Abstract

A rapid, reliable, and user-friendly electrochemical sensor was developed for the detection of xanthine (Xn), an important biomarker of food quality. The developed sensor is based on a nanocomposite comprised of molybdenum disulfide-molybdenum trioxide (MoS2/MoO3) and synthesized using a single-pot hydrothermal method. Structural analysis of the MoS2/MoO3 nanocomposite was conducted using X-ray diffraction (XRD) and Raman spectroscopy, while its compositional properties were evaluated through X-ray photoelectron spectroscopy (XPS). Morphological features were observed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Two-dimensional (2D) MoS2 offers advantages such as a high surface-to-volume ratio, biocompatibility, and strong light–matter interaction, whereas MoO3 serves as an effective electron transfer mediator and exhibits excellent stability in aqueous environments. The enzymatic biosensor derived from this nanocomposite demonstrates remarkable cyclic stability and a low limit of detection of 64 nM. It enables rapid, reproducible, specific, and reproducible detection over 10 cycles while maintaining a shelf life of more than 5 weeks. These findings highlight the potential of our proposed approach for the development of early detection devices for Xn.
Introduction
Keeping fish meat fresh is a significant challenge for the food and pharmaceutical industries. Because fish meat is perishable, it requires careful preservation to resolve food safety concerns and preserve product excellence. To preserve the safety and quality of seafood products, keeping the freshness of fish meat is crucial in the food sector.1 Once fish is handled or stored improperly, dangerous bacteria like Listeria, Salmonella, and Vibrio can quickly proliferate and pose a risk for foodborne illnesses for consumers. The industry uses a variety of strategies to address this problem, including proper storage, temperature management, and the application of preservatives. The pharmaceutical industry uses fish meat as a valuable ingredient in the manufacturing of pharmaceuticals like fish oil supplements and medications made from fish. In order to maintain the effectiveness and safety of these products, the freshness of the fish meat is essential. Thus, ensuring the freshness of fish meat stands as a substantial challenge in both the food and pharmaceutical sectors, necessitating adherence to proper handling, storage, and processing methods to guarantee the safety and quality of fish-based merchandise.
When a fish is sacrificed, the presence of endogenous enzymes in the intestine and the muscle tissues causes the decomposition of nucleotides and other autocatalytic reactions start immediately.2,3 The rapid decomposition of adenosine-5-triphosphate (ATP) into adeonosine-5-di-phosphate (ADP) and further breaks into adenosine-5-monophosphate (AMP), inosine-5-monophosphate (IMP), inosine (I), hypoxanthine (HXn), and xanthine (Xn), respectively.2,4,5 At the time of death, the autolysis causes the rotting of fish by ATP degradation to Xn (3,7-dihydro-purine-2,6-dione), which later ends in the formation of uric acid (UA) by the oxidation of Xn.6,7 The compound IMP majorly contributes toward the taste of the meat, and its degradation to HXn and Xn leads to the bitter taste of the fish meat.8 The level of chief metabolite Xn in the ATP degradation process in the dead fish increases with the storage time of fish meat.9 Therefore, keeping track of the Xn level in the fish meat can be used as an important parameter to determine the freshness of the fish meat.8
Xn is a purine base that is found in various biological samples, including urine, blood, and cerebrospinal fluid. The Xn finally breaks into the UA, which ultimately breaks into the blood and passes through the kidney to come out as urine. Elevated levels of Xn can indicate certain medical conditions, such as renal failure, diabetes influencer, hyperuricemia, liver, gout disease, cerebral ischemia, xanthinuria, and cardiac diseases.10−14 Therefore, sensitive and accurate methods for xanthine sensing are important for disease diagnosis and monitoring. There are several methods for xanthine sensing, including enzymatic and nonenzymatic approaches. Enzymatic methods involve the use of xanthine oxidase (XOD), an enzyme that catalyzes the conversion of xanthine to uric acid. Nonenzymatic methods involve the use of various materials that can selectively bind to xanthine and generate a measurable signal. Overall, xanthine sensing is an important area of research with many potential applications in medical diagnostics and monitoring. Further research is needed to optimize the performance of xanthine sensors and to develop practical applications for disease diagnosis and monitoring.
Previously, many techniques have been deployed to detect Xn and its metabolites, like spectrophotometry, mass spectrometry, chemo/electroluminescence, high-performance liquid chromatography, and capillary electrophoresis biosensors.15−21 The traditional techniques were supposedly time-consuming and had a limited range of detection toward the targeted analyte, which made researchers look out for other options. One such popular alternative is the electrochemical biosensors, which have an exquisite range of detection, simple processing, and high sensitivity and selectivity.22−28 Till now, many attempts have been made to immobilize the enzymes onto the electrode’s surface by means of adsorption, covalent, and intermediate molecular linkage, which affects the sensing ability of the electrochemical sensors.29,30 In the last decade, nanoparticles have been extensively used due to their unique properties and great potential in the field of biosensing.31−37 One such class of popular nanomaterials is transition metal dichalcogenides (TMDs). These are the two-dimensional (2D) nanomaterials having a high surface-to-volume ratio and good electronic response, which could be employed as potent electrodes for Xn detection. On the other side, the layered metal oxides have also gained attention as a new class of van der Waals material.
The combination of the materials of these two classes exhibits great potential for Xn detection. Molybdenum disulfide (MoS2) and molybdenum trioxide (MoO3) are very famous 2D materials with interesting electronic and optical properties.38−40 MoS2 exhibits semiconductor properties and possesses a direct band gap within the visible spectrum, rendering it highly promising for optoelectronic applications, whereas MoO3 functions as a wide-band-gap semiconductor, which acts as an effective electron transfer mediator and exhibits excellent stability in aqueous environments, and has found utility in gas sensing and other domains. The amalgamation of MoS2 and MoO3 in a nanohybrid configuration yields stimulating properties and unveils possibilities for novel applications.41 The MoS2/MoO3 nanohybrid has gained significant attention in diverse fields, including energy storage and electrocatalysis, among others. In energy storage applications, MoS2/MoO3 nanohybrids have been used as electrode materials for lithium-ion batteries and supercapacitors. The unique structure of the nanohybrid allows for efficient charge transfer and high capacitance, leading to improved performance compared to individual MoS2 or MoO3 materials.
Here, in this work, the heterostructure of a MoS2/MoO3 nanohybrid has been synthesized through a facile hydrothermal process, which enabled the large defect sites for Xn adsorption at the surface of the working electrode. No such work has been reported so far where the MoS2/MoO3 nanohybrid-based electrode has been used for Xn detection; therefore, the results hold great significance in this field. The high surface area of the nanohybrid allows for efficient enzyme adsorption, and the electronic properties of the MoS2 component can be tuned to enhance the sensitivity and selectivity of the sensor to develop a proficient enzymatic biosensor.
Experimental Method
Synthesis of MoS2 Nanosheets and the MoS2/MoO3 Nanocomposite
The synthesis of MoS2 nanosheets was performed using the hydrothermal method. Initially, the precursor solution was prepared by dissolving ammonium molybdate and thioacetamide in deionized water in a 1:2 ratio to form the precursor solution. After stirring the precursor solution for 30 min, 0.05 g of cetyltrimethylammonium bromide (CTAB) was added as a surfactant to the solution, which helps to stabilize the nanosheets and prevent agglomeration. After the addition of the surfactant, the mixture was stirred for a certain amount of time to ensure that the surfactant was evenly distributed throughout the solution. Then, the solution was transferred to a Teflon-lined autoclave and heated at 200 °C for 36 h. After the hydrothermal process was complete, the resulting solution was cooled to room temperature and then centrifuged to separate the MoS2 nanosheets from the solution. The nanosheets were then washed several times with deionized water and ethanol to remove any remaining impurities and surfactant. Finally, the MoS2 nanosheets were dried under a vacuum to remove any remaining solvent. The MoS2/MoO3 nanocomposite was prepared by adding MoO3 with ammonium molybdate in a certain ratio. The further process will be repeated as usual. The resulting powders were then characterized using various analytical techniques to determine their morphology and composition.
Electrophoretic Deposition (EPD) of MoS2/MoO3 Nanosheets
Before the electrodeposition of the MoS2/MoO3 nanocomposite over the indium tin oxide (ITO) surface, the electrode was cut into a 2 cm × 1 cm dimension and washed properly with an ethanol/deionized (DI) solution to remove the dirt and other impurities from its surface. The prepared nanosheets were deposited on a prehydrolyzed ITO electrode using the two-electrode EPD technique (Genetix, GX300C). Platinum was used as a counter electrode. In order to deposit films, the two electrodes were placed at a distance of 10 mm inside the sonicated solutions of the prepared samples in distilled water (DI) water. The deposition of films was achieved at 10 V for 25 s. Finally, the prepared films were kept in a vacuum oven for drying. Two deposited films have been retrieved, i.e., MoS2/ITO and MoS2/MoO3/ITO, respectively.
Fabrication of the MoS2/MoO3/ITO Nanohybrid Biosensor
The immobilization was done by treating electrodes with 0.1% glutaraldehyde and allowed to rest for some time (1–2 h) at ambient temperature in order to activate the functional groups attached to the surface of the electrodes.
Afterward, phosphate-buffered saline (PBS; pH 7.4, 100 mM, 0.9% NaCl) was used to wash the electrodes, and then the covalent immobilization of XOD (20 μL) was done. The XOD/MoS2/ITO and XOD/MoS2/MoO3/ITO electrodes were then kept in a moist condition at room temperature overnight. The scheme for the electrode formation is shown in Figure 1.
Figure 1.
Scheme describing the fabrication of the MoS2/MoO3 nanosheet-based enzymatic biosensor.
Results and Discussion
Structural and Morphological Studies
Raman spectroscopy serves as a potent technique for examining the structural and electronic characteristics of MoS2/MoO3, thereby offering valuable insights into the material’s properties. Through the analysis of Raman spectra, crucial information regarding the nanocomposite’s vibrational modes and crystalline structure was extracted, as illustrated in Figure 2.
Figure 2.

Raman spectrum of MoS2/MoO3 nanosheets; the inset shows the characteristic bands for MoS2 nanosheets.
The Raman spectrum of MoS2/MoO3 consists of several peaks corresponding to different vibrational modes. In MoS2/MoO3, the Raman spectrum of MoS2 shows peaks related to the E1g (396.28 cm–1) and A1g (424.42 cm–1) modes of the crystal lattice, which are related to the in-plane and out-of-plane vibrations of the sulfur atoms, respectively (as shown in the inset). The Raman spectrum of MoO3 shows peaks related to the symmetric and asymmetric stretching vibrations of the Mo–O bonds. The peaks in the spectrum include those at around 609 and 815 cm–1, which are assigned to the B2g, and Eg modes, respectively, depicting the occurrence of the D2h16 space group. The occurrence of the MoS2/MoO3 nanohybrid was confirmed through the Raman analysis, and the difference between E1g and A1g (28.14 cm–1) depicts the presence of a few layered composites.
X-ray photoelectron spectroscopy (XPS) is a powerful surface analysis technique that provides information about the elemental and chemical composition, chemical bonding, and electronic structure of a material. The XPS has been recorded on ULVAC-PHI, PHI5000, which depicts the chemical states of the MoS2/MoO3 sample (shown in Figure 3a). Here, the binding energy of the core electrons of the constituent elements, including Mo, S, and O, would be observed. The binding energy of the Mo 3d, S 2p, and O 1s core levels has provided information about the oxidation state of the elements and the nature of the chemical bonding. The wide spectrum of MoS2/MoO3 nanosheets describes the presence of Mo, C, O, and S elements on the surface of the prepared nanoparticles. In Figure 3b, the two Mo bands observed at 228.55 and 231.76 eV are attributed to Mo 3d5/2 and Mo d3/2, respectively, which signifies the occurrence of the Mo4+ state of molybdenum and their peak separation was measured to be 3.21 eV. The XPS spectra of the S 2p region, as shown in Figure 3c, represent two bands at 161.67 and 162.91 eV attributed to the occurrence of S 2p3/2 and S 2p1/2, respectively, which indicates the presence of the S2– oxidation state of sulfur. In Figure 3d, the O 1s oxidation state at 531.65 eV is attributed to the occurrence of MoO3. The XPS spectrum confirms the occurrence of the MoS2/MoO3 composites.
Figure 3.
XPS spectra of (a) MoS2/MoO3 nanosheets, (b) XPS map of the Mo 3d element, (c) XPS map of the S 2s element, and (d) XPS map of O 1s.
Figure 4 shows the field emission scanning electron microscopy (FESEM) images obtained using a JEOL JSM 6610LV instrument for MoS2/MoO3, which typically showed a layered structure consisting of MoS2 and MoO3 layers stacked on top of each other. The surface of the material appears rough and porous due to the presence of MoO3 nanoparticles. The size and distribution of these nanoparticles were analyzed using FESEM images, which came out to be 400–600 nm (Figure 4a,b). Energy-dispersive X-ray spectroscopy (EDS) was used to analyze the elemental composition of the MoS2/MoO3 nanohybrid, which confirmed the formation of the composite (Figure 4c), and the elemental mapping was done to verify the homogeneity of constituent elements across the sample, in addition to XPS results (Figure 4d–f).
Figure 4.
SEM images (a, b), EDX analysis (c), and elemental mapping of the components (d–f) for MoS2/MoO3 nanosheets.
Transmission electron microscopy (TEM) analysis is done on a TECNAI 200 kV, which has provided valuable information about the morphology of the MoS2/MoO3 composite. The sample was imaged at various magnifications to observe the structure and morphology of the MoS2/MoO3 composite, as shown in Figure 5. The TEM images were recorded for the sample dispersed in DI water, loaded on a copper TEM grid with a carbon coating. The TEM images illustrate the layered structure of the composite stacked over one another. The figure illustrates the formation of a few layers counted from the edges of the sheets. It shows the presence of the 4–6 nanosheets in the prepared sample, which lead to high adsorption due to a greater surface-to-volume ratio. This confirms the results obtained from the Raman analysis of the composite material. The selected area electron diffraction (SAED) pattern shown in Figure 5d illustrates the high crystalline nature of the composite.
Figure 5.
TEM images at different resolutions: (a–c) SAED patterns of the synthesized MoS2/MoO3 nanosheets.
Electrochemical Studies of the Fabricated Electrodes
The electrochemical properties were observed on instrument Autolab potentiostat (Eco-Chemie, the Netherlands) for the prepared MoS2/ITO and MoS2/MoO3/ITO heterostructure-based electrode. Electrochemical impedance spectroscopy (EIS; PBS, 5 mM [Fe(CN)6]3–/4–) within the frequency range of 0.01 to 105 Hz at a set potential of 0.01 V was carried out(Figure 6a).
Figure 6.
(a) EIS curves and (b) CV curves of prepared MoS2/ITO and XOD/MoS2/MoO3/ITO electrodes.
EIS is particularly useful for investigating the interface between an electrode and an electrolyte solution, which is important for a wide range of applications. The data obtained from EIS can be analyzed through the Nyquist plot, which is a linear plot observed at lower frequencies, whereas at higher frequencies, a semicircular plot was recorded. This is attributed to the resistance because of the diffusion-controlled electron transfer and electron transfer limited process, respectively. The equivalent circuit has been displayed in the inset of Figure 6a. The charge transfer resistance (Rct) of MoS2/ITO and MoS2/MoO3/ITO was recorded to be 98.511 and 66.165 Ω, respectively.
From the obtained Rct values, the exchange current per geometric unit area (io) and the apparent electron transfer rate constant (Kapp) for MoS2/ITO and MoS2/MoO3/ITO electrodes have been calculated using eqs 1 and 2
| 1 |
| 2 |
where R is the gas constant, F is the Faraday constant, T is the temperature in Kelvin, and A and C are the area of the electrode and the concentration of the redox probe, respectively. The io for the MoS2/ITO electrode was found to be 26.06 × 10–5 A cm–2, whereas, for the MoS2/MoO3/ITO electrode, the value of io was 38.80 × 10–5 A cm–2 respectively. Similarly, Kapp for the MoS2/ITO and MoS2/MoO3/ITO electrodes was calculated to be 2.14 × 10–6 and 3.19 × 10–6 cm s–1, respectively. This increase in the values of io and Kapp of the MoS2/MoO3/ITO electrode compared to the MoS2/ITO electrode clearly describes the precise diffusion of the redox couple through the electrode interface.
The technique of cyclic voltammetry (CV) is widely utilized in electrochemistry to examine the redox characteristics of materials. In this study, the CV analysis of ITO (curve i), MoS2/ITO (curve ii), and MoS2/MoO3/ITO (curve iii) electrodes was carried out by subjecting the working electrode to a voltage ramp ranging from −1 to 1 V while monitoring the resulting current (Figure 6b). There was a direct flow of electrons between the electrode and the ferro–ferri electrolyte, leading to an increase in the oxidation signal of MoS2/MoO3/ITO (curve iii). This finding suggests that the MoS2/MoO3/ITO electrode is a promising candidate for future biosensing applications. The charge transfer coefficient (α) and heterogeneous electron transfer coefficient (Ks) for MoS2/MoO3/ITO have been calculated to be 0.9073 and 0.0342, respectively, at a scan rate (v) of 50 mV/s (using Laviron’s model eq 3).47,48
| 3 |
For the MoS2/MoO3/ITO (Figure 7a) electrode, the performance of the electrodes was investigated by varying the scan rate (10 to 150 mV/s). It has been observed that the oxidation peak current increases linearly with a positive shift in the peak potential. Both the anodic peak current (Ipa), as well as cathodic peak current (Ipc) increase linearly with increasing the scan rate; the following eqs 4 and 5 show the surface adsorption-controlled process kinetics for the MoS2/MoO3/ITO electrode (Figure 7b).
| 4 |
| 5 |
Figure 7.

(a) CV at different scan rates, (b) anodic and cathodic peak current, and (c) anodic and cathodic peak voltage of the MoS2/MoO3/XOD/ITO electrode.
The anodic and cathodic peak potentials (Epa and Epc) also showed a linear relation with the natural logarithm of the scan rate (ln v). The corresponding linear regression equations eqs 6 and 7 have been given in Figure 7c.
| 6 |
| 7 |
Electrochemical Biosensing Response Studies of the XOD/MoS2/MoO3/ITO Electrode
Xanthine is typically detected through an enzymatic reaction catalyzed by xanthine oxidase (XOD) immobilized on the electrode surface. The MoS2/MoO3 nanocomposite can act as a mediator in the electron transfer process. The nanocomposite structure facilitates the efficient transfer of electrons between the immobilized enzyme (XOD) and xanthine, generating an electrochemical signal. To perform the biosensing studies, the XOD/MoS2/MoO3/ITO electrode was used to measure the electrochemical response under various concentrations of Xn (Figure 8a). The current response was recorded as the Xn concentration was increased from 1 nM to 1 μM. A linear decrease in current was observed with an increase in Xn concentration, indicating direct electron transfer between Xn and the XOD/MoS2/MoO3/ITO electrode (Figure 8b). The biosensor’s sensitivity toward Xn was measured using the regression equation y = 112.034 – 7.357 × 10–6x, where y represents the recorded peak current and x is the concentration of Xn, resulting in a sensitivity of 29.198 μA M–1 cm–2. The limit of detection (LOD) and the limit of quantization (LOQ) were found using the equation 3.3 σ/S and (10 × LOD)/3.3, respectively, where σ is the standard deviation of the bioelectrode and S is sensitivity.42,43 The calculated values for LOD and LOQ are 64 and 177 nM, respectively.
Figure 8.
(a) DPV studies showing the current response of the MoS2/MoO3/XOD/ITO electrode and (b) current calibration plot with increasing concentration of Xn.
Real-Sample (Rohu Fish Extract) Validation of the Biosensor
Differential pulse voltammetry (DPV) was used to evaluate the practicality of the MoS2/MoO3/ITO biosensor for the direct detection of Xn in fish meat samples.
Xn was extracted from Rohu (Labeo rohita) fish meat using the method of Watanabe et al. The meat was collected from a nearby market, washed, and chopped finely, and 7 g of the meat was centrifuged in 15 mL of DI water at 4500 rpm. The surfactant was filtered, and the resulting solution was diluted 100 times to achieve the desired concentration. Electrochemical studies demonstrated a linear decrease in the peak current with an increase in Xn concentration from 0.01 to 0.5 μM (Figure 9), similar to synthetic Xn samples. The XOD/MoS2/MoO3/ITO biosensor was compared to other Xn-based sensors for various biosensing parameters (Table 1). The results showed that the biosensor exhibited a better response toward the primary detection of Xn in fish meat samples with a lower limit of detection (LOD).
Figure 9.

DPV studies of the real sample from fish showing the current response of the XOD/MoS2/MoO3/ITO electrode with increasing concentration of Xn.
Table 1. Comparison of the Performance of XOD/MoS2/MoO3/ITO with Other Biosensors for Xn Detection.
| s.no. | immobilization matrix | detection technique | linear range (μM) | LOD | refs |
|---|---|---|---|---|---|
| 1. | Au-XOR/fMWCNT-PEDOT/GCE | DPV | 0.1–10 | 5.45 × 10–2 μM | (44) |
| 2. | XODNPs/Au | CV | 0.01–1 | 0.01 μM | (45) |
| 3. | XO/poly(l-asp)/MWCNT/GCE | DPV | 0.001–0.004 | 3.5 × 10–4 μM | (46) |
| 4. | XOD/ZnO/Ch/c-MWCNT/PANI/Pt | CV | 0.1–100 | 0.1 μM | (47) |
| 5. | Au-PPy | CV | 0.4–100 | 0.4 μM | (48) |
| 6. | XOD/c-MWCNT/PANI/Pt | CV | 0.6–58 | 0.6 μM | (49) |
| 7. | Ch/PPy/AuNPs/GCE | CV | 1–200 | 0.25 μM | (50) |
| 8. | PANI@TiO2/ITO | DPV | 1–100 | 0.1 μM | (3) |
| 9. | poly-TTCA/Au | CV | 5 – 0.001 | 1 μM | (51) |
| 10. | XOD/MoS2/MoO3/ITO | DPV | 0.001–1 | 64 nM | present work |
Selectivity, Reproducibility, Reusability, and Shelf-Life Studies
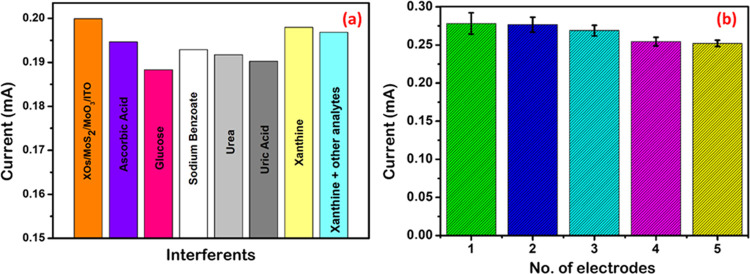
The nanohybrid biosensor (XOD/MoS2/MoO3/ITO) was evaluated for cross-reactivity against interfering analytes (glucose, sodium benzoate, urea, uric acid, and ascorbic acid) having a 10-fold higher concentration than Xn.
No significant changes were observed (Figure 10a); the current remained stable for all other analytes represented as a mixture. The reproducibility of the XOD/MoS2/MoO3/ITO electrodes was confirmed by fabricating five similar electrodes under the same conditions (Figure 10b). The response of the fabricated biosensors to 1 nM of Xn ranged from 90 to 99%, with a relative standard deviation (RSD) value of 12% (n = 5). Stability tests were conducted to evaluate the sensor’s reusability and shelf life, as shown in Figure 11. The single electrode was used 10 times after washing in saline, with no significant changes observed (Figure 11a).
Figure 10.
(a) Specificity and (b) reproducibility tests of the XOD/MoS2/MoO3/ITO electrode for Xn detection in the fish sample.
Figure 11.
(a) Reusability tests conducted upto 10 cycles and (b) shelf-life study of upto 5 weeks of the fabricated enzymatic biosensor.
The biosensor’s shelf life was monitored by recording the DPV response toward Xn every week while being stored at 4 °C. After 5 weeks, the electrochemical response decreased from its original activity, indicating its stability over a long period (Figure 11b).
Conclusions
Detecting Xn is highly important in the food industry as it can signal the spoilage of fish meat in its early stages. Therefore, here, an electrochemical enzymatic biosensor has been fabricated for the primary detection of Xn in fish meat by employing the 2D MoS2/MoO3 nanosheets. The 2D arrangement of MoS2 nanosheets provides a large active surface area, and the inclusion of MoO3 acts as an electron mediator and enhances the electrochemical response of the electrode, as well as it is more cost-effective than previously used materials such as gold nanoparticles. The developed enzymatic sensor demonstrated a fast response time of 10 s and a detection limit of approx. 64 nanomoles, whereas the limit of quantization came out to be 177 nM. The accuracy of the biosensor was validated using real samples of xanthine extracted from rohu fish meat purchased from a local market, and similar trends were observed in the results for xanthine detection. The biosensor was found to be stable, specific, and reproducible. The proposed enzymatic biosensing platform based on the MoS2/MoO3 nanohybrid has been validated successfully for fish meat and exhibited its reliable applicability toward fish freshness parameters.
Acknowledgments
The authors would like to acknowledge the Greater Noida Institute of Technology, Delhi Technological University, and the University of Delhi for providing the appropriate infrastructure and facilities required for promoting this research work. D.T. acknowledges the DST-INSPIRE for the SRF Award.
Author Contributions
P.S. contributed to conceptualization, data curation, formal analysis, investigation, methodology, and writing and editing—original draft. D.T. contributed to conceptualization, data curation, formal analysis, investigation, methodology, and draft editing. D.K. contributed to supervision, project administration, and draft editing.
The authors declare no competing financial interest.
References
- Reza K. K.; Singh M. K.; Yadav S. K.; Singh J.; Agrawal V. V.; Malhotra B. D. Quantum Dots Based Platform for Application to Fish Freshness Biosensor. Sens. Actuators, B 2013, 177, 627–633. 10.1016/J.SNB.2012.11.059. [DOI] [Google Scholar]
- Sharma N. K.; Monika; Kaushal A.; Thakur S.; Thakur N.; Sheetal; Kumar D.; Bhalla T. C. Nanohybrid Electrochemical Enzyme Sensor for Xanthine Determination in Fish Samples. 3 Biotech 2021, 11, 212 10.1007/S13205-021-02735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur D.; Pandey C. M.; Kumar D. Highly Sensitive Enzymatic Biosensor Based on Polyaniline-Wrapped Titanium Dioxide Nanohybrid for Fish Freshness Detection. Appl. Biochem. Biotechnol. 2022, 194, 3765–3778. 10.1007/S12010-022-03931-7. [DOI] [PubMed] [Google Scholar]
- Devi R.; Yadav S.; Nehra R.; Yadav S.; Pundir C. S. Electrochemical Biosensor Based on Gold Coated Iron Nanoparticles/Chitosan Composite Bound Xanthine Oxidase for Detection of Xanthine in Fish Meat. J. Food Eng. 2013, 115, 207–214. 10.1016/J.JFOODENG.2012.10.014. [DOI] [Google Scholar]
- Choi H. K.; Liu S.; Curhan G. Intake of Purine-Rich Foods, Protein, and Dairy Products and Relationship to Serum Levels of Uric Acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005, 52, 283–289. 10.1002/ART.20761. [DOI] [PubMed] [Google Scholar]
- Lawal A. T.; Adeloju S. B. Comparison of Enzyme Immobilisation Methods for Potentiometric Phosphate Biosensors. Biosens. Bioelectron. 2009, 25, 406–410. 10.1016/J.BIOS.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Ghanbari K.; Nejabati F. Ternary Nanocomposite-Based Reduced Graphene Oxide/Chitosan/Cr2O3 for the Simultaneous Determination of Dopamine, Uric Acid, Xanthine, and Hypoxanthine in Fish Meat. Anal. Methods 2020, 12, 1650–1661. 10.1039/D0AY00161A. [DOI] [Google Scholar]
- Rezaeinasab M.; Benvidi A.; Gharaghani S.; Abbasi S.; Zare H. R. Electrochemical Investigation of the Inhibition Effect of Carvacrol on Xanthine Oxidase Activity Merging with Theoretical Studies. Process Biochem. 2019, 83, 86–95. 10.1016/J.PROCBIO.2019.03.014. [DOI] [Google Scholar]
- Torres A. C.; Ghica M. E.; Brett C. M. A. Design of a New Hypoxanthine Biosensor: Xanthine Oxidase Modified Carbon Film and Multi-Walled Carbon Nanotube/Carbon Film Electrodes. Anal. Bioanal. Chem. 2013, 405, 3813–3822. 10.1007/s00216-012-6631-1. [DOI] [PubMed] [Google Scholar]
- Shih M. H.; Lazo M.; Liu S. H.; Bonekamp S.; Hernaez R.; Clark J. M. Association between Serum Uric Acid and Nonalcoholic Fatty Liver Disease in the US Population. J. Formosan Med. Assoc. 2015, 114, 314–320. 10.1016/J.JFMA.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T.; Tsuruta R.; Fujita M.; Aki H. S.; Kutsuna S.; Kawamura Y.; Wakatsuki J.; Aoki T.; Kobayashi C.; Kasaoka S.; Maruyama I.; Yuasa M.; Maekawa T. Xanthine Oxidase Is One of the Major Sources of Superoxide Anion Radicals in Blood after Reperfusion in Rats with Forebrain Ischemia/Reperfusion. Brain Res. 2009, 1305, 158–167. 10.1016/J.BRAINRES.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Davis S. N.; Lastra-Gonzalez G. Diabetes and Low Blood Sugar (Hypoglycemia). J. Clin. Endocrinol. Metab. 2008, 93, E2 10.1210/JCEM.93.8.9993. [DOI] [Google Scholar]
- Kim K. Y.; Schumacher H. R.; Hunsche E.; Wertheimer A. I.; Kong S. X. A Literature Review of the Epidemiology and Treatment of Acute Gout. Clin. Ther. 2003, 25, 1593–1617. 10.1016/S0149-2918(03)80158-3. [DOI] [PubMed] [Google Scholar]
- Kostić D. A.; Dimitrijević D. S.; Stojanović G. S.; Palić I. R.; Dordević A. S.; Ickovski J. D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015, 1–8. 10.1155/2015/294858. [DOI] [Google Scholar]
- Hlavay J.; Haemmerli S. D.; Guilbault G. G. Fibre-Optic Biosensor for Hypoxanthine and Xanthine Based on a Chemiluminescence Reaction. Biosens. Bioelectron. 1994, 9, 189–195. 10.1016/0956-5663(94)80121-5. [DOI] [PubMed] [Google Scholar]
- Kito M.; Tawa R.; Takeshima S.; Hirose S. Fluorometric Determination of Hypoxanthine and Xanthine in Biological Fluids by High-Performance Liquid Chromatography Using Enzyme Reactors. J. Chromatogr. B: Biomed. Sci. Appl. 1983, 278, 35–42. 10.1016/S0378-4347(00)84753-2. [DOI] [PubMed] [Google Scholar]
- Olojo R. O.; Xia R. H.; Abramson J. J. Spectrophotometric and Fluorometric Assay of Superoxide Ion Using 4-Chloro-7-Nitrobenzo-2-Oxa-1,3-Diazole. Anal. Biochem. 2005, 339, 338–344. 10.1016/J.AB.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Berti G.; Fossati P.; Tarenghi G.; Musitelli C.; d’Eril G. V. M. Enzymatic Colorimetric Method for the Determination of Inorganic Phosphorus in Serum and Urine. Clin. Chem. Lab. Med. 1988, 26, 399–404. 10.1515/CCLM.1988.26.6.399. [DOI] [PubMed] [Google Scholar]
- A quantitative method for the analysis of xanthine alkaloids in Paullinia cupana (guarana) by capillary column gas chromatography | Semantic Scholar, 2022. https://www.semanticscholar.org/paper/A-quantitative-method-for-the-analysis-of-xanthine-Pagliarussi-Freitas/06172977e6d1a1eb6b0182532a9e71245a7f37da. (accessed Novermber 03, 2022).
- Kock R.; Delvoux B.; Greiling H. A High-Performance Liquid Chromatographie Method for the Determination of Hypoxanthine, Xanthine, Uric Acid and Allantoin in Serum. Clin. Chem. Lab. Med. 1993, 31, 303–310. 10.1515/CCLM.1993.31.5.303. [DOI] [PubMed] [Google Scholar]
- Thakur D.; Pandey C. M.; Kumar D. Graphitic Carbon Nitride-Wrapped Metal-Free PoPD-Based Biosensor for Xanthine Detection. ACS Omega 2022, 8, 2328–2336. 10.1021/acsomega.2c06727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimuthu P.; Leimkühler S.; Bernhardt P. V. Low-Potential Amperometric Enzyme Biosensor for Xanthine and Hypoxanthine. Anal. Chem. 2012, 84, 10359–10365. 10.1021/ac3025027. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Deng P.; Tian Y.; Feng J.; Xiao J.; Li J.; Liu J.; Li G.; He Q. Simultaneous and Sensitive Determination of Ascorbic Acid, Dopamine and Uric Acid via an Electrochemical Sensor Based on PVP-Graphene Composite. J. Nanobiotechnol. 2020, 18, 112 10.1186/s12951-020-00672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q.; Wu Y.; Tian Y.; Li G.; Liu J.; Deng P.; Chen D. Facile Electrochemical Sensor for Nanomolar Rutin Detection Based on Magnetite Nanoparticles and Reduced Graphene Oxide Decorated Electrode. Nanomaterials 2019, 9, 115 10.3390/NANO9010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Deng P.; Tian Y.; Ding Z.; Li G.; Liu J.; Zuberi Z.; He Q. Rapid Recognition and Determination of Tryptophan by Carbon Nanotubes and Molecularly Imprinted Polymer-Modified Glassy Carbon Electrode. Bioelectrochemistry 2020, 131, 107393 10.1016/J.BIOELECHEM.2019.107393. [DOI] [PubMed] [Google Scholar]
- He Q.; Tian Y.; Wu Y.; Liu J.; Li G.; Deng P.; Chen D. Facile and Ultrasensitive Determination of 4-Nitrophenol Based on Acetylene Black Paste and Graphene Hybrid Electrode. Nanomaterials 2019, 9, 429 10.3390/NANO9030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q.; Liu J.; Liu X.; Li G.; Chen D.; Deng P.; Liang J. Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination. Nanomaterials 2018, 8, 194 10.3390/NANO8040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q.; Liu J.; Liu X.; Li G.; Chen D.; Deng P.; Liang J. A Promising Sensing Platform toward Dopamine Using MnO2 Nanowires/Electro-Reduced Graphene Oxide Composites. Electrochim. Acta 2019, 296, 683–692. 10.1016/J.ELECTACTA.2018.11.096. [DOI] [Google Scholar]
- Zeng R.; Wang W.; Cai G.; Huang Z.; Tao J.; Tang D.; Zhu C. Single-Atom Platinum Nanocatalyst-Improved Catalytic Efficiency with Enzyme-DNA Supermolecular Architectures. Nano Energy 2020, 74, 104931 10.1016/J.NANOEN.2020.104931. [DOI] [Google Scholar]
- Zeng R.; Huang Z.; Wang Y.; Tang D. Enzyme-Encapsulated DNA Hydrogel for Highly Efficient Electrochemical Sensing Glucose. ChemElectroChem 2020, 7, 1537–1541. 10.1002/CELC.202000105. [DOI] [Google Scholar]
- Huang L.; Cai G.; Zeng R.; Yu Z.; Tang D. Contactless Photoelectrochemical Biosensor Based on the Ultraviolet-Assisted Gas Sensing Interface of Three-Dimensional SnS2Nanosheets: From Mechanism Reveal to Practical Application. Anal. Chem. 2022, 94, 9487–9495. 10.1021/acs.analchem.2c02010. [DOI] [PubMed] [Google Scholar]
- Lv S.; Zhang K.; Zhu L.; Tang D. Zif-8-Assisted Nayf4:Yb,Tm@zno Converter with Exonuclease Iii-Powered Dna Walker for near-Infrared Light Responsive Biosensor. Anal. Chem. 2020, 92, 1470–1476. 10.1021/acs.analchem.9b04710. [DOI] [PubMed] [Google Scholar]
- Rahman M. M.; Balkhoyor H. B.; Asiri A. M. Ultra-Sensitive Xanthine Sensor Development Based on Wet-Chemically Prepared Co/ZnO Nanoparticles. Mater. Express 2017, 7, 93–103. 10.1166/MEX.2017.1356. [DOI] [Google Scholar]
- Rahman M. M.; Marwani H. M.; Algethami F. K.; Asiri A. M. Xanthine Sensor Development Based on ZnO–CNT, ZnO–CB, ZnO–GO and ZnO Nanoparticles: An Electrochemical Approach. New J. Chem. 2017, 41, 6262–6271. 10.1039/C7NJ00278E. [DOI] [Google Scholar]
- Alam M. M.; Asiri A. M.; Uddin M. T.; Islam M. A.; Rahman M. M. Wet-Chemically Prepared Low-Dimensional ZnO/Al 2 O 3 /Cr 2 O 3 Nanoparticles for Xanthine Sensor Development Using an Electrochemical Method. RSC Adv. 2018, 8, 12562–12572. 10.1039/C8RA01734D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. M.; Rahman M. M.; Asiri A. M.; Awual M. R. Non-Enzymatic Simultaneous Detection of l -Glutamic Acid and Uric Acid Using Mesoporous Co 3 O 4 Nanosheets. RSC Adv. 2016, 6, 80511–80521. 10.1039/C6RA12256F. [DOI] [Google Scholar]
- Alam M. M.; Asiri A. M.; Uddin M. T.; Islam M. A.; Awual M. R.; Rahman M. M. Detection of Uric Acid Based on Doped ZnO/Ag2O/Co3O4 Nanoparticle Loaded Glassy Carbon Electrode. New J. Chem. 2019, 43, 8651–8659. 10.1039/C9NJ01287G. [DOI] [Google Scholar]
- Sharma P.; Singh M. K.; Mehata M. S. Sunlight-Driven MoS2 Nanosheets Mediated Degradation of Dye (Crystal Violet) for Wastewater Treatment. J. Mol. Struct. 2022, 1249, 131651 10.1016/J.MOLSTRUC.2021.131651. [DOI] [Google Scholar]
- Sharma P.; Mehata M. S. Colloidal MoS2 Quantum Dots Based Optical Sensor for Detection of 2,4,6-TNP Explosive in an Aqueous Medium. Opt. Mater. 2020, 100, 109646 10.1016/J.OPTMAT.2019.109646. [DOI] [Google Scholar]
- Sharma P.; Mehata M. S. Rapid Sensing of Lead Metal Ions in an Aqueous Medium by MoS2 Quantum Dots Fluorescence Turn-Off. Mater. Res. Bull. 2020, 131, 110978 10.1016/J.MATERRESBULL.2020.110978. [DOI] [Google Scholar]
- Singh S.; Deb J.; Sarkar U.; Sharma S. MoS2/MoO3 Nanocomposite for Selective NH3Detection in a Humid Environment. ACS Sustainable Chem. Eng. 2021, 9, 7328–7340. 10.1021/acssuschemeng.1c01527. [DOI] [Google Scholar]
- Yu Z.; Qiu C.; Huang L.; Gao Y.; Tang D. Microelectromechanical Microsystems-Supported Photothermal Immunoassay for Point-of-Care Testing of Aflatoxin B1 in Foodstuff. Anal. Chem. 2023, 95, 4212–4219. 10.1021/acs.analchem.2c05617. [DOI] [PubMed] [Google Scholar]
- Zeng R.; Qiu M.; Wan Q.; Huang Z.; Liu X.; Tang D.; Knopp D. Smartphone-Based Electrochemical Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 15155–15161. 10.1021/acs.analchem.2c03606. [DOI] [PubMed] [Google Scholar]
- Sen S.; Sarkar P. An Interference-Free New Xanthine Biosensor Based on Immobilized Enzyme-Nanogold Conjugate on Carbon Nanotube Doped Poly(3,4-Ethylenedioxythiophene) Composite Film. Int. J. Biol. Macromol. 2022, 199, 275–286. 10.1016/J.IJBIOMAC.2021.12.094. [DOI] [PubMed] [Google Scholar]
- Joon A.; Ahlawat J.; Aggarwal V.; Jaiwal R.; Pundir C. S. An Improved Amperometric Determination of Xanthine with Xanthine Oxidase Nanoparticles for Testing of Fish Meat Freshness. Sens. Bio-Sens. Res. 2021, 33, 100437 10.1016/J.SBSR.2021.100437. [DOI] [Google Scholar]
- Yazdanparast S.; Benvidi A.; Abbasi S.; Rezaeinasab M. Enzyme-Based Ultrasensitive Electrochemical Biosensor Using Poly(l-Aspartic Acid)/MWCNT Bio-Nanocomposite for Xanthine Detection: A Meat Freshness Marker. Microchem. J. 2019, 149, 104000 10.1016/J.MICROC.2019.104000. [DOI] [Google Scholar]
- Devi R.; Yadav S.; Pundir C. S. Amperometric Determination of Xanthine in Fish Meat by Zinc Oxide Nanoparticle/Chitosan/Multiwalled Carbon Nanotube/Polyaniline Composite Film Bound Xanthine Oxidase. Analyst 2012, 137, 754–759. 10.1039/C1AN15838D. [DOI] [PubMed] [Google Scholar]
- Devi R.; Yadav S.; Pundir C. S. Au-Colloids-Polypyrrole Nanocomposite Film Based Xanthine Biosensor. Colloids Surf., A 2012, 394, 38–45. 10.1016/J.COLSURFA.2011.11.021. [DOI] [Google Scholar]
- Devi R.; Yadav S.; Pundir C. S. Electrochemical Detection of Xanthine in Fish Meat by Xanthine Oxidase Immobilized on Carboxylated Multiwalled Carbon Nanotubes/Polyaniline Composite Film. Biochem. Eng. J. 2011, 58–59, 148–153. 10.1016/J.BEJ.2011.09.008. [DOI] [Google Scholar]
- Dervisevic M.; Dervisevic E.; Çevik E.; Şenel M. Novel Electrochemical Xanthine Biosensor Based on Chitosan–Polypyrrole–Gold Nanoparticles Hybrid Bio-Nanocomposite Platform. J. Food Drug Anal. 2017, 25, 510–519. 10.1016/J.JFDA.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. A.; Won M. S.; Shim Y. B. Xanthine Sensors Based on Anodic and Cathodic Detection of Enzymatically Generated Hydrogen Peroxide. Electroanalysis 2007, 19, 631–637. 10.1002/ELAN.200603799. [DOI] [Google Scholar]