ABSTRACT
Gout arthritis is an inflammatory condition that occurs suddenly in joints affected by high uric acid levels (hyperuricemia). The uric acid levels in this disease fluctuate throughout its various phases, resulting in frequent or recurrent attacks. This study aims to review some aspects of gout arthritis, such as its pathophysiology, treatment goals, and adverse drug reactions. This study employs review literature using articles published between 2017 and 2022 as the research methodology. Furthermore, articles under 2017 are used as references if they are relevant to the study’s subject matter. The findings showed the importance of the pathogenesis of inflammation in the treatment of gout arthritis. It is also recommended to use anti-inflammatories such as colchicine and uric acid-lowering medications starting at a specific time to prevent unintended risks. Hence, pharmacotherapy management’s adverse effects include nausea, vomiting, myalgia, neuropathy, and stomach pain.
Keywords: Colchicine, gout arthritis, inflammatory, pathophysiology, pharmacology
INTRODUCTION
Gouty arthritis is a significant case commonly associated with inflammatory arthritis and occurs worldwide. Gout occurs when there is an increase in serum urate levels of more than 7 mg/dL or 420 μmol/L, which contributes to the formation of deposits of monosodium urate (MSU) crystals. This disease is distinguished by acute episodes of joint inflammation, which frequently affect a single joint and are preceded by symptom-free periods of varying duration.[1,2]
The prevalence of gout in the population varies widely, with ethnic and regional differences worldwide. The prevalence of gout arthritis is highest in the Oceania region, where the baseline uric acid in the population is high. It is commonly found in men than women, as in the study populations in Europe and North America find out that the male and female sexes have a ratio of 2:1 and 4:1 of the incidence of gout arthritis.[3] This occurs due to the response of pro-and anti-inflammatory cytokines in the inflammatory process in gout arthritis. Interleukin (IL)-1 β is an important cytokine that acts as a pro-inflammatory cytokine, which in its production and activation process involves three main phases: those are the pro-IL-1 β precursor production; the precursor maturation; and the maturation of IL-1 β before going to the extracellular space. The maturation process is a discrete process that needs the activation of a protease, caspase-1, through a member of the NOD-like receptor. Based on available research evidence, MSU crystals activate NALP3 or NLRP3 inflammasomes. Under conditions where the NLRP3 inflammasome has few macrophages, it cannot secrete active IL-1 β after MSU crystal stimulation.[1,2]
Gout risk factors include genetics, metabolism, and other comorbidities such as metabolic syndrome and heart and kidney disease. The health-care burden worldwide is increasing globally, with prevalence varying depending on the study population in the different countries and the assessment method, which ranges from <1% to 8%. The incidence is around 0,58–2,89/1000 persons per year.[4] The diagnosis established in the primary clinical setting is achieved through a scoring system, in which the score is composed of sex, then a previous gout attack, the day before onset, joint flushing, Metatarsophalangeal joint involvement, hypertension, or cardiac disease, as well as high serum levels tendon. In conditions where joint aspirations are doubtful or if joint aspiration is not found in a clinical setting, an ultrasound or computed tomography scan can be used.[3]
Several previous studies show global trends of gouty arthritis in decades using bibliometric analysis. The result revealed that the number of publications on gout arthritis fluctuated slightly, and overall, the number of publication (Np) increased.[5] Besides, Henderson found that potential medications to maintain and boost lubricin may reduce the occurrence and progression of gouty arthritis. A new biomarker called lubricin may be used to monitor a patient’s chance of developing gout.[6] Hongzhi and friends also studied treatment for acute flares of gout. However, they just analyzed several treatments using meta-analysis, which can help practitioners to gain more knowledge.[7] The researchers are interested in analyzing gout inflammation and its therapies based on those studies. It depends on the therapy management since it involves anti-inflammation, such as nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, or steroids. In addition, based on the inflammatory pathway involved in the acute process of gout, uric acid-lowering drugs need to be supplemented with anti-inflammatory drugs. Available drugs also vary depending on the therapeutic target of the molecules involved in the inflammatory pathway. However, further research is needed regarding drugs with minimal side effects in gout arthritis patients. Therefore, this study was conducted to determine pharmacological therapy in gout arthritis and its side effects.
MATERIALS AND METHODS
The method used in this article is a literature review which is a systematic method that can be used for the synthesis of works. The literature used is research work and ideas produced by researchers and practitioners which obtained through national and international journals. The author uses the keywords: gout arthritis, inflammatory pathway in gout arthritis, management in gout arthritis, colchicine, urate-lowering therapy (ULT), side effects, and adverse events of gout arthritis and uses the Boolean operator (AND and NOT). This literature review employs literature released between 2017 and 2022 that is available in complete text and scholarly (peer-reviewed journals). Even so, several articles under 2017 are still taken as references if the content and topic of the article are considered essential, and no other articles review it within the specified timeframe. The selected books and journal articles are in Indonesian and English. In addition, the types of article references can be original articles, meta-analyses, systematic reviews, and literature reviews, with keywords and topics that match the theme and title of the literature review.
DISCUSSION
Pathophysiology
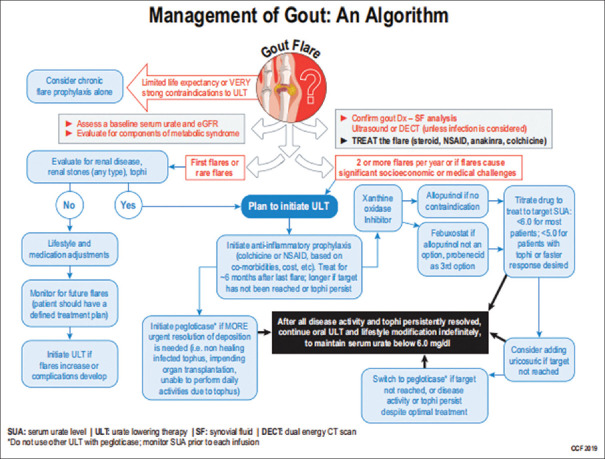
Based on the evidence of the involvement of inflammatory mechanisms in developing gout arthritis, anti-inflammatory drugs are the main treatment for acute gout attacks. Intra-articular steroids are more frequently used in patients with monoarticular or single-joint gout, especially if the patient cannot take oral therapy. Oral colchicine is the next option for patients with contraindications or who are intolerant of NSAIDs or oral systemic corticosteroids as the main treatment. When the acute attack has gradually improved, the use of colchicine is more suitable in addition to NSAIDs. Low-dose colchicine prevents recurrent attacks compared to other therapies, such as NSAIDs.[8,9] As an illustration, the following is a gout management chart [Figure 1]: The main hope in treating cases of gout arthritis is to reduce acute gout attacks ad save health costs, so it is essential to maintain uric acid levels on target using allopurinol at a dose of 100–800 mg/day or febuxostat 40–80 mg/day or one of the drugs combined with uricosuric to achieve therapeutic targets. The dose of allopurinol needs to be adjusted if there is renal failure.[11] Gout is mainly treated with anti-inflammatory drugs and lowering serum uric acid levels, such as allopurinol, benzbromarone, febuxostat, probenecid, and sulfinpyrazone. Some patients have no tolerance for continuous uric acid-lowering therapy, so patient compliance is reduced in the long term resulting in a gradual worsening of the response to therapy.[4] The essence of inflammation in gout arthritis is the activation of cytokines against gout-containing MSU crystals. The danger signal leading to the initiation of these crystals is the primary endogenous activator implicated in converting inflammatory cytokine precursors to their active form. Toll-like receptors require interactive signals and are receptors on the cell surface as they mediate the first signals of the assembly and activation phases which then provide app-regulated expression of inflammatory components. The therapeutic approach to gout is based on treating acute attacks to controlling hyperinflammation and preventing recurrent attacks using xanthine oxidase inhibitor (XOI)-lowering therapy, for example, allopurinol or febuxostat and uricosuric.[12]
Figure 1.
Algorithm for management of gout arthritis[10]
Anti-inflammation (colchicine)
Pharmacological treatment of anti-inflammatory administration should be started as soon as possible, preferably within 12–24 h of the onset of an acute attack of gout. The first treatment can use NSAIDs, glucocorticoids, and colchicine. Therapy resulting in hyperuricemia, such as diuretics and low doses of acetylcysteine, should not be started in patients with acute gout attacks. Long-term therapy should be started at least 2 weeks after the first acute gout attack and it is suggested for patients with these symptoms; there are two or more attacks of gout per year, there is both urolithiasis and gout, there is overproduction of uric acid at the time of chemotherapy, and preexisting gout.[13]
Colchicine is an alkaloid derivative derived from Crocus–Colchicum autumnale, which has been used to treat inflammatory diseases for more than 2000 years. Initially, colchicine was used to block microtubule arrangement in neutrophils and other inflammatory cells, an effect that could minimize phagocytosis and transport MSU crystals to lysosomes. Therefore, colchicine also alters the expression of cell surface proteins, including regulating tumor necrosis factor-alpha (TNF-α) receptors, insulin, and beta-adrenergic antagonists. Colchicine also inhibits the release of chemotactic factors, including peptide and lipid derivatives such as leukotriene B4 which originates from neutrophils. This also occurs in the process of acute gout arthritis and requires a reasonably high concentration of colchicine.[8,9]
In treating acute gout arthritis, about 0. 6 mg oral dose of colchicine is usually given, followed by 0. 6 mg at 1-h intervals until experience the effects such as nausea, vomiting, and diarrhea last up to a maximum of 6–8 h from the initiation of therapy. Patients only need about 3–4 doses of 6 mg of oral colchicine. For substantial improvement, limited doses of oral colchicine, especially when started in the first 24 h of an acute gout attack, are very beneficial because they reduce the frequency of gastrointestinal side effects, which can sometimes be severe. Colchicine probably has a very narrow therapeutic window compared to other drugs used to treat acute attacks of gouty arthritis.[11] Compared to oral colchicine, colchicine intravenous injection has fewer gastrointestinal side effects, but the most common and serious side effect is spinal cord suppression. Therefore, agents that have less toxic effects than colchicine are available but are rarely used in patients with acute gout attacks.[11]
Nowadays, colchicine is used as prophylaxis therapy for acute gout attacks. Colchicine is also a therapy for diseases caused by the deposition of MSU crystals due to its broad-spectrum anti-inflammatory properties. The mechanism itself is by binding to free tubulin dimers which then go to microtubules. After the microtubules, colchicine will bend and polymerize microtubules. Suppression of the release of leukotriene B4 chemotactic agents alters the deformability of the neutrophils.[14]
Overall, colchicine is well tolerated, although it has a narrow therapeutic window, and has some side effects such as gastrointestinal disturbances (diarrhea, nausea, or vomiting), which may occur in >20% of patients taking colchicine use, especially in patients with inappropriate dose adjustments, patients with renal disease. Symptoms from colchicine toxicity usually last 1 week to several months after withdrawal. Optimally, colchicine should be administered to patients as soon as possible during an acute attack.[14]
Urate-lowering therapy
ULT is conditional for patients with gout who are having their first attack. However, initiating ULT is recommended for patients with comorbidities such as chronic kidney disease (≥3), whose serum urate concentrations are >9 mg/dL, or who have urolithiasis. Initiating ULT therapy is also conditionally recommended in asymptomatic hyperuricemia patients. However, according to the guidelines, ULT should be initiated earlier to avoid the formation of crystallization if the uric acid condition does not improve. However, there is no strong evidence because allopurinol has several side effects and can also increase the incidence of acute attacks, especially in the early days of therapy. Hence, several journals suggest that the patient lowers uric acid levels in the initial phase through lifestyle changes. The increased frequency of acute attacks in the early phase of initiation of ULT may occur in the early weeks to 1st months when serum uric acid is reduced and uric acid deposits are mobilized out of the tissues.[13]
In patients who initiate ULT, there are three options for recommended therapy’s duration: 6 months, 3 months after reaching the target in patients with resolved tophus, and confirmed by physical examination [Figure 2]. EULAR guidelines are more straightforward in recommending colchicine as the first prophylaxis or ULT with a suggested dose of 0,5 – 0,6 mg/day.[14,15]
Figure 2.
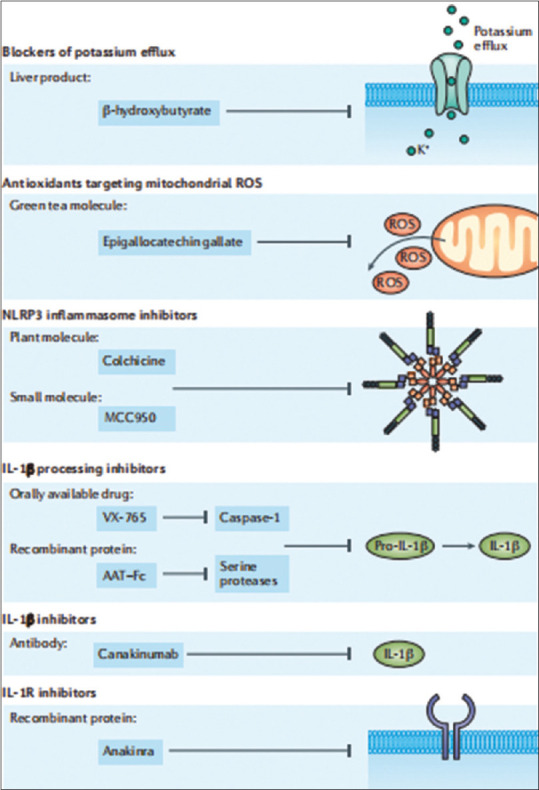
Therapeutic targets in inflammatory gout arthritis are related to the inflammatory pathways involved as therapeutic targets.[1] IL: Interleukin, ROS: Reactive oxygen species, AAT-Fc: Alpha-1-anti-trypsin-Fc fusion protein
Side effects
The most common side effects are gastrointestinal such as diarrhea, nausea, vomiting, abdominal pain, decreased appetite, upset stomach, constipation, melena, and peptic ulcers. The process by which colchicine affects diarrhea and gastrointestinal is not well known; It is thought that this is influenced by the level of intestinal prostaglandin synthesis and the increase in gastrointestinal motility due to the drug. Other studies also prove that colchicine can cause liver enzyme disorders, hepatitis, and hepatotoxicity. Side effects on the muscles include myalgia, muscle cramps, increased creatinine phosphokinase levels, and muscle weakness. In small amounts, anemia, bone marrow toxicity, leukopenia, and purpura have also been reported.[16]
Based on existing studies, the use of colchicine in pregnant and lactating women has a teratogenic effect that results in defects in the fetus in animal studies and inhibits the differentiation of human trophoblasts in vitro conditions. Colchicine has been detected in the breast milk of nursing mothers, but there are no known adverse effects in breastfed children. Clinical help is needed side effects or symptoms of toxicity are appeared, such as redness of the skin, itching, and swelling, especially on the face, tongue, and throat, as well as severe dizziness and shortness of breath.[17]
Colchicine should not be used again if the patient has a history of allergic reactions. Colchicine can result in a false positive on a urine test to be mistaken for red blood cells of hemoglobin. Grapefruit is a CYP3A4 cytochrome inhibitor, so consumption of this fruit juice increases colchicine levels and the risk of toxicity. Burnt food and cigarettes that use polycyclic aromatic hydrocarbons trigger cytochrome P1A2. Alcohol can increase the risk of toxicity from colchicine as much as three-fold through two mechanisms, first through a negative effect on the hepatic function where there is the metabolism of colchicine, and second by increasing the concentration of uric acid so it increases the risk of gout acute attacks.[17]
Colchicine should be controlled in the elderly; rare but dangerous toxicity may be encountered, and some of the signs are muscle weakness and pain, tingling, numbness in the toes or hands, abnormal bleeding or bruising, severe diarrhea, vomiting, weakness, fatigue, recurrent infections, pallor of the lips, tongue, palms, abdominal pain, alopecia or baldness, myopathy, peripheral neuritis, and vomiting. At the same time, other drugs taken with colchicine should be considered.[16]
Meanwhile, sudden ULT therapy may increase the risk for patients with gout who are being treated. However, abrupt discontinuation is unavoidable in specific contexts where multidrug use is contraindicated in severe liver disease, and cutaneous drug reactions can occur, especially in patients receiving allopurinol. If the patient is intolerant to allopurinol, there is a second line of XOI, which is more potent, namely, febuxostat with the required dose being between 80 and 120 mg/day.[13]
The occurrence of infection concomitant with attacks of gout arthritis is a contraindication to using IL-1 inhibitors. ULT is the highest priority in these circumstances. Sudden discontinuation of ongoing use of ULT at the time of a gout attack is not recommended since it may result in sudden changes of serum urate levels or deposition of urate crystals and gout attacks. A critical condition where the patient has been exposed to various triggers of gout attacks such as kidney disease, acute illness, and diuretics.[18]
CONCLUSION
This study aims to identify gout arthritis pharmacological therapy and its side effects. The results show that gout arthritis has gone through several phases with unstable uric acid levels, triggering repeated, or recurrent attacks. The inflammatory process continues chronically and changes the structures around the joints, forming tophi, tissue deformities, and other anatomical structures. The role of inflammatory pathways related to the pathophysiology of gout arthritis plays an important role in the target of therapy for this disease. Many of these cytokines respond specifically to acute gout attacks. Cytokines that play an essential role in inflammatory gout arthritis are IL-1 β, TNF-α, nuclear factor kappa B, and inflammasome NLRP3 which require activation signals from previously circulating cytokines. When an attack of gout arthritis occurs, it is recommended to use anti-inflammatory and uric acid-lowering drugs initiated at intervals to avoid unwanted risks to the patient due to a sudden decrease in serum urate levels. The anti-inflammatory that is generally used is colchicine, although other anti-inflammatories, such as NSAIDs and corticosteroids, can also be given. Pharmacotherapeutic management of gout arthritis patients with acute or established attacks has several side effects including stomach discomfort, nausea, vomiting, myalgia, and neuropathy. Therefore, further evaluation and development regarding new therapies with minimal side effects are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment/Fundin
This publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW009672. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.So AK, Martinon F. Inflammation in gout:Mechanisms and therapeutic targets. Nat Rev Rheumatol. 2017;13:639–47. doi: 10.1038/nrrheum.2017.155. [DOI] [PubMed] [Google Scholar]
- 2.Martinon F, Glimcher LH. Gout:New insights into an old disease. J Clin Invest. 2006;116:2073–5. doi: 10.1172/JCI29404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039–52. doi: 10.1016/S0140-6736(16)00346-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Wei K, Jiang P, Chang C, Xu L, Xu L, et al. Inflammatory response to regulated cell death in gout and its functional implications. Front Immunol. 2022;13:888306. doi: 10.3389/fimmu.2022.888306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng P, Wang S, Sun X, Qi Y, Ma Z, Pan X, et al. Global trends in research of gouty arthritis over past decade:A bibliometric analysis. Front Immunol. 2022;13:910400. doi: 10.3389/fimmu.2022.910400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsaid K, Merriman TR, Rossitto LA, Liu-Bryan R, Karsh J, Phipps-Green A, et al. Amplification of inflammation by lubricin deficiency implicated in incident, erosive gout independent of hyperuricemia. Arthritis Rheumatol. 2022;75:794–805. doi: 10.1002/art.42413. [doi:10.1002/art. 42413] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang H, Xu G, Zheng Q, Cheng Y, Zheng H, Li J, et al. Treatment for acute flares of gout:A protocol for systematic review. Medicine (Baltimore) 2020;99:e19668. doi: 10.1097/MD.0000000000019668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther. 2010;12:206. doi: 10.1186/ar2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(Suppl 1):S3. doi: 10.1186/ar1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillinger MH, Mandell BF. Therapeutic approaches in the treatment of gout. Semin Arthritis Rheum. 2020;50:S24–30. doi: 10.1016/j.semarthrit.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Singh JA. Gout:Will the “king of diseases”be the first rheumatic disease to be cured? BMC Med. 2016;14:180. doi: 10.1186/s12916-016-0732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galozzi P, Bindoli S, Doria A, Oliviero F, Sfriso P. Autoinflammatory features in gouty arthritis. J Clin Med. 2021;10:1880. doi: 10.3390/jcm10091880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel B, Just J, Bleckwenn M, Weckbecker K. Treatment options for gout. Dtsch Arztebl Int. 2017;114:215–22. doi: 10.3238/arztebl.2017.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on colchicine, Rheumatology (Oxford) 2017;2018;57:i4–11. doi: 10.1093/rheumatology/kex453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perhimpunan Reumatologi Indonesia. Pedoman Diagnosis dan Pengelolaan Gout. Jakarta: Perhimpunan Reumatologi Indonesia; 2018. [Google Scholar]
- 16.Stewart S, Yang KC, Atkins K, Dalbeth N, Robinson PC. Adverse events during oral colchicine use:A systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020;22:28. doi: 10.1186/s13075-020-2120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocco G, Jerie P. Colchicine:Efficacy and safety issues. J Symptoms Signs. 2014;3:179–87. [Google Scholar]
- 18.Satpanich P, Jatuworapruk K. Gout flare in the critical care setting:Diagnostic challenges and treatment options. Signa Vitae. 2021;18:9–17. [Google Scholar]