Abstract

Exposure to fine particulate matter (PM < 2.5 μm in diameter [PM2.5]) may accelerate human sperm quality decline, although research on this association is limited. Our objective was to investigate the relationship between exposure to the chemical constituents of PM2.5 air pollution and decreased sperm quality and to further explore the exposure–response relationship. We conducted a multicenter population-based cohort study including 78,952 semen samples from 33,234 donors at 6 provincial human sperm banks (covering central, northern, southern, eastern, and southwestern parts of China) between 2014 and 2020. Daily exposure to PM2.5 chemical composition was estimated using a deep learning model integrating a density ground-based measure network at a 1 km resolution. Linear mixed models with subject- and center-specific intercepts were used to quantify the harmful impacts of PM2.5 constituents on semen quality and explore their exposure–response relationships. Per interquartile range (IQR) increase in PM2.5 exposure levels during spermatogenesis was significantly associated with decreased sperm concentration, progressive motility, and total motility. For PM2.5 constituents, per IQR increment in Cl– (β: −0.02, 95% CI: [−0.03, −0.00]) and NO3– (β: −0.05, 95% CI: [−0.08, −0.02]) exposure was negatively associated with sperm count, while NH4 (β: −0.03, 95% CI: [−0.06, −0.00]) was significantly linked to decreased progressive motility. These results suggest that exposure to PM2.5 chemical constituents may adversely affect human sperm quality, highlighting the urgent need to reduce PM2.5 exposure.
Keywords: chemical constituents, exposure−response relationship, semen quality, multicenter study, particulate matter
Short abstract
This large-scale multi-center study demonstrates that certain constituents of PM2.5 adversely affect sperm quality parameters and for the first time explores the nonlinear exposure−response relationship between PM2.5 constituents and sperm quality.
Introduction
Ambient fine particulate matter (PM) (particulate matter with an aerodynamic diameter ≤ 2.5 μm, PM2.5) is widely recognized as a predominant hazardous air pollutant on a global scale, particularly in developing countries such as China, where ambient PM2.5 concentrations exceed the limits recommended by the World Health Organization (WHO).1 Prior research has indicated that PM2.5 can pose risks to a variety of health outcomes such as mortality, lung cancer, and respiratory and cardiovascular diseases. Additionally, in recent years, its detrimental impact on reproductive health has garnered widespread attention.2−5
As a major reproductive health problem, male infertility has drawn widespread concern. Previous studies suggest that at least 30 million men are affected by infertility.6 Male infertility is influenced by a variety of factors, among which semen quality plays a pivotal determinant.7−9 Over the past 50 years, there has been a notable decline in semen quality among men worldwide.6,7,10−12 Previous studies have linked PM2.5 exposure and sperm quality,13−16 while the magnitude of such associations varied across regions. Numerous studies have shown that the chemical constituents of PM2.5 vary spatiotemporally depending on various factors such as climate characteristics, population density, economic activities, and industrial structure,17−20 which may partially account for the observed disparities in the aforementioned associations. Distinct PM2.5 chemical composition may exert varied effects on human health through diverse pathways.21 Hence, the identification of toxic PM2.5 components could facilitate the development of targeted strategies aimed at mitigating the adverse impacts of PM2.5 pollution.
Till date, only two epidemiological studies have explored the association between the PM2.5 constituents and human sperm quality.22,23 However, the results of these studies are inconsistent. One underlying reason may be that these studies are conducted in a single geographical region, with a comparatively diminutive pool of participants, thereby constraining the generalizability of their findings. Moreover, an assumption of linear exposure–response association between PM2.5 constituents and sperm quality was made in these studies, which may not hold true on many occasions. In environmental epidemiological studies, understanding the exposure–response relationship of air pollution to health is essential for quantitative assessment. Hence, a multicenter population-based cohort study covering multiple geographical regions with a large sample size to investigate the association between PM2.5 chemical components and sperm quality is warranted and would provide an in-depth understanding of the harmful effects that PM2.5 constituents can have on male reproductive health.
Therefore, we conceived this large-scale, multicenter study based on 78,952 samples of 33,234 sperm donors from 6 representative regions across China. To reduce the influence of confounding factors, we utilized three different adjustment models to explore the association of exposure to the main chemical constituents [including black carbon (BC), nitrate (NO3–), chloride (Cl–), ammonium (NH4), and sulfate (SO42–)] of PM2.5 with male semen quality. Furthermore, we quantitatively described the exposure–response relationships between PM2.5 constituents and sperm quality.
Materials and Methods
Study Region
Semen quality data for this population-based multicenter study was obtained from 6 provincial human sperm banks in Henan, Hubei, Guangdong, Shanxi, Sichuan, and Zhejiang provinces of China. Guangdong, a coastal region in South China, it has a warm climate all year round. PM2.5 accounts for approximately 15% of the whole province’s air pollutants. In contrast, both Hubei and Henan are in central China where there are distinct seasons, with cold winters and hot summers. In 2019, only two cities in Hubei met the national secondary air quality standards for PM2.5, while Henan only met the standards for five months out of the year. Sichuan Province, located in southwest China, is an inland region with a great difference in climate. Nearly half of the cities in the province exceeded the PM2.5 standards in 2019. Shanxi, situated in North China, experiences warm and humid weather conditions throughout the year, but the PM2.5 concentration also exceeds the recommended standard. Zhejiang Province belongs to the eastern coastal area and has moderate temperatures with seasonal changes in precipitation. The six provinces covered different geographical areas of China with distinct meteorological and socioeconomic characteristics. Moreover, these provinces exhibit different levels of environmental fine particulate matter pollution, which increases the generalizability of the study to some extent.
Study Population
Participants in this study were recruited from 6 provincial human sperm banks in China during the study period of 2014–2020. Initially, 49,374 semen donation volunteers were included in this study, each of whom was required to undergo at least one semen analysis. These sperm donors come from the general male population whose fertility is unknown and are representative of the general men with reproductive age. After undergoing routine semen screening tests, the sperm donors are further required to have blood drawn to test for sexually transmitted diseases (hepatitis B, hepatitis C, syphilis, and AIDS), toxoplasma gondii, cytomegalovirus, herpes simplex virus, rubella virus, karyotype analysis, and thalassemia screening. In addition, pathogenic microorganisms such as gonococcus, chlamydia, and mycoplasma were examined in semen samples. If any abnormality was detected in the test results, the sperm donors were eliminated from the study.24 After that, a total of 97,451 semen samples were collected from these subjects. Due to the absence of ethnicity, age, semen parameters, and abnormal ejaculation or semen parameters, we eliminated the corresponding semen samples, and then we screened the rest of the semen samples according to certain inclusion and exclusion criteria. First, the samples should conform to the requirement that the corresponding semen donors should be between 19 and 45 years old (approximately covering the range of reproductive age). In addition, the subjects should live in the study area and have an abstinence period between 2 and 7 days. Finally, a total of 78,952 semen samples from 33,234 sperm donors were included in the analysis. The spatial distribution of the participants is illustrated in Figure 2.
Figure 2.
Spatial distributions of 33,234 sperm donors in 6 geographical regions of China, with the average exposure concentrations of PM2.5 and the chemical constituents included in the study in each region. Abbreviation: PM2.5, particulate matter ≤ 2.5 μm in aerodynamic diameter; BC, black carbon; Cl–, chloride; NH4+, ammonium; NO3, nitrate; SO42–, sulfate.
Semen Analysis
Each sperm donor was instructed to masturbate into a sterile plastic sample container. Semen samples were analyzed in strict accordance with the WHO fifth edition of the Human Semen Testing and Processing Laboratory Manual.25 The semen quality parameters assessed included semen volume, sperm count, concentration, and motility, which were then tested. Sperm count (×106) was determined by multiplying semen volume and semen concentration. Sperm motility consisted of progressive and non-progressive motility, and total motility was the summation of non-progressive plus progressive motility. Based on prior research,22,23 sperm count, concentration, progressive motility, and total motility were identified as the primary parameters for assessing sperm quality. However, data on total sperm motility in Sichuan and Shanxi were not available. All personal information involved in this study was anonymous. Each technician received regular training in laboratory quality control.
Exposure Assessment
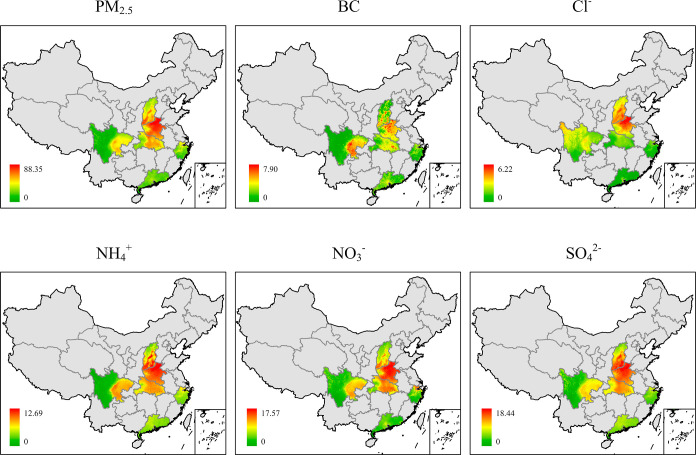
Air pollution data used in this study were obtained from the ChinaHighAirPollutant (CHAP) dataset with spatial resolution of 1 km (≈0.01° × 0.01°). The CHAP is an extensive, high-resolution, high-quality dataset of ground-level air pollutants within China that is generated by integrating artificial intelligence and big data, such as ground-based measurements, and satellite remote-sensing products, considering the spatial and temporal heterogeneity of air pollution. Its validity has been verified in previous studies.26,27 Daily concentrations of PM2.5 chemical constituents were extracted from this dataset based on subjects’ residential addresses to assess individual exposure. Since human sperm development takes an approximate 90 day period and PM2.5 constituents are usually not normally distributed over this period, we chose the median concentration from 0 to 90 days prior to the semen donation date to assess individual exposure to PM2.5 and its constituents. The spatial distribution of PM2.5 and its constituents across 6 provinces in China is presented in Figure 1.
Figure 1.
Average PM2.5 constituents’ concentration distribution in 6 regions from 2013 to 2020. Abbreviation: PM2.5, particulate matter ≤ 2.5 μm in aerodynamic diameter; BC, black carbon; Cl–, chloride; NH4+, ammonium; NO3, nitrate; SO42–, sulfate.
Covariates
Age (<30, 30–39, ≥40), ethnicity (Han or other), abstinence period (2–3, 4–5, 6–7 days), and sperm donation date data were obtained from the collected data, and year and month were further obtained from the sperm donation data. Previous studies showed that the impact of air pollutants may vary by season.28 Given that sperm development lasts approximately 90 days, it may not be entirely restricted to a specific season, so we considered adjusting month instead of season in the model. At present, many studies have proved that temperature will have different degrees of influence on semen quality, so exposure to ambient temperature of sperm donors during the period of sperm development will also be included in the model. The average daily temperatures of 6 regions in China during 2014–2020 were calculated using the Global Climate Atmosphere Reanalysis dataset of the European Centre for Medium Range Weather Forecasts (fifth generation, ERA5) with a spatial resolution of 0.1° × 0.1° and temporal resolution of 1 h. This is a state-of-the-art global land application reanalysis dataset.29 Previous studies validated the validity of grid-based data by comparing effect estimation of ERA5-Land data with observation-based effect estimation.30,31 Missing values (<0.4%) of meteorological data were estimated with the multiple imputation algorithm using the “mice” package in R software.
Statistical Analysis
Initially, we analyzed the basic characteristics of the sperm donors. Subsequently, the correlation between PM2.5 total mass and chemical component concentrations were assessed by the Spearman rank correlation coefficient (rs). Furthermore, we constructed a multivariate linear mixed model (LMM) with subject-specific and center-specific intercepts, taking into account repeated measures and geographic heterogeneity of semen samples, to explore the relationships between exposures to PM2.5 constituents and sperm quality parameters. Given that the sperm quality parameters examined in our study were found to be skewed, we employed the BoxCox transform to ensure normal distribution (or approximately normal distribution) of sperm quality parameters to meet the prerequisites for constructing LMM.32 In order to facilitate interpretation and comparability, we standardized the semen parameters, ensuring that both the standardized deviation (SD) and variance were equal to 1.33 Therefore, the pooled regression coefficients and corresponding 95% confidence intervals (CIs) for a relative change of each semen quality parameter in the scale of SD were estimated based on per interquartile range (IQR) increment in the exposure concentration of a pollutant.
A common analytical approach only uses a single PM2.5 constituent to explore the relationship between PM2.5 and semen quality. However, the estimation obtained from this approach is prone to bias as it can be easily affected by the correlation among PM2.5 and constituents. An easy way to consider the effect of PM2.5 is to include PM2.5 as a term in the model that explores the relationship between a constituent and semen quality. The constituent’s parameter represents the impact of its increased concentration on sperm quality when other constituents are held constant, and the PM2.5 parameter represents the effect of all other constituents on semen quality. However, since constituent concentration is often strongly correlated with PM2.5 total mass, the inclusion of two collinear terms may lead to instability and large variance of the coefficient. Therefore, we further considered calculating residuals that were not correlated with PM2.5 levels and represented changes in component levels independent of PM2.5 by developing an LMM with component concentrations as the dependent variable and PM2.5 levels as the independent variable. Finally, we employed three different models to estimate the association between PM2.5 component exposure and sperm quality: model 1, which solely adjusts for the concentration of a single constituent to estimate its specific effect; model 2, which adjusts for both the concentration of a single constituent and PM2.5 total mass, thereby estimating the effect of a particular constituent with other chemical components unchanged; and model 3, which adjusts for the concentration of a single constituent and the residuals calculated from a linear regression model, providing an estimate of the effect of a particular chemical component holding PM2.5 constant.22,34 All these models were adjusted for age, ethnicity, abstinence period, month, and year. However, some constituents may be highly correlated with PM2.5 total mass. When these constituents and PM2.5 total mass are included in the model at the same time, the corresponding results will be affected by the interference of PM2.5 to a large extent, and multicollinearity may exist. Therefore, we utilized variance inflation factor (VIF) to measure the multicollinearity between PM2.5 constituents in all LMMs. Typically, VIFs above 4 warrant further investigation, and those above 10 indicate serious multicollinearity.35
In addition, we incorporated natural cubic spline function of PM2.5 constituents in the LMM to further explore the nonlinear exposure–response relationship between the PM2.5 component and semen parameters, while adjusting for the covariates mentioned above. We combined biological rationality (such as monotonicity) and model fitting to select the optimal degree of freedom for the spline function, which was determined to be 3.
We performed multiple subgroup analyses to examine the robustness of our results. First, subgroup analyses of semen samples that satisfy or do not satisfy normal sperm concentration (≥15 × 106/mL), sperm count (≥39 × 106), total motility (≥40%), and progressive motility (≥32%) were performed according to the WHO human semen parameter reference level.25 Second, in order to understand how repeated measurements affected our results, we restricted the study to the initial semen sample of all participants. At last, we focused on the subjects enrolled prior to the COVID-19 pandemic (2014–2019) because the pandemic during 2020–2021 may have an impact on the exposure levels of PM2.5 and sperm quality. All of the statistical tests were 2-sided, and a P value of <0.05 was deemed as statistically significant. The statistical analysis was implemented in R software with version 4.1.2.
Results
Table 1 demonstrates the characteristics of 33,234 sperm donors with respect to 78,952 semen samples. Subjects enrolled in the study were predominantly under 30 years of age (72.0%), while only 3.3% were over 40 years old. A majority (97.5%) of the participants belonged to the Han ethnicity, and over half (51.4%) had abstinence periods ranging from 4 to 5 days. The mean PM2.5, BC, Cl–, NH4+, NO3, and SO42– exposures of the study were 43.69, 3.89, 1.78, 5.43, 8.31, and 9.21 μg/m3, respectively. Among the six regions, Henan had the highest average concentration of various PM2.5 constituents, while exceeding that of the whole study. During the study period, the average concentration of PM2.5 constituents exhibited a declining trend year by year. There was variation in the number of semen samples collected across different seasons, with the highest number of samples being collected during summer and the lowest number during winter. Furthermore, the exposure to the average concentration of PM2.5 constituents was relatively higher in spring and winter. The characteristics of the subgroup data meeting the WHO standards for semen quality parameters are presented in Table S1.
Table 1. Characteristics of the Study Population according to the Exposure Concentration of PM2.5 and Chemical Constituentsa.
| mean ± SD |
|||||||
|---|---|---|---|---|---|---|---|
| characteristic | n (%) | PM2.5 | BC | Cl– | NH4+ | NO3– | SO42– |
| total subjects | 33,234 (100.0) | ||||||
| total samples | 78,952 (100.0) | 43.69 ± 21.90 | 3.89 ± 1.81 | 1.78 ± 1.63 | 5.43 ± 2.98 | 8.31 ± 5.53 | 9.21 ± 3.44 |
| Ages, years | |||||||
| <30 | 56,879 (72.0) | 43.31 ± 21.57 | 3.83 ± 1.77 | 1.74 ± 1.60 | 5.37 ± 2.93 | 8.28 ± 5.50 | 9.13 ± 3.39 |
| 30–39 | 19,470 (24.7) | 44.80 ± 22.70 | 4.06 ± 1.90 | 1.88 ± 1.71 | 5.59 ± 3.10 | 8.45 ± 5.61 | 9.44 ± 3.56 |
| ≥40 | 2603 (3.3) | 43.78 ± 22.69 | 4.03 ± 1.84 | 1.79 ± 1.65 | 5.40 ± 3.10 | 7.92 ± 5.64 | 9.29 ± 3.40 |
| Ethnicity | |||||||
| Han | 77,007 (97.5) | 43.90 ± 22.03 | 3.90 ± 1.82 | 1.79 ± 1.65 | 5.46 ± 3.00 | 8.36 ± 5.55 | 9.25 ± 3.46 |
| Other | 1945 (2.5) | 35.53 ± 13.75 | 3.31 ± 1.04 | 1.25 ± 0.82 | 4.2 ± 1.78 | 6.27 ± 4.04 | 7.73 ± 1.92 |
| Abstinence Period, days | |||||||
| 2–3 | 22,072 (28.0) | 42.76 ± 21.53 | 3.89 ± 1.70 | 1.76 ± 1.59 | 5.25 ± 2.94 | 7.90 ± 5.49 | 9.11 ± 3.37 |
| 4–5 | 40,617 (51.4) | 42.62 ± 21.03 | 3.80 ± 1.75 | 1.71 ± 1.56 | 5.28 ± 2.86 | 8.11 ± 5.37 | 9.00 ± 3.27 |
| 6–7 | 16,263 (20.6) | 47.63 ± 23.99 | 4.10 ± 2.08 | 1.97 ± 1.84 | 6.04 ± 3.23 | 9.37 ± 5.85 | 9.88 ± 3.83 |
| Region | |||||||
| Guangdong | 34,219 (43.3) | 31.39 ± 7.52 | 3.36 ± 0.54 | 1.28 ± 0.68 | 3.64 ± 1.12 | 4.92 ± 2.44 | 7.42 ± 1.38 |
| Henan | 12,953 (16.4) | 76.91 ± 23.47 | 6.92 ± 2.10 | 4.38 ± 2.25 | 10.19 ± 3.03 | 15.59 ± 5.53 | 15.30 ± 2.81 |
| Hubei | 2655 (3.4) | 55.00 ± 23.38 | 4.44 ± 1.41 | 1.27 ± 0.76 | 6.62 ± 2.37 | 10.17 ± 5.83 | 11.70 ± 2.47 |
| Shanxi | 938 (1.2) | 47.18 ± 15.06 | 3.87 ± 1.38 | 2.21 ± 1.44 | 7.40 ± 2.54 | 8.09 ± 2.98 | 11.11 ± 3.33 |
| Sichuan | 1150 (1.5) | 50.74 ± 19.60 | 6.25 ± 2.19 | 1.88 ± 0.81 | 6.87 ± 2.41 | 8.53 ± 3.13 | 9.80 ± 2.59 |
| Zhejiang | 27,037 (34.2) | 41.82 ± 15.77 | 2.95 ± 0.86 | 1.18 ± 0.81 | 5.16 ± 1.89 | 8.92 ± 4.73 | 8.22 ± 1.87 |
| Year at Semen Examination | |||||||
| 2014 | 9103 (11.5) | 70.64 ± 19.25 | 5.44 ± 2.05 | 3.14 ± 2.14 | 9.02 ± 2.62 | 14.34 ± 5.04 | 13.97 ± 3.26 |
| 2015 | 7176 (9.1) | 71.04 ± 25.78 | 5.52 ± 2.42 | 3.15 ± 2.40 | 8.99 ± 3.31 | 13.96 ± 5.81 | 13.30 ± 3.71 |
| 2016 | 21,386 (27.1) | 37.05 ± 15.48 | 3.63 ± 1.46 | 1.63 ± 1.27 | 4.53 ± 2.26 | 6.25 ± 4.51 | 8.34 ± 2.43 |
| 2017 | 15,825 (20.0) | 39.46 ± 17.41 | 3.71 ± 1.42 | 1.44 ± 1.21 | 4.78 ± 2.47 | 7.45 ± 4.97 | 8.42 ± 2.53 |
| 2018 | 13,507 (17.1) | 35.83 ± 12.16 | 3.40 ± 1.15 | 1.30 ± 1.01 | 4.29 ± 1.72 | 6.58 ± 3.74 | 7.89 ± 1.74 |
| 2019 | 7590 (9.6) | 36.90 ± 16.21 | 3.35 ± 1.71 | 1.40 ± 1.30 | 4.84 ± 2.22 | 8.02 ± 4.45 | 7.87 ± 2.02 |
| 2020 | 4365 (5.5) | 26.58 ± 5.98 | 2.30 ± 0.69 | 0.69 ± 0.51 | 3.45 ± 1.27 | 5.47 ± 2.96 | 6.09 ± 1.05 |
| Season at Semen Examination | |||||||
| Spring (Mar, Apr, May) | 22,545 (28.6) | 53.87 ± 23.51 | 4.50 ± 1.93 | 2.48 ± 1.88 | 6.89 ± 3.01 | 11.67 ± 4.92 | 10.14 ± 3.17 |
| Summer (Jun, Jul, Aug) | 22,620 (28.7) | 34.27 ± 14.66 | 3.35 ± 1.04 | 1.15 ± 0.80 | 4.17 ± 2.14 | 5.57 ± 3.03 | 8.24 ± 3.42 |
| Autumn (Sep, Oct, Nov) | 18,675 (23.7) | 33.49 ± 12.04 | 3.21 ± 1.26 | 1.11 ± 0.88 | 4.14 ± 2.17 | 5.03 ± 3.57 | 8.49 ± 3.51 |
| Winter (Dec, Jan, Feb) | 15,112 (19.1) | 55.22 ± 25.13 | 4.63 ± 2.42 | 2.49 ± 2.09 | 6.73 ± 3.30 | 11.45 ± 6.56 | 10.16 ± 3.1 |
Abbreviation: SD, standardized deviation; PM2.5, particulate matter ≤ 2.5 μm in aerodynamic diameter; BC, black carbon; Cl–, chloride; NH4+, ammonium; NO3, nitrate; SO42–, sulfate.
The correlation coefficients (rs) between the average concentration of PM2.5 and the five constituents during the study period are demonstrated in Figure S1. PM2.5 exposure was highly correlated with NH4+, NO3, and SO42– (rs = 0.83–0.96), and BC and Cl– were moderately highly correlated with other constituents (rs = 0.63–0.73). Table 2 shows the distribution of semen parameters according to quintiles of PM2.5 constituents. In terms of PM2.5 constituents, the IQR concentrations ranged from 1.29 μg/m3 for BC to 7.01 μg/m3 for NO3. We found that the distribution of PM2.5 constituents was skewed, and the concentration span of the last quintile group is the largest. The sperm quality of individuals in the highest percentile of PM2.5 constituents’ exposure was considerably poorer than that of subjects in the other quintiles. The distribution of semen parameters in subjects related to the range of quantiles of PM2.5 constituents in subgroup is shown in Table S2.
Table 2. Distribution of Semen Parameters of Study Subjects according to the Range of Quintiles of Exposure Concentration of PM2.5 and Chemical Constituentsa.
| pollutants | IQR, μg/m3 | chemical constituents exposure range, μg/m3 | sperm concentration, 106/mL | sperm count, 106 | total motility, % | progressive motility, % |
|---|---|---|---|---|---|---|
| PM2.5 | 23.35 | 11.07–28.83 | 73.0 (45.0) | 257.6 (182.7) | 52.0 (14.0) | 55.0 (14.0) |
| 28.84–36.07 | 76.0 (49.0) | 268.6 (199.5) | 54.0 (16.0) | 58.0 (15.0) | ||
| 36.08–52.18 | 70.0 (44.0) | 244.2 (193.8) | 51.0 (15.0) | 55.0 (14.0) | ||
| 52.19–150.51 | 55.0 (35.0) | 150.0 (164.0) | 50.0(23.0) | 55.0 (20.0) | ||
| BC | 1.29 | 0.69–2.85 | 70.0 (40.0) | 255.0 (173.3) | 50.0 (11.0) | 53.0 (10.0) |
| 2.86–3.39 | 75.0 (46.0) | 262.7 (191.1) | 55.0 (14.0) | 58.0 (14.0) | ||
| 3.40–4.14 | 74.0 (47.0) | 255.0 (195.4) | 54.0 (15.0) | 58.0 (15.0) | ||
| 4.15–16.30 | 52.5 (39.0) | 141.0 (160.0) | 50.0 (24.0) | 58.0 (22.0) | ||
| Cl– | 1.36 | 0.16–0.74 | 70.0 (41.0) | 247.9 (177.6) | 50.0 (14.0) | 54.0 (13.0) |
| 0.75–1.29 | 73.0 (45.0) | 256.0 (186.1) | 52.0 (14.0) | 56.0 (13.0) | ||
| 1.30–2.09 | 72.0 (47.0) | 250.0 (204.0) | 54.0 (16.0) | 58.0 (15.0) | ||
| 2.10–11.39 | 60.0 (40.0) | 160.0 (178.8) | 50.0 (20.0) | 57.0 (21.0) | ||
| NH4+ | 3.31 | 0.92–3.41 | 73.0 (46.0) | 258.4 (182.7) | 52.0 (13.0) | 56.0 (14.0) |
| 3.42–4.59 | 75.0 (48.0) | 268.8 (199.4) | 54.0 (16.0) | 58.0 (15.0) | ||
| 4.60–6.72 | 69.0 (42.0) | 240.5 (189.4) | 51.0 (15.0) | 55.0 (14.0) | ||
| 4.72–20.26 | 55.0 (35.0) | 150.0 (164.9) | 50.0 (22.0) | 55.0 (20.0) | ||
| NO3– | 7.01 | 0.85–3.69 | 76.0 (48.0) | 263.9 (190.6) | 54.0 (14.0) | 58.0 (15.0) |
| 3.70–7.11 | 72.0 (48.0) | 258.3 (194.6) | 52.0 (16.0) | 56.0 (14.0) | ||
| 7.12–10.71 | 67.0 (42.0) | 233.1 (198.1) | 51.0 (15.0) | 55.0 (15.0) | ||
| 10.72–31.18 | 60.0 (35.0) | 165.0 (176.0) | 50.0 (20.0) | 55.0 (20.0) | ||
| SO42– | 3.27 | 3.21–6.98 | 73.0 (45.0) | 259.2 (180.6) | 51.0 (14.0) | 55.0 (13.0) |
| 6.99–8.31 | 76.0 (47.0) | 270.6 (192.5) | 53.0 (14.0) | 56.0 (14.0) | ||
| 8.32–10.24 | 73.0 (44.0) | 254.6 (192.0) | 53.0 (15.0) | 57.0 (14.0) | ||
| 10.25–25.66 | 50.0 (38.0) | 135.0 (145.0) | 50.0 (27.0) | 56.0 (20.0) |
Data were given as median (IQR) for the results of quintile of PM2.5 constituents exposure. Abbreviation: IQR, interquartile range; PM2.5, particulate matter ≤ 2.5 μm in aerodynamic diameter; BC, black carbon; Cl–, chloride; NH4+, ammonium; NO3, nitrate; SO42–, sulfate.
Figure S2 demonstrates the results of the relationship between each IQR increases of PM2.5 constituents and semen parameters using three LMMs. Among them, sperm concentration, progressive motility, and total motility were negatively associated with PM2.5 (β: −0.05, 95% CI: [−0.07,–0.02], β: −0.05, 95% CI: [−0.07,–0.02] and β: −0.06, 95% CI: [−0.09,–0.03]), but no significant association was observed between sperm count and PM2.5 (p > 0.05). For the PM2.5 chemical components, per IQR increment in Cl– (β: −0.02, 95% CI: [−0.03, −0.00] in model 3) and NO3– exposure (β: −0.05, 95% CI: [−0.08, −0.02] in model 3) was significantly linked to a decrease in sperm count, while NH4 (β: −0.03, 95% CI: [−0.06, −0.00] in model 3) exposure was negatively linked to progressive motility in all three models. For sperm concentration and total motility, there was no evidence suggesting associations in model 3, but NO3– exposure was negatively linked to sperm concentration in model 1 and model 2, and NH4 exposure was negatively related to total motility in model 1 and model 2. Figure S3 shows pooled regression coefficients and 95% CIs of semen parameters in the subgroup, corresponding to an IQR increase of PM2.5 constituent exposures in three models.
Table 3 presents the pooled regression coefficient associated with each IQR increases of PM2.5 constituents in the entire group and subgroup. Compared with the entire group, all PM2.5 constituents evaluated were significantly linked with declines in progressive motility and total motility according to the results of subgroup analyses in model 1. In addition, the result of the constituent was not materially different from that of the entire group, which was significantly correlated with sperm count. However, exposure to specific PM2.5 constituents revealed new associations with sperm quality parameters in subgroups. Specifically, Cl– exposure (β: −0.02, 95% CI: [−0.03, −0.00] in model 3) was significantly negatively associated with sperm concentration; SO42– exposure (β: −0.03, 95% CI: [−0.05, −0.01] in model 3) was significantly and negatively linked to progressive motility; each IQR increase in Cl– (β: −0.02, 95% CI: [−0.03, −0.00] in model 3), NH4 (β: −0.06, 95% CI: [−0.09, −0.03] in model 3), and SO42– (β: −0.03, 95% CI: [−0.05, −0.01] in model 3) exposure was linked to a decrease in total motility. It is noteworthy that the VIF results were less than 10 in the entire group or subgroup, and VIF values for model 3 were all below 4, indicating that there was no serious multicollinearity among variables.
Table 3. Pooled Regression Coefficients and 95% CIs of Semen Parameters Associated with Each IQR Increment in the Exposure Concentration of PM2.5 and Chemical Constituents by the Entire Group as Well as the Subgroupa.
| model
1 |
model 2 |
model 3 |
|||||
|---|---|---|---|---|---|---|---|
| semen quality parameter | PM2.5 constituents | entire group | subgroupb | entire group | subgroupb | entire group | subgroupb |
| sperm concentration | PM2.5 | –0.04(−0.07, −0.02)d | –0.01(−0.03, 0.01)d | ||||
| BC | –0.01(−0.02, 0.00)c | –0.00(−0.01, 0.01)c | 0.00(−0.01, 0.02)c | –0.00(−0.01, 0.01)c | 0.01(−0.01, 0.02)c | –0.00(−0.01, 0.01)c | |
| Cl– | –0.02(−0.03, −0.00)c | –0.02(−0.03, −0.01)c | –0.01(−0.02, 0.00)c | –0.02(−0.03, −0.01)c | –0.00(−0.02, 0.01)c | –0.02(−0.03, −0.00)c | |
| NH4+ | –0.03(−0.06, −0.01)c | –0.01(−0.04, 0.01)c | –0.01(−0.04, 0.03)d | –0.01(−0.04, 0.02)d | 0.01(−0.02, 0.04)c | –0.01(−0.04, 0.02)c | |
| NO3– | –0.06(−0.09, −0.03)d | –0.03(−0.06, −0.01)d | –0.05(−0.08, −0.01)d | –0.04(−0.08, −0.01)d | –0.01(−0.05, 0.02)c | –0.03(−0.06, 0.00)c | |
| SO42– | –0.01(−0.03, 0.00)c | –0.00(−0.02, 0.01)c | 0.00(−0.02, 0.02)c | –0.00(−0.02, 0.02)c | 0.01(−0.01, 0.03)c | 0.00(−0.02, 0.02)c | |
| sperm count | PM2.5 | –0.01(−0.03, 0.02)d | 0.02(0.00, 0.04)d | ||||
| BC | –0.01(−0.02, 0.00)c | –0.00(−0.01, 0.01)c | –0.01(−0.02, 0.01)c | –0.01(−0.02, 0.00)c | –0.01(−0.02, 0.01)c | –0.01(−0.02, 0.00)c | |
| Cl– | –0.02(−0.03, −0.00)c | –0.01(−0.02, −0.00)c | –0.02(−0.03, −0.00)c | –0.02(−0.03, −0.01)c | –0.02(−0.03, −0.00)c | –0.02(−0.04, −0.01)c | |
| NH4+ | –0.01(−0.03, 0.01)c | 0.01(−0.01, 0.03)c | –0.01(−0.04, 0.02)d | –0.00(−0.03, 0.03)d | –0.00(−0.03, 0.02)c | –0.01(−0.04, 0.02)c | |
| NO3– | –0.05(−0.07, −0.02)d | –0.03(−0.06, −0.01)d | –0.06(−0.10, −0.03)d | –0.07(−0.11, −0.04)d | –0.05(−0.08, −0.02)c | –0.07(−0.10, −0.04)c | |
| SO42– | 0.01(−0.00, 0.03)c | 0.02(0.01, 0.04)c | 0.02(0.00, 0.04)c | 0.02(0.00, 0.04)c | 0.02(0.00, 0.04)c | 0.01(−0.00, 0.03)c | |
| progressive motility | PM2.5 | –0.04(−0.07, −0.02)d | –0.03(−0.05, −0.01)d | ||||
| BC | –0.01(−0.02, 0.00)c | –0.02(−0.03, −0.01)c | 0.00(−0.01, 0.02)c | –0.01(−0.02, 0.00)c | 0.01(−0.01, 0.02)c | –0.01(−0.02, 0.00)c | |
| Cl– | –0.01(−0.03, 0.00)c | –0.02(−0.03, −0.01)c | –0.00(−0.02, 0.01)c | –0.01(−0.03, 0.00)c | 0.00(−0.01, 0.02)c | –0.01(−0.02, 0.01)c | |
| NH4+ | –0.06(−0.08, −0.04)c | –0.06(−0.08, −0.04)c | –0.06(−0.09, −0.02)d | –0.07(−0.11, −0.04)d | –0.03(−0.06, −0.00)c | –0.05(−0.08, −0.03)c | |
| NO3– | –0.04(−0.07, −0.01)d | –0.04(−0.07, −0.01)d | –0.01(−0.05, 0.02)d | –0.03(−0.06, 0.01)d | 0.01(−0.02, 0.05)c | –0.00(−0.03, 0.03)c | |
| SO42– | –0.03(−0.05, −0.01)c | –0.04(−0.06, −0.03)c | –0.02(−0.04, −0.00)c | –0.04(−0.06, −0.02)c | –0.01(−0.03, 0.01)c | –0.03(−0.05, −0.01)c | |
| total motility | PM2.5 | –0.06(−0.09, −0.03)d | –0.04(−0.06, −0.01)d | ||||
| BC | –0.01(−0.02, 0.01)c | –0.02(−0.03, −0.01)c | 0.01(−0.01, 0.02)c | –0.01(−0.03, 0.00)c | 0.01(−0.00, 0.03)c | –0.01(−0.02, 0.00)c | |
| Cl– | –0.02(−0.03, −0.00)c | –0.03(−0.04, −0.01)c | –0.01(−0.02, 0.01)c | –0.02(−0.04, −0.01)c | 0.00(−0.01, 0.02)c | –0.02(−0.03, −0.00)c | |
| NH4+ | –0.08(−0.10, −0.05)d | –0.08(−0.10, −0.05)d | –0.06(−0.10, −0.03)d | –0.09(−0.12, −0.05)d | –0.03(−0.06, 0.01)c | –0.06(−0.09, −0.03)c | |
| NO3– | –0.06(−0.09, −0.02)d | –0.06(−0.09, −0.02)d | –0.02(−0.06, 0.02)d | –0.04(−0.08, −0.01)d | 0.01(−0.02, 0.05)c | –0.01(−0.05, 0.02)c | |
| SO42– | –0.04(−0.06, −0.02)c | –0.05(−0.07, −0.03)c | –0.02(−0.04, 0.00)c | –0.04(−0.07, −0.02)c | –0.01(−0.03, 0.02)c | –0.03(−0.05, −0.01)c | |
Three different linear mixed models were used to associate PM2.5 constituent exposures with semen quality. model 1 included single constituent (or PM2.5) concentration; model 2 included single constituent concentration and PM2.5; model 3 included single constituent residual calculated by constructing a linear regression model with constituent concentration as the dependent variable and PM2.5 as the independent variable. All three models were adjusted for age, ethnicity, abstinence period, year, month, and a natural cubic spline function of ambient temperature (df = 3) during the exposure period. Abbreviation: CI, confidence interval; IQR, interquartile range; PM2.5, particulate matter ≤2.5 μm in aerodynamic diameter; BC, black carbon; Cl–, chloride; NH4+, ammonium; NO3, nitrate; SO42–, sulfate.
The subgroup only included subjects with normal sperm concentration, sperm count, and motility according to the WHO reference levels for human semen parameters.
VIF ≤ 4.
VIF > 4 and VIF≤ 10.
We can see from the expose–response relationship curve (Figure 3) that in the general male population, how do semen quality parameters respond to atmospheric exposure levels of PM2.5 and its chemical components. Among them, Cl– and NO3– showed a relatively obvious inverse J-shape nonlinear relationship to the four sperm quality parameters, which was consistent with the results shown in Figure 3. Additionally, the relationship between PM2.5, NH4, SO42–, and progressive motility and total motility showed an inverted J-shaped curve to varying degrees; with the increase of constituents’ concentration, the pooled regression coefficient showed an obvious downward trend, while BC had an S-shaped nonlinear trend with progressive motility and total motility. Overall, the curve clearly confirms a trend between exposure to PM2.5 chemical components and decreased sperm quality in men.
Figure 3.
Exposure–response relationship between PM2.5 constituent exposures and semen quality. With adjustment for age, ethnicity, abstinence period, year, month, and temperature during the exposure period, the regression coefficients and corresponding 95% CIs were estimated with linear mixed models by including a natural cubic spline function of ambient temperature (df = 3), while the below histogram presents the distribution of each constituent. Abbreviation: CI, confidence interval; PM2.5, particulate matter ≤2.5 μm in aerodynamic diameter; BC, black carbon; Cl–, chloride; NH4+, ammonium; NO3, nitrate; SO42–, sulfate.
As shown in Figures S4–S6, we observed similar results for sperm concentration and sperm count in the subgroup limited to unqualified donors compared to the main analysis, but sperm motility did not show significant results possibly due to the small sample size. Furthermore, the results of analysis excluding subjects enrolled during the COVID-19 pandemic and those limited to the first sperm donation were not materially different from the primary analysis. We noted some positive correlation results with sperm concentration and motility in model 2 or model 3 of subgroup analysis, which may be due to the relatively small sample size in some regions resulting in unstable results.
Discussion
In this large-scale, multicenter population-based study, we utilized LMM that included subject-specific and center-specific random intercepts to quantitatively explored the exposure–response relationship between PM2.5 total mass and its chemical components and semen quality among 33,234 sperm donors with 78,952 semen samples. As far as we know, this is the initial multicenter study to explore the potentially detrimental impact of PM2.5 constituents on sperm quality. The results suggested that exposure to PM2.5 total mass and certain constituents adversely affected sperm quality. More specifically, PM2.5 total mass was linked to reduced sperm concentration, progressive motility, and total motility. In addition, NH4+ exposure showed a negative association with progressive motility, while exposure to Cl– and NO3 was related to decreased sperm count. Furthermore, we observed an approximately inverse J-shape relationship between Cl– and NO3– exposure and sperm concentration, sperm count, progressive motility, and total motility.
Our study revealed inverse associations between PM2.5 exposure throughout spermatogenesis and sperm quality parameters such as sperm concentration, progressive motility, and total motility. Compared with our study, a study of 1759 men conducted by Wu et al. demonstrated that PM2.5 was linked to a reduction in both sperm concentration and sperm count.32 Hammoud et al. identified significant negative associations for sperm motility and PM2.5 exposure, but not sperm concentration.36 These studies supported the negative associations of sperm quality and exposure to PM2.5, yet the evidence on sperm quality in relation to specific PM2.5 constituents has been limited and inconsistent, wherein the evidence was derived from single regions with relatively small sample sizes.22,23 In this large-scale study, we observed consistent negative associations between decreased sperm count and exposure to Cl– and NO3– and an inverse relationship between decreased progressive motility and exposure to NH4 across three models. Among these constituents, NO3– is mainly generated during combustion processes, and a large body of evidence suggests that it has greater toxicity and health hazards compared to other components.37 In line with the current study, our previous study in Guangzhou also employed LMM and reported a negative correlation between exposure to NH4 and progressive motility.23 Nevertheless, our findings are inconsistent with a study conducted in Wuhan, China, which reported an association between reduced sperm concentration and exposure to SO42– and NH4.22 The discrepancy in the impact of PM2.5 constituents in studies might be explained by different study areas, population characteristics, sample sizes, and exposure assessment approaches.
As far as we know, this present study is the first attempt to examine the exposure–response relationships between PM2.5 constituents and human sperm quality. While no prior research has examined this relationship specifically, previous research has investigated the exposure–response relationship between PM2.5 constituents and a variety of health outcomes, including all-cause mortality and diabetes.3,38 These studies have revealed the harmful impacts of PM2.5 chemical components on human health. In this study, we observed approximately inverted J-shaped relationships between exposure to Cl– and NO3– with sperm count, sperm concentration, progressive motility, and total motility. Inverted J-shaped relationships were also observed between exposure to NH4 and SO42– with progressive motility and total motility. According to the results of the exposure–response curves, a slight decline in semen quality was observed at lower exposure levels, which may be attributed to the smaller sample size for specific PM2.5 constituents at these levels, as evidenced by the much wider confidence intervals. On the other hand, these results indicated that certain PM2.5 chemical constituents may be linked to reduced semen quality at higher exposure levels. Overall, our results suggest a distinct nonlinear exposure–response relationship for semen quality with certain PM2.5 constituents.
When analyzing the impact of PM2.5 constituents on health, a widely adopted method is to model each constituent separately. However, recent research have indicated that PM2.5 is linked to both its constituents and health outcomes.34 This means that strong associations between specific constituents and health effects may be due to their correlation with PM2.5 rather than their inherent toxicity. In our research, we implemented three models to examine the relationship between PM2.5 chemical components exposure and sperm quality. We initially constructed a single-constituent model (model 1) to estimate the effect of each chemical component. However, given the strong correlation between constituent exposures and PM2.5, we developed two additional models: model 2, where we adjusted the PM2.5 total mass to eliminate potential confounding by PM2.5, and model 3, where we adjusted the PM2.5-adjusted constituent residuals that were uncorrelated with PM2.5 total mass. In our study, we found that NO3– exposure was significantly linked to reduced progressive motility and total motility in model 1, while no significant associations were found in model 2 and model 3. This could be due to the high correlation between NO3 and PM2.5 (rs = 0.92, Figure S1), indicating that the observed associations of NO3– exposure with progressive motility and total motility might be attributed to the strong correlation with PM2.5 rather than the inherent toxicity of the NO3. It is worth noting that consistent associations across models are generally considered to be more reliable. Therefore, it is advisable to refrain from drawing conclusions based only the results of a singular model.
The biological mechanisms underlying the link between PM2.5 constituents and reduced semen quality have not yet been completely elucidated. Nevertheless, the prevailing theory is that the soluble components of PM2.5 can cross the gas–blood barrier and enter the bloodstream, where they can interact with other tissues and organs, resulting in oxidative stress, systemic inflammation, and endocrine disruption.39,40 Previous research has indicated that PM2.5 exposure can induce oxidative stress, which may result in sperm dysfunction.41 Exposure to PM2.5 is also linked to excessive reactive oxygen species, which can cause damage to the blood–testis barrier and ultimately reduce semen quality.42−44 In addition, exposure to particulate matter can induce systemic inflammation by elevating levels of tumor necrosis factor and interleukin 1β, thereby reducing sperm quality.41,45,46 Moreover, exposure to certain toxic chemical components of PM2.5 during spermatogenesis may suppress the hypothalamus–pituitary–adrenal axis.39,47 Overall, PM2.5 and its constituents’ exposure may impair testicular function, which in turn affects spermatogenesis, leading to a decrease in sperm quality. Further research is warranted to examine potential biological mechanisms linking PM2.5 constituents to decreased semen quality.
Considering the downward trend in semen quality,48,49 our study has important public health implications. As male infertility’s main cause, poor semen quality has garnered worldwide attention.50 The present study contributes additional evidence that PM2.5 exposure may result in diminished sperm quality, and specific PM2.5 components might contribute to this effect, highlighting the need to control ambient PM2.5 and certain constituents’ exposure. By identifying the accountable PM2.5 components, our results could facilitate the establishment of more specific and efficient regulations for mitigating fine particulate pollution. This is especially crucial in regions and countries that face substantial challenges related to air pollution, such as China. Furthermore, our study provides researchers with a better understanding of the potential mechanisms of decreased semen quality caused by PM2.5 exposure throughout spermatogenesis.
Our study has several strengths. First, the main advantages of our study were the wide study area and large sample size. We enrolled 33,234 sperm donors from several regions with different exposure levels in China, which has good population and geographical representation. Our study represents the first attempt to analyze the impact of PM2.5 chemical components on human sperm quality by a multicenter population-based cohort study with large sample sizes. Hence, we have sufficient statistical power to quantitatively examine the relationship between PM2.5 chemical components and human sperm quality. Second, we estimated individual exposure to PM2.5 and its constituents using grid datasets with high spatial resolution, which help reduce exposure misclassification and yielded more precise exposure estimates. Third, our study strictly followed the WHO manual for semen analysis with sufficient quality control, which ensures the reliability of our results and allows for comparability with other studies. Additionally, conducting repeated measurements on semen sample data enable us to account for within-subject variability and obtain more precise estimates. Fourth, we utilized three distinct models that accounted for the confounding effect of PM2.5 to assess the relationship between PM2.5 chemical components and sperm quality, which makes our results more reliable and robust.
It is imperative to acknowledge the limitations of our study. First, although we estimated individual exposure to PM2.5 and its chemical components based on the residential address and high-spatial-resolution gridded data, we did not take into account the mobility of sperm donors, which may result in exposure misclassification. Second, we adjusted for some important factors in the model such as age and abstinence period; however, there remains a possibility that unmeasured confounding factors played a role in the observed associations. Third, this study only investigated the relationship between PM2.5 chemical components and four main semen quality parameters, including sperm concentration, sperm count, progressive motility, and total motility. However, other semen quality parameters, such as the percentage of normal sperm morphology, were not analyzed due to lack of data, which will be investigated in our future study. Fourth, although the sample size included in this study was large, the study subjects were sperm donors from human sperm banks and this is likely to be one of the main sources of selection bias in this study. According to the human sperm bank’s screening criteria for sperm donors, this population may have high sperm quality and not be fully representative of the randomly selected male population.24 By contrast, other existing studies on the effect of air pollution exposure on semen quality generally recruited participants from medical institutions, and such populations are more likely to have potential infertility problems and more vulnerable to air pollution, which would introduce greater selection bias.51,52 Although the selection bias in our study may be relatively smaller, it still needs to be noted.
In summary, this multicenter cohort demonstrated the detrimental effects of PM2.5 and specific constituents during spermatogenesis on semen quality, particularly sperm concentration, progressive motility, and total motility, underscoring the need to reduce PM2.5 exposure. Our study provides comprehensive insights into the impact of fine particulate matter chemical compositions on semen quality across varying levels of fine particulate matter pollution. Furthermore, our findings could inform policymakers in the formulation and development of PM2.5 control strategies and the establishment of PM2.5 constituents’ monitoring stations to promote male reproductive health.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c03928.
Spearman’s rank correlation among PM2.5 and 5 constituents’ concentrations, results of subgroup and sensitivity analyses, characteristics of the study population by PM2.5 constituents in the subgroup, and distribution of semen parameters in subjects related to the range of quantiles of PM2.5 constituents in the subgroup (PDF)
Author Contributions
†† L.W., T.X., and Q.W. contributed equally to this work.
The study was partly funded by the Guangdong Provincial Natural Science Foundation of China (no. 2022A1515011517). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no competing financial interest.
Notes
The air pollution data are available at https://weijing-rs.github.io/product.html. The meteorological data are available at https://cds.climate.copernicus.eu. The clinical data are confidential.
Supplementary Material
References
- World Health Organization . WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, 2021. [PubMed] [Google Scholar]
- Lelieveld J.; Evans J. S.; Fnais M.; Giannadaki D.; Pozzer A. The Contribution of Outdoor Air Pollution Sources to Premature Mortality on a Global Scale. Nature 2015, 525, 367–371. 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Liu L.; Luo S.; Zhang Y.; Yang Z.; Zhou P.; Mo S.; Zhang Y. Longitudinal Impacts of PM2.5 Constituents on Adult Mortality in China. Environ. Sci. Technol. 2022, 56, 7224–7233. 10.1021/acs.est.1c04152. [DOI] [PubMed] [Google Scholar]
- Cai J.; Zhao Y.; Kan J.; Chen R.; Martin R.; van Donkelaar A.; Ao J.; Zhang J.; Kan H.; Hua J. Prenatal Exposure to Specific PM2.5 Chemical Constituents and Preterm Birth in China: A Nationwide Cohort Study. Environ. Sci. Technol. 2020, 54, 14494–14501. 10.1021/acs.est.0c02373. [DOI] [PubMed] [Google Scholar]
- Guarnieri M.; Balmes J. R. Outdoor Air Pollution and Asthma. Lancet 2014, 383, 1581–1592. 10.1016/s0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A.; Mulgund A.; Hamada A.; Chyatte M. R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 37. 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A.; Baskaran S.; Parekh N.; Cho C.-L.; Henkel R.; Vij S.; Arafa M.; Panner Selvam M. K.; Shah R. Male Infertility. Lancet 2021, 397, 319–333. 10.1016/s0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- Wang C.; Swerdloff R. S. Limitations of Semen Analysis as a Test of Male Fertility and Anticipated Needs from Newer Tests. Fertil. Steril. 2014, 102, 1502–1507. 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen H. E.; Jørgensen N.; Toppari J. Semen Quality in the 21st Century. Nat. Rev. Urol. 2017, 14, 120–130. 10.1038/nrurol.2016.261. [DOI] [PubMed] [Google Scholar]
- Levine H.; Jørgensen N.; Martino-Andrade A.; Mendiola J.; Weksler-Derri D.; Mindlis I.; Pinotti R.; Swan S. H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P.; Nwagha U.; Dutta S.; Krajewska-Kulak E.; Izuka E. Evidence for Decreasing Sperm Count in African Population from 1965 to 2015. Afr. Health Sci. 2017, 17, 418. 10.4314/ahs.v17i2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P.; Borges E.; Dutta S.; Krajewska-Kulak E. Decline in Sperm Count in European Men during the Past 50 Years. Hum. Exp. Toxicol. 2018, 37, 247–255. 10.1177/0960327117703690. [DOI] [PubMed] [Google Scholar]
- Radwan M.; Jurewicz J.; Polańska K.; Sobala W.; Radwan P.; Bochenek M.; Hanke W. Exposure to Ambient Air Pollution-Does It Affect Semen Quality and the Level of Reproductive Hormones?. Ann. Hum. Biol. 2016, 43, 50–56. 10.3109/03014460.2015.1013986. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Zhu Q.; Lin J.; Cai J. Association of Exposure to Particulate Matter Air Pollution With Semen Quality Among Men in China. JAMA Netw. Open 2022, 5, e2148684 10.1001/jamanetworkopen.2021.48684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.; Chen Y.; Cheng Y.; Tang Q.; Pan F.; Tang N.; Sun Z.; Wang X.; London S. J.; Xia Y. Association between Ambient Particulate Matter Exposure and Semen Quality in Fertile Men. Environ. Health 2022, 21, 16. 10.1186/s12940-022-00831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X.; Chen G.; Zhang M.; Mei K.; Liu Y.; Ding C.; Chang Y.; Wu Z.; Huang H. Exposure to Ambient Particulate Matter Affects Semen Quality: A Case Study in Wenzhou, China. Andrology 2023, 11, 444–455. 10.1111/andr.13326. [DOI] [PubMed] [Google Scholar]
- Jin H.; Zhong R.; Liu M.; Ye C.; Chen X. Spatiotemporal Distribution Characteristics of PM2.5 Concentration in China from 2000 to 2018 and Its Impact on Population. J. Environ. Manage. 2022, 323, 116273. 10.1016/j.jenvman.2022.116273. [DOI] [PubMed] [Google Scholar]
- Xu W.; Wang Y.; Sun S.; Yao L.; Li T.; Fu X. Spatiotemporal Heterogeneity of PM2.5 and Its Driving Difference Comparison Associated with Urbanization in China’s Multiple Urban Agglomerations. Environ. Sci. Pollut. Res. 2022, 29, 29689–29703. 10.1007/s11356-021-17929-x. [DOI] [PubMed] [Google Scholar]
- Gu K.; Zhou Y.; Sun H.; Dong F.; Zhao L. Spatial Distribution and Determinants of PM2.5 in China’s Cities: Fresh Evidence from IDW and GWR. Environ. Monit. Assess. 2021, 193, 15. 10.1007/s10661-020-08749-6. [DOI] [PubMed] [Google Scholar]
- Lu D.; Xu J.; Yang D.; Zhao J. Spatio-Temporal Variation and Influence Factors of PM2.5 Concentrations in China from 1998 to 2014. Atmos. Pollut. Res. 2017, 8, 1151–1159. 10.1016/j.apr.2017.05.005. [DOI] [Google Scholar]
- Yang Y.; Ruan Z.; Wang X.; Yang Y.; Mason T. G.; Lin H.; Tian L. Short-Term and Long-Term Exposures to Fine Particulate Matter Constituents and Health: A Systematic Review and Meta-Analysis. Environ. Pollut. 2019, 247, 874–882. 10.1016/j.envpol.2018.12.060. [DOI] [PubMed] [Google Scholar]
- Huang X.; Zhang B.; Wu L.; Zhou Y.; Li Y.; Mao X.; Chen Y.; Wang J.; Luo P.; Ma J.; Zhang H.; Peng Z.; Cui X.; Xie S.; Huo X.; Zhang M.; Bao W.; Shi T.; Liu Y. Association of Exposure to Ambient Fine Particulate Matter Constituents With Semen Quality Among Men Attending a Fertility Center in China. Environ. Sci. Technol. 2019, 53, 5957–5965. 10.1021/acs.est.8b06942. [DOI] [PubMed] [Google Scholar]
- Wu H.; Yu X.; Wang Q.; Zeng Q.; Chen Y.; Lv J.; Wu Y.; Zhou H.; Zhang H.; Liu M.; Zheng M.; Zhao Q.; Guo P.; Feng W.; Zhang X.; Tian L. Beyond the Mean: Quantile Regression to Differentiate the Distributional Effects of Ambient PM2.5 Constituents on Sperm Quality among Men. Chemosphere 2021, 285, 131496. 10.1016/j.chemosphere.2021.131496. [DOI] [PubMed] [Google Scholar]
- Ping P.; Zhu W.-B.; Zhang X.-Z.; Li Y.-S.; Wang Q.-X.; Cao X.-R.; Liu Y.; Dai H.-L.; Huang Y.-R.; Li Z. Sperm Donation and Its Application in China: A 7-Year Multicenter Retrospective Study. Asian J. Androl. 2011, 13, 644–648. 10.1038/aja.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization, 2010. [Google Scholar]
- Wei J.; Li Z.; Lyapustin A.; Sun L.; Peng Y.; Xue W.; Su T.; Cribb M. Reconstructing 1-Km-Resolution High-Quality PM2.5 Data Records from 2000 to 2018 in China: Spatiotemporal Variations and Policy Implications. Remote Sens. Environ. 2021, 252, 112136. 10.1016/j.rse.2020.112136. [DOI] [Google Scholar]
- Wei J.; Li Z.; Chen X.; Li C.; Sun Y.; Wang J.; Lyapustin A.; Brasseur G. P.; Jiang M.; Sun L.; Wang T.; Jung C. H.; Qiu B.; Fang C.; Liu X.; Hao J.; Wang Y.; Zhan M.; Song X.; Liu Y. Separating Daily 1 Km PM 2.5 Inorganic Chemical Composition in China since 2000 via Deep Learning Integrating Ground, Satellite, and Model Data. Environ. Sci. Technol. 2023, acs.est.3c00272. 10.1021/acs.est.3c00272. [DOI] [PubMed] [Google Scholar]
- Balhara K.; Singh S.; Sharma S. Seasonal Variation in Semen Quality: Effect of Temperature: A Study in North India. Int. J. Reprod. Contracept. Obstet. Gynecol. 2022, 11, 547. 10.18203/2320-1770.ijrcog20220186. [DOI] [Google Scholar]
- Muñoz-Sabater J.; Dutra E.; Agustí-Panareda A.; Albergel C.; Arduini G.; Balsamo G.; Boussetta S.; Choulga M.; Harrigan S.; Hersbach H.; Martens B.; Miralles D. G.; Piles M.; Rodríguez-Fernández N. J.; Zsoter E.; Buontempo C.; Thépaut J.-N. ERA5-Land: A State-of-the-Art Global Reanalysis Dataset for Land Applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. 10.5194/essd-13-4349-2021. [DOI] [Google Scholar]
- Mistry M. N.; Schneider R.; Masselot P.; Royé D.; Armstrong B.; Kyselý J.; Orru H.; Sera F.; Tong S.; Lavigne É.; Urban A.; Madureira J.; García-León D.; Ibarreta D.; Ciscar J.-C.; Feyen L.; De Schrijver E.; De Sousa Zanotti Stagliorio Coelho M.; Pascal M.; Tobias A.; Alahmad B.; Abrutzky R.; Saldiva P. H. N.; Correa P. M.; Orteg N. V.; Kan H.; Osorio S.; et al. Comparison of Weather Station and Climate Reanalysis Data for Modelling Temperature-Related Mortality. Sci. Rep. 2022, 12, 5178. 10.1038/s41598-022-09049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban A.; Di Napoli C.; Cloke H. L.; Kyselý J.; Pappenberger F.; Sera F.; Schneider R.; Vicedo-Cabrera A. M.; Acquaotta F.; Ragettli M. S.; Íñiguez C.; Tobias A.; Indermitte E.; Orru H.; Jaakkola J. J. K.; Ryti N. R. I.; Pascal M.; Huber V.; Schneider A.; De’ Donato F.; Michelozzi P.; Gasparrini A. Evaluation of the ERA5 Reanalysis-Based Universal Thermal Climate Index on Mortality Data in Europe. Environ. Res. 2021, 198, 111227. 10.1016/j.envres.2021.111227. [DOI] [PubMed] [Google Scholar]
- Wu L.; Jin L.; Shi T.; Zhang B.; Zhou Y.; Zhou T.; Bao W.; Xiang H.; Zuo Y.; Li G.; Wang C.; Duan Y.; Peng Z.; Huang X.; Zhang H.; Xu T.; Li Y.; Pan X.; Xia Y.; Gong X.; Chen W.; Liu Y. Association between Ambient Particulate Matter Exposure and Semen Quality in Wuhan, China. Environ. Int. 2017, 98, 219–228. 10.1016/j.envint.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Huang G.; Zhang Q.; Wu H.; Wang Q.; Chen Y.; Guo P.; Zhao Q. Sperm Quality and Ambient Air Pollution Exposure: A Retrospective, Cohort Study in a Southern Province of China. Environ. Res. 2020, 188, 109756. 10.1016/j.envres.2020.109756. [DOI] [PubMed] [Google Scholar]
- Mostofsky E.; Schwartz J.; Coull B. A.; Koutrakis P.; Wellenius G. A.; Suh H. H.; Gold D. R.; Mittleman M. A. Modeling the Association Between Particle Constituents of Air Pollution and Health Outcomes. Am. J. Epidemiol. 2012, 176, 317–326. 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsley D. A.; Kuh E.; Welsch R. E.. Regression Diagnostics: Identifying Influential Data and Sources of Collinearit; Wiley, 1980. [Google Scholar]
- Hammoud A.; Carrell D. T.; Gibson M.; Sanderson M.; Parker-Jones K.; Peterson C. M. Decreased Sperm Motility Is Associated with Air Pollution in Salt Lake City. Fertil. Steril. 2010, 93, 1875–1879. 10.1016/j.fertnstert.2008.12.089. [DOI] [PubMed] [Google Scholar]
- Liu C.; Cai J.; Qiao L.; Wang H.; Xu W.; Li H.; Zhao Z.; Chen R.; Kan H. The Acute Effects of Fine Particulate Matter Constituents on Blood Inflammation and Coagulation. Environ. Sci. Technol. 2017, 51, 8128–8137. 10.1021/acs.est.7b00312. [DOI] [PubMed] [Google Scholar]
- Zhou P.; Mo S.; Peng M.; Yang Z.; Wang F.; Hu K.; Zhang Y. Long-Term Exposure to PM2.5 Constituents in Relation to Glucose Levels and Diabetes in Middle-Aged and Older Chinese. Ecotoxicol. Environ. Saf. 2022, 245, 114096. 10.1016/j.ecoenv.2022.114096. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Chen R.; Xia Y.; Cai J.; Ying Z.; Lin Z.; Liu C.; Chen C.; Peng L.; Zhao Z.; Zhou W.; Chen J.; Wang D.; Huo J.; Wang X.; Fu Q.; Kan H. Fine Particulate Matter Constituents and Stress Hormones in the Hypothalamus–Pituitary–Adrenal Axis. Environ. Int. 2018, 119, 186–192. 10.1016/j.envint.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Xu F.; Shi X.; Qiu X.; Jiang X.; Fang Y.; Wang J.; Hu D.; Zhu T. Investigation of the Chemical Components of Ambient Fine Particulate Matter (PM2.5) Associated with in Vitro Cellular Responses to Oxidative Stress and Inflammation. Environ. Int. 2020, 136, 105475. 10.1016/j.envint.2020.105475. [DOI] [PubMed] [Google Scholar]
- Guan L.; Geng X.; Stone C.; Cosky E. E. P.; Ji Y.; Du H.; Zhang K.; Sun Q.; Ding Y. PM 2.5 Exposure Induces Systemic Inflammation and Oxidative Stress in an Intracranial Atherosclerosis Rat Model. Environ. Toxicol. 2019, 34, 530–538. 10.1002/tox.22707. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Cao X.-N.; Tang X.-L.; Shen L.-J.; Lin T.; He D.-W.; Wu S.-D.; Wei G.-H. Urban Fine Particulate Matter (PM2.5) Exposure Destroys Blood–Testis Barrier (BTB) Integrity through Excessive ROS-Mediated Autophagy. Toxicol. Mech. Methods 2018, 28, 302–319. 10.1080/15376516.2017.1410743. [DOI] [PubMed] [Google Scholar]
- Takeshima T.; Yumura Y.; Yasuda K.; Sanjo H.; Kuroda S.; Yamanaka H.; Iwasaki A. Inverse Correlation between Reactive Oxygen Species in Unwashed Semen and Sperm Motion Parameters as Measured by a Computer-Assisted Semen Analyzer. Asian J. Androl. 2017, 19, 350. 10.4103/1008-682x.173933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie H. D.; Welch G. R. Effects of Reactive Oxygen Species on Sperm Function. Theriogenology 2012, 78, 1700–1708. 10.1016/j.theriogenology.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Sanocka D.; Jędrzejczak P.; Szumała-Kaękol A.; Frączek M.; Kurpisz M. Male Genital Tract Inflammation: The Role of Selected Interleukins in Regulation of Pro-Oxidant and Antioxidant Enzymatic Substances in Seminal Plasma. J. Androl. 2003, 24, 448–455. 10.1002/j.1939-4640.2003.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Koçak I.; Yenisey Ç.; Dündar M.; Okyay P.; Serter M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor α levels with semen parameters in fertile and infertile men. Urol. Res. 2002, 30, 263–267. 10.1007/s00240-002-0269-y. [DOI] [PubMed] [Google Scholar]
- Qiu L.; Chen M.; Wang X.; Qin X.; Chen S.; Qian Y.; Liu Z.; Cao Q.; Ying Z. Exposure to Concentrated Ambient PM2.5 Compromises Spermatogenesis in a Mouse Model: Role of Suppression of Hypothalamus-Pituitary-Gonads Axis. Toxicol. Sci. 2018, 162, 318–326. 10.1093/toxsci/kfx261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M.-Q.; Ge P.; Zhang J.; Yang Y.-Q.; Zhou L.; Zhou D.-X. Temporal Trends in Semen Concentration and Count among 327 373 Chinese Healthy Men from 1981 to 2019: A Systematic Review. Hum. Reprod. 2021, 36, 1751–1775. 10.1093/humrep/deab124. [DOI] [PubMed] [Google Scholar]
- Chen Z. J.; Wang L.; Zhang L.; Song X. H.; Zhang H. B.; Xu C. Y. Decline of Semen Quality among Chinese Sperm Bank Donors within 7 Years (2008-2014). Asian J. Androl. 2017, 19, 521. 10.4103/1008-682x.179533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainberg J.; Kashanian J. A. Recent Advances in Understanding and Managing Male Infertility. F1000Research 2019, 8, 670. 10.12688/f1000research.17076.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q.; Chen S.; Wang B.; Dou X.; Lu Y.; Liang J.; Ni R.; Yang C.; Wang H.; Baktash M. B.; Wu W.; Wang X.; Fu G.; Xia Y. Effects of Particulate Matter Exposure on Semen Quality: A Retrospective Cohort Study. Ecotoxicol. Environ. Saf. 2020, 193, 110319. 10.1016/j.ecoenv.2020.110319. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Zhang J.; Cai G.; Xia Q.; Xu S.; Hu C.; Cao Y.; Pan F. Inverse Association between Ambient Particulate Matter and Semen Quality in Central China: Evidence from a Prospective Cohort Study of 15,112 Participants. Sci. Total Environ. 2022, 833, 155252. 10.1016/j.scitotenv.2022.155252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.