Significance
The vanilloid receptor TRPV1 is a prototype temperature receptor for detecting noxious heat among other pain-producing signals at peripheral nerve endings. Its strong temperature sensitivity requires a large energetic change in channel opening. But the underlying mechanism has not been elucidated. Here, we directly measured the heat uptake of TRPV1 by differential scanning calorimetry on reconstituted channels in vesicles. We show that the thermal transition in TRPV1 is accompanied with energetic changes on a scale sufficient to compromise protein stability. Our findings suggest that coupling functional activation to irreversible conformation transitions is important for TRPV1 to attain its exquisite temperature sensitivity, which, from a thermodynamic stand, may be a common mechanism for strongly temperature-dependent biological processes.
Keywords: Vanilloid receptor, temperature receptor, nociceptor, ion channel gating, protein denaturation
Abstract
The vanilloid receptor TRPV1 is an exquisite nociceptive sensor of noxious heat, but its temperature-sensing mechanism is yet to define. Thermodynamics dictate that this channel must undergo an unusually energetic allosteric transition. Thus, it is of fundamental importance to measure directly the energetics of this transition in order to properly decipher its temperature-sensing mechanism. Previously, using submillisecond temperature jumps and patch-clamp recording, we estimated that the heat activation for TRPV1 opening incurs an enthalpy change on the order of 100 kcal/mol. Although this energy is on a scale unparalleled by other known biological receptors, the generally imperfect allosteric coupling in proteins implies that the actual amount of heat uptake driving the TRPV1 transition could be much larger. In this paper, we apply differential scanning calorimetry to directly monitor the heat flow in TRPV1 that accompanies its temperature-induced conformational transition. Our measurements show that heat invokes robust, complex thermal transitions in TRPV1 that include both channel opening and a partial protein unfolding transition and that these two processes are inherently coupled. Our findings support that irreversible protein unfolding, which is generally thought to be destructive to physiological function, is essential to TRPV1 thermal transduction and, possibly, to other strongly temperature-dependent processes in biology.
Nociceptive ion channels are first-line responders of our nervous system to imminent danger. They are located on the body surface where their most critical goal is to initiate a “possible threat” signal. In particular, the vanilloid receptor subtype 1 (TRPV1) is specialized in the perception of low-threshold noxious heat (40 to 50 °C), as well as pain-producing substances such as proinflammatory components released during tissue injury, or natural products like capsaicin, the primary pungent ingredient in “hot” chili peppers (1). Its homologs, such as TRPV2 and TRPV3, are also responsive to heat but in different temperature ranges (2–5), whereas TRPM8 is activated by innocuous cold (6, 7). These temperature-sensitive channels belong to a group of thermal TRP channels that are principal candidates for temperature sensing in mammals (8, 9).
A hallmark of temperature receptors is their exquisite temperature sensitivity. The heat-sensitive TRPV1 reaches its maximum response over a temperature change less than 10 °C (10). While such a strong temperature dependence is essential to biological functions, it far exceeds that of other known ion channels. In principle, the channel may incur this temperature sensitivity from its environment, for instance, the membrane. However, the perturbation of membrane fluidity did not alter the heat sensitivity of TRPV1 (11). More recently, TRPV1 and its homologs were further found to retain their native temperature sensitivity in reconstituted vesicles (12–14). Models in favor of an intrinsic temperature dependence of proteins also exist, such as the heat capacity theory (15). This theory elegantly predicts a temperature-induced heat capacity change as a major energy source to drive channel activation. An implication of the model is that a relatively large number of nonpolar residues have to translocate into the aqueous environment, which may compromise protein stability. Intensive structure–function studies have also been undertaken to identify functionally important molecular sites pertinent to temperature sensing (16–34). So far, these studies have failed to converge to a consensus model or a consistent heat-sensor domain. Many molecular sites have been detected, but they are disparately located within channels and reveal no particular endothermic features required for strong temperature dependence.
In thermodynamics, the temperature dependence of a protein conformation transition originates from an enthalpy change—the steeper the temperature dependence, the larger the energetic change. Thus, to understand how thermal TRP channels such as TRPV1 sense temperature, it is fundamentally important to determine the energetics of these channels. By applying fast temperature jumps that resolve the activation time course, we found previously that the heat activation of TRPV1 incurs an enthalpy change about 100 kcal/mol (10). This energy is many times that of ligand or voltage-dependent gating (35). Furthermore, in allosteric proteins, the energy delivered to an effector site (in channels, the gate) is only a fraction of that detected by the sensor site(s). Hence, the actual amount of heat absorbed by TRPV1 is likely much larger. Although there is no theory predicting how large the enthalpy change in ion channel gating can be, that of an allosteric transition ultimately pertains to conformational changes that need to be limited in scale in order to maintain reversibility. Indeed, most ion channels oscillate between stable states under the influence of an external stimulus. But, while such a paradigm is generally necessary for channels to function continuously and repetitively over time, it does not exclude alternative mechanisms for other functional scenarios. Here, we show that the unusual energetic of TRPV1 reflects a departure from this apparent requirement of reversibility in channel gating.
In this paper, we measure directly the heat uptake in TRPV1 by differential scanning calorimetry (DSC). DSC directly monitors the energy (enthalpy) change accompanying temperature-induced conformational transitions in the protein. It reports energy of all transitions occurring in the channel and thus complements the patch-clamp detection of that of the closed-to-open transition in the last step of gating. The DSC measurements reveal that TRPV1 possesses inherent thermal instability. Temperatures that normally activate the channel also induce partial protein denaturation (unfolding). The thermal transitions underlying channel opening and denaturation are tightly coupled and share similar activation energy barriers, environmental dependences, and transition temperatures. We therefore hypothesize that the heat-evoked opening of TRPV1 incurs large conformational transitions that both allow an exotically large enthalpy change and also prime the channel for denaturation.
Results
Protein Purification and Reconstitution.
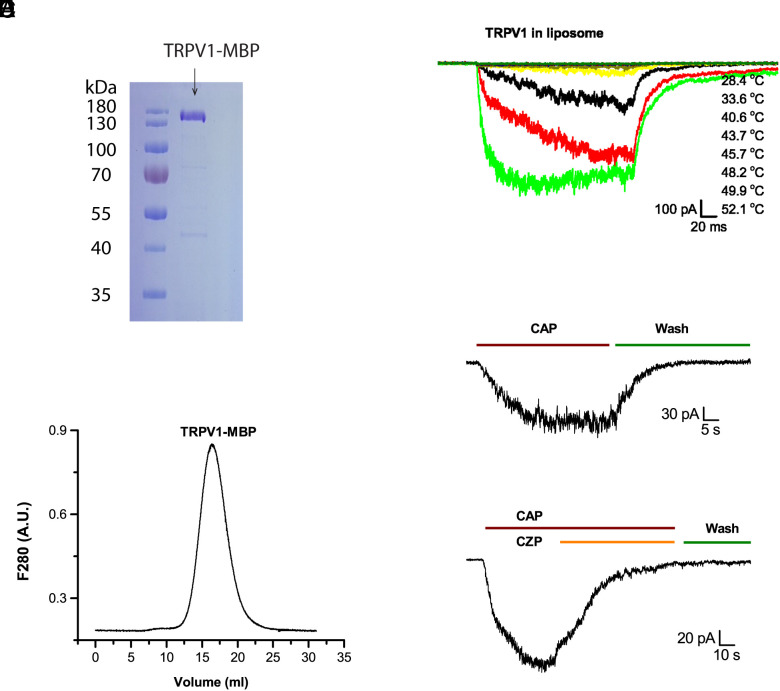
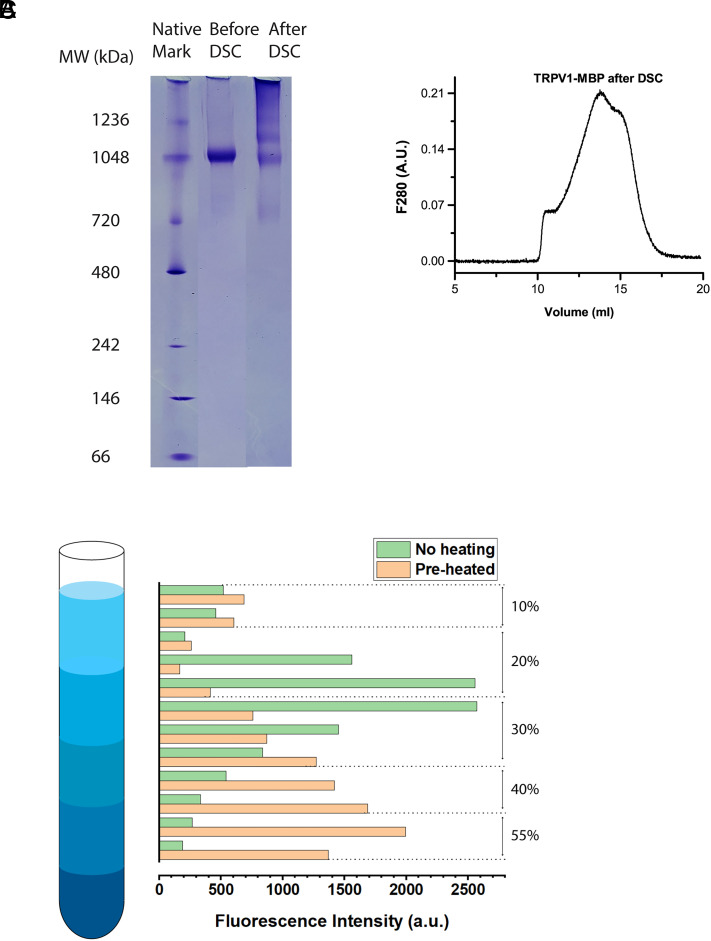
We exploited the maltose binding protein (MBP) fused to the N terminus of TRPV1 (12) as both an affinity tag and an internal caliber in subsequent DSC experiments to calibrate and quantify protein concentrations (see SI Materials and Methods and Discussions below). The amylose affinity chromatography allowed the TRPV1-MBP fusion protein to be purified in one step to biochemical homogeneity (Fig. 1A). Protein integrity of the sample was validated by the size-exclusion chromatography (SEC), which shows that the purified TRPV1-MBP proteins migrated predominantly as monodispersed tetramers (Fig. 1B).
Fig. 1.
Characterization of TRPV1-MBP proteins in vesicles. (A) Affinity-purified TRPV1 proteins visualized on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel by Coomassie blue staining. (B) Analysis of purified TRPV1 proteins by SEC, showing that protein elutes migrated mainly as a single peak on a Superose 6 column (home packed). (C) Patch-clamp recording of heat response of TRPV1 reconstituted in soybean lipids. The pulse duration was 200 ms. The temperature list in the legend follows the order of traces. The time interval between successive pulses ranged from half a minute to a minute. (D) Capsaicin-evoked response (CAP; 10 µM). (E) Inhibition of capsaicin response by capsazepine (CZP; 10 µM). Holding potential −60 mV.
For functional testing and DSC measurements, the purified TRPV1-MBP fusion protein was reconstituted into liposomes formed from extracted polar soybean lipids. The reconstitution produced functional channels (Fig. 1 C–E) that remained activated by both heat and capsaicin as well as inhibited by capsazepine, a specific antagonist of TRPV1. The heat activation profiles in vesicles resembled those in native cells, further supporting that the fusion of MBP did not interfere with channel functions (12).
Thermal Transitions of TRPV1 in Liposomes.
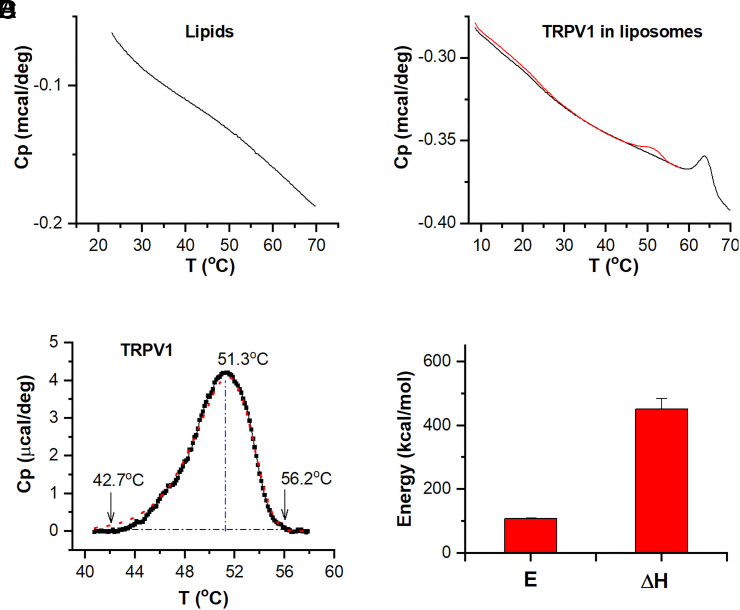
DSC scans detected no apparent peak in control liposomes over a broad temperature range (5 °C to 70 °C; Fig. 2A), indicating that the soybean lipids used for reconstitution were free of phase transitions. Fig. 2B shows exemplar DSC scans of proteoliposomes containing TRPV1-MBP proteins. The scans displayed two transition peaks (red) on the top of an instrument-dependent baseline. The first transition peak occurred at ∼50 °C and the second between 60 and 70 °C. These transition temperatures closely match the characteristic heat activation temperature of TRPV1 in cell membranes and the melting temperature of MBP in aqueous solutions (36), respectively. To corroborate that the low-temperature transition is indeed associated with the channel protein, we separately reconstituted TRPV1 by enzymatically cleaving off the MBP tag. The scans of the resultant proteoliposomes showed almost exclusively the first transition peak (SI Appendix, Fig. S1A), with a residual peak of MBP about 10% of that before cleavage (SI Appendix, Fig. S1B; c.f. SI Appendix, Fig. S2A). Thus, based on these observations, we assign the first peak of the DSC scan to TRPV1 and the second to MBP.
Fig. 2.
Thermal transitions of TRPV1 in liposomes. (A) Calorimetric scan of blank liposomes obtained in the reconstitution buffer. Lipid concentrations were 5 mg/mL. The downward shift of the scan was intrinsic to the instrument. (B) Original DSC scans of reconstituted TRPV1 proteins in liposomes. The channel protein was fused with the MBP on the N terminus for both affinity purification and concentration calibration. The initial scan was limited to the temperature range of functional heat activations, while the subsequent scans were continued to 70 °C to induce the melting transition of MBP. (C) Thermograph of TRPV1, extracted from original scan after baseline subtraction. The dotted red line represents the fit by a two-state model. The area of the peak corresponds to the total enthalpy change between states (ΔH), while the rising slope of the curve manifested the activation enthalpy (E). The vertical blue line indicates the peak temperature, and the arrows point to temperatures measured at approximately 1.0% of peak amplitude. (D) Summary plot of DSC enthalpy change (ΔH) and activation energy (E) resulting from model fits. Data are presented as mean ± SE. n = 8. DSC scanning rate was 1.0 °C/min.
Repeated DSC scans demonstrate that the DSC transitions were strongly irreversible. Both transition peaks of TRPV1 and MBP were seen only during the first run and vanished in all subsequent scans. Limiting the first run to a submaximum temperature (e.g., ∼55 °C as in Fig. 2B, which corresponds to the maximum opening temperature of TRPV1) did not eliminate the irreversibility of the TRPV1 transition. This observation suggests that moderate temperatures as often used for functional activation of TRPV1 in cell membranes suffices to abolish its signature DSC transition peak. Consistently, patch-clamp recording corroborates that following the maximum activation by heat, continued applications of heat pulses resulted in functional rundown, with no sign of recovery within the experimental time (SI Appendix, Fig. S3). Apparent rundown of TRPV1 was also observed in native cells when stimulated with slow temperature controls (37). Since the rate of temperature increments in DSC was slower than temperature ramps commonly used in patch clamp, the same rundown process was anticipated in DSC. Thereby, the apparent DSC irreversibility mechanistically concurs with the functional irreversibility of TRPV1 in patch clamp.
For quantitative analyses, we subtracted the baseline and isolated the transition peaks of both TRPV1 and MBP (Fig. 2C and SI Appendix, Fig. S2A), which are hereafter called thermographs. Sharpness of the thermographs contains information about activation enthalpy (energy barrier), while the areas underneath the peaks determine total enthalpy changes across transition states. These energetic terms were extracted by fitting the thermographs to appropriate kinetic models (SI Appendix, S7). A two-state model, A→B, proved adequate for TRPV1, yielding an activation enthalpy E = 108 ± 2 kcal/mol and an end-state enthalpy change ΔH = 452 ± 22 kcal/mol (n = 8; Fig. 2D). As an internal control, the MBP thermograph revealed the same energetics as those obtained in solutions (see SI Appendix, Fig. S2 & below) (36), corroborating that our energetic analysis is on the correct scale.
To further examine the functional relevance of the DSC transition, we exploited the phenomenological concept of temperature threshold (Th) as commonly used for characterizing functional heat activations of temperature receptors. We measured temperatures of DSC transitions at ∼1% of the peak amplitude, which was ∼42 °C for TRPV1 and thus similar to functional observations (e.g., Fig. 1C). These results indicate that in DSC scans, TRPV1 possessed an activation energy and a temperature threshold both nearly identical to those from patch-clamp measurements during heat activation (E∼96 kcal/mol and Th ∼42 °C) (1, 10). Such a close match is probably not coincidental but reflects that the onset of the DSC transition is parallel to channel activation in reconstituted vesicles. On the other hand, the overall DSC ΔH, as integrated from the peak, considerably exceeded, by >fourfolds, the patch-clamp estimate of the ΔH between the closed and open states (∼100 kcal/mol) (10). The excess energy manifested that the energy transduced to the channel gate makes only a small portion of the total heat absorbed by the channel. A significant amount of energy was lost to intermediate processes, including imperfect allosteric coupling between the sensor and the gate and the traversing of additional conformation states following opening, such as the undefined rundown states evident in patch clamp (Discussion).
Thermal Transitions of TRPV1 in Detergents.
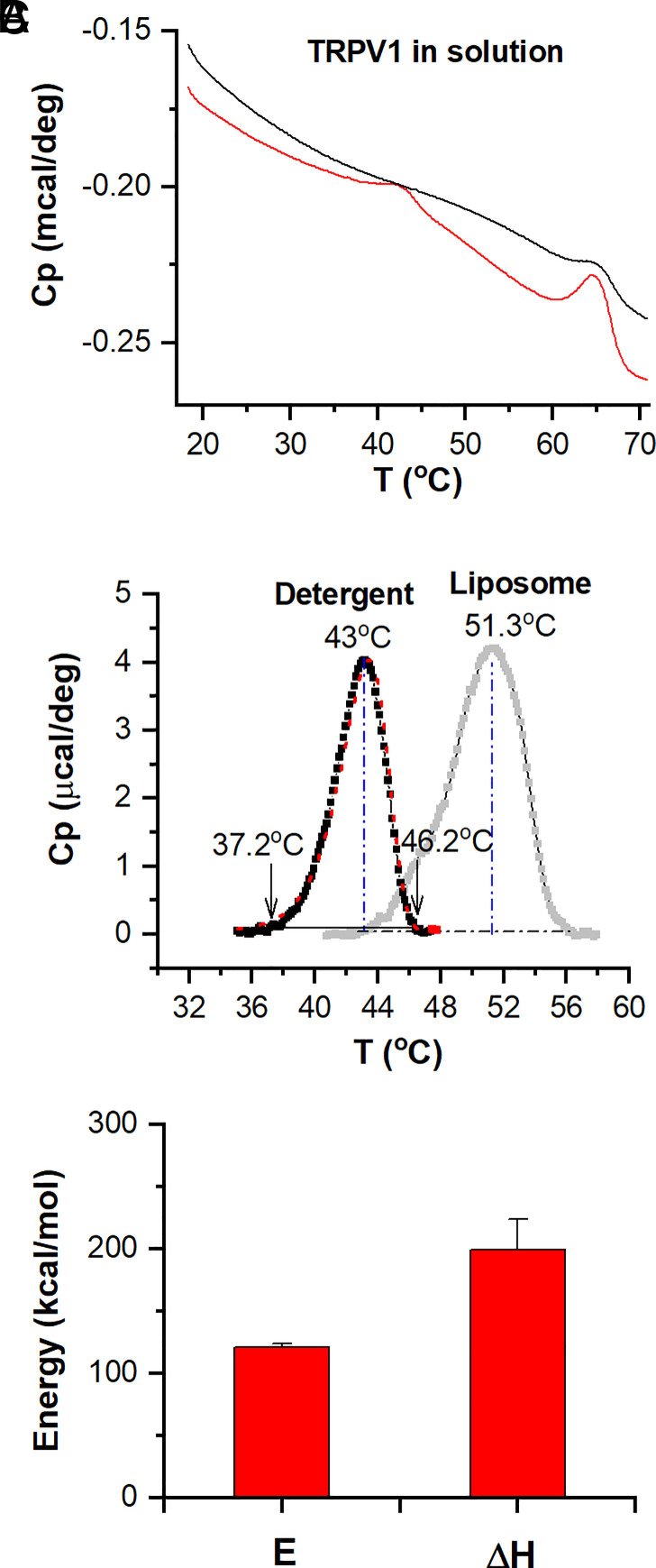
The large ΔH of the DSC transition peak indicates that heat induces complex thermal transitions in TRPV1 beyond channel opening. To resolve whether these transitions are mutually independent or mechanistically related, we resorted to perturbation experiments. In particular, we found that the replacement of lipids by detergents could produce a significant shift on the DSC transition of TRPV1. Thus, we purified channel proteins in detergents and measured their DSC transitions. The scan of TRPV1-MBP in the presence of 0.5 mM dodecyl maltoside (DDM) detected similarly two transition peaks that occurred only during the first run, too (Fig. 3). The MBP sometimes showed a residual peak in subsequent scans. But, compared to the large peak of the first scan, it was quite insignificant.
Fig. 3.
Thermal transitions of TRPV1 proteins in detergents. (A) Original DSC scans (red for initial run and black for repetition). Proteins were solubilized at a concentration of 0.41 mg/mL in the elution buffer containing 0.5 mM DDM. (B) Plot of excess heat capacity of TRPV1 (black color) extracted from the peak of the scan at lower temperatures. The curve shown in the background in gray color corresponds to the liposome measurement (Fig. 1C). The vertical blue lines indicate the peak temperatures, while the arrows point to the threshold temperatures at 1.0% peak amplitude. The dotted red lines represent the best fit by a two-state model. (C) Summary plot of DSC enthalpy change (ΔH) and activation energy (E) resulting from model fits (n = 7). DSC scanning rate at 1.0 K/min.
Fig. 3B compares the isolated thermograph of detergent-solubilized TRPV1-MBP with that of channels in membranes. Most noticeably, the DSC transitions of TRPV1 in detergents were profoundly shifted to a lower temperature range, by nearly 9 °C. Importantly, they remained to appear as a single concerted peak, indicating that the underlying component transitions of channel opening and rundown share a similar environmental dependence so that they were shifted concomitantly. The DSC transitions in detergents were fitted to a two-state model too, yielding an activation enthalpy E = 121 ± 3 kcal/mol and an end-state ΔH = 199 ± 3 kcal/mol (n = 7; Fig. 3C). The onset of the transition attained an activation enthalpy (energy barrier) comparable to that in lipids, whereas the ΔH across states became profoundly reduced (by ~56%). These results are consistent with the observations in liposomes that the early transitions were initiated by channel opening, while the overall ΔH involves additional processes, and the large shift in the temperature threshold implies an increase of the activation entropy.
In contrast to TRPV1, the DSC transitions of MBP in detergents were almost the same as in lipids (SI Appendix, Fig. S2B) with virtually identical Tm [65.6 ± 0.3 °C (n = 7) in detergents vs. 65.5 ± 0.2 °C (n = 8) in lipids]. The measured ΔH (222 ± 1.1 kcal/mol, n = 7) was also comparable to the reported values of free MBP in aqueous solutions (36). Thus, the presence of a relatively low concentration of detergents (0.5 mM) exerted negligible influence on the MBP transitions, which further supports our use of it for calibrating protein concentrations in proteoliposomes. The detergent environment appears to mainly affect the transitions of TRPV1 by reducing its thermal stability in the absence of a lipid bilayer. Similar environmental dependency of TRPV1 was observed in patch clamp where the channel in liposomes showed more rapid rundown at high temperatures than in native cell membranes (12, 37).
Intrinsicality of the DSC Transition of TRPV1.
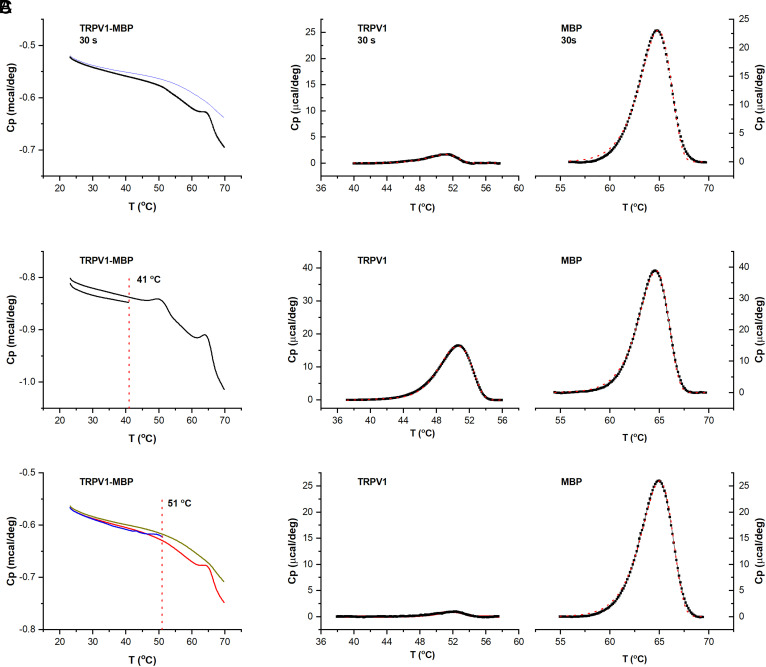
Compared to fast temperature control of single cells in patch clamp, DSC has a slow scan rate, thus inevitably introducing prolonged heating to proteins (∼10 min over a typical DSC peak). We asked whether the apparent irreversible transition of TRPV1 pertains to this slow heating rate. As an alternative, we pretreated samples by controlled, more rapid heating in a PCR machine before DSC. We reasoned that if a transition is intrinsic to TRPV1 and is irreversible, its peak would disappear in a subsequent DSC scan. Fig. 4A and SI Appendix, Fig. S5 show DSC scans of TRPV1-MBP after preheat treatment (51 °C). Three preheating durations were tested (2 min, 1 min, and 30 s). In all cases, the TRPV1 peak became nearly completely diminished, while the MBP peak remained intact. For comparison, the control sample left at room temperature exhibited both TRPV1 and MBP peaks as previously observed (SI Appendix, Fig. S5A). Thus, the irreversibility of the TRPV1 transition is apparently independent of the slow DSC heating rate. Moreover, for reliable heating of large volumes, we have limited the shortest heating duration to 30 s. Hence, the actual time course of the TRPV1 transition is likely more rapid than detected, as would be expected from the opening and rundown time course in patch clamp.
Fig. 4.
Intrinsicality of the DSC transition of TRPV1. (A) DSC scans of reconstituted TRPV1, showing that a preheating of the channel at 51 °C for 30 s in a PCR thermocycler largely abolished its DSC transition peak (see also SI Appendix, Fig. S5 for other heating durations). (B and C) DSC scans of reconstituted TRPV1, showing that an initial partial DSC scan up to 41 °C preserved the irreversible DSC transition of TRPV1 (B), while increasing temperature to 51 °C abrogated TRPV1 peak in subsequent scans (C). In all cases, the MBP transition peak was not affected. Data were representative of 2 to 3 experiments at each condition.
In another test of intrinsicality of the irreversible DSC transition, we further examined the threshold temperature of the irreversibility and its physiological relevance. In these two tandem scans, we limited the maximum up-scanning temperature in the first scan and detected its effect on the TRPV1 transition in the second scan (Fig. 4 B and C). Two temperatures were examined, one near the heat activation threshold of TRPV1 (41 °C) and the other close to its maximum activation temperature (∼51 °C). As evident in Fig. 4B, with prior heating limited to 41 °C, the TRPV1 transition was preserved in the second DSC scan. In contrast, the heating to 51 °C in the first scan abolished the TRPV1 peak in the second scan, while the MBP peak still remained intact (Fig. 4C). Thus, the experiment shows that the irreversible transition of TRPV1 occurs both at specific temperatures pertinent to its biological functions and independently of heating protocols.
Biochemical Changes to Heat-Treated Channel Proteins.
The strong irreversibility of the DSC transitions suggests that the protein integrity became severely compromised after heat stimulation. To test the hypothesis, we analyzed channel proteins after DSC scan with biochemical assays of native-gel electrophoresis and gel filtration chromatography. When TRPV1-MBP proteins after DSC scan in detergents were examined by native PAGE (Fig. 5A), they clearly became higher-order oligomers (heavier than the tetramers; apparent pentamers or hexamers in size based on Fig. 5A). When TRPV1-MBP after DSC scan was analyzed by gel filtration, its elution profile revealed the overwhelming presence of different oligomers that are heavier than the tetramers and of varied stoichiometry (Figs. 5B vs. 1B), suggesting that the channels undergo irreversible aggregation, a common feature of partially denatured proteins. Due to the size limit of the gel filtration column, aggregates heavier than 2 MDa were not detectable. Nonetheless, these data clearly demonstrate that the heat absorption by TRPV1 during a DSC scan causes irreversible biochemical changes that lead to severe protein aggregation.
Fig. 5.
Alteration of protein integrity by heat stimulation. (A) Native PAGE of TRPV1-MBP, showing that the sample (in detergent-solubilized form) migrated in a discrete sharp band prior to DSC, but in multiple, diffusive bands after DSC (final temperature 60 °C). (B) In gel filtration chromatography, the sample after DSC exhibited a complex, multiple peak elution profile, in contrast to a single monodisperse peak prior to DSC experiment. (C) Sucrose density gradient fractionation of cell lysates from transiently transfected HEK293 cells either preheated (48 °C for 60 s; orange color) or without heat treatment (green color). TRPV1 was detected by fluorescence of EGFP fused to the C terminus. The heat treatment caused a clear shift of TRPV1-EGFP distribution to higher-density bands. Fluorescence intensity distribution was normalized for display to same total fluorescence intensities. Data were representative of 2 to 3 runs for each experiment.
Finally, we examined whether TRPV1 in native cells also undergoes similar irreversible protein unfolding causing aggregation after heat stimulation. We detected the presence of TRPV1 aggregates by sucrose density gradient fractionation of cell lysates. Patch-clamp experiments show that strong rundown of TRPV1 occurs at temperatures above 47 °C after prolonged repetitive heat stimulation (37). Accordingly, to induce possible protein unfolding, we subjected cells to a preheating in a PCR thermocycler to 48 °C for 60 s before lysis and membrane solubilization for sedimentation. To facilitate the detection, we fused fluorescent protein EGFP to the C terminus of TRPV1. Fig. 5C illustrates the fluorescence distribution of TRPV1-EGFP across sucrose density gradient fractions of both control and preheated cells. In the control cells, almost all TRPV1 (>85%) showed up in the fractions of 10 to 30% sucrose. Strikingly, the cells after brief heat treatment exhibited quite a different distribution profile that was significantly shifted toward higher density of 35 to 50% sucrose, supporting the existence of a majority of TRPV1 (>80%) as high-order oligomers. As a negative control, we tested cells expressing TRPV1 and EGFP separately (SI Appendix, Fig. S6B). The fluorescence in this case was mainly distributed in the low-density fractions of 10 to 20% sucrose, independent of preheat treatment. Thus, the fractionation experiment supports that TRPV1 in cells is also prone to irreversible biochemical changes following heat stimulation.
Discussion
The DSC transition of TRPV1 shares essential characteristics of heat activation, supporting that the transition is functionally relevant. TRPV1 in liposomes preserved its gating properties in cells. Patch clamp predicts that its opening by heat involves a large enthalpy change (ΔH) as DSC detected. The DSC transition occurred in the same temperature range as the heat activation, and the two processes show a similar activation enthalpy (energy barrier; ∼100 kcal/mol), indicating that the DSC transition begins with heat activation. The DSC, however, measured directly the total heat uptake by the channel. Hence, the DSC ΔH is expectedly larger than the ΔH of channel opening (>400 kcal/mol vs. ∼100 kcal/mol). The excess energy in DSC can arise from at least two sources. One is the rundown transition following heat-evoked opening (SI Appendix, Figs. S3 and S4). This rundown process is strongly temperature-dependent and thus is expected to also make a large contribution to the DSC ΔH. On the other hand, patch clamp inevitably underestimates the ΔH of channel opening since it only detects the last step of gating. Accordingly, such estimates mainly pertain to the energy of the final closed-to-open transition at the gate, which can be substantially less than the heat input to the channel at the sensor site(s). If we assume that TRPV1 has an allosteric coupling efficiency similar to other channels (∼50%) (38), its opening would take ∼200 kcal/mol instead, about half of the DSC ΔH. This leaves other half of the DSC ΔH to postopening processes (e.g., rundown). Such a partition of the DSC ΔH seems consistent with the strongly temperature-dependent rundown rate (SI Appendix, Fig. S4). Hence, the DSC transition, despite its seemingly large ΔH, reconciles well with functional measurements.
The inherent thermal instability of TRPV1 as detected by DSC offers an unconventional perspective on how TRPV1 attains its large energetic for a high-temperature sensitivity. On a scale of ∼1 cal/g, the DSC ΔH of TRPV1 is close to the energy of protein unfolding (3 to 10 cal/g) (39). Compared to the unfolding of T7 lysozyme, a well-studied globular protein with 150 residues and a ΔH at 120 to 130 kcal/mol (40), the DSC ΔH of TRPV1 is equivalent to that of unfolding 3 to 4 lysozyme molecules, totaling 400 to 500 residues. Such a large scale of changes supports explicitly that the thermal transition in TRPV1 involves a strong component of protein unfolding. Indeed, DSC experiments show that the irreversibility of the DSC transition is intrinsic to channel proteins and independent of DSC heating protocols. Moreover, the unfolding transition depends on specific temperatures (40 to 50 °C) that overlap with the heat activation temperatures, and the two processes further share a similar environmental dependency, suggesting that they are inherently coupled. Taken together, the DSC experiments strongly argue that the channel protein is thermally unstable in its functional temperatures. It seems that TRPV1 apparently exploits this instability by coupling heat-evoked opening to protein unfolding. Without upholding the reversibility of the overall reaction, such a coupling permits the channel to explore more extreme conformational changes, thereby achieving a large enthalpy change. Irreversible changes have also been observed in other temperature receptors, such as the strong hysteresis of TRPV2 and TRPV3 (41, 42) and the C terminus unwinding of TRPM8 (43). Thus, the coupling of functional activation to irreversible conformational transitions may represent a more general paradigm to attain an exquisite temperature dependence by thermal receptors or other temperature-sensing processes in biology.
Biologically, coupling functional activation to protein unfolding seems consistent with the physiological roles of nociceptors whose primary task is to rapidly generate an “imminent threat” signal. TRPV1 possesses rapid kinetics in line with such a functional role, such as the very fast activation kinetics at high temperatures, on the order of a few ms (44), and a rundown time course in a range of a few hundreds of ms (SI Appendix, Fig. S3 and ref. 42) matching well with reflex time. The fast responsiveness of the channel can apparently mitigate the extent of protein denaturation. TRPV1 is also a cutaneous receptor. Intense heating causes tissue damage, in which case the fate of TRPV1 no longer matters since the channel will be regenerated along with local tissues. An exquisite temperature sensitivity under such conditions can be more beneficial for initiating a strong perception of imminent dangers than preserving channel integrity. In general, however, the model does restrict the lifespan of receptors and thus raises interesting questions on whether cells have deployed any mechanisms to detect and remove denatured channels or refold them to sustain the continuous function of thermal transduction at nerve endings.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by grant R01 GM132762 (F.Q.) from the NIH. Q.-X.J. was supported by an R21GM131231. We would like to thank Drs. Zhengrong Yan (University of Alabama at Birmingham), Kiran Andra, George Makhatadze (Rensselaer Polytechnic Institute), and Karl Magleby (University of Miami) for assistance and helpful discussions.
Author contributions
A.M. and F.Q. designed research; A.M., R.C., F.C., B.L., Q.-X.J., and F.Q. performed research; F.Q. contributed new reagents/analytic tools; A.M. and F.Q. analyzed data; and Q.-X.J. and F.Q. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix. The computer code of data analysis is available at Zenodo (https://zenodo.org/record/8239944) (45).
Supporting Information
References
- 1.Caterina M. J., et al. , The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Caterina M. J., Rosen T. A., Tominaga M., Brake A. J., Julius D., A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398, 436–441 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Peier A. M., et al. , A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Xu H. X., et al. , TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Smith G. D., et al. , TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190 (2002). [DOI] [PubMed] [Google Scholar]
- 6.McKemy D. D., Neuhausser W. M., Julius D., Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Peier A. M., et al. , A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Clapham D. E., TRP channels as cellular sensors. Nature 426, 517–524 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Patapoutian A., Peier A. M., Story G. M., Viswanath V., ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat. Rev. Neurosci. 4, 529–539 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Yao J., Liu B. L., Qin F., Kinetic and energetic analysis of thermally activated TRPV1 channels. Biophys. J. 99, 1743–1753 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B., Hui K., Qin F., Thermodynamics of heat activation of single capsaicin ion channels VR1. Biophys. J. 85, 2988–3006 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao E., Cordero-Morales J. F., Liu B., Qin F., Julius D., TRPV1 Channels Are Intrinsically Heat Sensitive and Negatively Regulated by Phosphoinositide Lipids. Neuron 77, 667–679 (2013), 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakharian E., Cao C., Rohacs T., Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 30, 12526–12534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng Z., et al. , Gating of human TRPV3 in a lipid bilayer. Nat. Struct. Mol. Biol. 27, 635–644 (2020), 10.1038/s41594-020-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clapham D. E., Miller C., A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proc. Natl. Acad. Sci. U.S.A. 108, 19492–19497 (2011), 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B., Qin F., Identification of a helix-turn-helix motif for high temperature dependence of vanilloid receptor TRPV2. J. Physiol. 599, 4831–4844 (2021), 10.1113/JP282073. [DOI] [PubMed] [Google Scholar]
- 17.Liu B., Qin F., Single-residue molecular switch for high-temperature dependence of vanilloid receptor TRPV3. Proc. Natl. Acad. Sci. U.S.A. 114, 1589–1594 (2017), 10.1073/pnas.1615304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao J., Liu B., Qin F., Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc. Natl. Acad. Sci. U.S.A. 108, 11109–11114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordero-Morales J. F., Gracheva E. O., Julius D., Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc. Natl. Acad. Sci. U.S.A. 108, E1184–E1191 (2011), 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brauchi S., Orta G., Salazar M., Rosenmann E., Latorre R., A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci. 26, 4835–4840 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlachova V., et al. , Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J. Neurosci. 23, 1340–1350 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong L., et al. , Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep. 1, 1–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang K., et al. , Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 481, 76–80 (2012), 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gracheva E. O., et al. , Molecular basis of infrared detection by snakes. Nature 464, 1006–1011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladron-de-Guevara E., et al. , The contribution of the ankyrin repeat domain of TRPV1 as a thermal module. Biophys. J. 118, 836–845 (2020), 10.1016/j.bpj.2019.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M., et al. , Evidence that the TRPV1 S1–S4 membrane domain contributes to thermosensing. Nat. Commun. 11, 4169 (2020), 10.1038/s41467-020-18026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A. K., et al. , Structural basis of temperature sensation by the TRP channel TRPV3. Nat. Struct. Mol. Biol. 26, 994–998 (2019), 10.1038/s41594-019-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadezhdin K. D., et al. , Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Nat. Struct. Mol. Biol. 28, 564–572 (2021), 10.1038/s41594-021-00615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon D. H., et al. , Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat. Struct. Mol. Biol. 28, 554–563 (2021), 10.1038/s41594-021-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grandl J., et al. , Pore region of TRPV3 ion channel is specifically required for heat activation. Nat. Neurosci. 11, 1007–1013 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grandl J., et al. , Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat. Neurosci. 13, 708–714 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang F., Cui Y., Wang K., Zheng J., Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 7083–7088 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S. E., Patapoutian A., Grandl J., Single residues in the outer pore of TRPV1 and TRPV3 have temperature-dependent conformations. PLoS One 8, e59593 (2013), 10.1371/journal.pone.0059593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F., et al. , Heat activation is intrinsic to the pore domain of TRPV1. Proc. Natl. Acad. Sci U.S.A. 115, E317–E324 (2018), 10.1073/pnas.1717192115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hille B., Ionic Channels of Excitable Membranes (Sinauer Associates, ed. 3, 2001). [Google Scholar]
- 36.Novokhatny V., Ingham K., Thermodynamics of maltose binding protein unfolding. Protein Sci. 6, 141–146 (1997), 10.1002/pro.5560060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Moreno A., et al. , Irreversible temperature gating in trpv1 sheds light on channel activation. Elife 7, e36372 (2018), 10.7554/eLife.36372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Indurthi D. C., Auerbach A., Agonist efficiency from concentration-response curves: Structural implications and applications. Biophys. J. 120, 1800–1813 (2021), 10.1016/j.bpj.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z., Brouillette C. G., A guide to differential scanning calorimetry of membrane and soluble proteins in detergents. Methods Enzymol. 567, 319–358 (2016), 10.1016/bs.mie.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Eriksson A. E., et al. , Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science 255, 178–183 (1992), 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- 41.Liu B. L., Yao J., Qin F., Hysteresis of gating underlines sensitization of TRPV3 channels. J. Gen. Physiol. 138, 509–520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B. Y., Qin F., Use dependence of heat sensitivity of vanilloid receptor TRPV2. Biophys. J. 110, 1523–1537 (2016), 10.1016/j.bpj.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz-Franulic I., Raddatz N., Castillo K., Gonzalez-Nilo F. D., Latorre R., A folding reaction at the C-terminal domain drives temperature sensing in TRPM8 channels. Proc. Natl. Acad. Sci. U.S.A. 117, 20298–20304 (2020), 10.1073/pnas.2004303117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao J., Liu B., Qin F., Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies. Biophys. J. 96, 3611–3619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mugo A., et al. , Matlab codes for DSC data fitting. Zenodo. https://zenodo.org/record/8239944. Accessed 11 August 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix. The computer code of data analysis is available at Zenodo (https://zenodo.org/record/8239944) (45).