Abstract
Muscle spindles, one of the two main classes of proprioceptors together with Golgi tendon organs, are sensory structures that keep the central nervous system updated about the position and movement of body parts. Although they were discovered more than 150 years ago, their function during movement is not yet fully understood. Here, we summarize the morphology and known functions of muscle spindles, with a particular focus on locomotion. Although certain properties such as the sensitivity to dynamic and static muscle stretch are long known, recent advances in molecular biology have allowed the characterization of the molecular mechanisms for signal transduction in muscle spindles. Building upon classic literature showing that a lack of sensory feedback is deleterious to locomotion, we bring to the discussion more recent findings that support a pivotal role of muscle spindles in maintaining murine and human locomotor robustness, defined as the ability to cope with perturbations. Yet, more research is needed to expand the existing mechanistic understanding of how muscle spindles contribute to the production of robust, functional locomotion in real world settings. Future investigations should focus on combining different animal models to identify, in health and disease, those peripheral, spinal and brain proprioceptive structures involved in the fine tuning of motor control when locomotion happens in challenging conditions.
Keywords: locomotion, muscle spindles, perturbations, proprioception
Graphical Abstract
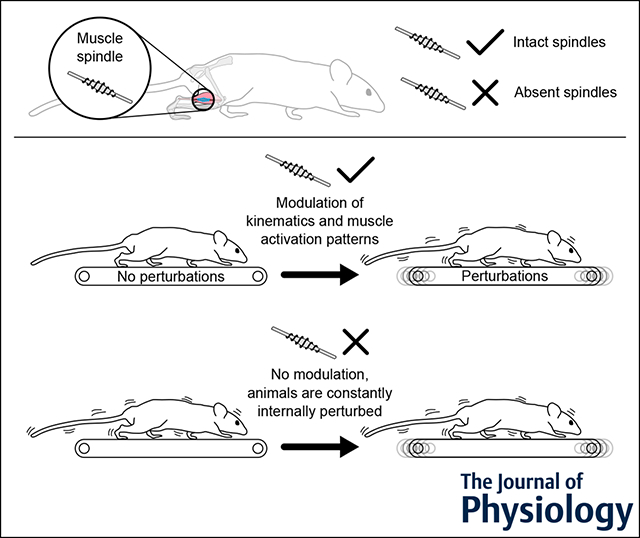
Vertebrates with intact muscle spindles respond to locomotor perturbations with increased kinematic variability and reduced accuracy of muscle activation patterns. When muscle spindles, but not Golgi tendon organs, are genetically removed in mice, the animals fail to modulate kinematics and muscle activity in response to perturbations and appear to be in a constantly perturbed state.
Brief history of muscle spindles
Muscle spindles were first discovered in 1862 by Kölliker (1862) and received the name ‘Muskelspindeln’ (i.e. muscle spindles in German) by Kühne (1863). Only three decades later, thanks to the work of Sherrington (1894) and Ruffini (1898), their sensory function was uncovered and shown to contribute to the already-existing concept of ‘muscular sense’ (Sherrington, 1900), also defined by Bastian as ‘kinaesthesis’, or ‘sense of movement’ (Bastian, 1880). Finally, after the first hypotheses on what is now is called proprioception (Sherrington, 1906), it was shown that these sensory structures are sensitive to muscle stretch (Adrian & Zotterman, 1926; Matthews, 1933). Today, it is established that muscle spindles are part of the proprioceptive system, defined as a network of sensory receptors that receive their stimuli by the organism itself (Sherrington, 1907). They are part of the kinesthetic sensory system that informs the central nervous system about the position and movement of the body (Proske & Gandevia, 2009, 2012). In the following, we briefly review the overall structure and function of muscle spindles, followed by their role in terrestrial mammalian locomotor behaviour.
The morphology of muscle spindles.
Studies in humans and other mammals have shown that muscle spindles are present in the vast majority of striated muscles, with numbers ranging from 1 to 100 spindles per gram of muscle and with a typical human body containing around 30 000 spindles (Matthews, 1972; Prochazka, 2021). By contrast to extrafusal (i.e. outside the spindle) muscle fibres that generate force through their contraction, intrafusal (i.e. inside the spindle) muscle fibres modulate the transduction properties of the spindle without contributing to the overall muscle force generation (Ruffini, 1898; Sherrington, 1894). The overall structure of muscle spindles consists of contractile fibres in the two polar regions flanking an encapsulated equatorial (i.e. central) non-contractile zone (Fig. 1). The sensory terminals in the equatorial region are responsible for signal transduction (Bewick & Banks, 2015). Contraction of the polar regions adjusts the length of the muscle spindle and therefore the sensitivity of sensory afferents and is regulated by the activity of beta innervation originating from alpha motor neurons that innervate both intrafusal and extrafusal fibres (Banks, 1994, 2015; Bessou et al., 1963) and specialized motor neurons called gamma motor neurons, which exclusively innervate intrafusal fibres (Barker, 1974; Banks, 1994, 2015; Cooper & Daniel, 1956; Kanning et al., 2010; Matthews, 1933; Proske & Gandevia, 2012).
Figure 1. The morphology of muscle spindles.
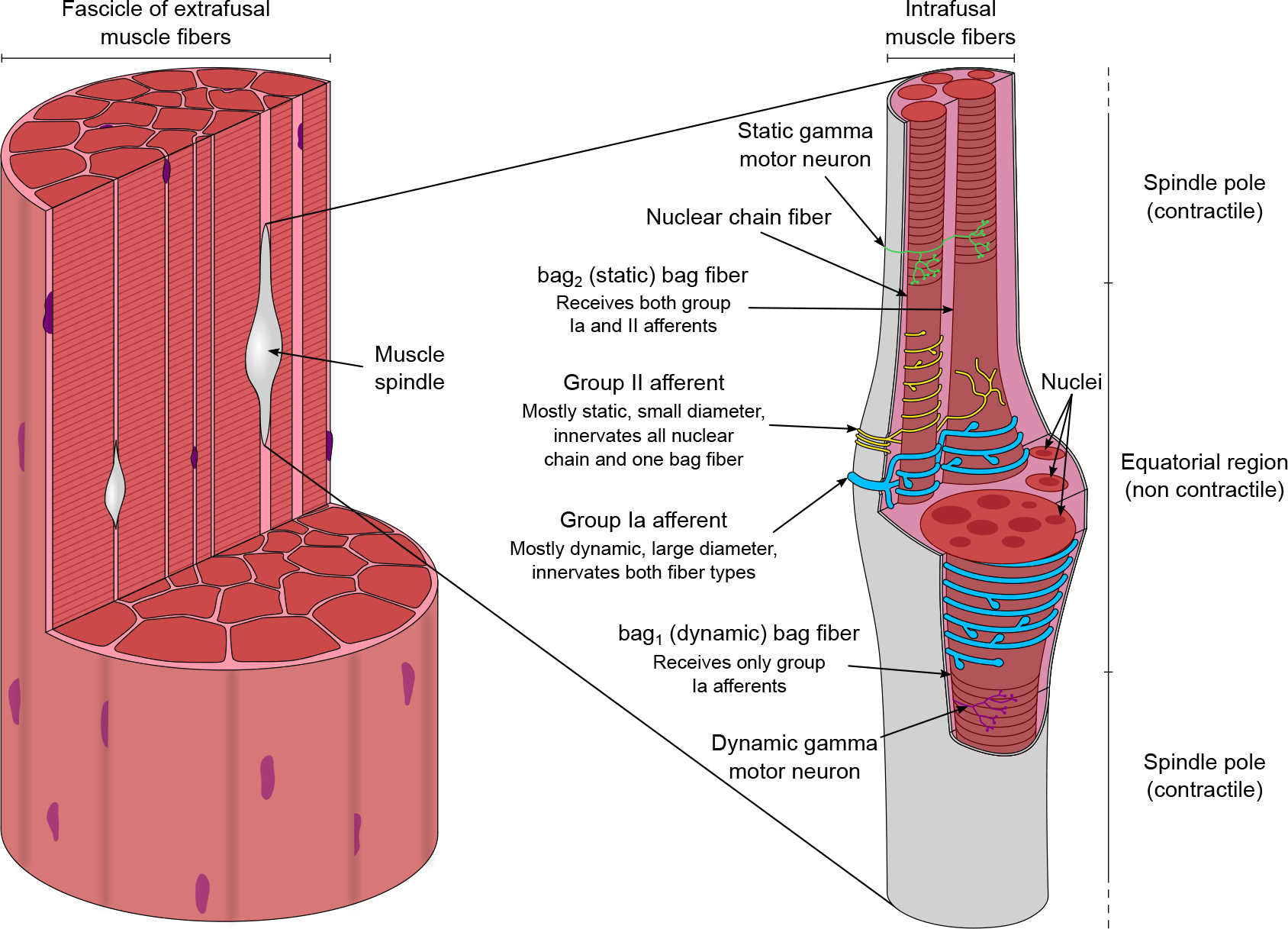
Found within fascicles of muscle fibres (left), muscle spindles (right) are sensory organs that detect muscle stretch and length and are built from contractile fibres in the two poles and non-contractile sensory fibres in the equatorial region. Nuclear chain fibres and static bag fibres (bag2) are innervated by both the unique primary (group Ia) and the several secondary (group II) afferents, whereas dynamic bag fibres (bag1) only receive endings of the primary afferent. The contractile portions of the intrafusal fibres are innervated by the specialized gamma motor neurons that aid the fine-tuning of overall sensitivity and avoid potential slack in the sensory component if the spindle length is reduced by unloading.
Within the encapsulated central region of the intrafusal muscle fibres, there are two morphologically distinct fibre types (Boyd, 1956, 1958; Cooper & Daniel, 1956; Tourtellotte et al., 2001): the nuclear bag fibres and nuclear chain fibres (Fig. 1), named after the arrangement of myonuclei in the equatorial region (Boyd, 1960). The nuclear bag fibres are often longer and larger in diameter, especially in the equatorial and juxtaequatorial regions, where a cluster of nuclei disposed side by side is present (Boyd, 1958; Matthews, 1972; Tourtellotte et al., 2001). By contrast, the nuclear chain fibres are usually shorter and thinner, with their nuclei distributed in a single file (Boyd, 1958; Banks et al., 1977; Matthews, 1972; Tourtellotte et al., 2001). Two different types of afferents innervate these fibres (Fig. 1): a single, larger diameter primary or group Ia afferent and several smaller diameter secondary or group II afferents, both entering the spinal cord through the dorsal roots (Banks et al., 2021; Boyd, 1962; Eccles & Sherrington, 1930; Kucera et al., 1988; Matthews, 1972; Pierrot-Deseilligny & Burke, 2012; Ruffini, 1898; Sherrington, 1894). Although only one type of nuclear chain fibre exists, nuclear bag fibres are of two types, depending on whether they receive only group Ia afferent innervation (bag1 nuclear bag fibres) or both group Ia and group II afferent innervation (bag2 nuclear bag fibres). Thus, the group Ia afferents innervate all of the nuclear bag and nuclear chain fibres, specifically in the equatorial region (Hulliger, 1984). By contrast, the group II afferents innervate all of the nuclear chain fibres and only bag2 fibres near to the equatorial region around what are called the juxtaequatorial regions (Hulliger, 1984). Because the group Ia afferents respond primarily, yet not exclusively, to the dynamic aspect of muscle stretch, the bag1 fibres are also called ‘dynamic bag fibres’ (Boyd et al., 1977). Accordingly, the group II afferents primarily respond to the static, or steady-state, aspect of stretch, and therefore the bag2 fibres are also called ‘static bag fibres’ (Boyd et al., 1977). A typical mammalian muscle spindle consists of up to eight fibres: two nuclear bag fibres and a varying number of nuclear chain fibres (Banks et al., 1982; Banks, 1986, 1994). Proprioceptive information is elaborated not only locally in the spinal cord, but also possesses collateral projections to the brain in the dorsal column–medial lemniscus pathway, which transmits to the somatosensory cortex through the brainstem (Niu et al., 2013). Additionally, proprioceptive information is conveyed to the cerebellum through the dorsal and ventral spinocerebellar tracts (Bosco & Poppele, 2001).
The contraction of the contractile polar regions of the intrafusal muscle receives beta and gamma efferent innervation (Barker, 1948; Hunt & Kuffler, 1951). Gamma motor neurons are located in the ventral horn of the spinal cord grey matter and leave the spinal cord through the ventral roots (Burke et al., 1977). The main structural difference with the alpha motor neurons is that gamma motor neurons typically have smaller cell body and smaller axon diameter, translating into a slower conduction velocity (Barker, 1974; Banks, 1994). In addition, the alpha motor neurons and the gamma motor neurons differ in many ways that are not discussed further here in terms of presynaptic drive and gene expression patterns. A detailed review has been given elsewhere (Kanning et al., 2010). Based on the muscle fibre type that they innervate, gamma motor neurons can also be divided into dynamic (reaching only bag1 fibres) and static (reaching both bag2 and nuclear chain fibres) gamma motor neurons (Fig. 1) (Matthews, 1962). The different intrafusal fibre and afferent types combined with this complex motor innervation make muscle spindles one of the most complex sensory systems.
The function of muscle spindles.
Muscle spindles were recognized as specialized sensory organs contributing to the muscle sense in that they detect muscle stretch (Matthews, 1933; Sherrington, 1900). By doing so, they contribute to the sensation of angular position and movement of joints that are moved by the relevant muscles. For example, if a joint extends and flexor muscle fibres are stretched, flexor muscle spindles are stimulated (Fig. 2). Because the group Ia afferents are predominantly influenced by the dynamic aspect of stretch, they mainly signal the angular velocity of the joint movement. On the other hand, the group II afferents that are mostly affected by the static aspects of stretch signal the angular joint position (Hunt & Ottoson, 1975). Yet, the functional distinction between primary and secondary afferents is not always dichotomous (Wilkinson et al., 2012) and recent evidence suggests the existence of new spindle afferent subtypes (Wu et al., 2021). If the same joint flexes and the flexor muscle fibres shorten, the muscle spindles of that muscle shorten as well. This relaxation, called unloading, leads to diminished sensitivity of the spindles. When unloading occurs, activation of gamma motor neurons causes contraction of the intrafusal muscle fibres, restoring the tension and, accordingly, the sensitivity of the spindles (Crowe & Matthews, 1964a, 1964b; Hunt & Kuffler, 1951). These mechanisms show that the way muscle spindles sense angular displacement during movement is very complex. Detailed reviews, also describing the role of muscle spindles in the various phases of the gait cycle, have been given elsewhere (Dietz & Duysens, 2000; Frigon et al., 2021; Pearson, 2008; Proske, 1997, 2006; Proske & Gandevia, 2018).
Figure 2. Activation of gamma motor neurons maintains the sensitivity of unloaded muscle spindles.
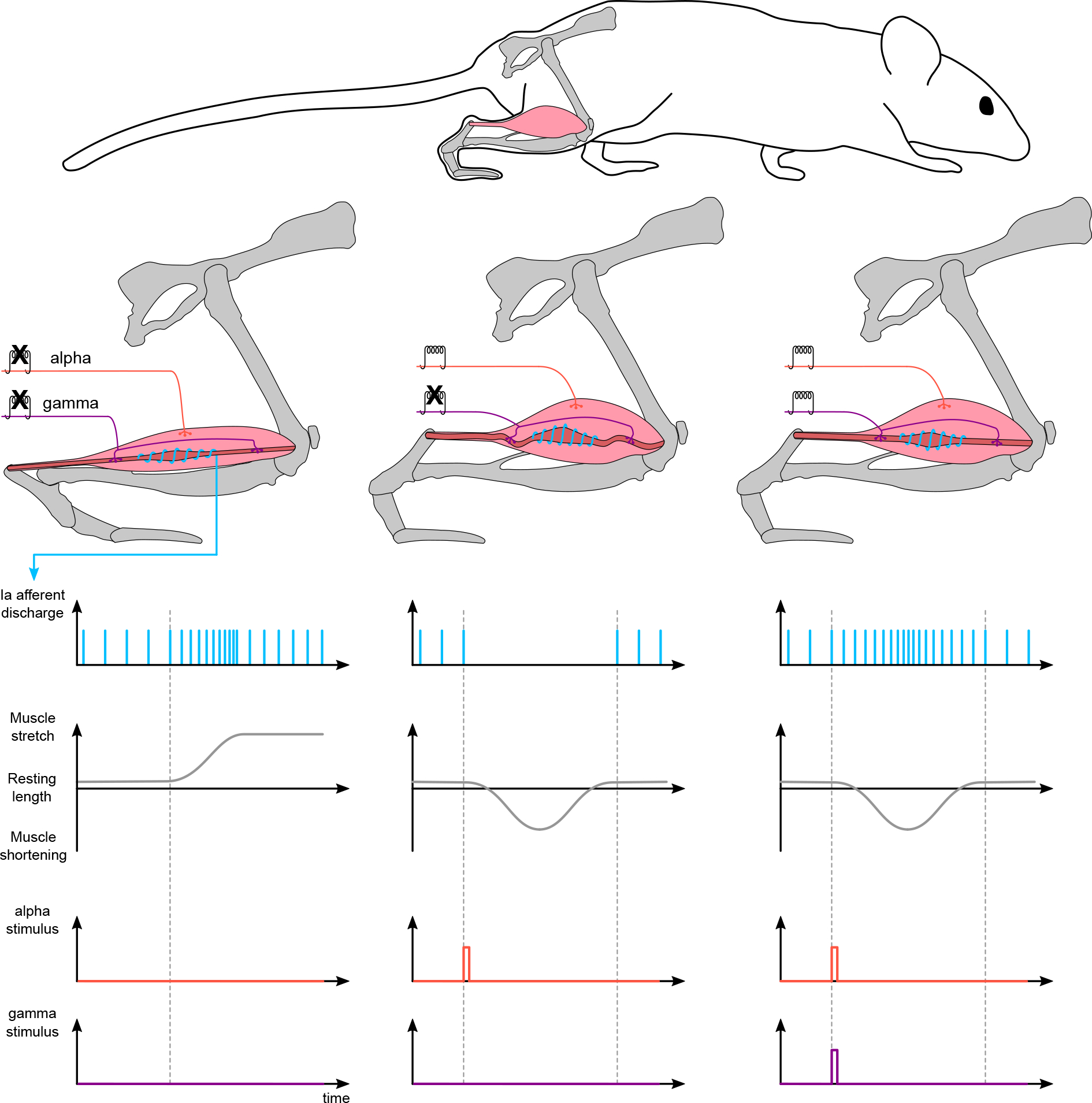
When the muscle is further stretched (left), primary Ia afferents increase their firing rate until a steady-state is reached. When the muscle is shortened after activation of alpha motor neurons, muscle spindles unload and undergo relaxation (middle). Activation of gamma motor neurons (right) re-tensions spindles to the extrafusal fibres to ensure that their transduction capabilities are maintained within the appropriate range.
How is the stretch of intrafusal fibres translated into neuronal signalling? The stretch of muscle spindles causes deformation of the sensory terminals (Banks, 1986; Bendeich et al., 1978). The mechanical deformation is primarily detected by the mechanosensitive nonselective cation channel protein PIEZO2 at the afferent endings (Kefauver et al., 2020; Woo et al., 2015). Yet, current evidence does not exclude that other candidate channels might be involved in the mechanotransduction process (Bewick & Banks, 2021). For example, the voltage-gated sodium channel NaV1.1 has been recently shown to be essential for proprioceptive signaling (Espinoetal.,2022). Stretch-induced opening of the PIEZO2 channels and influx of Na+ and Ca2+ ions causes depolarization of the membrane of the afferent endings, whereas K+ efflux is responsible for repolarization, collectively shaping the stretch-induced receptor potential at the afferent endings (Hunt & Ottoson, 1975; Hunt et al., 1978). The stretch-induced receptor potential is then translated into action potential signals that are conveyed to the central nervous system (Bewick & Banks, 2015). This sensory transduction may be facilitated in an axon-autonomous manner: the discovery of synaptic-like vesicles containing glutamate (Banks et al., 2002) and the vesicular glutamate transporter 1 (VGLUT1) (Fig. 3) protein (Wu et al., 2004) suggested that the afferent endings can release glutamate in the spindle, increasing the firing rate of the autonomous afferent (Bewick et al., 2005; Bewick & Banks, 2015) and improving the static but not the dynamic sensitivity (Than et al., 2021). But whether this positive feedback loop is differentially activated in a context-dependent manner and what role it has in behaviour remain unknown. Considering all these aspects of sensory transduction, muscle spindles are structurally and functionally complex organs that detect muscle stretch. Therefore, muscle spindles indirectly encode the angular position and velocity of joints and the force, stiffness, velocity and acceleration of muscles (Blum et al., 2020).
Figure 3. Immunofluorescence histochemistry staining of a murine muscle spindle.
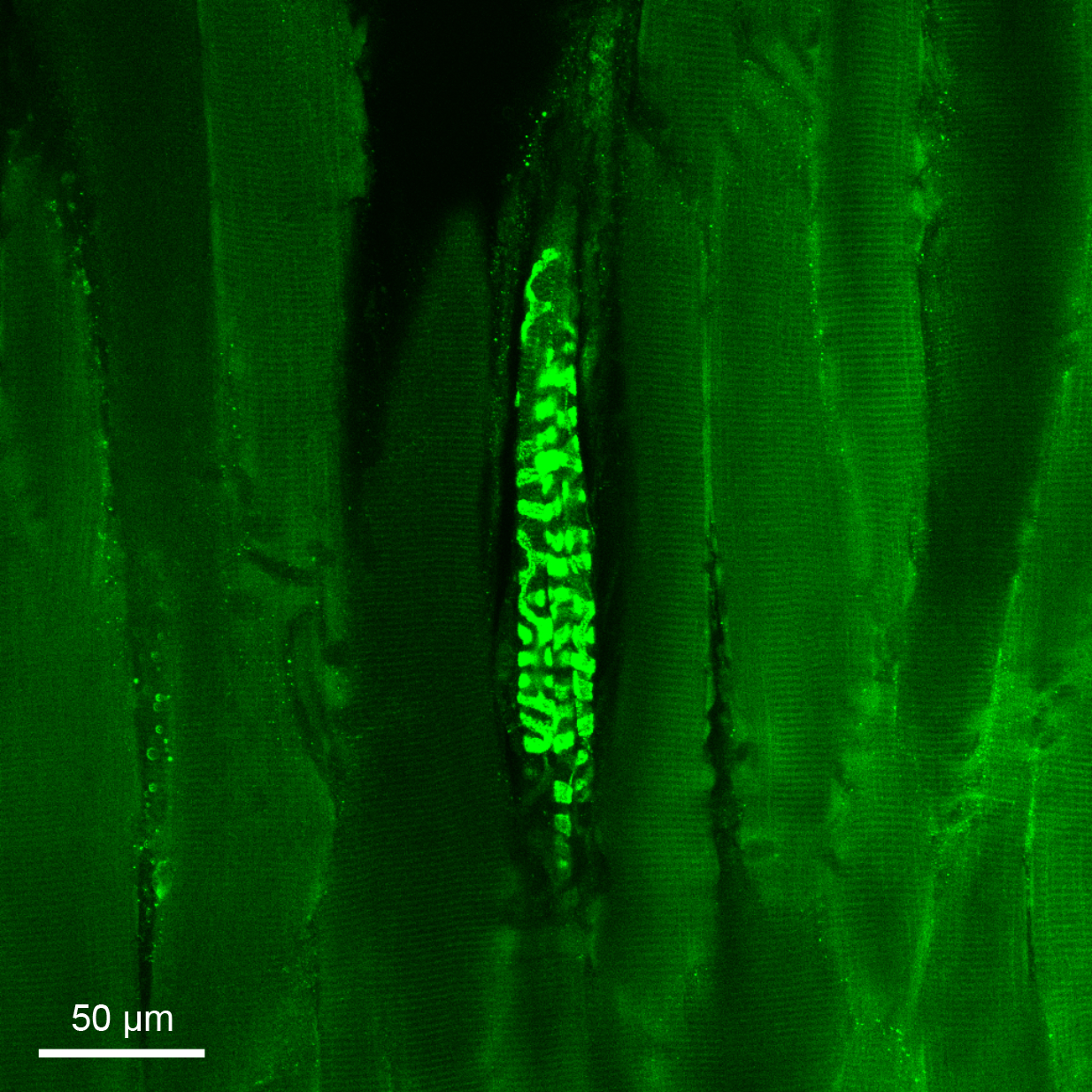
The muscle spindle and surrounding tissue were isolated from the tibialis anterior hindlimb muscle of a wild-type mouse with C57BL/6 background. VGLUT1 is expressed in the central and peripheral sensory endings of muscle-spindle afferents, allowing staining of their spiralling terminations and leaving the extrafusal and intrafusal fibres uncoloured.
Muscle spindles contribute to locomotor robustness.
All biological systems are inherently modular, complex and robust (Csete & Doyle, 2002; Kitano, 2007). Modularity can be seen at most scale levels, from genes and proteins to the co-ordinated muscle activations needed to achieve movement (Bizzi & Cheung, 2013; Csete & Doyle, 2002). Complexity, found not only in biological systems, but also in artificial structures such as modern airplanes, computers or grand pianos, describes how different parts of the same system are organized and hierarchically ordered at multiple scales (Carlson & Doyle, 2002). Robustness is the ability to cope with perturbations or errors of execution (Santuz et al., 2018; Stelling et al., 2004) and it allows maintenance of function or performance in the face of external and internal perturbations (Kitano, 2007). Biological systems such as mammals achieve the ultimate goal of robustness thanks to a fine interplay between complexity, modularity and feedback mechanisms: for a specific task, each ingredient needs to be present in the correct amount to maintain function (Csete & Doyle, 2002). The systemic or partial absence of proprioceptive feedback is known to disrupt locomotor patterns in frogs (Kargo & Giszter, 2000), mice (Akay et al., 2014; Takeoka & Arber, 2019), cats (English, 1980, 1985), guinea fowls (Gordon et al., 2020) and humans (Lajoie et al., 1996). Ageing, cancer, inflammation, infection, pain, trauma and many musculoskeletal, neurodegenerative, neurodevelopmental and peripheral nervous system disorders are known to be associated with impaired proprioception (Blanche et al., 2012; Blecher et al., 2018; Camdessanche et al., 2009; Dietz, 2002; D’Silva et al., 2016; Hammond et al., 2013; Kröger & Watkins, 2021; Prochazka, 2021; Röijezon et al., 2015; Sheikh & Amato, 2010). The disruption can be seen in the resulting movements (e.g. via analysis of the animal’s kinematics) and also in the muscle activations required to perform those movements (e.g. recorded via electromyography). For example, the genetic removal of muscle spindles in mice produces, amongst other symptoms, evident and irreversible gait ataxia (Tourtellotte & Milbrandt, 1998). The ataxia manifests itself during locomotion in an exaggerated lift of the paw achieved by prolonged activation of the ankle flexor muscles, especially in the hindlimb (Akay et al., 2014), and this is dramatically worsened by the concurrent removal of Golgi tendon organs (Takeoka & Arber, 2019). The same is true in humans, where purely sensory neuropathy is known to have detrimental effects on gait fluidity that are compensated for by reducing the overall degrees of freedom (e.g. locking the knee joint during the stance phase), increasing the width of the base of support and relying on visual feedback to assess the position of the lower limbs (Lajoie et al., 1996). Thus, locomotion without feedback from muscle spindles is possible, yet aberrant.
Locomotor drive modularity and muscle spindles.
Deeper insight into the role of sensory feedback can be gained by analysing the modular organization of muscle activity. One prominent theory in motor control states that the central nervous system might benefit from controlling multiple muscles synchronously rather than individually (Bernstein, 1967), in finely orchestrated sets of activation patterns that have been called muscle synergies (Bizzi et al., 1991). Following this concept, the great number of muscle activations needed to execute a certain movement could result from the combination of a small number of synergies, which would drastically reduce the dimensionality of the control strategy (Tresch et al., 1999). In muscle spindle-deficient mice (Santuz et al., 2019, 2022), the synergistic activation motifs on average undergo a change in timing compared to the wild-type, mostly resulting in their widening (i.e. activations are of longer duration in the absence of muscle spindles). This outcome is similar to that previously found for individual muscles (Akay et al., 2014) and fits previous findings in humans where the widening of muscle activity emerged in response to pathology (Cappellini et al., 2016; Janshen et al., 2020; Martino et al., 2014), development stage (Cappellini et al., 2016; Dominici et al., 2011), aging (Dewolf et al., 2021; Santuz et al., 2020) and external mechanical perturbations (Martino et al., 2015; Santuz et al., 2018). The parallelism between muscle spindle-deficiency and other internal or external perturbed states shows that proprioceptive feedback is needed to regulate the locomotor output.
To support the connection between muscle spindles and locomotor robustness, it is possible to consider what happens when external mechanical perturbations are administered to genetically modified mice lacking proprioceptive feedback. Wild-type mice (Santuz et al., 2019, 2022), similarly to healthy humans (Chvatal & Ting, 2012; Martino et al., 2015; Santuz et al., 2018, 2020), respond to external perturbations by increasing the duration of muscle activation patterns relative to the gait cycle. This is true for both species independently of whether the stimulus is a sudden acceleration or a mediolateral displacement of the treadmill’s belt (Santuz et al., 2020, 2022), a mechanically-evoked trip in mice (Santuz et al., 2019) or the unevenness (Santuz et al., 2018) or slipperiness (Martino et al., 2015) of the locomotion surface in humans. The increased duration reflects the adaptive strategies (i.e. anticipatory, predictive and reactive) used by the central nervous system to cope with perturbations (Patla, 2003). In muscle spindle-deficient mice, however, activation patterns are wider compared to the wild-type in both the presence and absence of external perturbations, showing a loss of capability in the regulation of timing. Similarly, a lack of regulation is true for kinematic-related variables (Santuz et al., 2022). Moreover, the simultaneous ablation of both muscle spindles and Golgi tendon organs induces such a remarkable hindering of function that the animals lose the ability to adapt to fast speeds and, similar to muscle spindle-deficient mice, to regulate the timing of activation patterns (Santuz et al., 2022; Takeoka & Arber, 2019). Thus, the role of muscle spindles in maintaining locomotor function is more pronounced in the presence of external perturbations and intimately connected with the modularity of the locomotor output.
Locomotor drive complexity and muscle spindles.
As mentioned above, the ultimate goal of robustness in biological systems is achieved by the dense interplay of modularity and complexity. Amongst the numerous possibilities of addressing complexity from a numerical standpoint, methods stemming from fractal theory (Mandelbrot, 1983) have recently been used in behavioural studies and have been reviewed elsewhere (Jelinek et al., 2005; Kesić & Spasić, 2016; Müller et al., 2017). In sum, biological signals such as those recorded from muscles via electromyography possess fractal properties in that oscillations of those time series are visible at various scales (or, in other words, at different levels of magnification) and are largely similar to one another (Mandelbrot, 1985). Fractal measures of complexity can be used to detect properties of the signal that would be difficult to quantify otherwise: its roughness, its repeatability over various cycles, its noise content, and so on (Santuz & Akay, 2020). Fractal analysis reveals that muscle activation patterns undergo a decrease in complexity when locomotion happens in the presence of both internal and external perturbations. This outcome stands for mice as well as for humans: wild-type mice (Santuz & Akay, 2020; Santuz et al., 2022) and healthy humans of young and older age (Santuz et al., 2020) decrease the complexity of synergistic activation motifs when exposed to external mechanical perturbations. Similarly, the genetic removal of muscle spindles in mice produces decreased complexity of synergistic activations when the animals are compared with their wild-type littermates, independently of the administration of external mechanical disturbances (Santuz et al., 2022). This constitutes further confirmation that feedback from muscle spindles is essential to allow the fine tuning of motor output depending on the environmental demands and its absence leaves the system in a constantly perturbed state. Moreover, studying the complexity of muscle synergies, it is possible to show that supraspinal integration of proprioceptive information is needed to cope with perturbations during locomotion (Santuz et al., 2022). Wild-type mice in which the dorsal column of the spinal cord is lesioned lose the ability to cope with external perturbations, often coming to a complete stop, indicating failure to maintain locomotor function and thus robustness. This is probably because synergistic activation patterns do not undergo any modulation of complexity if proprioceptive pathways to the brain are partially interrupted. Indeed, in those animals, the complexity of synergistic activities during perturbed locomotion does not differ from that observed in intact littermates and remains undiscernible from that of unperturbed locomotion. Taken together, these recent results highlight an important role of muscle spindles in the regulation of the intrinsic complexity of the locomotor drive, aimed at the production of robust locomotion.
Conclusions
We have briefly summarized the vast body of knowledge on the structure and function of muscle spindles, with particular attention to the role of these sensory organs in assisting vertebrate locomotion. Subsequent to their discovery 160 years ago, the scientific interest in muscle spindles has intensified, but a complete characterization of their function is still a long way off. Recent findings based on the analysis of muscle activity’s modularity and complexity support a crucial role of muscle spindles in maintaining murine and human locomotor robustness: a fundamental feature for avoiding loss of function during locomotion. Yet, there still remain the questions of how this regulation is controlled and which peripheral, spinal and brain structures are responsible for the fine tuning required to face locomotion in real world environments. Answering these questions, possibly by the concurrent use of both non-human and human models, will provide new insight into the fundamental mechanisms of proprioception. Only by grasping those mechanisms will it be possible to fully decode the function of muscle spindles during locomotion, in both health and disease.
Funding
A.S. was supported by the Deutsche Forschungsgemeinschaft (Research Fellowships Program, grant SA 3695/1-1). The Akay lab is supported by the Canadian Institutes of Health Research (CIHR, grant PJT-162357) and the National Institute of Health (NIH, R01 NS119268 and R01 NS115900).
Biography

Alessandro Santuz received his bachelor’s and master’s degree in Aerospace Engineering at the University of Padua (Italy). He completed his PhD in Sport Science at the Humboldt-Universität zu Berlin (Germany) and was a postdoctoral fellow at the Department of Medical Neuroscience, Dalhousie University (Canada) and at the Institute for Biomechanics, ETH (Switzerland). His research focusses on the neural control of locomotion through sensory feedback in mice and humans. Alessandro uses special treadmills and mouse genetics to produce external and internal perturbations during locomotion and linear/nonlinear analysis tools to investigate how the central nervous system controls muscles and uses proprioceptive sensory feedback for the production of robust locomotion. He is currently employed at the Max Delbrück Center for Molecular Medicine in Berlin (Germany)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the HTML view of the article. Supporting information files available:
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP282563#support-information-section).
References
- Adrian ED, & Zotterman Y (1926). The impulses produced by sensory nerve-endings. Part 2. The response of a single end-organ. The Journal of Physiology, 61(2), 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akay T, Tourtellotte WG, Arber S, & Jessell TM (2014). Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proceedings of the National Academy of Sciences of the United States of America, 111(47), 16877–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW (1986). Observations on the primary sensory ending of tenuissimus muscle spindles in the cat. Cell and Tissue Research, 246(2), 309–319. [DOI] [PubMed] [Google Scholar]
- Banks RW (1994). The motor innervation of mammalian muscle spindles. Progress in Neurobiology, 43(4–5), 323–362. [DOI] [PubMed] [Google Scholar]
- Banks RW (2015). The innervation of the muscle spindle: A personal history. Journal of Anatomy, 227(2), 115–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Barker D, & Stacey MJ (1982). Form and distribution of sensory terminals in cat hindlimb muscle spindles. Philosophical Transactions of the Royal Society of London B, Biological Sciences, 299, 329–364. [DOI] [PubMed] [Google Scholar]
- Banks RW, Bewick GS, Reid B, & Richardson C (2002). Evidence for activity-dependent modulation of sensory-terminal excitability in spindles by glutamate release from synaptic-like vesicles. In, Gandevia SC, Proske U, & Stuart DG (Eds.), Sensorimotor control of movement and posture (pp. 13–18). Springer US, Boston, MA. [DOI] [PubMed] [Google Scholar]
- Banks RW, Ellaway PH, Prochazka A, & Proske U (2021). Secondary endings of muscle spindles: Structure, reflex action, role in motor control and proprioception. Experimental Physiology, 106(12), 2339–2366. [DOI] [PubMed] [Google Scholar]
- Banks RW, Harker DW, & Stacey MJ (1977). A study of mammalian intrafusal muscle fibres using a combined histochemical and ultrastructural technique. Journal of Anatomy, 123, 783–796. [PMC free article] [PubMed] [Google Scholar]
- Barker D (1948). The innervation of the muscle-spindle. The Quarterly Journal of Microscopical Science, 89, 143–186. [PubMed] [Google Scholar]
- Barker D (1974). The morphology of muscle receptors. In Hunt CC (Ed.), Muscle receptors (pp. 1–190). Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- Bastian HC (1880). Phrenology: old and new. In The brain as an organ of mind (p. 543). D. Appleton and Co., New York. [Google Scholar]
- Bendeich EG, Hooker WM, & Karlsson UL (1978). Sensory nerve deformation in the stimulated frog muscle spindle. Journal of Ultrastructure Research, 62(2), 137–146. [DOI] [PubMed] [Google Scholar]
- Bernstein NA (1967). The co-ordination and regulation of movements. Pergamon Press Ltd., Oxford. [Google Scholar]
- Bessou P, Emonet-Denand F, & Laporte E (1963). Mechanisms of neuromuscular spindle discharge triggered by alpha-motoneurons in the cat. Journal de Physiologie, 55, 114–115. [PubMed] [Google Scholar]
- Bewick GS, & Banks RW (2015). Mechanotransduction in the muscle spindle. Pflügers Archiv - European Journal of Physiology, 467(1), 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, & Banks RW (2021). Mechanotransduction channels in proprioceptive sensory nerve terminals: Still an open question? Current Opinion in Physiology, 20, 90–104. [Google Scholar]
- Bewick GS, Reid B, Richardson C, & Banks RW (2005). Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: Evidence from the rat muscle spindle primary sensory ending. The Journal of Physiology, 562(2), 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, & Cheung VC-K (2013). The neural origin of muscle synergies. Frontiers in Computational Neuroscience, 7, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, Mussa-Ivaldi FA, & Giszter SF (1991). Computations underlying the execution of movement: A biological perspective. Science, 253(5017), 287–291. [DOI] [PubMed] [Google Scholar]
- Blanche EI, Reinoso G, Chang MC, & Bodison S (2012). Proprioceptive processing difficulties among children with autism spectrum disorders and developmental disabilities. American Journal of Occupational Therapy, 66(5), 621–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher R, Heinemann-Yerushalmi L, Assaraf E, Konstantin N, Chapman JR, Cope TC, Bewick GS, Banks RW, & Zelzer E (2018). New functions for the proprioceptive system in skeletal biology. Philosophical Transactions of the Royal Society B, 373(1759), 20170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KP, Campbell KS, Horslen BC, Nardelli P, Housley SN, Cope TC, & Ting LH (2020). Diverse and complex muscle spindle afferent firing properties emerge from multiscale muscle mechanics. eLife, 9, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, & Poppele RE (2001). Proprioception from a spinocerebellar perspective. Physiological Reviews, 81(2), 539–568. [DOI] [PubMed] [Google Scholar]
- Boyd IA (1956). The tenuissimus muscle of the cat. The Journal of Physiology, 133, 35–36. [Google Scholar]
- Boyd IA (1958). The innervation of mammalian neuromuscular spindles. The Journal of Physiology, 140, 14–15. [PubMed] [Google Scholar]
- Boyd IA (1960). The diameter and distribution of the nuclear bag and nuclear chain muscle fibres in the muscle spindles of the cat. In Proceedings of the Physiological Society, pp. 23–24. Available at: 10.1113/jphysiol.1960.sp006558. [DOI] [Google Scholar]
- Boyd IA (1962). The structure and innervation of the nuclear bag muscle fibre system and the nuclear chain muscle fibre system in mammalian muscle spindles. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 245, 81–136. [Google Scholar]
- Boyd IA, Gladden MH, McWilliam PN, & Ward J (1977). Control of dynamic and static nuclear bag fibres and nuclear chain fibres by gamma and beta axons in isolated cat muscle spindles. The Journal of Physiology, 265(1), 133–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Strick PL, Kanda K, Kim CC, & Walmsley B (1977). Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. Journal of Neurophysiology, 40(3), 667–680. [DOI] [PubMed] [Google Scholar]
- Camdessanche J-P, Jousserand G, Ferraud K, Vial C, Petiot P, Honnorat J, & Antoine J-C (2009). The pattern and diagnostic criteria of sensory neuronopathy: A case-control study. Brain, 132(7), 1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Martino G, MacLellan MJ, Sacco A, Morelli D, & Lacquaniti F (2016). Immature spinal locomotor output in children with cerebral palsy. Frontiers in Physiology, 7, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, & Doyle J (2002). Complexity and robustness. Proceedings of the National Academy of Sciences of the United States of America, 99(suppl_1), 2538–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, & Ting LH (2012). Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. The Journal of Neuroscience, 32(35), 12237–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, & Daniel PM (1956). Human muscle spindles. The Journal of Physiology, 133, 1–3P. [PubMed] [Google Scholar]
- Crowe A, & Matthews PBC (1964a). The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. The Journal of Physiology, 174(1), 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A, & Matthews PBC (1964b). Further studies of static and dynamic fusimotor fibres. The Journal of Physiology, 174(1), 132–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csete ME, & Doyle JC (2002). Reverse engineering of biological complexity. Science, 295(5560), 1664–1669. [DOI] [PubMed] [Google Scholar]
- Dewolf AH, Sylos-Labini F, Cappellini G, Ivanenko YP, & Lacquaniti F (2021). Age-related changes in the neuromuscular control of forward and backward locomotion ed. Masani K PLoS ONE, 16(2), e0246372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V (2002). Proprioception and locomotor disorders. Nature Reviews Neuroscience, 3(10), 781–790. [DOI] [PubMed] [Google Scholar]
- Dietz V, & Duysens J (2000). Significance of load receptor input. Gait & Posture, 11, 102–110. [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, D’Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, & Lacquaniti F (2011). Locomotor primitives in newborn babies and their development. Science, 334(6058), 997–999. [DOI] [PubMed] [Google Scholar]
- D’Silva LJ, Lin J, Staecker H, Whitney SL, & Kluding PM (2016). Impact of diabetic complications on balance and falls: Contribution of the vestibular system. Physical Therapy, 96(3), 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, & Sherrington CS (1930). Numbers and contraction-values of individual motor-units examined in some muscles of the limb. Proceedings of the Royal Society of London Series B, Containing Papers of a Biological Character, 106, 326–357. [Google Scholar]
- English AW (1980). Interlimb coordination during stepping in the cat: effects of dorsal column section. Journal of Neurophysiology, 44(2), 270–279. [DOI] [PubMed] [Google Scholar]
- English AW (1985). Interlimb coordination during stepping in the cat: The role of the dorsal spinocerebellar tract. Experimental Neurology, 87(1), 96–108. [DOI] [PubMed] [Google Scholar]
- Espino CM, Lewis CM, Ortiz S, Dalal MS, Garlapalli S, Wells KM, O’Neil DA, Wilkinson KA, & Griffith TN (2022). NaV1.1 is essential for proprioceptive signaling and motor behaviors. eLife, 11, e79917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Akay T, & Prilutsky BI (2021). Control of mammalian locomotion by somatosensory feedback. Comprehensive Physiology, 12, 2877–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JC, Holt NC, Biewener AA, & Daley MA (2020). Tuning of feedforward control enables stable muscle force-length dynamics after loss of autogenic proprioceptive feedback. eLife, 9, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond N, Wang Y, Dimachkie MM, & Barohn RJ (2013). Nutritional neuropathies. Neurologic Clinics, 31(2), 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M (1984). The mammalian muscle spindle and its central control. In Reviews of physiology, biochemistry and pharmacology (Vol., 101, pp. 1–110). Springer Berlin Heidelberg. [DOI] [PubMed] [Google Scholar]
- Hunt CC, & Kuffler SW (1951). Further study of efferent small-nerve fibres to mammalian muscle spindles. Multiple spindle innervation and activity during contraction. The Journal of Physiology, 113(2–3), 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, & Ottoson D (1975). Impulse activity and receptor potential of primary and secondary endings of isolated mammalian muscle spindles. The Journal of Physiology, 252(1), 259–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, Wilkinson RS, & Fukami Y (1978). Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. Journal of General Physiology, 71(6), 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janshen L, Santuz A, Ekizos A, & Arampatzis A (2020). Fuzziness of muscle synergies in patients with multiple sclerosis indicates increased robustness of motor control during walking. Scientific Reports, 10(1), 7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek HF, Elston N, & Zietsch B (2005). Fractal analysis: Pitfalls and revelations in neuroscience. In Losa GA, Merlini D, Nonnenmacher TF & Weibel ER, Fractals in biology and medicine (pp. 85–94). Birkhäuser-Verlag, Basel, Boston, Berlin. [Google Scholar]
- Kanning KC, Kaplan A, & Henderson CE (2010). Motor neuron diversity in development and disease. Annual Review of Neuroscience, 33(1), 409–440. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, & Giszter SF (2000). Afferent roles in hindlimb wipe-reflex trajectories: Free-limb kinematics and motor patterns. Journal of Neurophysiology, 83(3), 1480–1501. [DOI] [PubMed] [Google Scholar]
- Kefauver JM, Ward AB, & Patapoutian A (2020). Discoveries in structure and physiology of mechanically activated ion channels. Nature, 587(7835), 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesić S, & Spasić SZ (2016). Application of Higuchi’s fractal dimension from basic to clinical neurophysiology: A review. Computer Methods and Programs in Biomedicine, 133, 55–70. [DOI] [PubMed] [Google Scholar]
- Kitano H (2007). Towards a theory of biological robustness. Molecular Systems Biology, 3(1), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker A (1862). Untersuchungen über die letzten Endigungen der Nerven. Zeitschrift Für Wissenschaftliche Zoologie, 12, 149–164. [Google Scholar]
- Kröger S, & Watkins B (2021). Muscle spindle function in healthy and diseased muscle. Skeletal Muscle, 11(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera J, Walro JM, & Reichler J (1988). Motor and sensory innervation of muscle spindles in the neonatal rat. Anatomy and Embryology, 177(5), 427–436. [DOI] [PubMed] [Google Scholar]
- Kühne W (1863). Die muskelspindeln. Archiv für Pathologische Anatomie und Physiologie und für Klinische Medicin, 28, 528–538. [Google Scholar]
- Lajoie Y, Teasdale N, Cole JD, Burnett M, Bard C, Fleury M, Forget R, Paillard J, & Lamarre Y (1996). Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology, 47(1), 109–115. [DOI] [PubMed] [Google Scholar]
- Mandelbrot BB (1983). The fractal geometry of nature. W. H. Freeman and Co., New York. [Google Scholar]
- Mandelbrot BB (1985). Self-affine fractals and fractal dimension. Physica Scripta, 32(4), 257–260. [Google Scholar]
- Martino G, Ivanenko YP, D’Avella A, Serrao M, Ranavolo A, Draicchio F, Cappellini G, Casali C, & Lacquaniti F (2015). Neuromuscular adjustments of gait associated with unstable conditions. Journal of Neurophysiology, 114(5), 2867–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Ivanenko YP, Serrao M, Ranavolo A, D’Avella A, Draicchio F, Conte C, Casali C, & Lacquaniti F (2014). Locomotor patterns in cerebellar ataxia. Journal of Neurophysiology, 112(11), 2810–2821. [DOI] [PubMed] [Google Scholar]
- Matthews BHC (1933). Nerve endings in mammalian muscle. The Journal of Physiology, 78(1), 1–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC (1962). The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences, 47(4), 324–333. [DOI] [PubMed] [Google Scholar]
- Matthews PBC (1972). The structure of receptors. In Mammalian Muscle Receptors and Their Central Actions, Issue 23 of the Monographs of the Physiological Society, pp. 1–55. Edward Arnold, London. [Google Scholar]
- Müller W, Jung A, & Ahammer H (2017). Advantages and problems of nonlinear methods applied to analyze physiological time signals: Human balance control as an example. Scientific Reports, 7(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, Moberly A, Badea TC, Duncan ID, Son YJ, Scherer SS, & Luo W (2013). Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. Journal of Neuroscience, 33(45), 17691–17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patla AE (2003). Strategies for dynamic stability during adaptive human locomotion. Engineering in Medicine and Biology Magazine, IEEE, 22(2), 48–52. [DOI] [PubMed] [Google Scholar]
- Pearson KG (2008). Role of sensory feedback in the control of stance duration in walking cats. Brain Research Reviews, 57(1), 222–227. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, & Burke D (2012). Fusimotor mechanisms, muscle spindles and their role in the control of movement. In The circuitry of the human spinal cord (pp. 110–137). Cambridge University Press, Cambridge. [Google Scholar]
- Prochazka A (2021). Proprioception: Clinical relevance and neurophysiology. Current Opinion in Physiology, 23, 100440. [Google Scholar]
- Proske U (1997). The mammalian muscle spindle. Physiology, 12(1), 37–42. [Google Scholar]
- Proske U (2006). Kinesthesia: The role of muscle receptors. Muscle & Nerve, 34, 545–558. [DOI] [PubMed] [Google Scholar]
- Proske U, & Gandevia SC (2009). The kinaesthetic senses. The Journal of Physiology, 587(17), 4139–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, & Gandevia SC (2012). The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiological Reviews, 92(4), 1651–1697. [DOI] [PubMed] [Google Scholar]
- Proske U, & Gandevia SC (2018). Kinesthetic Senses. In Comprehensive physiology (pp. 1157–1183). Wiley. [DOI] [PubMed] [Google Scholar]
- Röijezon U, Clark NC, & Treleaven J (2015). Proprioception in musculoskeletal rehabilitation. Part 1: Basic science and principles of assessment and clinical interventions. Manual Therapy, 20(3), 368–377. [DOI] [PubMed] [Google Scholar]
- Ruffini A (1898). On the minute anatomy of the neuromuscular spindles of the cat, and on their physiological significance. The Journal of Physiology, 23(3), 190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santuz A, & Akay T (2020). Fractal analysis of muscle activity patterns during locomotion: Pitfalls and how to avoid them. Journal of Neurophysiology, 124(4), 1083–1091. [DOI] [PubMed] [Google Scholar]
- Santuz A, Akay T, Mayer WP, Wells TL, Schroll A, & Arampatzis A (2019). Modular organization of murine locomotor pattern in the presence and absence of sensory feedback from muscle spindles. The Journal of Physiology, 597(12), 3147–3165. [DOI] [PubMed] [Google Scholar]
- Santuz A, Brüll L, Ekizos A, Schroll A, Eckardt N, Kibele A, Schwenk M, & Arampatzis A (2020). Neuromotor dynamics of human locomotion in challenging settings. iScience, 23(1), 100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santuz A, Ekizos A, Eckardt N, Kibele A, & Arampatzis A (2018). Challenging human locomotion: Stability and modular organisation in unsteady conditions. Scientific Reports, 8(1), 2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santuz A, Laflamme OD, & Akay T (2022). The brain integrates proprioceptive information to ensure robust locomotion. The Journal of Physiology, 600(24), 5267–5294. [DOI] [PubMed] [Google Scholar]
- Sheikh SI, & Amato AA (2010). The dorsal root ganglion under attack: The acquired sensory ganglionopathies. Practical Neurology, 10(6), 326–334. [DOI] [PubMed] [Google Scholar]
- Sherrington CS (1894). On the anatomical constitution of nerves of skeletal muscles; with remarks on recurrent fibres in the ventral spinal nerve-root. The Journal of Physiology, 17(3–4), 210–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS (1900). The muscular sense. In Sharpey-Schäfer EA (Ed.), Textbook of physiology (pp. 1002–1025). Young J. Pentland, Edinburgh, London. [Google Scholar]
- Sherrington CS (1906). Interaction between reflexes. In The integrative action of the nervous system (pp. 114–149). Yale University Press, New Haven, CT. [Google Scholar]
- Sherrington CS (1907). On the proprio-ceptive system, especially in its reflex aspect. Brain, 29(4), 467–482. [Google Scholar]
- Stelling J, Sauer U, Szallasi Z, Doyle FJ, & Doyle J (2004). Robustness of cellular functions. Cell, 118(6), 675–685. [DOI] [PubMed] [Google Scholar]
- Takeoka A, & Arber S (2019). Functional local proprioceptive feedback circuits initiate and maintain locomotor recovery after spinal cord injury. Cell Reports, 27(1), 71–85.e3. [DOI] [PubMed] [Google Scholar]
- Than K, Kim E, Navarro C, Chu S, Klier N, Occiano A, Ortiz S, Salazar A, Valdespino SR, Villegas NK, & Wilkinson KA (2021). Vesicle-released glutamate is necessary to maintain muscle spindle afferent excitability but not dynamic sensitivity in adult mice. The Journal of Physiology, 599(11), 2953–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte WG, Keller-Peck C, Milbrandt J, & Kucera J (2001). The transcription factor Egr3 modulates sensory axon-myotube interactions during muscle spindle morphogenesis. Developmental Biology, 232(2), 388–399. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, & Milbrandt J (1998). Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nature Genetics, 20(1), 87–91. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, & Bizzi E (1999). The construction of movement by the spinal cord. Nature Neuroscience, 2(2), 162–167. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Kloefkorn HE, & Hochman S (2012). Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation ed. Jones KE. PLoS ONE, 7(6), e39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-H, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, & Patapoutian A (2015). Piezo2 is the principal mechanotransduction channel for proprioception. Nature Neuroscience, 18(12), 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Petitpré C, Fontanet P, Sharma A, Bellardita C, Quadros RM, Jannig PR, Wang Y, Heimel JA, Cheung KKY, Wanderoy S, Xuan Y, Meletis K, Ruas J, Gurumurthy CB, Kiehn O, Hadjab S, & Lallemend F (2021). Distinct subtypes of proprioceptive dorsal root ganglion neurons regulate adaptive proprioception in mice. Nature Communications, 12(1), 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-X, Koshimizu Y, Feng Y-P, Okamoto K, Fujiyama F, Hioki H, Li Y-Q, Kaneko T, & Mizuno N (2004). Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Research, 1011(2), 247–251. [DOI] [PubMed] [Google Scholar]


