Abstract
Background
Emergence delirium (ED) is generally occurred after anesthesia associated with increased risks of long-term adverse outcomes. Therefore, this study aimed to evaluate the efficacy of preconditioning with nasal splint and mouth-breathing training on prevention of ED after general anesthesia.
Methods
This randomized controlled trial enrolled 200 adult patients undergoing ESS. Patients were randomized to receive either nasal splinting and mouth breathing training (n = 100) or standard care (n = 100) before surgery. The primary outcome was the occurrence of ED within 30 min of extubation, assessed using the Riker Sedation-Agitation Scale. Logistic regression identified risk factors for ED.
Results
Totally 200 patients were randomized and 182 aged from 18 to 82 years with 59.9% of males were included in the final analysis (90 in C-group and 92 in P-group). ED occurred in 16.3% of the intervention group vs. 35.6% of controls (P = 0.004). Male sex, smoking and function endoscopic sinus surgery (FESS) were independent risk factors for ED.
Conclusions
Preoperative nasal splinting and mouth breathing training significantly reduced the incidence of emergence delirium in patients undergoing endoscopic sinus surgery.
Trial Registration
ChiCTR1900024925 (https://www.chictr.org.cn/index.aspx) registered on 3/8/2019.
Keywords: Emergence delirium, Functional endoscopic sinus surgery, General anesthesia, Randomized controlled trial
Background
Emergence delirium (ED, aka emergence agitation, EA), which referring to a short-term state of dissociation of consciousness during the recovery from general anesthesia, manifested with motor agitation, incoherence, inconsolability, and unresponsiveness, is the most common neuropsychiatric complications after surgery [1–3]. The incidences of ED in patients underwent different surgeries varied from less than 10% up to 80% [4–6]. Although ED is often considered self-limited without the necessity of medical treatment, the agitated behavior sometimes may result in post-anesthesia care unit (PACU) accidents such as loss of catheters and tubes, accidental injuries, and elongated occupation [7, 8]. In addition, ED may be associated with increased risks of long-term adverse outcomes, including cognitive dysfunction and negative behavior that cause lower quality of life, readmission to hospitals, and even deaths [9–11]. Therefore, application of interventions in reducing the incidence of ED is still of great clinical significance.
Multiple factors related to characteristics of patients, anesthesia, and surgery have been linked with ED risks [12]. Generally, ED may be generated by an imbalance between the patient’s arousal state and the recovery of consciousness [13]. Therefore, factors related to patients’ cognitive functions and those cause agitation during induction and emergence of anesthesia may be associated with ED risks, for example, age, pre-operative anxiety, types of operation, postoperative pain, differential types and clearance methods of anesthetic agents [6, 14–16]. Accordingly, interventional strategies aiming at controlling various risk factors have been developed to reduce the incidence of ED, including medical interventions such as gabapentin [13], dexmedetomidine [17–19], melatonin [20] and ramelteon [21], as well as non-pharmacologic interventions such as time and place orientation, transcranial direct current stimulation or aided hearing and vision optimization [22].
Functional endoscopic sinus surgery (FESS) is a minimally invasive technique used to restore sinus ventilation and normal function [23]. Absorbable foams are regularly left in the nasal cavity after FESS until self-dissolvement to prevent postoperative adhesions and bleeding [24]. During the emergence from anesthesia, obstruction by these foams, in combination with postoperative mucosal edema in the nasal cavity, can cause breathing difficulties and feelings of suffocation which forced patients to breathe through mouth [25, 26]. These sensory abnormalities may cause an emergence agitation and increase the risk of ED. Previous studies have shown that preoperative adaption to postoperative abnormal sensory agitations may reduce the incidence of ED. Recent study showed that visual preconditioning for visual disturbance with an eye mask effectively reduced ED after eye surgery [27, 28]. Therefore, we designed a randomized controlled study to evaluate the efficacy of preconditioning by nasal splint and mouth-breathing training on preventing ED after FESS.
Methods
Study design
This is a randomized, single blind, parallel, controlled study to evaluate the efficacy of nasal splinting and mouth-breathing training in preventing ED in adults after FESS. Adult patients were 1:1 randomly allocated to receive preconditioning treatment or control treatment. The random sequences were generated by computer and concealed until the patients were included. Patients in the preconditioning treatment group received nasal splint and mouth-breathing training before FESS, and no any interventions were performed in the control group. The study protocol has been approved by the Shanghai General Hospital Institutional Review Board (IRB 2019KY039) and was registered prior to patient enrollment at chictr.org.cn (ChiCTR1900024925) [29].
Patients
Patients scheduled for FESS at the Shanghai General Hospital, China from September 18th, 2019 to December 16th, 2020 due to chronic sinusitis were screened for eligibility. Inclusion criteria were: age over 18 years old; American Society of Anesthesiologists Classification (ASA) class I–II with no serious cardiovascular disease; and normal preoperative liver and kidney function. Patients were excluded if they met the following criteria: ASA III-IV, severe cardiovascular disease, or poor blood pressure control; history of mental illness, neurological diseases, or usage of sedative or antipsychotic drug; nasal malformation, history of nasal trauma or implantation of nasal prosthesis. Written informed consent was obtained from all participants.
Interventions
Patients in preconditioning group underwent nasal splint preconditioning and mouth breath training in pre-anesthesia room 1 h before surgery. In details, a nasal splint (patent No. 202020982582.9, China) was used to clamp the subnasal cartilage in front of the nasal cavity to block breathing through nose (Fig. 1). Patients were instructed to stay calm and adapt to breathing through mouth. Oxygen saturation (SpO2), heart rate (HR) and non-invasive blood pressure (NBP) were monitored during the process. If SpO2 level was below 95% of that at baseline and last for at least 5 min, the patient was considered to fail the adaption of mouth breathing training. For these patients, 4 L/min O2 was supplied until the SpO2 level returns to the normal level. Unless patients asked due to intolerance, the nasal splint was not removed until the anesthesia-induced loss of consciousness was detected. Patients in control group did not receive any interventions before the general anesthesia.
Fig. 1.
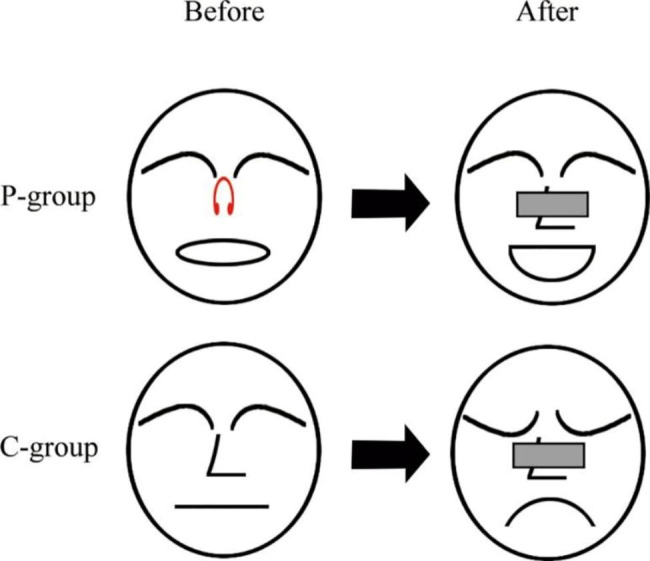
A graphic summary displaying that preconditioning by nasal splint and mouth-breathing training reduces emergence delirium after FESS
For general anesthesia induction, all patients administrated midazolam (0.05 mg/kg), propofol (2 mg/kg), and sufentanil (0.25 µg/kg). Rocuronium(0.6 mg/kg) was then administered. After satisfactory muscle relaxation, tracheal intubation was carried out, followed by 60% O2 ventilation and EtCO2 maintaining between 35 and 45 mmHg. Intraoperative anesthesia was maintained with desflurane inhalation, and the minimum alveolar concentration (MAC) value was controlled between 0.8 and 1.0. Before performing the nasal endoscopy, a dose of 5 or 10 µg sufentanil was administered depending on patient’s weight and circulation status. Due to the short duration of FESS, dose of sufentanil is usually not increased intraoperatively. In case the operation lasted for more than 1 h or the level of NBP and HR raised from inadequate anesthesia, another 5 or 10 µg sufentanil was further administered. If the operation wound was relatively large, 5 µg sufentanil was added at the end of the operation before nasal packing with absorbable foam. The package was composed of a 2 × 2 × 4 cm cuboid absorbable swelling sponge, which was inserted into the nasal cavity from the nostrils. Neostigmine antagonist was given according to the level of patient’s muscle relaxation. When tidal volume (Vt) reached to 5 ml/kg, the respiratory frequency (f) reached to 12/min, obvious swallowing reflex occurred, and the oxygen saturation maintained at 95% and above after 5 min of breathing, it was considered indication for extubation. In case sympathetic excitement caused by adrenaline infiltration in the nasal cavity, nimodipine and esmolol were used to control blood pressure and HR within the normal range.
Outcomes
Outcomes assessed followed the statements of previously published protocol [29]. The primary outcome was the occurrence of ED, which was assessed within 30 min after extubation according to the Riker Sedation-Agitation scale [30] and a score of 5–7 points was considered as occurrence of ED.
The main secondary outcome was the severity of ED, which was determined based on the presences of combative behavior, thrashing, and hyperactive motor behavior [31]. If the above mentioned behavior occurred during stimulation, such as phlegm, but stopped at the removal of stimulation, the severity of ED was rated as mild. ED which occurred without stimulation that lasted for at least 5 min, but did not require any interventions, was considered as moderate. ED lasted for at least 5 min and had to be controlled with medication and/or physical restraint was rated as severe.
Other secondary outcomes included intraoperative monitored parameters, such as duration of surgery, duration and dosage of anesthesia, usage of nasal package; as well as those recorded in post anesthesia care unit (PACU), including time to extubation, physical and biochemical characteristics when leaving PACU and length of hospital stay. Pain was assessed using Numerical Rating Scale (NRS) every 5 min immediately after extubation by a trained anesthesia nurse. A reassessed score of 4 or above indicated substantial pain, which was managed by an intravenous injection of 3–5 µg sufentanil.
Sample size
Based on the results from our pilot study, the incidence of ED after FESS was 21.3%. We assumed that the preconditioning would result in reduction of ED occurrence with an odds ratio (OR) of 1/3. At the setting of α = 0.05, an estimated sample size of 164 could provide a 90% of statistical power. Considering a potential dropout rate of 20%, 197 patients were required. Therefore, recruitment of 200 patients was planned.
Randomization and blinding
Random number sequence was generated by computer. Single-blind ED assessment was performed by an independent anesthesiologist who was not aware of the grouping information (started observation until after the removal of nasal clip) and a nurse anesthetist (Yuyu Gu) at PACU, with necessary consultation with the intraoperative anesthesiologist.
Statistical analyses
According to the intention-to-treat principle, we used full analysis data set to analyze the primary outcome. The full analysis set (FAS), including all patients underwent randomization and at least once training and with once measurement after surgery, was used for analysis of primary and secondary outcome. Normal distributed continuous data were presented as mean ± standard deviation (SD), and difference between groups was tested by using student t-test. Skew-distributed data was presented as median and the 25th percentile and 75th percentile, and between-group differences were tested by using Mann-Whitney test. Categorical variables were described as number (n) and percentage (%) and chi-square test or Fisher’s exact test was used for comparisons between the two groups. Multivariable logistic regression model was performed to identify independent associated factors of ED. All statistical analyses were conducted by using IBM SPSS Statistics (Version 20.0, IBM, Armonk, NY, USA). Two-sided P-value less than 0.05 indicated statistical significance.
Results
After screening of 232 patients, 200 met the inclusion and exclusion criteria and finally randomized. Among them, 18 patients were withdrawn (Fig. 2) due to cancellation of surgery (n = 3), changes of anesthesia (7 changed to local anesthesia, 2 changed to total intravenous anesthesia, and 1 performed no endotracheal intubation), or changes of surgery (2 with ear surgery, 1 with nasopharyngeal carcinoma radical operation, 1 with inferior turbinate and palatopharyngoplasty, and 1 with tonsil surgery). A total of 182 patients (90 in the control group and 92 in the preconditioning group) were included in the final FAS of analysis. Among patients in preconditioning, nasal splint was removed for 1 before anesthesia due to intolerance to mouth breathing training. In addition, extubation was failed for 1 patient in preconditioning group due to high risk of reflux aspiration, and 1 in the control group was observed with blindness as surgical complication. All these 3 patients were retained in PACU for more than 1 h.
Fig. 2.
Recruitment, randomization, and patient flow diagram of the trial. P-group, group with preconditioning of nasal splint and mouth breathing training; C-group, control group
The median age of 182 patients was 51.5 years and 59.8% were male (Table 1). The baseline demographic and clinical characteristics were comparable in two groups (all P-values > 0.05). In addition, no significant differences were observed in preoperative vital signs of HR, MAP and SpO2 between the two groups (All P-values > 0.05).
Table 1.
Baseline characteristics of patients between preconditioning group and control group
| Characteristics | Total (n = 182) | Control group (n = 90) | Preconditioning group (n = 92) | P-value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, year | 51.1(18, 82) | 53(18, 82) | 51(20, 78) | 0.547 |
| Gender, N (%) | > 0.999 | |||
| Male | 109(59.9) | 54(60.00) | 55(59.78) | |
| Female | 73(40.1) | 36(40.00) | 37(40.22) | |
| BMI, kg/m2 | 24.6 ± 3.95 | 24.7 ± 4.32 | 24.5 ± 3.57 | 0.767 |
| Current smoking, N (%) | 37(20.3) | 15(16.7) | 22(23.9) | 0.270 |
| History of HBP, N (%) | 43(23.6) | 20(22.2) | 23(25.0) | 0.728 |
| History of DM, N (%) | 14(7.7) | 10(11.1) | 4(4.35) | 0.101 |
| Disease conditions | ||||
| MAP at admission, mmHg | 98.4 ± 11.8 | 97.4 ± 11.3 | 99.5 ± 12.2 | 0.221 |
| ASA, N (%) | > 0.999 | |||
| I | 112(61.5) | 55(61.1) | 57(62.0) | |
| II | 70(38.5) | 35(38.9) | 35(38.0) | |
| Head ache, N (%) | > 0.999 | |||
| Negative | 94(72.3) | 45(72.6) | 49(72.1) | |
| Positive | 36(27.7) | 17(27.4) | 19(27.9) | |
| Nasal obstruction, N (%) | 0.374 | |||
| Negative | 103(56.6) | 54(60.0) | 49(53.3) | |
| Positive | 79(43.4) | 36(40.0) | 43(46.7) | |
| Nasal obstruction side, N (%) | 0.786 | |||
| Single | 23(40.4) | 11(44.0) | 12(37.5) | |
| Both | 34(59.6) | 14(56.0) | 20(62.5) | |
| Nasal obstruction description, N (%) | 0.455 | |||
| Intermittent | 9(81.8) | 5(100) | 4(66.7) | |
| Persistent | 2(18.2) | 0(0) | 2(33.3) | |
| Nasal obstruction with nasal polyps, N (%) | 0.373 | |||
| Negative | 44(55.7) | 18(50.0) | 26(60.8) | |
| Positive | 35(44.3) | 18(50.0) | 17(39.5) | |
| Nasal obstruction with septum deviation, N (%) | 0.405 | |||
| Negative | 63(79.7) | 27(75.0) | 36(83.7) | |
| Positive | 16(20.3) | 9(25.0) | 7(16.3) | |
| Chronic rhinosinusitis with nasal polyps, N (%) | 0.749 | |||
| Negative | 126(69.2) | 61(67.8) | 65(70.7) | |
| Positive | 56(30.8) | 29(32.2) | 27(29.5) | |
| Preoperative vital signs | ||||
| HR before Nasal splint, bpm | 75.5 ± 11.2 | 74.4 ± 9.82 | 76.6 ± 12.4 | 0.184 |
| MAP before Nasal splint, mmHg | 95.1 ± 11.2 | 95.1 ± 10.4 | 95.0 ± 11.9 | 0.936 |
| SpO2 before Nasal splint, % | 99.0(98.0,99.0) | 99.0 (98.0, 99.0) | 98.5(98.0, 99.0) | 0.052 |
| h after Nasal splint, bpm | - | - | 76.0(65.3, 81.0) | - |
| MAP after Nasal splint, mmHg | - | - | 93.0(88.0, 98.0) | - |
| SpO2 after Nasal splint, % | - | - | 99.0(98.0, 99.0) | - |
Note: Continuous data were presented as mean ± SD or median (the 25th percentile, the 75th percentile). Categorical variables were displayed in number (percentage). BMI: body mass index; MAP: mean arterial pressure; ASA: American Society of Anesthesiologists; HBP: high blood pressure; DM: diabetes mellitus; HR: heart rate; SpO2: oxygen saturation. A P-value < 0.05 indicates significant difference between the two groups
The incidence of ED in the preconditioning group was 16.30% (n = 15) and significantly lower than control group (n = 32, 35.56%, P = 0.004) (Table 2). The severity of ED in precondition group appeared to be similar in two groups (control vs. preconditioning: 6.25% vs. 6.67 for severe and 21.9% vs. 13.3% for moderate degree, P = 0.449). The median intraoperative sufentanil dosage used in preconditioning group was 20 µg (P25, P75: 15, 20), which was significantly higher than 15 µg (P25, P75: 15, 20) in control group (P = 0.020). Other intraoperative measurements, including duration of surgery, duration of anesthesia, intraoperative sufentanil per unit anesthesia time, FESS with septoplasty, nasal package, and package side were similar in two groups (all P-values > 0.05). 2 patients in the control group (2.22%) and 1 patient in the preconditioning group (1.09%) had used the medication in PACU (P = 0.619). The time to extubation and the time of PACU were similar in two groups (all P-values > 0.05), the median time to extubation were 16.0 min (the 25th percentile, the 75th percentile: 12.0, 17.8) for preconditioning group and 17.0 min (12.0, 20.0) for control group respectively; and the time of PACU were 55.0 min (47.0, 59.0) and 56.0 min (47.0, 59.0), respectively. All other postoperative measurements, such as NRS in PACU, HR when left PACU, MAP when left PACU, SpO2 when left PACU, and postoperative hospital stays, were similar between two groups (All P-values > 0.05).
Table 2.
Comparisons of outcomes between preconditioning group and control group
| Outcomes | Preconditioning group(n = 92) | Control group (n = 90) |
P-value |
|---|---|---|---|
| Major outcomes | |||
| ED, N (%) | 0.004 | ||
| Negative | 77(83.7) | 58(64.4) | |
| Positive | 15(16.3) | 32(35.6) | |
| Degree of ED, N (%) | 0.449 | ||
| Mild | 12(80.0) | 23(71.9) | |
| Moderate | 2(13.3) | 7 (21.9) | |
| Severe | 1(6.67) | 2(6.25) | |
| Intraoperative measurements | |||
| Duration of surgery, min | 45.0(30.0, 68.8) | 40.0(30.0, 60.0) | 0.225 |
| Duration of anesthesia, min | 68.0(50.0, 88.0) | 60.0(50.0, 79.3) | 0.161 |
| Intraoperative sufentanil dosage, µg | 20.0(15.0, 20.0) | 15(15.0, 20.0) | 0.028 |
| Dosage of sufentanil per unit anesthesia time, µg/min | 0.27(0.27, 0.36) | 0.27(0.22, 0.36) | 0.562 |
| FESS with septoplasty, N (%) | 0.645 | ||
| Without | 82(89.1) | 78(86.7) | |
| With | 10(10.9) | 12(13.3) | |
| Nasal package, N (%) | > 0.999 | ||
| Negative | 25(27.2) | 24(26.7) | |
| Positive | 67(72.8) | 66(73.3) | |
| Package Side, N (%) | 0.666 | ||
| Single | 13(25.0) | 16(29.6) | |
| Both | 39(75.0) | 38(70.4) | |
| Postoperative measurements | |||
| Medication in PACU, N/P | 0.619 | ||
| Negative | 91(98.9) | 88(97.8) | |
| Positive | 1(1.09) | 2(2.22) | |
| Time to extubation, min | 16.0(12.0, 17.8) | 17.0(12.0, 20.0) | 0.714 |
| PACU time, min | 55.0(47.0,59.0) | 56.0(47.0,59.0) | 0.689 |
| NRS in PACU | 0(0, 6.00) | 0(0, 6.00) | 0.949 |
| h when left PACU, bpm | 71.0 (64.0, 77.5) | 71.0(61.0, 78.0) | 0.689 |
| MAP when left PACU, mmHg | 84.0(80.0, 91.5) | 86.5 (79.0, 94.0) | 0.815 |
| SpO2 when left PACU, % | 99.0(98.0, 99.0) | 99.0(98.0, 99.0) | 0.235 |
| Postoperative hospital stays, day | 3.00(3.00, 4.00) | 4.00(3.00, 4.00) | 0.308 |
Note: Data were presented as median (IQR) or number (percentage). ED: emergence delirium; PACU: post-anesthesia care unit; NRS: numerical rating scale; HR: heart rate; MAP: mean arterial pressure; SpO2: oxygen saturation. A P-value < 0.05 indicates significant difference between the two groups
A post-hoc sensitivity analysis identifying risk factors for ED was performed by using the univariate and multivariate models. After univariate models, we found totally 10 factors, including age, gender, BMI, smoking status, nasal obstruction with septum deviation, nasal splint, FESS with septoplasty, time to left PACU, medication use in PACU, and postoperative hospital stays, were significantly related with ED incidence (P < 0.05) (Table 3). Considering the risk factors being confirmed by previous evidence and clinical experience, we further added duration of surgery and anesthesia, intraoperative sufentanil dosage, minutes to extubation, NAS in PACU, HR, MAP, and SpO2 when left PACU into our multivariable model. Since there was no patient with ED having used the medication in PACU, we excluded this factor from the multivariable model (Table 4). After controlling all above mentioned factors, the patients with ED were more likely to be younger (OR: 0.977, 95% CI: 0.954, 1.000, P = 0.028), males (OR: 2.762, 95% CI: 6.757, 1.134, P = 0.001), current smokers (OR: 2.547, 95% CI: 1.065, 6.091, P = 0.019), FESS with septoplasty (OR: 2.770, 95% CI: 1.108, 6.925, P = 0.029), with longer postoperative hospital stays (OR: 1.672, 95% CI: 1.121, 12.49, P = 0.012), and with nasal septum deviation with obstruction (OR: 6.000, 95% CI: 1.793, 20.07, P = 0.004) compared with patients without ED. Nasal splint was a protective factor for ED, with the OR of 0.270 (95% CI: 0.123, 0.591, P = 0.001).
Table 3.
Univariate analysis of potential associated factors of emergence delirium
| Characteristics | With ED (N = 135) |
Without ED (N = 47) |
P-value |
|---|---|---|---|
| Baseline characteristics | |||
| Age, year | 54(18–82) | 45(19–76) | 0.028 |
| Gender, N (%) | 0.001 | ||
| Male | 71(52.6) | 38(80.9) | |
| Female | 64(47.4) | 9(19.2) | |
| BMI, kg/m2 | 24.2(21.5, 26.5) | 25.9(22.7, 27.7) | 0.042 |
| MAP at admission, mmHg | 97.8 (12.1) | 100 (10.6) | 0.180 |
| ASA, N (%) | > 0.999 | ||
| I | 83 (61.5) | 29(61.7) | |
| II | 52(38.5) | 18(38.3) | |
| Head ache, N (%) | 0.183 | ||
| Negative | 67(69.1) | 27(81.8) | |
| Positive | 30(30.9) | 6(18.2) | |
| Nasal obstruction, N (%) | 0.306 | ||
| Negative | 73(54.1) | 30(63.8) | |
| Positive | 62(45.9) | 17(36.2) | |
| Nasal obstruction side, N (%) | 0.750 | ||
| Single | 17(38.6) | 6(46.2) | |
| Both | 27(61.4) | 7(53.8) | |
| Nasal obstruction description, N (%) | 0.491 | ||
| Intermittent | 7(87.5) | 2(66.7) | |
| Persistent | 1(12.5) | 1(33.3) | |
| Nasal obstruction with nasal polyps, N (%) | 0.270 | ||
| Negative | 37(59.7) | 7(41.2) | |
| Positive | 25(40.3) | 10(58.8) | |
| Nasal obstruction with septum deviation, N (%) | 0.004 | ||
| Negative | 54(87.1) | 9(52.9) | |
| Positive | 8(12.9) | 8(47.1) | |
| Chronic rhinosinusitis with nasal polyps, N (%) | 0.354 | ||
| Negative | 96(71.1) | 20(63.8) | |
| Positive | 39(28.9) | 17(36.2) | |
| Current smoker, N (%) | 0.011 | ||
| Negative | 114(83.8) | 31(66.0) | |
| Positive | 21(16.2) | 16(34.0) | |
| History of HBP, N (%) | 0.686 | ||
| Negative | 104(77.0) | 35(74.5) | |
| Positive | 31(23.0) | 12(25.5) | |
| History of DM, N (%) | 0.525 | ||
| Negative | 123(91.1) | 45(95.7) | |
| Positive | 12(8.89) | 2(4.26) | |
| Preoperative measurements | |||
| Nasal splint, N/P | 0.004 | ||
| Negative | 58(43.0) | 32(68.1) | |
| Positive | 77(57.0) | 15(31.9) | |
| HR before Nasal splint, bpm | 76.0(68.0, 82.0) | 76.0(68.0, 81.0) | 0.918 |
| MAP before Nasal splint, mmHg | 94.10(11.051) | 94.9(11.6) | 0.933 |
| SpO2 before Nasal splint, % | 99.0(98.0, 99.0) | 99.0(98.0, 99.0) | 0.512 |
| h after Nasal splint, bpm | 76.0(65.0, 81.0) | 78.0 (69.5, 79.0) | 0.903 |
| MAP after Nasal splint, mmHg | 93.3 (10.4) | 91.7 (13.3) | 0.625 |
| SpO2 after Nasal splint, % | 99.0(98.0, 99.0) | 99.0(98.0, 99.0) | 0.353 |
| Intraoperative measurements | |||
| Duration of surgery, min | 45.0(30.0, 60.0) | 45.0(25.0, 70.0) | 0.970 |
| Duration of anesthesia, min | 64.0(50.0, 84.0) | 65.0(49.0, 89.0) | 0.931 |
| Intraoperative sufentanil dosage, µg | 20.0(15.0, 23.0) | 20.0(15.0, 20.0) | 0.567 |
| Dosage of sufentanil per unit anesthesia time, µg/min | 0.27(0.22, 0.33) | 0.27 (0.22, 0.33) | 0.660 |
| FESS with septoplasty, N (%) | 0.036 | ||
| Without | 123(91.1) | 37(78.2) | |
| With | 12(8.89) | 10(21.3) | |
| Nasal package, N (%) | 0.851 | ||
| Negative | 37(27.4) | 12(25.5) | |
| Positive | 98(72.6) | 35(74.5) | |
| Package Side, N (%) | 0.805 | ||
| Single | 21(26.6) | 8(29.6) | |
| Both | 58(73.4) | 19(70.4) | |
| Postoperative measurements | |||
| Minutes to extubation, min | 17.0(12.0, 19.0) | 16.0(12.0, 17.0) | 0.816 |
| Medication in PACU, N (%) | 0.016 | ||
| Negative | 135(100) | 44(93.6) | |
| Positive | 0(0) | 3(6.38) | |
| Time to left PACU, min | 54.0(46.0,58.0) | 58.0(49.0,60.0) | 0.003 |
| NRS in PACU, Sum | 0(0, 6.00) | 0(0, 6.00) | 0.619 |
| h when left PACU, bpm | 70.0(63.0, 78.0) | 72.0(64.0, 80.0) | 0.098 |
| MAP when left PACU, mmHg | 84.0(79.0, 93.0) | 86.0(81.0, 92.0) | 0.654 |
| SpO2 when left PACU, % | 99.0(98.0, 99.0) | 99.0(98.0, 99.0) | 0.593 |
| Postoperative hospital stays, day | 3.00(3.00, 4.00) | 4.00(3.00, 4.00) | < 0.001 |
Note: Continuous data were presented as mean ± SD or median (the 25th percentile, the 75th percentile). Categorical variables were displayed in number (percentage). ED: emergence delirium; BMI: body mass index; MAP: mean arterial pressure; ASA: American Society of Anesthesiologists; HBP: high blood pressure; DM: diabetes mellitus; HR: heart rate; SpO2: oxygen saturation; PACU: post-anesthesia care unit; NRS: numerical rating scale; FESS: function endoscopic sinus surgery. A P-value < 0.05 indicates significant difference between the two groups
Table 4.
Multivariate logistic regression analysis of independent associated variables of emergence delirium
| Characteristics | OR | 95% CI | P-value |
|---|---|---|---|
| Preoperative | |||
| Age, year | 0.977 | 0.954, 1.000 | 0.053 |
| Male | 2.762 | 6.757, 1.134 | 0.026 |
| BMI, kg/m2 | 1.081 | 0.985, 1.186 | 0.099 |
| Current smoker | 2.547 | 1.065, 6.091 | 0.036 |
| Nasal splint | 0.270 | 0.123, 0.591 | 0.001 |
| FESS with septoplasty | 2.770 | 1.108, 6.925 | 0.029 |
| Nasal septum deviation with obstruction | 6.000 | 1.793, 20.07 | 0.004 |
| Intraoperative & postoperative | |||
| Duration of surgery, min | 0.987 | 0.939, 1.037 | 0.594 |
| Duration of anesthesia, min | 1.014 | 0.996, 1.064 | 0.581 |
| Intraoperative sufentanil dosage | 0.980 | 0.927, 1.036 | 0.474 |
| min to extubation | 1.014 | 0.966, 1.064 | 0.581 |
| NRS in PACU | 0.988 | 0.918, 1.064 | 0.753 |
| PACU time, min | 1.012 | 0.986, 1.039 | 0.367 |
| Postoperative hospital stays, days | 1.672 | 1.121, 12.49 | 0.012 |
| h when left PACU, bpm | 1.027 | 0.992, 1.063 | 0.134 |
| MAP when left PACU, mmHg | 0.986 | 0.948, 1.026 | 0.495 |
| SpO2 when left PACU, % | 0.799 | 0.618, 1.032 | 0.085 |
Note: OR, odds ratio; 95% CI, 95% confidence interval; BMI, body mass index
Discussion
This randomized controlled trial showed that preconditioning with nasal splint and mouth-breathing training preparation significantly decreased the incidence of ED after FESS by more than 50%. The intraoperative sufentanil dosage of the preconditioning group was significantly improved by 20.0 µg. Post-hoc analysis suggested that patients with ED required more postoperative hospital stay, and younger, males, with smoking status, with FESS with septoplasty, with aasal septum deviation with obstruction and without nasal splint were more likely to present ED.
Otorhinolaryngology operations, or surgeries involving the ear, nose, and throat (ENT), were reported to be associated with increased risks of postoperative agitation and ED [6, 32]. Previous studies have shown that incidence of ED after surgery of ENT almost tripled as that in all other surgery types [6, 33]. Although the underlying pathological mechanism of ED remains opaque, the agitation caused by sensory abnormalities during anesthesia emergence, which were often encountered for patients undergo ENT surgeries, may play an important role [33, 34]. Preconditioning to these sensory abnormalities may help to reduce the agitation. For example, it was suggested that feeling of suffocation during emergence from anesthesia was responsible for ED after head and neck surgery [35], and prophylactic conditioning with eyepatch could reduce ED for children undergoing cataract surgery [27]. The patient’s hypopharyngeal airspace is constrained during FESS [36]. During emergence from anesthesia, the suffocating sense and compulsory mouth breathing may be an agitating factor for patients that increases ED risk. Thus, preoperative conditioning to nasal obstruction and training of mouth breathing may help the adaption to the postoperative conditions therefore decrease the chance of developing ED. The effect of nasal preconditioning on ED reduction was consistent with the results from a prior randomized controlled trial, where the incidence of ED was reduced from 60 to 30% in patients underwent nasal surgery by preoperative nasal closure for 30 min [37, 38].
The identified incidence of ED in this study was 25.8%, which was consistent with the previously evidence. For example, a retrospective study reviewing 792 adults who underwent general anesthesia for nasal surgery reported the overall incidence of emergence agitation at 22.2% [39]. Most moderate and severe ED cases were in the control group, with moderate for 7 of total 9 and severe for 2 of total 3. Therefore, the efficacy of preconditioning might decrease the severity of ED while this effect need to be confirmed in future.
This randomized trial potentially balanced many baseline characteristics and preoperative parameters between two groups; however, the occurrence of ED could be influenced by numerous unknown factors. For example, the intraoperative dosage of sufentanil was significantly higher in preconditioning group than that in the control group. To further eliminate the possibility that the observed difference in ED incidence was due to differences in other factors rather than the preconditioning treatment, we applied a post-hoc case-control analysis to confirmed the independent risk factors of ED. Our results identified age, gender, smoking, FESS with septoplasty and surgery category were independent risk factors of ED, which were consistent from previous evidences [6, 39].
There were several limitations in this study. Firstly, the study was a single center study with relatively limited samples size, hence our conclusion might not be stable and extension of our conclusion to other patients should be verified in further multi-center or multi-regional, well-designed, large-scaled randomized trials. Secondly, in this study the identification of ED mainly depended on the subjective assessment from physicians based on the postoperative evens and intraoperative signs. Also, the blindness of intervention for intraoperative anesthesiologist could not be achieved. All these might raise the uncontrolled bias for outcome assessment and introduced the misclassification bias. More objective evaluation of ED by anesthesiologist and nurse with blindness at PACU was necessary. Thirdly, although this is a drug-free intervention and had almost no side effects, this study did not include a positive parallel control to eliminate the non-drug modality with pharmacological treatment (e.g., dexmedetomidine) [28, 38]. Fourthly, we observed a higher sufentanil dose in the preconditioning group, which might attenuate the treatment effect, however, we concluded a significant effect of nasal splint conditioning and mouth-breathing training before anesthesia on reducing the occurrence of ED incidence. Finally, due to the limited number of participants, the subgroups analysis is less statistical powerful to be applying, thus we can not to further detect the specific target population for the treatment. A RCT found clonidine preconditioning effective in reducing ED in opium abusers, which suggested that our nasal splint conditioning and mouth-breathing training before anesthesia might have a specific target population, which warrant further well-designed large-scale study.
Conclusions
Our single-center randomized controlled study compare the patients with nasal splint conditioning and mouth-breathing training before anesthesia with patients without intervention and found that this non-pharmacological intervention was an efficacy strategy for reducing the occurrence of ED by 50% among adults undergoing FESS. This non-pharmacological intervention is safe and cost-effective and can be easily implemented in practice, which could be a potential benefit strategy for patients prepared for surgery, although the conclusion on other surgery and population should be firstly verified before applying.
Acknowledgements
Not applicable.
Abbreviations
- ASA
American Society of Anesthesiologists Classification
- ED
emergence delirium
- f
frequency
- FESS
Functional Endoscopic Sinus Surgery
- HR
heart rate
- IQR
interquartile range
- MAC
minimum alveolar concentration
- NBP
non-invasive blood pressure
- NRS
Numerical Rating Scale
- OR
odds ratio
- PACU
post-anesthesia care unit
- SD
standard deviation
- SpO2
Oxygen saturation
- Vt
tidal volume
Author contributions
H.X. contributed to drafting of the manuscript. Z.S. contributed to data curation. Y.G. contributed to supervision. Y.H. contributed to data collection. J.J., X.L. and M.Z. contributed to the operation of the study. Y.Z. contributed to statistical analysis. J.L. contributed to study design and reviewing of the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by Clinical Research Innovation Plan of Shanghai General Hospital [grant number: KD031-ly01to Jinbao Li, CNY 600,000; CTCCR-2021C21 to Hongjiao Xu, CNY 50,000]. The funding only gave financial support.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted according to the Declaration of Helsinki and study protocol was approved by the Shanghai General Hospital Institutional Review Board (IRB 2019KY039). Written informed consent to participate was obtained from participants.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongjiao Xu, Zhenyuan Shen These authors are co-first authors and contributed equally to this work.
Contributor Information
Minmin Zhu, Email: zhu_mm@126.com.
Jinbao Li, Email: lijinbaoshanghai@163.com.
References
- 1.Nair S, Wolf A. Emergence delirium after paediatric anaesthesia: new strategies in avoidance and treatment. BJA Educ. 2018;18:30–3. doi: 10.1016/j.bjae.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy M, Rivest MP, Namian D, Moreau N. The critical reception of the DSM-5: towards a typology of audiences. Public Underst Sci. 2019;28:932–48. doi: 10.1177/0963662519868969. [DOI] [PubMed] [Google Scholar]
- 4.Doerrfuss JI, Kramer S, Tafelski S, Spies CD, Wernecke KD, Nachtigall I. Frequency, predictive factors and therapy of emergence delirium: data from a large observational clinical trial in a broad spectrum of postoperative pediatric patients. Minerva Anestesiol. 2019;85:617–24. doi: 10.23736/S0375-9393.19.13038-6. [DOI] [PubMed] [Google Scholar]
- 5.Munk L, Andersen G, Møller AM. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol Scand. 2016;60:1059–66. doi: 10.1111/aas.12717. [DOI] [PubMed] [Google Scholar]
- 6.Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anesthesia/Journal Canadien d’anesthésie. 2010;57:843–8. doi: 10.1007/s12630-010-9338-9. [DOI] [PubMed] [Google Scholar]
- 7.Banchs RJ, Lerman J. Preoperative anxiety management, emergence delirium, and postoperative behavior. Anesthesiol Clin. 2014;32:1–23. doi: 10.1016/j.anclin.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 9.Crocker E, Beggs T, Hassan A, Denault A, Lamarche Y, Bagshaw S, et al. Long-Term Effects of postoperative delirium in patients undergoing Cardiac Operation: a systematic review. Ann Thorac Surg. 2016;102:1391–9. doi: 10.1016/j.athoracsur.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, He S-T, Nie B, Li X-Y, Wang D-X. Emergence delirium is associated with increased postoperative delirium in elderly: a prospective observational study. J Anesth. 2020;34:675–87. doi: 10.1007/s00540-020-02805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houben A, Ghamari S, Fischer A, Neumann C, Baehner T, Ellerkmann RK. Pediatric emergence delirium is linked to increased early postoperative negative behavior within two weeks after adenoidectomy: an observational study. Braz J Anesthesiol. 2021. [DOI] [PubMed]
- 12.Reynolds T, Sankaran S, Chimbira WT, Phan T, Nafiu OO. Severe obesity and sleep-disordered breathing as risk factors for Emergence Agitation in Pediatric ambulatory surgery. J Perianesth Nurs. 2018;33:304–11. doi: 10.1016/j.jopan.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menser C, Smith H. Emergence agitation and delirium: considerations for Epidemiology and Routine Monitoring in Pediatric Patients. Local Reg Anesth. 2020;13:73–83. doi: 10.2147/LRA.S181459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahmani S, Delivet H, Hilly J. Emergence delirium in children: an update. Curr Opin Anaesthesiol. 2014;27:309–15. doi: 10.1097/ACO.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 15.Munk L, Andersen LP, Gögenur I. Emergence delirium. J Perioper Pract. 2013;23:251–4. doi: 10.1177/175045891302301103. [DOI] [PubMed] [Google Scholar]
- 16.Gooden R, Tennant I, James B, Augier R, Crawford-Sykes A, Ehikhametalor K, et al. [The incidence of emergence delirium and risk factors following sevoflurane use in pediatric patients for day case surgery, Kingston, Jamaica] Rev Bras Anestesiol. 2014;64:413–8. doi: 10.1016/j.bjan.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Chen JY, Jia JE, Liu TJ, Qin MJ, Li WX. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth. 2013;60:385–92. doi: 10.1007/s12630-013-9886-x. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Zhu M, Gao Z, Zhao S, Feng X, Chen J, et al. Dexmedetomidine for prevention of postoperative delirium in older adults undergoing oesophagectomy with total intravenous anaesthesia: a double-blind, randomised clinical trial. Eur J Anaesthesiol. 2021;38:9–s17. doi: 10.1097/EJA.0000000000001382. [DOI] [PubMed] [Google Scholar]
- 19.Jun JH, Kim KN, Kim JY, Song SM. The effects of intranasal dexmedetomidine premedication in children: a systematic review and meta-analysis. Can J Anaesth. 2017;64:947–61. doi: 10.1007/s12630-017-0917-x. [DOI] [PubMed] [Google Scholar]
- 20.Aiello G, Cuocina M, La Via L, Messina S, Attaguile GA, Cantarella G et al. Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: a systematic review and Meta-analysis of Randomized controlled trials. J Clin Med. 2023;12. [DOI] [PMC free article] [PubMed]
- 21.Barnes J, Sewart E, Armstrong RA, Pufulete M, Hinchliffe R, Gibbison B, et al. Does melatonin administration reduce the incidence of postoperative delirium in adults? Systematic review and meta-analysis. BMJ Open. 2023;13:e069950. doi: 10.1136/bmjopen-2022-069950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318:1161–74. doi: 10.1001/jama.2017.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slack R, Bates G. Functional endoscopic sinus surgery. Am Fam Physician. 1998;58:707–18. [PubMed] [Google Scholar]
- 24.Berlucchi M, Castelnuovo P, Vincenzi A, Morra B, Pasquini E. Endoscopic outcomes of resorbable nasal packing after functional endoscopic sinus surgery: a multicenter prospective randomized controlled study. Eur Arch Otorhinolaryngol. 2009;266:839–45. doi: 10.1007/s00405-008-0841-3. [DOI] [PubMed] [Google Scholar]
- 25.Szczygielski K, Rapiejko P, Wojdas A, Jurkiewicz D. Use of CMC foam sinus dressing in FESS. Eur Arch Otorhinolaryngol. 2010;267:537–40. doi: 10.1007/s00405-009-1117-2. [DOI] [PubMed] [Google Scholar]
- 26.Tafelmeier M, Knapp M, Lebek S, Floerchinger B, Camboni D, Creutzenberg M et al. Predictors of delirium after cardiac surgery in patients with sleep disordered breathing. Eur Respir J. 2019;54. [DOI] [PubMed]
- 27.Lin Y, Shen W, Liu Y, Wang Q, Chen Q, Fang Z, et al. Visual preconditioning reduces emergence delirium in children undergoing ophthalmic surgery: a randomised controlled trial. Br J Anaesth. 2018;121:476–82. doi: 10.1016/j.bja.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Cocuzza S, Maniaci A, Di Luca M, La Mantia I, Grillo C, Spinato G, et al. Long-term results of nasal surgery: comparison of mini-invasive turbinoplasty. J Biol Regul Homeost Agents. 2020;34:1203–8. doi: 10.23812/19-522-L-4. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Li X, Yang B, Shen Z, Li W, Zhou Y, et al. Effects of preconditioning by nasal splint and mouth breathing on emergence delirium after functional endoscopic sinus surgery in chinese adults: a study protocol for a randomised controlled trial. BMJ Open. 2020;10:e033803. doi: 10.1136/bmjopen-2019-033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27:1325–9. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Cohen IT, Hannallah RS, Hummer KA. The incidence of emergence agitation associated with desflurane anesthesia in children is reduced by fentanyl. Anesth Analg. 2001;93:88–91. doi: 10.1097/00000539-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96:1625–30. doi: 10.1213/01.ANE.0000062522.21048.61. [DOI] [PubMed] [Google Scholar]
- 33.Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96:747–53. doi: 10.1093/bja/ael094. [DOI] [PubMed] [Google Scholar]
- 34.Maniaci A, Merlino F, Cocuzza S, Iannella G, Vicini C, Cammaroto G, et al. Endoscopic surgical treatment for rhinogenic contact point headache: systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2021;278:1743–53. doi: 10.1007/s00405-021-06724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckenhoff JE, Kneale DH, Dripps RD. The incidence and etiology of postanesthetic excitment. A clinical survey. Anesthesiology. 1961;22:667–73. doi: 10.1097/00000542-196109000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Friedman M, Maley A, Kelley K, Leesman C, Patel A, Pulver T, et al. Impact of nasal obstruction on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2011;144:1000–4. doi: 10.1177/0194599811400977. [DOI] [PubMed] [Google Scholar]
- 37.Kasem A, Abdelkader A. Preoperative external nasal compression: does it decrease emergence agitation after nasal surgery? Ain-Shams J Anaesthesiol. 2016;9:593–7. doi: 10.4103/1687-7934.198250. [DOI] [Google Scholar]
- 38.Maniaci A, Lechien JR, Calvo-Henriquez C, Iannella G, Leigh S, Ingrassia A, et al. Long-term stability of outcomes of endoscopic surgery for rhinogenic contact point headache (Sluder’’s neuralgia) Am J Otolaryngol. 2022;43:103368. doi: 10.1016/j.amjoto.2021.103368. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Kim DK, Kim HY, Kim JK, Choi SW. Risk factors of emergence agitation in adults undergoing general anesthesia for nasal surgery. Clin Exp Otorhinolaryngol. 2015;8:46–51. doi: 10.3342/ceo.2015.8.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.



