Abstract
Dermal substitutes are well established in the reconstructive ladder. MatriDerm® (Dr. Otto Suwelack Skin & Health Care AG, Billerbeck, Germany) is a single-layer dermal substitute composed of a bovine collagen (type I, III, and V) and elastin hydrolysate, that allows for immediate split-thickness skin grafting (SSG). The aim of this study was to histologically characterize the integration of MatriDerm® when used during burns surgery reconstruction. Eight subjects with nine burn scars and one acute burn wound underwent reconstruction with MatriDerm® and an immediate SSG. MatriDerm® integration and skin graft take were assessed with serial biopsies performed at weeks 1, 2, 3, and 4 and months 2, 3, 6, 9, and 12. Biopsies were assessed with standard special stains and immunohistochemistry, and representative slides were imaged with a transmission electron microscope. Patient satisfaction and clinical scar outcome were assessed with the Vancouver Scar Scale and a patient questionnaire. Histological analysis showed similar stages of wound healing as shown in other dermal templates but on a different timescale. There is early evidence of vascularization and an inflammatory infiltrate in the first 2 weeks. MatriDerm® is resorbed earlier than other dermal substitutes, with evidence of resorption at week 3, to be completely replaced by a neodermis at 2 months. The use of MatriDerm® in reconstruction with immediate skin grafting is supported histologically with early evidence of vascularization to support an epidermal autograft. Future histological studies may help further characterize the ideal dermal substitute.
Global mortality secondary to burn injury continues to decrease,1 making the attainment of satisfactory functional outcomes and acceptable aesthetic appearance now paramount. Dermal substitutes were originally developed as a solution to the challenge of limited donor sites by providing alternative wound cover, but have been increasingly used to address scar quality, with favorable functional and aesthetic outcomes.2–6
MatriDerm® (Dr. Otto Suwelack Skin & Health Care AG, Billerbeck, Germany) is a single-layer dermal substitute composed of bovine collagen (types I, III, and V) and elastin hydrolysate. The collagen–elastin template creates a porous matrix facilitating the immediate application of a skin graft onto the 1 mm thick sheet. Immediate application of skin graft over a dermal matrix is a new concept. The matrix can act as a barrier for vascularization and may lead to skin graft desiccation and failure. A delay in revascularisation of the skin graft due to the presence of MatriDerm®, was not observed in high-resolution episcopic microscopy, and it was suggested that nutritional support of the skin graft is provided through diffusion, preventing graft desiccation.7
MatriDerm® collagen is not cross-linked, unlike other dermal substitutes. Cross-linking of a dermal matrix results in a more stable structure that has increased resistance to biodegradation, which can be observed clinically with MatriDerm® having been found to absorb more quickly when compared to other dermal substitutes.8 However, increasing a dermal substitute’s rigidity through cross-linking may cause increased differentiation of fibroblasts to myofibroblasts, which could lead to increased wound contracture.9 A study examining the effect of cross-linking MatriDerm® using the same technique as that used to cross-link Integra® found that cross-linked MatriDerm® had reduced pore size and density, and delayed evidence of angiogenesis when compared to noncross-linked MatriDerm®. Biodegradability may have a relationship to angiogenesis, with vessel formation primarily observed in areas where the scaffold had biodegraded. Ultimately this did not affect graft take or wound contraction in an in vivo murine model.10 MatriDerm® matrix design has been shown to be crucial in influencing neovascularization, with a large pore size and relatively low density, allowing diffusion of nutrition and faster ingrowth of host fibroblasts and endothelial cells forming new vascular channels and neodermis.11–13
Other dermal substitutes such as bi-layered Integra® (Integra LifeSciences Corporation, Plainsboro, USA)14–16 and Pelnac (Gunze, Osaka, Japan),17–20 use a temporary silicon layer which protects the dermal substitute from fluid loss and infection whilst matrix vascularization and neodermis formation take place. The silicon layer is replaced with autologous split-thickness skin graft (SSG) when wound bed integration is complete, as a two-stage reconstruction procedure. More recently the development of NovoSorb™ Biodegradable Temporizing Matrix (Polynovo, Adelaide, Australia), also a bi-layer dermal substitute, has shown that animal products can be avoided, being a fully synthetic matrix composed of a biodegradable polyurethane foam.21–24
MatriDerm® provides a scaffold for the formation of a neodermis when applied to full-thickness wounds, in the context of acute burn excision, skin cancer, trauma, or scar resurfacing.6,25,26 In the acute burn setting, MatriDerm® can be prioritized for areas of cosmetic or functional importance.6,27–29 It has also been shown to have a role in areas in which skin grafting would not be effective, such as bone without periosteum and tendon without paratenon.30 The long-term clinical and histological benefits of MatriDerm® over skin grafting alone are yet to be established.31,32
We have previously examined the histological profile of Integra® and its ability to form a neodermis, providing evidence for the optimal timing of grafting in the use of Integra®.33 A similar study has not been conducted for MatriDerm®. The aim of this study was to characterize the histological changes of MatriDerm® during the various stages of its integration into the wound bed and to assess how this process differs from that of Integra®. Our current surgical protocol for use of MatriDerm® is also described.
METHODS
Ethical approval was obtained from the Regional Ethics Committee (NRES Committee West Midlands-South Birmingham—REC reference 12/WM/0024) prior to commencement of the study.
Patient Recruitment and Selection
Patients requiring reconstruction for acute burn injuries or burn scars were screened from inpatient multidisciplinary ward rounds and outpatient clinics. Patients with a total of ten reconstructed sites meeting the eligibility criteria (Table 1) were approached using patient information sheets to obtain informed consent. Baseline data including demographics, comorbidities, and Fitzpatrick skin type were collected. Wound mapping was performed with clinical photographs and the dimensions were recorded according to the surface area.
Table 1.
Patient eligibility criteria
| Patient inclusion criteria | Patient exclusion criteria |
|---|---|
| Age: 18–65 y Full-thickness wounds following burn wound/scar excision Deemed compliant with complex after-care Capacity to give informed consent for the research trial, photography, and skin biopsies |
Allergy to bovine collagen Inhalation injury at the time of burn Deemed unlikely to survive study period Risk factors that can affect healing Auto-immune disorders or immunosuppressive states Long-term antiplatelet or anticoagulation therapy Inclusion in another research trial Pregnant or breastfeeding |
Procedure
The surgical protocol is demonstrated in Figure 1A–D. Surgical excision of burn scar tissue (Figure 1A) was performed in depth to a healthy wound bed of either fat or in some cases fascia, and breadth to soft wound boundaries (Figure 1B).34 After meticulous haemostasis, Artiss® (Baxter Healthcare Ltd., Norfolk, UK) a fibrin sealant, was sprayed onto the wound bed to improve adherence and aid haemostasis. MatriDerm® of 1 mm thickness was then applied dry to the wound bed and moistened with saline in-situ to improve conformity (Figure 1C). MatriDerm® becomes impossible to handle if wetted before application and should only be moistened after it is placed well onto the wound. A second spray of Artiss® was then applied over the top of the matrix before the skin graft was applied. A sheet of epidermal autograft (0.06- to 0.08-inch thickness) was then applied (Figure 1D) and dressed with at least six layers of Bactigras® (Smith and Nephew plc, Watford, UK) and Polyfax® antimicrobial ointment (Elaiapharm, Valbonne, France). This particular primary dressing provides a sealed moist environment to prevent desiccation of the skin graft until the MatriDerm® is vascularized enough to support it. To prevent sheer and accumulation of fluid under the matrix or the skin graft, a foam cylinder or a tie-over dressing were applied according to the location of reconstruction. Patients were allowed home either on the same day or the day after for reconstructive cases.
Figure 1.
Surgical application of MatriDerm from a single patient (M05): A. Preoperative appearance with scar contracture; B. Wound bed preparation; C. MatriDerm® application; D. SSG application; and E. Month 3.
Primary graft inspection was performed on day 7 postoperation, with utmost care to prevent sheer or displacement. Skin graft overlying the MatriDerm® matrix is extremely fragile. At this stage, the skin graft is usually adherent, if not, the graft was handled with extreme care and dabbed clean and the dressings replaced.
Patients were closely monitored by a senior occupational therapist, before, during, and after the procedure. Scar management commenced the moment the skin graft was stable and solidly adherent to the underlying neodermis (Figure 1E).
Clinical Assessments
All patients were scheduled for 12 months of follow-up (weeks 1, 2, 3, and 4; months 2, 3, 6, 9, and 12). Clinical images were taken and a modified Vancouver Scar Scale (mVSS) was documented at each visit. Patient satisfaction in subjects undergoing scar reconstruction was assessed using a questionnaire.33 The mVSS and Patient Satisfaction Scores were documented by either a surgeon, senior research nurse, or occupational therapist experienced in scar management but not involved in the care of the study patients.
Biopsies
At each visit, a single punch biopsy (3 mm) was performed under local anesthesia from the skin-grafted site, allowing at least a 1 cm border of MatriDerm® to be left around the edge of the site. The specimen was placed in 10% formalin saline solution and paraffin sections were processed with hematoxylin and eosin (H&E), Elastic-Van-Gieson (EVG), and Orcein staining. Immunohistochemical analysis was performed to determine neovascularization, using antibodies to the vascular endothelial cell marker CD31. MatriDerm® thickness, vessel size, and density were measured. The neodermis and MatriDerm® thickness were measured in the H&E, Orcein, or EVG stained slides (with an average of 9 thickness measurements per slide); whilst vessel size and vessel density measurements were obtained in the CD31 stained slides using Image J software. Additionally, representative slides were imaged with transmission electron microscope (TEM) and collagen fiber (native and MatriDerm®) thickness was measured. All measurements were performed by two independent assessors and the mean readings were used.
Statistical Analysis
Longitudinal data were analyzed using the Statistical Package for Social Sciences Software version 19 (Armonk, NY: IBM Corp) using Wilcoxon signed rank tests and T-tests. A P-value of <0.05 was considered significant.
RESULTS
Ten sites of MatriDerm® reconstruction were performed on eight patients. The patients were mostly male, with a mean age of 34.5 years (range 18–55 years) and no significant preexisting physical comorbidities. Fitzpatrick skin type and anatomical site of reconstruction varied across the cohort (Table 2). With the exception of one case for acute burn wound cover, all cases were performed for burn scar reconstruction.
Table 2.
Patient baseline characteristics
| Patient | Age | Gender | Comorbidities | Fitzpatrick Skin Type | Anatomical Site of Reconstruction | Type of Reconstruction |
|---|---|---|---|---|---|---|
| M1 | 33 | Male | None | 3 | Right upper thigh | Acute burn |
| M2* | 53 | Male | None | 4 | Left arm | Scar reconstruction |
| M3 | 38 | Male | Psychiatric disorder | 3 | Left popliteal fossa | Scar reconstruction |
| M4 | 38 | Female | None | 1 | Left anterior and lateral thigh | Scar reconstruction |
| M5# | 25 | Male | None | 3 | Left axilla, radial upper arm, forearm | Scar reconstruction |
| M6 | 25 | Female | None | 4 | Left anterior torso | Scar reconstruction |
| M7 | 30 | Male | None | 5 | Left forearm | Scar reconstruction |
| M8* | 55 | Male | None | 4 | Right popliteal fossa | Scar reconstruction |
| M9# | 26 | Male | None | 3 | Right arm | Scar reconstruction |
| M10 | 18 | Female | None | 5 | Right breast | Scar reconstruction |
*M2 and M8 are the same patient operated on at different anatomical sites on different dates;
#M5 and M9 are the same patient operated on at different anatomical sites on different dates.
Epidermis
The epidermis showed reconstitution of the rete ridges as early as 2 weeks, but the rete ridges seen at this stage were flattened and sparse. By week 4 all specimens showed relatively well-developed rete ridges.
Dermis
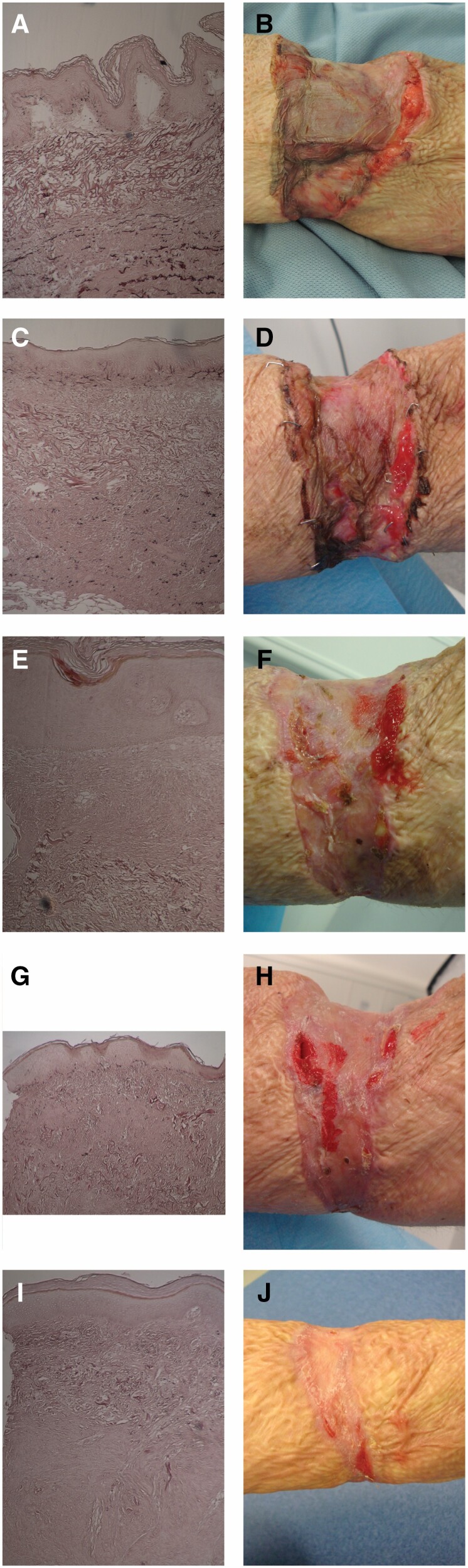
The gradual change in the histological and clinical appearance of the MatriDerm® reconstructed wound of one patient is shown in Figure 2A–J, demonstrating by month 2 MatriDerm® has been resorbed and replaced by a neodermis. A significant increase in the thickness of the dermis from the skin graft (SSG dermis) is seen between week 1 (average 0.03 mm) and month 12 (0.38 mm) (P < 0.05) (Figure 3A). However, the relative contribution of the SSG dermis to the total dermal thickness is small, 13% at week 1 and 23% at month 12 (Figure 3B). Mean MatriDerm® thickness is seen to slightly increase from 0.26 mm (week 1) to 0.30 mm (week 4, P = 0.427) (Figure 3A). Significant resorption of MatriDerm® and replacement by neodermis is observed around weeks 3 to 4, when it becomes difficult to identify histologically (Figure 2G). As the MatriDerm collagen disappears, it is replaced with host collagen forming the neodermis that increases thickness from month 2 onwards. The dermal layer changes from three distinct layers (SSG dermis, MatriDerm, neodermis) to two layers (Figure 3B). No residual MatriDerm® is seen in any of the specimens at 12 months (Figure 2I).
Figure 2.
MatriDerm® histology at ×10 magnification using Orcein staining with accompanying clinical appearance from a single patient (M05): A. Week 1 histology; B. Week 1 clinical appearance; C. Week 2 histology; D. Week 2 clinical appearance; E. Week 3 histology; F. Week 3 clinical appearance; G. Week 4 histology; H. Week 4 clinical appearance; I. Month 2 histology; and J. Month 2 clinical appearance. MatriDerm® layer integrity is preserved up to week 2 and shows distortion and fragmentation from week 3 onwards. At month 2, MatriDerm is entirely replaced by neodermis.
Figure 3.
Total dermal thickness with varying contributions of the skin graft dermis, MatriDerm® and neodermis in: A. Actual thickness (mm); B. Percentage of total dermal thickness (%).
Vascularization and Inflammvatory Cells
A high proportion of CD31 positive endothelial buds was observed in weeks 1 to 2 throughout the full thickness of MatriDerm®, with gradual vessel size increase (45.1 × 10–5 mm2, week 1 versus 79.3 × 10–5 mm2, week 4; P = 0.027) accompanied by no significant change in vessel density (529 vessels/mm2, week 1 vs 316 vessels/mm2, week 4; P = 0.161) (Figure 4). Inflammatory infiltrate of predominantly neutrophils with eosinophils, lymphocytes, and fibroblasts, was observed up to week 4, but seen only sporadically following this.
Figure 4.
Mean vessel size and density over the initial four weeks after its application.
Collagen and Elastin
All specimens showed increased collagen density in the lower reticular dermis. The collagen in the SSG dermis retains its random collagen arrangement at 12 months, however, the MatriDerm® layer and neodermis exhibit a nodular pattern of arrangement.
With TEM, individual MatriDerm® collagen fibers were found to be significantly thicker than native collagen fibers (mean diameter: 172 nm vs 53 nm, P < 0.001) (Figure 5A). As the scar matures MatriDerm® collagen fibers are seen to have fragmented and dispersed (Figure 5B). Macrophages are seen phagocytosing MatriDerm® derived collagen (Figure 5C).
Figure 5.
Transmission electron microscope images of MatriDerm® within the wound at direct magnification ×1900: A. MatriDerm® collagen bundles are larger in size compared to native collagen; B. Fragments of MatriDerm® are seen dispersed within the extracellular matrix during late stage of scar maturation; C. Macrophage observed engulfing bundles of MatriDerm® collagen.
Elastin fibers were present in 78% of specimens (7/9), however, the fibers seen were short and thickened with reduced density in all specimens. Mature elastic fibers could not be identified with the TEM.
Clinical Outcomes
There was no significant change in mean mVSS over the study period (preoperative, 7 ± 3 vs month 12, 7 ± 3; P = 0.310). The majority of patients reported improved outcomes following reconstruction with regards to their experience of itching, pain, appearance, dryness, pliability, sensation, and activities of daily living (Table 3). All patients who had difficulties in performing activities of daily living at screening reported improvement after surgery.
Table 3.
Patient self-assessment survey of scar improvement; significance = P < 0.05
| Parameter (Total n) | Improved (%) | No Change (%) | Worsened (%) | P |
|---|---|---|---|---|
| Itch (8) | 62.5 | 25.0 | 12.5 | 0.62 |
| Pain (9) | 55.6 | 22.2 | 22.2 | 1.00 |
| Appearance (9) | 66.7 | 11.1 | 11.1 | 0.35 |
| Dryness (9) | 77.8 | 22.2 | 0.0 | 0.06 |
| Softness (9) | 55.6 | 33.3 | 11.1 | 1.00 |
| Sensation (9) | 66.7 | 66.7 | 0.0 | 0.35 |
| ADL (9) | 110 | 0 | 0.0 | <0.01 |
DISCUSSION
The invention of the first dermal substitute, Integra® in the 1960s was a remarkable step change in wound management of acute major burns, reconstructive surgery, trauma, and chronic wounds. Improvements in surgical techniques have widened the scope of its application and avoided otherwise complex procedures in frail patients. Clear histologic understanding of how dermal substitutes are integrated onto the wound bed should improve day to day management of our patients and gives an insight of future dermal substitute developments. This study was designed to mirror the methodology of previously published work investigating the histological integration of Integra® into the wound bed.3 One-millimeter thick MatriDerm® was applied to nine postburn reconstruction sites, and one case of acute burn wound coverage. Sequential histological examination to evaluate the integration of MatriDerm® was performed. This was correlated with the Integra® previous histologic study (Table 4). At the time of study design single layer Integra® had not yet received a “Conformite Europeenne” certification and was therefore not available for inclusion. A comparative study may give an insight into the different vascularization patterns of the two matrices, which might help future dermal substitute characterization.
Table 4.
Phases of integration of dermal regeneration templates MatiDerm® and Integra® within the reconstructed burn scar
| Timeline | MatriDerm® | Integra® |
|---|---|---|
| Week 1 | Imbibition Inflammatory infiltrate of predominantly neutrophils. MatriDerm® remains of equal thickness in the wound |
Imbibition Matrix interstices fill with wound fluid and matrix swells Fibrin fosters adherence of matrix to wound |
| Week 2 | Lymphocytes, macrophages, occasional eosinophil, and fibroblasts are seen Rete ridges appear CD31 seen with patent vascular channels that traverse the MatriDerm® matrix |
Fibroblasts seen in the wound using Integra® collagen as a scaffold Endothelial cell migration seen from the end of the second week |
| Week 3 | Beginning of resorption and replacement by neodermis A sharp increase in vessel size with no increase in vessel density |
Fibroblasts seen settled along the interstices of the matrix and producing host collagen |
| Week 4 | Immature collagen bundles seen | Solid columns form that stain positive for CD31 Lumen formation is seen by the fourth week New vessel formation is occasionally seen by the end of the fourth week |
| Week 4 onwards | MatriDerm® continues to resorb and by 2 months is completely by neodermis | New collagen indistinguishable from the normal dermis Initially, neodermis is thicker than native dermis but thins over time Autograft becomes adherent to neodermis Rete ridges form |
Histological analysis of this study demonstrated similarities between MatriDerm® vascularization, and Integra® (Table 4).33 The cellular infiltration and vascularization of the MatriDerm® matrix seemed to be accelerated, however the matrix loses its thickness soon after it is applied to the wound bed, a feature that was not seen when examining Integra® matrix histologically. Vascularization followed the same pattern in both matrices with increased vascular lumen size and reduced vessel density as the neodermis matures.
By the end of the second week, fibroblasts begin to infiltrate the MatriDerm® matrix, together with some inflammatory cells, predominantly neutrophils. Macrophages were noted in the third week as new collagen was laid down by invading fibroblasts and the matrix collagen was resorbed. There was early evidence of angiogenic activity with a high density of patent endothelial channels traversing the entire MatriDerm® scaffold, identified with CD31 cell markers. By the third week, during MatriDerm® resorption, there was a significant increase in vessel size, with no increase in vessel density. Similarly, endothelial cells identified with CD31 were present in Integra® at the beginning of the second week, with lumen formation seen during the third week. When examining Integra®, neovascularization was only well established at the end of the fourth week, corresponding with increased graft take when skin grafting was delayed to the fourth week.33
A major difference observed was the early resorption of the MatriDerm®, starting by the fourth week and almost complete at 2 months. Integra® matrix traces were still histologically present at 2 years postsurgery.35 This could be explained by the cross-linkage of the Integra® matrix that maintains its structure, avoiding collapse of the scaffolding whilst cellular infiltration into its porous structure is progressing. MatriDerm® collagen matrix, on the other hand, is not cross-linked, a design to promote faster cell infiltration into the matrix. The lack of cross-linkage may also explain the early reduction of the matrix thickness seen at weeks 1 and 2, and the early resorption of the collagen by month 2. The inflammatory cellular response demonstrated in this study was not seen in the previous Integra® histological study and previous animal study.3,36 This could be explained by the need for inflammatory cell infiltration to clear the debris of the collapsing scaffold and prepare the wound for the deposition of new collagen by the native fibroblasts. Alternatively, the addition of Glycosaminoglycan GAGs to Integra® collagen matrix may explain the lack of the inflammatory response and the resistance to early resorption.
The histological findings of this study support the use of 1 mm thick MatriDerm® as a single-stage procedure. The senior author’s experience with 2 mm MatriDerm® with immediate application of skin graft was disappointing, with significant skin graft loss. The 2 mm thick matrix is too wide a barrier to the imbibition required to maintain skin graft viability. This can be mitigated by applying an occlusive dressing to prevent skin graft desiccation till vascularization is enough to maintain skin graft viability. The presence of wide pores on the MatriDerm® matrix and its ability to hold wound exudate enhances early graft nutrition and survival prior to early vascularization, allowing one-stage reconstruction.
In this study, with the use of electron microscopy, elastin fibers seen in MatriDerm® were different to normal elastic fiber architecture, and were short, thickened, and reduced in density in all specimens. A recent study compared elastin fiber density in biopsies taken from patients who had reconstruction with MatriDerm®, full-thickness skin graft (FTG), SSG, Alloderm®, and normal skin. Elastin contents in MatriDerm® samples were almost half those of normal skin or FTG but more than SSG or Alloderm®.37 These results and the histological findings in our current study, question the effectiveness of the elastic fibers in the MatriDerm® matrix.
Only one patient experienced worsening of their symptoms of itch, pain, appearance, and softness. Without a comparison of scar reconstructed with SSG only it is difficult to conclude whether the patient-reported improvement is due to MatriDerm® or the nature of scar release. We found that reconstruction of a chronic burn scar with MatriDerm® did not alter mVSS at 12 months, in keeping with literature. In an intraindividual comparison of MatriDerm® reconstruction to standard skin grafting reconstruction, there is a documented early improvement in VSS at 3 to 4 months after reconstruction.38,39 However, this is not seen at 12 months, in keeping with our study.40
Study Limitations
There are a number of limitations to this study. Histological analysis of revascularisation is a two-dimensional assessment of a complex three-dimensional process, and provides limited data in comparison to other techniques such as corrosion casting and the use of high-resolution episcopic microscopy.7,41,42 We did not use scar assessment tools such as the Cutometer® (Courage + Khazaka, Germany) which give an objective assessment of the clinical outcome of MatriDerm®.43,44 Instead, we used patient-reported outcomes and the Vancouver Scar Scale (VSS), the same clinical assessment methodology as for the previous study of Integra®.3 Scar assessment is complex, with currently no defined validated “gold standard”.45 Outcomes that are important to patients encompass more than scar characteristics, making patient-reported outcomes the most clinically relevant.46 The mVSS, despite questionable reliability, is a straightforward and low-cost tool that is widely used, making its inclusion useful for comparison amongst the literature.
Conclusion
This study demonstrates the stages through which MatriDerm® integrates within the reconstructed burn scar. The early angiogenic activity observed in this study gives evidence to support the one-stage reconstruction model of MatriDerm®. It was found to have resorbed from the tissue bed and was absent from the reconstructed site completely by the end of the second month. Future biomechanical and immunological studies can investigate the mechanism of neo-dermal synthesis and help characterize the ideal dermal substitute matrix.
Contributor Information
Kathryn Dickson, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK.
Kwang Chear Lee, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Scar Free Foundation Centre for Conflict Wound Research, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Institute of Inflammation and Aging, University of Birmingham, Birmingham B15 2TT, UK.
Abdulrazak Abdulsalam, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Scar Free Foundation Centre for Conflict Wound Research, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK.
Ezekwe Amirize, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Scar Free Foundation Centre for Conflict Wound Research, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK.
Hadyn K N Kankam, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Institute of Inflammation and Aging, University of Birmingham, Birmingham B15 2TT, UK.
Britt ter Horst, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Scar Free Foundation Centre for Conflict Wound Research, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK.
Fay Gardiner, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Scar Free Foundation Centre for Conflict Wound Research, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK.
Amy Bamford, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Scar Free Foundation Centre for Conflict Wound Research, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK.
Rahul K Hejmadi, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK.
Naiem Moiemen, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Scar Free Foundation Centre for Conflict Wound Research, University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Mindelsohn Way, Edgbaston, Birmingham B15 2WB, UK; Institute of Inflammation and Aging, University of Birmingham, Birmingham B15 2TT, UK.
REFERENCES
- 1. Roth GA, Abate D, Abate KHet al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stern R, McPherson M, Longaker MT.. Histologic study of artificial skin used in the treatment of full-thickness thermal injury. J Burn Care Rehabil 1990;11:7–13. [DOI] [PubMed] [Google Scholar]
- 3. Moiemen NS, Staiano JJ, Ojeh NO, Thway Y, Frame JD.. Reconstructive surgery with a dermal regeneration template: clinical and histologic study. Plast Reconstr Surg 2001;108:93–103. [DOI] [PubMed] [Google Scholar]
- 4. Dantzer E, Braye FM.. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg 2001;54:659–64. [DOI] [PubMed] [Google Scholar]
- 5. Petersen W, Rahmanian-Schwarz A, Werner JOet al. The use of collagen-based matrices in the treatment of full-thickness wounds. Burns 2016;42:1257–64. [DOI] [PubMed] [Google Scholar]
- 6. Phillips GSA, Nizamoglu M, Wakure A, Barnes D, El-Muttardi N, Dziewulski P.. The use of dermal regeneration templates for primary burns surgery in a UK regional burns centre. Ann Burns Fire Disasters 2020;33:245–52; available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7680195/. [PMC free article] [PubMed] [Google Scholar]
- 7. Wiedner M, Tinhofer IE, Kamolz LPet al. Simultaneous dermal matrix and autologous split-thickness skin graft transplantation in a porcine wound model: a three-dimensional histological analysis of revascularization. Wound Repair Regen 2014;22:749–54. [DOI] [PubMed] [Google Scholar]
- 8. Philandrianos C, Andrac-Meyer L, Mordon Set al. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012;38:820–9. [DOI] [PubMed] [Google Scholar]
- 9. Harrison CA, MacNeil S.. The mechanism of skin graft contraction: an update on current research and potential future therapies. Burns 2008 1;34:153–63. [DOI] [PubMed] [Google Scholar]
- 10. Maitz J, Wang Y, Fathi Aet al. The effects of cross-linking a collagen-elastin dermal template on scaffold bio-stability and degradation. J Tissue Eng Regen Med 2020;14(9):1189–200. [DOI] [PubMed] [Google Scholar]
- 11. Auger FA, Berthod F, Moulin V, Pouliot R, Germain L.. Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol Appl Biochem 2004;39:263–75. [DOI] [PubMed] [Google Scholar]
- 12. Calcagni M, Althaus MK, Knapik ADet al. In vivo visualization of the origination of skin graft vasculature in a wild-type/GFP crossover model. Microvasc Res 2011;82:237–45. [DOI] [PubMed] [Google Scholar]
- 13. Ring A, Langer S, Homann HHet al. Analysis of neovascularization of PEGT/PBT-copolymer dermis substitutes in balb/c-mice. Burns 2006;32:35–41. [DOI] [PubMed] [Google Scholar]
- 14. Yannas IV, Lee E, Orgill DPet al. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A 1989;86:933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burke JF, Yannas IV, Quinby WC Jr, Bondoc CC, Jung WK.. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981;194:413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heimbach DA, Luterman AR, Burke JOet al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg 1988;208:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki S, Matsuda K, Isshiki N, Tamada Y, Ikada Y.. Experimental study of a newly developed bilayer artificial skin. Biomaterials 1990;11:356–60. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki S, Matsuda K, Maruguchi Tet al. Further applications of “bilayer artificial skin”. Br J Plast Surg 1995;4:222–9. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki S, Isshiki N, Taira Tet al. The clinical use of a new, refreezed and dried, bilayer artificial skin. Jpn J Plast Reconstr Surg 1993;36:479–87. [Google Scholar]
- 20. Suzuki S, Kawai K, Ashoori F, Morimoto N, Nishimura Y, Ikada Y.. Long-term follow-up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br J Plast Surg 2000;53:659–66. [DOI] [PubMed] [Google Scholar]
- 21. Greenwood JE, Li A, Dearman BLet al. Evaluation of NovoSorb novel biodegradable polymer for the generation of a dermal matrix part 1: in-vitro studies. Wound Pract Res 2010;18:14–22; available from https://search.informit.org/doi/abs/10.3316/INFORMIT.984461070794232. [Google Scholar]
- 22. Greenwood JE, Li A, Dearman BLet al. Evaluation of NovoSorb novel biodegradable polymer for the generation of a dermal matrix part 2: in-vivo studies. Wound Pract Res: 2010;18(1); 24, 26, 28, 30, 32–34. available from https://search.informit.org/doi/abs/10.3316/informit.984479703765490. [Google Scholar]
- 23. Greenwood JE, Dearman BL.. Split skin graft application over an integrating, biodegradable temporizing polymer matrix: immediate and delayed. J Burn Care Res J 2012;33:7–19. [DOI] [PubMed] [Google Scholar]
- 24. Greenwood JE, Schmitt BJ, Wagstaff MJ.. Experience with a synthetic bilayer biodegradable temporising matrix in significant burn injury. Burns Open 2018;2:17–34. [Google Scholar]
- 25. Kang SW, Park JK, Shon HC, Choi E-S, Kim D-S, Min K-T.. Skin graft using MatriDerm® for plantar defects after excision of skin cancer. Cancer Manag Res 2019;11:2947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Min JH, Yun IS, Lew DH, Roh TS, Lee WJ.. The use of MatriDerm and autologous skin graft in the treatment of full thickness skin defects. Arch Plast Surg 2014;41:330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haslik W, Kamolz L-P, Nathschläger G, Andel H, Meissl G, Frey M.. First experiences with the collagen-elastin matrix Matriderm® as a dermal substitute in severe burn injuries of the hand. Burns 2007;33:364–8. [DOI] [PubMed] [Google Scholar]
- 28. Ryssel H, Germann G, Kloeters O, Gazyakan E, Radu CA.. Dermal substitution with Matriderm® in burns on the dorsum of the hand. Burns 2010;36:1248–53. [DOI] [PubMed] [Google Scholar]
- 29. Atherton DD, Tang R, Jones I, Jawad M.. Early excision and application of matriderm with simultaneous autologous skin grafting in facial burns. Plast Reconstr Surg 2010;125:60e–1e. [DOI] [PubMed] [Google Scholar]
- 30. Heckmann A, Radtke C, Rennekampff HO, Jokuszies A, Weyand B, Vogt PM.. One-stage defect closure of deperiosted bone and exposed tendons with MATRIDERM® and skin transplantation. Possibilities and limitations. Unfallchirurg 2012;115:1092–8. [DOI] [PubMed] [Google Scholar]
- 31. Bloemen MC, van Leeuwen MC, van Vucht NEet al. Dermal substitution in acute burns and reconstructive surgery: a 12-year follow-up. Plast Reconstr Surg 2010;125:1450–9. [DOI] [PubMed] [Google Scholar]
- 32. Van Zuijlen PP, Lamme EN, Van Galen MJ, van Marle J, Kreis RW, Middelkoop E.. Long-term results of a clinical trial on dermal substitution: a light microscopy and Fourier analysis based evaluation. Burns 2002;28:151–60. [DOI] [PubMed] [Google Scholar]
- 33. Moiemen NS, Vlachou E, Staiano JJ, Thawy Y, Frame JD.. Reconstructive surgery with Integra dermal regeneration template: histologic study, clinical evaluation, and current practice. Plast Reconstr Surg 2006;117(7 Suppl):160S–74S. [DOI] [PubMed] [Google Scholar]
- 34. Moiemen NS, Lee KC.. The role of alternative wound substitutes in major burn wounds and burn scar resurfacing. In: Herndon D. N. (ed.) Total burn care. 5th ed. Elsevier; Edinburgh. 2018. pp. 633–39.e1. [Google Scholar]
- 35. Moiemen N, Yarrow J, Hodgson Eet al. Long-term clinical and histological analysis of Integra dermal regeneration template. Plast Reconstr Surg 2011;127:1149–54. [DOI] [PubMed] [Google Scholar]
- 36. Böttcher-Haberzeth S, Biedermann T, Schiestl Cet al. Matriderm® 1 mm versus Integra® Single Layer 1.3 mm for one-step closure of full thickness skin defects: a comparative experimental study in rats. Pediatr Surg Int 2012;28:171–7. [DOI] [PubMed] [Google Scholar]
- 37. Lee MY, Kim H, Kwak ISet al. Immunohistochemical analysis of postburn scars following treatment using dermal substitutes. Anal Cell Pathol Amst 2022;2022:3686863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Zuijlen PP, van Trier AJ, Vloemans JF, Groenevelt F, Kreis RW, Middelkoop E.. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one-stage grafting model. Plast Reconstr Surg 2000;106:615–23. [DOI] [PubMed] [Google Scholar]
- 39. Ryssel H, Gazyakan E, Germann G, Ohlbauer M.. The use of MatriDerm® in early excision and simultaneous autologous skin grafting in burns—A pilot study. Burns 2008;34:93–7. [DOI] [PubMed] [Google Scholar]
- 40. van Zuijlen PP, Vloemans JF, van Trier AJet al. Dermal substitution in acute burns and reconstructive surgery: a subjective and objective long-term follow-up. Plast Reconstr Surg 2001;108:1938–46. [DOI] [PubMed] [Google Scholar]
- 41. Geyer SH, Tinhofer IE, Lumenta DBet al. High-resolution episcopic microscopy (HREM): a useful technique for research in wound care. Ann Anat 2015;197:3–10. [DOI] [PubMed] [Google Scholar]
- 42. Conrad M. Abnormalities of the digital vasculature as related to ulceration and gangrene. Circulation 1968;38:568–81. [DOI] [PubMed] [Google Scholar]
- 43. Lee KC, Bamford A, Gardiner Fet al. Burns objective scar scale (BOSS). Burns 2020;46:110–20. [DOI] [PubMed] [Google Scholar]
- 44. Lee KC, Dretzke J, Grover L, Logan A, Moiemen N.. A systematic review of objective burn scar measurements. Burns Trauma 2016;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price K, Moiemen N, Nice L, Mathers J.. Patient experience of scar assessment and the use of scar assessment tools during burns rehabilitation: a qualitative study. Burns Trauma 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones LL, Calvert M, Moiemen Net al. ; PEGASUS team. Outcomes important to burns patients during scar management and how they compare to the concepts captured in burn-specific patient reported outcome measures. Burns 2017;43:1682–92. [DOI] [PubMed] [Google Scholar]