Abstract
Background
Evidence from functional and structural research suggests that abnormal brain activity plays an important role in the pathophysiology of schizophrenia (SZ). However, limited studies have focused on post-treatment changes, and current conclusions are inconsistent.
Study Design
We recruited 104 SZ patients to have resting-state functional magnetic resonance imaging scans at baseline and 8 weeks of treatment with second-generation antipsychotics, along with baseline scanning of 86 healthy controls (HCs) for comparison purposes. Individual regional homogeneity (ReHo), amplitude of low-frequency fluctuations (ALFF), and degree centrality values were calculated to evaluate the functional activity. The Positive and Negative Syndrome Scale (PANSS) and MATRICS Consensus Cognitive Battery were applied to measure psychiatric symptoms and cognitive impairment in SZ patients.
Results
Compared with HCs at baseline, SZ patients had higher ALFF and ReHo values in the bilateral inferior temporal gyrus, inferior frontal gyrus, and lower ALFF and ReHo values in fusiform gyrus and precuneus. Following 8 weeks of treatment, ReHo was increased in right medial region of the superior frontal gyrus (SFGmed) and decreased in the left middle occipital gyrus and the left postcentral gyrus. Meanwhile, ReHo of the right SFGmed was increased after treatment in the response group (the reduction rate of PANSS ≥50%). Enhanced ALFF in the dorsolateral of SFG correlated with improvement in depressive factor score.
Conclusions
These findings provide novel evidence for the abnormal functional activity hypothesis of SZ, suggesting that abnormality of right SFGmed can be used as a biomarker of treatment response in SZ.
Keywords: schizophrenia, regional homogeneity, resting-state functional magnetic resonance imaging, prediction, treatment response
Introduction
Schizophrenia (SZ) is a serious psychiatric disorder that affects 1% of the world’s population and the main symptoms can be classified as positive, negative, and general psychopathology symptoms.1,2 Several hypotheses have been proposed to explain the etiology of SZ, the most widely accepted being neurodevelopmental abnormalities.3 Evaluation of brain activity may therefore aid in clarifying the pathophysiological mechanisms underlying SZ.4
Resting-state functional magnetic resonance imaging (rs-fMRI) can be effectively used to assess regional brain function and functional connectivity (FC) and is readily accepted by patients with neuropsychiatric disorders since a complex experimental design is not required.5 Regional brain function is measured by assessing the amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo), and degree centrality (DC).6–8 ALFF measures spontaneous low-frequency neural activity fluctuations in a voxel. ReHo reflects the regional homogeneity of neural activity between adjacent voxels, and DC is the most direct measure of node centrality in network analysis to portray a stable property of cortical network architecture at the voxel level. These 3 voxel-based metrics define brain functional characteristics from different perspectives and show a progressive relationship that allows for more sensitive identification of regional abnormalities.
Many studies have reported functional changes in multiple brain regions of patients with SZ.9,10 An earlier meta-analysis showed that in SZ, foci with decreased ALFF and ReHo in SZ were mainly located in the somatosensory, posterior parietal, and occipital cortexes. In contrast, foci with increased ALFF and ReHo were predominant in the bilateral striatum, medial temporal cortex, and medial prefrontal cortex.11 Patients with SZ exhibit extensive emotional and cognitive dysfunction, which may be attributable to changes in brain activity. A significant correlation between abnormal local activity with the neuropathological mechanism of auditory verbal hallucination has been demonstrated.12 The ReHo values in the right inferior frontal gyrus/insula are reported to be positively correlated with negative symptom scores and negatively correlated with Hopkins verbal learning test-revised/verbal learning.13 However, no consensus has been reached on alterations of these indexes and their relationship with symptom changes in the disorder.11 More importantly, the researchers also found that the specific changes in ReHo, ALFF, and DC before and after treatment may provide markers for differentiating SZ from healthy controls (HCs),13,14 and the prediction of treatment effects.15–17
To date, few relevant studies have focused on the changes in local brain activities in patients with SZ after short-term treatment. Moreover, evaluation of limited sample sizes has led to inconsistent conclusions. Therefore, we propose the hypothesis that patients with SZ have abnormal local activity in multiple brain regions and exhibit corresponding clinical symptoms according to the function of this brain region. And after treatment, there may be a relationship between the improvement of brain activity and the relief of clinical symptoms.
Materials and Methods
Participants
We recruited patients with SZ between March 2021 and February 2022 from the Second Affiliated Hospital of Xinxiang Medical University, China. All HCs were enlisted from the surrounding community. The inclusion criteria were as follows: (1) age range of 18–55 years, (2) Han Chinese origin, (3) right-handed, (4) normal intelligence, and (5) diagnostic criteria were met according to structured clinical interviews for DSM-IV-TR disorders (SCID), (6) HCs and their paternal or maternal relatives within 3 generations did not meet the diagnostic criteria for Axis I disorders in DSM-IV based on SCID. The exclusion criteria were as follows: (1) severe and unstable physical symptoms and/or diabetes, thyroid disease, hypertension, and heart disease, (2) history of head injury, (3) history of epileptic seizures, (4) meeting the DSM-IV-TR diagnostic criteria for alcohol and drug dependence (methamphetamine, ketamine, and cocaine), (5) having been treated using modified electroconvulsive therapy for 1 month prior to selection, (6) having suffered or currently suffering from the neuroleptic malignant syndrome or severe tardive dyskinesia, (7) state of pregnancy or lactation, and (8) presence of implanted metal frames or electronic devices that could prevent MRI scanning. Positive and Negative Syndrome Scale (PANSS) and MATRICS Consensus Cognitive Battery (MCCB)18 were applied to assess the symptoms and neurocognitive functioning of SZ. Five factors were derived from PANSS, specifically, positive symptoms, negative symptoms, cognition, depression, and excitement.2 Patients were treated with second-generation antipsychotic drugs for 8 weeks before magnetic resonance reexamination, with dose and drug selection conducted by the psychiatrist. The Ethics Committee approved this study at the Second Affiliated Hospital of Xinxiang Medical University, and all patients and control subjects provided written informed consent.
Data Acquisition and Preprocessing
MRI data were acquired using a 3.0-T MR scanner (Siemens, Verio). rs-fMRI data were obtained using an echo planar imaging sequence sensitive to BOLD contrast (repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, matrix size = 64 × 64, resolution of axial slice 3.4 × 3.4 mm2, slice thickness = 4 mm, gap between slices = 0.6 mm). Resting-state data were acquired for 8 min (240 time points).
rs-fMRI data were processed using Resting-State fMRI Data Analysis Toolkit plusv1.24 (RESTplus v1.24, http://restfmri.net/forum/restplus) using the following steps: (1) removing the first 10 time points, (2) slice-timing, (3) head motion correction, (4) spatial normalization to the Montreal Neurological Institute (MNI) space, (5) spatial smoothing with an isotropic Gaussian kernel with full width at half-maximum (FWHM) of 6 mm, (6) eliminating the linear trend of the time course, (7) regression of head motion effect, gray matter, white matter, and cerebrospinal fluid signals from the fMRI data, and (8) bandpass filtering (0.01–0.08 Hz). Participants with head motion exceeding 3 mm or rotation exceeding 3°C during scanning were excluded.
ALFF Calculation
After data preprocessing, the time course of each voxel was transformed into the frequency domain using a fast Fourier transform, and the power spectrum was subsequently obtained. The square root was calculated at each frequency of the power spectrum, and the average square root was obtained as the ALFF value in the range of 0.01–0.08 Hz for each voxel, which was further divided by the global mean ALFF of each individual for group comparison.6
ReHo Calculation
A single ReHo map was generated by calculating the Kendall’s coefficient of concordance (KCC) of the time series of a given voxel and its nearest neighbor (26 voxels) in a voxel-wise way.7 The formula for calculating the KCC value has been clarified in previous studies. For standardization, the ReHo value of every voxel was divided by the global mean ReHo of each individual. The spatial smoothing (FWHM = 6 mm) was performed after ReHo calculation.
DC Calculation
Pearson’s correlation of time series was performed between each voxel and every other voxel in the entire brain to calculate a correlation matrix R = (rij), j = 1 … N (N is the number of voxels), i ≠ 1. The correlation coefficients with rij ≥ 0.32 (P < .05, Bonferroni-corrected over whole-brain voxels) were summed up for each voxel and then a weighted DC was obtained for each voxel. The weighted DC of each voxel was further divided by the global mean weighted DC of each individual for group comparison.8,19
Statistical Analysis
Statistical analyses were conducted using the Statistical Package for Social Science version 26.0 (SPSS 26.0). Age and years of education for the 2 groups were compared using 2-sample t-tests. The sex composition ratio was compared using the Pearson Chi-square test. PANSS and MCCB scores before and after treatment in the patient group were compared with paired t-tests. RESTplus software was employed to analyze fMRI data. ALFF, ReHo, and DC maps were compared at baseline and after treatment, respectively. Individual age, sex, and years of education were treated as covariates in the group comparison. The false discovery rate (FDR) theory was applied to correct for multiple comparisons. Significance was set at P < .05 (FDR corrected and cluster size >10). Partial correlation analyses with age, sex, illness duration, and years of education as covariates were conducted between change values and the change of PANSS/MCCB scores. In addition, in order to further evaluate whether the changes in brain function activity before and after treatment were related to the efficacy, SZ patients were divided into the response and nonresponse group based on the reduction rate (RR) of PANSS. Improvement in clinical symptoms was calculated by the RR of the PANSS total scores. RR ≥50% represent responders, and RR <50% represent nonresponders.20 The clinical information of the 2 subgroups and the changes of ALFF, ReHo, and DC before and after treatment were compared.
Results
Analysis of Demographics and Clinical Characteristics
The flowchart of the study subjects is shown in figure 1. 104 SZ patients and 86 HCs were recruited for the study. Finally, 88 SZ patients and 81 HCs were included for statistical analyses. Sociodemographic and clinical data of study groups are presented in table 1. The demographic data and clinical characteristics separately for response and nonresponse group of SZ in supplementary table 1. Of the 88 patients with SZ enrolled in this study, 7 of them had not taken medication in baseline, 81 of them were adherent to second-generation antipsychotics prior to enrollment. Specific antipsychotic and comorbid medication use are shown in supplementary table 2. Antipsychotic drug doses were converted to the chlorpromazine equivalent.21
Fig. 1.
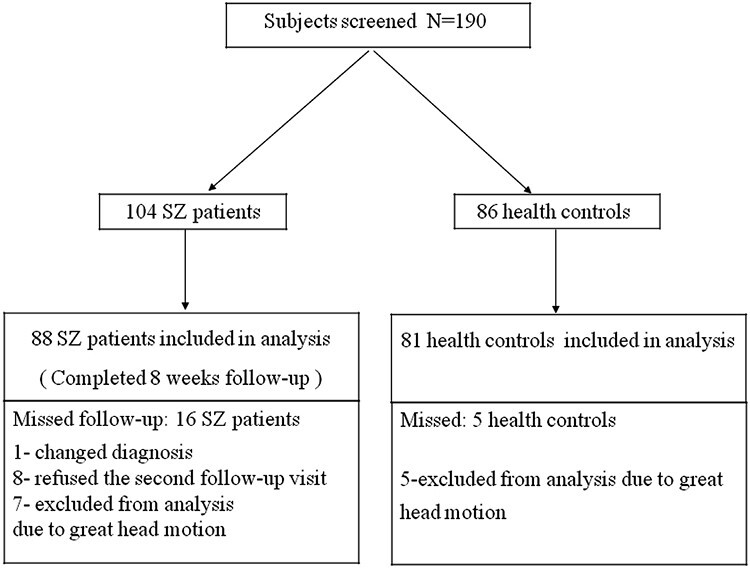
Chart flow for the study subjects.
Table 1.
Demographic Data and Clinical Characteristics of All Subjects
| Variables | SZ (n = 88) | HC (n = 81) | t/Z/χ2 | P | |
|---|---|---|---|---|---|
| Age (y) | 28.76 ± 8.29 | 30.93 ± 10.75 | −0.735 | .462 | |
| Sex (M/F) | 38/43 | 47/41 | 0.712 | .399 | |
| Education (y) | 10.93 ± 3.15 | 13.52 ± 4.12 | −4.156 | .000 | |
| Disease duration (mo) | 74.84 ± 65.80 | N/A | N/A | N/A | |
| Response/nonresponse | 47/41 | N/A | N/A | N/A | |
| Baseline | 8 wk | ||||
|---|---|---|---|---|---|
| PANSS total | 93.16 ± 14.99 | 59.83 ± 14.18 | N/A | 21.290 | .000 |
| PANSS positive | 13.22 ± 3.97 | 7.20 ± 3.12 | N/A | 15.558 | .000 |
| PANSS negative | 25.75 ± 6.25 | 18.35 ± 6.36 | N/A | 11.473 | .000 |
| PANSS cognitive | 20.52 ± 4.73 | 14.22 ± 3.65 | N/A | 11.616 | .000 |
| PANSS depressive | 8.35 ± 3.33 | 5.59 ± 2.22 | N/A | 9.783 | .000 |
| PANSS excitement | 19.83 ± 5.59 | 10.39 ± 3.53 | N/A | 17.097 | .000 |
| TMT | 72.07 ± 38.00 | 52.77 ± 24.03 | N/A | 6.500 | .000 |
| Symbol coding | 37.71 ± 12.07 | 38.73 ± 12.60 | N/A | −1.174 | .243 |
| HVLT-R | 16.16 ± 6.06 | 20.51 ± 6.11 | N/A | −8.051 | .000 |
| Spatial span | 11.60 ± 3.15 | 12.34 ± 3.26 | N/A | −2.345 | .021 |
| Mazes | 7.84 ± 4.75 | 10.00 ± 6.12 | N/A | −4.640 | .000 |
| BVMT-R | 17.10 ± 8.73 | 21.75 ± 10.15 | N/A | −4.982 | .000 |
| Category fluency | 14.99 ± 5.50 | 17.35 ± 6.06 | N/A | −3.953 | .000 |
| CPT-IP | 1.28 ± 0.66 | 1.28 ± 0.72 | N/A | −0.051 | .959 |
| Cpz (mg/d) | 345.73 ± 235.49 | 622.64 ± 275.43 | N/A | −9.026 | .000 |
Note: Unless otherwise indicated, data are means ± SD; BVMT-R, The revised Brief Visuospatial Memory Test; CPT-IP, The Continuous Performance Test Identical Pairs; Cpz, chlorpromazine equivalents; HC, healthy control; HVLT-R, The revised Hopkins Verbal Learning Test; N/A, not applicable; PANSS, Positive and Negative Syndrome Scale; SZ, schizophrenia; TMT, trail making test.
Abnormalities in Brain ALFF, ReHo, and DC at Baseline
Compared with HCs at baseline, SZ patients had higher ALFF values in the bilateral inferior temporal gyrus (ITG) (t = 5.570/5.274, P < .05), right orbital part of inferior frontal gyrus (ORBinf) (t = 3.803, P < .05), right amygdala (t = 4.384, P < .05), bilateral caudate (t = 5.288/7.093, P < .05), right triangular part of inferior frontal gyrus (IFGtriang) (t = 4.383, P < .05), right cerebellar hemisphere, lobule 8 (t = 4.094, P < .05), and lower ALFF values in left precentral gyrus (PreCG) (t = −3.751, P < .05), left supramarginal gyrus (t = −4.978, P < .05), right fusiform gyrus (FFG) (t = −3.581, P < .05), left precuneus (PCUN) (t = −5.747, P < .05), left superior parietal gyrus (t = −4.451, P < .05) (supplementary table 3 and supplementary figure 1).
SZ patients exhibited higher ReHo in the bilateral ORBinf (t = 5.363/3.852, P < .05), bilateral ITG (t = 5.725/4.973, P < .05), left cerebellar hemisphere, lobule 9 (t = 4.809, P < .05), right thalamus (t = 4.519, P < .05), left IFGtriang (t = 4.612, P < .05), vermis (t = 4.946, P < .05), left hippocampus (t = 4.251, P < .05), left putamen (t = 4.729, P < .05), and lower ReHo in the left ventromedial prefrontal cortex (t = −3.830, P < .05), right middle temporal gyrus (t = −4.358, P < .05), left PCUN (t = −5.570, P < .05), right inferior parietal gyrus (t = −4.557, P < .05), left supplementary motor area (t = −4.157, P < .05), left median cingulate and paracingulate gyri (t = −4.032, P < .05), and right FFG (t = −3.861, P < .05) compared with HCs (supplementary table 4 and supplementary figure 2). No regions showed significant differences between SZ and HCs in terms of DC.
Changes in Brain ALFF, ReHo, and DC After Treatment of SZ
After treatment, SZ patients showed increased ALFF in the right dorsolateral region of superior frontal gyrus (SFGdor) (t = 5.622, P < .05). Conversely, ALFF was decreased in the bilateral middle occipital gyrus (MOG) (t = −4.898/−4.745, P < .05), left paracentral lobule (t = −5.354, P < .05), light lingual (t = −4.727, P < .05), and right PreCG (t = −5.058, P < .05) (figure 2A; supplementary table 3).
Fig. 2.
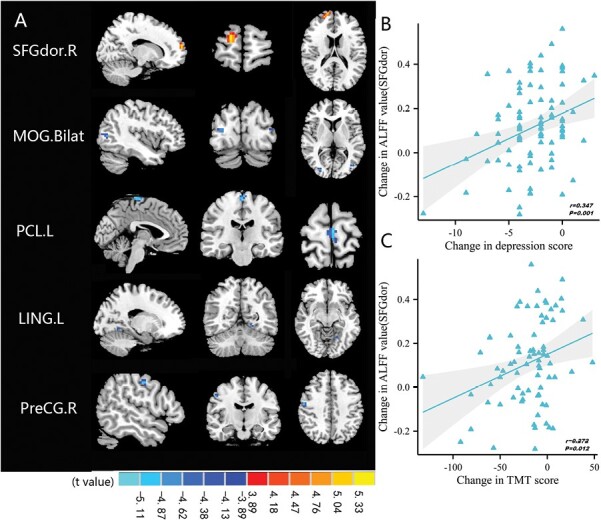
Comparison and correlation results of ALFF. (A) Differences in ALFF before and after treatment (FDR-corrected P < .05). (B) After 8 weeks of treatment, altered ALFF of SFGdor.R significantly correlated with reduction in depression score. (C) After treatment, altered ALFF of SFGdor.R significantly correlated with reduction in TMT score. Note: ALFF, amplitude of low-frequency fluctuations; Bilat, bilateral; FDR, false discovery rate; L, left; LING, lingual; MOG, middle occipital gyrus; PCL, paracentral lobule; PreCG, precentral gyrus; R, right; SFGdor, superior frontal gyrus, dorsolateral; TMT, trail making test; Post->pretreatment in SFGdor.R; Post-<pretreatment in MOG.Bilat, PCL.L, LING.L, and PreCG.R.
Following 8 weeks of treatment, ReHo was increased in the right medial region of superior frontal gyrus (SFGmed) (t = 4.721, P < .05) and decreased in the left MOG (t = −4.922, P < .05) and the left postcentral gyrus (PoCG) (t = −5.213, P < .05) (figure 3A; supplementary table 4). In contrast, no differences in DC were observed.
Fig. 3.
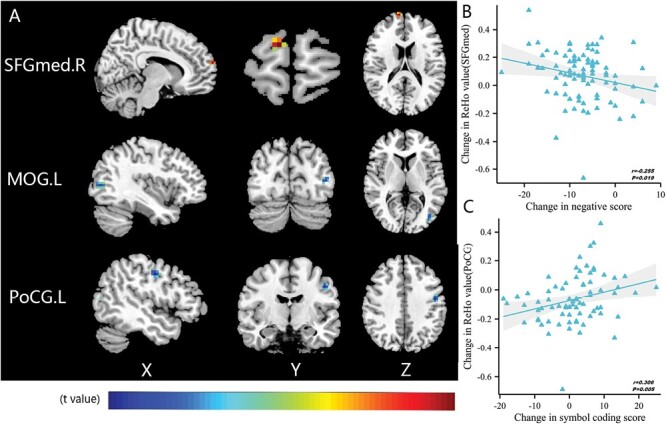
Comparison and correlation results of ReHo. (A) Differences in ReHo before and after treatment (FDR-corrected P < .05). (B) After 8 weeks of treatment, altered ReHo of SFGmed.R significantly correlated with reduction in negative score. (C) After treatment, altered ReHo of PoCG.L significantly correlated with increase in symbol-coding score. Note: FDR, false discovery rate; L, left; MOG, middle occipital gyrus; PoCG, postcentral gyrus; R, right; ReHo, regional homogeneity; SFGmed, superior frontal gyrus, medial; Post->pretreatment in SFGmed.R; Post-<pretreatment in MOG.L and PoCG.L.
Correlation Analysis Between PANSS and ALFF or ReHo
The degree of ALFF increase in SFGdor after treatment was significantly and positively related to the magnitude of decrease in depression score (r = .347, P = .001, PFDR-corrected = .014) (figure 2B, supplementary tables 5). Longitudinal increases of ReHo in SFGmed were negatively correlated with the change in negative scores (r = −.255, P = .019) (figure 3B), this correlation was not found when corrected by FDR (P = .266, supplementary tables 6). The remaining brain regions showing significant differences in regional activity were not correlated with changes in other subscores of PANSS (supplementary tables 5 and 6). We further performed correlation analysis between CPZ and PANSS RR, ALFF, and ReHo. There was significant negatively correlation only between CPZ and PANSS RR (r = −.355, P = .001) (supplementary table 7).
Analysis of Correlations Among MCCB, ALFF, and ReHo
The increase in ALFF in SFGdor after treatment was positively correlated with a decrease in the trail making test score to a significant extent (r = .272, P = .012) (figure 2C). A longitudinal decrease in ReHo in the left PoCG was positively correlated with changes in symbol-coding scores (r = .306, P = .005) (figure 3C). Those correlations were not found when corrected by FDR. No associations were observed between the remaining brain regions showing significant changes before and after treatment and changes in MCCB (supplementary tables 5 and 6).
Specific ReHo Differences Between the Response and Nonresponse Group
Eighty-eight patients with SZ were divided into the response (53.41%) and nonresponse (46.59%) group (table 1). There were significant differences in PANSS symptoms between the 2 groups after treatment (P < .05), but no differences in MCCB cognitive function (P > .05) (supplementary table 1). No significant changes were found in ALFF and DC in both groups before and after treatment (P > .05). We only found that the ReHo in the right SFGmed increased after treatment in the response group (cluster size = 53, t = 5.741, Pun-corrected = .000, PFDR-corrected = .004, figure 4). At the same time, no such change was observed in the nonresponse group.
Fig. 4.
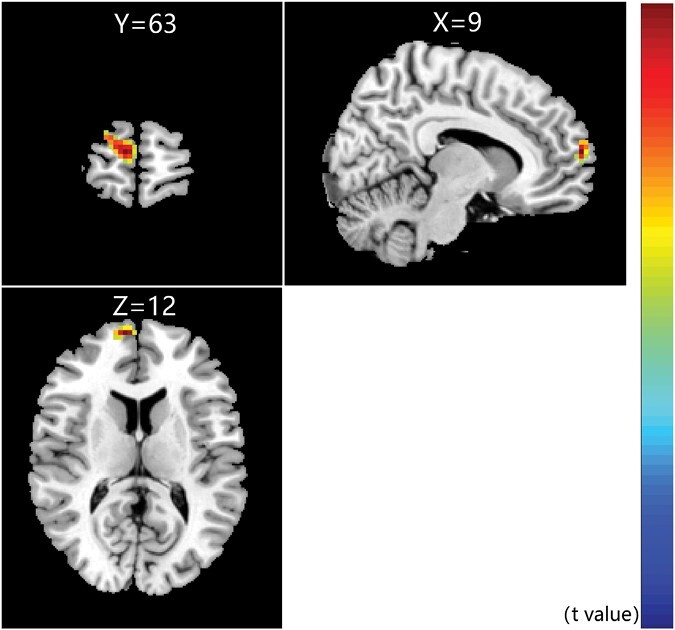
Specific ReHo differences in the response group (FDR-corrected P < .05). ReHo increased in the right medial of superior frontal gyrus in response group after treatment. Note: FDR, false discovery rate; ReHo, regional homogeneity.
Discussion
In this study, we analyzed the differences in ALFF, ReHo, and DC between SZ patients and HCs at baseline and changes in SZ after treatment. Compared with ALLF and DC, the change of ReHo is closely related to antipsychotic drug treatment, and serves as a sensitive indicator of drug efficacy. We observed a marked decrease or increase in local activity of multiple brain regions at baseline and after treatment, indicating extensive alterations in SZ brain. Moreover, the present study provides novel evidence that the increased ReHo in the right SFGmed may serve as a predictor for treatment response in SZ.
We observed overlaps between brain regions with ALFF and ReHo abnormalities at baseline, including ITG, ORBinf, IFGtriang, FFG, PCUN, and cerebellar hemisphere. Those findings provided complementary information on regional spontaneous brain activity, further validating the results.22,23 Brain activity changes in the frontal and temporal lobe regions are common in patients with SZ.24 Altered temporal lobe function is associated with hallucinations and delusional symptoms in SZ.25 ITG and FFG have long been associated with object and face/body recognition,26,27 and changes in these parameters may contribute to the development of hallucination symptoms in SZ. Damage to the orbitofrontal gyrus cortex is closely associated with apathy symptoms28 and may play an important role in the development of negative symptoms in patients with SZ. IFGtriang is an important part of the prefrontal lobe. The prefrontal lobe is associated with sensory information processing, memory, thinking, emotional function and is an important brain area for processing emotions.29 The PCUN is one of the core areas of the default network and is involved in contextual memory and emotional processing.30 During negative emotional stress, increased activity in the precuneus can facilitate distraction and thus alleviate negative emotions.31 This study found reduced spontaneous brain activity in the PCUN of SZ patients, which may lead to reduced modulation of negative emotions.
MOG is an important part of the occipital lobe, and its functional changes are closely related to cognitive impairment and visual hallucinations.32 However, the results of previous studies are inconsistent.23,33,34 No changes in the MOG were observed at baseline in our study, which may be attributable to population heterogeneity and different experimental parameters. However, local brain activity in the MOG decreased after treatment, consistent with data from a previous 1-year follow-up functional MRI study on SZ.14 Higher ALFF values in the MOG at baseline were recovered toward normal levels at 1 year of follow-up.35 Our results suggest that brain activity in the MOG is altered from the beginning of the treatment period, indicating that potential pathological changes are triggered in SZ during treatment.
PoCG is the central node of the somatosensory network showing a high degree of integration.36 In the present study, decreased ReHo in PoCG after treatment was positively correlated with an increase in the symbol-coding score. Symbol-coding test in the MCCB reflects the information processing speed of subjects. Our findings are consistent with previous results showing that the activity of PoCG is negatively correlated with processing speed in SZ.37,38 Abnormalities in PoCG affect the speed of information processing through effects on the reaction time,39 suggesting that PoCG could serve as a critical target of antipsychotic drugs to improve cognitive impairment.
The prefrontal lobe has been identified as one of the critical regions affecting affective and cognitive disorders in SZ.40,41 Spontaneous neuronal activity changes in the frontal cortex are evident in the early stages of SZ.42,43 Moreover, the FC also exhibits abnormalities. One study found that decreased FC between left SFG and bilateral PCUN, right hippocampus, right parahippocampal gyrus, left thalamus, left caudate, insula, and right superior parietal lobule, whereas increased FC was seen between the left SFG and right middle frontal gyrus in the youth-onset drug-naive SZ. The dysfunctional connectivity of the left SFG may be a potential pathophysiological mechanism in youth-onset drug-naive SZ.44 At present, rs-fMRI longitudinal studies of SZ are still lacking, including antipsychotic treatment for 1-week,17 12-week,16 and 1-year follow-up.14 We observed enhanced regional brain activity in SFG after 8 weeks of treatment, which was correlated with improvement in symptoms. Moreover, the changes of SFGmed were only observed in the response group after treatment. Previous study found that early reduction of functional activity in the right putamen17 and inter-hemispheric connectivity15 may be the predictor for treatment response in SZ. Our results suggest that different brain regions of the prefrontal lobe contribute differentially to SZ, validating an essential role of the prefrontal lobe in the pathophysiological mechanism of the disease, and the changes of SFGmed may be related to the prediction of efficacy, which needs further study.
Previous study has demonstrated that DC is sensitive to the functional disconnectivity of SZ.45 The nonresponders differed from the responders in dynamic DC not only at baseline but in the characteristics of changes before and after treatment.16 However, the majority of subjects in our study were patients with chronic SZ, which may be the underlying reason for inconsistency with previous results.
The current study has several potential limitations. Firstly, we did not strictly limit the drugs used by the subjects to exclude potential confounding effects caused by variable mechanisms of action of the different drugs. Secondly, brain function data of HCs were not collected after treatment for multivariate ANOVA analysis. Thirdly, the possible problem is that the partial volume effects are particularly salient for voxels close to the boundaries between different tissues in the ReHo calculation, which may have some impact.
Conclusion
In conclusion, SZ patients exhibit abnormal functional activities in a wide range of brain regions, further supporting the pathological features identified with neuroimaging. Increased regional brain activity in right SFGmed may predict efficacy in SZ. Increased functional activity of SFG brain regions could be effectively used as an indicator to assess the improvement of symptoms. Our collective findings provide further insights into the regional changes in brain functional activities before and after SZ treatment. Further studies are warranted to identify biomarkers that serve as reliable predictors of efficacy.
Supplementary Material
Acknowledgments
The authors thank the patients, their families, and the healthy volunteers for their participation, as well as the physicians and technicians who helped us collect clinical data in the Second Affiliated Hospital of Xinxiang Medical University. We thank the International Science Editing (http://www.internationalscienceediting.com) for editing our manuscript. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Xue Li, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Qing Liu, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Zhaonian Chen, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Yalin Li, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Ying Yang, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Xiujuan Wang, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Xiaoge Guo, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Binbin Luo, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Yan Zhang, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Han Shi, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Luwen Zhang, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Xi Su, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Minglong Shao, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Meng Song, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Suqin Guo, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Lingzhong Fan, Brainnetome Center & National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing, China.
Weihua Yue, Institute of Mental Health, Peking University, Beijing, China; Key Laboratory for Mental Health, Ministry of Health, Beijing, China.
Wenqiang Li, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Luxian Lv, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China.
Yongfeng Yang, Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China; Henan Key Lab of Biological Psychiatry, Xinxiang Medical University, Xinxiang, China; International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang, China; Henan Collaborative Innovation Center of Prevention and treatment of mental disorder, Xinxiang, China.
Funding
This work was supported by the National Natural Science Foundation of China (81971252 to L.L.; U1904130 and 82171498 to W.L., and 82001407 to X.S., 81825009 to W.Y.), Major science and technology projects of Henan Province (201300310200 to W.Y.), Science and technology research project of Henan Province (212102310587 to Me.S., 222102310130 to X.S.), Open program of Henan clinical research center for mental disorders (2021-zxkfkt-006 to L.Z.), Medical science and technology research project of Henan Province (LHGJ20210541 to X.W.), and the Open Program of the Henan Biological Psychiatry Key Laboratory (ZDSYS2016002 to L.F.).
References
- 1. Marder SR, Cannon TDS.. Schizophrenia. N Engl J Med. 2019;381(18):1753–1761. [DOI] [PubMed] [Google Scholar]
- 2. Kim JH, Kim SY, Lee J, Oh KJ, Kim YB, Cho ZH.. Evaluation of the factor structure of symptoms in patients with schizophrenia. Psychiatry Res. 2012;197(3):285–289. [DOI] [PubMed] [Google Scholar]
- 3. Pino O, Guilera G, Gomez-Benito J, Najas-Garcia A, Rufian S, Rojo E.. Neurodevelopment or neurodegeneration: review of theories of schizophrenia. Actas Esp Psiquiatr. 2014;42(4):185–195. [PubMed] [Google Scholar]
- 4. Gao B, Wang Y, Liu W, et al. Spontaneous activity associated with delusions of schizophrenia in the left medial superior frontal gyrus: a resting-state fMRI study. PLoS One. 2015;10(7):e0133766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox MD, Raichle ME.. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. [DOI] [PubMed] [Google Scholar]
- 6. Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. [DOI] [PubMed] [Google Scholar]
- 7. Zang Y, Jiang T, Lu Y, He Y, Tian L.. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. [DOI] [PubMed] [Google Scholar]
- 8. Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang N, Niu Y, Sun J, et al. Altered complexity of spontaneous brain activity in schizophrenia and bipolar disorder patients. J Magn Reson Imaging. 2021;54(2):586–595. [DOI] [PubMed] [Google Scholar]
- 10. Gerretsen P, Menon M, Mamo DC, et al. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr Res. 2014;160(1–3):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Y, Zhuo C, Qin W, Zhu J, Yu C.. Altered spontaneous brain activity in schizophrenia: a meta-analysis and a large-sample study. Biomed Res Int. 2015;2015:204628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C, Wang GH, Wu SH, Zou JL, Zhou Y, Wang HL.. Abnormal local activity and functional dysconnectivity in patients with schizophrenia having auditory verbal hallucinations. Curr Med Sci. 2020;40(5):979–984. [DOI] [PubMed] [Google Scholar]
- 13. Gao S, Ming Y, Wang J, et al. Enhanced prefrontal regional homogeneity and its correlations with cognitive dysfunction/psychopathology in patients with first-diagnosed and drug-naive schizophrenia. Front Psychiatry. 2020;11:580570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yin P, Zhao C, Li Y, Liu X, Chen L, Hong N.. Changes in brain structure, function, and network properties in patients with first-episode schizophrenia treated with antipsychotics. Front Psychiatry. 2021;12:735623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui LB, Fu YF, Liu L, et al. Baseline structural and functional magnetic resonance imaging predicts early treatment response in schizophrenia with radiomics strategy. Eur J Neurosci. 2020;53(6):1961–1975. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Jiang Y, Su W, et al. Temporal dynamics in degree centrality of brain functional connectome in first-episode schizophrenia with different short-term treatment responses: a longitudinal study. Neuropsychiatr Dis Treat. 2021;17:1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu R, Ou Y, Liu F, et al. Reduced brain activity in the right putamen as an early predictor for treatment response in drug-naive, first-episode schizophrenia. Front Psychiatry. 2019;10:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56(5):301–307. [DOI] [PubMed] [Google Scholar]
- 19. Zuo XN, Ehmke R, Mennes M, et al. Network centrality in the human functional connectome. Cereb Cortex. 2012;22(8):1862–1875. [DOI] [PubMed] [Google Scholar]
- 20. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR.. What does the PANSS mean? Schizophr Res. 2005;79(2–3):231–238. [DOI] [PubMed] [Google Scholar]
- 21. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC.. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turner JA, Damaraju E, van Erp TG, et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci. 2013;7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170(11):1308–1316. [DOI] [PubMed] [Google Scholar]
- 24. Yan W, Zhang R, Zhou M, et al. Relationships between abnormal neural activities and cognitive impairments in patients with drug-naive first-episode schizophrenia. BMC Psychiatry. 2020;20(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun J, Maller JJ, Guo L, Fitzgerald PB.. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61(1):14–32. [DOI] [PubMed] [Google Scholar]
- 26. Gerlach C, Aaside CT, Humphreys GW, Gade A, Paulson OB, Law I.. Brain activity related to integrative processes in visual object recognition: bottom-up integration and the modulatory influence of stored knowledge. Neuropsychologia. 2002;40(8):1254–1267. [DOI] [PubMed] [Google Scholar]
- 27. Kim NY, Lee SM, Erlendsdottir MC, McCarthy G.. Discriminable spatial patterns of activation for faces and bodies in the fusiform gyrus. Front Hum Neurosci. 2014;8:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knutson KM, Dal Monte O, Raymont V, Wassermann EM, Krueger F, Grafman J.. Neural correlates of apathy revealed by lesion mapping in participants with traumatic brain injuries. Hum Brain Mapp. 2014;35(3):943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiao L, Wei DT, Li WF, et al. Rumination mediates the relationship between structural variations in ventrolateral prefrontal cortex and sensitivity to negative life events. Neuroscience. 2013;255:255–264. [DOI] [PubMed] [Google Scholar]
- 30. Du L, Liu H, Du W, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry. 2018;7(11):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferri J, Schmidt J, Hajcak G, Canli T.. Emotion regulation and amygdala-precuneus connectivity: focusing on attentional deployment. Cogn Affect Behav Neurosci. 2016;16(6):991–1002. [DOI] [PubMed] [Google Scholar]
- 32. Tao H, Guo S, Ge T, et al. Depression uncouples brain hate circuit. Mol Psychiatry. 2013;18(1):101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu R, Hsieh MH, Wang HL, et al. Frequency dependent alterations in regional homogeneity of baseline brain activity in schizophrenia. PLoS One. 2013;8(3):e57516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoptman MJ, Zuo XN, Butler PD, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li F, Lui S, Yao L, et al. Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional MR imaging study. Radiology. 2016;279(3):867–875. [DOI] [PubMed] [Google Scholar]
- 36. Adhikari BM, Hong LE, Sampath H, et al. Functional network connectivity impairments and core cognitive deficits in schizophrenia. Hum Brain Mapp. 2019;40(16):4593–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bogler C, Vowinkel A, Zhutovsky P, Haynes JD.. Default network activity is associated with better performance in a vigilance task. Front Hum Neurosci. 2017;11:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang P, Yang J, Yin Z, et al. Amplitude of low-frequency fluctuation (ALFF) may be associated with cognitive impairment in schizophrenia: a correlation study. BMC Psychiatry. 2019;19(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M.. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115(Pt 4):1045–1059. [DOI] [PubMed] [Google Scholar]
- 40. Li HJ, Chan RC, Gong QY, et al. Facial emotion processing in patients with schizophrenia and their non-psychotic siblings: a functional magnetic resonance imaging study. Schizophr Res. 2012;134(2–3):143–150. [DOI] [PubMed] [Google Scholar]
- 41. Ellison-Wright I, Bullmore E.. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. [DOI] [PubMed] [Google Scholar]
- 42. Guo W, Liu F, Chen J, et al. Hyperactivity of the default-mode network in first-episode, drug-naive schizophrenia at rest revealed by family-based case-control and traditional case-control designs. Medicine. 2017;96(13):e6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu XB, Zhang Y, Yang DY, et al. Analysis of first-episode and chronic schizophrenia using multi-modal magnetic resonance imaging. Eur Rev Med Pharmacol Sci. 2018;22(19):6422–6435. [DOI] [PubMed] [Google Scholar]
- 44. Qiu X, Lu S, Zhou M, et al. The relationship between abnormal resting-state functional connectivity of the left superior frontal gyrus and cognitive impairments in youth-onset drug-naive schizophrenia. Front Psychiatry. 2021;12:679642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lo CY, Su TW, Huang CC, et al. Randomization and resilience of brain functional networks as systems-level endophenotypes of schizophrenia. Proc Natl Acad Sci U S A. 2015;112(29):9123–9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


