The heterocyclic fused ring geometry of the title compound coincides with the geometries of related molecules but without directed intermolecular contacts determining the crystal packing.
Keywords: crystal structure, fused ring, heterocycle, dithiine, anhydride
Abstract
In the title compound (systematic name: 2,3-dihydro-1,4-dithiino[2,3-c]furan-5,7-dione), C6H4O3S2, the observed geometry agrees well with those of its phthalamide, thieno and hydroxy analogs, and with a calculated geometry obtained by density functional theory (DFT) calculations. Specific structural features are an S—C—C—S torsion angle of −70.39 (17)° and S—C bonds to sp
2-hybridized C atoms approximately 0.1 Å shorter than those to sp
3-hybridized C atoms. Unlike the extended structures of the analogs, there are no directed intermolecular interactions and the head-to-tail rows of molecules that are a prominent structural motif of the packing can be rationalized in terms of optimized dipole–dipole interactions.
Structure description
The unit-cell parameters for the title compound have been reported previously [Grabowski, 1968 ▸; Cambridge Structural Database (CSD; Groom et al., 2016 ▸) refcode QQQDIA], but atomic coordinates are not available. Related compounds with reported three-dimensional atomic coordinates are the phthalamide (DTHPIM; Kirfel et al., 1975 ▸), the thieno (ZUHQUQ; Skabara et al., 2003 ▸) and the monohydroxy (USUMOL; Kurbangalieva et al., 2010 ▸) analogs, all of which are reported to crystallize in the monoclinic space groups P21/c or P21/n. We report here the three-dimensional structure of 5,6-dihydro-1,4-dithiine-2,3-dicarboxylic anhydride, which crystallizes in the triclinic space group P
 with unit-cell parameters in agreement with those reported by Grabowski.
with unit-cell parameters in agreement with those reported by Grabowski.
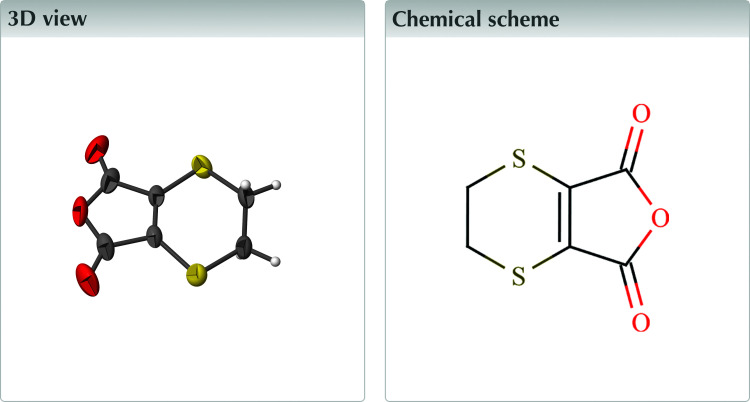
The molecule (Fig. 1 ▸) consists of furandione and dihydro-1,4-dithiine rings fused by a common carbon–carbon double bond (atoms C3 and C4) and is largely planar (r.m.s. deviation of 0.044 Å from the mean plane for all atoms except the CH2 groups). The CH2 groups are twisted about the molecular plane in order to reduce angle strain, with C1 0.323 (3) Å above and C2 0.528 (3) Å below, and an S1—C1—C2—S2 torsion angle of −70.39 (17)°. The S—C bond lengths are 0.096 (7) Å shorter for bonds to sp 2-hybridized C atoms than to those with sp 3-hybridization (Table 1 ▸). The interior bond angles within the furandione ring are close to idealized values for a uniform pentagon, ranging from 107.44 (17) to 108.36 (18)°. The O=C—O angles have expected values of 121–122° for an sp 2-hybridized center, while the external O=C—C angles average 130.2 (7)° in order to accommodate the geometry of the planar ring. These details agree well with the geometrical parameters for maleic anhydride [MLEICA (Marsh et al., 1962 ▸) and MLEICA01 (Lutz, 2001 ▸)].
Figure 1.
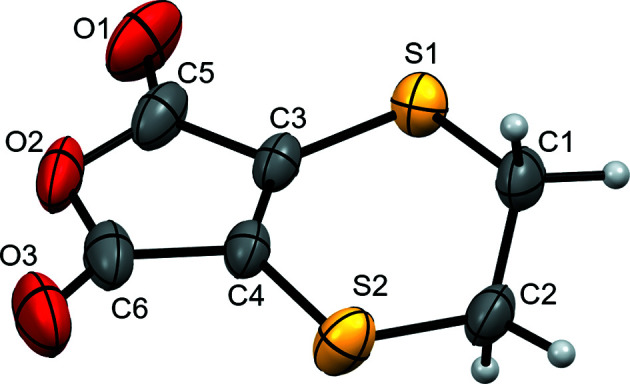
Displacement ellipsoid plot at the 50% probability level of the formula unit of the title compound, showing labels for non-H atoms.
Table 1. Selected geometric parameters (Å, °).
| S1—C1 | 1.805 (2) | C3—C5 | 1.469 (3) |
| S1—C3 | 1.7136 (19) | C4—C6 | 1.472 (3) |
| S2—C2 | 1.817 (2) | O1—C5 | 1.193 (3) |
| S2—C4 | 1.7160 (19) | O2—C5 | 1.376 (3) |
| C1—C2 | 1.510 (3) | O2—C6 | 1.386 (3) |
| C3—C4 | 1.345 (3) | O3—C6 | 1.186 (3) |
| C1—S1—C3 | 99.54 (10) | C3—C4—C6 | 107.44 (17) |
| C2—S2—C4 | 98.30 (10) | O1—C5—C3 | 129.7 (2) |
| S1—C1—C2 | 114.41 (15) | O2—C5—C3 | 108.36 (18) |
| C1—C2—S2 | 115.01 (15) | O1—C5—O2 | 121.98 (19) |
| S1—C3—C4 | 131.57 (14) | O2—C6—C4 | 108.13 (18) |
| S1—C3—C5 | 120.60 (16) | O3—C6—C4 | 130.7 (2) |
| C4—C3—C5 | 107.83 (17) | O2—C6—O3 | 121.2 (2) |
| C3—C4—S2 | 129.27 (14) | C5—O2—C6 | 108.14 (15) |
| C6—C4—S2 | 123.28 (16) |
The geometrical details for the related compounds listed above agree closely with those of the title compound. In particular, the S—C—C—S torsion-angle magnitudes range from 68.10 to 70.75° and the S—C bond lengths to sp 2-hybridized C atoms average 0.087 (14) Å shorter than those to sp 3-hybridized C atoms, with the phthalamide analog providing the closest agreement [average sp 3–sp 2 bond length difference = 0.0995 (7) Å]. A DFT geometry optimization in vacuo [B3LYP functional, cc-pTVZ basis set; GAMESS (Schmidt et al., 1993 ▸)] yields similar results, with an S—C—C—S torsion angle of −69.6° and S—C bond lengths of 1.730 and 1.829 Å to sp 2- and sp 3-hybridized C atoms, respectively. An electrostatic potential plot with the optimized molecule visible is presented in Fig. 2 ▸.
Figure 2.
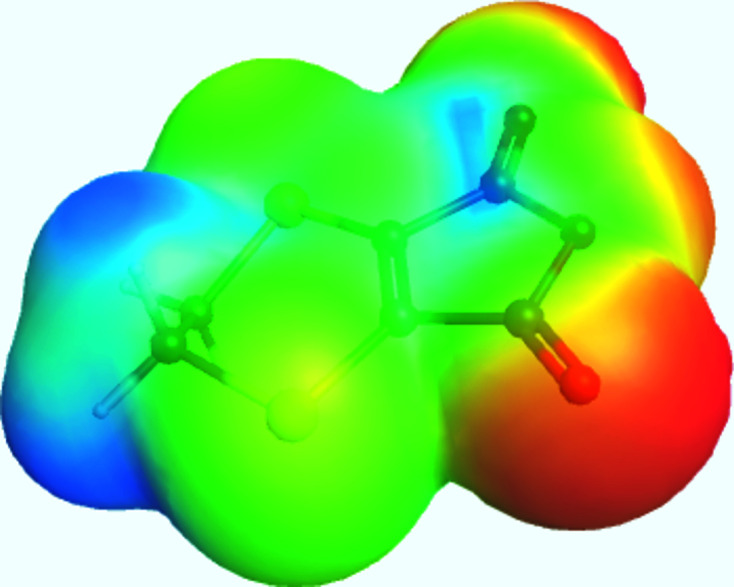
Electrostatic potential plot of the title molecule with the optimized geometry visible. Red represents the most negatively charged regions, while blue represents the most positively charged.
The unit-cell packing of the title compound consists of sheets of molecules lying parallel to (11
 ), with neighboring sheets related by inversion. The molecular planes are approximately coplanar with the sheet, with molecules forming head-to-tail rows parallel to [1
), with neighboring sheets related by inversion. The molecular planes are approximately coplanar with the sheet, with molecules forming head-to-tail rows parallel to [1
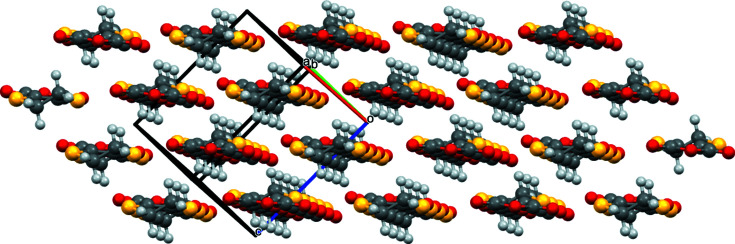
 0] within the sheet. Neighboring rows within the sheet have opposite orientations, while rows on neighboring sheets straddle each other. This packing differs from that of similar molecules, where directed hydrogen bonding or short S⋯O contacts feature prominently, with the head-to-tail rows of molecules in the title compound rationalized in terms of optimized dipole–dipole interactions. A ball-and-stick diagram of a sheet is presented in Fig. 3 ▸ and a unit-cell packing diagram viewed edge on to the sheets is presented in Fig. 4 ▸.
0] within the sheet. Neighboring rows within the sheet have opposite orientations, while rows on neighboring sheets straddle each other. This packing differs from that of similar molecules, where directed hydrogen bonding or short S⋯O contacts feature prominently, with the head-to-tail rows of molecules in the title compound rationalized in terms of optimized dipole–dipole interactions. A ball-and-stick diagram of a sheet is presented in Fig. 3 ▸ and a unit-cell packing diagram viewed edge on to the sheets is presented in Fig. 4 ▸.
Figure 3.
Ball-and stick diagram of the sheet structure viewed perpendiciular to (11
 ).
).
Figure 4.
Ball-and-stick packing diagram of a unit cell, with axis labels viewed along [1
 0], showing the stacking of four sheets to generate the three-dimensional structure.
0], showing the stacking of four sheets to generate the three-dimensional structure.
Synthesis and crystallization
5,6-Dihydro-1,4-dithiin-2,3-dicarboxylic anhydride (98+%) was purchased from Fisher Scientific and recrystalized by slow evaporation at room temperature from tetrahydrofuran solution to yield yellow block-like crystals.
Refinement
Crystal data, data collection, and structure refinement details are listed in Table 2 ▸. The final structure refinement was carried out within the OLEX2 system via Hirshfeld atom refinement with nonspherical atomic form factors using NoSpherA2 (Kleemiss et al., 2021 ▸; Midgley et al., 2021 ▸) derived from electron density from DFT calculations using ORCA (B3LYP functional, def2-SVP basis set; Neese, 2022 ▸). All atoms were refined anisotropically.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C6H4O3S2 |
| M r | 188.23 |
| Crystal system, space group | Triclinic, P
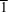
|
| Temperature (K) | 295 |
| a, b, c (Å) | 5.398 (1), 7.5537 (15), 9.2566 (18) |
| α, β, γ (°) | 89.273 (6), 87.361 (6), 75.701 (5) |
| V (Å3) | 365.36 (12) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.68 |
| Crystal size (mm) | 0.42 × 0.38 × 0.17 |
| Data collection | |
| Diffractometer | Bruker D8 Quest Eco |
| Absorption correction | Multi-scan (SADABS; Bruker, 2016 ▸) |
| T min, T max | 0.648, 0.746 |
| No. of measured, independent and observed [I ≥ 2u(I)] reflections | 16978, 1669, 1338 |
| R int | 0.046 |
| (sin θ/λ)max (Å−1) | 0.651 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.033, 0.074, 1.11 |
| No. of reflections | 1669 |
| No. of parameters | 136 |
| H-atom treatment | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.45, −0.25 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314623006478/hb4438sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623006478/hb4438Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314623006478/hb4438Isup3.cml
CCDC reference: 2284875
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| C6H4O3S2 | Z = 2 |
| Mr = 188.23 | F(000) = 192.603 |
| Triclinic, P1 | Dx = 1.711 Mg m−3 |
| a = 5.398 (1) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 7.5537 (15) Å | Cell parameters from 7641 reflections |
| c = 9.2566 (18) Å | θ = 2.8–27.4° |
| α = 89.273 (6)° | µ = 0.68 mm−1 |
| β = 87.361 (6)° | T = 295 K |
| γ = 75.701 (5)° | Plate, yellow |
| V = 365.36 (12) Å3 | 0.42 × 0.38 × 0.17 mm |
Data collection
| Bruker D8 Quest Eco diffractometer | 1338 reflections with I≥ 2u(I) |
| φ and ω scans | Rint = 0.046 |
| Absorption correction: multi-scan (SADABS; Bruker, 2016) | θmax = 27.6°, θmin = 3.6° |
| Tmin = 0.648, Tmax = 0.746 | h = −7→7 |
| 16978 measured reflections | k = −9→9 |
| 1669 independent reflections | l = −12→12 |
Refinement
| Refinement on F2 | 0 constraints |
| Least-squares matrix: full | Primary atom site location: dual |
| R[F2 > 2σ(F2)] = 0.033 | All H-atom parameters refined |
| wR(F2) = 0.074 | w = 1/[σ2(Fo2) + (0.0229P)2 + 0.1706P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.11 | (Δ/σ)max = 0.0002 |
| 1669 reflections | Δρmax = 0.45 e Å−3 |
| 136 parameters | Δρmin = −0.25 e Å−3 |
| 0 restraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.87227 (9) | 0.35661 (7) | 0.38382 (6) | 0.04127 (16) | |
| S2 | 0.45568 (10) | 0.23942 (8) | 0.13318 (6) | 0.04824 (17) | |
| C1 | 0.8444 (5) | 0.1333 (3) | 0.3315 (2) | 0.0440 (5) | |
| H1a | 1.028 (5) | 0.039 (4) | 0.347 (3) | 0.091 (10) | |
| H1b | 0.700 (5) | 0.098 (3) | 0.400 (3) | 0.072 (8) | |
| C2 | 0.7794 (4) | 0.1195 (3) | 0.1758 (2) | 0.0440 (5) | |
| H2a | 0.793 (6) | −0.019 (4) | 0.152 (3) | 0.086 (9) | |
| H2b | 0.909 (5) | 0.169 (4) | 0.102 (3) | 0.064 (8) | |
| C3 | 0.6129 (3) | 0.4890 (2) | 0.3010 (2) | 0.0338 (4) | |
| C4 | 0.4505 (3) | 0.4463 (2) | 0.2093 (2) | 0.0347 (4) | |
| C5 | 0.5328 (4) | 0.6869 (3) | 0.3268 (2) | 0.0457 (5) | |
| C6 | 0.2545 (4) | 0.6150 (3) | 0.1802 (2) | 0.0478 (5) | |
| O1 | 0.6268 (4) | 0.7808 (2) | 0.3987 (2) | 0.0682 (5) | |
| O2 | 0.3162 (3) | 0.75751 (18) | 0.25156 (17) | 0.0558 (4) | |
| O3 | 0.0695 (3) | 0.6399 (2) | 0.11090 (19) | 0.0695 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0364 (3) | 0.0389 (3) | 0.0489 (3) | −0.0100 (2) | −0.0021 (2) | −0.0005 (2) |
| S2 | 0.0401 (3) | 0.0441 (3) | 0.0610 (4) | −0.0107 (2) | −0.0014 (2) | −0.0144 (3) |
| C1 | 0.0523 (14) | 0.0263 (10) | 0.0484 (13) | −0.0007 (10) | 0.0016 (11) | 0.0030 (9) |
| H1a | 0.08 (2) | 0.057 (19) | 0.10 (2) | 0.045 (17) | −0.031 (18) | 0.019 (17) |
| H1b | 0.09 (2) | 0.033 (15) | 0.10 (2) | −0.030 (15) | 0.015 (18) | −0.009 (15) |
| C2 | 0.0464 (12) | 0.0299 (11) | 0.0496 (13) | 0.0014 (9) | 0.0061 (10) | −0.0076 (10) |
| H2a | 0.13 (3) | 0.038 (16) | 0.09 (2) | −0.012 (17) | 0.007 (18) | −0.021 (15) |
| H2b | 0.053 (17) | 0.076 (19) | 0.060 (17) | −0.013 (15) | 0.022 (13) | 0.014 (15) |
| C3 | 0.0335 (9) | 0.0236 (9) | 0.0427 (10) | −0.0055 (7) | 0.0074 (8) | −0.0008 (8) |
| C4 | 0.0306 (9) | 0.0281 (9) | 0.0415 (10) | −0.0011 (7) | 0.0060 (8) | 0.0003 (8) |
| C5 | 0.0551 (13) | 0.0245 (10) | 0.0556 (13) | −0.0096 (9) | 0.0171 (10) | −0.0021 (9) |
| C6 | 0.0370 (11) | 0.0442 (12) | 0.0528 (12) | 0.0054 (9) | 0.0076 (10) | 0.0118 (10) |
| O1 | 0.0867 (13) | 0.0358 (9) | 0.0862 (12) | −0.0262 (9) | 0.0178 (10) | −0.0185 (9) |
| O2 | 0.0611 (10) | 0.0270 (7) | 0.0673 (10) | 0.0084 (7) | 0.0161 (8) | 0.0070 (7) |
| O3 | 0.0467 (9) | 0.0712 (12) | 0.0784 (12) | 0.0086 (8) | −0.0076 (9) | 0.0218 (10) |
Geometric parameters (Å, º)
| S1—C1 | 1.805 (2) | C2—H2b | 1.08 (2) |
| S1—C3 | 1.7136 (19) | C3—C4 | 1.345 (3) |
| S2—C2 | 1.817 (2) | C3—C5 | 1.469 (3) |
| S2—C4 | 1.7160 (19) | C4—C6 | 1.472 (3) |
| C1—H1a | 1.08 (2) | O1—C5 | 1.193 (3) |
| C1—H1b | 1.06 (2) | O2—C5 | 1.376 (3) |
| C1—C2 | 1.510 (3) | O2—C6 | 1.386 (3) |
| C2—H2a | 1.06 (2) | O3—C6 | 1.186 (3) |
| C1—S1—C3 | 99.54 (10) | S1—C3—C4 | 131.57 (14) |
| C2—S2—C4 | 98.30 (10) | S1—C3—C5 | 120.60 (16) |
| S1—C1—H1a | 107.2 (15) | C4—C3—C5 | 107.83 (17) |
| S1—C1—H1b | 108.1 (12) | C3—C4—S2 | 129.27 (14) |
| H1a—C1—H1b | 110 (2) | C6—C4—S2 | 123.28 (16) |
| S1—C1—C2 | 114.41 (15) | C3—C4—C6 | 107.44 (17) |
| C2—C1—H1a | 107.8 (16) | O1—C5—C3 | 129.7 (2) |
| C2—C1—H1b | 108.9 (15) | O2—C5—C3 | 108.36 (18) |
| C1—C2—S2 | 115.01 (15) | O1—C5—O2 | 121.98 (19) |
| S2—C2—H2a | 105.1 (17) | O2—C6—C4 | 108.13 (18) |
| S2—C2—H2b | 107.5 (13) | O3—C6—C4 | 130.7 (2) |
| C1—C2—H2a | 108.8 (16) | O2—C6—O3 | 121.2 (2) |
| C1—C2—H2b | 111.8 (14) | C5—O2—C6 | 108.14 (15) |
| H2a—C2—H2b | 108 (2) | ||
| S1—C1—C2—S2 | −70.39 (17) | S2—C4—C6—O3 | 4.1 (2) |
| S1—C3—C4—S2 | −3.9 (2) | C3—C4—C6—O2 | 3.05 (16) |
| S1—C3—C4—C6 | 176.69 (19) | C3—C4—C6—O3 | −176.50 (17) |
| S1—C3—C5—O1 | 1.95 (19) | C3—C5—O2—C6 | 0.24 (18) |
| S1—C3—C5—O2 | −177.86 (15) | C4—C6—O2—C5 | −1.95 (18) |
| S2—C4—C3—C5 | 176.57 (18) | C5—O2—C6—O3 | 177.65 (17) |
| S2—C4—C6—O2 | −176.39 (17) |
References
- Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2015). Acta Cryst. A71, 59–75. [DOI] [PMC free article] [PubMed]
- Bruker (2016). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2018). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Grabowski, M. (1968). Soc. Sci. Lodz. Acta Chim. 13, 43.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Kirfel, A., Will, G. & Fickentscher, K. (1975). Acta Cryst. B31, 1973–1975.
- Kleemiss, F., Dolomanov, O. V., Bodensteiner, M., Peyerimhoff, N., Midgley, M., Bourhis, L. J., Genoni, A., Malaspina, L. A., Jayatilaka, D., Spencer, J. L., White, F., Grundkötter-Stock, B., Steinhauer, S., Lentz, D., Puschmann, H. & Grabowsky, S. (2021). Chem. Sci. 12, 1675–1692. [DOI] [PMC free article] [PubMed]
- Kurbangalieva, A. R., Lodochnikova, O. A., Devyatova, N. F., Berdnikov, E. A., Gnezdilov, O. I., Litvinov, I. A. & Chmutova, G. A. (2010). Tetrahedron, 66, 9945–9953.
- Lutz, M. (2001). Acta Cryst. E57, o1136–o1138.
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Marsh, R. E., Ubell, E. & Wilcox, H. E. (1962). Acta Cryst. 15, 35–41.
- Midgley, L., Bourhis, L. J., Dolomanov, O. V., Grabowsky, S., Kleemiss, F., Puschmann, H. & Peyerimhoff, N. (2021). Acta Cryst. A77, 519–533. [DOI] [PubMed]
- Neese, F. (2022). WIREs Comput. Mol. Sci. 12, e1606.
- Schmidt, J. R. & Polik, W. F. (2016). WebMO Enterprise. Version 20.0.011e. WebMO LLC, Holland, MI, USA.
- Schmidt, M. W., Baldridge, K. K., Boatz, J. A., Elbert, S. T., Gordon, M. S., Jensen, J. H., Koseki, S., Matsunaga, N., Nguyen, K. A., Su, S., Windus, T. L., Dupuis, M. & Montgomery, J. A. (1993). J. Comput. Chem. 14, 1347–1363.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Skabara, P. J., Coles, S. J. & Hursthouse, M. B. (2003). CCDC deposition No. 1057366. CCDC, Cambridge, UK.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314623006478/hb4438sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623006478/hb4438Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314623006478/hb4438Isup3.cml
CCDC reference: 2284875
Additional supporting information: crystallographic information; 3D view; checkCIF report