 .004 |
| Cocaine and/or stimulant use, n (%) | 244 (16.30) | 358 (31.68) | <.001 | 2.18 (1.79–2.66) | <.001 | 1.82 (1.45–2.29) | <.001 |
Note: Raw ORs are adjusted only for age, sex, and ethnicity, whereas fully adjusted ORs are additionally adjusted for current cannabis use in recent stimulant use models or lifetime cannabis use in lifetime use models and education level.
FEP, first-episode psychosis; OR, odds ratio; SD, standard deviation; CI, confidence intervals.
†Reference group for both raw and fully adjusted ORs is abstainers (recent or lifetime) unless otherwise specified.
*Out of the 89 FEP patients who used stimulants last year, 62.9% also used cocaine in the past year. Out of the 217 FEP patients who have used stimulants during their lifetime, 72.8% also used cocaine during their lifetime.
**No missing data for Recent use. 26 missing data for lifetime use (13 controls, 13 FEP). None of them had used stimulants in the last year. These participants were not excluded from the analyses, and we did not observe any difference in age or sex between these 26 participants and the rest of the population (χ2P values > .05).
Fully adjusted logistic regression models showed that those with recent (ORfully adjusted = 1.74, 95% CI 1.20–2.54, P = .004) or lifetime illicit stimulant use (ORfully adjusted = 1.62, 95% CI 1.25–2.09, P < .001) had greater odds of FEP than abstainers. These findings were consistent in both raw and fully adjusted models (table 2). Figure 1 shows raw and fully adjusted ORs values according to lifetime patterns of illicit stimulant use. Sensitivity analyses showed comparable results overall in terms of the direction and magnitude of the effects for lifetime and recent stimulant use after adjusting for type (high vs low potency) and frequency (daily vs other) of cannabis use and after adding first-degree family history mental illness covariate to the fully adjusted model, although recent stimulant use no longer reached statistical significance after controlling for frequency of cannabis use (P = .086) (Supplementary table 1). Also, comparable results were obtained after running analyses using an age-and sex-matched control sample (Supplementary table 2).
Fig. 1.
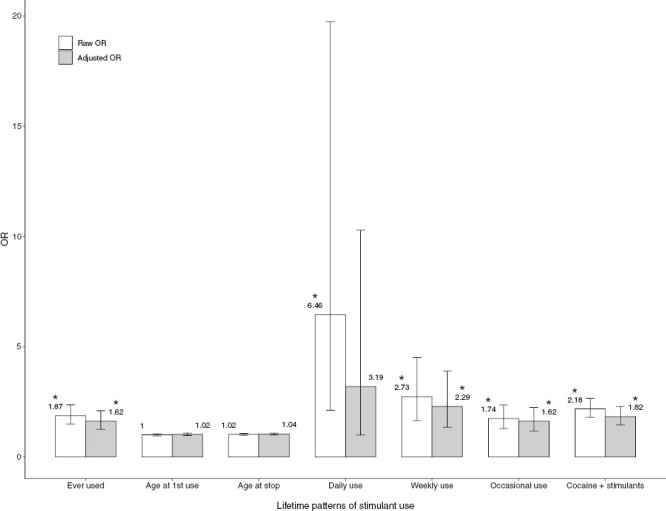
Raw and fully adjusted ORs of first-episode psychosis for lifetime patterns of stimulant use and the combined measure of cocaine and/or stimulant use for the whole sample. Raw ORs are adjusted for age, sex, and ethnicity, whereas fully adjusted ORs were additionally adjusted for recent and lifetime cannabis use and education level. Error bars represent 95% of CIs. The reference group for both raw and fully adjusted ORs is abstainers (recent or lifetime, accordingly). Asterisks represent significant ORs (P < .05). CI, confidence interval; OR, odds ratio.
We also estimated the ORs for recent and lifetime cocaine and/or stimulant use for the whole sample, by country and by the site (results can be found in table 2 and Supplementary results1).
When considering the prevalence of exposure to stimulants by country, both prevalence and ORs of FEP for recent or lifetime illicit stimulant use varied across countries. Fully adjusted ORs of FEP for last year's use ranged from 1.04 (95% CI 0.28–3.84) in Spain to 8.89 (95% CI 0.95–83.47) in Brazil. Fully adjusted ORs of FEP for lifetime illicit stimulant use ranged from 1.01 (95% CI 0.60–1.68) in the United Kingdom to 6.07 (95% CI 2.16–17.07) in Italy. All results are presented in Supplementary table 3.
Analyses for recent and lifetime illicit stimulant use by site can be found in Supplementary results 2 and table 4. Results for cocaine and/or stimulant use for the whole sample, by country and site, identified differences in the ORs and can be found in Supplementary tables 5 and 6 and results 3.
PAFs of FEP Associated With Stimulant Use
Assuming causality, for fully adjusted models, the proportion of new cases of psychotic disorder in the whole sample attributable to recent use was 3.35 % (95% CI 1.31–4.78) and 7.61% (95% CI 3.68–10.54) for lifetime use. Supplementary table 3 shows raw and fully adjusted models of PAF.
In addition, the PAF analysis revealed variations by country (figure 2 and Supplementary table 3). PAFs in fully adjusted models ranged from 4.95% (95% CI −8.96 to 12.37) of new cases of FEP in The Netherlands being attributable to recent use to just 0.13% (95% CI −1.91 to 2.43) of cases in Spain. Furthermore, the PAF for lifetime stimulant use ranged from 19.61% (95% CI 3.70–28.59) of cases in The Netherlands to 0.25% (95% CI −16.67–10.12) estimated in the United Kingdom (see Supplementary table 3).
Fig. 2.
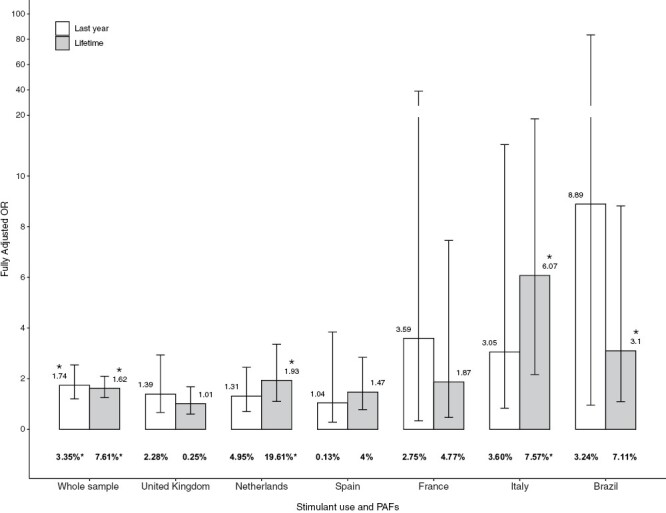
Fully adjusted ORs and PAFs of psychotic disorder for recent or lifetime use for the whole sample and across countries. In bold, PAFs are shown in percentages. Error bars represent 95% of CIs. CI, confidence interval; OR, odds ratio; PAF, population attributable fraction. *P < .05.
In addition, PAFs were also estimated for each of the 17 sites for recent and lifetime illicit stimulant use. Also, PAFs were calculated for the whole sample, for each country, and for each site for recent and lifetime cocaine and/or stimulant use (results can be found in Supplementary results 1, 2, and 3, and tables 4, 5, and 6).
We did not find any significant association between raw incidence rates of FEP and the prevalence of illicit stimulant use in controls across countries (rrecent = 0.399, P = .433; rlifetime = .303, P = .559, Supplementary figure).
Analyses in the Subsample of Participants With FES
Analyses of the subsample of participants with FES (NFES = 573, Supplementary table 7) showed that those who had used illicit stimulants at least once in their lifetimes (ORfully adjusted = 1.70, 95% CI 1.24–2.32, P < .001) had greater odds of FES than abstainers. Recent use was not statistically significantly related to the odds of FES (ORfully adjusted = 1.57, 95% CI 0.99–2.50, P = .054). These findings were consistent using both raw and fully adjusted models (see Supplementary table 8). PAFs in fully adjusted models were 2.85% (95%CI −0.08– 4.71) for recent use and 8.56 % (95% CI 4.02–11.83) for lifetime use for the whole sample (Supplementary table 9 for all details).
Discussion
This study suggests that less illicit stimulant use could reduce psychosis incidence. Assuming causality, stopping illicit stimulant use could prevent 3.4% of new cases of psychosis. An additional finding is that rates and patterns of stimulant use, the strength of their association with odds of FEP, and the PAF varied notably across geographical areas.
Prevalence of recent illicit stimulant use in people with FEP was about 3% while the prevalence of lifetime illicit stimulant use was about 20%. These figures are greater than those previously reported. A meta-analysis of 64 studies of stimulant use in more than 22 000 people with psychosis found a lifetime prevalence of stimulant use in people with psychosis of about 9%, with significant differences across regions.7 Our study also showed that the prevalence of recent and lifetime stimulant use in controls was around 4% and 10%, respectively. These figures are higher than those found in adults in the European Drug Report 2021 from the EMCDDA (around 0.5% for recent use and around 4.5% for lifetime use).3 In addition, our reported prevalence rates in the control group were also higher than those found by a study conducted during 2015–2016 in the adult (≥18 years) general population of the United States (which had a prevalence of 1.9% misused without use disorders, and 0.2% use disorders for recent use).26
The high prevalence rates found in our study in both FEP cases and controls could possibly imply that our data represents a more realistic approach to the measurement of illicit stimulant use or that we overestimated stimulant use by using self-reported questionnaires.
Our results also highlight substantial between study and between-country heterogeneity. This finding is supported by previous studies reporting significant between-country differences in illicit stimulant use in both the general population3 and in the population with psychosis, ranging in the latter from 0.5 to 37%.7 Furthermore, we found heterogeneity by site within each country (Supplementary table 4), so an important caveat to these national numbers is the degree to which they mask regional variability. Geographical heterogeneity may be related to stimulant availability in the illegal market or to legal issues such as different social policies or legal penalties by country.3,26
Importantly, our results showed that recent and lifetime use prevalence was greater in people with FEP than in controls. Both were associated with increased odds of FEP, with excess risk of 74% and 62% (OR 1.74 and 1.62), respectively, compared to abstainers. These results were similar even after controlling for cannabis use. Our results are in accordance with the European Drug Emergencies Network, which reported in 2016 (using data collected in 2013 and 2014) an OR of psychosis of 3.0 for lifetime illicit stimulant use (including both FEP and chronic psychosis).15 The results of the 95% CI, with overlap between groups, showed that there were no significant differences in the odds of FEP related to the frequency of illicit stimulant use. This may be due, at least in part, to the fact that the dominant frequency of illicit stimulant use was less than weekly. We found comparable results in a less heterogeneous subsample of participants with FES, suggesting that the impact of illicit stimulant use on the risk of psychosis is similar across the psychosis spectrum.
In this study, we report for the first time the extent to which a lack of illicit stimulants may affect the incidence of psychosis. We found that such a lack could prevent a substantial number of FEP cases (3.35% recent and 7.61% lifetime use) (table 2). As mentioned before, these numbers are higher than those previously reported in the EMCDDA.3 This could be due to reasons associated with differences in methodology and sampling procedures. In comparison with the EU-GEI interview and questionnaire, EMCDDA conducted general population surveys at a national level. Surveys could present limitations in estimating the prevalence of intensive forms of drugs due to low prevalence figures and non-probabilistic errors (exclusion from the sampling frame, non-response). Moreover, inEU-GEI project, although recruitment followed a mixture of random and quota sampling strategies, the participating sample could have traits different from the general population, which could mask or strengthen effects. Finally, in EMCDDA, most countries included 15–64 age range, but occasionally countries used wider age ranges. As the EU-GEI project included subjects 18–64 years old, and substance use is less common at very young and very old ages, this could also be influencing results.
Although there is evidence supporting the relationship between illicit stimulant use and psychotic symptoms, we cannot confirm causality. Therefore, PAF indexes derived from this study should be interpreted with caution. The high PAF in The Netherlands (4.95% for recent and 19.61% for lifetime use) are a consequence of the high prevalence of exposure to stimulants in FEP (20.9% for last year's use and 40.7% for lifetime use). The 2015 Dutch National Drug Report also reported a greater prevalence of ecstasy and amphetamine use in The Netherlands as compared with the general population in the European Union member states and Norway.27
Contrary to the correlation found between incidence rates for psychotic disorder and prevalence of cannabis use in controls across sites,19 we did not find a significant correlation between recent and lifetime stimulant use in controls and variation in the raw incidence rates for FEP across countries. However, this could be influenced by the low number of countries (6 countries were included in this study) and the large heterogeneity among rates by country (Supplementary figure).
Our findings need to be appraised in the context of some limitations. First, data on illicit stimulant use are self-reported and not validated by biological measures, such as urine, blood, or hair samples. However, other studies with laboratory and self-reported information have shown that substance users report the frequency and type of substance used with enough accuracy to be useful.28,29 Second, we were not able to report stimulant doses. Moreover, the study included subjects with substance-induced psychosis and we did not differentiate them from stimulant users with FEP. These 2 groups may have different biological and clinical characteristics.30 Stimulant-induced psychosis develops more frequently in stimulant users with higher doses and a family history of psychosis.31 Nevertheless, we found comparable effect sizes in the subsample with FES to those found in the FEP sample, suggesting that the association may not be attributable only to the presence of substance-induced psychosis in the group of FEP. Also, although the present study analyzed a large sample of 1130 FEP patients, it cannot be guaranteed that the results are representative of the whole FEP population. Additionally, in the case-control comparison, we should consider possible sources of unmeasured or residual confounding. Substantial evidence supports an association between stimulant use and prior risk factors including use of alcohol, cannabis, or other drugs, family history of mental illness and comorbidity, or developmental issues, as reflected in higher rates of learning disorders.32 A recent systematic review reported cannabis use and a family history of mental illness but not sociodemographic variables as risk factors for methamphetamine-related psychosis.33 In the present study, we tried to account for some of these confounding variables by adjusting for age, sex, ethnicity, education level, use of cannabis (recent use, lifetime use, use of high potency, and daily use), and first-degree family history of mental illness. We also conducted supplementary analyses using an age- and sex-matched control sample. The greater odds of FEP found in stimulant users vs abstainers remained significant after controlling for the additional covariates (Supplementary table 1) and after repeating the analysis in an age- and sex-matched sample of patients and controls (Supplementary table 2). However, the effects of recent stimulant use no longer reached statistical significance after controlling for the frequency of cannabis use. Furthermore, 192 cases (17%) reported duration of psychosis longer than 52 weeks at the date of first contact with a healthcare professional. Although we found comparable effects for recent stimulant use after excluding these participants (Supplementary table 1), long duration of psychosis in some of the participants could have affected our results. Finally, we do not have information on the use of stimulants with a medical prescription, which may be a confounding variable. Methylphenidate, the most common medication for children with attention-deficit/hyperactivity disorder in many countries, is often prescribed for long periods of time. An estimated 16 million (6.6%) US adults26 and 2.8 million (3.5 %) children34 use stimulants annually. Any long-term psychotropic treatment in childhood raises concerns about possible adverse neurological and psychiatric outcomes. An association between a history of childhood attention-deficit/hyperactivity disorder and schizophrenia-spectrum disorders later in life has been described,35 and therefore FEP group is more likely to have received stimulants for this condition. Although a recent systematic review of observational studies of prescribed stimulants and psychosis risk concluded that observational studies do not support a clear-cut effect of prescribed methylphenidate on psychosis risk,36 we cannot rule out a potential effect of prescribed stimulants on our results.
In conclusion, our findings confirm previous evidence of the harmful effect of illicit stimulant use on mental health, increasing the odds of a first episode of psychosis by 74% (for recent use) and 62% (for lifetime use). For the first time, this study shows that if illegal stimulants were no longer available, the number of new cases of psychosis could be reduced. It is important for public health to acknowledge the adverse effects associated with stimulant use and promote early intervention and prevention programs.
EU-GEI WP2 Group Author
Silvia Amoretti1, Alvaro Andreu-Bernabeu2, Grégoire Baudin3,4, Stephanie Beards5, Elena Bonora, Chiara Bonetto6, Bibiana Cabrera1, Angel Carracedo7, Thomas Charpeaud8,9,10, Javier Costas7, Doriana Cristofalo6, Pedro Cuadrado11, Giuseppe D’Andrea, Aziz Ferchiou3,4, Nathalie Franke12, Flora Frijda13, Paz Garcia-Portilla14, Emiliano González2, Kathryn Hubbard5, Stéphane Jamain3,8,15, Estela Jiménez-López16, Marion Leboyer3,4,8,15, Esther Lorente-Rovira17, Camila Marcelino Loureiro18,19, Giovanna Marrazzo20, Mario Matteis2, Elles Messchaart21, Gisela Mezquida22, Baptiste Pignon3,4,8, Marta Rapado2, Jean-Romain Richard3,8, José Juan Rodríguez Solano23, Mirella Ruggeri6, Emilio Sánchez24, Crocettarachele Sartorio20,25, Franck Schürhoff3,4,8,15, Fabio Seminerio25, Marco Seri, Rosana Shuhama18,19, Lucia Sideli25, Simona A. Stilo26, Fabian Termorshuizen21,27, Giada Tripoli26, Anne-Marie Tronche8,9,10, Daniella van Dam12, Elsje van der Ven21,27, Simona Stilo26.
1Barcelona Clínic Schizophrenia Unit, Neuroscience Institute, Hospital Clínic of Barcelona; Bipolar and Depressive Disorder Unit, Neuroscience Institute, Hospital Clínic de Barcelona; CIBERSAM, ISCIII, Barcelona, Spain; Group of Psychiatry, Mental Health and Addictions, Psychiatric Genetics Unit, Vall d’Hebron Research Institute (VHIR); University of Barcelona, Spain; 2Department of Child and Adolescent Psychiatry, Hospital General Universitario Gregorio Marañón, School of Medicine, Universidad Complutense, IiSGM (CIBERSAM), C/Doctor Esquerdo 46, 28007 Madrid, Spain; 3INSERM, U955, Equipe 15, 51 Avenue de Maréchal de Lattre de Tassigny, 94010 Créteil, France; 4AP-HP, Groupe Hospitalier “Mondor”, Pôle de Psychiatrie, 51 Avenue de Maréchal de Lattre de Tassigny, 94010 Créteil, France; 5Department of Health Service and Population Research, Institute of Psychiatry, King’s College London, De Crespigny Park, Denmark Hill, London SE5 8AF, UK; 6Section of Psychiatry, Department of Neuroscience, Biomedicine and Movement, University of Verona, Piazzale L.A. Scuro 10, 37134 Verona, Italy; 7Fundación Pública Galega de Medicina Xenómica, Hospital Clínico Universitario, Choupana s/n, 15782 Santiago de Compostela, Spain; 8Fondation Fondamental, 40 Rue de Mesly, 94000 Créteil, France; 9CMP B CHU, BP 69, 63003 Clermont Ferrand, Cedex 1, France; 10Université Clermont Auvergne, EA 7280, Clermont-Ferrand, 63000, France; 11Villa de Vallecas Mental Health Department, Villa de Vallecas Mental Health Centre, Hospital Universitario Infanta Leonor / Hospital Virgen de la Torre, C/San Claudio 154, 28038 Madrid, Spain; 12Department of Psychiatry, Early Psychosis Section, Academic Medical Centre, University of Amsterdam, Meibergdreef 5, 1105 AZ Amsterdam, The Netherlands; 13Etablissement Public de Santé Maison Blanche, Paris 75020, France; 14Department of Medicine, Psychiatry Area, School of Medicine, Universidad de Oviedo, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), C/Julián Clavería s/n, 33006 Oviedo, Spain; 15Faculté de Médecine, Université Paris-Est, 51 Avenue de Maréchal de Lattre de Tassigny, 94010 Créteil, France; 16Department of Psychiatry, Servicio de Psiquiatría Hospital “Virgen de la Luz”, C/Hermandad de Donantes de Sangre, 16002 Cuenca, Spain; 17Department of Psychiatry, School of Medicine, Universidad de Valencia, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), C/Avda. Blasco Ibáñez 15, 46010 Valencia, Spain; 18Division of Psychiatry, Department of Neuroscience and Behaviour, Ribeirão Preto Medical School, University of São Paulo, São Paulo, Brazil; 19Department of Preventative Medicine, Faculdade de Medicina FMUSP, University of São Paulo, São Paulo, Brazil; 20Unit of Psychiatry, “P. Giaccone” General Hospital, Via G. La Loggia n.1, 90129 Palermo, Italy; 21Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg Mental Health Research and Teaching Network, Maastricht University Medical Centre, P.O. Box 616, 6200 MD Maastricht, The Netherlands; 22Barcelona Clínic Schizophrenia Unit, Hospital Clínic of Barcelona; Departament de Fonaments Clínics, Institut de Neurociències (UBNeuro), Universitat de Barcelona (UB); Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS); CIBERSAM, ISCIII, Barcelona, Spain; 23Puente de Vallecas Mental Health Department, Hospital Universitario Infanta Leonor / Hospital Virgen de la Torre, Centro de Salud Mental Puente de Vallecas, C/Peña Gorbea 4, 28018 Madrid, Spain; 24Department of Psychiatry, Hospital General Universitario Gregorio Marañón, School of Medicine, Universidad Complutense, IiSGM (CIBERSAM), C/Doctor Esquerdo 46, 28007 Madrid, Spain; 25Department of Experimental Biomedicine and Clinical Neuroscience, University of Palermo, Via G. La Loggia 1, 90129 Palermo, Italy; 26Department of Psychosis Studies, Institute of Psychiatry, King’s College London, De Crespigny Park, Denmark Hill, London SE5 8AF, UK; 27Rivierduinen Institute for Mental Health Care, Sandifortdreef 19, 2333 ZZ Leiden, The Netherlands.
Supplementary Material
Contributor Information
Elisa Rodríguez-Toscano, Institute of Psychiatry and Mental Health, Hospital Clínico San Carlos, IdISSC, School of Medicine, Universidad Complutense, Madrid, Spain; Faculty of Psychology, Universidad Complutense, Madrid, Spain; Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain.
Clara Alloza, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain; Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid, Spain.
David Fraguas, Institute of Psychiatry and Mental Health, Hospital Clínico San Carlos, IdISSC, School of Medicine, Universidad Complutense, Madrid, Spain; Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain; Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid, Spain.
Manuel Durán-Cutilla, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain.
Laura Roldán, Faculty of Psychology, Universidad Complutense, Madrid, Spain.
Teresa Sánchez-Gutiérrez, Faculty of Health Science, Universidad Internacional de la Rioja (UNIR), Madrid, Spain.
Gonzalo López-Montoya, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain; Faculty of Health Science, Universidad Internacional de la Rioja (UNIR), Madrid, Spain.
Mara Parellada, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain; Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid, Spain.
Carmen Moreno, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain; Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid, Spain.
Charlotte Gayer-Anderson, Department of Health Services and Population Research, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Hannah E Jongsma, PsyLife Group, Division of Psychiatry, University College London, London, UK; Department of Psychiatry, University of Cambridge, Cambridge, UK.
Marta Di Forti, Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Diego Quattrone, Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Eva Velthorst, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, USA; Seaver Autism Center for Research and Treatment, Icahn School of Medicine at Mount Sinai, New York, USA; Early Psychosis Section, Department of Psychiatry, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Lieuwe de Haan, Early Psychosis Section, Department of Psychiatry, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Jean-Paul Selten, Institute for Mental Health, GGZ Rivierduinen, Leiden, The Netherlands; Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre, Maastricht, The Netherlands.
Andrei Szöke, Institut National de la Santé et de la Recherche Médicale, U955, Equipe 15 Neuro-Psychiatrie Translationnelle, Créteil, France; AP-HP, Pôle de Psychiatrie des Hôpitaux Universitaires Henri Mondor, Créteil, France; Fondation FondaMental, Créteil, France.
Pierre-Michel Llorca, EA 7280 Npsydo, Université Clermont Auvergne, Clermont-Ferrand, France.
Andrea Tortelli, Pôle Psychiatrie Précarité, Groupe Hospitalier Universitaire Paris Psychiatrie and Neurosciences, 75014 Paris, France.
Julio Bobes, Department of Medicine, Psychiatry Area, School of Medicine, Universidad de Oviedo, ISPA, INEUROPA, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Oviedo, Spain.
Miguel Bernardo, Barcelona Clinic Schizophrenia Unit, Hospital Clínic of Barcelona; Departament de Medicina, Institut de Neurociències (UBNeuro), Universitat de Barcelona (UB); Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS); CIBERSAM, ISCIII, Barcelona, Spain.
Julio Sanjuán, Department of Psychiatry, School of Medicine, Universidad de Valencia, Centro de Investigación Biomédica en Red de Salud Mental, Valencia, Spain.
José Luis Santos, Department of Psychiatry, Hospital “Virgen de la Luz”, Cuenca, Spain.
Manuel Arrojo, Department of Psychiatry, Psychiatric Genetic Group, Instituto de Investigación Sanitaria de Santiago de Compostela, Complejo Hospitalario Universitario de Santiago de Compostela, Santiago, Spain.
Ilaria Tarricone, Department of Biomedical and NeuroMotor Sciences, Psychiatry Unit, Alma Mater Studiorium Università di Bologna, Bologna, Italy.
Domenico Berardi, Alma Mater Studiorium Università di Bologna, Bologna, Italy.
Mirella Ruggeri, Section of Psychiatry, Department of Neuroscience, Biomedicine and Movement, University of Verona, Verona, Italy.
Antonio Lasalvia, Section of Psychiatry, Department of Neuroscience, Biomedicine and Movement, University of Verona, Verona, Italy; Section of Psychiatry, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
Laura Ferraro, Department of Biomedicine, Neuroscience and advanced Diagnostic (BiND), Psychiatry section, University of Palermo, Palermo, Italy.
Caterina La Cascia, Department of Biomedicine, Neuroscience and advanced Diagnostic (BiND), Psychiatry section, University of Palermo, Palermo, Italy.
Daniele La Barbera, Department of Biomedicine, Neuroscience and advanced Diagnostic (BiND), Psychiatry section, University of Palermo, Palermo, Italy.
Paulo Rossi Menezes, Department of Preventive Medicine, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil.
Cristina Marta Del-Ben, Division of Psychiatry, Department of Neuroscience and Behaviour, Ribeirão Preto Medical School, Universidade de São Paulo, São Paulo, Brazil.
Bart P Rutten, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre, Maastricht, The Netherlands.
Jim van Os, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre, Maastricht, The Netherlands; Department of Psychiatry, Brain Center Rudolf Magnus, Utrecht University Medical Centre, Utrecht, The Netherlands; Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Peter B Jones, Department of Psychiatry, University of Cambridge, Cambridge, UK; CAMEO Early Intervention Service, Cambridgeshire and Peterborough National Health Service Foundation Trust, Cambridge, UK.
Robin M Murray, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
James B Kirkbride, PsyLife Group, Division of Psychiatry, University College London, London, UK.
Craig Morgan, Department of Health Services and Population Research, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Covadonga M Díaz-Caneja, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain; Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid, Spain.
Celso Arango, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, School of Medicine, Universidad Complutense, Madrid, Spain; Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid, Spain.
EU-GEI WP2 Group:
Silvia Amoretti, Alvaro Andreu-Bernabeu, Grégoire Baudin, Stephanie Beards, Elena Bonora, Chiara Bonetto, Bibiana Cabrera, Angel Carracedo, Thomas Charpeaud, Javier Costas, Doriana Cristofalo, Pedro Cuadrado, Giuseppe D’Andrea, Aziz Ferchiou, Nathalie Franke, Flora Frijda, Paz Garcia-Portilla, Emiliano González, Kathryn Hubbard, Stéphane Jamain, Estela Jiménez-López, Marion Leboyer, Esther Lorente-Rovira, Camila Marcelino Loureiro, Giovanna Marrazzo, Mario Matteis, Elles Messchaart, Gisela Mezquida, Baptiste Pignon, Marta Rapado, Jean-Romain Richard, José Juan Rodríguez Solano, Mirella Ruggeri, Emilio Sánchez, Crocettarachele Sartorio, Franck Schürhoff, Fabio Seminerio, Marco Seri, Rosana Shuhama, Lucia Sideli, Simona A Stilo, Fabian Termorshuizen, Giada Tripoli, Anne-Marie Tronche, Daniella van Dam, Elsje van der Ven, and Simona Stilo
Funding
Supported by the Spanish Ministry of Science and Innovation. Instituto de Salud Carlos III (SAM16PE, PI14/00397, PI17/00481, PI20/00216, PI20/00721, JR19/00024), CIBER-Consorcio Centro de Investigación Biomédica en Red- (CB/07/09/0023); Madrid Regional Government (S2022/BMD-7216 AGES 3-CM), European Union Structural Funds, European Union Seventh Framework Programme, European Union H2020 Programme under the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement No.101034377 (Project PRISM-2), and grant agreement No 777394 (Project AIMS-2-TRIALS)), European Union Horizon Europe, the National Institute of Mental Health of the National Institutes of Health under Award Number 1U01MH124639-01 (Project ProNET) and Award Number 5P50MH115846-03 (project FEP-CAUSAL), Fundación Familia Alonso, and Fundación Alicia Koplowitz. The European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI) Project is funded by grant agreement FP7-HEALTH-2009-2.2.1-2-241909 (Project EU-GEI) from the European Community’s Seventh Framework Programme. The Brazilian study was funded by grant 2012/0417-0 from the São Paulo Research Foundation. The study sponsors did not play any role in the study design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interests
Dr. Fraguas has been a consultant and/or has received fees from Angelini, Casen-Recardati, Janssen, Lundbeck, and Otsuka. He has also received grant support from Instituto de Salud Carlos III (Spanish Ministry of Science and Innovation) and from Fundación Alicia Koplowitz. Dr. Díaz-Caneja has received honoraria from Exeltis and Angelini. Dr. Arango has been a consultant to or has received honoraria or grants from Acadia, Angelini, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda. Dr. Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Janssen-Cilag, Menarini, Rovi, and Takeda. Dr. Murray has received honoraria from Janssen, Sunovian, Otsuka, and Lundbeck. Dr. Moreno has received honoraria as a consultant and/or advisor and/or for lectures from Angelini, Esteve, Exeltis Janssen, Lundbeck, Neuraxpharm, Nuvelution, Otsuka, Pfizer, Servier, and Sunovion outside the submitted work. The other authors have no conflicts of interest to declare. Dr. Jones has received an honorarium from MSD.
References
- 1. UNODC, United Nations Office on Drugs and Crime. Core team: Beate Hammond (coordination), Juan Carlos Araneda, Conor Crean, Jakub Gregor, Alice Hamilton, Raggie Johansen, Kristina Kuttnig, Sabrina Levissianos, Shawn Kelley, Tun Nay Soe. Global Amphetamine-Type Stimulant Assessment: Amphetamine and Ecstasy. New York, NY: United Nations Publication; 2011. [Google Scholar]
- 2. UNODC, United Nations Office on Drugs and Crime. World Drug Report. New York, NY: United Nations Publication; 2009. [Google Scholar]
- 3. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Schizophrenia Bulletin 2021: Prevalence of Drug Use. 2021. [Google Scholar]
- 4. Brunette MF, Mueser KT, Babbin S, et al. Demographic and clinical correlates of substance use disorders in first episode psychosis. Schizophr Res. 2018;19.:4–12. doi: 10.1016/j.schres.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 5. Barnes TRE, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM.. Comorbid substance use and age at onset of schizophrenia. Br J Psychiatry J Ment Sci. 2006;18.:237–242. doi: 10.1192/bjp.bp.104.007237. [DOI] [PubMed] [Google Scholar]
- 6. Barnett JH, Werners U, Secher SM, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007;19.(6):515–520. doi: 10.1192/bjp.bp.106.024448. [DOI] [PubMed] [Google Scholar]
- 7. Sara GE, Large MM, Matheson SL, et al. Stimulant use disorders in people with psychosis: a meta-analysis of rate and factors affecting variation. Aust N Z J Psychiatry. 2015;.(49):106–117. [DOI] [PubMed] [Google Scholar]
- 8. Sara G, Burgess P, Malhi GS, Whiteford H, Hall W.. Differences in associations between cannabis and stimulant disorders in first admission psychosis. Schizophr Res. 2013;14.(2–3):216–222. doi: 10.1016/j.schres.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 9. McKetin R, Leung J, Stockings E, et al. Mental health outcomes associated with of the use of amphetamines: a systematic review and meta-analysis. EClinicalMedicine. 2019;1.:81–97. doi: 10.1016/j.eclinm.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hermens DF, Lubman DI, Ward PB, Naismith SL, Hickie IB.. Amphetamine psychosis: a model for studying the onset and course of psychosis. Med J Aust. 2009;19.(S4). doi: 10.5694/j.1326-5377.2009.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 11. Angrist B, Sathananthan G, Wilk S, Gershon S.. Amphetamine psychosis: behavioral and biochemical aspects. J Psychiatr Res. 1974;1.:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- 12. McKetin R, McLaren J, Lubman DI, Hides L.. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;10.(10):1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 13. Curran C, Byrappa N, McBride A.. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;18.(3):196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- 14. McKetin R, Lubman DI, Baker AL, Dawe S, Ali RL.. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;7.(3):319–324. doi: 10.1001/jamapsychiatry.2013.283. [DOI] [PubMed] [Google Scholar]
- 15. Vallersnes OM, Dines AM, Wood DM, et al. ; Euro-DEN Research Group. Psychosis associated with acute recreational drug toxicity: a European case series. BMC Psychiatry. 2016;1.:293. doi: 10.1186/s12888-016-1002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gayer-Anderson C, Jongsma HE, Di Forti M, et al. The EUropean Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI): incidence and first-episode case-control programme. Soc Psychiatry Psychiatr Epidemiol. 202;5.(5):645–657. [DOI] [PubMed] [Google Scholar]
- 17. McGuffin P, Farmer A, Harvey I.. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;4.(8):764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 18. Jongsma HE, Gayer-Anderson C, Lasalvia A, et al. ; European Network of National Schizophrenia Networks Studying Gene-Environment Interactions Work Package 2 (EU-GEI WP2) Group. Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry. 2018;7.(1):36–46. doi: 10.1001/jamapsychiatry.2017.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Forti M, Quattrone D, Freeman TP, et al. ; EU-GEI WP2 Group. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;.(5):427–436. doi: 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mallett R, Leff J, Bhugra D, Pang D, Zhao JH.. Social environment, ethnicity and schizophrenia. A case-control study. Soc Psychiatry Psychiatr Epidemiol. 2002;3.(7):329–335. doi: 10.1007/s00127-002-0557-4. [DOI] [PubMed] [Google Scholar]
- 21. Power BD, Stefanis NC, Dragovic M, Jablensky A, Castle D, Morgan V.. Age at initiation of amphetamine use and age at onset of psychosis: the Australian Survey of High Impact Psychosis. Schizophr Res. 2014;15.(1):300–302. doi: 10.1016/j.schres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 22. Ho D, Imai K, King G, Stuart EA.. 2011. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;4.(8):1–28. [Google Scholar]
- 23. Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;9.(5):325–332. doi: 10.1093/oxfordjournals.aje.a121617 [DOI] [PubMed] [Google Scholar]
- 24. Jedynak J, Hearing M, Ingebretson A, et al. Cocaine and amphetamine induce overlapping but distinct patterns of AMPAR plasticity in nucleus accumbens medium spiny neurons. Neuropsychopharmacology. 2016;4.(2):464–476. doi: 10.1038/npp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primer. 2015;.(1):15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- 26. Compton WM, Han B, Blanco C, Johnson K, Jones CM.. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;17.(8):741–755. doi: 10.1176/appi.ajp.2018.17091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wetenschappelijk Inderzoek- en Documentatiecentrum. Ministerie van Veiligheid en Justicie. Trimbos instituut. Nationale Drug Monitor. 2015. [Google Scholar]
- 28. Curran HV, Hindocha C, Morgan CJA, Shaban N, Das RK, Freeman TP.. Which biological and self-report measures of cannabis use predict cannabis dependency and acute psychotic-like effects? Psychol Med. 2019;4.(09):1574–1580. doi: 10.1017/S003329171800226X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freeman TP, Morgan CJA, Hindocha C, Schafer G, Das RK, Curran HV.. Just say “know”: how do cannabinoid concentrations influence users’ estimates of cannabis potency and the amount they roll in joints?: cannabis potency. Addiction. 2014;10.(10):1686–1694. doi: 10.1111/add.12634. [DOI] [PubMed] [Google Scholar]
- 30. Chiang M, Lombardi D, Du J, et al. Methamphetamine-associated psychosis: clinical presentation, biological basis, and treatment options. Hum Psychopharmacol Clin Exp. 2019;3.(5). doi: 10.1002/hup.2710. [DOI] [PubMed] [Google Scholar]
- 31. Rabe-Jabłońska J, Mirek M, Pawelczy T.. Risk factors of schizophrenia development in patients with amphetamines dependence and psychosis (amphetamine-induced psychosis and schizophrenia), and without psychosis. Psychiatry Pol. 2012;.(46):571–584. [PubMed] [Google Scholar]
- 32. Bramness JG, Rognli EB.. Psychosis induced by amphetamines. Curr Opin Psychiatry. 2016;2.:236–241. [DOI] [PubMed] [Google Scholar]
- 33. Arunogiri S, Foulds JA, McKetin R, Lubman D.. A systematic review of risk factors for methamphetamine-associated psychosis. Aust N Z J Psychiatry. 2018;5.(6):514–529. doi: 10.1177/0004867417748750. [DOI] [PubMed] [Google Scholar]
- 34. Zuvekas SH, Vitiello B.. Stimulant medication use in children: a 12-year perspective. Am J Psychiatry. 2012;16.(2):160–166. doi: 10.1176/appi.ajp.2011.11030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dalsgaard S, Mortensen PB, Frydenberg M, Maibing CM, Nordentoft M, Thomsen PH. . Association between attention-deficit hyperactivity disorder in childhood and schizophrenia later in adulthood. Eur Psychiatry. 2014;2.(4):259–263. [DOI] [PubMed] [Google Scholar]
- 36. Gallagher KE, Funaro MC, Woods SW.. Prescription stimulants and the risk of psychosis: a systematic review of observational studies. J Clin Psychopharmacol. 2022;4.(3):308–314. doi: 10.1097/JCP.0000000000001552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


