Abstract
During adolescence, the prefrontal cortex (PFC) undergoes substantial structural development, including cortical thinning, a process associated with improvements in behavioral control. The cingulate cortex is among the regions recruited in response inhibition and mounting evidence suggests cingulate function may be sensitive to availability of an essential dietary nutrient, omega-3 fatty acids (N3; i.e. EPA + DHA). Our primary aim was to investigate the relationship between a biomarker of omega-3 fatty acids -- percent of whole blood fatty acids as EPA + DHA (N3 Index) -- and cingulate morphology, in typically developing adolescent males (n = 29) and females (n = 33). Voxel-based morphometry (VBM) was used to quantify gray matter volume (GMV) in the dorsal region of the cingulate (dCC). Impulse control was assessed via caregiver report (BRIEF) and Go/No-Go task performance. We predicted that greater N3 Index in adolescents would be associated with less dCC GMV and better impulse control. Results revealed that N3 Index was inversely related to GMV in males, but not in females. Furthermore, males with less right dCC GMV exhibited better caregiver-rated impulse control. A simple mediation model revealed that, in males, N3 Index may indirectly impact impulse control through its association with right dCC GMV. Findings suggest a sex-specific link between levels of N3 and dCC structural development, with adolescent males more impacted by lower N3 levels than females. Identifying factors such as omega-3 fatty acid levels, which may modulate the neurodevelopment of response inhibition, is critical for understanding typical and atypical developmental trajectories associated with this core executive function.
Keywords: Omega-3 fatty acids, Blood, Adolescence, Neurodevelopment, Gray matter volume, Cingulate cortex, Impulse control, Response inhibition, Sex differences
Introduction
The long-chain omega-3 (N3) fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are essential nutrients in the diet that are critical for normal development and function of the central nervous system.1 Found in cold-water marine sources, these fatty acids, particularly DHA, accrue in the neuronal phospholipid membrane between the perinatal period and young adulthood.2 One of their functions is as an integral component of membrane phospholipids, where they promote membrane fluidity and neurotransmission,3 dendritic arborization,4 neuronal size,5 synaptogenesis,6 pruning of superfluous synapses7 and ultimately development of large scale functional networks.8 Brain levels of DHA are correlated with blood levels of DHA, and changing intake of dietary essential fatty acids is reflected in a change in both blood levels and brain levels.9–12 Blood levels of EPA + DHA are highly influenced by dietary intake,13 and as such, display a high level of variability (20%–30% in adults) in the general population.14
Animal evidence suggests that the prefrontal cortex (PFC) may be particularly sensitive to changes in dietary essential fatty acid intake.15 The PFC undergoes substantial structural development during adolescence when gray matter volume peaks and begins to decline through young adulthood.16–18 Across cortex, the process of gray matter thinning, thought to be due at least in part to pruning of superfluous synapses,19 is associated with cognitive ability.18,20 Within the PFC, the protracted developmental period of cortical thinning is associated with improvements in impulse control,21 a core executive function. Among cortical regions recruited during response inhibition,22 a region on the dorsal border of the cingulate cortex is implicated in performance monitoring/reactive cognitive control.23 As a part of the medial prefrontal cortex, this region follows a cubic developmental trajectory, attaining peak thickness in early adolescence.17 Periods of aggressive cortical growth may render regions under development susceptible to DHA insufficiency,24 potentially impacting normative development of impulse control.
The adolescent diet is often poor in sources of N3 fatty acids.25 Studies directly measuring N3 availability via blood report that greater N3 levels are associated with lower self-reported cognitive impulsivity in adults,26 although N3 levels are not significantly related to objectively measured impulse control in boys.27–29 Blood levels of N3 are associated with functional connectivity between anterior cingulate and regions recruited during sustained attention.27 Moreover, N3 blood levels are specifically associated with anterior cingulate (but not dlPFC) metabolic function (via myo-inositol levels).28 Indeed, work from our lab reveals that within the prefrontal cortex, cingulate activity during successful response inhibition is greater among adolescents who reported lower intake of long-chain omega-3 fatty acids,30 though this activity is in a region more dorsal than the studies previously described. These findings suggest that the cingulate cortex, a region implicated in response inhibition and under development during the adolescent period, may be particularly sensitive to inadequate intake of long chain N3 fatty acids.
To date, most studies of task-based response inhibition in typically developing youth have focused on boys,27–29 which may be due in part to the greater prevalence of neurodevelopmental disorders involving high degrees of impulsivity, such as ADHD, in males.31 Also, there is evidence of sexual dimorphism of PFC morphology and its association with ADHD.32 As a result, data on the nature of the relationship between long chain N3 fatty acids, brain architecture and behavior in girls is lacking. The influence of sex may be an important consideration in tissue levels of N3 fatty acids, however, as sex hormones have been demonstrated to modulate the conversion between precursor forms of omega-3 (alpha-linolenic acid, EPA, DPA) and the neurally-relevant product, DHA.33 These findings highlight the importance of contrasting the nature of this relationship between adolescent females and males.
The present study addresses this empirical gap through a cross-sectional investigation of the relationship between blood levels of N3 fatty acids, gray matter volume of cingulate gyrus and behavioral control in adolescent boys and girls. We used voxel-based morphometry (VBM), which is sensitive to morphological characteristics of gray matter such as thickness and cortical folding,34 to quantify differences in gray matter volume (GMV). Based on the evidence reviewed above as well as results of an analysis of greater BOLD activity during successful inhibition associated with reduced dietary omega-3 intake reported in a preprint elsewhere,30 we restricted our analyses to an a priori area of interest, the dorsal region of the cingulate (dCC). As a primary objective, we sought to explore the relationship between a biomarker of long chain N3 fatty acids – percent of whole blood fatty acids comprised of EPA + DHA (hereafter, N3 Index) – and structural characteristics of the cingulate cortex. We hypothesize that whole blood N3 Index would be associated with dCC gray matter volume in a sample of male and female adolescents, though we expect the magnitude of this relationship to be greater in males than in females.32,33 Our secondary aim was to explore whether whole blood N3 Index relates to response inhibition behavior, and hypothesize that N3 Index is positively related to impulse control.
Materials and methods
Participants
Participants were recruited as a part of a longitudinal neuroimaging study, the Adolescent Development Study, described in detail elsewhere.35 In brief, adolescents in a narrow age range (11–13 years old) were recruited. Main exclusions included prior substance use, left-handedness, conditions rendering MRI unsafe, history of head trauma, and neurodevelopmental disorders (e.g. Autism spectrum disorders, Tourette’s). Participants taking psychostimulant (centrally acting) medications were not enrolled if study visits could not be scheduled during normally occurring medication “holidays”. Demographic, neurocognitive, drug and alcohol use surveys, and imaging assessments were conducted at baseline and repeated 18- and 36-months later. The data reported here were collected at the 18-month follow-up visit. The university Institutional Review Board approved all study procedures and adolescents and their caregivers provided assent and consent, respectively, prior to data collection.
Upon enrollment into the study, intelligence was assessed using the Kauffman Brief Intelligence Questionnaire (K-BIT).36 Additionally, family socioeconomic status index was estimated using parental education and household income provided by one of the caregivers (method adapted from37). Caregivers reported their own educational level as well as that of the alternate biological parent, if known. Mean parental cumulative years of education was standardized and then averaged with total annual household income level (standardized). SES index was computed by re-standardizing the mean of two standard scores (mean parental cumulative years of education and total annual household income level) to achieve a distribution with a 0-centered mean and a standard deviation of 1 for the full sample (n = 135).
At the time of the visit, physical maturation and pubertal status were assessed via the Pubertal Development Scale, which consists of a series of questions about the degree to which progress of physical change has occurred (e.g. breast development, skin/voice changes)38 and is highly correlated with Tanner stage.39 Responses are allocated a score of 1–4 and averaged. Resulting scores range from (1) prepubertal to (4) postpubertal. In addition, body mass index (BMI) (kg/m2) sex and age-specific z-scores and percentiles were calculated using weight measured with a digital scale (Health-O-Meter Professional 394KLX) and height measured via stadiometer (SECA 216 Wall-mount Mechanical measuring rod; triplicate measures within 0.5 cm, averaged) applied to 2000 CDC Growth Charts.40 These indices of physical development were used to determine growth characteristics of males and females in the sample.
Dried blood spot collection and analysis
Blood collection and analysis procedures followed those described in Bell et al. (2011).41 In brief, adolescents were asked to provide blood samples at the end of their scan visit (non-fasted condition). Droplets of whole blood were absorbed onto two circular collection spots on Whatman 903 blood collection cards from the middle finger tip using a blood lance (Accu-Chek®, Safe-T-Pro Plus, Roche Diagnostics GmbH, Mannheim, Germany). The procedure lasted approximately 10 min and was collected just prior to the end of visit on the same day neuroimaging and behavioral data were collected. Blood spot cards were air dried for 3 h, sealed in polythene bags with desiccant packets, and stored at −80°C until shipment (up to 6 months). Samples were shipped for analysis via first overnight delivery at ambient temperature. Samples were subjected to transmethlyation and fatty acid methyl esters were separated and quantified by gas liquid chromatography (as detailed by).41 Fatty acids, including EPA and DHA, were classified using reference standards and expressed as percentage of total fatty acids. A log-base10 transformation of this value successfully corrected the originally skewed distribution.
Assessments of inhibitory control
As impulsivity is a multidimensional construct (e.g.42), we used two measures to assess different dimensions43 of impulse control in this adolescent sample: caregiver ratings of impulse control in real world context and ability to control prepotent responses on a laboratory task.
Behavior rating inventory of executive function (BRIEF)
The BRIEF44 is an 86-item psychometrically validated questionnaire to assess facets of executive abilities and was administered to the caregiver who came with the participant for the study visit. Each item asks the caregiver to rate the frequency that their child’s behaviors were “never”, “sometimes”, or “often” a problem over the 6 months prior to their visit. This assessment of general inhibitory control temperament45 yields 8 non-overlapping scales, including the Inhibit subscale, reflecting the ability to control impulses or stop behavior of relevance to the current study. Higher scores suggest higher levels of impulsivity. Normative values for age and sex (t-scores) are reported.
Go/No-Go task
To measure impulsive action, adolescents completed a simple Go/No-Go task while in the MRI scanner. Only behavioral data are presented in the current study. Alternating blocks of event-related Go/No-Go (45 sec) and rest (12–16 sec) were each repeated 5 times. During the Go/No-Go blocks, a series of 30 letters was presented for 200 ms each, followed by a 1300 ms rest. Subjects were instructed to press the button in their right hand as quickly as possible for every letter (“Go” trials) except the letter ‘Q’ (“No-Go” trials). A total of 150 trials were presented in this design of which 18% were No-Go trials. Trials were included in analyses if response time (processing speed) was at least 150ms, indicative of an intentional, rather than anticipatory, response. Ability to inhibit response for the No-Go trials was used as an indication of ability to control impulsive action (i.e. ability to inhibit a prepotent response).46 The task was implemented in E-prime. Calculated metrics of interest include false alarms (Incorrect No-Go/Total No-Go) (reflecting impulsivity), and reaction time to Go trials (reflecting processing speed). Hit rate (Correct Go/Total Go) was used to determine whether the participant was adequately engaged with the task (at least 70% of Go trials with a response).
MR image acquisition and analysis
All scanning was performed using a Siemens TIM Trio 3 T scanner with a 12-channel head coil. The high-resolution structural magnetic resonance images were collected using a T1-weighted MPRAGE. A total of 176 sagittal slices were collected with the following sequence parameters: TR/TE/TI = 1920/2.25/900 ms, flip angle = 9°, slices thickness = 1.0 mm, FOV = 250 × 250 mm2 and a matrix of 256 × 256 for an effective spatial resolution of 0.97 × 0.97 × 1.0 mm3.
Quality control for MPRAGEs
Three independent raters blind to the participant identity visually verified quality of structural scans for inclusion in a Voxel-Based Morphometry (VBM) analysis. Raters scored structural scans for degree of artifacts, which may impact the tissue classification algorithm (i.e. gross quality problems such as phase wrap or clipped brain, ringing, and other motion related artifacts) on a 0–5 point scale, where 0 indicates a scan free from visible artifact and 5 represents a scan of the poorest quality due to the severe presence of that specific artifact. For each of the 3 raters, a weighted summary score was computed. Summary scores were in good to excellent agreement (intraclass correlation coefficient = 0.873, 95% confidence interval = 0.798–0.919 [SPSS 24 based on mean-rating (k = 3), absolute-agreement, 2-way mixed-effects model]).47 An average weighted score of the 3 raters was assigned to each structural scan. Scans with an average weighted quality score > 9 were considered to be of extremely poor quality. These scans were subsequently reviewed/confirmed by the authors and excluded from the pool of scans suitable for VBM analysis.
Preprocessing
Preprocessing for VBM was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Tissues were segmented using the New Segment option using the default parameters to generate the native tissue segmentation as well as lower resolution versions for the DARTEL registration. Individual images were spatially normalized to a study-specific whole-brain template (i.e. semi-optimized) using DARTEL toolbox to increase accuracy of inter-subject alignment. Images were spatially normalized to Montreal Neurological Institute (MNI) space and modulated by the determinant of the Jacobian of the transformation to preserve tissue volume and create images corresponding to gray matter volume (GMV). The GMV images were smoothed using convolution with a Gaussian kernel with a FWHM of 12 mm to reduce spatial noise and minor differences in anatomic variability following spatial normalization.48 Measures of GMV were normalized by total intracranial volume (ICV) in order to account for inter-individual differences in brain size.
Region of interest (ROI) selection
Given an association between total calorie-adjusted dietary N3 Index and BOLD activation in the dorsal cingulate at adolescents’ baseline visit,30 separate left and right dorsal/mid- cingulate masks were generated using AAL via the Wake Forest Pick Atlas (Fig. 1).49 Masks were imported into MarsBaR and used to extract mean gray matter volume from these regions. GMV from bilateral dorsal/mid-cingulate ROIs were subsequently analyzed in the statistical analyses as described below.
Figure 1.
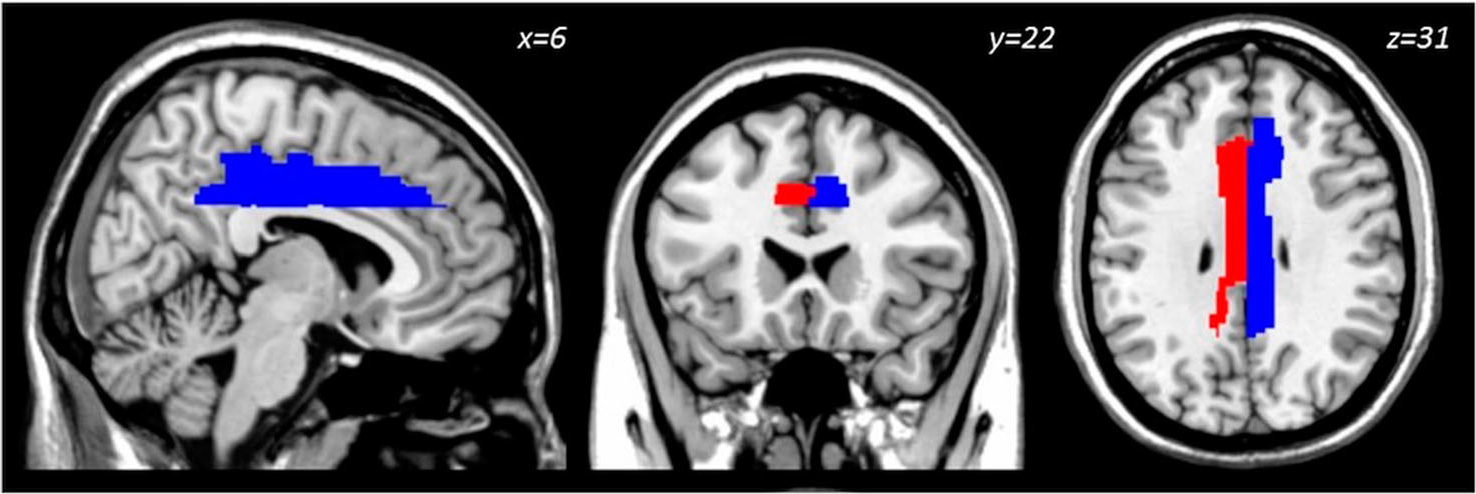
Location of a priori dorsal/mid-Cingulate ROI used to extract gray matter volume for analysis. Right hemisphere ROI (blue), left hemisphere ROI (red).
Statistical analysis
Distributions of dependent variables were confirmed prior to analysis to ensure assumptions of normality. Examination of dependent variables confirmed no outliers were present in any of the measures.50 As stated previously, our main predictor variable, N3 Index, was log-base10 transformed before being included in anyanalyses. Males and females were compared on characteristics listed in Table 1 and dependent variables (cingulate GMV and response inhibition measures) using the independent samples Student’s T-test and Mann–Whitney U-test where noted. Pubertal status, which differed by sex, was controlled for in whole group correlations between N3 Index and the dependent variables. Finally, we tested the effect of sex on the association between blood N3 and dependent variables using ANCOVA. Data were analyzed in SPSS 24 (IBM Statistics).
Table 1.
Participant characteristics
| Whole sample | Males | Females | p | |
|---|---|---|---|---|
|
| ||||
| N | 62 | 29 | 33 | |
| Age | 14.4(0.7) | 14.3(0.6) | 14.4(0.8) | 0.514 |
| Race | 0.202 | |||
| Caucasian % | 56.5% | 55.2% | 57.6% | |
| African American | 32.3% | 41.4% | 24.2% | |
| Hispanic/Latino | 3.2% | 0% | 6.1% | |
| Other | 8.1% | 3.4% | 12.1% | |
| Socioeconomic status index (z-score)a | 0.024(0.980) | 0.016(1.025) | 0.031(0.956) | 0.972 |
| Parental education (years, mean) | 16.3(2.7) | 16.6(2.9) | 16.1(2.5) | |
| Household income (median) | $100,00-$149,999 | $75,000-$99,999 | $100,00-$149,999 | |
| Pubertal development | 2.82(0.58) | 2.59(0.46) | 3.03(0.61) | 0.003 |
| BMI z-score | 0.34(1.04) | 0.19(1.03) | 0.47(1.05) | 0.294 |
| Percentilea | 59.9(29.6) | 55 1(30 5) | 64.1(28.6) | 0.244 |
| Intelligence (K-BIT) | 110.2(14.2) | 111.4(17.7) | 109.0(10.0) | 0.528 |
| Blood DHA (% of total fatty acids)a | 1.87(0.50) | 1.81(0.42) | 1.93(0.56) | 0.521 |
| N3 Index (% of total fatty acids)a | 2.17(0.60) | 2.10(0.47) | 2.24(0.70) | 0.667 |
Mann-Whitney U-test used to examine difference between male and female means.
Note: Group means and standard deviations listed, except where indicated.
The distribution of two dependent variables, BRIEF Inhibit t-score and Correct Go reaction time, were non-normal (Shapiro–Wilk 0.875, df = 62, p < 0.001, and Shapiro–Wilk 0.867, df = 62, p < 0.001, respectively). As standard transformations were unsuccessful at normalizing distributions, these dependent variables were used in non-parametric Spearman’s rank correlations and rank-transformed for use in linear modeling.
Results
Of the 135 participants enrolled in the parent study, 93 were asked to provide blood samples; this sub-study was added after the parent study was underway. Ten participants declined to provide blood. The remaining 83 assented and caregivers provided informed consent for their child to provide blood samples. A total of 81 participants’ blood samples were successfully collected (n = 2 failed due to insufficient blood extracted for analysis).
Of the 81 participants providing blood, 4 were excluded from analysis (n = 3 not attending to task - hit rate <70%; n = 1 technical data collection issue). Of the remaining 77 participants, 13 did not have structural images (due to braces at time of visit) and an additional 2 did not meet quality control standards for the MPRAGE, resulting in 62 participants (33 females, 29 males) eligible for analysis.
Participant characteristics as a whole group and by sex are presented in Table 1. Males and females were similar in characteristics except for pubertal development, with females reporting greater development than males (t = −3.068, df = 55, p = 0.003).
Blood N3 Index was unrelated to potentially confounding variables amongst the whole group (age, r = 0.18, p = 0.890; pubertal development, r = −0.134, p = 0.321; SES Index z-score rs = −0.91, p = 0.483; BMI z-score r = −0.104, p = 0.419; IQ r = −0.157, p = 0.232, n = 60). Blood N3 Index was also not significantly different between males and females (t = −0.685, df = 60, p = 0.496) (Table 1).
As a whole group, pubertal status was related to a dependent variable (Go/No-Go False Alarms r = 0.350, p = 0.008, n = 57; but not Go/No-Go reaction time rs = 10.108, n = 57, p = 0.425; BRIEF Inhibit t-score rs = −0.24, p = 0.093, n = 57, or dCC volumes left r = −0.051, p = 0.704, n = 57; right r = −0.090, p = 0.509, n = 57); and pubertal status was significantly different between sexes (Table 1). Thus, pubertal development score was entered as a covariate in the subsequent whole group correlation analyses.
Dorsal/mid-cingulate (dCC) GMV
Among the entire sample (N = 62), N3 Index was not related to dCC GMV, controlling for pubertal status (left: r = −0.107, df = 54, p = 0.431; right: r = −0.161, df = 54, p = 0.234).
Males and females were similar in ICV-adjusted GMV for dCC volumes on both left (t = −0.895, df = 60, p = 0.374) and right (t = −1.295, df = 60, p = 0.475) (Table 2). However, while N3 Index was unrelated to cingulate GMV in females (left: r = 0.253, n = 33, p = 0.156; right: r = 0.124, n = 33, p = 0.439), N3 Index was significantly inversely related to dCC gray matter volume in males (left: r = −0.556, n = 29, p = 0.002; right: r = −0.503, n = 29, p = 0.005) (Fig. 2). The slope of the regression lines was significantly different between sexes (left: F1, 59 = 15.21, p < 0.001; right: F1, 59 = 9.53, p = 0.003). Separately, we performed a voxel-wise analysis exploring associations (puncorr 0.001; cluster extent 10 voxels) between blood N3 Index and gray matter volume across a bilateral prefrontal gray matter cortical mask generated using AAL via the Wake Forest Pick Atlas (consisting of superior, middle and inferior frontal gyri, precentral gyrus, supplementary motor area, Rolandic operculum, gyrus rectus, and anterior and middle cingulate gyri), but no clusters survived non-stationary correction.
Table 2.
Outcome variables: Whole group and by sex
| Whole sample | Males | Females | P | |
|---|---|---|---|---|
|
| ||||
| N | 62 | 29 | 33 | |
| BRIEF Inhibit subscale | ||||
| t-scorea | 50.4(9.7) | 49.9(9.6) | 50.8(9.9) | 0.832 |
| median | 48 | 48 | 50 | |
| range | 40–87 | 40–72 | 41 –87 | |
| Percentilea | 59.5(23.0) | 57.9(24.7) | 60.9(21.7) | 0.916 |
| median | 60 | 59 | 65 | |
| range | 23–99 | 23–96 | 28–99 | |
| Go/No-Go False Alarm Rate (% Incorrect No go) | 32.5(15.4) | 28.7(13.3) | 35.8(16.5) | 0.070 |
| Go/No-Go Response time, ms (Correct Go)a | 331.1(62.7) | 331.4(63.6) | 330.9(63.0) | 0.983 |
| Median | 311.0 | 310.0 | 320.4 | |
| Range | 248.5–558.4 | 255.0–558.4 | 243.5–519.8 | |
| Total Intracranial Volume (cubic liters) | 1.53(0.14) | 1.61(0.10) | 1.44(0.12) | <0.001 |
| Mid-cingulum GMV (ICV adjusted) | ||||
| Mid, left | 0.296(0.016) | 0.294(0.019) | 0.297(0.013) | 0.374 |
| Mid, right | 0.287(0.015) | 0.284(0.018) | 0.289(0.013) | 0.475 |
Mann-Whitney U-test used to examine difference between male and female means.
Figure 2.
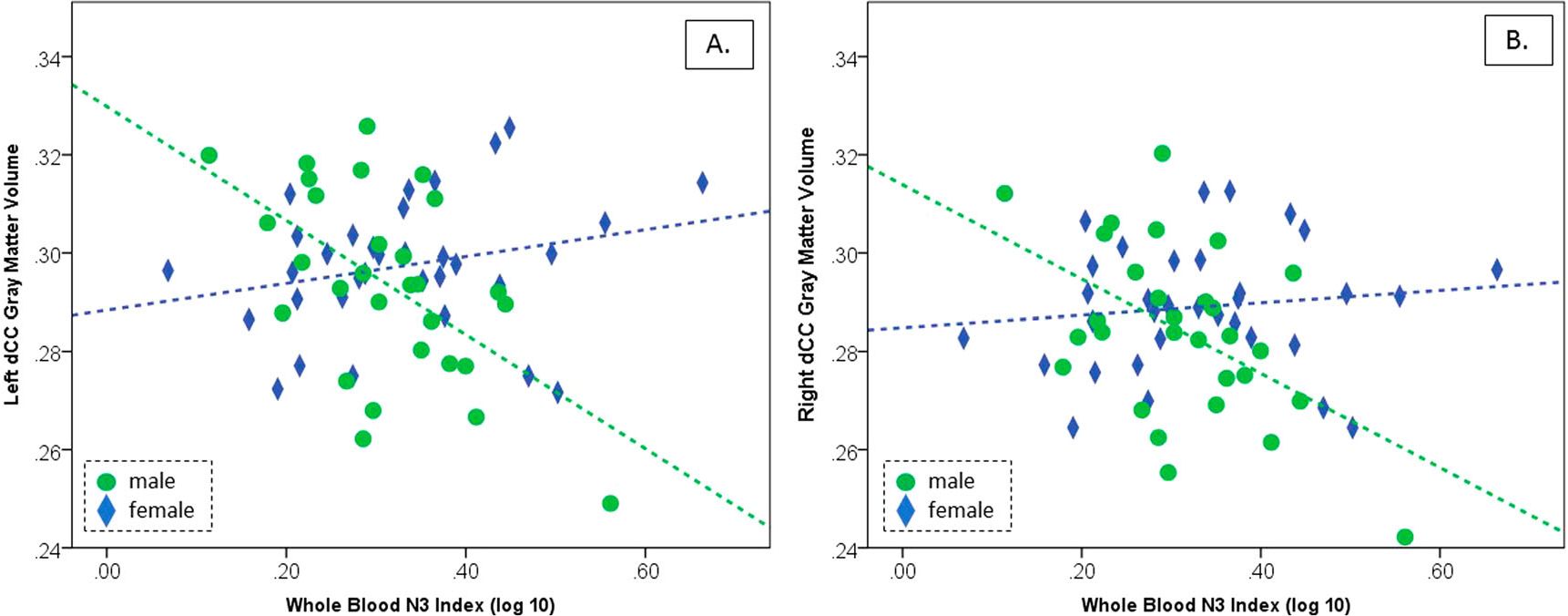
Pearson’s correlations between whole blood N3 Index and gray matter volume by sex in (A) left mid-cingulate and (B) right mid-cingulate. While no test reached significance in females (blue diamonds), both left and right male mid-cingulate GMV was significantly inversely related to N3 Index (green circles).
Inhibitory control
Go/No-Go performance
Among the whole sample, controlling for pubertal status, blood N3 Index was neither related to false alarm rate (r = −0.174, df = 54, p = 0.199) nor processing speed (Correct Go reaction time, rank-transformed; r = 0.074, df = 54, p = 0.588). Males and females were similar in processing speed (Mann–Whitney U = 480.0, p = 0.983), and the difference between sexes for false alarms did not reach statistical significance (t = −1.843, df = 60, p = 0.070) (Table 2). There were no significant relationships between N3 Index and false alarms or processing speed for either sex (false alarms, females: r = −0.178, n = 33, p = 0.321 and males: r = −0.291, n = 29, p = 0.125; processing speed, females: rs = 0.273, n = 33, p = 0.124 and males: rs = −0.111, n = 29, p = 0.566). Neither the slope nor intercept were significantly different between males and females for the association between N3 Index and task processing speed (slope, p = 0.375; intercept, p = 0.921). However, for the association between N3 Index and false alarm rate, while the slopes of the regression lines are similar for males and females (p = 0.618), the significant difference in Y-intercepts (F1,60 = 4.06, p = 0.048) indicating that females displayed a significantly greater false-alarm rate than males.
BRIEF Inhibit subscale
Within the whole sample, controlling for pubertal status, the relationship between log-base10 transformed blood N3 Index and the rank-transformed BRIEF Inhibit subscale did not reach statistical significance (r = −0.231; df = 54 p = 0.087). Males and females were similar in BRIEF inhibit t-score (Mann–Whitney U = 493.5; p = 0.832) (Table 2). Inhibit t-scores were not related to N3 Index in either females (rs = −0.061, n = 33, p = 0.738) or males (rs = −0.220, n = 29, p = 0.253), and there were no differences in either the slopes (p = 0.438) or intercepts (0.741) of these regression lines.
Of note, males and females differed in their association between BRIEF Inhibit t-scores and right dCC GMV (slope, F1,59 = 4.22, p = 0.044) but not left (slope, p = 0.347; intercept, p = 0.389). Specifically, among males only, BRIEF Inhibit t-scores were positively related to GMV in the right dCC (rs = 0.440, n = 29, p = 0.017) (but not left, p = 0.264) such that less GMV was associated with lower t-scores, indicative of better impulse control.
Mediation analysis
Significant relationships were found in males between N3 Index and right dCC GMV, and between GMV and impulse control as measured by the BRIEF Inhibit subscale. Although no significant direct relationship was found between N3 Index and this behavioral measure, we implemented a post-hoc mediation analysis to investigate whether N3 exerts an indirect effect on behavior, mediated by dCC.51 A simple mediation model was estimated using the PROCESS macro (v3)52 implemented in SPSS 24. We found a significant mediation, such that whole-blood N3 Index was indirectly related to impulse control through its relationship with right dCC. Adolescent males with less whole-blood N3 Index exhibited greater GMV in the right dCC (a = −0.0959, SE = 0.0317) and adolescent males with greater GMV in right dCC exhibit increased impulsiveness (b = 14.1976, SE = 7.7390) (Fig. 3). A bootstrap confidence interval (CI) for the indirect effect based on 10,000 bootstrap samples was entirely below zero (ab = −1.3615, 95% CI −3.3537 to −0.0678) indicating significance of the indirect effect. There was no evidence that N3 Index was related to impulsivity independent of its association with right dCC GMV (c’ = −0.5839, p = 0.6957).
Figure 3.
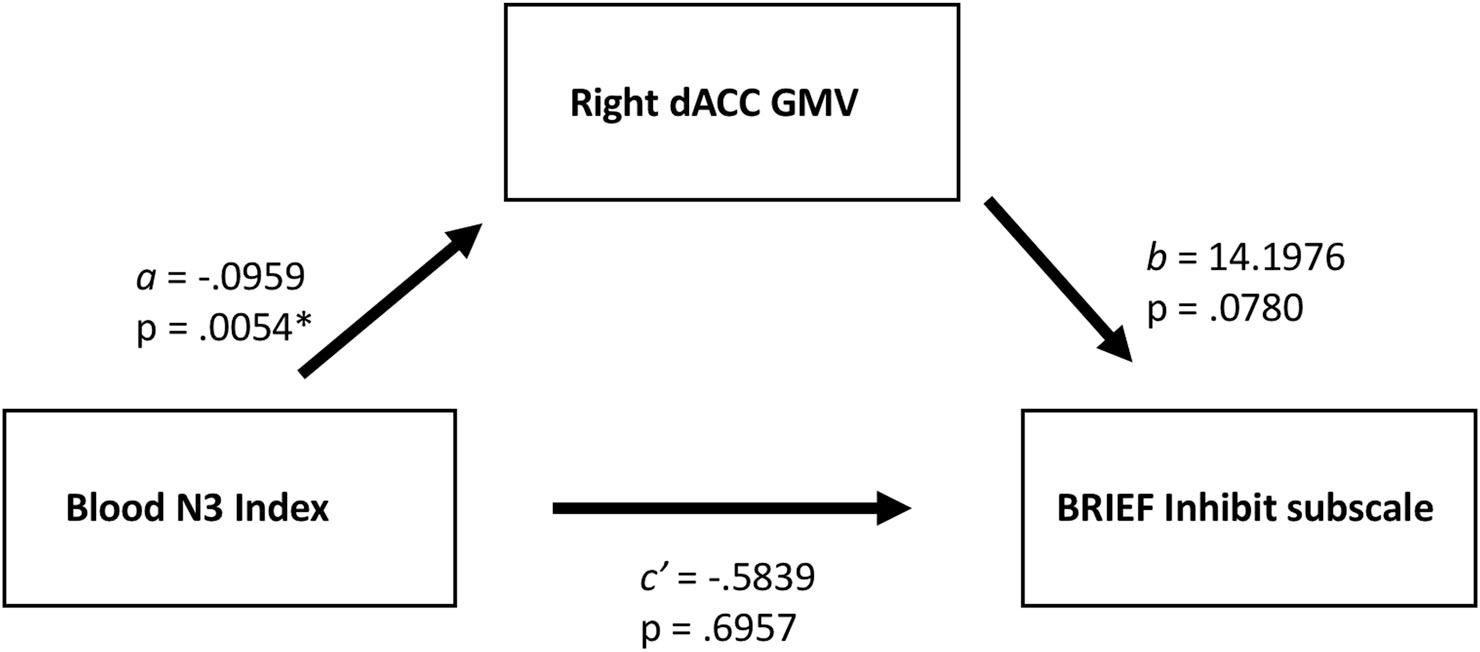
Greater right dCC GMV mediates an indirect relationship between low levels of whole blood N3 Index and poor inhibitory control in adolescent males. A bootstrap confidence interval (CI) for the indirect effect based on 10,000 bootstrap samples was entirely below zero (ab = −1.3615, 95% CI −3.3537 to −0.0678) indicating significance of the indirect effect.
Discussion
This study aimed to explore the relationship between circulating N3 fatty acid levels, structural development of the dorsal cingulate gyrus, and inhibitory control in both adolescent males and females. We found that, while there was no relationship between whole blood N3 Index and GMV of the dorsal cingulate among the sample as a whole or in females separately, there was a significant inverse relationship in males such that higher N3 Index was associated with less dorsal cingulate GMV bilaterally, which in turn was related to improved impulse control as rated by caregivers. Therefore, variations in N3 Index intake may play a role in cingulate development in male adolescents, with implications for development of impulse control.
Our main finding was that greater blood N3 Index was associated with less dorsal cingulate GMV in male adolescents. N3 Index explained a sizeable portion of the variance in left (30.9%) and right (25.3%) dCC volumes. In adults, anterior cingulate GMV has been positively associated with long-chain omega-3 fatty acids in both the blood53 and in the diet.54 The different direction in these relationships may be may be explained by the different ages of participants in the other studies. Long chain N3 fatty acids, particularly DHA, are involved not only in trophic support of gray matter over the entire life span,5,55,56 but are also critically involved in the developmental process of pruning superfluous axonal connections.7 Subsequent to the general increase in cortical gray matter volume in childhood, peak volume is reached in late childhood/early adolescence and then declines into young adulthood,16,57,58 partially reflecting synaptic pruning and refinement.19,59 The medial prefrontal and cingulate cortices attain peak thickness relatively late, with the dorsal supracallosal anterior cingulate estimated to reach peak cortical thickness at 13.8 years,17 suggesting that dCC volume for adolescents in the current study may be on the descent.
A possible implication of the main finding reported here is that adolescent boys with greater blood N3 Index levels may be further along in this aspect of neuroanatomical development of the dCC than those with lower N3 Index levels. Accordingly, we found that males with less right dCC GMV (but not left, p = 0.264) had better impulse control as rated by caregivers. Previous studies have found that the relationship between cingulate morphology and response inhibition varies with participant age, diagnosis, and assessment method: children with behavioral disorders have less anterior cingulate surface area32 and thickness60 compared to controls and in adults less anterior cingulate GMV is associated with higher self-reported impulsivity.61 However, in college-aged participants, greater anterior cingulate GMV is associated with impulsive action.62 Our findings lend support to the notion that, in typically developing adolescent males, less cingulate GMV is associated with better impulse control, and less cingulate GMV is associated with higher blood levels of long chain N3 fatty acids. Furthermore, a mediation analysis revealed an indirect relationship between N3 Index and the BRIEF Inhibit subscale mediated by GMV in the right dCC in males. Although not a direct test of causality, these results suggest N3 fatty acids may impact inhibitory control in typically developing male adolescents by influencing development of the dCC.
The current study is largely consistent with previously reported findings demonstrating an association between long chain N3 fatty acids and cingulate characteristics. The anterior region of the cingulate gyrus of boys with low blood levels of DHA was reported to have reduced indices of metabolic activity,28 and also reduced functional connectivity during sustained attention with regions including ventrolateral prefrontal cortex and insula.27 Together with the current finding that boys with lower blood N3 Index have greater GMV in the dorsal cingulate, these studies suggest that long-chain omega-3 fatty acids are related to the structural and metabolic development and ensuing network development of the cingulate gyrus in males.
The present study also extends the literature in humans demonstrating that, contrary to our hypotheses, dorsal cingulate cortical structural development in female adolescents is not related to long-chain N3 fatty acids. The finding that dorsal cingulate volume is related to N3 Index only in boys is consistent with animal evidence that brain volume in males may be particularly sensitive to N3 variation (e.g.11). While male and female adolescents had similar levels of whole blood N3 Index, consistent with other studies,63–66 levels of individual N3 Index components reportedly vary by sex.64,67 Given evidence that estrogen facilitates68 and testosterone downregulates69 synthesis of long chain polyunsaturated fatty acids, conversion of precursor omega-3 fatty acids (alpha-linolenic acid, EPA and DPA) to DHA is more efficient in young females than males.70 This effect may render males more sensitive to variation in dietary N3 intake and thus blood N3 Index level which, in turn, could contribute to the disparity between rates of certain neuropsychiatric disorders between males and females (as speculated by).71 Moreover, given that females in the present study were significantly more advanced in pubertal status, it is possible that advancing sexual maturity and thus estrogen status may have contributed to the relative insensitivity of female cingulate GMV to variation in blood N3 Index level. Alternatively, or in addition, females reach peak gray matter volume earlier than males57 and this may account for the lack of a relationship between omega-3 levels and dCC structure in the girls in our study. Overall, the disparity between sexes observed in the present study supports the conclusion by Crowe et al. (2008)67 that males and females “may need to be considered separately when examining the association between disease risk and biomarkers of [N3] fatty acids.” These results suggest a sex-specific link between blood levels of long-chain N3 fatty acids and the structural development of cingulate cortex in boys but not girls.
Contrary to our hypothesis, we did not find a significant direct relationship between blood levels of the N3 Index and impulse control in either adolescent boys or girls, as measured by caregiver report (BRIEF Inhibit subscale) or by ability to inhibit a prepotent response during a Go/No-Go task. While there is evidence that behavioral control is related to long chain N3 fatty acids in animal models,72,73 patient populations74 and in cognitively healthy adults via self-report,26 we did not detect a direct relationship between task-based response inhibition and blood N3 Index levels, consistent with other studies using younger participants.27,28 Given that improved performance on PFC-based cognitive tasks after omega-3 supplementation has been demonstrated in adults,75,76 it is unclear why blood N3 Index was unrelated to either assessment of impulse control. It is possible that, in the present study, the range of N3 Index observed, potentially insufficient task difficulty (median hit rate 99.2%; mean successful response inhibition rate 66.2%±16.6%, data not shown), and/or less variability in the behavioral abilities of this typically developing adolescent sample, precluded detection of a relationship with N3 fatty acids without a larger sample size. Moreover, given that there are other facets to impulse regulation (e.g.77), it is also possible that other facets of impulse control may be more substantially impacted, although these were not tested in the current study. Further exploration in clinical adolescent samples with the phenotype of interest (i.e. disinhibitory syndromes) may be warranted.
The present results should be interpreted in the context of the strengths and limitations of this study. Use of a biomarker for long-chain N3 Index may be considered a strength in that it provides an objective measure of long-chain N3 status. In general, the whole blood collection technique used here produced results comparable to similar studies.63,64 The ease of obtaining the whole blood sample facilitates data collection78, and in the present study enabled collection of this biomarker in a neuroimaging study of adolescents. Some caution, however, that the strength in this measurement of N3 Index levels is best realized when used in conjunction with dietary intake data.79 We used VBM to study relationships between GMV and whole blood N3 Index. Although selection of a large smoothing kernel was methodologically strong, there may be the potential to further optimize the VBM analyses,80 thereby improving sensitivity to relationships between VBM and whole blood N3 Index. Furthermore, GMV is a composite of two other cortical traits (surface area and thickness), so it is at present difficult to determine if the nature of the relationship between N3 Index and dCC structure in males is due surface area, thickness, or some combination of the two. Future studies may use alternate analysis techniques to delineate specific contributions to these associations. Lastly, we studied a cross sectional sample of adolescents and so are unable to explore the trajectory of dCC gray matter change or contribution of long chain N3 fatty acids to variance in this trajectory over time. A longitudinal study is necessary to further investigate whether low N3 boys are delayed in their neurodevelopmental progress compared to high N3 boys. Future studies using accelerated longitudinal designs may be better able to delineate the nature of long chain N3 fatty acid contribution to variance in medial PFC structure over time.
During adolescence, the brain undergoes aggressive cortical development in regions implicated in executive function. Identifying modulatory factors in these neurodevelopmental changes is critical for mitigating poor outcomes. To our knowledge, this is the first large-scale neuroimaging study of typically developing adolescents to investigate a relationship between one such potential modulatory factor – a biomarker of omega-3 fatty acids, whole blood N3 Index – and GMV in the dCC in both boys and girls. Our results showed a relationship between N3 Index and dorsal cingulate gray matter volume in males, but not in females. We further demonstrated that males with less right dCC GMV had better impulse control as rated by caregivers. These results suggest a sex-specific link between intake of long chain Omega-3 fatty acids and structural development of the dCC, potentially indicating adolescent males are more susceptible to negative effects of a diet low in Omega-3 fatty acids compared to females. Importantly, this increased susceptibility to Omega-3 fatty acid status in males may play a role in the sex-differences in prevalence of neuropsychiatric disorders involving high degrees of impulsivity.
Acknowledgments
The authors thank Dr. Robert McNamara (University of Cincinnati) for his comments on previous drafts and Melissa Avalos (Georgetown University) for her contributions to the data collection and analysis.
Funding
This work was supported by the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism [grant numbers 1R01AA019983–01; 3R01AA019983–02S1; 5F31AA023462–02], the Georgetown-Howard Universities Center for Clinical and Translational Science [grant number 1UL1TR001409–01] and National Institute of Child Health and Human Development [grant number 5P30HD040677–15].
Footnotes
Conflicts of interest None.
Contributors None
Ethics approval None
References
- 1.Mitchell DC, Gawrisch K, Litman BJ, Salem N. Why is docosahexaenoic acid essential for nervous system function? Biochem Soc Trans 1998;26:365–70. [DOI] [PubMed] [Google Scholar]
- 2.Carver J, Benford V, Han B, Cantor A. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull 2001;56:79–85. [DOI] [PubMed] [Google Scholar]
- 3.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 2003;126:1–27. [DOI] [PubMed] [Google Scholar]
- 4.Calderon F, Kim H-Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 2004;90:979–88. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr Neurol 2002;26:210–218. [DOI] [PubMed] [Google Scholar]
- 6.Wurtman RJ, Cansev M, Ulus IH. Synapse formation is enhanced by oral administration of uridine and DHA, the circulating precursors of brain phosphatides. J Nutr Health Aging 2009;13:189–97. [DOI] [PubMed] [Google Scholar]
- 7.de Velasco PC, et al. Nutritional restriction of omega-3 fatty acids alters topographical fine tuning and leads to a delay in the critical period in the rodent visual system. Exp Neurol 2012;234:220–9. [DOI] [PubMed] [Google Scholar]
- 8.Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. J Neurosci 2014;34:2065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res 1990;31:237–47. [PubMed] [Google Scholar]
- 10.Moriguchi T, Salem N. Recovery of brain docosahexaenoate leads to recovery of spatial task performance. J Neurochem 2003;87:297–309. [DOI] [PubMed] [Google Scholar]
- 11.Galli C, White HB, Paoletti R. Brain lipid modifications induced by essential fatty acid deficiency in growing male and female rats. J Neurochem 1970;17:347–55. [DOI] [PubMed] [Google Scholar]
- 12.Hulbert A, Turner N, Storlien L, Else P. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev 2005;80:155–69. [DOI] [PubMed] [Google Scholar]
- 13.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 2005;40:343–7. [DOI] [PubMed] [Google Scholar]
- 14.Harris WS. Assessing fatty acid biostatus: red blood cells or plasma? Lipid Technol 2013;25:179–81. [Google Scholar]
- 15.Favrelière S, Barrier L, Durand G, Chalon S, Tallineau C. Chronic dietary n-3 polyunsaturated fatty acids deficiency affects the fatty acid composition of plasmenylethanolamine and phosphatidylethanolamine differently in rat frontal cortex, striatum, and cerebellum. Lipids 1998;33:401–7. [DOI] [PubMed] [Google Scholar]
- 16.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through earlyadulthood. Proc Natl Acad Sci 2004;101:8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 2008;28:3586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sowell ER, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 2004;24:8223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huttenlocher P, Dabholkar A. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997;387:167–78. [DOI] [PubMed] [Google Scholar]
- 20.Shaw P, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2006;63:540–9. [DOI] [PubMed] [Google Scholar]
- 21.Steinbeis N, Bernhardt BC, Singer T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron 2012;73:1040–51. [DOI] [PubMed] [Google Scholar]
- 22.Simmonds D, Pekar J, Mostofsky S. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 2008;46:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heilbronner SR, Hayden BY. Dorsal anterior cingulate cortex: a bottom-up view. Annu Rev Neurosci 2016;39:149–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diau G-Y, et al. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med 2005;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler GJ, Flood A, Hannan P, Neumark-Sztainer D. Major patterns of dietary intake in adolescents and their stability over time. J Nutr 2009;139:323–8. [DOI] [PubMed] [Google Scholar]
- 26.Conklin SM, et al. Serum omega-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry Res 2007;152:1–10. [DOI] [PubMed] [Google Scholar]
- 27.Almeida DM, Jandacek RJ, Weber WA, McNamara RK. Docosahexaenoic acid biostatus is associated with event-related functional connectivity in cortical attention networks of typically developing children. Nutr Neurosci 2017;20:246–54. [DOI] [PubMed] [Google Scholar]
- 28.McNamara RK, et al. Low docosahexaenoic acid status is associated with reduced indices in cortical integrity in the anterior cingulate of healthy male children: a 1H MRS study. Nutr Neurosci 2013;16:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamara RK, et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr 2010;91:1060–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darcey VL, McQuaid GA, Fishbein DH, VanMeter JW. Dietary long-chain omega-3 fatty acids are related to impulse control and anterior cingulate function in adolescents. bioRxiv 2018. doi: 10.1101/379263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child Adolesc Psychiatr Clin N Am 2000;9:541–55, vii. [PubMed] [Google Scholar]
- 32.Dirlikov B, et al. Distinct frontal lobe morphology in girls and boys with ADHD. NeuroImage Clin 2015;7:222–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva V, Barazzoni R, Singer P. Biomarkers of fish oil omega-3 polyunsaturated fatty acids intake in humans. Nutr Clin Pract 2014;29:63–72. [DOI] [PubMed] [Google Scholar]
- 34.Hutton C, De Vita E, Ashburner J, Deichmann R, Turner R. Voxel-based cortical thickness measurements in MRI. Neuroimage 2008;40:1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fishbein DH, Rose EJ, Darcey VL, Belcher AM, VanMeter JW. Neurodevelopmental precursors and consequences of substance use during adolescence: promises and pitfalls of longitudinal neuroimaging strategies. Front Hum Neurosci 2016;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman A, Kaufman N. Kaufman brief intelligence test. 1st ed. Circle Pines (MN): American Guidance Service; 1990. [Google Scholar]
- 37.Manuck SB, Phillips JE, Gianaros PJ, Flory JD, Muldoon MF. Subjective socioeconomic status and presence of the metabolic syndrome in midlife community volunteers. Psychosom Med 2010;72:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988;17:117–33. [DOI] [PubMed] [Google Scholar]
- 39.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Heal 1993;14:190–95. [DOI] [PubMed] [Google Scholar]
- 40.Kuczmarski RJ, et al. CDC growth charts: United States. Adv Data 2000. Jun 8;(314):1–27. [PubMed] [Google Scholar]
- 41.Bell GJ, et al. Using a fingertip whole blood sample for rapid fatty acid measurement: method validation and correlation with erythrocyte polar lipid compositions in UK subjects. Br J Nutr 2011;106:1408–15. [DOI] [PubMed] [Google Scholar]
- 42.Evenden JL. Varieties of impulsivity. Psychopharmacology 1999;146:348–61. [DOI] [PubMed] [Google Scholar]
- 43.McAuley T, Chen S, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J Int Neuropsychol Soc 2010;16:495–505. [DOI] [PubMed] [Google Scholar]
- 44.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol 2000;6:235–8. [DOI] [PubMed] [Google Scholar]
- 45.Petersen I, Hoyniak CP, McQuillan ME, Bates JE, Staples AD. Measuring the development of inhibitory control: the challenge of heterotypic continuity. Dev Rev 2016;40:25–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol 2002;17:235–72. [PubMed] [Google Scholar]
- 47.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridgway GR, et al. Ten simple rules for reporting voxel-based morphometry studies. Neuroimage 2008;40:1429–35. [DOI] [PubMed] [Google Scholar]
- 49.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–9. [DOI] [PubMed] [Google Scholar]
- 50.Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc 1987;82:1147–9. [Google Scholar]
- 51.Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr 2009;76:408–20. [Google Scholar]
- 52.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Publications; 2017. [Google Scholar]
- 53.Zamroziewicz MK, Paul EJ, Rubin RD, Barbey AK. Anterior cingulate cortex mediates the relationship between O3PUFAs and executive functions in APOE e4 carriers. Front Aging Neurosci 2015;7(87):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conklin SM, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett 2007;421:209–12. [DOI] [PubMed] [Google Scholar]
- 55.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006;139:991–7. [DOI] [PubMed] [Google Scholar]
- 56.Salem N, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001;36:945–59. [DOI] [PubMed] [Google Scholar]
- 57.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–3. [DOI] [PubMed] [Google Scholar]
- 58.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 2006;30:718–29. [DOI] [PubMed] [Google Scholar]
- 59.Tamnes CK, et al. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex 2010;20:534–48. [DOI] [PubMed] [Google Scholar]
- 60.Fahim C, et al. Neuroanatomy of childhood disruptive behavior disorders. Aggress Behav 2011;37:326–37. [DOI] [PubMed] [Google Scholar]
- 61.Matsuo K, et al. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp 2009;30:1188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, et al. Dissociated neural substrates underlying impulsive choice and impulsive action. Neuroimage 2016;134:540–9. [DOI] [PubMed] [Google Scholar]
- 63.van der Wurff ISM, et al. Association between blood omega-3 index and cognition in typically developing Dutch adolescents. Nutrients 2016;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marangoni F, Colombo C, Martiello A, Negri E, Galli C. The fatty acid profiles in a drop of blood from a fingertip correlate with physiological, dietary and lifestyle parameters in volunteers. Prostaglandins Leukot Essent Fat Acids 2007;76:87–92. [DOI] [PubMed] [Google Scholar]
- 65.Johnston DT, Deuster PA, Harris WS, Macrae H, Dretsch MN. Red blood cell omega-3 fatty acid levels and neurocognitive performance in deployed U.S. Servicemembers. Nutr Neurosci 2013;16:30–8. [DOI] [PubMed] [Google Scholar]
- 66.Ogura T, et al. Fatty acid composition of plasma, erythrocytes and adipose: their correlations and effects of age and sex. Lipids 2010;45:137–144. [DOI] [PubMed] [Google Scholar]
- 67.Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br J Nutr 2008;99:168–74. [DOI] [PubMed] [Google Scholar]
- 68.Mason JK, et al. 17beta-estradiol increases liver and serum docosahexaenoic acid in mice fed varying levels of alpha-linolenic acid. Lipids 2014;49:745–56. [DOI] [PubMed] [Google Scholar]
- 69.Marra CA, de Alaniz MJ. Influence of testosterone administration on the biosynthesis of unsaturated fatty acids in male and female rats. Lipids 1989;24:1014–9. [DOI] [PubMed] [Google Scholar]
- 70.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 2005;45:581–97. [DOI] [PubMed] [Google Scholar]
- 71.Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr 2010;169:149–64. [DOI] [PubMed] [Google Scholar]
- 72.Levant B, Zarcone TJ, Fowler SC. Developmental effects of dietary n-3 fatty acids on activity and response to novelty. Physiol Behav 2010;101:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vancassel S, et al. Hyperactivity in the rat is associated with spontaneous low level of n-3 polyunsaturated fatty acids in the frontal cortex. Behav Brain Res 2007;180:119–26. [DOI] [PubMed] [Google Scholar]
- 74.Raz R, Gabis L. Essential fatty acids and attention-deficit-hyperactivity disorder: a systematic review. Dev Med Child Neurol 2009;51:580–92. [DOI] [PubMed] [Google Scholar]
- 75.Fontani G, et al. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest 2005;35:691–9. [DOI] [PubMed] [Google Scholar]
- 76.Narendran R, Frankle WG, Mason NS, Muldoon MF, Moghaddam B. Improved working memory but no effect on striatal vesicular monoamine transporter type 2 after omega-3 polyunsaturated fatty acid supplementation. PLoS One 2012;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Wit H Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 2009;14:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stark KD, Van Elswyk ME, Higgins MR, Weatherford CA, Salem N Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res 2016;63:132–52. [DOI] [PubMed] [Google Scholar]
- 79.Vandevijvere S, et al. Evaluation of food and nutrient intake assessment using concentration biomarkers in European adolescents from the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr 2012;12:1–12. [DOI] [PubMed] [Google Scholar]
- 80.Radua J, Canales-rodríguez EJ, Pomarol-clotet E, Salvador R. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage 2014;86:81–90. [DOI] [PubMed] [Google Scholar]


