Abstract
Research exploring the links between the microbiome and autism has inadequately considered the contribution of diet diversity. Recently in Cell, Yap et al. addressed the contribution of restrictive dietary patterns to microbiome diversity in autism and have found that decreased dietary diversity shapes the microbiome more than previously appreciated.
The concept of dietary diversity is joyfully embodied by the Eric Carle classic, The Very Hungry Caterpillar, where the caterpillar eats a different fruit each day of the week: on Monday an apple; Tuesday pears; Wednesday plums; Thursday strawberries; Friday oranges; Saturday a smorgasbord of new foods; and on Sunday a nice green leaf. Objectively, this caterpillar has a highly diverse diet. This book is a staple of many homes and at its core is a message about diverse eating patterns that children may emulate. But, for some families of children diagnosed with autism spectrum disorder (ASD), meal times are filled with tension from avoidant and selective food preferences due to strong sensitivities to food appearance, taste, texture, and smell.
ASD is a complex developmental disorder characterized by alterations in communication and social interactions, sensory anomalies, and increased incidence of repetitive behaviors and restricted interests. Signs of ASD can be observed during the first year of life, despite diagnosis not typically occurring until toddlerhood (Constantino and Marrus, 2017). The etiology of ASD is thought to have much deeper roots, beginning very early in life, involving both genetic and environmental factors (Constantino and Marrus, 2017). Genetic studies show a network of genes whose dysfunction during development contributes to the ASD phenotypes observed. Environmental exposures, and other factors such as increased parental age and preterm delivery, also play important roles. Based on both animal and human work, deviations from exposures to the typical host of microbes early in life associated with these risk factors may contribute to the etiology of ASD (Roussin et al., 2020).
The most compelling evidence that the microbiome contributes to the development of ASD comes from animal studies. When mice born without microbial exposure (germ-free) are colonized with stool from human donors with ASD post-weaning, these mice show altered behavior that appears to mirror that observed in autistic humans. Further, altering the microbiome of the dam during pregnancy can also impact ASD relevant behaviors (Roussin et al., 2020). These studies and others provide evidence that early microbial disruptions contribute greatly to the behavioral alterations observed. Combined, these animal studies support a role of early microbiome exposures in the etiology of ASD.
Recently in Cell, Yap et al. (2021) comprehensively explored microbiome-ASD associations in a highly detailed and rigorous observational study in humans. Yap et al. sequenced the fecal microbiomes of 247 children, including 99 with diagnosed ASD and 51 of their undiagnosed siblings, using metagenomic sequencing. Models using this data showed that children’s microbiome composition was associated with age, stool consistency, and diet, but not ASD diagnosis; with only one species, Romboutsia timonensis, identified as differentially abundant and lower in those with ASD. These results differ from previous human studies that have reported multiple ASD-microbiome associations (Ho et al., 2020). However, few of these previous studies address the potential critical confounding role of diet in ASD-microbiome relationships.
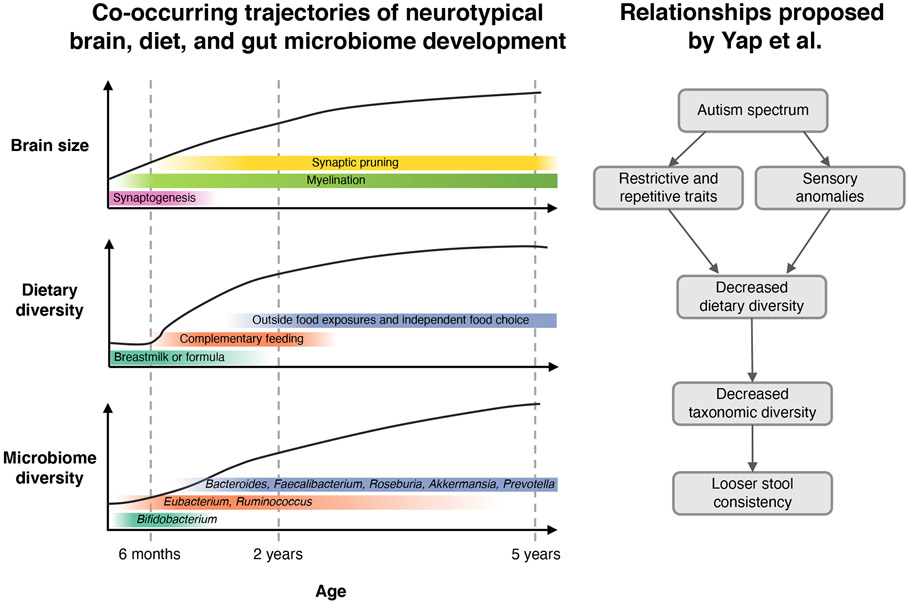
The developing microbiome undergoes enormous restructuring in concert with the period of development between infancy and childhood (Figure 1). An expansion of species in the gut begins around 4 months corresponding with increased hand-to-mouth actions, then continues with the introduction of solids around 6 months of age (Roswall et al., 2021). It is tempting and convenient to assume that diet drives much of the expansion in microbiome diversity during this developmental period and into adulthood. While this hypothesis is an appealing one (Heiman and Greenway, 2016), a relationship between dietary diversity and microbiome diversity has not been causally demonstrated. In fact, dietary diversity has been minimally explored in relation to the human microbiome and only as a component of dietary scoring indices that also incorporate overall dietary quality (Claesson et al., 2012). Isolating the impact of dietary diversity, exclusive of overall eating pattern, on microbiome diversity has not been explored, in part, due to challenges operationalizing dietary diversity.
The complex relationships between brain development, dietary intake, and microbiome diversity in neurotypical development are still poorly understood, making alterations in these pathways that contribute to the development of ASD nearly impossible to identify without consideration and careful concurrent measurement of these potential influential factors. The first 5 years of life are characterized by rapid brain growth and underlying cellular neurodevelopmental processes. During the same period dietary exposures change from exclusive diets of breastmilk and/or formula to the introduction of complementary foods and the development of independent food choices. These changes in diet have also been linked with variation in microbiome composition with a transition from a Bifidobacterium-dominant community to a more adult-like composition corresponding with intake diverse dietary fibers (Roswall et al., 2021). Yap et al. propose a model where autism spectrum disorder is upstream of the development of restrictive eating traits and sensory preferences that result in lowered dietary diversity and ultimately lead to a reduction in taxonomic diversity and changes in stool consistency. According to this model optimal ASD interventions may benefit from strategies to increase diet diversity to improve GI symptoms. Although this approach may successfully alleviate GI discomfort, it is unclear from this work whether it would also improve the core behavioral phenotypes, or when such strategies should be implemented (i.e., during infancy) to prevent severe symptoms.
Yap et al. explore relationships between dietary diversity and the microbiome in ASD by applying the Shannon diversity index to measure alpha diversity of diet. Ecological metrics for alpha diversity are common in analysis of microbiomes but have rarely been applied to dietary data (Johnson et al., 2019). Yap et al. report that children with ASD had less-diverse diets than both their undiagnosed siblings and the undiagnosed children. This reduced dietary diversity corresponded with reduced microbiome diversity. Overall, they found that ASD-associated dietary restriction, and not ASD diagnosis or energy intake, was associated with reduced microbiome diversity and stool consistency.
Only prospective, longitudinal data and fecal sample collection from a group of infants at increased risk to develop ASD will ultimately be able to answer questions regarding how specific early exposures, including diet, shape ASD phenotypes. However, these observational findings raise important questions about the role of the microbiome in the pathology of ASD and the potential for microbial interventions to manage ASD symptoms in children. It brings into question the validity of intestinal microbiota transplants in autistic populations (Kang et al., 2019), especially outside of very early childhood. If microbiota transplants are to be implemented successfully, then it is likely those interventions will need to be accompanied by intensive behavioral and dietary interventions to increase dietary diversity to support a new diverse microbiome.
Just as the very hungry caterpillar undergoes an amazing metamorphosis emerging from a chrysalis and becoming a butterfly, a symbol of the beauty in neurodiversity, incorporation of advanced dietary metrics into analysis of microbiome data has the potential to reveal previously obscured relationships. This research highlights how important it is to incorporate detailed dietary data into observational studies of complex conditions, particularly where there are feasible dietary contributions to microbiome outcomes.
Acknowledgements
AJJ is supported in part by the National Institutes of Health under Award Numbers P30CA077598 and R21DK125933. BRH is an iTHRIV Scholar. The iTHRIV Scholars Program is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR003015 and KL2TR003016.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O’Sullivan O, et al. (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184. [DOI] [PubMed] [Google Scholar]
- Constantino JN, and Marrus N (2017). The Early Origins of Autism. Child Adolesc. Psychiatr. Clin. N. Am 26, 555–570. [DOI] [PubMed] [Google Scholar]
- Heiman ML, and Greenway FL (2016). A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab 5, 317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LKH, Tong VJW, Syn N, Nagarajan N, Tham EH, Tay SK, Shorey S, Tambyah PA, and Law ECN (2020). Gut microbiota changes in children with autism spectrum disorder: a systematic review. Gut Pathogens 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Walter J, et al. (2019). Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host & Microbe 25, 789–802.e5. [DOI] [PubMed] [Google Scholar]
- Kang D-W, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, and Krajmalnik-Brown R (2019). Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep 9, 5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roswall J, Olsson LM, Kovatcheva-Datchary P, Nilsson S, Tremaroli V, Simon M-C, Kiilerich P, Akrami R, Krämer M, Uhlén M, et al. (2021). Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 29, 765–776.e3. [DOI] [PubMed] [Google Scholar]
- Roussin L, Prince N, Perez-Pardo P, Kraneveld AD, Rabot S, and Naudon L (2020). Role of the Gut Microbiota in the Pathophysiology of Autism Spectrum Disorder: Clinical and Preclinical Evidence. Microorganisms 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap et al. (2021). Autism-related dietary preferences mediate autism-gut microbiome associations. Cell, DOI: 10.1016/j.cell.2021.10.015 [DOI] [PubMed] [Google Scholar]