Abstract
We developed a new simple assay for the quantitation of the activities of drugs against intracellular Legionella pneumophila. The cells of a murine macrophage-like cell line (J774.1 cells) allowed the intracellular growth and replication of the bacteria, which ultimately resulted in cell death. The infected J774.1 cell monolayers in 96-well microplates were first treated with antibiotics and were further cultured for 72 h. The number of viable J774.1 cells in each well was quantified by a colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and an enzyme-linked immunosorbent assay reader. The number of growing bacteria in each well was also determined by counting the numbers of CFU on buffered charcoal yeast extract-α agar plates. Viable J774.1 cell counts, determined by the colorimetric assay, were inversely proportional to the number of intracellular replicating bacteria. The minimum extracellular concentrations (MIECs) of 24 antibiotics causing inhibition of intracellular growth of L. pneumophila were determined by the colorimetric assay system. The MIECs of beta-lactams and aminoglycosides were markedly higher than the MICs in buffered yeast extract-α broth. The MIECs of macrolides, fluoroquinolones, rifampin, and minocycline were similar to the respective MICs. According to their intracellular activities, clarithromycin and sparfloxacin were the most potent among the macrolides or fluoroquinolones tested in this study. Our results indicated that the MTT assay system allows comparative and quantitative evaluations of the intracellular activities of antibiotics and efficient processing of a large number of samples.
Legionella pneumophila is a facultative intracellular pathogen known to cause both community-acquired pneumonia (9, 22) and nosocomial pneumonia (23) of variable severity, including fatal infections (1). The pathogenesis of Legionella infections is due to the survival and replication of the microorganism within phagocytic cells (20) as well as to the cytotoxic by-products resulting from the intracellular multiplication of the bacteria (3). These facts, together with clinical observations (4), suggest that effective treatment of Legionella infections requires the use of drugs active against both intracellular and extracellular bacteria. Furthermore, laboratory evaluation of the activities of drugs against Legionella spp. should include determination of their intracellular activities against the bacteria (4), in addition to determination of the MICs. Unfortunately, in contrast to MIC determinations, most assays for the activities of drugs against intraphagocytic Legionella are laborious and expensive and are not usually quantitative or suitable for use in comparative studies of several antibiotics. As an indicator of the intracellular activity of a drug, the minimum extracellular concentration (MIEC) inhibiting intracellular multiplication has been described previously (31), but the technique used to determine intracellular bacterial growth involves counting of the numbers of CFU and is time-consuming.
In this report we describe a simple quantitative method for measuring the intracellular activities of drugs against L. pneumophila by a colorimetric assay of the remaining J774.1 cells infected with L. pneumophila.
MATERIALS AND METHODS
Bacterial strains.
L. pneumophila serogroup 1 (SG1) 80-045 was isolated from the first patient in Japan identified to have Legionnaires’ disease (30). The other strains used in this study were isolated in our laboratory from the bronchial wash specimens of different patients with pneumonia. These strains were kept in sterile skim milk supplemented with 1% (wt/vol) sodium glutamate at −80°C. The clinical isolates were identified as L. pneumophila SG1 by their biochemical profile, the results of slide agglutination tests with polyclonal rabbit antibodies (Denka Seiken, Osaka, Japan), and the DNA hybridization technique with photobiotin-labeled bacterial DNA (8). The bacteria were first cultured on buffered charcoal yeast extract-α (BCYE-α; Difco Laboratories, Detroit, Mich.) plates at 35°C for 72 h and were then suspended at 1.5 × 108 CFU/ml in 5 ml of buffered yeast extract supplemented with α-ketoglutarate (BYE-α) broth (5) and incubated at 35°C with vigorous shaking for 12 h. The bacteria were harvested by centrifugation at 3,000 rpm for 20 min and were resuspended in sterile pyrogen-free distilled water; their concentration was adjusted to approximately 2.9 × 108 CFU/ml.
Reagents and antimicrobial agents.
Yellow tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), was purchased from Research Organics (Cleveland, Ohio). Levofloxacin (Dai-ichi Pharmaceutical Co., Tokyo, Japan), ciprofloxacin (Bayer Pharmaceuticals Japan, Tokyo, Japan), sparfloxacin (Dainippon Pharmaceuticals, Osaka, Japan), erythromycin base (Dainippon Pharmaceuticals), clarithromycin (Taisho Pharmaceutical Co., Tokyo, Japan); roxithromycin (Nippon Roussel Co., Tokyo, Japan), azithromycin (Pfizer Pharmaceuticals Japan, Tokyo, Japan), and ceftizoxime (Fujisawa Pharmaceuticals, Osaka, Japan) were donated by the respective commercial suppliers. Other antimicrobial agents were also donated by respective commercial suppliers.
MICs.
The MICs of the antimicrobial agents were determined by the microdilution method (25) in BYE-α broth. For this purpose, the bacteria were inoculated with a MIC-2000 inoculator (Dynatech Laboratories Inc., Alexandria, Va.) into 100 μl of BYE-α broth containing serial dilutions of the antibiotics in the wells of a microtiter plate (inoculum size, 105 CFU/well). The MIC was defined as the minimum concentration of the drug that inhibited visible bacterial growth after culture at 35°C for 2 days.
Cell line.
The cells of a murine macrophage-like cell line (J774.1 cells) were suspended in a mixture of 10% dimethyl sulfoxide and 90% fetal calf serum (FCS; Whittaker, Walkersville, Md.) and stocked in liquid nitrogen. An aliquot of the J774.1 cell suspension was thawed and cultured in a medium consisting of RPMI 1640 (pH 7.2; Gibco Laboratories, Grand Island, N.Y.), 10 mM HEPES (Dojin Chemicals Co. Ltd., Kumamoto, Japan), and 10% heat-inactivated FCS (Whittaker) in a 75-cm2 culture flask (Falcon 3084; Nippon Becton Dickinson Co., Tokyo, Japan) in humidified air with 5% CO2 at 37°C. The cells were harvested at the logarithmic growth phase and were suspended in RPMI 1640 medium at 2 × 105 cells/ml. In the next step, 100 μl of the cell suspension was allowed to adhere to a 96-well flat-bottom tissue culture plate (Falcon 3072; Becton Dickinson, Lincoln Park, N.J.) in humidified air with 5% CO2 at 37°C for 12 h.
Infection of J774.1 cells.
Cultured cell monolayers in 96-well microplates were infected with various concentrations of bacteria suspended in RPMI 1640 medium containing 5% FCS and were incubated for 12 h. In the next step, the extracellular fluid and bacteria were removed by decanting the contents of the microplate containing Legionella-infected J774.1 cell monolayers, and then antimicrobial agents were added to the wells and the plate was incubated for an additional 72 h. Finally, the infected cell monolayer and the supernatant in each well were harvested in 9.8 ml of sterile distilled water, and the mixture was then vortexed for 20 s to lyse the cells completely and to prevent an antibiotic carryover effect. These bacterial suspensions were appropriately diluted, and aliquots (50 μl) of the dilutions were inoculated onto BCYE-α agar. The number of viable Legionella in each well was determined by counting the CFU after incubation at 35°C for 3 days.
DFA staining.
After culture of J774.1 cells on sterile chamber slides (Nalge Nunc International, Naperville, Ill.) in 5% CO2–air at 37°C, they were infected with bacteria for 12 h as described above. The infected cells were cultured in fresh RPMI 1640 medium without any antibiotics for an additional 0, 36, or 72 h. The upper structure of the chamber slide was removed according to the instructions provided by the manufacturer, and the cell monolayers were fixed in ethanol for at least 5 min to allow the antibody to penetrate the macrophage membrane (26). The monolayers were then stained with fluorescein-conjugated anti-L. pneumophila rabbit polyclonal antibody (MerDx, Scotch Plains, N.J.) for 30 min in humidified air at 35°C, washed sufficiently with 10 mM phosphate-buffered saline (pH 7.4), and observed with a confocal laser scanning microscope (Fluoview; Olympus Co. Ltd., Tokyo, Japan).
Colorimetric assay of remaining J774.1 cells.
After incubation of the J774.1 cells with the bacteria for 72 h, the remaining J774.1 macrophages were quantified by the rapid colorimetric assay by the tetrazolium dye procedure (2, 27), with minor modifications. The contents of the plate were decanted to remove the supernatants, the extracellular bacteria, and the lysed macrophages. Then, 100 μl of RPMI 1640 medium containing 5% FCS and 0.5 μg of MTT per ml was added to each well of the plate. After incubation at 37°C in humidified air with 5% CO2 for 90 min, the supernatants were removed and 100 μl of isopropyl alcohol containing 0.4 N HCl and 0.5% sodium dodecyl sulfate was added to each well. After vigorous shaking of the microplates to lyse the cell monolayers, the optical density of each well was measured at 550 nm with an automatic plate reader.
Viable Legionella cells also can cleave MTT, and Gebran et al. (12) used the MTT assay to quantify bacterial growth itself. In a series of preliminary experiments, we examined the effect of cleavage of MTT by bacteria in this assay. When bacteria were present in this assay system at less than 107 CFU/well, the bacteria did not affect the absorbance at 550 nm. The number of bacteria associated with cell monolayers decreased to less than 2.8 × 104 CFU/ml after the contents of the microplate were decanted. Thus, cleavage of MTT by bacteria did not influence this assay. To determine the precise cytopathic effect of the bacteria, we examined cell death in two groups of control cell monolayers. The first group consisted of uninfected cell monolayers (control cells with no cytopathic effect), while the second group consisted of cell monolayers treated with 0.1% saponin (control cells with the maximum cytopathic effect). The average cell death of these two controls was defined as 50% cell death, and the corresponding infective dose resulting in 50% cell death (CPED50) was determined.
Statistical analysis.
Data were expressed as means ± standard deviations (SDs). Differences between groups were tested for statistical significance by analysis of variance and Schaffe’s test. A P value of less than 0.01 denoted the presence of a statistically significant difference.
RESULTS
Intracellular growth of Legionella in J774.1 cells.
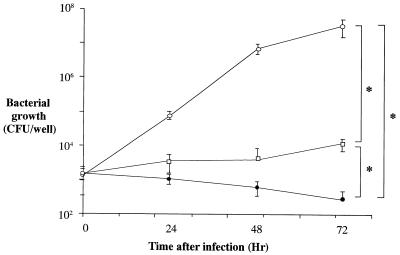
A clinical isolate of L. pneumophila, strain 80-045 SG1, was cultured in 10% FCS-supplemented RPMI 1640 medium alone, medium with J774.1 cell lysates (the contents corresponding to 1 × 106 cells/ml), or medium with live J774.1 cells (5 × 105 cells/ml). Bacterial growth was enhanced in the medium containing live J774.1 cells, while the number of viable bacteria progressively decreased with time when the cells were cultured in medium only (Fig. 1). The presence of J774.1 cell lysates maintained the number of viable bacteria at the baseline level (Fig. 1). The other four clinical isolates (isolates 90-001, 90-002, 91-003, and 97-001) also grew in cultures containing viable J774.1 cells, while FCS-supplemented RPMI 1640 medium only did not support the growth of these strains (data not shown).
FIG. 1.
Multiplication of L. pneumophila in J774.1 cells. L. pneumophila SG1 80-045 was cultured in medium alone (RPMI 1640 plus 5% FCS), the same medium and a lysate of J774.1 cells, or the same medium and live J774.1 cells. The number of viable bacteria in each well was determined by counting the number of CFU on BCYE-α agar after culture at 35°C for 3 days. Each datum point represents the mean ± SD for five wells. ∗, significant differences (P < 0.01) at 72 h of culture tested by Schaffe’s test. Open circles, medium with live J774.1 cells; open squares, medium with lysate of J774.1 cells; closed circles, medium alone.
Cytopathic effect of L. pneumophila on J774.1 macrophage cells.
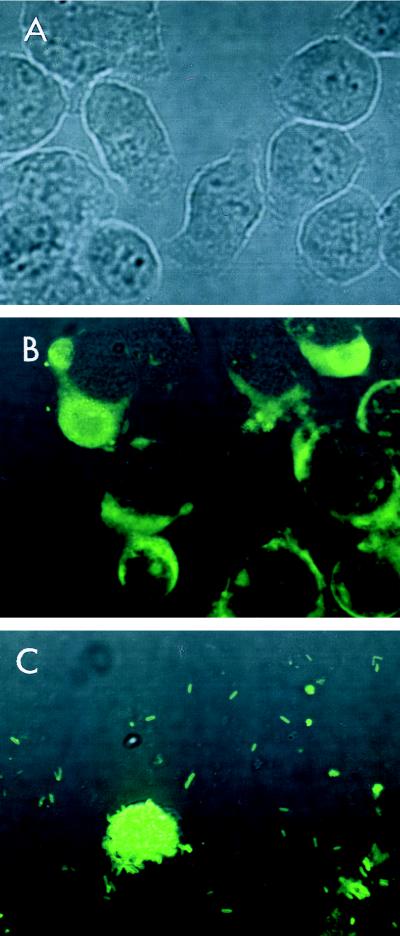
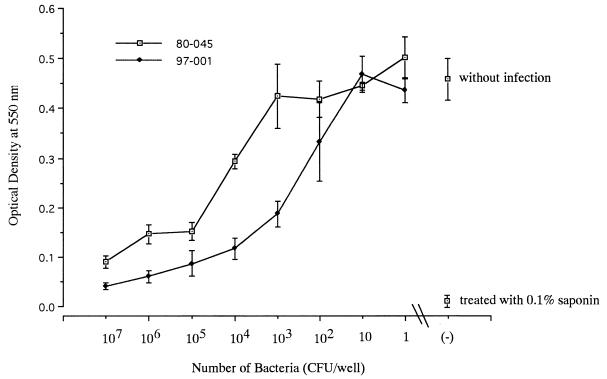
To identify the exact site of bacterial growth in this culture system, the infected J774.1 cell monolayers were stained with DFA for L. pneumophila SG1 (Fig. 2). Multiplying L. pneumophila was found within the host cells after 36 h of culture (Fig. 2B). The peak growth of Legionella occurred after 72 h of incubation and resulted in the destruction of the host cells (Fig. 2C). The cytopathic effect of L. pneumophila against J774.1 cells was dose dependent (Fig. 3). The CPED50 of strain 80-045 was approximately 4 × 104 CFU/well, while other strains showed much stronger cytopathic effects (data not shown).
FIG. 2.
DFA staining of J774.1 cell monolayers. The cell monolayers were infected with L. pneumophila SG1 80-045 at 1.6 × 106 CFU/well and were cultured for another 72 h. At the indicated time intervals, the culture medium was discarded and the cell monolayers were stained with fluorescein-conjugated anti-L. pneumophila SG1 antibody. The stained samples were examined under a confocal laser scanning microscope (original magnification, ×600). (A) Control at the time of infection of J774.1 cell monolayers with L. pneumophila; (B) 36 h after infection; note the presence of replicating bacteria within the cytoplasms of the J774.1 cells; (C) 72 h after infection. Note that the majority of the J774.1 cells are destroyed and that only a few remaining cells are present. Note also that these cells are filled with bacteria.
FIG. 3.
Cytopathic effect of L. pneumophila SG1 against J774.1 cell monolayers. J774.1 cells (2 × 104/well) were cultured in a 96-well microplate for 12 h. The cell monolayers were then infected with clinical isolates of L. pneumophila SG1 at various inoculum sizes. The microplates were cultured at 37°C for 12 h, and the contents of the plates were then decanted to remove extracellular medium and bacteria and new culture medium (200 μl) without any antibiotics was added to each well. The numbers of viable J774.1 cells after 72 h of incubation were quantified by the MTT assay and were expressed as the optical density at 550 nm. Representative results for two strains are shown. Each point represents the mean ± SD for three wells.
Comparison of assay of CFU counts and colorimetric MTT assay for evaluation of intracellular activity against L. pneumophila.
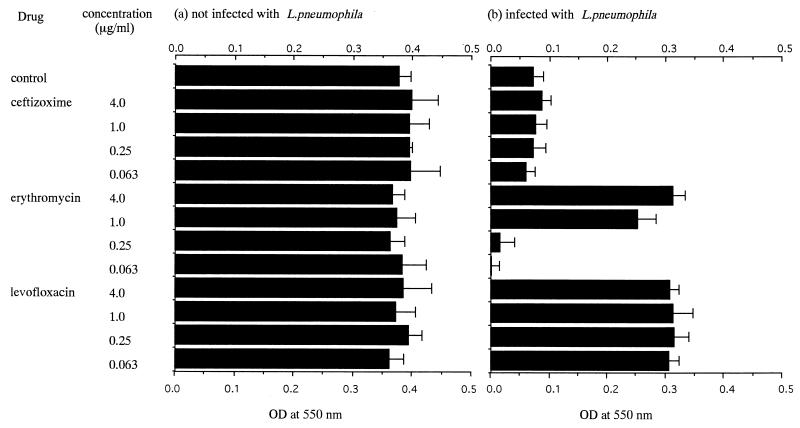
J774.1 macrophage cells were cultured in 96-well microplates and were infected with L. pneumophila 80-045 SG1 at a concentration of 8 × 105 CFU/well (20 times the CPED50). In the next step, a serial fourfold concentration of erythromycin, levofloxacin, or ceftizoxime was added to Legionella-infected macrophage monolayers. After culture for 72 h, the number of viable bacteria in each well was determined (Fig. 4). Ceftizoxime at concentrations of ≤4 μg/ml did not inhibit the intracellular growth of the bacteria, and the viable bacterial count was almost similar to that for the antibiotic-free control. Levofloxacin (≥0.063 μg/ml) was effective in inhibiting bacterial growth, and the CFU counts in all wells containing levofloxacin were lower than the detection limit of this assay. The intracellular activity of erythromycin against the bacteria was concentration dependent, and erythromycin (≥4 μg/ml) significantly inhibited bacterial growth. After 72 h of incubation the remaining J774.1 cells were also evaluated by the MTT colorimetric assay under the same conditions used for the CFU count assay, and the results were expressed as optical densities at 550 nm (Fig. 5). J774.1 cells treated with levofloxacin (at a concentration of ≥0.063 μg/ml) survived well, while treatment with ceftizoxime failed to prevent the death of J774.1 cells. Erythromycin increased the survival of J774.1 macrophage cells in a dose-dependent manner. As a control for drug-induced cytotoxicity, these three drugs were also added to macrophage monolayers without bacterial infection. No direct cytotoxicity of these drugs was observed in this study (Fig. 5). The optical densities at 550 nm for MTT-treated J774.1 cells in each experimental group correlated inversely with the number of viable bacteria, indicating that drugs that are effective against the intracellular growth of Legionella also inhibited the cytopathic effect of intracellularly replicating bacteria.
FIG. 4.
Inhibition of intracellular multiplication of L. pneumophila by antibiotics. Live J774.1 cells were infected with L. pneumophila SG1 80-045 at 7.8 × 105 CFU/well (20 times the CPED50) for 12 h. After the extracellular bacteria were washed out, antibiotics were added to the wells at the indicated concentrations. Bacterial growth after 72 h of incubation was quantified by counting the numbers of CFU on BCYE-α agar plates. Each column represents the mean ± SD for triplicate wells. ∗, significantly different from the control by Schaffe’s test (P < 0.01).
FIG. 5.
Inhibition by antibiotics of cytopathic effect of intracellular L. pneumophila. (a) Uninfected live J774.1 cells were treated with antibiotics. (b) Live J774.1 cells were infected with L. pneumophila SG1 80-045 at 8 × 105 CFU/well (20 times the CPED50) for 12 h. After the extracellular bacteria were washed out, antibiotics were added to the wells at the indicated concentrations. After 72 h of culture the remaining J774.1 cells were stained with MTT and the optical density (OD) of the purple formazan product was quantified at 550 nm. Each column represents the mean ± SD for quadruplicate wells.
Determination of intracellular activity of antibiotics against L. pneumophila.
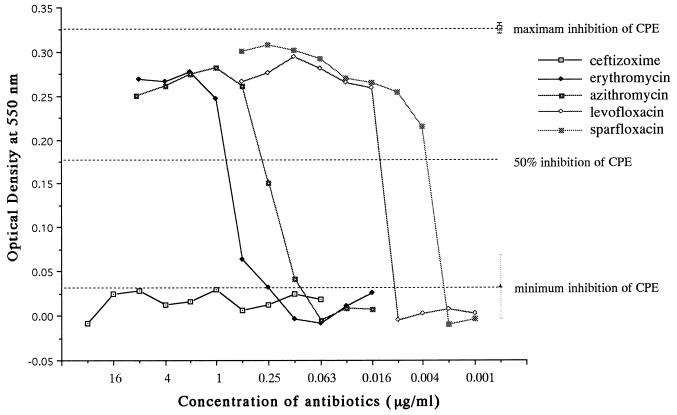
The intracellular activity of each antibiotic was evaluated by its ability to inhibit the bacterial cytopathic effect as described above. J774.1 macrophage cell monolayers infected with clinical isolates of L. pneumophila SG1 (at 20 times the CPED50) were treated with twofold dilutions of various antibiotics. On the basis of the results of the experiment described above, the maximum inhibition of the cytopathic effect bacterial was obtained when J774.1 cells were treated with levofloxacin at a concentration of 4 μg/ml, while the minimum inhibition occurred in the absence of antibiotics. Treatment of Legionella-infected J774.1 cells with twofold dilutions of the antibiotics allowed measurement of the dose-response curve of each drug against the cytopathic effect of the bacteria and determination of the 50% inhibitory concentration of each drug for the bacterial cytopathic effect (Fig. 6). As described above, the MIEC resulting in >50% inhibition of the bacterial cytopathic effect was defined as the MIEC of each drug, with the MIEC representing a quantitative indicator of the intracellular activity of the antibiotic. The MIEC was used to compare the potencies of the various antibiotics. We compared the MICs and the MIECs of 24 different drugs for strain 80-045 (Table 1). The MIECs of the beta-lactams and aminoglycosides were markedly higher than the MICs, while the MIECs of the macrolides, the fluoroquinolones, minocycline, rifampin, and clindamycin were almost similar to the corresponding MICs. Table 2 summarizes the MICs and MIECs of the macrolides and fluoroquinolones for strain 80-045 and four other clinical isolates. These results indicate that clarithromycin was the most potent among the macrolides tested in this study, with an MIEC of approximately 0.031 μg/ml. All three fluoroquinolones tested had good intracellular activity against five clinical isolates of L. pneumophila SG1, and sparfloxacin was the most potent drug among the fluoroquinolones.
FIG. 6.
Dose-response curves demonstrating the effect of different concentrations of various antimicrobial agents on the cytopathic effect (CPE) of L. pneumophila 80-045 SG1. J774.1 cells (2 × 104/well) were cultured in 96-well microplates for 12 h. The cell monolayers were infected with L. pneumophila 80-045 SG1 (at 8 × 105 CFU/well; 20 times the CPED50) for 12 h. The contents of the microplates were decanted to remove extracellular medium and bacteria after culture at 37°C for 12 h, and then new culture medium (200 μl) containing twofold dilution series of different antibiotics was added to each well. The viability of the J774.1 cells was quantified 72 h later by the MTT assay and was expressed as the optical density at 550 nm. Maximum inhibition of the cytopathic effect was obtained when J774.1 cells were treated with levofloxacin (4 μg/ml); minimum inhibition of the cytopathic effect was recorded when no antibiotic was used. The MIEC resulting in >50% inhibition of the bacterial cytopathic effect was defined as the MIEC of each drug, representing an indicator of the intracellular activity of the antibiotic.
TABLE 1.
Extracellular and intracellular activities of various drugs against L. pneumophila 80-045 SG1
| Drug | MIC (μg/ml)a | MIEC (μg/ml)b |
|---|---|---|
| Ampicillin | 2 | >64 |
| Methicillin | 4 | 64 |
| Piperacillin | 4 | 64 |
| Cefazolin | 64 | >64 |
| Cefotiam | 2 | >64 |
| Ceftizoxime | 0.125 | >64 |
| Cefotaxime | 0.25 | >64 |
| Ceftazidime | 0.125 | >64 |
| Imipenem | 0.125 | >64 |
| Panipenem | 0.125 | >64 |
| Gentamicin | 2 | 32 |
| Tobramycin | 4 | 64 |
| Erythromycin | 0.125 | 1 |
| Josamycin | 0.125 | 0.063 |
| Rokitamycin | 0.063 | 0.031 |
| Clarithromycin | 0.031 | 0.031 |
| Roxithromycin | 0.063 | 0.125 |
| Azithromycin | 0.063 | 0.5 |
| Levofloxacin | 0.063 | 0.016 |
| Ciprofloxacin | 0.031 | 0.063 |
| Sparfloxacin | 0.004 | 0.004 |
| Rifampin | 0.002 | 0.004 |
| Minocycline | 2 | 0.25 |
| Clindamycin | 16 | 8 |
MICs were determined by the microdilution method in BYE-α broth, and the inoculum size was 106 CFU/ml.
MIECs represent the intracellular antimicrobial activities of antibiotics and were determined by the methods described in the text. Triplicate examinations showed that each drug had identical MIECs.
TABLE 2.
Drug activities against five clinical isolates of L. pneumophila SG1a
| Drug | MIC range (μg/ml)b | MIEC range (μg/ml)c |
|---|---|---|
| Ceftizoxime | 0.125–0.5 | >64 |
| Erythromycin | 0.063–0.5 | 1.0–4.0 |
| Azithromycin | 0.031–0.5 | 0.5–4.0 |
| Roxithromycin | 0.125–0.5 | 0.25–0.5 |
| Clarithromycin | 0.031–0.063 | 0.031–0.063 |
| Levofloxacin | 0.031–0.063 | 0.016 |
| Ciprofloxacin | 0.016–0.031 | 0.016–0.063 |
| Sparfloxacin | 0.004 | 0.002–0.004 |
The MICs and MIEC for five clinical isolates (isolates 80-045, 90-001, 90-002, 93-001, and 97-001) were evaluated.
MICs were determined by the microdilution method in BYE-α broth, and the inoculum size was 106 CFU/ml.
The MIECs represent the intracellular antimicrobial activity and were determined by the methods described in the text.
DISCUSSION
In the present study, we demonstrated that J774.1 cells, a murine macrophage-like cell line, allowed the intracellular growth of L. pneumophila, as shown previously by Kura et al. (19). DFA staining of infected monolayers indicated that the bacteria replicated within these cells, which subsequently resulted in the destruction of the host cells. Marra et al. (20) also described the cytopathic effect of intracellularly replicating Legionella against host cells using HL-60-derived macrophages. Our results indicate that the cytopathic effect is dose dependent and seems to be due to the increased volume of bacteria replicating within macrophages, as evident on examination of DFA-stained infected J774.1 monolayers. In addition to the physical damage caused by replicating bacteria, it is also possible that the death of these cells was due to a variety of cytotoxins (16) (e.g., peptide toxin [11]), metalloprotease (28), and/or apoptosis (24). The number of remaining host cells, as quantified by the MTT assay, was inversely proportional to the number of viable replicating bacteria, as evaluated by CFU assay. These results confirm that the intracellular growth of Legionella results in host cell death.
Because the cytopathic effect of bacteria on host cells is relevant to the severity of the disease in vivo (21), the direct assay system which examines the cytopathic effect of Legionella and its inhibition by antibiotics should provide valuable information and should be a better predictor of the effect of the drug in vivo than assays that evaluate bacterial growth itself. Thus, the use of the cytopathic effect assay should be clinically useful for measuring the in vivo effect of any drug against legionellosis. Although the use of a human-derived cell line would have been more relevant in the evaluation and prediction of antibiotic efficacy in humans, our experience indicates that human cell lines, such as HL-60-derived macrophages (20) or the U-937 cell line (26), are less adherent to the bottoms of 96-well plates than the murine macrophage cell line used in this study (data not shown), making their use inconvenient when the supernatants were decanted or aspirated from the cell monolayers.
In this study, we compared the intracellular activities of several drugs against different strains of L. pneumophila SG1. Our results indicated that the intraphagocytic activities of beta-lactams and aminoglycosides are markedly lower than the MICs, while the intraphagocytic activities of macrolides, fluoroquinolones, rifampin, and minocycline were comparable. While similar observations have previously been made by other investigators (14, 15, 17, 31, 32), the present study is the first to provide quantitative and comparative evaluations of these drugs. The activities of aminoglycosides against intracellular Legionella are controversial (13, 17, 32); however, the present study clearly indicated a lack of intracellular activity of aminoglycosides against Legionella. Aminoglycosides failed to cure animals infected with L. pneumophila (5), and the extracellular activities of these drugs described in previous studies (13, 32) may have been misinterpreted as the intracellular killing of bacteria. This study showed the superior activity of clarithromycin relative to those of the other macrolides tested, thus confirming the results of previous in vivo studies of experimental legionellosis in guinea pigs (10, 18). Our results also indicated that the intracellular activity of azithromycin is similar to that of erythromycin, as reported by Edelstein and Edelstein (7). All fluoroquinolones examined in this study had good intracellular activity against Legionella, a finding consistent with the results of previous studies (13, 30). Sparfloxacin was most potent among the fluoroquinolones tested in this study, in agreement with our previous study (29) and that of Edelstein et al. (6). Thus, the results obtained with the cytopathic effect assay system used in the present study are consistent with those of previous in vitro and in vivo studies.
Differences between the results of the MTT assay and those of the CFU assay may exist under certain circumstances. One or more of the following factors should be considered in such situations: (i) modulation of cellular viability by antibiotics, (ii) differences in antibiotic effect on bacterial growth and bacterial cytopathic effect, and (iii) methodological errors. Methodological errors can be easily identified when assays are performed three or more times. When methodological errors are ruled out, the two other possibilities should be investigated. Further examination of the differences between the two assays may lead to new information on disease pathogenicity and the mechanism of drug activity against intracellular L. pneumophila.
In conclusion, the MTT assay described in the present study allows quantitative and comparative evaluations of intracellular activities of drugs against Legionella. It may also serve as a valuable tool for investigating the relationships among bacteria, host phagocytes, and antimicrobial agents.
ACKNOWLEDGMENTS
We thank F. G. Issa, University of Sydney, Sydney, Australia, for help in editing the manuscript.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.American Thoracic Society. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. Am Rev Respir Dis. 1993;148:1418–1426. doi: 10.1164/ajrccm/148.5.1418. [DOI] [PubMed] [Google Scholar]
- 2.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 3.Dowling J N, Saha S K, Glew R H. Virulence factors of the family Legionellaceae. Microbiol Rev. 1992;56:32–60. doi: 10.1128/mr.56.1.32-60.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelstein, P. H. 1995. Antimicrobial chemotherapy for Legionnaires’ disease: a review. Clin. Infect. Dis. 21(Suppl. 3):S265–S276. [DOI] [PubMed]
- 5.Edelstein P H, Calarco K, Yasui V K. Antimicrobial therapy of experimental Legionnaires’ disease in guinea pigs. Am Rev Respir Dis. 1984;130:849–856. doi: 10.1164/arrd.1984.130.5.849. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein P H, Edelstein M A C, Weidenfeld J, Dorr M B. In vitro activity of sparfloxacin (CI-978; AT-4140) for clinical Legionella isolates, pharmacokinetics in guinea pigs, and use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob Agents Chemother. 1990;34:2122–2127. doi: 10.1128/aac.34.11.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelstein P H, Edelstein M A C. In vitro activity of azithromycin against clinical isolates of Legionella species. Antimicrob Agents Chemother. 1991;35:180–181. doi: 10.1128/aac.35.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezaki T, Hashimoto Y, Yamamoto H, Lucida M L, Liu S L, Kusunoki S, Asano K, Yabuuchi E. Evaluation of the microplate hybridization method for rapid identification of Legionella species. Eur J Clin Microbiol Infect Dis. 1990;9:213–217. doi: 10.1007/BF01963841. [DOI] [PubMed] [Google Scholar]
- 9.Falco V, Sevilla T F, Alegre J. Legionella pneumophila: a cause of severe community-acquired pneumonia. Chest. 1991;100:1007–1011. doi: 10.1378/chest.100.4.1007. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes P B, Bailer R, Swanson R, Hanson C W, McDonald E, Ramer N, Hardy D, Shipkowitz N, Bower R R, Gade E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986;30:865–873. doi: 10.1128/aac.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman R L, Iglewski B H, Miller R D. Identification of a cytotoxin produced by Legionella pneumophila. Infect Immun. 1980;29:271–274. doi: 10.1128/iai.29.1.271-274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebran S J, Newton C A, Yamamoto Y, Klein T W, Friedman H. A rapid colorimetric assay for evaluating Legionella pneumophila growth in macrophages in vitro. J Clin Microbiol. 1994;32:127–130. doi: 10.1128/jcm.32.1.127-130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlichek D, Saravolatz L, Pohlod D. Effect of quinolones and other antimicrobial agents on cell-associated Legionella pneumophila. Antimicrob Agents Chemother. 1987;31:1529–1534. doi: 10.1128/aac.31.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higa F, Saito A, Inadome J, Kusano N, Kitsukawa K. Influence of methylprednisolone on the intracellular antimicrobial activity of erythromycin and clindamycin against Legionella pneumophila. J Antimicrob Chemother. 1993;31:901–908. doi: 10.1093/jac/31.6.901. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz M A, Silverstein S C. Intracellular multiplication of Legionnaires’ disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J Clin Invest. 1982;71:15–26. doi: 10.1172/JCI110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitsukawa K, Hara J, Saito A. Inhibition of Legionella pneumophila in guinea pig peritoneal macrophages by new quinolone, macrolide and other antimicrobial agents. J Antimicrob Chemother. 1991;27:343–353. doi: 10.1093/jac/27.3.343. [DOI] [PubMed] [Google Scholar]
- 18.Kohno S, Koga H, Yamaguchi K, Masaki M, Inoue Y, Dotsu Y, Masuyama Y, Hayashi T, Hirota M, Saito A, Hara K. A new macrolide, TE-031 (A-56268), in treatment of experimental Legionnaires’ disease. J Antimicrob Chemother. 1989;24:397–405. doi: 10.1093/jac/24.3.397. [DOI] [PubMed] [Google Scholar]
- 19.Kura F, Suzuki K, Watanabe H, Akamatsu Y, Amano F. Difference in Legionella pneumophila growth permissiveness between J774.1 murine macrophage-like JA4 cells and lipopolysaccharide (LPS)-resistant mutant cells, LPS1916, after stimulation with LPS. Infect Immun. 1994;62:5419–5426. doi: 10.1128/iai.62.12.5419-5423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marra A, Horwitz M A, Shuman H A. The HL-60 model for the interaction of human macrophages with the Legionnaires’ disease bacterium. J Immunol. 1990;144:2738–2744. [PubMed] [Google Scholar]
- 21.Marra A, Blander S J, Horwitz M A, Shuman H A. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrie J T, Durant H, Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis. 1989;11:586–599. doi: 10.1093/clinids/11.4.586. [DOI] [PubMed] [Google Scholar]
- 23.Marrie J T, MacDonald S, Haldane D. Nosocomial Legionnaires’ disease: lessons from a four-year prospective study. Am J Infect Control. 1990;19:79–85. doi: 10.1016/0196-6553(91)90043-c. [DOI] [PubMed] [Google Scholar]
- 24.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 2nd ed. Tentative standard. NCCLS document M7-T2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 26.Pearlman E, Jiwa A H, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 27.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985;82:131–140. doi: 10.1016/0022-1759(85)90232-7. [DOI] [PubMed] [Google Scholar]
- 28.Quinn F D, Tompkins L S. Analysis of a cloned sequence of Legionella pneumophila encoding a 38 kD metalloprotease possessing hemolytic and cytotoxic activities. Mol Microbiol. 1989;3:797–805. doi: 10.1111/j.1365-2958.1989.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 29.Saito, A., and M. Gaja. 1995. In vitro and in vivo activities of sparfloxacin in Legionella infection. Drugs 49(Suppl. 2):250–252. [DOI] [PubMed]
- 30.Saito A, Sawatari K, Fukuda Y, Nagasawa M, Koga H, Tomonaga A, Nakazato H, Fujita Y, Shigeno Y, Suzuyama K, Yamaguchi K, Izumikawa K, Hara K. Susceptibility of Legionella pneumophila to ofloxacin in vitro and in experimental legionella pneumonia in guinea pigs. Antimicrob Agents Chemother. 1985;28:15–20. doi: 10.1128/aac.28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilde J L, Dournon E, Rajagopalan P. Inhibition of Legionella pneumophila multiplication within human macrophages by antimicrobial agents. Antimicrob Agents Chemother. 1986;30:743–748. doi: 10.1128/aac.30.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida S, Mizuguchi Y. Antibiotic susceptibility of Legionella pneumophila Philadelphia-1 in cultured guinea pig peritoneal macrophages. J Gen Microbiol. 1984;130:901–906. doi: 10.1099/00221287-130-4-901. [DOI] [PubMed] [Google Scholar]