Abstract
This study evaluates the short- and long-term effect of burns on children’s height and weight, by comparing their pre and postburn growth trajectory. We invited children (≤17 years old), who sustained a burn requiring surgical treatment or admission at one of the Dutch burn centers in 2013 (n = 175). As well as children who sustained a severe burn, covering >10% of the total body surface area (TBSA), throughout 2009–2018 (n = 228). Data was collected from a survey on health-related topics, Youth Health Care records, and the Dutch Burn Repository R3. For all participants, height and weight were converted to Z-scores using Dutch reference values. Linear mixed modeling, nested on the individual level, was used to examine the associations between burns and children’s height and weight Z-scores. Children’s height and weight Z-scores remained within the normal range throughout the study period. During the first-year postburn, children’s height and weight Z-scores decreased by −0.21 (95% CI −0.41, −0.01) and −0.23 (95% CI −0.46, −0.04), respectively. Beyond the first-year postburn, estimates were consistent with a positive linear association between burn size and the overall effect of burns on participants’ height and weight Z-scores. This included a modest, but statistically significant, effect among participants with a burn covering ≤4.5% and >14.0% of the TBSA. Sensitivity analyses did not alter our findings. In conclusion, children were on track or even surpassed their growth potential. Our findings could therefore be considered reassuring to patients, parents, and clinicians.
Over the last few decades, child mortality associated with burn injury has significantly decreased.1 A development that was strongly driven by the advances in acute burn care, including early management, fluid resuscitation, infection control, and enteral nutrition.2,3 The focus in clinical care and research has since shifted towards long-term health outcomes.
In children, linear growth and weight gain are important markers of overall health and well-being.4,5 Linear growth and weight gain are both dependent on environmental, genetic, and hormonal factors.6 A deficiency, may result in growth retardation—also known as growth faltering and stunting. Growth retardation can be defined as a failure to reach one’s growth potential, implying a child is too short or light for its age. It is considered stunting when a child’s height- or weight-for-age Z-score drops to more than two standard deviations (SD) below the median value.5,7 Growth retardation, especially during early childhood, is associated with undesirable short- and long-term health outcomes, including delayed cognitive and motor development, reduced physical strength and work capacity, increased risk of noncommunicable diseases in adulthood, increased risk of cephalopelvic disproportion, and even undesirable birth outcomes in the next generation.5,8
Burns are a common injury in children and induce a pathophysiologic response that may impede growth. As the release of stress hormones and pro-inflammatory mediators contribute to hypermetabolism, increased catabolism, and insulin resistance.9,10 Previous studies have reported growth retardation in children with extensive burns covering ≥30% of the total body surface area (TBSA), as demonstrated by an increase in the prevalence of low height- and weight-for-age, following discharge and up to 3.0 and 10.0 years postburn, respectively.11–14 However, in the Netherlands, children with extensive burns (≥30% TBSA) account for less than 5% of the general pediatric burn population.15
For the remainder, 95% of the pediatric burn population, it remains unclear whether the growth trajectory is affected. The current study therefore aimed to describe the short- and long-term effect of burn injury on children’s height and weight, by comparing the pre and postburn growth trajectory of children with a wide range of burn sizes and characteristics.
MATERIAL AND METHODS
Study Population
In this nationwide cohort study, eligible patients included children who sustained a burn injury requiring hospital admission of ≥1 day or surgical treatment at one of the Dutch burn centers in 2013. The sample was further enriched with children who sustained a severe burn injury, defined as a burn size of >10% TBSA, throughout 2009–2018. Exclusion criteria included death, incomplete or outdated contact details, living outside the Netherlands, and insufficient command of the Dutch language.
The Medical Ethics Review Committee of VU University Medical Center ruled that the Medical Research Involving Human Subjects Act (WMO) was not applicable to the current study. The Medical Ethics Review Committee of VU University Medical Center is registered with the U.S. Office for Human Research Protections as IRB00002991 (FWA00017598). Written informed consent was provided by either the participant if aged ≥16 years old, by both a legal guardian and the participant if aged ≥12 years old, or by a legal guardian if aged ≤11 years old.
Data Collection
Between June and November 2020, eligible patients received a postal invitation, including an information letter, informed consent form, and survey. If 3 weeks passed without response, eligible patients received a one-time reminder to consider study participation.
Participants completed a survey on the following health-related topics: 1) Origin, defined as the country of birth of the participant and biological parents; 2) Participant’s current height, participant’s current weight, and parental height reported in centimeters and kilograms; 3) General health, based on the Burns Outcome Questionnaire for 0- to 4-year-olds and 5- to 18-year-olds16,17; 4) Preexisting medical conditions; 5) Physical activity, based on the Dutch Healthy Physical Activity Guidelines18; 6) The Strengths and Difficulties Questionnaire, a brief behavioral screening instrument consisting of 25 items across five domains (emotional symptoms, conduct problems, hyperactivity, peer relationship problems, and prosocial behavior).19,20
Participants were also asked to consent to a review of their Youth Health Care Records. In the Netherlands, Youth Health Care is a free-of-charge nationwide program monitoring the health and general development of all children aged ≤18 years old. Children are invited for 18 regular checkups, 14 of which are scheduled throughout infancy and toddlerhood (≤4 years old). Attendance is voluntary and estimated to be 95% among children aged 0 to 4 years old, and 90% among children aged ≥5 years old. Record linkage was carried out using the participant’s name, date of birth, postal code of residence, and sex. Pre and postburn data collected from the Youth Health Care Records, included gestational age, preexisting medical conditions, the Dutch Development Instrument (Dutch: Van Wiechenschema), the Baecke-Fassaert Motor Test, height in centimeters, and weight in kilograms. The Dutch Development Instrument and Baecke-Fassaert Motor Test are the standard instruments used to assess development in 0 to 4- and 5 to 6-year-olds, respectively.21,22 Both are performance based, focusing on communication, fine and gross motor skills. Height and weight were measured by a trained physician, nurse, or assistant, at each checkup. In accordance with the Dutch Center for Youth Health guidelines, height was measured as the recumbent length in infants (≤2 years old) using a measuring board, and standing height in children using a fixed stadiometer. Weight was measured using either a mechanic or digital (infant) scale, to the nearest 100 g in children and adolescents, or 10 g in infants.
Demographics and clinical characteristics were derived from the Dutch Burn Repository R3; a national health care register, covering every patient admitted for ≥2 hours to one of three Dutch burn centers since 2009. Demographics and clinical characteristics included age, sex, hospital, burn size, full-thickness burn, etiology, date of injury, date of admission, admission height, admission weight, length of stay, number of surgeries, enteral nutrition, mechanical ventilation, and reconstructive surgery. To compare the characteristics of participants and nonparticipants, a subset of this information was also gathered—anonymously for nonparticipants.
Statistical Analysis
The baseline characteristics of the study population were described using summary statistics. Continuous variables were summarized using the mean and SD if the distribution was normal, otherwise the median and interquartile range (IQR) were reported. Binary and categorical variables were summarized using the frequency count and percentage.
A nonresponse analysis was performed using a Mann–Whitney U test or chi2 test, depending on the level of measurement and distribution of the observations. Between participants and nonparticipants comparisons were made on sex, age, burn size, full-thickness burn, etiology, length of stay, mechanical ventilation, number of surgeries, and reconstructive surgery.
For all participants, height and weight were converted to Z-scores using Dutch reference values. Calculation was based on sex, origin, age, gestational age, birth weight, birth height, and parental height. For children of Turkish, Moroccan, or Hindustan origin, Z-scores were calculated using reference values of their nationality.23,24 For children with one parent of nonDutch origin, Z-scores were calculated under the assumption of Dutch origin.25,26
We used linear mixed modeling with maximum likelihood estimation, to examine associations between burn injury and study participants’ height and weight Z-scores. Mixed model analyses were used to adjust for the dependency of repeated observations within the participant. The linear mixed models included the exposure variable (burn), time relative to burn (years), and the interaction between the exposure variable and time. As participants received treatment at one of the Dutch burn centers, the inclusion of burn center as an additional level was considered. However, based on the likelihood ratio test, the inclusion of burn center as an additional level did not provide a better fit. Thus, a 1-level nesting, observations nested on participant level, was adopted in the linear mixed model. Possible confounding and effect modification was investigated and adjusted for if necessary. The patient, burn, and treatment characteristics that were considered for confounding and effect modification: sex, age at the time of burn, preexisting medical conditions, self-reported physical activity, burn size, full-thickness burn, length of stay, nutrition support, therapeutic agents, mechanical ventilation, number of surgical procedures, excision and skin grafting, amputation, and reconstructive surgery. In the models presented in this paper, adjustments were made for age at the time of injury, burn size, and reconstructive surgery.
Additionally, a series of subgroup and sensitivity analyses were conducted. First, the main analyses were repeated with stratification of burn size into quartiles. Second, the main analyses were repeated among study participants aged 0 to 4 years old at the time of injury. Study participants within this age category go through important stages of postnatal growth and development. Furthermore, 14 out of 18 regular checkups in the Youth Health Care program are scheduled throughout infancy and toddlerhood (≤4 years old), suggesting study participants in this age category may have the most detailed pre and postburn data available. Third, the main analyses were repeated including only the study participants for whom Youth Health Care records could be retrieved. This is to establish the influence of missing values on the estimates reported in the main analyses.
Statistical significance was defined as a value of P < .05. All statistical analyses were performed using Stata 16.0 (Stata Corp., College Station, TX, USA).
STATISTICS
Study Population
A total of 403 patients were identified through the Dutch Burn Repository R3. Of these, 77 were excluded because they did not meet the inclusion criteria (incomplete or outdated contact details [n = 12], living outside the Netherlands [n = 16], insufficient command of the Dutch language [n = 31], different reasons [n = 18]). Leaving a total of 326 eligible patients, who received a postal invitation. The mean response rate was 34.4% across the three Dutch burn centers. In total, 112 children agreed to study participation, 68 (60.8%) of whom were male. At the time of injury, participants had a median (IQR) age of 2.0 (1.0–10.0) years old. Burn size ranged from 1% to 72% TBSA, with a median (IQR) burn size of 10.5% (5.0–15.0). Participants with a severe burn injury (>10% TBSA) accounted for 50.8% of the study population (n = 57). Among all study participants, 59.9% of burn injuries resulted from scald, 25.9% from flame or fire, 10.7% from fat, and 3.6% from other causes. Participants’ median (IQR) length of stay was 12 (5.0–21.0) days. During the hospital admission, 43 (38.4%) participants received enteral or parenteral nutrition. Therapeutic agents with an anabolic or anti-catabolic effect (eg, oxandrolone, recombinant human growth hormone, propranolol) were not commonly administered to pediatric burn patients in the Dutch burn centers. Propranolol, a β-adrenergic receptor blocker, was administered to reduce tachycardia in one participant (>40% TBSA). The mean (SD) time duration between injury and completion of the survey was 7.0 (±2.3) years (Table 1).
Table 1.
Demographics and clinical characteristics of study participants and nonparticipants
| Participants N = 112 |
Nonparticipants N = 292 |
P | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 68 (60.8) | 170 (58.2) | .65 |
| Female | 44 (39.2) | 122 (41.8) | |
| Age, median (IQR) | |||
| At time of burn | 2.0 (1.0–10.0) | 2.0 (1.0–6.0) | .70 |
| At time of study | 8.0 (6.0–15.0) | 8.0 (6.0–12.0) | .92 |
| Time since burn, mean (SD) | 7.0 (2.3) | – | – |
| % TBSA, median (IQR) | 10.5 (5.0–15.0) | 10.0 (4.0–13.8) | .16 |
| % TBSA, n (%) | |||
| ≤10% TBSA | 55 (49.1) | 156 (53.4) | .89 |
| 10% ≤ 19% TBSA | 43 (38.4) | 100 (34.2) | |
| 20% ≤ 29% TBSA | 7 (6.2) | 21 (7.2) | |
| 30% ≤ 39% TBSA | 4 (3.6) | 9 (3.1) | |
| 40% ≤ 72% TBSA | 3 (2.7) | 6 (2.1) | |
| % Full-thickness burn, median (IQR) | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | .80 |
| % Full-thickness burn, n (%) | |||
| ≤10% TBSA | 109 (97.3) | 272 (93.2) | .17 |
| 10% ≤ 19% TBSA | 0 (0.0) | 10 (3.4) | |
| 20% ≤ 29% TBSA | 0 (0.0) | 5 (1.7) | |
| 30% ≤ 39% TBSA | 1 (0.9) | 1 (0.3) | |
| 40% ≤ 72% TBSA | 2 (1.8) | 4 (1.4) | |
| Etiology, n (%) | |||
| Scald | 67 (59.9) | 200 (68.5) | .28 |
| Flame | 17 (15.2) | 28 (9.6) | |
| Flash fire | 12 (10.7) | 23 (7.9) | |
| Fat | 12 (10.7) | 26 (8.9) | |
| Contact | 4 (3.6) | 6 (2.1) | |
| Other | 0 (0.0) | 7 (2.4) | |
| Length of stay in days, median (IQR) | 12 (5.0-21.0) | 11 (4.0–21.0) | .39 |
| Nutrition support, n (%) | |||
| Nasogastric tube | 41 (36.6%) | – | – |
| Percutaneous endoscopic gastronomy | 1 (0.9%) | – | |
| Parenteral nutrition | 1 (0.9%) | – | |
| Therapeutic agents, n (%) | |||
| Anabolic agents | 0 (0.0%) | – | – |
| Hormonal therapies | 0 (0.0%) | – | |
| Beta-blockade | 1 (0.9%) | – | |
| Mechanical ventilation, n (%) | |||
| Yes | 10 (8.9) | 6 (2.1) | .001 |
| No | 102 (91.1) | 285 (97.9) | |
| Number of surgeries, median (IQR) | 0 (0.0–1.0) | 1 (0.0–1.0) | .15 |
| Excision and skin grafting, n (%) | |||
| Yes | 59 (52,7) | – | – |
| No | 53 (47.3) | – | |
| Amputation, n (%) | |||
| Yes | 0 (0.0) | – | – |
| No | 112 (100.0) | – | |
| Reconstructive surgery, n (%) | |||
| Yes | 12 (10.7) | 22 (7.6) | .29 |
| No | 100 (89.3) | 269 (92.4) | |
N, Number; IQR, Interquartile range; SD, Standard deviation; TBSA, Total body surface area.
The demographics and clinical characteristics of the study participants (n = 112) were comparable to those of nonparticipants (n = 292), with the exception that study participants more frequently required mechanical ventilation during their hospital stay (10.7% vs 7.6%; P = .001) (Table 1).
Overall Growth Trajectories
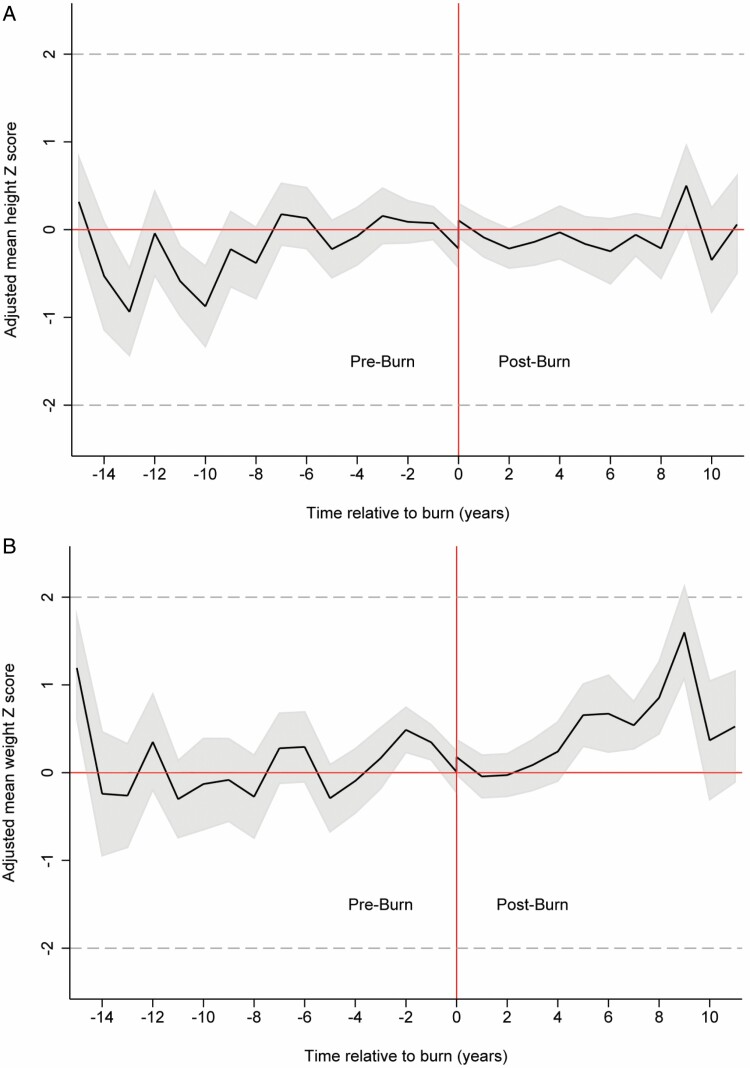
The number of height and weight measurements available for each study participant varied between 1 and 21, with an average of 9.3 and 9.6, respectively. The measurements were performed between 15 years preburn and 11 years postburn. Throughout the entire time, children’s height and weight Z-scores remained within the normal range (±1.96 SD); the adjusted mean height Z-score ranged from −0.94 SD to 0.50 SD and the adjusted mean weight Z-score ranged from −0.28SD to 1.59SD (Figure 1A and B).
Figure 1.
A. The adjusted mean height Z-score over the 26-year study period. B. The adjusted mean weight Z-score over the 26-year study period.
Following the injury, there was a small instantaneous shift in the adjusted mean height Z-score of 0.31 SD (95% CI 0.10, 0.51). This was followed by a mean decrease of −0.21 SD (95% CI −0.41, −0.01) during the first-year postburn. Thereby, the height Z-score returned to preburn values and the instantaneous effect was diminished. The overall trend in height Z-score seemed to be largely unaffected by the burn injury, with a mean difference of 0.002SD (95% CI −0.10, 0.11) between the pre and postburn height trajectory (Figure 1A).
The adjusted mean weight Z-score temporarily decreased by −0.23 SD (95% CI −0.46, −0.01) during the first 12 months postburn. Beyond 1-year postburn, the adjusted mean weight Z-score seemed to increase over time. Yet, there was no statistically significant difference between the overall pre and postburn weight trajectory (−0.03SD; 95% CI −0.15, 0.09) (Figure 1B).
Subgroup and Sensitivity Analyses
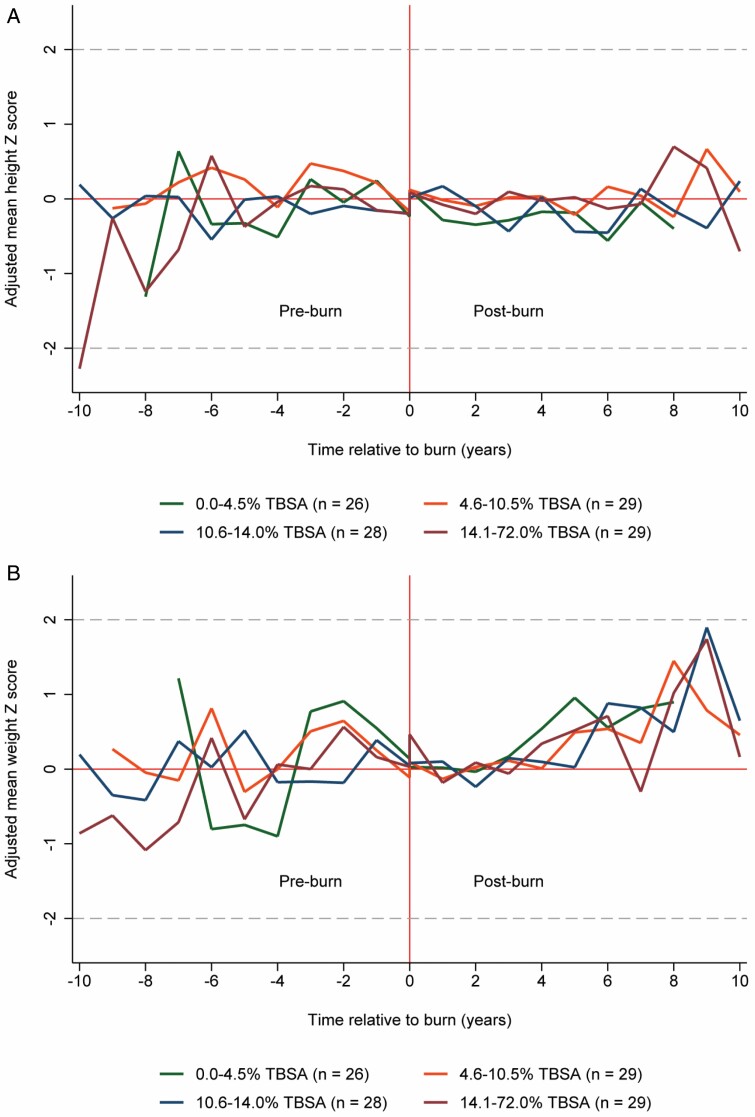
First, the main analyses were repeated with stratification of burn size into quartiles of ≤4.5% (n = 26); 4.6–10.5% (n = 29); 10.6–14.0% (n = 28); and >14.0% TBSA (n = 29) (Figure 2A and B). During the first-year postburn, the effect of burn injury on the height Z-score was most pronounced among those with a burn injury covering ≤4.5% TBSA (−0.40 SD; 95% CI −0.75, −0.05). On the contrary, the short-term effect of burn injury on the weight Z-score was most pronounced among those with a burn injury covering >14.0% TBSA (−0.64 SD; 95% CI −1.09, −0.19) (Table 2). Beyond the first-year postburn, we observed a modest, but statistically significant, effect among participants with a burn injury covering ≤4.5% and >14.0% TBSA. Namely, if burn size was ≤4.5% TBSA, the height and weight Z-scores decreased by −0.27 SD (95% CI −0.46, −0.08) and −0.23 SD (95% CI −0.46, −0.01), respectively. If the burn size was >14.0% TBSA, the height and weight Z-scores increased by 0.26 SD (95% CI 0.06, 0.46) and 0.28 SD (95% CI 0.06, 0.51), respectively. Moreover, the estimates were consistent with a positive linear association between burn size and the overall effect of burn injury on participants’ height and weight Z-scores (Table 2).
Figure 2.
A. The adjusted mean height Z-score—stratified by burn size. B. The adjusted mean weight Z-score—stratified by burn size.
Table 2.
Subgroup analyses by burn size
| ≤4.5% TBSA a n = 26 |
4.6% ≤ 10.5% TBSA a n = 29 |
10.6% ≤ 14.0% TBSA a n = 28 |
14.1% ≤ 72.0% TBSA a n = 29 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | 95% CI | P | Effect | 95% CI | P | Effect | 95% CI | P | Effect | 95% CI | P | |
| Height | ||||||||||||
| ≤12 months | −0.40 | (−0.75, −0.05) | .025 | −0.13 | (−0.45, 0.18) | .398 | 0.15 | (−0.24, 0.55) | .447 | −0.16 | (−0.56, 0.24) | .432 |
| Overall | −0.27 | (−0.46, −0.08) | .005 | −0.13 | (−0.32, 0.06) | .204 | 0.15 | (−0.06, 0.37) | .166 | 0.26 | (0.06, 0.46) | .011 |
| Weight | ||||||||||||
| ≤12 months | −0.008 | (−0.42, 0.39) | .968 | −0.21 | (−0.56, 0.14) | .235 | 0.025 | (−0.41, 0.46) | .910 | −0.64 | (−1.09, −0.19) | .005 |
| Overall | −0.23 | (−0.46, −0.01) | .045 | −0.14 | (−0.32, 0.04) | .134 | 0.06 | (−0.19, 0.30) | .657 | 0.28 | (0.06, 0.51) | .012 |
n, Number; CI, Confidence interval; TBSA, Total body surface area.
aData were generated with use of a linear mixed model, adjustments were made for age at the time of injury and reconstructive surgery.
Second, we performed sensitivity analyses including only study participants aged 0 to 4 years old at the time of injury (n = 68). The number of height and weight measurements available for each of the remaining study participants varied between 1 and 21, with an average of 10.4 and 11.0, respectively. Although the sensitivity analyses may have been underpowered, there was no statistically significant difference between our main analyses and when excluding those aged ≥5 years old at the time of injury (Table 3).
Table 3.
Sensitivity analyses
| Main analysesa N = 112 |
Aged 0–4 years olda, b n = 68 |
Youth Health Care recordsa n = 72 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | 95% CI | P | Effect | 95% CI | P | Effect | 95% CI | P | |
| Height | |||||||||
| ≤12 months | −0.21 SD | (−0.41, −0.01) | .040 | −0.22 SD | (−0.43, −0.01) | 0.040 | −0.15 SD | (−0.35, 0.05) | 0.134 |
| Overall | 0.002 SD | (−0.10, 0.11) | .974 | −0.04 SD | (−0.17, 0.09) | 0.551 | −0.07 SD | (−0.19, 0.06) | 0.326 |
| Weight | |||||||||
| ≤12 months | −0.23 SD | (−0.46, −0.01) | 0.040 | −0.15 SD | (−0.38, 0.09) | 0.226 | −0.22 SD | (−0.44, 0.02) | 0.070 |
| Overall | −0.03 SD | (−0.15, 0.09) | 0.637 | −0.12 SD | (−0.27, 0.02) | 0.101 | −0.07 SD | (−0.21, 0.08) | 0.346 |
n, Number; CI, Confidence interval; TBSA, = Total body surface area.
aData were generated with use of a linear mixed model, adjustments were made for burn size, age at the time of injury, and reconstructive surgery.
bAge at the time of injury.
Third, we performed sensitivity analyses including only study participants for whom Youth Health Care records could be retrieved (n = 72). The number of height and weight measurements available for each of the remaining study participants varied between 2 and 21, with an average of 13.3 and 13.9, respectively. Although the sensitivity analyses may have been underpowered, there was no statistically significant difference between our main analyses and when excluding those whose Youth Health Care records could not be retrieved (Table 3).
DISCUSSION
Our study provided a longitudinal series of children’s growth following a burn injury of any size. Children’s height and weight Z-scores remained within the normal range throughout the study period. Following the burn incident, however, there was a statistically significant short-term effect on children’s growth. As indicated by a decrease of −0.21 SD (95% CI −0.41, −0.01) and −0.23 SD (95% CI −0.46, −0.04) in children’s height and weight Z-scores during the first-year postburn. Stratification by burn size, showed that the association between burn size and the short-term effect of burn injury was opposite for height and weight. Beyond the first-year postburn, estimates were consistent with a positive linear association between burn size and the overall effect of burn injury on participants’ height and weight Z-scores. Including a modest, but statistically significant, negative effect among participants with a burn injury covering ≤4.5% TBSA. A similar, but positive, effect was observed among those with a burn injury covering >14.0% TBSA.
Previous reports studying the effect of burn injury on children’s growth, have indicated growth deceleration in both height and weight up to 3.0 and 10.0 years following a severe burn injury (≥30% TBSA).11–14 This effect was most profound at the time of discharge and during the first-year postburn.12–14,27 Though the latter is consistent with our findings, the effect size reported by previous studies is considerably larger. For example, Prelack et al. observed a mean decrease in children’s height-for-age Z-score of 0.50 to 0.76 SD (P < .0001) following a burn injury covering ≥50% TBSA.12 The difference in duration and magnitude of growth deceleration can probably be explained by the difference in burn size between study populations. As research in children with burns covering 2.0% to 87.0% TBSA identified a positive linear association between burn size and the pathophysiological response to burn injury.28–32 More comparable might be the cross-sectional study by Disseldorp et al., that aimed to describe the anthropometrics, muscular strength, and aerobic capacity in children admitted to one of the three Dutch burn centers between 0.5 and 5.0 years prior—with burns covering >10% TBSA or a length of stay of more than 6 weeks.15 Disseldorp et al. observed no significant deviations in children’s height and weight Z-scores compared to national norm values. A significant deviation was defined as a Z-score smaller than −1.96 SD or greater than 1.96 SD. Consistent with the findings by Disseldorp et al., children’s height and weight Z-scores remained within the normal range throughout the study period (Figure 1A and B).
In our study, we observed a positive linear association between burn size and the overall effect of burn injury on children’s height and weight Z-scores. This association may partly be explained by differences in both local and systemic physiological responses.28–32 Secondly, there may be a difference in nutrition support or intake based on burn size. Throughout recovery, nutrition support aids patients to meet their increased energy expenditure through high-caloric and high-protein intake. In case of a severe burn injury or insufficient oral intake during their hospital stay, parenteral, or enteral nutrition will be initiated and administered in a continuous fashion.33 Furthermore, following their recovery, children and adolescents may engage in disordered eating—be it through the social and psychosocial impact that burn scars may have on their body image and self-esteem, or the inability to readapt their oral intake to their caloric needs. Lastly, parents of children with more extensive burns may choose to restrict the participation in physical activity based on perceptions of fragility. This would, however, be inconsistent with recent findings by Akkerman et al., who reported that burn size was not predictive for time spent in physical activity, moderate-to-vigorous physical activity, or sedentary behavior.34
Stratification by burn size further showed that the negative effect of burn injury on height Z-score was most pronounced among children with a burn injury covering ≤4.5% TBSA. A possible explanation includes the significantly higher prevalence of infants (≤2 years) within this group (n = 18; 69.2%). Linear growth is not a continuous process, rather, it is a saltatory process.35 Extended periods of statis are punctuated by short periods of growth or “growth spurts.” Postnatal growth velocity is at its highest during infancy, resulting in a rapid increase in height and weight during the first year of life. It is also during this stage of development that the factors influencing an infant’s linear growth, shift from maternal to genetic and environmental; the most important environmental factor being nutritional intake.6
Our study has several strengths, including the inclusion of less extensive burn injuries, long-term follow-up, and the availability and use of preburn data. Yet, some limitations should be considered in the interpretation of our results. First, the response rate of 34.4% was somewhat low. Factors that may have affected the willingness to participate in the present study include the time elapsed since burn (7.0 ± 2.3 years), postal invitation, the path to recovery, burn size, and age at the time of injury. For example, an adolescent who sustained less extensive burn injuries and has since recovered, may not see the need for research. Consequently, there was a potential risk of selection bias, where the inclusion of children with more extensive burns could lead to an overestimation of the effect on postburn growth. However, the nonresponse analysis underlined that our study population was representative of an unselected sample, both in terms of demographics and burn characteristics. Second, Youth Health Care records were not available for approximately 35% of all study participants. The records could either not be located, or had been destroyed if the time limit for storage was reached. Consequently, pre and postburn measurements of these study participants were only available from the Dutch Burn Repository R3 and the survey. Sensitivity analyses including only the study participants for whom Youth Health Care records could be retrieved, revealed that the estimates reported in the main analyses were not significantly affected by these missing values. Third, as part of the Youth Health Care program, children are invited for 18 regular checkups, 14 of which are scheduled throughout infancy and toddlerhood (≤4 years old). Consequently, there was more detailed pre and postburn data available for study participants aged ≤4 years old at the time of injury (60.7%). Yet again, sensitivity analyses including only those aged ≤4 years old at the time of injury, confirmed the estimates reported in the main analyses. Fourth, participants current height and weight were self-reported as part of the survey. Despite the provided measurement instructions, there may have been variations in the measurement procedure. However, such variations in the measurement procedure would likely result in random rather than systemic measurement error. Fifth, it was not possible to determine the participants’ nutritional intake or dietary habits beyond the duration of their hospital admission.
In conclusion, we observed a modest, but statistically significant, negative effect of burn injuries on children’s growth trajectory. This effect was dependent on burn size both in terms of magnitude and duration. Nevertheless, children’s height and weight Z-scores remained within the normal range throughout the study period. Our findings could therefore be considered reassuring to patients, parents, and clinicians. Further studies should investigate the causal pathway and determinants of growth following a burn injury.
ACKNOWLEDGEMENTS
The authors would like to thank H. Eshuis, H. Hofland, J. Hiddingh, J. Meijer, M. van Baar, M. Nieuwenhuis, M. van Dinteren-Heijblom, M. Stoop, M. Fokke-Akkerman, and S. Singh, for their assistance and dedication to this study; the Dutch Burn Repository Group; Red Cross Hospital, Martini Hospital, and Maasstad Hospital.
Funding: This work was funded by the Dutch Burns Foundation (18.102 to A. Pijpe). The Dutch Burns Foundation was not involved in the study design, research conduct, or manuscript preparation.
Conflict of interest statement: The authors declare no conflicts of interest.
Contributor Information
Maxime D Cuijpers, Red Cross Hospital, Burn Centre Beverwijk, Vondellaan 13, Beverwijk, The Netherlands; Department of Plastic, Reconstructive and Hand Surgery, Amsterdam Movement Sciences, Amsterdam University Medical Centre, Location VU University Medical Centre, de Boelelaan 1117, Amsterdam, The Netherlands; Association of Dutch Burn Centres, Zeestraat 27-29, Beverwijk, The Netherlands.
Pauline J H van de Sande, Red Cross Hospital, Burn Centre Beverwijk, Vondellaan 13, Beverwijk, The Netherlands.
Charlotte I Cords, Association of Dutch Burn Centres, Zeestraat 27-29, Beverwijk, The Netherlands; Maasstad Hospital, Burn Centre Rotterdam, Maasstadweg 21, Rotterdam, The Netherlands.
Sonja M H J Scholten-Jaegers, Department of Surgery, Martini Hospital, Burn Centre Groningen, van Swietenlaan 4, Groningen, The Netherlands.
Paul P M van Zuijlen, Red Cross Hospital, Burn Centre Beverwijk, Vondellaan 13, Beverwijk, The Netherlands; Department of Plastic, Reconstructive and Hand Surgery, Amsterdam Movement Sciences, Amsterdam University Medical Centre, Location VU University Medical Centre, de Boelelaan 1117, Amsterdam, The Netherlands; Department of Plastic Surgery, Reconstructive and Hand Surgery, Red Cross Hospital, Vondellaan 13, Beverwijk, The Netherlands; Department of Paediatric Surgery, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam and Vrije Universiteit Amsterdam, Amsterdam Reproduction and Development Research Institute, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Martin G A Baartmans, Department of Paediatrics, Maasstad Hospital, Maasstadweg 21, Rotterdam, The Netherlands.
Anouk Pijpe, Red Cross Hospital, Burn Centre Beverwijk, Vondellaan 13, Beverwijk, The Netherlands; Department of Plastic, Reconstructive and Hand Surgery, Amsterdam Movement Sciences, Amsterdam University Medical Centre, Location VU University Medical Centre, de Boelelaan 1117, Amsterdam, The Netherlands; Association of Dutch Burn Centres, Zeestraat 27-29, Beverwijk, The Netherlands.
REFERENCES
- 1. Barrow RE, Przkora R, Hawkins HK, Barrow LN, Jeschke MG, Herndon DN.. Mortality related to gender, age, sepsis, and ethnicity in severely burned children. Shock 2005;23:485–7. [PubMed] [Google Scholar]
- 2. Herndon DN, Tompkins RG.. Support of the metabolic response to burn injury. Lancet 2004;363:1895–902. [DOI] [PubMed] [Google Scholar]
- 3. Wilmore DW, Aulick LH.. Metabolic changes in burned patients. Surg Clin North Am 1978;58:1173–87. [DOI] [PubMed] [Google Scholar]
- 4. WHO Expert Committee on Physical Status: The Use and Interpretation of Anthropometry (1993: Geneva). World Health Organization (1995). Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. Published online 1995. [PubMed]
- 5. Leroy JL, Frongillo EA.. Perspective: what does stunting really mean? A critical review of the evidence. Adv Nutr 2019;10:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei C, Gregory JW.. Physiology of normal growth. Paediatr Child Health 2009;19:236–40. [Google Scholar]
- 7. World Health Organization. Stunting in a Nutshell. World Health Organization. Published 2015; available from https://www.who.int/news/item/19-11-2015-stunting-in-a-nutshell; accessed 10 Aug. 2021 [Google Scholar]
- 8. Sudfeld CR, McCoy DC, Danaei Get al. Linear growth and child development in low- and middle-income countries: a meta-analysis. Pediatrics 2015;135:e1266–75. [DOI] [PubMed] [Google Scholar]
- 9. Jeschke MG, Baar ME, Choudhry MAet al. Burn injury. Nat Rev Dis Primers. 2020. Feburary 13;6(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Fact sheet: burns. WHO [online]. Published 2018; available from https://www.who.int/news-room/fact-sheets/detail/burns; accessed 15 Aug. 2020.
- 11. Jeschke MG, Gauglitz GG, Kulp GAet al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prelack K, Dwyer J, Dallal GEet al. Growth deceleration and restoration after serious burn injury. J Burn Care Res 2007;28:262–8. [DOI] [PubMed] [Google Scholar]
- 13. Rutan RL, Herndon DN.. Growth delay in postburn pediatric patients. Arch Surg 1990;125:392–5. [DOI] [PubMed] [Google Scholar]
- 14. Mittendorfer B, Hildreth MA, Desai MH, Herndon DN.. The 1995 Clinical Research Award. Younger pediatric patients with burns are at risk for continuing postdischarge weight loss. J Burn Care Rehabil 1995;16:589–95. [PubMed] [Google Scholar]
- 15. Disseldorp LM, Mouton LJ, van der Woude LHV, Van Brussel M, Nieuwenhuis MK.. Anthropometry, muscular strength and aerobic capacity up to 5 years after pediatric burns. Burns 2015;41:1839–46. [DOI] [PubMed] [Google Scholar]
- 16. van Baar ME, Essink-Bot ML, Oen IMMHet al. Reliability and validity of the Health Outcomes Burn Questionnaire for infants and children in The Netherlands. Burns 2006;32:357–65. [DOI] [PubMed] [Google Scholar]
- 17. van Baar ME, Essink-Bot ML, Oen IMMHet al. Reliability and validity of the Dutch version of the American Burn Association/Shriners Hospital for Children Burn Outcomes Questionnaire (5-18 years of age). J Burn Care Res 2006;27:790–802. [DOI] [PubMed] [Google Scholar]
- 18. Health Council of the Netherlands. Physical activity guidelines 2017; The Hague: Health Council of the Netherlands, 2017; Publication no.:2017/08e. [Google Scholar]
- 19. van Widenfelt BM, Goedhart AW, Treffers PDA, Goodman R.. Dutch version of the Strengths and Difficulties Questionnaire (SDQ). Eur Child Adolesc Psychiatry 2003;12:281–9. [DOI] [PubMed] [Google Scholar]
- 20. Muris P, Meesters C, van den Berg F.. The Strengths and Difficulties Questionnaire (SDQ)—further evidence for its reliability and validity in a community sample of Dutch children and adolescents. Eur Child Adolesc Psychiatry 2003;12:1–8. [DOI] [PubMed] [Google Scholar]
- 21. Jacobusse G, van Buuren S, Verkerk PH.. An interval scale for development of children aged 0-2 years. Stat Med 2006;25:2272–83. [DOI] [PubMed] [Google Scholar]
- 22. de Kroon MLA, van Kernebeek WG, Neve BFet al. Concurrent validity and discriminative ability of Dutch performance-based motor tests in 5 to 6 years old children. PLoS One 2019;14:e0224722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schönbeck Y, van Dommelen P, Hira Sing RAet al. Trend in height of Turkish and Moroccan children living in the Netherlands. PLoS One 2015;10:e0124686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Wilde JA, van Dommelen P, van Buuren S, Middelkoop BJC.. Height of South Asian children in the Netherlands aged 0-20 years: secular trends and comparisons with current Asian Indian, Dutch and WHO references. Ann Hum Biol 2015;42:38–44. [DOI] [PubMed] [Google Scholar]
- 25. Schönbeck Y, Talma H, van Dommelen Pet al. The world’s tallest nation has stopped growing taller: the height of Dutch children from 1955 to 2009. Pediatr Res 2013;73:371–7. [DOI] [PubMed] [Google Scholar]
- 26. Bocca-Tjeertes IFA, van Buuren S, Bos AF, Kerstjens JM, Ten Vergert EM, Reijneveld SA.. Growth of preterm and full-term children aged 0-4 years: integrating median growth and variability in growth charts. J Pediatr 2012;161:460–465.e1. [DOI] [PubMed] [Google Scholar]
- 27. Childs C, Hall T, Davenport PJ, Little RA.. Dietary intake and changes in body weight in burned children. Burns 1990;16:418–22. [DOI] [PubMed] [Google Scholar]
- 28. Abdel-Hafez NM, Saleh Hassan Y, El-Metwally TH.. A study on biomarkers, cytokines, and growth factors in children with burn injuries. Ann Burns Fire Disasters 2007;20:89–100. [PMC free article] [PubMed] [Google Scholar]
- 29. Caldwell FT, Jr, Wallace BH, Cone JB.. The effect of wound management on the interaction of burn size, heat production, and rectal temperature. J Burn Care Rehabil 1994;15(2):121–129. [DOI] [PubMed] [Google Scholar]
- 30. Jeschke MG, Mlcak RP, Finnerty CCet al. Burn size determines the inflammatory and hypermetabolic response. Crit Care 2007;11:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dehne MG, Sablotzki A, Hoffmann A, Mühling J, Dietrich FE, Hempelmann G.. Alterations of acute phase reaction and cytokine production in patients following severe burn injury. Burns 2002;28:535–42. [DOI] [PubMed] [Google Scholar]
- 32. Sarginson JH, Hollén L, Emond A, Mackie I, Young AE.. Multicentre observational study describing the systemic response to small-area burns in children. Burns 2021;47:560–8. [DOI] [PubMed] [Google Scholar]
- 33. Rousseau AF, Losser MR, Ichai C, Berger MM.. ESPEN endorsed recommendations: nutritional therapy in major burns. Clin Nutr 2013;32:497–502. [DOI] [PubMed] [Google Scholar]
- 34. Akkerman M, Mouton LJ, Disseldorp LMet al. Physical activity and sedentary behavior following pediatric burns—a preliminary investigation using objective activity monitoring. BMC Sports Sci Med Rehabil 2018;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lampl M, Veldhuis JD, Johnson ML.. Saltation and stasis: a model of human growth. Science 1992;258:801–3. [DOI] [PubMed] [Google Scholar]