Summary
Background
PD-1-based immune checkpoint inhibition (ICI) is the major backbone of current melanoma therapy. Tumor PD-L1 expression represents one of few biomarkers predicting ICI therapy outcome. The objective of the present study was to systematically investigate whether the type of tumor tissue examined for PD-L1 expression has an impact on the correlation with ICI therapy outcome.
Methods
Pre-treatment tumor tissue was collected within the prospective DeCOG cohort study ADOREG/TRIM (CA209-578; NCT05750511) between February 2014 and May 2020 from 448 consecutive patients who received PD-1-based ICI for non-resectable metastatic melanoma. The primary study endpoint was best overall response (BOR), secondary endpoints were progression-free (PFS) and overall survival (OS). All endpoints were correlated with tumor PD-L1 expression (quantified with clone 28–8; cutoff ≥5%) and stratified by tissue type.
Findings
Tumor PD-L1 was determined in 95 primary tumors (PT; 36.8% positivity), 153 skin/subcutaneous (34.0% positivity), 115 lymph node (LN; 50.4% positivity), and 85 organ (40.8% positivity) metastases. Tumor PD-L1 correlated with BOR if determined in LN (OR = 0.319; 95% CI = 0.138–0.762; P = 0.010), but not in skin/subcutaneous metastases (OR = 0.656; 95% CI = 0.311–1.341; P = 0.26). PD-L1 positivity determined on LN metastases was associated with favorable survival (PFS, HR = 0.490; 95% CI = 0.310–0.775; P = 0.002; OS, HR = 0.519; 95% CI = 0.307–0.880; P = 0.014). PD-L1 positivity determined in PT (PFS, HR = 0.757; 95% CI = 0.467–1.226; P = 0.27; OS; HR = 0.528; 95% CI = 0.305–0.913; P = 0.032) was correlated with survival to a lesser extent. No relevant survival differences were detected by PD-L1 determined in skin/subcutaneous metastases (PFS, HR = 0.825; 95% CI = 0.555–1.226; P = 0.35; OS, HR = 1.083; 95% CI = 0.698–1.681; P = 0.72).
Interpretation
For PD-1-based immunotherapy in melanoma, tumor PD-L1 determined in LN metastases was stronger correlated with therapy outcome than that assessed in PT or organ metastases. PD-L1 determined in skin/subcutaneous metastases showed no outcome correlation and therefore should be used with caution for clinical decision making.
Funding
Bristol-Myers Squibb (ADOREG/TRIM, NCT05750511); German Research Foundation (DFG; Clinician Scientist Program UMEA); Else Kröner-Fresenius-Stiftung (EKFS; Medical Scientist Academy UMESciA).
Keywords: Melanoma, Tumor PD-L1, Biomarker, Immune checkpoint inhibition, Therapy outcome
Highlights
• Pre-treatment tumor tissue from 448 melanoma patients who received PD-1-based immunotherapy was tested for PD-L1 expression.
• PD-L1 in lymph node metastases was stronger correlated with therapy outcome than PD-L1 in primaries or organ metastases.
• Tumor PD-L1 determined in skin or subcutaneous metastases showed no correlation with immunotherapy outcome.
Research in context.
Evidence before this study
Tumor PD-L1 is a biomarker of immune checkpoint inhibition therapy outcome. In melanoma its predictive value is discussed controversially.
Added value of this study
This study investigated whether the type of tumor tissue examined for PD-L1 expression has an impact on the correlation with immunotherapy outcome in melanoma patients. Pre-treatment tumor tissue was prospectively collected within the multicenter study Tissue Registry in Melanoma (ADOREG/TRIM) from 448 consecutive patients who received PD-1-based immunotherapy for non-resectable metastatic melanoma.
Implications of all the available evidence
Tumor PD-L1 determined in lymph node metastases was stronger correlated with therapy outcome than that assessed in primary tumors or organ metastases. PD-L1 determined in skin or subcutaneous metastases showed no correlation with immunotherapy outcome. Further comparative analyses of different tissue types are required to provide further insights into the mechanisms underlying these differences in outcome prediction.
Introduction
Background/rationale
Melanoma is one of the most lethal types of skin cancer and shows early lymphogenic and hematogenic metastasis.1 Fortunately, modern therapeutic options such as inhibition of the RAS-RAF-MEK-ERK pathway (BRAF/MEK-targeted therapy, TT) or PD-1-based immune checkpoint inhibition (ICI) have significantly improved the prognosis of advanced melanoma patients.2, 3, 4, 5 Yet, 30–60% of patients show primary therapy resistance. Clinical parameters that correlate with the response and survival to systemic therapies with TT or ICI include tumor burden, serum LDH, ECOG overall performance status, patient age, and site and number of organs involved in metastasis.6, 7, 8 Specific biomarkers predicting the outcome of ICI therapy like blood neutrophil-lymphocyte ratio or serum PD-1/PD-L1 are rare, so that models have been described combining several unspecific clinical and blood parameters to enhance the usefulness for clinical decision making.9
For predicting treatment success of PD-1-based ICI based on tumor tissue, besides tumor mutational burden (TMB) and expression of IFNγ-related genes,10 the expression of PD-L1 on the surface of tumor cells (tumor PD-L1) is the only immunohistochemical marker that provides a reliable predictive value in a number of cancer types. Tumor PD-L1 has therefore already been an integral part of treatment decision making in non-small cell lung cancer (NSCLC) and urothelial carcinoma.11 In melanoma, however, its predictive value is still discussed controversially.12, 13, 14 Two large pivotal clinical trials investigating PD-1-based ICI in melanoma demonstrated that patients with PD-L1 positive tumors had a significantly better treatment response and superior survival compared with patients with PD-L1 negative tumors.5,15 Other studies and clinical trials could not confirm tumor PD-L1 expression as a useful predictor of ICI treatment outcome.16,17 Notably, these trial publications did not report on type or origin of the tumor tissue samples used for PD-L1 expression quantification.15
The prospective multicenter translational study Tissue Registry in Melanoma (ADOREG/TRIM; CA209-578) aimed to investigate the PD-L1 expression from a pre-therapeutically obtained tumor tissue sample for its correlation with the outcome of a PD-1-based ICI therapy in a large real-world cohort of metastatic melanoma patients.18 During the work-up of tissue samples sent in for molecular analysis within the study, we frequently observed a discordant PD-L1 expression in tissues obtained from different tumor sites of the same patient. This led to inconclusive results for tumor PD-L1 classification in a substantial number of patients, who would have been classified as PD-L1 positive based on one tumor lesion, but PD-L1 negative based on another, or vice versa. This observation of discordant tumor PD-L1 expression resulting from the examination of different types of tumor tissue has high clinical impact, since PD-L1 expression results have recently gained even more importance. The results of the RELATIVITY trial led to the recent approval of the PD-1 inhibitor nivolumab plus the LAG-3 inhibitor relatlimab, but was restricted by the EMA in Europe to patients with PD-L1 negative tumors only.19 This means, that patients with incorrect tumor PD-L1 classification depending on an inappropriate type of tumor tissue examined, may not receive this new drug combination.
Objectives
The present study aimed to investigate whether the type of tissue sample used for PD-L1 quantification, and thus for the classification of tumors as PD-L1 positive or negative, has an impact on its correlation with the outcome of PD-1-based ICI therapy in melanoma patients.
Methods
Study design, setting and participants
Formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples from melanoma patients were prospectively collected within the multicenter translational study Tissue Registry in Melanoma (ADOREG/TRIM; CA209-578; ClinicalTrials.gov identifier: NCT05750511) performed within the framework of the skin cancer registry ADOREG of the German Dermatologic Cooperative Oncology Group (DeCOG), annotated with clinical parameters at basline and during follow-up. From this consecutive cohort, patients were selected for the present analysis according to the following criteria: Histologically confirmed diagnosis of melanoma of the skin, mucosa, or unknown primary; tumor tissue sample taken prior to the start of the first PD-1-based ICI therapy for non-resectable stage III or IV metastatic disease (AJCCv8)20; and complete documentation of ICI treatment outcome and follow-up. The patients received either anti-PD1 monotherapy at an approved dosage (Nivolumab 3 mg/kg every 2 weeks or Nivolumab 480 mg every 4 weeks or Pembrolizumab 200 mg every 3 weeks) or dual ICI therapy at an approved dosage (Ipilimumab 3 mg/kg and Nivolumab 1 mg/kg). Response and survival outcome to ICI therapy was correlated with PD-L1 expression quantified on pre-treatment tumor tissue from each patient, taking into account the type of tumor tissue examined (primary tumor, LN metastasis, skin/subcutaneous metastasis, organ metastasis). Best overall response (BOR) was assessed according to RECIST version 1.1.21 Progression-free survival (PFS) and overall survival (OS) were defined as the time from therapy start to disease progression or death, respectively; if no such event occurred, the date of last patient contact was used as the endpoint for survival assessment (censored observation). The study was approved by the Ethics Committee of the University of Duisburg-Essen (15-6566-BO); informed consent was obtained from all participating patients.
Study endpoints/outcomes
The primary study endpoint was best overall response (BOR), secondary study endpoints were progression-free survival (PFS) and overall survival (OS); all endpoints were correlated with tumor PD-L1 expression (clone 28–8; cutoff ≥5%) and stratified by tissue type.
Data sources/measurement: PD-L1 staining and quantification
PD-L1 expression was assessed in FFPE tumor tissue samples using a rabbit anti-human PD-L1 monoclonal antibody (clone 28–8) and an analytically validated automated immunohistochemical assay (PD-L1 IHC 28–8 pharmDx for Autostainer Link 48; Dako, Glostrup, Denmark). For each sample, a comparable tissue slide of the same specimen was stained with non-specific IgG and used as negative control. PD-L1 expression in tumor tissue was quantified as the percentage of vital tumor cells that exhibit a specific membrane staining of the cell surface of any intensity in a section containing at least 100 evaluable tumor cells, with ≥5% defined as positive staining. The quantification of PD-L1 expression was performed by either a pathologist or a dermatologist experienced in histopathology, or both, using conventional bright-field microscopy.
Sample size calculation
The TRIM study is designed as a registry study consecutively enrolling patients from multiple clinical centers throughout Germany. This study design as well as the explorative nature of the statistical methods planned make a formal sample size calculation inapplicable.
Statistical methods
The survival endpoints (PFS and OS) were calculated using the Kaplan–Meier method for censored failure time data. The two-sided log-rank test was used to compare survival rates between groups. Differences in BOR was calculated by chi-square test. Multivariable analyses were performed using the Cox proportional hazards model and multinomial regression. Multiple testing was not allowed. For multivariable analyses, tumor PD-L1 expression (positive, ≥5% versus negative, <5%) was tested together with known prognostic and predictive parameters of metastatic melanoma that were considered as potential confounders: age (<65 versus ≥65 years), sex (male versus female), stage of disease (III, IV M1a/b versus IV M1c/d), LDH serum activity (normal versus elevated), number of organs involved in metastasis (≤2 versus ≥3), and ICI therapy type (anti-PD-1 monotherapy versus anti-PD-1 plus anti-CTLA-4 combination therapy). In order to examine the relationship between two categorical variables, the chi-square test was applied. One-way ANOVA (analysis of variance) was used to determine whether a statistically significant relationship existed between more than two independent groups. Statistical analysis was performed using SPSS (Version 25, IBM, Armonk, NY, USA) and Graphpad Prism (Version 9, GraphPad Software, CA, USA). P < 0.05 was considered statistically significant.
Results
Participants, descriptive data, and outcome data
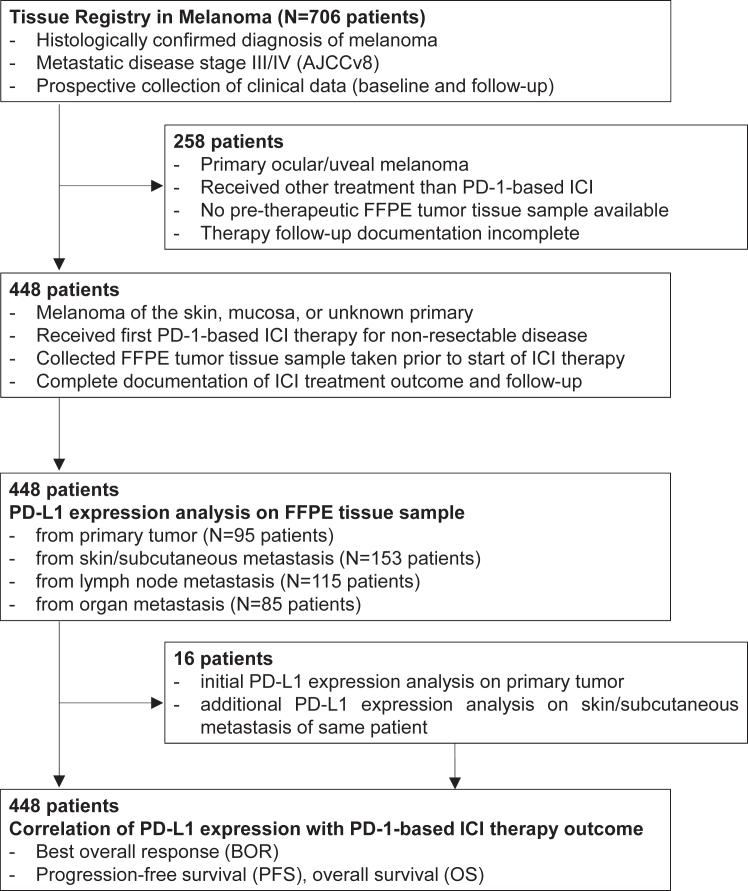
Of 706 patients participating in the TRIM project, 448 patients (62.2% male; mean age 65.2 years) from 14 German skin cancer centers (Table S1) started an anti-PD-1-based ICI therapy between February 2014 and May 2020 and met the above-mentioned selection criteria. Of these, the sent in pre-treatment tumor tissue specimens examined for PD-L1 expression were obtained from primary tumors (PT) in 95/448 (21.2%) patients, and from metastases in 353/448 (78.8%) patients. In the patient group tested for PD-L1 expression in metastatic tissue, 153/353 (43.3%) tissues were from skin or subcutaneous (SC) metastases, 115/353 (32.6%) from lymph node (LN) metastases, and 85/353 (24.1%) from organ metastases. In 16 patients initially tested in primary tumors, a second tissue sample from skin/SC metastasis was available for a second PD-L1 expression testing. An overview on the study patient flow is provided in Fig. 1. First non-adjuvant PD-1-based ICI therapy consisted of single-agent PD-1 ICI in 314/448 (70.1%), and of PD-1 plus CTLA-4 ICI combination therapy in 134/448 (29.9%) patients. At data cut-off on July 15, 2022, after a median follow-up time of 18.5 months, the BOR to a first PD-1-based ICI therapy was objective response (CR/PR) in 120 (26.8%) patients, stable disease (SD) in 127 (28.3%) patients, and progressive disease (PD) in 196 (43.8%) patients. 295/448 patients (65.8%) progressed at any time after starting PD-1-based ICI therapy, and 252/448 patients (56.3%) died. The median PFS of the total cohort of 448 patients after start of PD-1-based ICI therapy was 6.2 months; the median OS was 26.0 months. For detailed patient characteristics including possible confounders and missing data see Table 1.
Fig. 1.
Schematic presentation of the study flow.
Table 1.
Patient characteristics at start of the first PD-1-based immune checkpoint inhibition (index therapy).
| Total patient cohort |
Primary tumor |
Lymph node metastasis |
Skin/Subcutaneous metastasis |
Organ metastasis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | N (%) | N all (%) | N PD-L1<5% (%) | N PD-L1≥5% (%) | N all (%) | N PD-L1<5% (%) | N PD-L1≥5% (%) | N all (%) | N PD-L1<5% (%) | N PD-L1≥5% (%) | N all (%) | N PD-L1<5% (%) | N PD-L1≥5% (%) |
| Total | 448 (100%) | 95 (100%) | 60 (63.2%) | 35 (36.8%) | 115 (100%) | 57 (49.6%) | 58 (50.4%) | 153 (100%) | 101 (66.0%) | 52 (34.0%) | 85 (100%) | 51 (60.0%) | 34 (40.0%) |
| Mean age (range), years | 65.2 (17.6–93.4) | 65.4 (19.9–89.8) | 64.9 (25.6–89.8) | 66.2 (19.9–89.6) | 65.9 (17.6–85.2) | 66.1 (17.6–85.2) | 65.7 (31.0–85.2) | 65.3 (27.4–91.2) | 66.4 (27.4–91.2) | 68.5 (28.3–88.5) | 61.9 (26.4–93.4) | 62.0 (26.4–93.4) | 61.9 (39.3–81.0) |
| Sex | |||||||||||||
| Female | 279 (62.2%) | 66 (69.5%) | 37 (61.7%) | 29 (82.9%) | 72 (62.6%) | 36 (63.2%) | 36 (62.1%) | 92 (60.1%) | 61 (60.4%) | 31 (59.6%) | 36 (42.4) | 25 (49.0%) | 11 (32.4%) |
| Male | 169 (37.8%) | 29 (30.5%) | 23 (38.3%) | 6 (17.1%) | 43 (37.4%) | 21 (36.8%) | 22 (37.9%) | 61 (39.9%) | 40 (39.6%) | 21 (40.4%) | 49 (57.6%) | 26 (50.1%) | 23 (67.4%) |
| Localisation of primary | |||||||||||||
| Skin | 372 (83.0%) | 79 (83.2%) | 48 (80.0%) | 31 (88.6%) | 97 (84.3%) | 48 (84.2%) | 49 (84.5%) | 141 (92.2%) | 92 (91.1%) | 49 (94.2%) | 55 (64.7) | 31 (60.8%) | 24 (70.6%) |
| Mucosa | 37 (8.3%) | 16 (16.8%) | 12 (20.0%) | 4 (11.4%) | 6 (5.2%) | 3 (5.3%) | 3 (5.2%) | 4 (26.1%) | 1 (1.0%) | 3 (5.8%) | 14 (16.5%) | 9 (17.6%) | 5 (14.7%) |
| Occult (unknown primary) | 39 (5.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (10.4%) | 6 (10.5%) | 6 (10.3%) | 8 (5.2%) | 8 (7.9%) | 0 (0.0%) | 16 (18.8%) | 11 (21.6%) | 5 (14.7%) |
| BRAF V600 mutation (tumor) | |||||||||||||
| Yes | 134 (29.9%) | 30 (31.6%) | 20 (33.3%) | 10 (28.6%) | 33 (28.7%) | 18 (31.6%) | 15 (25.8%) | 37 (24.2%) | 23 (22.8%) | 14 (26.9%) | 38 (44.7%) | 19 (37.3%) | 19 (55.9%) |
| No | 314 (70.1%) | 65 (68.4%) | 40 (66.7%) | 25 (71.4%) | 82 (71.3%) | 39 (68.4%) | 43 (74.1%) | 116 (75.8%) | 78 (77.2%) | 38 (73.1%) | 47 (55.3%) | 32 (62.7%) | 15 (44.1%) |
| AJCC stage and M category | |||||||||||||
| III, IV M1a/b | 262 (58.5%) | 49 (51.6%) | 35 (58.3%) | 14 (40.0%) | 76 (66.1%) | 42 (73.7%) | 34 (58.6%) | 109 (71.2%) | 72 (71.3%) | 37 (71.1%) | 28 (32.9%) | 17 (33.3%) | 11 |
| IV M1c/d | 174 (38.8%) | 34 (35.8%) | 19 (31.7%) | 15 (42.9%) | 39 (33.9%) | 15 (26.3%) | 24 (41.4%) | 44 (28.8%) | 29 (28.7%) | 15 (28.9%) | 57 (67.1%) | 34 (66.7%) | 23 |
| Unknown | 12 (2.7%) | 12 (12.6%) | 6 (10%) | 6 (17.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Number of organs involved in metastasis | |||||||||||||
| ≤2 | 329 (73.4%) | 65 (68.4%) | 44 (73.3%) | 21 (60.0%) | 86 (74.8%) | 45 (78.9%) | 41 (70.7%) | 118 (77.1%) | 76 (75.2%) | 42 (80.8%) | 60 (70.6%) | 37 (72.5%) | 23 (67.4%) |
| ≥3 | 102 (22.8%) | 13 (13.7%) | 7 (16.7%) | 6 (17.1%) | 29 (25.2%) | 12 (21.1%) | 17 (29.3%) | 35 (22.9%) | 25 (24.8%) | 10 (19.2%) | 25 (29.4%) | 14 (27.5%) | 11 (32.4%) |
| Unknown | 17 (3.8%) | 17 (17.9%) | 9 (15.0%) | 8 (22.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| LDH (serum) | |||||||||||||
| Normal (≤ULN) | 190 (42.4%) | 39 (41.1%) | 27 (45.0%) | 12 (34.3%) | 53 (46.1%) | 24 (42.1%) | 29 (50.0%) | 66 (43.1%) | 47 (46.5%) | 19 (36.5%) | 32 (37.6%) | 17 (33.3%) | 15 (44.1%) |
| Elevated (>ULN) | 156 (34.8%) | 25 (26.3%) | 16 (26.7%) | 9 (25.7%) | 37 (32.2%) | 19 (33.3%) | 18 (31.0%) | 61 (39.9%) | 40 (39.6%) | 21 (40.4%) | 33 (38.9%) | 23 (29.4%) | 10 (29.4%) |
| Unknown | 102 (22.8%) | 31 (32.6%) | 17 (28.3%) | 14 (40.0%) | 25 (21.7%) | 14 (24.6%) | 11 (19.0%) | 26 (17.0%) | 14 (13.9%) | 12 (23.1%9 | 20 (23.5%) | 11 (26.5%) | 9 (26.5%) |
| ECOG overall performance status | |||||||||||||
| 0 | 280 (62.5%) | 67 (70.5%) | 45 (75.0%) | 22 (62.9%) | 67 (58.3%) | 35 (61.4%) | 32 (55.2%) | 98 (64.1%) | 64 (63.4%) | 34 (65.4%) | 49 (57.6%) | 28 (54.9%) | 21 (61.8%) |
| ≥1 | 82 (18.3%) | 19 (20.0%) | 11 (18.3%) | 8 (22.9%) | 17 (14.8%) | 8 (14.0%) | 9 (15.5%) | 31 (20.3%) | 20 (19.8%) | 11 (21.2%) | 15 (17.6%) | 10 (19.6%) | 5 (14.7%) |
| Unknown | 86 (19.2%) | 9 (9.5%) | 4 (6.7%) | 5 (14.3%) | 31 (26.9%) | 14 (24.6%) | 17 (29.3%) | 24 (15.7%) | 17 (16.8%) | 7 (13.5%) | 21 (24.7%) | 13 (25.5%) | 8 (23.5%) |
| Non-adjuvant pre-treatment | |||||||||||||
| Yes | 110 (24.5%) | 27 (28.4%) | 23 (38.3%) | 4 (11.4%) | 26 (22.6%) | 11 (19.3%) | 15 (25.9%) | 37 (24.2%) | 23 (22.8%) | 14 (26.9%) | 35 (68.6%) | 22 (43.1%) | 13 (38.2%) |
| No | 304 (67.9%) | 60 (63.2%) | 32 (53.3%) | 28 (80%) | 84 (73.0%) | 44 (77.2%) | 40 (69.0%) | 105 (68.6%) | 70 (69.3%) | 35 (67.3%) | 37 (72.5%) | 24 (47.1%) | 13 (38.2%) |
| Unknown | 34 (7.6%) | 8 (8.4%) | 5 (8.3%) | 3 (8.6%) | 5 (4.4%) | 2 (3.5%) | 3 (5.1%) | 11 (7.2%) | 8 (7.9%) | 3 (5.7%) | 13 (25.4%) | 5 (9.8%) | 8 (23.6%) |
| Type of ICI therapy | |||||||||||||
| PD-1 | 314 (70.1%) | 62 (65.3%) | 35 (58.3%) | 27 (77.1%) | 94 (81.7%) | 48 (84.2%) | 46 (79.3%) | 113 (73.9%) | 72 (71.3%) | 41 (78.8%) | 33 (38.8%) | 24 (47.1%) | 19 (55.9%) |
| PD-1 plus CTLA-4 | 134 (29.9%) | 33 (34.7%) | 25 (41.7%) | 8 (22.9%) | 21 (18.3%) | 9 (15.8%) | 12 (20.7%) | 40 (26.1%) | 29 (28.7%) | 11 (21.2%) | 52 (61.2%) | 27 (52.9%) | 15 (44.1%) |
| Therapy Outcome | |||||||||||||
| Total | 442 (100%) | 95 (100%) | 60 (100%) | 35 (100%) | 112 (100%) | 56 (100%) | 56 (100%) | 150 (100%) | 100 (100%) | 50 (100%) | 85 (100%) | 51 (60.0%) | 34 (40.0%) |
| CR | 45 (10.2%) | 9 (9.5%) | 5 (8.3%) | 4 (11.4%) | 10 (8.9%) | 4 (7.1%) | 6 (10.7%) | 18 (12.0%) | 10 (10.0%) | 8 (16.0) | 8 (9.4%) | 4 (7.8%) | 4 (11.8%) |
| PR | 74 (16.7%) | 13 (13.7%) | 6 (10.0%) | 7 (20.0%) | 20 (17.8%) | 5 (8.9%) | 15 (26.7%) | 27 (18.0%) | 17 (17.0%) | 10 (20.0) | 14 (16.5%) | 6 (11.8%) | 8 (23.5%) |
| SD | 127 (28.7%) | 33 (34.7%) | 25 (41.7%) | 8 (22.9%) | 36 (32.1%) | 16 (28.6%) | 20 (35.7%) | 37 (24.7%) | 23 (23.0%) | 14 (28.0%) | 21 (24.7%) | 13 (25.5%) | 8 (23.5%) |
| PD | 196 (44.3%) | 40 (42.1%) | 24 (40.0%) | 16 (45.7%) | 46 (41.1) | 31 (54.4%) | 15 (26.7%) | 68 (45.3%) | 50 (50.0%) | 18 (36.0%) | 42 (49.1%) | 28 (54.9%) | 14 (41.2%) |
| ORR | |||||||||||||
| CR/PR | 119 (26.9%) | 22 (23.2%) | 11 (18.3%) | 11 (31.4%) | 30 (26.7%) | 9 (16.1%) | 21 (37.5%) | 45 (30.0%) | 27 (27.0%) | 18 (36.0%) | 22 (25.9% | 10 (19.6%) | 12 (35.3%) |
| SD/PD | 323 (73.1%) | 73 (76.8%) | 49 (81.7%) | 24 (68.6%) | 82 (73.3%) | 47 (83.9%) | 35 (62.5%) | 105 (70.0%) | 73 (73.0%) | 32 (64.0%) | 63 (74.1%) | 41 (80.4%) | 22 (64.7%) |
| DCR | |||||||||||||
| CR/PR/SD | 246 (55.6%) | 55 (57.9%) | 36 (60%) | 19 (54.3%) | 66 (58.9%) | 25 (44.6%) | 41 (73.2%) | 82 (54.7%) | 50 (50.0%) | 32 (64.0%) | 43 (50.6%) | 23 (45.1%) | 20 (58.8%) |
| PD | 196 (44.4%) | 40 (42.1%) | 24 (40.0%) | 16 (45.7%) | 46 (41.1) | 31 (55.3%) | 15 (26.8%) | 68 (45.3%) | 50 (50.0%) | 18 (36.0%) | 42 (49.1%) | 28 (54.9%) | 14 (41.2%) |
| Median PFS in months (95% CI) | 5.0 (3.5–6.6) | 5.8 (0.0–12.7) | 3.5 (1.3–5.6) | 22.0 (11.9–32.2) | 6.0 (2.5–9.5) | 7.5 (1.4–13.7) | 3.5 (2.0–4.9) | 6.3 (0.0–23.5) | |||||
| HR (95% CI) | 0.757 (0.467–1.226) | 0.490 (0.310–0.775) | 0.825 (0.555–1.226) | 0.614 (0.368–1.023) | |||||||||
| Median OS in months (95% CI) | 15.5 (9.4–21.6) | Not reached | 16.6 (9.4–23.8) | 68.9 (29.8–107.9) | 28.7 (11.5–45.8) | 31.1 (15.6–46.6) | 13.3 (4.6–21.9) | 31.5 (17.2–45.8) | |||||
| HR (95% CI) | 0.528 (0.305–0.913) | 0.519 (0.307–0.880) | 1.083 (0.698–1.681) | 0.643 (0.380–1.085) | |||||||||
Patient characteristics at start of the first non-adjuvant PD-1-based immune checkpoint inhibition (ICI) therapy of the total patient cohort and of the subgroups of patients with PD-L1 assessment in primary tumors, lymph node metastases, and skin/subcutaneous metastases. Statistical analysis of total subgroups with.
∗One-way ANOVA and ∗∗chi-square test.
AJCCv8 was used for disease classification. LDH, lactate dehydrogenase; ULN, upper limit of normal.
Main results: different PD-L1 expression in primary tumors and corresponding skin/subcutaneous metastases of the same patient
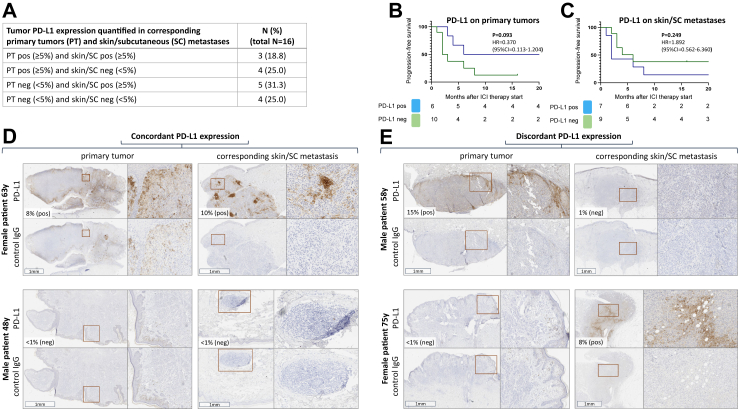
In a first small hypothesis-generating cohort, 16 of the total of 448 patients were identified, whose initial tumor PD-L1 expression analysis was done in primary tumor tissue, and of whom additional pre-therapeutically obtained FFPE tissue specimen from a skin/SC metastasis were available (see Fig. 1). Among these 16 patients, PD-L1 expression testing from primary tumors and skin/SC metastases revealed concordant results (PT pos and skin/SC pos; PT neg and skin/SC neg) in n = 7 (43.8%), and discordant results (PT pos and skin/SC neg; PT neg and skin/SC pos) in n = 9 (56.2%) cases (Fig. 2A). PD-L1 stainings of four representative patients are shown in Fig. 2D and E. Of the primary tumors, 7/16 (43.8%) were classified as PD-L1 positive, and 9/16 (56.2%) as PD-L1 negative. Patients whose primary tumors were classified as PD-L1 negative had a median PFS of only 2 months upon ICI therapy, whereas patients whose primary tumors were classified as PD-L1 negative had a longer median PFS of 6 months (Fig. 2B, HR = 0.369; 95% CI = 0.113–1.204; P = 0.093). The quantification of PD-L1 expression in skin/SC metastases of the same patients revealed similar proportions with 8/16 (50.0%) metastases tested as PD-L1 positive, and 8/16 (50.0%) as PD-L1 negative (Fig. 2A). However, patients with PD-L1 positive skin/SC metastases did not show a superior PFS compared to patients with PD-L1 negative skin/SC metastases (Fig. 2C, HR = 1.892; 95% CI = 0.562–6.360; P = 0.42).
Fig. 2.
Different tumor PD-L1 expression in primary tumors and corresponding skin/subcutaneous metastases of 16 melanoma patients. Tumor PD-L1 expression was quantified on pre-therapeutically obtained tissue samples. (A) different combinations of PD-L1 expression; (B, C) progression-free survival by PD-L1 expression; (D, E) examples of concordant and discordant tumor PD-L1 expression of 4 representative patients. Coloured boxes indicate areas of magnification.
Main results: PD-L1 in primary tumors versus metastases and its correlation with PD-1-based ICI therapy outcome
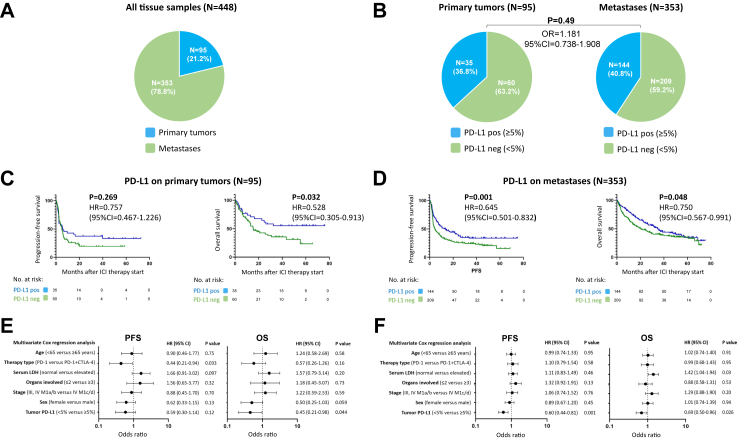
Of n = 448 pre-treatment tumor samples, 21.2% (n = 95) were from primary tumors (Table S2), and 78.8% (n = 353) were from different metastatic sites; Fig. 3A. A total of 269 (60.0%) tumor tissues were classified as PD-L1 negative and 179 (40.0%) as PD-L1 positive. Of patients whose tumors were classified as PD-L1 positive, 66 (36.9%) showed an objective response (CR/PR) to ICI therapy, whereas of patients whose tumors were classified as PD-L1 negative, 60 (22.3%) showed an objective response (OR = 0.492; 95% CI = 0.325–0.739; P = 0.008; Figure S1A). Patients with PD-L1 positive tumors showed a median PFS of 11.2 months (95% CI = 5.3–17.2) and a median OS of 35.4 months (95% CI = 23.2–47.6). In comparison, patients with PD-L1 negative tumors showed a median PFS of 4.6 months (95% CI = 3.3–5.9) and a median OS of 19.2 months (95% CI = 13.2–25.3). Overall, patients with PD-L1 positive tumors showed a statistically significant better PFS (HR = 0.656; 95% CI = 0.524–0.821; P < 0.001; Figure S1B) and OS (HR = 0.688; 95% CI = 0.537–0.882; P = 0.004; Figure S1C).
Fig. 3.
Tumor PD-L1 assessed on primary tumors and metastases and its association with survival outcome of the first PD-1-based ICI therapy. (A) Frequencies of tissue types used for tumor PD-L1 quantification in the total patient cohort; (B) frequencies of tumor PD-L1 expression determined on primary tumors versus metastases. (C, D) Kaplan–Meier survival curves showing progression-free (PFS) and overall survival (OS) dependent on tumor PD-L1 expression assessed on primary tumors (C) and metastases (D). (E, F) Multivariate Cox regression analysis of PFS and OS considering tumor PD-L1 expression determined on primary tumors (E) and metastases (F).
Next, we considered the subgroups depending on which tissue localization was used for PD-L1 analysis. Primary tumors were classified as PD-L1 positive in 36.8%, and metastases in 40.8%, revealing no significantly different frequency between groups (OR = 1.181; 95% CI = 0.738–1.908; P = 0.49; Fig. 3B). The median PFS after start of PD-1-based ICI in patients with PD-L1 positive primary tumors was 5.8 months (95% CI = 0.0–12.7 months); the median OS was not reached. In patients with PD-L1 negative primary tumors, the median PFS was 5.0 months (95% CI = 3.5–6.6 months), and the median OS was 15.5 months (95% CI = 9.4–21.6 months). Thus, patients with PD-L1 positive primary tumors revealed a superior survival compared to patients with PD-L1 negative primary tumors, with differences between groups were statistically significant for OS (HR = 0.528; 95% CI = 0.305–0.913; P = 0.032), but not for PFS (HR = 0.757; 95% CI = 0.467–1.226 P = 0.269); Fig. 3C. In patients whose metastases were classified as PD-L1 positive, the median PFS after ICI therapy start was 13.4 months (95% CI = 6.8–32.6 months), and the median OS was 34.8 months (95% CI = 27.3–42.3 months). In patients with metastases tested as PD-L1 negative, the median PFS was 4.4 months (95% CI = 2.5–6.2 months), and the median OS was 20.3 months (95% CI = 13.4–27.1 months). Patients with PD-L1 positive metastases showed a statistically significantly improved PFS (HR = 0.645; 95% CI = 0.501–0.832; P = 0.001) and OS (HR = 0.750; 95% CI = 0.567–0.991; P = 0.048) compared to patients with PD-L1 negative metastases; Fig. 3D.
Applying multivariable Cox regression analysis, PD-L1 expression quantified in primary tumors was independently correlated with OS (HR = 0.45; 95% CI = 0.21–0.98; P = 0.044), but not with PFS (HR = 0.59; 95% CI = 0.30–1.14; P = 0.12); Fig. 3E. The PD-L1 expression quantified in metastases was independently correlated with PFS (HR = 0.60; 95% CI = 0.44–0.81; P = 0.001) and OS (HR = 0.69; 95% CI = 0.50–0.96; P = 0.026), Fig. 3F.
Main results: PD-L1 in LN metastases, but not in skin or subcutaneous metastases, correlates with therapy outcome
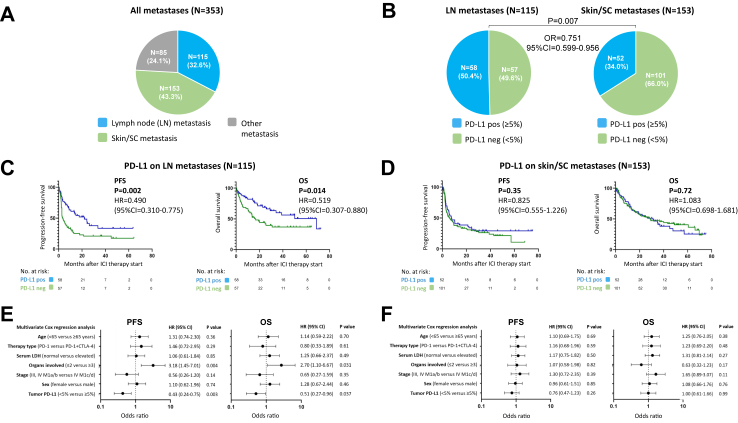
We then investigated whether, in the group of 353 patients classified according to PD-L1 expression in tissue samples from metastases, there were differences in the correlation with therapy outcome of PD-L1 expression depending on the metastatic site. Among these tissue samples, the majority originated from lymph node (LN) (115/353; 32.6%; Table 1) and skin/SC (153/353; 43.3%; Table 1) metastases. All other metastatic sites (lung, liver, brain, bone, soft tissue, renal) whose tissue samples were used for PD-L1 expression quantification summed up to 85/353 (24.1%), Fig. 4A. When considering the two largest groups of metastatic sites investigated in this study, LN and skin/SC metastasis, we observed that LN metastases were classified as PD-L1 positive more frequently than skin/SC metastases (50.4% versus 34.0%; OR = 0.506; 95% CI = 0.311–0.839, P = 0.007), Fig. 4B. The median PFS after initiation of a PD-1-based ICI in patients with PD-L1 positive LN metastases was 22.0 months (95% CI = 11.9–32.2 months), and the median OS was 68.9 months (95% CI = 29.8–107.9 months). In patients with PD-L1 negative LN metastases, the median PFS was 3.5 months (95% CI = 1.3–5.6 months), and the median OS was 16.6 months (95% CI = 9.4–23.8 months). The differences in survival by tumor PD-L1 expression determined in LN metastases were statistically significant for PFS (HR = 0.490; 95% CI = 0.310–0.775; P = 0.002) and OS (HR = 0.519; 95% CI = 0.307–0.880; P = 0.014); Fig. 4C. In contrast, no statistically significant differences in survival after start of a PD-1-based ICI could be detected by tumor PD-L1 expression assessed in skin/SC metastases (PFS= HR=0.825; 95% CI = 0.555–1.226; P = 0.35; OS= HR=1.083; 95% CI = 0.698–1.681; P = 0.72), Fig. 4D. Here, in patients with PD-L1 positive skin/SC metastases, the median PFS after ICI therapy start was 7.5 months (95% CI = 1.4–13.7 months), and the median OS was 31.1 months (95% CI = 15.6–46.6 months). In patients with PD-L1 negative skin/SC metastases, the median PFS was 6.0 months (95% CI = 2.5–9.5 months), and the median OS was 28.7 months (95% CI = 11.5–45.8 months).
Fig. 4.
Tumor PD-L1 assessed on lymph node (LN) metastases and skin/subcutaneous (SC) metastases and its association with survival outcome of the first PD-1-based ICI therapy. (A) Frequencies of different types of tissue samples used for tumor PD-L1 quantification in the cohort of patients assessed on metastases; (B) frequencies of tumor PD-L1 expression determined on LN versus skin/SC metastases. (C, D) Kaplan–Meier survival curves showing progression-free (PFS) and overall survival (OS) dependent on tumor PD-L1 expression assessed on LN (C) and skin/SC (D) metastases. (E, F) Multivariate Cox regression analysis of PFS and OS considering tumor PD-L1 expression determined on LN (E) and skin/SC (F) metastases.
Multivariable Cox regression analysis revealed that tumor PD-L1 expression determined on LN metastases was statistically significant independently correlated with PFS (HR = 0.43; 95% CI = 0.24–0.75; P = 0.003) and OS (HR = 0.51; 95% CI = 0.27–0.96; P = 0.037), Fig. 4E. No statistically significant independent correlation with survival could be found for tumor PD-L1 expression assessed in skin/SC metastases (PFS, HR = 0.76, 95% CI = 0.47–1.23, P = 0.26; OS, HR = 1.00, 95% CI = 0.61–1.66, P = 0.99), Fig. 4F.
Main results: best overall response by tumor PD-L1 determined in different metastatic sites
Analysing the correlation of BOR upon PD-1-based ICI therapy with tumor PD-L1 expression assessed in tissue samples from different metastatic sites, tumor PD-L1 quantified from LN metastases revealed the highest correlation compared to PD-L1 quantified from skin/SC metastases or organ metastases (Figure S2 and S3B, Table S2).
Patients with an evaluable BOR upon first PD-1-based ICI, whose PD-L1 expression was quantified in tissue from LN metastases (n = 112), were classified as PD-L1 positive in 56 cases (50%). Of these, 21/56 (37.5%) showed an objective response (CR/PR), and 35/56 (62.5%) did not respond (SD/PD). Of the 56 patients who were classified as PD-L1 negative based on tissue from LN metastases, 9/56 (16.1%) showed an objective response and 47/56 (83.9%) showed no response. Overall, patients with PD-L1 positive LN metastases were statistically significantly more likely to respond to a PD-1-based ICI therapy (OR = 0.319; 95% CI = 0.138–0.762; P = 0.010), Figure S2A. Accordingly, multivariate analysis revealed tumor PD-L1 expression determined in LN metastases as statistically significant independently correlated with therapy response (HR = 0.32; 95% CI = 0.11–0.93; P = 0.037), Figure S2C.
Out of n = 150 patients with an evaluable BOR, whose PD-L1 expression was quantified in tissue from skin/SC metastases, 50 cases (33.3%) were classified as PD-L1 positive. Of these, 18/50 patients (36.0%) showed an objective response (CR/PR) and 32/50 (64.0%) did not respond. Among the n = 100 patients classified as PD-L1 negative on tissue from skin/SC metastases, 27 (27.0%) showed an objective response and 73 (73.0%) showed no response. Taken together, we found no statistically significant difference in response to a PD-1-based ICI therapy between patients with PD-L1 positive versus negative skin/SC metastases (OR = 0.656; 95% CI = 0.311–1.341; P = 0.26), Figure S2B. Also, multivariable analysis did not show tumor PD-L1 expression assessed in skin/SC metastases as statistically significant independently correlated with therapy response (HR = 0.69; 95% CI = 0.30–1.58; P = 0.38), Figure S2D.
In n = 85 patients with an evaluable BOR, pre-treatment tumor PD-L1 expression was quantified in tissue from organ metastases (lung, liver, brain, bone, soft tissue, renal; for details see Figure S3A). Among these patients, 34 (40.0%) cases were classified as PD-L1 positive, and showed an objective response rate of 35.3%. The n = 51 patients classified as PD-L1 negative had an objective response rate of 19.6%. There was a trend towards better treatment response in patients tested as PD-L1 positive in tissue from organ metastases which did not reach statistical significance (OR = 0.447; 95% CI = 0.177–1.235; P = 0.13; Figure S3B). Accordingly, the PFS and OS of this patient group revealed a trend, but no significant difference, by PD-L1 expression determined in tissue from organ metastases (PFS=HR=0.614; 95% CI = 0.369–1.023; P = 0.068; Figure S3C; OS=HR=0.642; 95% CI = 0.380–1.085; P = 0.105; Figure S3D).
Other analyses: PD-L1 in other distant organ metastases and its correlation with survival outcomes upon PD-1-based ICI therapy
Less frequently, organ metastases (n = 85) were sent in for PD-L1 analysis. PFS and OS were calculated based on PD-L1 expression for patients tested in lung metastases (n = 35), liver metastases (n = 13), brain metastases (n = 20), and soft tissue metastases (n = 12). Calculation of PFS of bone metastases (n = 3) and renal metastases (n = 2) was waived due to very low case numbers. Overall, there was no statistically significant longer PFS or OS for any of the metastatic sites studied (Figure S4A–H). However, relevant differences in survival (PFS and OS) by PD-L1 expression were seen for patients in whom PD-L1 was quantified in liver or brain metastases (Figure S4C–F) though not reaching statistical significance due to small numbers, whereas no relevant differences were observed for patients in whom PD-L1 was quantified in lung or soft tissue metastases (Figure S4A, B, G and H).
Discussion
The clinical value of tumor PD-L1 expression as a predictive marker of therapy outcome to PD1-based ICI in melanoma remains controversial. Difficulties in the quantification of PD-L1 in melanoma arise from the high melanin content of a significant number of tumor specimens and from the spatial heterogeneity of PD-L1 expression leading to a high interobserver variability. We recently reported that these difficulties could at least in part be overcome by digital quantification of PD-L1 expression in melanoma tissue samples, clearly demonstrating a superior treatment outcome for patients with PD-L1 positive tumors.22 Our results are in line with those of large clinical trials examining the efficacy of ICI in melanoma patients which also showed a moderate but significant treatment benefit for patients whose tumor tissue was classified as PD-L1 positive. However, the extent to which the type of tumor tissue studied (primary tumor, different sites of metastasis) affects the predictive value of the tumor PD-L1 expression detected, has not been investigated systematically in melanoma so far. For NSCLC it has been described that the PD-L1 expression differs significantly between biopsies and thereafter surgically resected whole tumor specimens of the same patient.23 Additionally, NSCLC tumors were shown to harbour different PD-L1 expression in different metastatic sites.24 A study comparing PD-L1 expression determined in primary tumors and paired metastases of the same patient in various cancer entities revealed a high frequency of discordance, whereas the TMB was found to be unchanged.25
In melanoma, it has already been described that tumor PD-L1 expression is frequently discordant between primary tumors and metastases of the same patient, as well as between intra-patient metastases,26 an observation which we can confirm with the results of our present study. To address this relevant issue, we compared the correlation of tumor PD-L1 determined in different types of pre-therapeutically obtained tumor tissue with PD1-based ICI therapy outcome. For this purpose we chose the large tissue collection of the prospective real-world multicenter patient cohort of the ADOREG/TRIM study of the DeCOG, because for this study various types of tumor tissue samples, from primary tumors and from different sites of metastases, were sent in for pre-treatment molecular analysis.
Key results
First, we compared tumor PD-L1 assessed in primary tumors with that determined in metastases. Here, we found similar frequencies of PD-L1 positivity and only moderate differences in the correlation with the outcome of PD1-based ICI between the two groups. In a next step, we compared the outcome correlation of tumor PD-L1 determined in different sites of metastases. Here we observed that, in addition to primary tumors, LN metastases and skin/SC metastases were the most frequently sent in tissue materials for pre-treatment PD-L1 quantification. Overall, LN metastases were significantly more often classified as PD-L1 positive than skin/SC metastases. Unexpectedly, there were marked differences in the outcome correlation of tumor PD-L1 assessed in these two metastatic sites. Patients in whom tumor PD-L1 examination had been performed in LN metastases showed significantly longer PFS and OS if they were classified as positive, whereas patients with tumor PD-L1 positivity determined in skin/SC metastases showed no survival benefit compared to patients who were classified as negative in the same tissue type. A similar correlation was found when examining tumor PD-L1 for PD-1-based ICI treatment response. Again, tumor PD-L1 classification assessed in LN metastases revealed a good correlation with treatment response, whereas tumor PD-L1 quantified in skin/SC metastases did not appear to be associated with response. Interestingly, our findings detected in melanoma did not match previous findings made in NSCLC. Here, tumor PD-L1 determined in primary tumors of the lung as well as in distant organ metastases was associated with ICI therapy outcome, whereas PD-L1 measured in LN metastases was not associated with either response or survival.24
The question now is, why in melanoma the treatment outcome association of tumor PD-L1 determined in LN metastases and skin/SC metastases shows such large differences. One explanation may lie in the high intratumoral heterogeneity of melanoma and its distinct metastatic niches, as evidenced by genetic, epigenetic, and metabolic heterogeneity of tumor cell clones, among other factors.27 Following this notion, it is conceivable that skin metastases originate from specific cell clones that, due to their specific characteristics exhibit exclusively skin metastases and might be unable to settle to other organs. In contrast, tumor cells that reached the LNs have the ability to spread to internal organs. In this context, it appears comprehensible that the PD-L1 expression of the tumor cell clones in the skin/SC metastases has no predictive value for survival in comparison to the PD-L1 expression of the tumor cell clones in LN metastases. Another factor contributing to the diffferences observed might be the long-standing observation that a tumor's PD-L1 expression depends on its infiltration by lymphocytes.28 To this regard, it can be assumed that the tumor microenvironment of skin/SC metastases differs significantly from that of primary tumors or LN metastases, particularly with concern to an overall lower infiltration with T lymphocytes. Differences in immune cell infiltration and antigen expression according to the metastatic site have been reported for melanoma patients.29,30 Since T cell infiltration and PD-L1 expression of tumors are known to be correlated, this hypothesis fits well with the fact that skin/SC metastases are less likely to be PD-L1 positive. A recent study on melanoma patients treated with the CTLA-4 inhibitor ipilimumab revealed that the HLA-class-I expression on tumor cells significantly differs between LN and skin/SC metastases.31 Herein, the authors describe that the HLA-class-I expression level in LN metastases, but not in skin/SC metastases, was significantly correlated to the density of tumor-infitrating T cells and to the outcome of ICI therapy with ipilimumab. Further comparative immunohistochemical and molecular studies are needed to further investigate this notion and uncover the underlying mechanisms. Another study demonstrated that high glycosylation of PD-L1 in different cancer entities might decrease the predictive value of this marker because it cannot be properly detected.32 It would therefore be interesting to investigate whether PD-L1 is glycosylated in differential extent in lymph node versus skin metastases.
Limitations/interpretation
The present study has limitations to be noted. First, tumor PD-L1 analysis was performed in a semiquantitative mode, as positive (≥5%) or negative (<5%). Furthermore, the PD-L1 analysis for this study was not performed digitally but by physicians/histopathologists, which reflects the current routine in the clinical setting, but means that interpersonal heterogeneity in the assessment cannot be ruled out. Moreover, some covariates such as overall performance status by ECOG were missing in a relevant number of patients, resulting in a limitation of these covariates for the multivariate analyses. For some subgroup analyses patient numbers were small, but are still shown due to their importance as hypothesis-generating cohorts. Due to the retrospective nature of the study, the subgroups showed a different distribution of possible confounders such as the location of the primary tumor, for example, mucosal melanomas showed cutaneous metastasis significantly less frequently. Furthermore, patients whose lymph node metastases were analyzed received anti-PD1 monotherapy statistically significantly more often than patients whose primary tumors were analyzed. However, there was no statistically significant difference in the type of ICI administered between patients analyzed on lymph node metastases versus skin metastases.
Generalisability/interpretation
The major strengths of this study are its high number of patients enrolled with pre-treatment tumor tissue samples evaluable for molecular analysis, as well as the real-world nature of the study. The latter ensures that not only the often highly selected trial populations but also trial-ineligible patients better reflecting the situation in the clinics are represented.33 Most importantly, the site of origin of the tumor tissue sample sent in for analysis was documented for every patient enrolled, enabling the detailed analysis of a correlation between the type of tumor tissue examined and the predictive value of its tumor PD-L1 expression. This advantage highlights our study compared to regular clinical trial reports on the association between therapy outcome and tumor PD-L1 status, which usually do not mention the tissue type used for PD-L1 classification. Finally, this study was designed multicentric, which ensures that the methods used cannnot only be applied to tissue samples collected at one single institution, but showed to provide valid results also if sent in from multiple institutions.
In summary, the present study shows that the correlation of pre-treatment tumor PD-L1 with the outcome of a subsequent PD-1-based ICI therapy varies considerably depending on the type of tumor tissue examined. While tumor PD-L1 determined in LN metastases, organ metastases and primary tumors is well suitable for stratifying patients with respect to ICI therapy outcome, PD-L1 assessed in skin/SC metastases shows only limited outcome correlation. We therefore conclude, that in the clinical setting metastases to the skin or subcutis, despite their ease of access to surgical procedures, can not be recommended for use in therapy decision making based on their tumor PD-L1 expression. However, further comparative molecular analyses of primary tumors, skin/SC metastases and LN metastases are required to provide further insights into the tissue-specific mechanisms underlying these differences in therapy outcome prediction.
Contributors
Study design: Selma Ugurel.
Data collection: Mona Kimmig, Klaus Griewank, Rudolf Herbst, Patrick Terheyden, Jochen Utikal, Claudia Pföhler, Jens Ulrich, Alexander Kreuter, Peter Mohr, Ralf Gutzmer, Friedegund Meier, Edgar Dippel, Julia Welzel, Daniel Robert Engel, Sophia Kreft, Antje Sucker, Georg Lodde, Frederik Krefting, Ingo Stoffels, Joachim Klode, Alexander Roesch, Lisa Zimmer, Elisabeth Livingstone, Eva Hadaschik, Jürgen C. Becker, Michael Weichenthal, Alpaslan Tasdogan, Dirk Schadendorf.
Verification of underlying data: Selma Ugurel, Mona Kimmig, Jan-Malte Placke.
Data analysis and interpretation: Selma Ugurel, Jan-Malte Placke, Mona Kimmig, Lisa Zimmer, Elisabeth Livingstone, Jürgen C. Becker.
Writing of manuscript: Jan-Malte Placke, Selma Ugurel.
Approval of final manuscript: All authors.
Data sharing statement
The data sets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Declaration of interests
Jürgen C. Becker is receiving speaker's bureau honoraria from Amgen, Pfizer, Recordati and Sanofi, and is a paid consultant/advisory board member/DSMB member for Almirall, Boehringer Ingelheim, InProTher, ICON, MerckSerono, Pfizer, 4SC, and Sanofi/Regeneron. His group received research grants from Bristol-Myers Squibb, Merck Serono, HTG, IQVIA, and Alcedis.
Ralf Gutzmer reported Personal Honoraria from Roche, BMS, MSD, Novartis, Amgen, Merck Serono, Almirall, SUN, Sanofi, Pierre-Fabre; Consultant or Advisory Role (personal) for BMS, Roche, Novartis, Almirall, MSD, Amgen, SUN, Sanofi, Pierre-Fabre, 4SC, Immunocore; Research Funding (to institution) from Novartis, Pfizer, Amgen, Merck-Serono, SUN, KyowaKirin, Almirall; and Travel, Accommodations, Expenses from SUN, Pierre-Fabre, Boehringer-Ingelheim; outside the submitted work.
Rudolf Herbst is employee of Helios Klinikum Erfurt GmbH.
Joachim Klode reported grants and or personal fees from Novartis, LaVision Bio Tec and Sastomed.
Sophia Kreft has received honoraria from Sun Pharma and reports travel support from Sanofi Genzyme.
Frederik Krefting received travel support for participation in congresses and/or (speaker) honoraria from Novartis, Almirall and Boehringer Ingelheim outside the submitted work.
Elisabeth Livingstone received honoraria from Novartis, Medac, Bristol Myers Squibb, Sanofi, Sun Pharma, and Pierre Fabre, reports consulting/advisory roles with Bristol Myers Squibb, Pierre Fabre and Novartis; and received travel/accommodations/expenses from Pierre Fabre, Bristol Myers Squibb, Medac, and Sun Pharma.
Georg Lodde received travel support from Sun Pharma.
Friedegund Meier has received travel support or/and speaker's fees or/and advisor's honoraria by Novartis, Roche, BMS, MSD, Pierre Fabre, Sanofi and Immunocore and research funding from Novartis and Roche.
Peter Mohr declares research support from Bristol Myers Squibb, Merck Sharp & Dohme and Novartis; speakers and advisory board honoraria from Bristol Myers Squibb, Beiersdorf, Merck Sharp & Dohme, Pierre Fabre, Sun Pharma, Immunocore, Sanofi and Novartis, and travel support from Bristol Myers Squibb, Merck Sharp & Dohme, Sanofi, Sun Pharma, and Pierre Fabre, outside the submitted work.
Claudia Pföhler received honoraria (speaker honoraria or honoraria as a consultant) and travel support from Novartis, BMS, MSD, Merck Serono, MSD, Celgene, AbbVie, Sunpharma, Pierre Fabre, UCB, Nutricia Milupa, Janssen and LEO, outside the submitted work.
Jan-Malte Placke served as consultant and/or has received honoraria from Bristol-Myers Squibb, Novartis, Sanofi and received travel support from Bristol-Myers Squibb, Novartis, Pierre Fabre and Therakos.
Alexander Roesch reported grants from Novartis, Bristol Myers Squibb, and Adtec; personal fees from Merck Sharp & Dohme; and nonfinancial support from Amgen, Roche, Merck Sharp & Dohme, Novartis, Bristol Myers Squibb, and Teva.
Dirk Schadendorf reports partial financial support from Bristol Myers Squibb for the conduct of this study and drug supply (nivolumab and ipilimumab) support; grants (or contracts) from Amgen, Array/Pfizer, Bristol-Myers Squibb, MSD, Novartis and Roche; consulting fees from 4SC, Amgen, Array Biopharma, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Haystick, Immunocore, InFlarX, Innocent, LabCorp, Merck Serono, MSD, Nektar, NeraCare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Replimune, Roche, Sandoz, Sanofi/Regeneron, Sun Pharma; honoraria from Bristol-Myers Squibb, MSD/Merck, Merck Serono, Novartis, Roche, Sanofi and Sun Pharma; support for attendings meetings or travel support from Bristol-Myers Squibb, MSD, Merck Serono, Novartis, Pierre Fabre and Sanofi; participation on drug safety monitoring or advisory boards for 4SC, Amgen, Array Biopharma, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Immunocore, InFlarX, Merck Serono, MSD, Nektar, NeraCare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Replimune, Roche, Sandoz, Sanofi/Regeneron and SunPharma; leadership roles for DeCOG, German Cancer Society, Hiege-Stiftung, Deutsche Hautkrebsstiftung, NVKH e.V. and EuMelaReg.
Patrick Terheyden has received honoraria from Bristol Myers Squibb, Novartis, Merck Sharp & Dohme, Pierre Fabre, CureVac, Merck Serono, Sanofi, Roche, Kyowa Kirin and Biofrontera and travel support from Bristol-Myers Squibb and Pierre Fabre.
Selma Ugurel declares research support from Bristol Myers Squibb and Merck Serono; speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, and Novartis, and travel support from Bristol Myers Squibb, Merck Sharp & Dohme, and Pierre Fabre.
Jens Ulrich received honoraria (speaker honoraria or honoraria as a consultant) and travel support from Bristol-Myers Squibb, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi/Regeneron, Sunpharma outside the submitted work.
Jochen Utikal is on the advisory board or has received honoraria and travel support from Amgen, Bristol Myers Squibb, GSK, Immunocore, LeoPharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, Sanofi outside the submitted work.
Michael Weichenthal received grants from Bristol-Myers Squibb and Merck Sharp & Dohme, consulting fees from Merck Sharp & Dohme, Immunocore and Novartis, lecture honoraria from Bristol-Myers Squibb and Merck Sharp & Dohme and Pierre-Fabre, and advisory board honoraria from Merck Sharp & Dohme.
Julia Welzel received travel grants from Bristol-Myers Squibb and Almriall as well as lecture honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis and Almirall.
Lisa Zimmer declares speakers and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi, Sunpharma, research support from Novartis and travel support from Merck Sharp & Dohme, Bristol-Myers Squibb, Pierre Fabre, Sanofi, Sunpharma and Novartis; outside the submitted work.
The other authors did not report conflicts of interest.
Acknowledgements
We thank the patients who participated in this study, their families, and the staff members at the study sites who cared for them.
Role of funders: This work was in part supported by Bristol Myers Squibb for the multicenter translational study Tissue Registry in Melanoma (TRIM) within the framework of the skin cancer registry ADOREG of the German Dermatologic Cooperative Oncology Group (DeCOG); role: support in data collection.
JMP was supported by the German Research Foundation (DFG; Clinician Scientist Program UMEA, FU 356/12–1); role: support in data analysis, interpretation, and writing of manuscript.
SU, JCB and DS received funding from the Else-Kröner-Fresenius Stiftung (EKFS; University Medicine Essen Medical Scientist Academy UMESciA); role: support in data analysis, interpretation, and writing of manuscript.
DRE received funding from the German Research Foundation (DFG; FOR5427 SP4, EN984/15–1 and 16–1, TR296 P09; TR332 A3 and Z1); role: support in data analysis and interpretation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104774.
Contributor Information
Jan-Malte Placke, Email: Jan-Malte.Placke@uk-essen.de.
Mona Kimmig, Email: MonaKimmig@gmail.com.
Klaus Griewank, Email: Klaus.Griewank@uk-essen.de.
Rudolf Herbst, Email: rudolf.herbst@helios-gesundheit.de.
Patrick Terheyden, Email: patrick.terheyden@uksh.de.
Jochen Utikal, Email: Jochen.Utikal@umm.de.
Claudia Pföhler, Email: claudia.pfoehler@uks.eu.
Jens Ulrich, Email: Jens.Ulrich@harzklinikum.com.
Alexander Kreuter, Email: alexander.kreuter@helios-gesundheit.de.
Peter Mohr, Email: peter.mohr@elbekliniken.de.
Ralf Gutzmer, Email: Ralf.Gutzmer@ruhr-uni-bochum.de.
Friedegund Meier, Email: Friedegund.Meier@uniklinikum-dresden.de.
Edgar Dippel, Email: DIPPELE@klilu.de.
Julia Welzel, Email: Julia.Welzel@uk-augsburg.de.
Daniel Robert Engel, Email: DanielRobert.Engel@uk-essen.de.
Sophia Kreft, Email: sophia.kreft@charite.de.
Antje Sucker, Email: Antje.Sucker@uk-essen.de.
Georg Lodde, Email: Georg.Lodde@uk-essen.de.
Frederik Krefting, Email: Frederik.Krefting@uk-essen.de.
Ingo Stoffels, Email: Ingo.Stoffels@uk-essen.de.
Joachim Klode, Email: Joachim.Klode@uk-essen.de.
Alexander Roesch, Email: Alexander.Roesch@uk-essen.de.
Lisa Zimmer, Email: Lisa.Zimmer@uk-essen.de.
Elisabeth Livingstone, Email: Elisabeth.Livingstone@uk-essen.de.
Eva Hadaschik, Email: Eva.Hadaschik@uk-essen.de.
Jürgen C. Becker, Email: j.becker@dkfz.de.
Michael Weichenthal, Email: mweichenthal@dermatology.uni-kiel.de.
Alpaslan Tasdogan, Email: Alpaslan.Tasdogan@uk-essen.de.
Dirk Schadendorf, Email: Dirk.Schadendorf@uk-essen.de.
Selma Ugurel, Email: selma.ugurel@uk-essen.de.
Appendix A. Supplementary data
Supplementary Files
References
- 1.Schadendorf D., van Akkooi A.C.J., Berking C., et al. Melanoma. Lancet. 2018;392(10151):971–984. doi: 10.1016/S0140-6736(18)31559-9. [DOI] [PubMed] [Google Scholar]
- 2.Dummer R., Ascierto P.A., Gogas H.J., et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–1327. doi: 10.1016/S1470-2045(18)30497-2. [DOI] [PubMed] [Google Scholar]
- 3.Robert C., Karaszewska B., Schachter J., et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 4.Robert C., Long G.V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 5.Robert C., Schachter J., Long G.V., et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Gassenmaier M., Lenders M.M., Forschner A., et al. Serum S100B and LDH at baseline and during therapy predict the outcome of metastatic melanoma patients treated with BRAF inhibitors. Target Oncol. 2021;16(2):197–205. doi: 10.1007/s11523-021-00792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershenwald J.E., Scolyer R.A., Hess K.R., et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J., Zhao J., Wang J., et al. Prognostic value of lactate dehydrogenase for melanoma patients receiving anti-PD-1/PD-L1 therapy: a meta-analysis. Medicine (Baltimore) 2021;100(14) doi: 10.1097/MD.0000000000025318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pires da Silva I., Ahmed T., McQuade J.L., et al. Clinical models to define response and survival with anti-PD-1 antibodies alone or combined with ipilimumab in metastatic melanoma. J Clin Oncol. 2022;40(10):1068–1080. doi: 10.1200/JCO.21.01701. [DOI] [PubMed] [Google Scholar]
- 10.Newell F., Pires da Silva I., Johansson P.A., et al. Multiomic profiling of checkpoint inhibitor-treated melanoma: identifying predictors of response and resistance, and markers of biological discordance. Cancer Cell. 2022;40(1):88–102.e107. doi: 10.1016/j.ccell.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Patel S.P., Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 12.Gibney G.T., Weiner L.M., Atkins M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu S., Stein J.E., Rimm D.L., et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195–1204. doi: 10.1001/jamaoncol.2019.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok J.D., Hoos A., O'Day S., et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 15.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D., Schilling B., Liu D., et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25(12):1916–1927. doi: 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obeid J.M., Erdag G., Smolkin M.E., et al. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology. 2016;5(11) doi: 10.1080/2162402X.2016.1235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ugurel S., Griewank K., Horn S., et al. Tumor PD-L1 expression and gene panel mutational profile as outcome predictors of PD-1-based checkpoint inhibition therapy in metastatic melanoma: a prospective multicenter DeCOG study. J Clin Oncol. 2021;39(15_suppl):9568. [Google Scholar]
- 19.Tawbi H.A., Schadendorf D., Lipson E.J., et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keung E.Z., Gershenwald J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18(8):775–784. doi: 10.1080/14737140.2018.1489246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz L.H., Litière S., de Vries E., et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Placke J.M., Soun C., Bottek J., et al. Digital quantification of tumor PD-L1 predicts outcome of PD-1-based immune checkpoint therapy in metastatic melanoma. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.741993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilie M., Long-Mira E., Bence C., et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27(1):147–153. doi: 10.1093/annonc/mdv489. [DOI] [PubMed] [Google Scholar]
- 24.Hong L., Negrao M.V., Dibaj S.S., et al. Programmed death-ligand 1 heterogeneity and its impact on benefit from immune checkpoint inhibitors in NSCLC. J Thorac Oncol. 2020;15(9):1449–1459. doi: 10.1016/j.jtho.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Zou Y., Hu X., Zheng S., et al. Discordance of immunotherapy response predictive biomarkers between primary lesions and paired metastases in tumours: a systematic review and meta-analysis. eBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madore J., Vilain R.E., Menzies A.M., et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28(3):245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 27.Grzywa T.M., Paskal W., Włodarski P.K. Intratumor and intertumor heterogeneity in melanoma. Transl Oncol. 2017;10(6):956–975. doi: 10.1016/j.tranon.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumeh P.C., Harview C.L., Yearley J.H., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartlett E.K., Fetsch P.A., Filie A.C., et al. Human melanoma metastases demonstrate nonstochastic site-specific antigen heterogeneity that correlates with T-cell infiltration. Clin Cancer Res. 2014;20(10):2607–2616. doi: 10.1158/1078-0432.CCR-13-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdag G., Schaefer J.T., Smolkin M.E., et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladányi A., Papp E., Mohos A., et al. Role of the anatomic site in the association of HLA class I antigen expression level in metastases with clinical response to ipilimumab therapy in patients with melanoma. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H.-H., Wang Y.-N., Xia W., et al. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 2019;36(2):168–178.e164. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rzeniewicz K., Larkin J., Menzies A.M., et al. Immunotherapy use outside clinical trial populations: never say never? Ann Oncol. 2021;32(7):866–880. doi: 10.1016/j.annonc.2021.03.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Files