Abstract
Background
Preterm birth interferes with brain maturation, and subsequent clinical events and interventions may have additional deleterious effects. Music as therapy is offered increasingly in neonatal intensive care units aiming to improve health outcomes and quality of life for both preterm infants and the well‐being of their parents. Systematic reviews of mixed methodological quality have demonstrated ambiguous results for the efficacy of various types of auditory stimulation of preterm infants. A more comprehensive and rigorous systematic review is needed to address controversies arising from apparently conflicting studies and reviews.
Objectives
We assessed the overall efficacy of music and vocal interventions for physiological and neurodevelopmental outcomes in preterm infants (< 37 weeks' gestation) compared to standard care. In addition, we aimed to determine specific effects of various interventions for physiological, anthropometric, social‐emotional, neurodevelopmental short‐ and long‐term outcomes in the infants, parental well‐being, and bonding.
Search methods
We searched Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, PsycINFO, Web of Science, RILM Abstracts, and ERIC in November 2021; and Proquest Dissertations in February 2019. We searched the reference lists of related systematic reviews, and of studies selected for inclusion and clinical trial registries.
Selection criteria
We included parallel, and cluster‐randomised controlled trials with preterm infants < 37 weeks` gestation during hospitalisation, and parents when they were involved in the intervention. Interventions were any music or vocal stimulation provided live or via a recording by a music therapist, a parent, or a healthcare professional compared to standard care. The intervention duration was greater than five minutes and needed to occur more than three times.
Data collection and analysis
Three review authors independently extracted data. We analysed the treatment effects of the individual trials using RevMan Web using a fixed‐effects model to combine the data. Where possible, we presented results in meta‐analyses using mean differences with 95% CI. We performed heterogeneity tests. When the I2 statistic was higher than 50%, we assessed the source of the heterogeneity by sensitivity and subgroup analyses. We used GRADE to assess the certainty of the evidence.
Main results
We included 25 trials recruiting 1532 infants and 691 parents (21 parallel‐group RCTs, four cross‐over RCTs). The infants gestational age at birth varied from 23 to 36 weeks, taking place in NICUs (level 1 to 3) around the world. Within the trials, the intervention varied widely in type, delivery, frequency, and duration. Music and voice were mainly characterised by calm, soft, musical parameters in lullaby style, often integrating the sung mother's voice live or recorded, defined as music therapy or music medicine. The general risk of bias in the included studies varied from low to high risk of bias.
Music and vocal interventions compared to standard care
Music/vocal interventions do not increase oxygen saturation in the infants during the intervention (mean difference (MD) 0.13, 95% CI ‐0.33 to 0.59; P = 0.59; 958 infants, 10 studies; high‐certainty evidence). Music and voice probably do not increase oxygen saturation post‐intervention either (MD 0.63, 95% CI ‐0.01 to 1.26; P = 0.05; 800 infants, 7 studies; moderate‐certainty evidence). The intervention may not increase infant development (Bayley Scales of Infant and Toddler Development (BSID)) with the cognitive composition score (MD 0.35, 95% CI ‐4.85 to 5.55; P = 0.90; 69 infants, 2 studies; low‐certainty evidence); the motor composition score (MD ‐0.17, 95% CI ‐5.45 to 5.11; P = 0.95; 69 infants, 2 studies; low‐certainty evidence); and the language composition score (MD 0.38, 95% CI ‐5.45 to 6.21; P = 0.90; 69 infants, 2 studies; low‐certainty evidence). Music therapy may not reduce parental state‐trait anxiety (MD ‐1.12, 95% CI ‐3.20 to 0.96; P = 0.29; 97 parents, 4 studies; low‐certainty evidence).
The intervention probably does not reduce respiratory rate during the intervention (MD 0.42, 95% CI ‐1.05 to 1.90; P = 0.57; 750 infants; 7 studies; moderate‐certainty evidence) and post‐intervention (MD 0.51, 95% CI ‐1.57 to 2.58; P = 0.63; 636 infants, 5 studies; moderate‐certainty evidence). However, music/vocal interventions probably reduce heart rates in preterm infants during the intervention (MD ‐1.38, 95% CI ‐2.63 to ‐0.12; P = 0.03; 1014 infants; 11 studies; moderate‐certainty evidence). This beneficial effect was even stronger after the intervention. Music/vocal interventions reduce heart rate post‐intervention (MD ‐3.80, 95% CI ‐5.05 to ‐2.55; P < 0.00001; 903 infants, 9 studies; high‐certainty evidence) with wide CIs ranging from medium to large beneficial effects. Music therapy may not reduce postnatal depression (MD 0.50, 95% CI ‐1.80 to 2.81; P = 0.67; 67 participants; 2 studies; low‐certainty evidence). The evidence is very uncertain about the effect of music therapy on parental state anxiety (MD ‐0.15, 95% CI ‐2.72 to 2.41; P = 0.91; 87 parents, 3 studies; very low‐certainty evidence). We are uncertain about any further effects regarding all other secondary short‐ and long‐term outcomes on the infants, parental well‐being, and bonding/attachment. Two studies evaluated adverse effects as an explicit outcome of interest and reported no adverse effects from music and voice.
Authors' conclusions
Music/vocal interventions do not increase oxygen saturation during and probably not after the intervention compared to standard care. The evidence suggests that music and voice do not increase infant development (BSID) or reduce parental state‐trait anxiety. The intervention probably does not reduce respiratory rate in preterm infants. However, music/vocal interventions probably reduce heart rates in preterm infants during the intervention, and this beneficial effect is even stronger after the intervention, demonstrating that music/vocal interventions reduce heart rates in preterm infants post‐intervention. We found no reports of adverse effects from music and voice. Due to low‐certainty evidence for all other outcomes, we could not draw any further conclusions regarding overall efficacy nor the possible impact of different intervention types, frequencies, or durations. Further research with more power, fewer risks of bias, and more sensitive and clinically relevant outcomes are needed.
Keywords: Child; Female; Humans; Infant; Infant, Newborn; Drug-Related Side Effects and Adverse Reactions; Gestational Age; Infant, Premature; Music; Premature Birth; Quality of Life
Plain language summary
Can music and vocal interventions benefit preterm infants and their parents?
Key messages
• Music and vocal interventions probably reduce heart rates in preterm infants compared to standard care during the intervention. This beneficial effect was even more substantial and confident after the intervention suggesting a long‐lasting relaxing and stabilising effect.
• We found no harmful effects from music and voice. However, many studies did not explicitly explore the possibility of unwanted effects.
• We found no evidence of any other clear beneficial or harmful effects of the interventions on the infants, their parents, and parent‐infant bonding. More good‐quality evidence is needed to draw further clear conclusions.
What is a preterm infant?
Preterm infants are newborns born before the gestational age of 37 weeks and often have to be treated for weeks to months in the stressful environment of a neonatal intensive care unit to survive.
Why examine the potential benefits of music and vocal interventions for preterm infants and their parents?
Preterm infants are at risk for various health issues. Preterm birth is a traumatic event for the parents as well. Therefore, complementary approaches such as music and vocal interventions are increasingly used in neonatal care to improve physical and mental health in preterm infants and their parents. However, various studies and reviews show ambiguous results in the efficacy of a variety of music and vocal interventions. A more comprehensive and rigorous systematic review is needed to address conflicting data and reviews.
What did we want to find out?
We wanted to find out if music and vocal interventions benefit:
• the health and development of the preterm infant
• the mental health of the parents and their bonding with the infant
We wanted to know which types, delivery, duration, and frequency of music and vocal interventions would best support infants and parents. We aimed to find out if the intervention can cause any harmful effects.
What did we do?
We searched for studies that compared:
• music and vocal interventions for preterm infants (and parents) compared to usual standard care in the neonatal unit that did not include any music or vocal interventions.
We compared and summarised their results and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 25 studies that involved 1532 preterm infants and 691 parents. The biggest study was in 272, and the smallest was in 17 preterm infants. Within the studies from around the world, mainly the immediate effect of music and voice was examined in the moments of intervention and minutes post‐intervention, whereas two studies wanted to know if there would be a beneficial effect on long‐term development at two years. Most studies were funded by University/Health Department/Hospital research funds and local medical/health foundations. The reported music and vocal interventions varied widely in type, delivery, frequency, and duration. They were mainly characterised by calm, soft, musical parameters in lullaby style, often integrating the mother's voice live or recorded, defined as music therapy when provided by a music therapist within a therapeutic relationship or music medicine when delivered as "medicine" by medical and healthcare professionals.
Main results
In preterm infants (and their parents), compared to standard care without any music and vocal interventions:
• Music and voice make no difference to the oxygen saturation during the intervention (10 studies with 958 infants) and may make no difference after the intervention (7 studies with 800 infants).
• Music and voice may make no difference in the respiratory rate during the intervention (7 studies with 750 infants) and after the intervention (5 studies with 636 infants).
• Music and voice may lead to a beneficial reduction in infants' heart rates (11 studies with 1014 infants). This beneficial effect was even more substantial and confident after the intervention, leading to a medium‐to‐large beneficial reduction in the heart rate (5 studies with 636 infants).
• We are uncertain if the intervention may influence infant long‐term development at two years of age (2 studies with 69 infants).
• We are uncertain about the possible effect of music therapy on parental state‐trait anxiety (4 studies with 97 participants) and postnatal depression (2 studies with 67 infants).
• We are very uncertain about a possible effect on parental state anxiety (3 studies with 87 parents).
• We found no studies which reported harmful effects of music or voice.
What are the limitations of the evidence?
We are confident that music and voice do not reduce oxygen saturation during the intervention compared to standard care. We are confident in our results of the substantial beneficial effect on the heart rate in preterm infants after the intervention. There are not enough rigorous studies (many small studies with poor recording standards) to be certain about the results of all other outcomes that we assessed in the infants and their parents. There is further uncertainty about music delivery and for which duration and frequency music works best.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to 12 November 2021.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Music and vocal interventions for preterm infants and their parents.
| Music and vocal interventions for preterm infants and their parents | ||||||
| Patient or population: preterm infants and their parents Setting: NICUs level 1‐3 in Europe, Middle East, USA, Asia, Australia, South America Intervention: music/vocal interventions Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with music/vocal interventions | |||||
| OXYGEN SATURATION DURING intervention (higher = favourable) | The mean OXYGEN SATURATION DURING intervention (higher = favourable) ranged from 92.76 to 98.2 | MD 0.13 higher (0.33 lower to 0.59 higher) | ‐ | 958 (10 RCTs) | ⊕⊕⊕⊕ Higha | Music/vocal interventions do not increase oxygen saturation during intervention.a |
| OXYGEN SATURATION POST‐intervention (higher = favourable) assessed with: up to follow‐up: 30 minutes | The mean OXYGEN SATURATION POST‐intervention (higher = favourable) ranged from 92.33 to 96.54 | MD 0.63 higher (0.01 lower to 1.26 higher) | ‐ | 800 (7 RCTs) | ⊕⊕⊕⊝ Moderateb | Music/vocal interventions probably do not increase oxygen saturation post‐intervention.b |
| INFANT DEVELOPMENT: Bayley Scales of Infant and Toddler Development‐III (BSID‐III) assessed with: COGNITIVE composition score Scale from: 0 to 200 (higher = favourable) | The mean INFANT DEVELOPMENT: Bayley Scales of Infant and Toddler Development‐III ranged from 98.6 to 102.8 | MD 0.35 higher (4.85 lower to 5.55 higher) | ‐ | 69 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | Music/ vocal interventions may not increase infant development.b,c |
| INFANT DEVELOPMENT: Bayley Scales of Infant and Toddler Development‐III (BSID III) assessed with: MOTOR composition score Scale from: 0 to 200 (higher = favourable) follow‐up: 2 patient years | The mean INFANT DEVELOPMENT: Bayley Scales of Infant and Toddler Development‐III ranged from 97.6 to 102.1 | MD 0.17 lower (5.45 lower to 5.11 higher) | ‐ | 69 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | Music/vocal interventions may not increase infant development.b,c |
| INFANT DEVELOPMENT: Bayley Scales of Infant and Toddler Development‐III (BSID III) assessed with: LANGUAGE composition score Scale from: 9 to 200 (higher = favourable) follow‐up: 2 patient years | The mean INFANT DEVELOPMENT: Bayley Scales of Infant and Toddler Development‐III ranged from 91.62 to 92.4 | MD 0.38 higher (5.45 lower to 6.21 higher) | ‐ | 69 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | Music/vocal interventions may not increase infant development.b,c |
| PARENTAL ANXIETY: STAI‐T assessed with: State Trait Anxiety Inventory Scale from: 6 to 80 (lower = favourable) | The mean PARENTAL ANXIETY: STAI‐T ranged from 12 to 38.85 | MD 1.12 lower (3.2 lower to 0.96 higher) | ‐ | 97 (4 RCTs) | ⊕⊕⊝⊝ Lowc,d | Music/ vocal interventions may not reduce parental state‐trait anxiety.c,d |
| RESPIRATORY RATE DURING intervention (lower = favourable) | The mean RESPIRATORY RATE DURING intervention (lower = favourable) ranged from 41.2 to 53 | MD 0.42 higher (1.05 lower to 1.9 higher) | ‐ | 750 (7 RCTs) | ⊕⊕⊕⊝ Moderateb | Music/vocal interventions probably do not reduce respiratory rate during intervention.b |
| RESPIRATOTY RATE POST‐intervention (lower = favourable) assessed with: up to follow‐up: 30 minutes | The mean RESPIRATOTY RATE POST‐intervention (lower = favourable) ranged from 48.5 to 60.33 | MD 0.51 higher (1.57 lower to 2.58 higher) | ‐ | 636 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | Music/vocal interventions probably do not reduce respiratory rate postintervention.b |
| HEART RATE DURING intervention (lower = favourable) | The mean HEART RATE DURING intervention (lower = favourable) ranged from 131.4 to 158.81 | MD 1.38 lower (2.63 lower to 0.12 lower) | ‐ | 1014 (11 RCTs) | ⊕⊕⊕⊝ Moderatee | Music/vocal interventions probably reduce heart rate during the intervention period.e |
| HEART RATE POST‐intervention (lower = favourable) assessed with: up to follow‐up: 30 minutes | The mean HEART RATE POST‐intervention (lower = favourable) was 138.19 to 161.4 | MD 3.8 lower (5.05 lower to 2.55 lower) | ‐ | 903 (9 RCTs) | ⊕⊕⊕⊕ Higha | Music/vocal interventions reduce heart rate postintervention.a |
| PARENTAL WELL‐BEING: EPDS assessed with: Edinburgh Postnatal Depression Scale Scale from: 0 to 30 (higher = favourable) | The mean PARENTAL WELL‐BEING: EPDS ranged from 7.83 to 8.08 | MD 0.5 higher (1.8 lower to 2.81 higher) | ‐ | 67 (2 RCTs) | ⊕⊕⊝⊝ Lowc,f | Music/ vocal interventions may not reduce postnatal depression.c,f |
| PARENTAL ANXIETY: STAI‐SKD assessed with: State Anxiety Inventory Scale from: 6 to 80 (lower = favourable) | The mean PARENTAL ANXIETY: STAI‐SKD ranged from 8.5 to 43.79 | MD 0.15 lower (2.72 lower to 2.41 higher) | ‐ | 87 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,c,g | The evidence is very uncertain about the effect of music/ vocal interventions on parental state anxiety.b,c,g |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_433263637383142806. | ||||||
a The evidence is certain. We did not downgrade since sensitivity analysis demonstrates that reducing analysis to studies without high risk of bias did not change the overall results. b The confidence intervals for the effect are consistent with both an appreciable benefit and appreciable harm. We downgraded by one level for imprecision. c Under 100 participants in total. We downgraded by one level. d Three of four studies have a high risk of bias. We downgraded by one level. e Half of the studies have a high risk of bias. We downgraded by one level. f A high risk of bias in detection bias in one of two studies. We downgraded by one level. g Two of three studies have a high risk of bias. We downgraded by one level.
Background
Approximately 15 million infants are born preterm yearly, constituting more than 10% of all infants born worldwide (Chawanpaiboon 2019). Advances in technology and treatments have increased survival rates and reduced morbidity in preterm infants. However, preterm birth interferes with brain maturation, and subsequent clinical events and interventions may have additional deleterious effects (Ment 2008; Stoll 2015). Therefore, various non‐pharmacological, therapeutic, or individual developmental care interventions have emerged that aim to improve health outcomes and quality of life for preterm infants and their parents (Aita 2021; Symington 2006). Music as therapy is one such intervention and is used increasingly in neonatal intensive care units (NICU). This has been studied in observational and experimental designs (Van der Heijden 2016; Yue 2021).
The sense of foetal hearing has been shown to develop as early as 16 weeks' gestation (Hepper 1994). Auditory perception has already developed when preterm infants are born. Studies suggest that the foetus responds to sound at least as early as 25 weeks to 27 weeks of gestational age (Clark‐Gambelunghe 2015; Hepper 1994; Monson 2018). Intrauterine sounds encompass characteristics of organised sounds that are highly musical in nature. The maternal heartbeat, for instance, is rhythmic, and the foetus primarily hears the musical parameters of speech: melody, rhythm, prosody (patterns of stress and intonation), phonemes (sounds that distinguish one word from another), and pitch contour of the maternal voice and external voices (Moon 2013; Partanen 2013; Philbin 2017). Music promotes neuronal activation, and many researchers suggest that musical learning starts prior to birth (Huotilainen 2010; Perani 2010). In preterm birth, the enclosed intrauterine environment optimal for foetal growth and maturation is abandoned too early. Aside from other stressful experiences, such as separation from the mother and father, preterm infants must also adjust to the unusual ‐ and potentially noxious ‐ sound environment of an intensive care unit (Kuhn 2013; Park 2014; Rossetti 2013).
Appropriate auditory stimulation and social contact for preterm infants are desirable (Anderson 2018). Music therapy may provide environmental and socio‐emotional enrichment through meaningful auditory stimulation and social contact (Anderson 2018; Haslbeck 2018; Loewy 2015; Shoemark 2015). This may be particularly warranted following preterm birth, as preterm infants are at risk of neurodevelopmental impairment, parents are at risk of post‐traumatic stress disorders, and parents and preterm babies risk attachment and bonding difficulties (Forcada‐Guex 2006; Korja 2011; Ruiz 2018). However, the precise effects of various musical and vocal stimulation types on short‐ and long‐term outcomes in preterm infants and their parents remain ambiguous.
Description of the condition
Preterm birth is a significant determinant of neurodevelopmental delay, and the resulting impairment can have adverse long‐term health effects (Pierrat 2017; Twilhaar 2018). It is sometimes associated with negative quality of life consequences, and an increased financial burden for the family and healthcare system (Lakshmanan 2021). Preterm infants face a range of morbidities, such as bradycardia, apnoea, anaemia, and respiratory distress syndrome. These infants are at risk of brain injury, and may have reduced white‐ and grey‐matter volumes (Inder 2005). Such brain‐structure abnormalities are associated with long‐term neurodevelopmental impairments, including motor dysfunction, cerebral palsy, cognitive and behavioural problems, and deficits in executive function (Twilhaar 2018; Woodward 2006). Factors such as environmental noise and sensory deprivation (e.g. the lack of the regular intrauterine rhythms of the maternal heartbeat and the maternal voice) may also impact neurodevelopment negatively (Heim 1999; Lahav 2014; McMahon 2012). For many parents, preterm birth is a traumatic and lasting experience. They struggle with numerous problems and concerns, such as the uncertainty of the infant’s future, feelings of fear, guilt, loss, grief, and confusion (Flacking 2007; Jotzo 2005; Roque 2017). These reactions may increase parental stress, adversely affect the stress‐coping behaviour of their infant, and impair the formation of a secure attachment (Forcada‐Guex 2006; Korja 2011; Malouf 2022).
Description of the intervention
Various musical and vocal interventions have been evaluated for efficacy in preterm infants (Haslbeck 2012; Mohan 2021; Van der Heijden 2016). They can be directed towards the infant (with or without parental involvement), to an entire family, or even applied within the whole NICU. The interventions aim to relax, stabilise and stimulate the infant and their parents (Hanson‐Abromeit 2008).
Auditory stimulation for preterm infants and their parents incorporates calm music sung softly or played on an instrument. Examples include lullabies; improvised music; popular, New Age, classical, or family indigenous music; or song‐of‐kin, songs or sounds entrained to infant vital signs (i.e. synchronised with breathing or heart rate pattern) or based on the acoustic intrauterine environment (womb sounds, heartbeats, and parents' voices) (Hanson‐Abromeit 2008; Haslbeck 2012; Loewy 2015; Mondanaro 2016). Music therapists, parents, nurses, doctors, nurses, and other healthcare professionals deliver the specific stimulation to the infants (and sometimes to their parents). These interventions are provided in addition to standard care in the NICU and are either performed live or recorded. The intervention is defined as music therapy when a trained music therapist provides the music within a therapeutic relationship and process facilitating personally tailored music experiences (Bradt 2015). Family‐integrating music therapy approaches (Haslbeck 2020; Loewy 2015; Shoemark 2015), may be most appropriate to address family‐centred recommendations in neonatal care, where the parents are seen as the most valuable resource for the infant (Lancet Child Adolescent Health 2019). In contrast, in music medicine, the music is administered by medical or healthcare professionals for passive listening (Dileo 1999).
How the intervention might work
The quality of early auditory experiences may have a direct influence on the plasticity of the brain’s auditory regions and may affect cortex development in infants (Yan 2003). Both auditory overstimulation and sensory deprivation in the NICU may adversely affect preterm infants' short‐ and long‐term neurobehavioural development, as the infants are already susceptible to neurodevelopmental impairment (Pineda 2014; Wachman 2011). Studies at the interface of music science and neuroscience suggest that music might promote neurobiological processes in humans, including the modulation of synaptic plasticity (linked to learning and memory), and might facilitate the differentiation, activation, readjustment, and growth of neurones (Abbott 2002; Rickard 2005; Sacks 2007). For instance, music can alter brain activity in core structures involved in processing emotions (Koelsch 2014). Auditory stimulation, therefore, is recommended to enhance psychological and physiological health in preterm infants (Jobe 2014; Shoemark 2015).
Several systematic reviews suggest that musical and vocal interventions may stabilise and soothe preterm infants demonstrating beneficial effects on their behavioural states, physiological parameters, sleep quality, oral feeding, pain, and maternal anxiety (Anderson 2018; Hartling 2009; Haslbeck 2012; Hodges 2010; Mohan 2021; Standley 2012; Tramo 2011; Van der Heijden 2016; Yue 2021). The Van der Heijden 2016 review suggested that music may improve heart rate, sleep, feeding, and sucking outcomes in preterm infants. A meta‐analysis by Bieleninik 2016 could not confirm or refute beneficial effects on those outcomes but did find a favourable impact of music on the infants' respiratory rate, and additionally demonstrated a reduction of maternal anxiety when parents were integrated into the music intervention process.
Why it is important to do this review
A number of systematic reviews have demonstrated ambiguous results for the efficacy of various types of auditory stimulation on preterm infants. Most of the reviews focused on a specific topic (e.g. maternal voice (Krueger 2010); music (Hartling 2009); or music interventions carried out by or in consultation with a trained music therapist (Bieleninik 2016)). The authors of these reviews concluded that the heterogeneity and clinical diversity of the included studies prevented the drawing of definitive conclusions about the impact of auditory stimulation on preterm infants (Hartling 2009; Haslbeck 2012; Hodges 2010; Krueger 2010; Standley 2012; Van der Heijden 2016). A more recent meta‐analysis (Yue 2021), reported the significant positive influence of any music intervention on preterm infants respiratory rate, heart rate, oral feeding volume, stress level, and maternal anxiety. However, detailed reported criteria for considering studies for inclusion and assessment of the certainty of evidence are missing. Therefore, a more comprehensive and rigorous systematic review is needed to address existing controversies arising from apparent conflicting studies and reviews. Firstly, we evaluate the overall efficacy of auditory stimulation. Then, by analysing the impact of various types of auditory stimulation systematically with subgroup analysis, and by focusing on the methodological quality of the included studies, we may be able to provide better guidance. We may be able to determine how to use these interventions most effectively to promote specific outcomes in preterm infants and their parents (e.g. live versus recorded versions; sung versus instrumental; choices made in rendering decisions regarding length and time of intervention, associated keys, etc.). The current review should assist health professionals in neonatal care to make practical, evidence‐based decisions about the use of musical and vocal interventions for preterm infants and their parents. If such a low‐cost, low‐risk intervention is demonstrated to be effective in supporting preterm infants' neurodevelopment and parental well‐being, the findings could have significant clinical implications for this vulnerable patient population.
Objectives
We assessed the overall efficacy of music and vocal interventions for physiological and neurodevelopmental outcomes in preterm infants (< 37 weeks' gestation), compared to standard care. In addition, we aimed to determine specific effects of various music and vocal interventions for physiological, anthropometric, social‐emotional, neurodevelopmental short‐ and long‐term outcomes in preterm infants, parental well‐being, and bonding.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel, cluster, and factorial randomised controlled trials. To avoid bias by carry‐over effects, we included only the first phase of cross‐over trials for short‐term outcomes.
Types of participants
We included preterm infants of less than 37 weeks' gestational age, during hospitalisation and parents, only when they were involved in the music or vocal intervention (listening to it with their infant, or providing it for their infant or themselves, in relation to their infant, e.g. to support singing to their infant).
Types of interventions
We included any music or vocal stimulation, provided live or by recording, addressing either the infant alone or also the parents. The music intervention could be combined with another intervention, such as skin‐to‐skin care, but only if both arms of the study received the additional intervention. We included studies that examined a combination of interventions versus only music or voice. We also included studies that compared one type of music or voice to another type of music or voice and analysed them separately. Music interventions during painful procedures were included and would have been analysed separately. A parent, music therapist, musician, doctor, nurse, or other health professional or caregiver could deliver the intervention. The intervention must include musical elements, such as rhythm and melody, or sounds based on the acoustic intrauterine environment, e.g. womb sounds, heartbeats, and the human voice.
The intervention duration must comprise at least five minutes and the intervention must be delivered/administered at least three times for inclusion in the review. The intervention period may include any time from birth to hospital discharge. We compared the interventions with standard care without musical or vocal stimulation. We excluded auditory stimulation with white noise (noise with constant amplitude throughout the audible frequency range) or digital signals. These stimulation types lack musical parameters such as melody and prosody.
Types of outcome measures
Primary outcomes
Short‐term outcome in preterm infants:
Change in mean oxygen saturation during and post‐interventiona
Long‐term outcome in preterm infants:
Infant development (assessed using Bayley Scales of Infant and Toddler Development (BSID‐II and III),b focusing on mean mental development index (MDI) scores and psychomotor development index (PSI) scores at two years of corrected age (Johnson 2008))
Outcomes in parents:
Change in anxietyb, defined as mean State‐Trait Anxiety Inventory Score with 20 items and a four‐point Likert Scale (Spielberger 1983).
Secondary outcomes
Short‐term outcomes in preterm infants:
Heart rate: beats per minute during and post‐interventiona (measured by pulse oximetry or electrocardiogram);
Respiratory rate during and post‐interventiona: inspiration per minutea (measured by, e.g. electric strain‐gauges, thoracic impedance plethysmography, nasal air‐flow sensor and spirometers);
Heart rate variability during and post‐interventiona (measured by low‐frequency power (ms2/Hz); high‐frequency power (ms2/Hz); low frequency/high‐frequency ratio, reflecting the balance between sympathetic and parasympathetic tone);
Behavioural outcomesb (measured with behavioural numerical scores or scales for neonates, e.g. Assessment of Preterm Infant Behaviour (Als 2005));
Hospitalisation (days);
Adverse effects, including severe apnoea during the intervention requiring stimulation by the neonatal care team; and
Weight gain (kg/day).
Long‐term outcomes in preterm infants:
Neurodevelopment (assessed by standardised follow‐up examinations, e.g. intelligence quotient (Wechsler Preschool and Primary Scale of Intelligence‐Revised (WPPSI‐R) (Park & Demakis 2017); Kaufmann Assessment Battery for Children (K‐ABC II) at five years of corrected age (Melchers 2009)
Outcomes in parents:
Well‐being (measured with e.g. the Edinburgh Postnatal Depression Scale);
Attachmentb (measured with standardised scales, e.g. Postpartum Bonding Questionnaire (Hoffenkamp 2015)).
aassessed up to 30 minutes before, during, and 30 minutes after each musical intervention or control condition; reported at study level as mean changes or assessed after the last measurement round of musical intervention or control condition
bassessed before and after the whole intervention or control period
Search methods for identification of studies
The Neonatal Group Information Specialist developed search strategies in consultation with the authors. The MEDLINE strategy was translated, using appropriate syntax, for other databases. Search strategies combine intervention terms with standard terms for the neonatal population. Methodological filters were used to limit retrieval to randomised controlled trials and systematic reviews. Searches were conducted without date, language, or publication status limits.
Database search results were reduplicated using a combination of methods as follows: deduplication of MEDLINE and Embase results in OVID; all results reduplicated in EndNote and Covidence.
Trial registries reference lists of included studies and related systematic reviews, and conference proceedings were searched.
Note: The timeline for this publication was disrupted by the COVID‐19 pandemic and staffing issues at the Cochrane Neonatal editorial base. As a result, publication of this review has been delayed, and the literature search is more than one year old. We will endeavour to undertake an updated search within the next calendar year.
Electronic searches
The following databases were searched November 1 to 12, 2021. Search strategies and dates of each search are presented in: Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9.
Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue #11) via CRS (Cochrane Register of Studies);
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to October 29, 2021);
Embase (OVID) (1974 to October 29, 2021);
CINAHL (1981 to November 1, 2021);
PsycINFO (1806 to November 1, 2021);
Web of Science (1982 to November 1, 2021);
RILM Abstracts of Music Literature (1967 to November 1, 2021);
ERIC (Educational Resources Information Center; 1966 to 15 November 1, 2021).
The following databases were searched in 2019 and strategies are presented in Appendix 10.
Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue #11) in the Cochrane Library;
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 15 November 2019);
CINAHL (1981 to 15 November 2019);
PsycINFO (1806 to 15 November 2019);
Web of Science (1982 to 15 November 2019);
RILM Abstracts of Music Literature (1967 to 15 November 2019);
ProQuest Dissertations & Theses A&I (1637 to 15 November 2019);
ERIC (Educational Resources Information Center; 1966 to 15 November 2019).
The search strategies and sources used in 2021 differ slightly from those run in 2019 ‐ mainly by the addition of field qualifiers in Cochrane Central, CINAHL, PsycINFO, Web of Science, and RILM Abstracts; the addition of Embase; and the omission of ProQuest Dissertations, which we were unable to access in 2021 due to technical difficulties. Results of the 2021 search were reduplicated against results from 2019 and results of both searches are represented in the PRISMA flow diagram (Figure 1).
1.
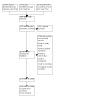
Study flow diagram
Searching other resources
The following trial registers were searched on 01 November 2021 and search strategies are presented in:
International Clinical Trials Registry Platform (ICTRP), The World Health Organisation (https://trialsearch.who.int/Default.aspx);
U.S. National Library of Medicine's https://clinicaltrials.gov;
ISRCTN Registry (https://www.isrctn.com).
Conference abstracts were searched for the following conferences:
Paediatric Academic Societies;
European Society for Paediatric Research;
European Association of Music Therapy;
American Music Therapy Association;
World Federation of Music Therapy.
We reviewed the reference lists of all included studies and related systematic reviews in an effort to identify studies not captured by database searches.
Data collection and analysis
We used the standard methods and criteria of the Cochrane Collaboration and its Neonatal Group to assess the methodological quality of the trials:
FH and DB were not involved in those activities for Haslbeck 2020a. TK and KM assessed this study instead. JL was not engaged in assessing methodological quality of the trials in general, so she was not involved in any assessment of her research (Loewy 2013). JM determined the final overall inclusion and exclusion criteria.
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the review management software Covidence to manage the results of our search. Two review authors (FH, KM) independently assessed study eligibility for inclusion in the review according to the prespecified selection criteria. They screened titles and abstracts to remove obviously irrelevant reports. The review authors linked together multiple reports of the same study so that each study rather than each report was the unit of interest in the review. They examined full‐text reports to establish the compliance of studies with the eligibility criteria. If trial eligibility was unclear, they resolved discrepancies through discussion with the other review authors to reach a consensus. They listed all excluded studies with reasons for exclusion. They recorded the selection process in sufficient detail to complete a flow diagram (Figure 1) and Characteristics of excluded studies table.
Data extraction and management
Three review authors (FH, KM, TK) independently conducted data extraction using and adapting the most recent version of the Cochrane data collection form (Higgins 2019), in Covidence. They pretested the adapted version with a subset of five studies before general application. They used the adapted form to decide trial inclusion or exclusion and to extract data from eligible trials.
They (FH, KM, TK) extracted the following characteristics from each included study.
Administrative details: study authors; published or unpublished, year of publication, CRS ID, sponsorship source;
Study characteristics: study design type, study grouping, study setting, number of study centres, location, and contact;
Participants: number randomised, the number lost to follow‐up/withdrawn, number analysed, age, gender and further baseline characteristics in the infants and parents, inclusion and exclusion criteria, the reason for dropouts, reasons for exclusion, sample size calculation;
Interventions: detailed description of music and voice type (music therapy or music medicine, musical parameters, instruments, music genre or music piece/voice genre or particular chosen voice, music selection, intervention alone or combined), dose, duration, frequency, mode of delivery (live, infant‐directed entrained/recorded or standardised and dB level);
Outcomes as mentioned under Types of outcome measures.
If any queries arose or when data appeared to be missing, they requested additional information from the authors of the original reports, e.g. when outcomes of interest were not reported. They described ongoing studies identified by their search, when available, detailing the authors, study reference, study name, methods, participants, interventions, outcomes, starting date, and contact information. If there were disagreements when comparing extracted data, they resolved them in consultation with the other review authors. Two review authors (FH, KM) entered and cross‐checked data using Review Manager Web (RevMan Web 2022).
Assessment of risk of bias in included studies
Three review authors (FH, KM, TK) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane Risk of bias tool (Higgins 2017), for the following domains.
• Sequence generation (selection bias);
• Allocation concealment (selection bias);
• Blinding of participants and personnel (performance bias);
• Blinding of outcome assessment (detection bias);
• Incomplete outcome data (attrition bias);
• Selective reporting (reporting bias);
• Any other bias.
We looked for evidence of bias or methodological differences between trials.
We resolved any disagreements through discussion with the other review authors to reach a consensus. See Appendix 11 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
The treatment effects of the individual trials were analysed using Review Manager Web (RevMan Web 2022). For dichotomous data, we planned to use risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CIs). If the difference between groups had been statistically significant, we would have calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), with their respective CIs. We evaluated continuous data by assessing the mean difference (MD) with its 95% CI when measured in the same way between trials. If studies had reported the same outcome but measured it in different ways, we would have used the standardised mean difference (SMD) with its 95% CI. Where summary statistics would have been missing, we would have derived them from the accompanying P values. We analysed short‐term cross‐over trials to determine if there would be no significant risk of a carry‐over effect. We calculated an effect estimate using the generic inverse variance method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We incorporated cross‐over trials into meta‐analyses using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). If we had identified cluster trials, we would have incorporated them using generic variance methods for analysis (Higgins 2019).
Unit of analysis issues
We performed the primary analysis per individual randomised. If the following issues had occurred, we planned to address them according to the methods described below.
Cluster‐randomised trials
In cluster‐randomised trials, groups of participants rather than individuals are randomised to different interventions. Because of this, participant data can no longer be assumed to be independent of one another. Unfortunately, some cluster‐randomised trials are not analysed correctly, i.e. do not take into account that the unit of allocation (the group) is different from the unit of analysis (the individual). If this clustering is ignored, there is a unit of analysis error, which means that the resulting P values and 95% CIs will be artificially small and lead to an inappropriately increased weight in the meta‐analysis. If cluster‐randomised trials had failed to report results based on appropriate analyses such as the multi‐level model or variance component analysis, we would have used the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 16.3.3) (Higgins 2019), to reanalyse these trials with appropriate consideration of the intra‐cluster (or intraclass) correlation coefficient (ICC) to estimate the effective sample size. Sensitivity analyses would have been performed to explore whether there were any differences in effects between cluster‐ and individually randomised trials.
Cross‐over trials
Cross‐over trials are suitable for evaluating interventions with a temporary effect in the treatment of stable conditions. The principal problem is that of carry‐over (a type of period‐by‐intervention interaction). Since we believe that some carry‐over from period one to period two cannot be precluded in these trials in our setting, we included only the data from the first period (as suggested in chapter 16.4.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019)). We requested the data from the first phase of the cross‐over trial from the authors to avoid bias in the carry‐over effect. In cross‐over trials, we assessed whether the short‐term cross‐over design is suitable, whether there is a carry‐over effect, whether only first‐period data are available, whether the analysis is correct, and whether the results are comparable with those from parallel‐group trials.
Studies with more than two intervention groups (multi‐arm studies)
If more than one comparison arm from the same trial was eligible for inclusion in the same meta‐analysis, we combined the live or recorded music intervention groups to create a single pairwise comparison so that the same participants did not contribute data to the meta‐analysis more than once according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 16.5.4) (Higgins 2019). We included all music and vocal intervention groups meeting our inclusion criteria in our synthesis since all interventions were relevant to our review. We (FH & KM) used the formulae in Table 6.5.a (6.5.2.10 Combining groups#section‐6‐5‐2‐10) to combine numbers into a single sample size, mean, and SD for each intervention group. We calculated independently, compared results, and where differences occurred, we calculated again to reach a consensus. To reflect the fact that comparisons within multi‐arm studies are correlated, we adjusted the standard error of each two‐arm comparison from a multi‐arm study. We used the method proposed by Rücker and Schwarzer which uses back‐calculated standard errors in the weighted least‐square estimator to reflect the within‐study correlation (Rücker 2012; Rücker 2014; Rücker 2016). In one study (Namjoo 2021), which compared a recorded versus a live lullaby intervention, we appropriately reduced the sample size of the control group so that the same participants did not contribute data to the meta‐analysis more than once according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 16.5.4) (Higgins 2019).
Multiple measurements of outcomes
When primary outcomes were assessed at more than one time point in our time ranges, we used the data from the latest time point available in our analyses. We did not plan to adjust for multiplicity in our review based on multiple outcome measurements. Considering the heterogeneity of time points for short‐time outcomes, we identified two different, but overall used time points: a) during intervention; b) post‐intervention. Since both time points are clinically relevant and were available in most of the included studies, we decided to conduct these two analyses: a) during intervention; b) post‐intervention in parallel. Since not all authors reported the data for the different time points in detail, the first author (FH) contacted and requested the missing data.
Dealing with missing data
We contacted the authors whenever we detected that data and statistics were missing or incomplete to request further information. However, when data were missing due to dropouts, we included the reported infants and examined the effect of losses in a sensitivity analysis according to the risk of bias. We contacted the primary investigators. If authors were unable or unwilling to provide the data, we still included the study in the review and explicitly stated that data were missing.
Assessment of heterogeneity
We describe the clinical diversity and methodological variability of the evidence in the review text and with study tables, which describe study characteristics including design features, population characteristics, and intervention details. To assess statistical heterogeneity, we visually inspected forest plots and described the direction and magnitude of the effect and the degree of overlap between confidence intervals. We estimated treatment effects in individual trials and examined heterogeneity between trials by inspecting forest plots and quantifying the impact of heterogeneity by using the I2 statistic, a measure that describes the proportion of variation in point estimates that is due to variability across studies rather than sampling error (Deeks 2017). We interpreted the results as follows:
Less than 25%: no heterogeneity;
25% to 49%: low heterogeneity;
50% to 74%: moderate heterogeneity;
75% to 100%: high heterogeneity.
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes and reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. Studies using the interventions in a potentially eligible infant population but not reporting on any of the primary and secondary outcomes would have been documented in the Characteristics of included studies table.
For outcomes reported by more than 10 studies, we planned to prepare a funnel plot to assess possible reporting bias. If publication bias had been suggested by a significant asymmetry of the funnel plot on visual assessment, we would have incorporated this in our assessment of the certainty of evidence (Egger 1997). If our review included few studies eligible for meta‐analysis, the ability to detect publication bias would be largely diminished, and we would simply note our inability to rule out possible publication bias or small study effects.
Data synthesis
We used the standard methods of Cochrane and Cochrane Neonatal to perform statistical analysis (neonatal.cochrane.org/resources-review-authors). The treatment effects of all infants in the eligible trials were analysed. If we identified multiple studies that we considered to be sufficiently similar, we performed meta‐analysis using Review Manager Web (RevMan Web 2022). We used a fixed‐effects model to combine the data. We planned to calculate average estimates of RR and RD with 95% CIs for any meta‐analyses where required. We used the MD with 95% CIs for continuous outcomes that were measured in the same way between trials. We planned to calculate the standardised mean difference (SMD) with 95% CIs to combine trials that would have measured the same outcome but used different scales where required. Individual trials were interpreted separately when a meta‐analysis appeared to be inappropriate based on clinical judgement and the I2 heterogeneity test (i.e. when I2 > 80%). When the I2 statistic was higher than 50%, we reported the finding and assessed the source of the heterogeneity (e.g. differences in study quality, participants, intervention regimens, or outcome assessments) by sensitivity and subgroup analysis (Subgroup analysis and investigation of heterogeneity).
Subgroup analysis and investigation of heterogeneity
Tests for subgroup differences in effects were interpreted with caution given the potential for confounding with other study characteristics. In particular, subgroup analyses with fewer than five studies per category are unlikely to be adequate to ascertain the valid differences in effects and were not highlighted in our results. When subgroup comparisons were possible, stratified meta‐analysis and a formal statistical test for interaction were conducted to examine subgroup differences that could account for effect heterogeneity (e.g. Cochran’s Q test, meta‐regression) (Higgins 2019).
According to the heterogeneity of auditory stimulation types, we planned to compare the following modalities separately, if a sufficient number of studies were identified.
-
Auditory stimulation
Spoken voice;
Sung voice;
Music without a voice;
Womb sounds;
Rhythmic sounds; or
Breathing sounds.
-
Auditory stimulation
Live, infant‐directed, or entrained music; or
Recorded or standardised music.
-
Musical decision or selection
By parent; or
Random, unidentified, or unknown.
-
Duration of intervention
Between five and 10 minutes; or
More than 10 minutes.
-
Frequency of intervention
Between three and seven times; or
At least eight times.
-
Auditory stimulation
Alone; or
Combined with other interventions (e.g. skin‐to‐skin care).
-
Painful procedure
With auditory stimulation; or
Without auditory stimulation.
-
Gestational age
Extremely preterm (less than 28 weeks gestation);
Very preterm (28 to 32 weeks gestation);
Moderate to late preterm (32 to 37 weeks gestation).
Given that studies in a variety of settings may not have reliable gestational age and may therefore use birth weight categories, we planned to include infants categorised as follows.
Low birth weight (LBW) infants – defined as infants with birth weight < 2500 g;
Very low birth weight (VLBW) infants – defined as infants with birth weight < 1500 g;
Extremely low birth weight (ELBW) infants – defined as infants with birth weight < 1000 g.
We planned to group infants with birth weights 1500 to 2499 g with moderate preterm infants, infants 1000 to 1499 g with very preterm infants, and infants < 1000 g with extremely preterm infants.
However, since there were insufficient studies or details to be able to distinguish the listed modalities clearly, we only assessed possible differences between subgroups of recorded versus live music and frequency of intervention by using the formal test for subgroup differences in Review Manager Web (RevMan Web 2022).
Sensitivity analysis
We planned to perform sensitivity analyses where sufficient data were available to explore methodological heterogeneity. We considered characteristics of bias (Assessment of risk of bias in included studies). It is worthwhile to note that double‐blinding has scarcely been possible in intervention designs with live music. Consequently, it is all the more crucial that the outcome assessors are blind to the data. In our sensitivity analyses, we excluded trials with a high risk of bias for any of the following: allocation concealment, adequate randomisation, and blinding of outcome assessment (Schulz 1994; Schulz 2000). Additionally, we considered the characteristics of participants (e.g. participants with high morbidities). Given that there is no formal statistical test that can be used for sensitivity analysis, we made informal comparisons between the different ways of estimating the effect under different assumptions. Changes in the P values should not be used to judge whether there is a difference between the main analysis and sensitivity analysis, since statistical significance may be lost with fewer studies included. We reported sensitivity analysis results narratively.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes.
Oxygen saturation during and post‐intervention;
Infant development assessed using the Bayley Scales of Infant and Toddler Development at two years;
Parental anxiety assessed using the State‐Trait‐Anxiety Inventory Scores;
Respiration rate during and post‐intervention;
Heart rate during and post‐intervention;
Parental well‐being assessed with the Edinburgh Postnatal Depression Scale;
Parental anxiety assessed using the State‐Anxiety Inventory Scores.
Two review authors (FH, KM) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high certainty, downgrading the evidence to one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEproGDT Guideline Development Tool to create Table 1 to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence in one of the following four grades.
High: we are very certain that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately certain in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our certainty in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little certainty in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
For a full description of studies please see the Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
Database searches identified 14,487 records; trial registry searches identified 73 records; and conference abstract searching identified 32 records (14,592 total). After removing 7059 duplicates, 7533 records were screened. We excluded 7412 records during screening and reviewed 121 full texts for eligibility. During the full‐text screening, 33 ongoing studies were identified and no awaiting classification studies found. We excluded 63 studies, finally including 25 trials; for details see Figure 1.
Included studies
Participants
Twenty‐five studies recruiting 1532 infants and 691 parents, of which 122 mothers of two studies (Namjoo 2021; Wirth 2016), were included to use their (recorded) voice as an intervention without assessing additional parental outcomes. Study sample sizes ranged from 17 (Calabro 2003; Caparros‐Gonzalez 2018), to 272 (Loewy 2013). Most of the studies included preterm infants, whereas 10 studies additionally included parents, of which only three music therapy studies (Kehl 2021; Loewy 2013; Menke 2021), included fathers in the analysis. The gestational age at birth for recruitment varied from 23 to 36 weeks, with only four music therapy studies including extremely preterm infants (Epstein 2021; Haslbeck 2021; Kraft 2021; Menke 2021) (Table 2).
1. Summary characteristics of included studies primary outcomes.
| Study | Design | Country | Infant/parent•• |
Infant category |
Intervention | Control condition | SPO2 |
Infant develop‐ ment |
State‐Trait‐anxiety |
| Calabro 2003 | Parallel | Australia | 22/0 | n.a. | Recorded music | Standard care | x | ||
| Caparros‐Gonzalez 2018 | Parallel | Spain | 22/0 | Moderately preterm infant | Recorded music | Standard care | x | ||
| Cevasco 2008 | Parallel | USA | 25/21 | Moderately preterm infant | Recorded music | Standard care | |||
| Portugal 2017 | Parallel | Portugal | 18/0 | Very preterm infant | Recorded auditory stimulation | Standard care | x | ||
| Epstein 2021 | Cross‐over | Israel | 40/40 | Extremely preterm infant | Live music | Standard care | x | Narratively reported | |
| Ettenberger 2014 | Parallel | Colombia | 30/27 | Moderately preterm infant | Live music/live music + kangaroo | Standard care | n.a. | x | |
| Farhat 2010 | Parallel | Iran | 44/0 | Very preterm infant | Recorded music | Standard care | x | ||
| Haslbeck 2021 | Parallel | Switzerland | 82/0 | Extremely preterm infant | Live music | Standard care | x | ||
| Jabraeili 2016 | Parallel | Iran | 75/0 | Extremely preterm infant | Recorded music | Standard care | x | ||
| Johnston 2007 | Cross‐over | Canada | 65/0 | Moderately preterm infant | Recorded auditory stimulation during heal lance | Standard care heal lance | x | ||
| Kehl 2021* | Parallel | Switzerland | 0/46 | Extremely preterm infant | Live music | Standard care | x | ||
| Kraft 2021 | Cross‐over | Netherlands | 59/23 | Extremely preterm infant | Live music | Standard care | x | ||
| Kucuk Alemdar 2020 | Parallel | Turkey | 136/0 | Very preterm infant | Recorded mothers spoken voice/breast mild odour/incubator cover | Standard care | x | ||
| Lafferty 2021 | Parallel | USA | 40/0 | Very preterm infant | Recorded music | Standard care | |||
| Lejeune 2019 | Parallel | Switzerland | 39/0 | Very preterm infant | Recorded music | Standard care | x | ||
| Liao 2021 | Parallel | China | 103/0 | Very preterm infant | Recorded music/white noise | Standard care | x | ||
| Loewy 2013 | Cross‐over | USA | 284/284 | Very preterm infant | Live music | Standard care | x | ||
| Menke 2021 | Parallel | Germany | 65/65 | Extremely preterm infant | Live music | Standard care | x | ||
| Nakhwa 2017 | Parallel | India | 40/0 | Not available | Recorded music & developmental care | Control + developmental program | |||
| Namjoo 2021 | Parallel | Iran | 90/90 | Moderately preterm infant | Recorded music/live music | Standard care | x | ||
| Tandoi 2015 | Parallel | Italy | 34/0 | Moderately preterm infant | Recorded music | Standard care | x | ||
| Vastani 2017 | Parallel | Iran | 60/0 | Moderately preterm infant | Live music | Standard care | |||
| White‐Traut 1988 | Parallel | USA | 33/33 | Moderately preterm infant | Live music / massage, talking, eye contact and rocking mother | Standard care | |||
| Wirth 2016 | Parallel | Germany | 62/62 | Moderately preterm infant | Recorded auditory stimulation/recorded music | Standard care | |||
| Yu 2021 | Parallel | Taiwan | 64/0 | Moderately preterm infant | Recorded auditory stimulation during heal lance | Standard care heal lance | x | ||
| Total | 1109/691 |
*Kehl 2021 is the same trial as Haslbeck 2020 with two different publications ‐ for a) Haslbeck 2020: infant long‐term outcomes and b) Kehl 2021: parental outcomes
**recruited
n.a.: not available
SPO2: Oxygen saturation
Setting
We identified 21 parallel‐group RCTs and 4 cross‐over RCTs (Epstein 2021; Johnston 2007; Kraft 2021; Loewy 2013). All trials were single‐centre studies except one multi‐centre trial (Loewy 2013). Nine RCTs examined two intervention arms, two RCTs evaluated three intervention arms (Loewy 2013; Kucuk Alemdar 2020), and the remaining RCTs compared one intervention with the control condition. The trials took place in NICUs from levels one to three. Most of the studies have been conducted since the 2010s except for four studies from earlier years (Calabro 2003; Cevasco 2008; Johnston 2007; White‐Traut 1988). The studies were performed by researchers from around the world, mainly from Europe (n = 8), followed by the Middle East (n = 6), USA (n = 4), Asia (n = 3), Australia (n =1), South America (n = 1) and Canada (n = 1) (see Table 2). Seven studies reported being funded by University/Health Department/Hospital research funds (Farhat 2010; Jabraeili 2016; Johnston 2007; Kraft 2021; Kucuk Alemdar 2020; Liao 2021; Namjoo 2021). In addition, local medical/health foundations funded five projects (Calabro 2003; Kehl 2021; Loewy 2013; White‐Traut 1988; Wirth 2016), and National Science Foundations supported two studies (Caparros‐Gonzalez 2018; Lafferty 2021); the remaining studies did not mention the sponsorship source.
Intervention
We identified a range of music and vocal interventions varying widely in intervention type, delivery, frequency, and duration across studies mainly characterised by calm, soft, musical parameters in lullaby style, often integrating the sung mother's voice live or recorded. Seventeen music medicine studies were conducted (we also included the vocal intervention delivered by medical personnel as music medicine), and seven studies provided music therapy of whom six studies were based on Creative Music Therapy with preterm infants and their parents (Haslbeck 2020), or the Rhythm, Breath, and Lullaby approach (Loewy 2013). In these family‐centred live music approaches, entrainment to the infant’s breathing rhythm is consciously deployed as a technique. The music therapist continually adapts and tailors the music to the infant’s and parents' needs and musical heritage in an ongoing individualised therapeutic reciprocal, family‐integrating, and interactive process. Additional to these six live music therapy studies, two music medicine studies provided live music for the infants (n = 8), whereas the remaining two‐thirds of the studies (n = 16) played recorded music and one study (n = 1) recorded the spoken voice. One of the studies (Namjoo 2021), compared a recorded lullaby to a live version. In a little over half of the studies (n = 14), the musical selection was random, unidentified, or unknown. In contrast, the remaining studies (n = 9) indicated that the parents selected the song/music in at least one intervention arm. More than two‐thirds of the studies (n =17) used the sung voice (mostly lullabies) in at least one intervention arm whereas the remaining one‐third used (additionally) spoken voice as the choice of intervention type (n = 7). Pure recorded instrumental music was rare (n = 4) characterised as calm and sedative music played by wind and string instruments (harp, string, flute) (Calabro 2003; Caparros‐Gonzalez 2018; Lejeune 2019). One study (Lafferty 2021), provided piano music (Mozart’s double piano sonata), and a few studies played womb sounds and rhythmic or breathing sounds. Most of the interventions were delivered exclusively whereas four studies provided music therapy during skin‐to‐skin care as an integral part of standard care in the unit (Epstein 2021; Ettenberger 2014; Haslbeck 2021; Menke 2021). Two studies were conducted while the infants were lying in the arm of the mother (Namjoo 2021; White‐Traut 1988). Two studies examined the effects of music on pain (Johnston 2007; Yu 2021). Moreover, the dose of the interventions ranged from five to 30 minutes with most studies (n = 20) providing the intervention for more than 10 minutes and more than two‐thirds of studies (n = 16) at least eight times. For further details see Table 3; Table 4; Table 5.
2. Summary first music/vocal intervention characteristics of included studies.
| Study | Intervention 1: Type & delivery (MM/MT): live, infant‐directed, entrained/recorded or standardised | Musical selection by parents/random, unidentified or unknown | Spoken voice/sung voice/music without voice | Womb sound/rhythmic sound | Intervention alone/combined with SSC/laying in mother's arm/during pain | Frequency & duration/dose (min) | 5‐10 min/ > 10 min |
3‐7 times/ ≥ 8 times |
| Calabro 2003 | MM: recorded sedative instrumental lullabies (strings, flute & harps: Brahms & Sandman) | random | music | alone | 4 x over 4 consecutive days (daily), 60‐70 dB/20 min | > 10 | 3‐7 | |
| Caparros‐Gonzalez 2018 | MM: recorded sedative instrumental music, composed by artificial intelligence | random | music | alone | 8 x (7: first not for analysis) over three consecutive days, 30 dB/20 min | > 10 | ≥ 8 | |
| Cevasco 2008 | MT: recorded mothers singing voice accompanied by guitar playing of music therapist/lullabies picked by mother from list or Brahms lullaby | parent | spoken voice | alone | 3‐5 per week until discharge; 65 dB/20 min | > 10 | ≥ 8 | |
| Portugal 2017 | MM: recorded mothers voice (spoken and sung) and her heartbeats | parent | spoken & sung voice |
rhythmic sound | alone | 4 x the day until moved to cradle or discharged; 60‐65 dB/45 min | > 10 | ≥ 8 |
| Epstein 2021 | MT: live, infant‐directed, entrained vocal & instrumental music (parents preferred) | parent | sung voice | SSC | 3 sessions over two weeks; dB: n.a./20 min | > 10 | 3‐7 | |
| Ettenberger 2014 | MT: SSC & live, infant‐directed entrained mothers` singing in lullaby style (song chosen by mother) accompanied by a music therapist with voice and or guitar | parent | sung voice | SSC | 17 sessions/13.7 min (range 8‐25) | > 10 | ≥ 8 | |
| Farhat 2010 | MM: commercially recorded lullabies sung by Iranian female vocalists | random | sung voice | SSC | 8 x for 8 consecutive days (daily); 60‐65 dB/20 min | > 10 | ≥ 8 | |
| Haslbeck 2021 | MT: live, infant‐directed, entrained singing accompanied by monochord, parents integrated, individualised therapy with or without parents, parental musical preferences integrated into the improvisation | parent | sung voice | womb sound | alone & SSC |
8 to 30 x (2‐3 per week until discharge)/20 min | > 10 | ≥ 8 |
| Kehl 2021 | MT: live, infant‐directed, entrained singing accompanied by monochord, parents integrated, individualised therapy with or without parents, parental musical preferences integrated into the improvisation | parent | sung voice | womb sound | alone & SSC |
8 to 30 x (2‐3 per week until discharge)/20 min | > 10 | ≥ 8 |
| Kraft 2021 | MT: live, infant‐directed, entrained vocal & instrumental music (parents preferred) | parent | sung voice | womb sound | alone | 6 x over two weeks/15 min | > 10 | 3‐7 |
| Kucuk Alemdar 2020 | MM: recorded mothers' voice expressing their thoughts and feelings and anything they wanted to say | parent | spoken voice | (alone) | 3 x a day until discharge/30 min | > 10 | ≥ 8 | |
| Lafferty 2021 | MM: recorded Mozart’s double piano sonata | random | music | alone | 2 x the day for 14 days/24 min | > 10 | ≥ 8 | |
| Jabraeili 2016 | MM: recorded Brahms lullaby | random | sung voice | alone | 3 x for 3 consecutive days (daily); 65 dB/15 min | > 10 | 3‐7 | |
| Johnston 2007 | MM: mother's sung or spoken filtered voice during pain | random | spoken & sung voice |
during pain |
6 x over 2 days (3 daily); 60‐65 dB/10 min | 5‐10 | 3‐7 | |
| Lejeune 2019 | MM: recorded instrumental especially composed calm music | random | music | alone | 5 per week until discharge; 30‐65 dB/8 min | 5‐10 | ≥ 8 | |
| Liao 2021 | MM: recorded mother's sung Chinese version of Schubert's Lullaby | random | sung voice | alone | 3 x a day for 4 consecutive days/20 min | > 10 | ≥ 8 | |
| Loewy 2013 | MT: live lullaby singing (preferred song of parents) with guitar | parent | sung voice | alone | 6 x over two consecutive weeks; 55‐65 dB/10 min | 5‐10 | 3‐7 | |
| Menke 2021 | MT: live, infant‐directed, entrained singing accompanied by monochord, parents integrated, individualised therapy with or without parents, parental musical preferences integrated into the improvisation | parent | sung voice | womb sound | alone & SSC |
at least 6 times (2 x per week until discharge)/20‐30 min | > 10 | ≥ 8 |
| Nakhwa 2017 | MM: recorded lullaby | random | sung voice | alone | 9 x over 3 weeks; 30‐40 dB/30 min | > 10 | ≥ 8 | |
| Namjoo 2021 | MM: recorded Persian lullaby sang by strange woman, played to infant while laying in the mother's arm using headphones | random | sung voice | arm | once a day for 14 days/20 min | > 10 | ≥ 8 | |
| Tandoi 2015 | MM: recorded womb sounds & voices | parent | spoken voice | womb & rhythmic sound | alone | 10 x in the first 10 days (daily); 55‐70 dB/20‐30 min | > 10 | ≥ 8 |
| Vastani 2017 | MM: live mothers` singing of standardised lullaby | random | sung voice | alone | 3 x for 3 consecutive days (daily)/10 min | 5‐10 | 3‐7 | |
| White‐Traut 1988 | MM: live talking or singing mother | parent | spoken & sung voice |
alone & arm |
6 x over three consecutive days; dB: n.a./15 min | > 10 | ≥ 8 | |
| Wirth 2016 | MM: recorded reading mother's voice | random | spoken & sung voice |
alone | 14 x over 14 consecutive days (daily); 55‐65 DB/ 30 min | > 10 | ≥ 8 | |
| Yu 2021 | MM: recorded mother's spoken voice reading standardised story and adding personal individual words | random & parent |
pain | once a day for 3 consecutive days/13 min | > 10 | 3‐7 | ||
| Total |
MM: 17 MT: 7* Recorded: 16 Live: 8* |
Parent: 12* Random: 13 |
Spoken voice: 7 Sung voice: 17* Music: 4 |
Rhythmic sound: 2 Womb sound: 4* |
Alone: 19* SSC: 4* Arm: 2 Pain: 2 |
Frequency: 3 ‐ n.a. Min: 13‐45 |
5‐10 min: 3 > 10 min: 20* |
3‐7 times: 8 ≥ 8 times: 16* |
*minus 1 since Kehl 2021 is an additional publication of the same intervention study of Haslbeck 2021
dB: decibel level
MM: music medicine
MT: music therapy
Min: minute
n.a.: not available
SSC: Skin‐to‐Skin Care
3. Summary second music/vocal intervention characteristics of included studies.
| Study | Intervention 2: type & delivery (MM/MT): live, infant‐directed, entrained/recorded or standardised | Musical selection by parents/random, unidentified or unknown | Spoken voice/sung voice/music without voice |
Womb sound/ Rhythmic sound/ Breathing sound |
Music alone/ Combined with SSC/ Laying mother's arm/ Combined with RISS |
Frequency/ Dose (minutes) |
5‐10 minutes/ > 10 minutes |
3‐7 times/ ≥ 8 times |
| Ettenberger 2014 | MT: Live entrained vocal & instrumental music (parents preferred); dB n.a. | parent | spoken voice | SSC | 2‐4 x over two weeks/13.7 min (8‐25) | > 10 | 3‐7 | |
| Kucuk Alemdar 2020 | MM: Live Mum's picked sang lullaby; 65 dB | parent | spoken voice | SSC | Per day for 3 consecutive days/15 min | > 10 | 3‐7 | |
| Loewy 2013 | MT: Live entrained ocean disc sound; 55‐65 dB | random | music | womb sound | alone | 6 x over two consecutive weeks/10 min | 5‐10 | ≥ 8 |
| Namjoo 2021 | MM: Live singing lullabies by mother, baby placed in her arms | parent | spoken voice | arm | once a day for 14 days/20 min | > 10 | ≥ 8 | |
| Vastani 2017 | MM: Live nurses` singing of standardised lullaby; 60‐70 dB | random | spoken voice | alone | 3 x for 3 consecutive days (daily)/30 min | > 10 | 3‐7 | |
| White‐Traut 1988 | MM: Live tactile, vestibular motion, auditory & visual stimulation; dB: n.a. | parent | spoken & sung voice |
RISS | 6 x over three consecutive days/15 min | > 10 | ≥ 8 | |
| Wirth 2016 | MM: Recorded sung lullabies; 55‐65 | random | spoken voice | alone | 14 x over 14 consecutive days (daily)/30 min | > 10 | ≥ 8 | |
| Total |
7 x intervention 2 MT: 2 MM: 5 Live: 6 Recorded: 1 |
Parent: 5 Random: 3 |
Spoken voice: 1 Sung voice: 6 |
Womb sound: 1 |
Alone: 3 SSC: 2 Arm: 1 RISS: 1 |
Frequency: 3 ‐ n.a. Min: 14‐30 |
5 ‐10 min: 1 > 10 min: 6 |
3‐7 times: 3 ≥ 8 times: 4 |
dB: decibel level
MM: music medicine
MT: music therapy
Min: minute
n.a.: not available
RISS: massage, talking, eye contact, and rocking
SSC: Skin‐to‐Skin Care
4. Summary third music/vocal intervention characteristics of included studies.
| Study | Intervention 3 | Intervention type & delivery (MT), live, infant‐directed, entrained | Musical selection by parents/random, unidentified or unknown | Spoken voice/sung voice/music without voice | Womb sounds/rhythmic sounds | Music intervention alone/combined with skin‐to skin | Frequency/dose (minutes) | 5‐10 minutes/ > 10 minutes | Between 3‐7 times |
| Loewy 2013 | live music | MT: Live entrained gato box; 55‐65 dB | By music therapist | only music | rhythmic sound | alone | 6 times over two consecutive weeks/10 min | 5‐10 min | 3‐7 times |
dB: decibel level
MT: music therapy
Min: minute
Excluded studies
After the abstract/title and records screening, we excluded 63 full‐text studies for the following reasons.
Eight of the 63 studies were identified as not suitable due to their study design.
Four studies displayed unmet criteria of the patient population.
Forty‐four studies used an intervention outside our study purpose; for example, the intervention was delivered only once.
Six studies were identified as not suitable due to the comparator.
One study, was excluded, due to unmet criteria of the data outcomes.
For further information see the Characteristics of excluded studies and Figure 1.
Ongoing studies
There are 33 ongoing studies. For further details of those studies, please see the Characteristics of ongoing studies.
Risk of bias in included studies
The general risk of bias in the included studies varied from low (e.g. for allocation concealment) to high risk of bias (e.g. for blinding outcome assessors), often remaining unclear since detailed descriptions were missing for many risk of bias areas. The risk of bias is described in detail in the Characteristics of included studies and summarised in Figure 2; Figure 3.
2.

Risk of bias graph
3.

Risk of bias summary
Allocation
Most of the studies clearly reported random sequence generation, except for five studies (Cevasco 2008; Lejeune 2019; Tandoi 2015; White‐Traut 1988; Wirth 2016), in which the sequence generation was not clearly described, and three studies with a high risk of bias concerning random sequencing (Farhat 2010; Nakhwa 2017; Namjoo 2021).
For concealment of allocation, the risk of bias was low in 11 studies and unclear in 13 studies not reporting sufficient detail on if/how allocation concealment was achieved. Only one study displayed a high risk of allocation concealment (Nakhwa 2017) (see Figure 2; Figure 3).
Blinding
Blinding of both participants and personnel could not be achieved through the study design in 22 of the 25 studies, leading to a high risk of performance bias as anticipated in our study protocol (Haslbeck 2019). Three studies using prerecorded music involved delivering a 'sham' intervention (silent prerecorded track) to the control group to achieve blinding of personnel (Caparros‐Gonzalez 2018; Jabraeili 2016; Lejeune 2019). Twelve studies reported blinding of outcome assessment. Nevertheless, nine studies demonstrated a high risk of detection bias by not blinding outcome assessors and, in four studies, there were insufficient details to determine if detection bias occurred (Kucuk Alemdar 2020; Liao 2021; Nakhwa 2017; Namjoo 2021).
Incomplete outcome data
Four studies were at high risk of attrition bias because of incomplete assessment of the trial cohort (Cevasco 2008; Ettenberger 2014; Johnston 2007; Lejeune 2019) and, in four studies, completeness of outcome data remained unclear (Farhat 2010; Kraft 2021; White‐Traut 1988). The remaining studies displayed a low risk for attrition bias.
Selective reporting
In more than half of the included studies, we were not able to assess reporting bias since we could not identify study protocols or other sources of prespecified outcomes of most of the trials. We assessed eleven studies as having a low risk of selective reporting and considered one study to have a high risk of reporting bias due to missing outcome data (Kraft 2021), compared with the trial register entry of the trial.
Other potential sources of bias
Many studies showed no other sources of bias (n =11). We considered twelve studies to have an unclear risk of bias and two studies to have a high risk of bias due to limited baseline characteristics of the included mothers (Kraft 2021), and due to differences in reporting of the number of randomised preterm infants in the paper (Cevasco 2008).
Effects of interventions
See: Table 1
See: Table 1
We included 25 studies, of which 23 studies were included in the meta‐analyses. In two studies, data were incompletely reported and therefore not eligible to be included (Nakhwa 2017; White‐Traut 1988). For short‐term infant physiological outcomes, we used two different endpoints (during the intervention and post‐intervention). We analysed studies during painful procedures separately. Where sufficient studies were available, we conducted subgroup analysis for recorded versus live musical/vocal interventions or for intervention frequency differences. There were insufficient studies for building further subgroups meaningfully.
Primary outcomes
Short‐term outcome in preterm infants
Oxygen saturation
We identified 14 studies that reported measuring oxygen saturation. In one study (Ettenberger 2014), outcome data were not sufficiently reported. After receiving the requested data from the authors, the amount of missing data did not allow for inclusion of the study in an analysis, so we conducted meta‐analyses using 13 studies (see Table 2).
During intervention
Ten studies provided data for this outcome (Calabro 2003; Epstein 2021; Farhat 2010; Jabraeili 2016; Kucuk Alemdar 2020; Liao 2021; Loewy 2013; Namjoo 2021; Portugal 2017; Tandoi 2015). Music and vocal interventions did not increase oxygen saturation in preterm infants during the intervention compared to standard care (mean difference (MD) 0.13, 95% CI ‐0.33 to 0.59; P = 0.59; 958 participants, 10 studies; high‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Music versus control, Outcome 1: Oxygen saturation during intervention
As heterogeneity was moderate (Chi² = 24.68, df = 10 (P = 0.006); I² = 59%), we conducted a sensitivity analysis excluding data from four studies with high selection or detection bias (Farhat 2010; Namjoo 2021; Portugal 2017; Tandoi 2015) so that heterogeneity was reduced to a low level (Chi² = 5.99, df = 5 (P = 0.31); I² = 17%). However, after sensitivity analysis, the overall effect on oxygen saturation showed no substantial changes (P = 0.45 without high‐risk bias studies versus P = 0.59 with all included studies). Subgroup analysis comparing recorded versus live music showed no substantial changes either (recorded music: P = 0.40, live music: P = 0.24) nor clear subgroup differences (Chi² = 1.78, df = 1 (P = 0.18), I² = 43.9%; Analysis 1.1).
During intervention with heel lance
One study (Yu 2021) with 64 infants compared oxygen saturation during recorded maternal voice during heel lance with standard care without discovering a significant difference.
Post‐intervention
Seven studies assessed oxygen saturation post‐intervention (Calabro 2003; Caparros‐Gonzalez 2018; Jabraeili 2016; Liao 2021; Loewy 2013; Namjoo 2021; Portugal 2017). Music and vocal interventions probably do not increase oxygen saturation post‐intervention compared to standard care (MD 0.63, 95% CI ‐0.01 to 1.26; P = 0.05; 800 infants, 7 studies; moderate‐certainty evidence; Analysis 1.2). Heterogeneity was low (Chi² = 7.65, df = 7 (P = 0.36); I² = 8%) and subgroup analysis comparing recorded versus live intervention revealed no clear subgroup differences (Chi² = 0.00, df = 1 (P = 0.98), I² = 0%) (see Analysis 1.2).
1.2. Analysis.

Comparison 1: Music versus control, Outcome 2: Oxygen saturation post‐intervention
Post‐intervention with heel lance
Two studies evaluated oxygen saturation after heel lance (Johnston 2007; Yu 2021). The evidence suggested that music and vocal interventions result in no substantial difference in oxygen saturation after the intervention compared to standard care after heel lance (MD 0.75, 95% CI ‐0.02 to 1.51; P = 0.06; 100 infants, 2 studies; Analysis 1.3). Heterogeneity was high (Chi² = 3.51, df = 1 (P = 0.06); I² = 71%). After removing the study with high attrition bias (Johnston 2007), the remaining study (Yu 2021), suggested a favourable effect of the music intervention on oxygen saturation compared to standard care (MD 0.93, 95% CI 0.14 to 1.72; P = 0.02; 64 infants; one study; Analysis 1.3).
1.3. Analysis.

Comparison 1: Music versus control, Outcome 3: Oxygen saturation after heel lance
Long‐term outcome in preterm infants
Infant development
Two studies provided data on all three Bayley Scale composition scores (cognitive, motor, language) at two years of age (Haslbeck 2021; Lejeune 2019) (Table 2). Music/vocal interventions may not increase infant development or the cognitive composition score (MD 0.35, 95% CI ‐4.85 to 5.55; P = 0.90; 69 infants, 2 studies; low‐certainty evidence; Analysis 1.4), the motor composition score (MD ‐0.17, 95% CI ‐5.45 to 5.11; P = 0.95; 69 infants, 2 studies; low‐certainty evidence; Analysis 1.4), or the language composition score (MD 0.38, 95% CI ‐5.45 to 6.21; P = 0.90; 69 infants, 2 studies; low‐certainty evidence; Analysis 1.4), although the CIs of all subscores included meaningful effects in both directions. Heterogeneity between the studies was minimal (I2 = 0%).
1.4. Analysis.

Comparison 1: Music versus control, Outcome 4: Infant Development: Bayley Scales of Infant and Toddler Development
Outcomes in parents
Change in state‐trait‐anxiety
Four music therapy studies (Ettenberger 2014; Kehl 2021; Kraft 2021; Menke 2021), reported data on parental anxiety after the whole treatment period, measured with the State‐Trait‐Anxiety Inventory (Table 2). Music and vocal interventions may not reduce parental state‐trait anxiety (MD ‐1.12, 95% CI ‐3.20 to 0.96; P = 0.29; 97 parents, 4 studies; low‐certainty evidence; Analysis 1.5), although the CIs included meaningful effects in favour of the music therapy group. Heterogeneity between the studies was minimal (I2 = 0%) (see Analysis 1.5).
1.5. Analysis.

Comparison 1: Music versus control, Outcome 5: Parental anxiety: STAI‐T
Secondary outcomes
Short‐term outcomes in preterm infants
Respiratory rate
We identified 10 studies that reported measuring respiratory rate by e.g. electric strain‐gauges, thoracic impedance plethysmography, nasal air‐flow sensor, and spirometers. We conducted meta‐analyses using nine studies. One study (Yu 2021), evaluated music during and after heel lance; these results are reported narratively (see Table 6).
5. Summary characteristics of included studies secondary outcomes.
| Study |
Secondary outcome infant heart rate |
Secondary outcome infant respiratory rate |
Secondary outcome infant heart rate variabilty |
Secondary outcome infant behavioural outcomes (Als) |
Secondary outcome infant hospitalisation (days) |
Secondary outcome infant adverse effects |
Secondary outcome infant weight gain (kg/day) |
Secondary outcome parents' well‐being |
Secondary outcome parents attachment |
| Calabro 2003 | x | x | |||||||
| Caparros‐Gonzalez 2018 | x | x | |||||||
| Cevasco 2008 | x | x | x (Parental Adaption Inventory) | ||||||
| Portugal 2017 | x | x | |||||||
| Epstein 2021 | x | x | narratively reported | x | x (STAI directly after intervention) | ||||
| Ettenberger 2014 | no results reported | x | x | x (STAI‐T directly after intervention) | x (mother‐to‐infant bonding) | ||||
| Farhat 2010 | x | x | x | ||||||
| Haslbeck 2021 | |||||||||
| Jabraeili 2016 | |||||||||
| Johnston 2007 | x | ||||||||
| Kehl 2021 | x (EPDS; PSS; STAI‐T) | x (PRAM) | |||||||
| Kraft 2021 | |||||||||
| Kucuk Alemdar 2020 | x | x | |||||||
| Lafferty 2021 | x | ||||||||
| Lejeune 2019 | |||||||||
| Liao 2021 | x | x | narratively reported | ||||||
| Loewy 2013 | x | x | x (parental stress) | ||||||
| Menke 2021 | x | x | x (EPDS; PSQ; PCQ; STAI‐T) | ||||||
| Nakhwa 2017 | |||||||||
| Namjoo 2021 | x | ||||||||
| Tandoi 2015 | x | x | x | ||||||
| Vastani 2017 | x | ||||||||
| White‐Traut 1988 | |||||||||
| Wirth 2016 | x | x | |||||||
| Yu 2021 | x | x during pain | |||||||
| Total | 14 | 10 | 1 | 2 | 3 | 2 | 4 (5) | (6) | (2) |
EPDS: Edinburgh Postnatal Depression Scale
n: number
n.a.: not available
PCQ: Parental Competences Questionnaire
PRAM: Pictorial Representation of Attachment Measure
PSS: Parental Stressor Scale
PSQ: Parental Stress Questionnaire
RISS: massage, talking, eye contact and rocking
STAI‐T: State‐Trait Anxiety Inventory
During intervention
Seven studies provided data on respiratory rate during the intervention demonstrating that music and vocal interventions probably do not reduce respiratory rate compared to standard care (Calabro 2003; Epstein 2021; Kucuk Alemdar 2020; Loewy 2013; Portugal 2017; Tandoi 2015; Wirth 2016) (MD 0.42, 95% CI ‐1.05 to 1.90; P = 0.57; 750 infants, 7 studies; moderate‐certainty evidence; Analysis 1.9). Heterogeneity was low (Chi² = 11.72, df = 6 (P = 0.07); I² = 49%; Analysis 1.9).
1.9. Analysis.

Comparison 1: Music versus control, Outcome 9: Respiratory rate during intervention
During intervention with heel lance
One study (Yu 2021) with 64 infants compared the respiratory rate during heel lance with the recorded maternal voice to standard care without discovering significant differences.
Post‐intervention
Five studies (Calabro 2003; Caparros‐Gonzalez 2018; Loewy 2013; Portugal 2017; Wirth 2016) provided data on respiratory rate post‐intervention demonstrating that music and vocal interventions probably do not reduce respiratory rate compared to standard care (MD 0.51, 95% CI ‐1.57 to 2.58; P = 0.63; 636 infants, 5 studies; moderate‐certainty evidence; Analysis 1.10). Heterogeneity was low (Chi² = 7.66, df = 4 (P = 0.10); I² = 48%; Analysis 1.10).
1.10. Analysis.

Comparison 1: Music versus control, Outcome 10: Respiratory rate post‐intervention
Post‐intervention with heel lance
One study (Yu 2021) with 64 infants compared the respiratory rate after heel lance with the recorded maternal voice to standard care without discovering significant differences.
Heart rate
We identified 14 studies that reported measuring heart rate by pulse oximetry or electrocardiogram. In one of these studies (Ettenberger 2014), outcome data were not sufficiently reported. After receiving the requested data from the authors the amount of missing data did not allow us to include the study in an analysis, so we conducted meta‐analyses using 13 studies (see Table 6).
During intervention
Eleven studies (Calabro 2003; Epstein 2021; Farhat 2010; Kucuk Alemdar 2020; Liao 2021; Loewy 2013; Namjoo 2021; Portugal 2017; Tandoi 2015; Vastani 2017; Wirth 2016) provided data on this outcome. Music and vocal interventions probably reduce heart rates in preterm infants during the intervention compared to standard care (MD ‐1.38, 95% CI ‐2.63 to ‐0.12; P = 0.03; 1014 infants, 11 studies; moderate‐certainty evidence; Analysis 1.6) with the CI ranging from small to medium effects favouring the intervention. Heterogeneity was moderate (Chi² = 23.15, df = 10 (P = 0.01); I² = 57%). Significant subgroup differences (Chi² = 8.66, df = 1 (P = 0.003), I² = 88.5%) indicated a greater reduction in the heart rate for music/vocal intervention with a frequency of at least 8 times (P = 0.0003). Investigating the related funnel plot in Figure 4 did not yield clear asymmetry, thus there was no clear indication of a risk of non‐reporting bias.
1.6. Analysis.

Comparison 1: Music versus control, Outcome 6: Heart rate during intervention
4.

During intervention with heel lance
One study (Yu 2021) with 64 infants compared the heart rate during heel lance with the recorded maternal voice to standard care without discovering a significant difference.
Post‐intervention
Nine studies (Calabro 2003; Caparros‐Gonzalez 2018; Farhat 2010; Liao 2021; Loewy 2013; Namjoo 2021; Portugal 2017; Vastani 2017; Wirth 2016), provided data on heart rate post‐intervention. Music and vocal interventions reduced heart rate post‐intervention compared to standard care (MD ‐3.80, 95% CI ‐5.05 to ‐2.55; P = P < 0.00001; 903 infants, 9 studies; high‐certainty evidence; Analysis 1.7). Wide confidence intervals ranged from a medium to large effect favouring the intervention (Analysis 1.7). Heterogeneity was moderate (Chi² = 18.50, df = 9 (P = 0.03); I² = 51%). Sensitivity analysis excluding studies with high selection or detection bias reduced heterogeneity (Chi² = 4.68, df = 4 (P = 0.32); I² = 15%) and the overall effect size (P < 0.0001), but still demonstrated a substantial beneficial effect.
1.7. Analysis.

Comparison 1: Music versus control, Outcome 7: Heart rate post‐intervention period
Subgroup differences (Chi² = 5.20; df = 1; P = 0.02; I² = 80.8%) indicated a greater reduction in the heart rate post‐intervention for recorded music and vocal intervention than for live music without substantial changes in the favouring of music and vocal interventions (Analysis 1.7). However, after sensitivity analysis, excluding studies with high selection or detection bias, we found no subgroup differences (Chi² = 0.09, df = 1 (P = 0.76), I² = 0%).
Post‐intervention with heel lance
Two studies (Johnston 2007; Yu 2021) with 100 infants, evaluated heart rate post‐intervention with heel lance showing no substantial difference in heart rate after the intervention compared to standard care after heel lance (MD 1.11, 95% CI ‐3.45 to 5.67; P = 0.63; 200 infants, 2 studies; Analysis 1.8). Heterogeneity was high (Chi² = 3.41, df = 1 (P = 0.06); I² = 71%) and, after removing the study with high attrition bias (Johnston 2007), the remaining study (Yu 2021) showed no favourable effect of the music intervention on heart rate compared to standard care (MD ‐2.6, 95% CI ‐7.72 to 3.60; P = 0.48; 64 infants, 1 study; Analysis 1.8).
1.8. Analysis.

Comparison 1: Music versus control, Outcome 8: Heart rate after heel lance
Heart rate variability
One study (Epstein 2021), of 35 infants with severe brain injury (grade 3 or 4 intraventricular haemorrhage or periventricular leukomalacia), measured heart rate variability by low‐frequency power (ms2/Hz); high‐frequency power (ms2/Hz); and low frequency/high‐frequency ratio, reflecting the balance between sympathetic and parasympathetic tone. A higher mean ± standard deviation (SD) LF/HF ratio (1.8 ± 0.7 vs. 1.1 ± 0.25, P = 0.01), were reported in maternal skin‐to‐skin care combined with maternal singing during music therapy as compared to maternal skin‐to‐skin‐care alone.
Behavioural outcomes: behavioural state (Als)
Two studies (Epstein 2021; Tandoi 2015) provided data on behavioural outcomes measured with the behavioural numerical scales for neonates by Als 2005 (Table 6). We found no effect of music and vocal interventions compared to standard care (MD ‐0.12, 95% CI ‐0.52 to 0.27; P = 0.54; 69 infants, 2 studies; Analysis 1.11). Heterogeneity was very high (Chi² = 12.93, df = 1 (P = 0.0003); I² = 92%; Analysis 1.11) and, due to the small number of studies, subgroup analysis and sensitivity analysis were not indicated.
1.11. Analysis.

Comparison 1: Music versus control, Outcome 11: Behavioural outcomes: Behavioural state (Als)
Other behavioural outcomes in the infants were reported by four small‐scale studies (Calabro 2003; Kraft 2021; Nakhwa 2017; White‐Traut 1988), measuring with different outcome scales which will be reported narratively: Calabro 2003 and colleagues, showed no significant changes in organised/disorganised infant behaviour between recorded sedative music and standard care group analysing 17 infants. Nakhwa 2017 provided data of 36 infants on the infant motor performance score showing a significant beneficial effect in favour of the music group (music group: MD 21.16 ± 0.5145; control group: MD 20.78 ± 0.4278; P < 0.0001). They reported significantly improved Infant Neurological International Battery (INFANIB) scores in favour of the music group (MD 65.11 ± 1.568) compared to the control group (MD 63.22 ± 2.756) with a P‐value of 0.0163. Kraft 2021 provided data of 21 infants on the Neonatal Infant Stressor Scale showing no significant differences between the music and control groups. White‐Traut 1988 showed no significant changes between the mother's live talking/singing group compared to standard care measured with the Nursing Child Assessment Feeding Scale (NCAFS) in 22 infants.
Hospitalisation
Three studies (Cevasco 2008; Ettenberger 2014; Menke 2021), provided data on oxygen hospitalisation in days (Table 6). The evidence suggested no effect of the intervention (MD ‐1.57, 95% CI ‐7.64 to 4.50; P = 0.61; 89 infants, 3 studies; Analysis 1.12) compared to standard care with wide CIs both favouring music and the control group. Heterogeneity was very high (Chi² = 9.78, df = 2 (P = 0.008); I² = 80%; Analysis 1.12) and all studies displayed high risk of bias.
1.12. Analysis.

Comparison 1: Music versus control, Outcome 12: Hospitalisation in days
Adverse effects
None of the included studies reported adverse effects from the music and vocal intervention including severe apnoea during the intervention requiring stimulation by the neonatal care team. However, only in two included studies (Lafferty 2021; Liao 2021) were adverse effects measured as explicit outcomes of interest.
Weight gain
Four studies (Cevasco 2008; Ettenberger 2014; Farhat 2010; Menke 2021), provided data on weight gain in grams per day (Table 6). We found no effect of the intervention (MD 3.88, 95% CI ‐1.61 to 9.38; P = 0.17; 137 infants, 4 studies; Analysis 1.13) compared to standard care with wide CIs both favouring the music group more than the control group. Heterogeneity was moderate (Chi² = 6.19, df = 3 (P = 0.10); I² = 52%; Analysis 1.13) and all studies displayed high risk of bias.
1.13. Analysis.

Comparison 1: Music versus control, Outcome 13: Weight gain
Long‐term outcomes in preterm infants
No study provided data on neurodevelopment in preterm infants at five years of age assessed by standardised follow‐up examinations.
Outcomes in parents
Parental well‐being
Postnatal depression
Two music therapy studies (Kehl 2021; Menke 2021) provided data on parental depression measures with the Edinburgh Postnatal Depression Scale (EPDS) (Table 6). Music therapy may not reduce parental depression compared to standard care with the CIs ranging from no effect to a favourable effect (MD 0.50, 95% CI ‐1.80 to 2.81; P = 0.67; 67 participants; 2 studies; low‐certainty evidence; Analysis 1.14). Heterogeneity between studies was moderate (Chi² = 2.05, df = 1 (P = 0.15); I² = 51%; Analysis 1.14).
1.14. Analysis.

Comparison 1: Music versus control, Outcome 14: Postnatal depression: EPDS
State anxiety
Three music therapy studies (Kehl 2021; Kraft 2021; Menke 2021), provided data on parental state anxiety measured with the Parental State Anxiety Inventory (Table 6). The evidence is very uncertain about the effect of music therapy on parental state anxiety compared to standard care with the CIs ranging from no effect to a favourable effect (MD ‐0.15, 95% CI ‐2.72 to 2.41; P = 0.91; 87 parents, 3 studies; very low‐certainty evidence; Analysis 1.15). Heterogeneity between studies was very low (Chi² = 1.11, df = 2 (P = 0.57); I² = 0%; Analysis 1.15).
1.15. Analysis.

Comparison 1: Music versus control, Outcome 15: Parental state anxiety: STAI‐SKD
Perception and stress outcomes
We identified three more small‐scale studies (Cevasco 2008; Kehl 2021; Menke 2021), that reported measuring parental well‐being after the whole intervention period with other outcomes (see Table 6) which we report narratively. Cevasco 2008 assessed maternal well‐being in 16 mothers by measuring with the Parental Perception Inventory (PPI) showing no significant differences between the music and control groups. Kehl 2021 provided data on parental stress measured by the Parental Stressor Scale: Neonatal Intensive Care Unit (PSS: NICU) in 32 parents identifying no significant differences between the intervention and the standard groups after the whole intervention period. Menke 2021 provided data from 30 parents on parental stress measured by the Parental Stress Questionnaire: Stress (PSQ) with no evidence of a difference between the intervention and control groups after the whole intervention period.
Attachment/bonding
We identified one music therapy study (Kehl 2021), with 32 parents providing data on the Pictorial Representation of Attachment Measurement (PRAM). There was no significant difference between music therapy and standard care after the intervention period. Another music therapy study (Ettenberger 2014) provided data on the Mother‐Infant Bonding Scale with 16 mothers displaying no significant differences between the groups after the last intervention (Table 6).
Discussion
Summary of main results
Our review evaluated the effectiveness of music and vocal interventions compared to standard care to improve neurodevelopmental outcomes in preterm infants and the well‐being of parents. We included 25 RCTs, involving 1532 randomised infants and 691 parents. The music and vocal interventions varied widely in intervention type, delivery, frequency, and duration across studies mainly characterised by calm, soft, and musical parameters in lullaby style, often integrating the sang mother's voice live or recorded delivered by neonatal staff, parents, or music therapists. The included studies compared the intervention to standard care in extremely to late‐preterm infants.
We found no substantial effects on our primary outcomes in the infants and parents. Music and vocal interventions do not increase oxygen saturation in preterm infants during the intervention compared to standard care and probably not post‐intervention either. The evidence suggests that the intervention does not increase infant development. The certainty of the evidence was rated as 'low' due to imprecision since data on infant development were only available from two trials involving 69 infants in total meaning that our confidence in these results is limited (Table 1). Music therapy compared to standard care may not reduce our primary outcome of parental state‐trait anxiety, measured with the State‐Trait Anxiety Inventory. However, the certainty of the evidence was rated again as 'low' meaning that these results should be considered with caution.
A substantial effect in favour of music and vocal interventions was retained in one of our secondary outcomes. Music and vocal interventions likely result in a substantial small‐to‐medium reduction in heart rate in preterm infants compared to standard care during the intervention, with moderate certainty, meaning we are moderately confident in the effect estimate. Greater reduction of the heart rate was associated with the music/vocal intervention with at least eight sessions of music therapy. Post‐intervention, this effect was even greater with a medium‐to‐large substantial effect in favour of the music and vocal interventions on heart rate, with high certainty, meaning we are highly certain that there is evidence of a beneficial effect of music/vocal interventions on heart rate in the post‐intervention period (see Table 1). However, this beneficial effect on the heart rate is of questionable clinical significance.
Music and vocal interventions compared to standard care probably do not reduce respiratory rate. The evidence is uncertain of any effect on heart rate variability, behavioural outcomes, hospitalisation, weight gain, neurodevelopmental outcomes at five years, and further parental well‐being and attachment outcomes. We found no certain evidence of any effect of musical and vocal interventions during and after painful procedures. For most of these outcomes, the certainty of the evidence was 'low' meaning that our confidence in these results is limited. We could not identify reported adverse effects of musical and vocal interventions on preterm infants and their parents. Study numbers were not sufficient to evaluate any possible differences between any of the intervention types, duration, frequency, or gestational age in the infants.
Overall completeness and applicability of evidence
Our review results suggest that the evidence base for the use of musical and vocal interventions in neonatal care is emerging, but still limited in number while displaying high heterogeneity in the intervention types, frequency, and duration. Physiological short‐term outcomes in the infants comparing recorded music and vocal interventions with standard care inherent in a short time frame received the most attention. One reason for this may be that these outcomes and interventions are most feasible for research purposes since physiological data, such as oxygen saturation, respiratory rate, and heart rate are assessed in preterm infants during standard routine care anyway. Music therapy trials with individualised family‐integrating live music are still limited in number whereas music medicine trials with recorded interventions received more attention. It is possible that a recorded intervention appears more feasible for research since only a recorded delivery can be double‐blinded, e.g. by a sham recording in the control group. However, in the last decades, more and more music therapy services have been applied in clinical neonatal practice around the world (Bieleninik 2016; Haslbeck 2012; Loewy 2013; Standley 2012) and the first randomised controlled trials were conducted on live family‐centred music therapy including parental outcomes (Haslbeck 2021; Kraft 2021; Loewy 2013; Menke 2021). Family‐centred or family‐integrated care programmes are highly endorsed by several organisations including the American Academy of Pediatrics (AAP) and the European Foundation for the Care of Newborn Infants (EFCNI) (Mushtaq 2019). Therefore, it would be important to evaluate further the possible effects of integrating parents as primary caregivers of their infant with the approach of family‐centred music therapy on infant and parental short‐ and long‐term outcomes in big‐scale trials.
Notably, clinically relevant long‐term developmental outcomes in preterm infants, such as the Bayley Scales of Infants and Toddler Development at two years of age were only assessed by two small‐scale trials, and five years neurodevelopmental outcomes are completely missing, meaning that the current evidence is too limited to guarantee certainty of the evidence of musical and vocal interventions on clinically relevant outcomes in preterm infants. Moreover, the outcomes addressed in the included studies cover clinically pathological‐oriented outcomes. However, resource‐oriented outcomes that assess quality of life, resilience, empowerment, self‐efficacy, and mental health may enhance the relevance of the review in the future since particularly music therapy approaches are based on health‐ and resource‐oriented approaches.
It was not possible to determine if certain types, frequencies, durations, and delivery modes of musical and vocal interventions have a differential impact on certain outcomes since the provided interventions varied widely in all aspects. Neither was it possible to distinguish between certain participant characteristics, such as birth weight categories or gender. Including clinically stable preterm infants without severe malformation or congenital diseases, intraventricular haemorrhages (IVH) (III‐IV) and periventricular leukomalacia (PVL) received the most attention. However, there was one exceptional small‐scale study (Epstein 2021) that included only preterm infants who developed IVH grades 3 or 4 or PVL diagnosed by brain ultrasound demonstrating unstable physiological responses during maternal singing with music therapy. Therefore, it would be important to evaluate further if music and vocal interventions may have a different effect on preterm infants with severe brain injury. Notably, most studies which included parents were only able to include mothers or fewer fathers than mothers. Thus, we were are not certain if the current evidence is applicable to fathers as well.
Our review results may reflect moderate generalisability since the review evidence comes from neonatal intensive care settings from around the world with cultural variations in treatment practices, musical choice, neonatal standard care, and parental attitudes towards the intervention. Additionally, an interdisciplinary variety was given by researchers from various disciplines (e.g. medicine, nursing, psychology, and music therapy) that conducted the studies. Moreover, the relevance of our findings will vary from healthcare systems which value music and music therapy to healthcare systems which decline or do not have the resources for complementary medicine approaches. However, parental lullaby singing is known in almost every culture and available to everyone so that health equity should play no substantial role in empowering parents to sing for their preterm infant.
Quality of the evidence
We found moderate‐to‐high‐certainty evidence for physiological outcomes in the infants in this review. The certainty of the evidence was rated as 'low' to 'very low' for other outcomes in the infants and parents (see Table 1) which means that further research is likely to change the effect estimates and our confidence that they are precise. These outcome results should therefore be considered with caution. The methodological quality of included studies varied. As anticipated in our Cochrane protocol (Haslbeck 2019), most studies did not blind the intervention for participants and personnel due to the nature of the intervention itself, except for three studies with recorded music which provided a scam intervention for the control group (Caparros‐Gonzalez 2018; Jabraeili 2016; Lejeune 2019). Selection bias was mostly low risk for adequate random sequence generation, but mainly unclear or low for allocation concealment (Figure 3). Many studies demonstrated a high risk of detection bias or insufficient details to determine if detection bias occurred. The overall likelihood of all other biases was low or unclear.
Our assessments of the certainty of the evidence mainly reflect concerns about the risk of bias and imprecision due to wide CIs and small sample sizes. Although we judged the studies to be at varying risks of bias overall, the evidence for our physiological outcomes is drawn from studies at low risk of bias demonstrating moderate‐to‐high certainty. We have demonstrated a high certainty of the evidence, particularly for the substantial effect in favour of music and vocal interventions on the infants' heart rates. One primary outcome, oxygen saturation, demonstrated a high certainty of the evidence while the two other primary outcomes of long‐term infant development and change in parental anxiety reflected low‐to‐very low certainty of the evidence. We downgraded the quality of evidence for these two main outcomes, due mainly to imprecision (Table 1).
Potential biases in the review process
We performed an extensive search of databases and additional sources and applied no restrictions concerning nationality or language within the search process; and relied additionally on an existing international network of leading researchers in the field. Thus, we consider that the probability that we have missed an eligible trial is low. There was no evidence of publication bias either. Study investigators were contacted directly to request missing data and most authors replied and delivered the missing trial details.
We conducted study selection, data extraction, and risk of bias assessments in duplicate and independently, and we reached a consensus by discussing any discrepancies. Some published trial reports did not provide enough details to extract outcomes and adequately assess risks of bias. Although we contacted the authors of the trials to request missing data, we could not avoid some bias assessments in the review process due to incomplete reporting of trial details or results, or both. Furthermore, we found 38 ongoing studies, meaning that incorporating these studies in a future update may alter the conclusions of the current review.
Agreements and disagreements with other studies or reviews
The findings of our review are partially consistent with other meta‐analyses of music interventions for preterm infants and their parents. Our identified substantial beneficial effect of music and vocal interventions on heart rate is consistent with the recent meta‐analysis results of Yue 2021 which found that the heart rate in the music group was significantly higher than in the control group (MD = −3.21; 95% CI = −5.22 to −1.19; P = 0.002). Our findings are further in line with the meta‐analysis results of Standley 2012 suggesting a beneficial effect on heart rate and the results of Mohan 2021 that provided an overview of systematic reviews demonstrating a beneficial heart rate effect as well. In contrast, our results are inconsistent with the review of Bieleninik 2016 which found no substantial effect of music therapy on the infants' heart rate, but a large effect favouring music therapy for infant respiratory rate similar to Yue 2021 which we cannot confirm by our meta‐analysis.
Our results that music and vocal interventions do not increase oxygen saturation are consistent with the meta‐analysis of Bieleninik 2016, Mohan 2021 and Yu 2021 but inconsistent with the review of Standley 2012 which suggests a significant difference in favour of music therapy. Interestingly Van Dokkum 2021 identified two distinct reactions in oxygenations to music therapy. These individually different reactions in the infants to music therapy may explain our identified lack of evidence of an effect on a group‐average level and may need further exploration for possible clinical relevance.
Notably, Bieleninik 2016 and Yu 2021 reported a significant positive influence of music therapy on maternal anxiety in contrast to our findings. However, we included only anxiety outcomes that had been assessed after the whole intervention or control period in contrast to the included studies in these two reviews (Bieleninik 2016; Yu 2021) evaluating the immediate short‐term effect on maternal anxiety directly after the music intervention. These contrary results at different time points are in line with the recently published multi‐centre study by Gaden 2022, which found no significant differences after the music therapy intervention period at discharge between the music therapy and the control groups. Interestingly, the two studies (Kehl 2021; Menke 2021) which assessed maternal anxiety over the whole music therapy period in neonatal care at various time points showed a significant decrease in parental anxiety levels over the first weeks in neonatal care that was only apparent in the music therapy group and not in the control group. Similarly, Kraft 2021 suggested that early provision of family‐centred music therapy may accelerate the reduction of maternal anxiety particularly in the first stressful weeks in the intensive care unit. Moreover, maternal anxiety levels were strongly related to infant stress, as indicated by Ettenberger 2014; and Kraft 2021 which claimed that paternal anxiety is heavily dependent on particular stress factors during the neonatal intensive care period and, thereby, should be considered as possible confounders in future studies.
Music and vocal interventions may result in little to no difference in infant development. To our knowledge, to date, only two small‐scale studies (Haslbeck 2021; Lejeune 2019) assessed possible long‐term effects at two years, and the available data may be insufficient in amount to demonstrate efficacy. Interestingly, both studies reported in further publications (Haslbeck 2020a; Lordier 2019) improved brain functional connectivity measured by resting‐state functional magnet resonance imaging at term‐equivalent age suggesting neurodevelopmental maturation effects. These pilot results indicate that further research is needed with big‐scale studies correlating short‐term brain imaging outcomes with neurodevelopmental long‐term outcomes. Notably, a multi‐centre study (Ghetti 2019) is ongoing assessing whether music therapy is superior to standard care by measuring Bayley Scale Scores at two years of age and one of the small‐scale brain imaging studies (Haslbeck 2018) will examine neurodevelopment in preterm infants at five years to provide further insights of a possible influence of music therapy on long‐term neurodevelopment in preterm infants.
Due to a further lack of evidence, we cannot yet determine a dose‐, type‐ or frequency‐response relationship for musical and vocal interventions, but our finding of a greater beneficial effect on heart rate with music concurs with the findings of Haslbeck 2020a, demonstrating a dose‐dependent effect in favour of music therapy indicating further research in this direction.
Moreover, other reviews of various types of musical and vocal interventions for preterm infants (and their caregivers) (Anderson 2018; Hartling 2009; Haslbeck 2012; Hodges 2010; Mohan 2021; Standley 2012; Tramo 2011; Van der Heijden 2016; Yue 2021) show much higher heterogeneity amongst studies than in our meta‐analysis. One reason may be that we restricted the inclusion of studies to a minimum duration of at least five minutes and a minimum frequency of at least three times. In addition, we divided time points into during and after the intervention and restricted parental well‐being and attachment outcomes to a time point after the whole intervention period which was not reported in the other reviews (Mohan 2021).
Authors' conclusions
Implications for practice.
Music and vocal interventions do not increase oxygen saturation during, and probably not after, the intervention compared to standard care. The evidence suggests that the intervention does not increase infant development (BSID) or parental state‐trait anxiety. However, we have high confidence in our findings that music and vocal interventions results in a reduction in heart rate in preterm infants, particularly post‐intervention. Although the reduction of four heartbeats per minute may not be clinically relevant, it may be meaningful, as the intervention may contribute to overall physiological stabilisation in preterm infants. It remains unknown if the intervention has any further beneficial effects on the infants and their parents. Further evidence suggests that the intervention has no adverse effects and is associated with more beneficial than harmful influences in general.
Based on our current review findings, it is not possible to express explicit implications for clinical practice yet, nor to implement music and vocal interventions in general, nor determine whether specific types, durations, frequencies, and modes of music or voice may be superior to others. However, the central role of the voice emerged, whether sung live, recorded, or with an accompanying instrument, as argued in the literature, to be the most attractive and meaningful auditory stimulus for a baby (Filippa 2017). It is strongly recommended that close observation of the immediate effect of any music and vocal interventions in neonatal care be undertaken in order to not harm, overstimulate or overwhelm this vulnerable group. Music and voice should never be played unobserved and with too high a duration, volume, and frequency. Moreover, identical vocal and music interventions may induce various effects depending upon the individual health condition, developmental stage, and age of the infant and upon the well‐being and cultural background of the parents, so caution and precise observation, sensitivity, and overall responsiveness are indicated for best clinical decision‐making with any kind of musical provision.
Implications for research.
Further examination of music and vocal interventions for preterm infants and their parents with larger power, fewer risks of bias, and more sensitive and clinically relevant outcomes are needed. Research may be improved by: reducing risks of bias, e.g. allocation concealment and blinding of the outcomes assessors while decreasing heterogeneity within and between studies; evaluating the impact of type, length, and frequency of treatment; carefully considering possible confounders such as infant and parental stress on parental anxiety, and evaluating long‐term clinically relevant outcomes.
Decreasing risks of bias and imprecision
It is valuable to increase precision by conducting studies which include power calculations and bigger sample sizes. Allocation concealment should be conducted, and detection bias should be avoided particularly when blinding participants and personnel is not possible due to the nature of the intervention itself. In general, future investigations need to pay more attention to reporting the risk of bias in more detail accordingly to the Consort guidelines (Hopewell 2008).
Decreasing heterogeneity
Future research should take the high level of heterogeneity in the intervention into account and may entail comparisons between various types, duration, and frequencies while continuing to examine music and vocal interventions compared to other interventions or standard care. Since preterm infants differ in sensory maturation depending on their gestational age at birth and clinically relevant baseline characteristics, future trials might entail more precise reporting of these demographic data. Future trials may investigate possible differences in age, diagnoses, and gender in the infants as well as in culture, socioeconomics, and gender of the parents.
Considering possible confounders
In this review, music and voice were mostly delivered during routine care and possible other auditory influences were hardly reported. Parental well‐being was assessed at different time points or without assessing the general mental health status of the parents which could have influenced parental well‐being outcomes. Therefore, future trials should consider assessing possible confounders for outcomes such as additional stressors, diagnoses, challenges, and socio‐emotional circumstances.
Increasing relevant outcomes measures
Future trials should consider investigating long‐term outcomes in infants and their parents. Despite clinically relevant outcomes, future research should include outcomes that are relevant for the users themselves, and should consult with parents during trial development to guarantee that outcomes are pertinent and of value for the infants, parents, and families (for example, quality of life and sensitive long‐term outcomes, such as executive functioning in the infants).
Parental advice should be obtained on study feasibility, intervention implementation, the parental informed consent procedure, and dissemination of results. In addition, neurological outcomes should be integrated to gain deeper insight into possible mechanisms of music and voice for the infants, the parents, and the bonding/attachment process. Brain imaging techniques, such as MRI, EEG, NIRS, and neuropsychological outcomes seem to be promising to deepen our understanding of music processing and the possible advantages of music and voice on socio‐emotional development (Chorna 2019). Moreover, implementation science should play a central role in future investigations to increase the adoption and dissemination of research findings into evidence‐based practice.
History
Protocol first published: Issue 11, 2019
Acknowledgements
The methods section of this review is based on a standard template used by Cochrane Neonatal.
We would like to thank Cochrane Neonatal: Fiona Russell, Jane Cracknell and Michelle Fiander, Managing Editors; and Roger Soll and Bill McGuire, Co‐coordinating Editors, who provided editorial and administrative support. We thank Anne Lethaby for copy‐editing.
We thank Information Specialists, Carol Friesen and Michelle Fiander for developing and running searches in 2019 and 2021, respectively.
We thank Mohan Pammi, Professor, Section of Neonatology, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA, and Alice R. Rumbold, SAHMRI Women and Kids, South Australian Health and Medical Research Institute, Adelaide, South Australia, Australia, for peer reviewing the manuscript.
Appendices
Appendix 1. Cochrane CENTRAL strategy 2021
| Cochrane CENTRAL via CRS | ||
| Search date: November 1, 2021 | ||
| # | Terms | Results |
| 1 | MESH DESCRIPTOR Music Therapy EXPLODE ALL AND CENTRAL:TARGET | 914 |
| 2 | MESH DESCRIPTOR Music EXPLODE ALL AND CENTRAL:TARGET | 722 |
| 3 | MESH DESCRIPTOR Singing EXPLODE ALL AND CENTRAL:TARGET | 56 |
| 4 | MESH DESCRIPTOR Acoustic Stimulation EXPLODE ALL AND CENTRAL:TARGET | 1154 |
| 5 | MESH DESCRIPTOR Auditory Perception EXPLODE ALL AND CENTRAL:TARGET | 1863 |
| 6 | MESH DESCRIPTOR Speech Perception EXPLODE ALL AND CENTRAL:TARGET | 667 |
| 7 | MESH DESCRIPTOR Sound EXPLODE ALL AND CENTRAL:TARGET | 730 |
| 8 | MESH DESCRIPTOR Voice EXPLODE ALL AND CENTRAL:TARGET | 404 |
| 9 | (acoustic and intrauterine) AND CENTRAL:TARGET | 10 |
| 10 | ((intrauterine or womb or heart* or breathing or maternal or mother* or parent*) and sound*) AND CENTRAL:TARGET | 1584 |
| 11 | (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart ADJ3 beat*) or lullab*):ti,ab,kw AND CENTRAL:TARGET | 14,407 |
| 12 | ((auditory or acoustic) and (stimul* or cue*)):ti,ab,kw AND CENTRAL:TARGET | 3249 |
| 13 | ((speech or language or auditory) and (perception or exposure)):ti,ab,kw AND CENTRAL:TARGET | 2226 |
| 14 | #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 | 22,002 |
| 15 | MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET | 17,083 |
| 16 | MESH DESCRIPTOR Intensive Care, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 353 |
| 17 | MESH DESCRIPTOR Intensive Care Units, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 841 |
| 18 | (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU):ti,ab,kw AND CENTRAL:TARGET | 71,944 |
| 19 | #15 OR #16 OR #17 OR #18 | 75,013 |
| 20 | #14 AND #19 | 1624 |
Appendix 2. MEDLINE strategy 2021
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) 1946 to October 29, 2021 | ||
| Search date: November 1, 2021 | ||
| # | Searches | Results |
| 1 | Music Therapy/ | 3912 |
| 2 | Music/ | 15,172 |
| 3 | Singing/ | 1074 |
| 4 | Acoustic Stimulation/ | 45,149 |
| 5 | auditory perception/ or auditory threshold/ or auditory fatigue/ or loudness perception/ or perceptual masking/ or pitch perception/ or pitch discrimination/ or sound localization/ or speech perception/ or timbre perception/ or voice recognition/ | 80,655 |
| 6 | voice/ or voice quality/ | 14,378 |
| 7 | (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart adj3 beat*) or lullab*).mp. | 147,967 |
| 8 | ((auditory or acoustic) and (stimul* or cue*)).mp. | 81,587 |
| 9 | ((speech or language or auditory) and (perception or exposure)).mp. | 88,836 |
| 10 | (acoustic and intrauterine).mp. | 100 |
| 11 | ((intrauterine or womb or heart* or breathing or maternal or mother* or parent*) and sound*).mp. | 13,901 |
| 12 | or/1‐11 [Music: Sensitive set to combine with RCT Filter] | 292,178 |
| 13 | Music/ or Music Therapy/ or Singing/ | 19,087 |
| 14 | (music* or singing or song or songs or sing? or lullab*).ti,ab,kw,kf. | 36,408 |
| 15 | ((vocal* or speaking or talking) adj2 (intervention? or parent* or father? or mother?)).ti,ab,kw,kf. | 1060 |
| 16 | or/13‐15 [Precise Music set to search for related Systematic Reviews] | 41,979 |
| 17 | exp infant, newborn/ or Intensive Care, Neonatal/ or Intensive Care Units, Neonatal/ | 64,0467 |
| 18 | (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU).ti,ab,kw,kf. | 947,761 |
| 19 | or/17‐18 [Filter: Neonatal Population 2021‐‐MEDLINE] | 1,226,768 |
| 20 | randomized controlled trial.pt. | 548,945 |
| 21 | controlled clinical trial.pt. | 94,499 |
| 22 | (randomized or randomised).ti,ab. | 696,149 |
| 23 | placebo.ab. | 222,732 |
| 24 | drug therapy.fs. | 2,395,933 |
| 25 | randomly.ab. | 368,952 |
| 26 | trial.ab. | 574,229 |
| 27 | groups.ab. | 2,266,078 |
| 28 | (quasirandom* or quasi‐random*).ti,ab. | 5245 |
| 29 | exp animals/ not humans/ | 4,908,071 |
| 30 | (or/20‐28) not 29 [RCT Filter‐Based on Cochrane‐ Box 6.4.c: Cochrane Highly Sensitive Search Strategy] | 4,521,264 |
| 31 | meta‐analysis/ or "systematic review"/ or network meta‐analysis/ [/ finds same as.pt. syntax] | 246,324 |
| 32 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kf,kw. | 245,404 |
| 33 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kf,kw. | 32,232 |
| 34 | (data synthes* or data extraction* or data abstraction*).ti,ab,kf,kw. | 32,619 |
| 35 | (hand search* or handsearch*).ti,ab,kf,kw. | 10,128 |
| 36 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kf,kw. | 30,350 |
| 37 | meta‐analysis as topic/ or network meta‐analysis/ | 23,318 |
| 38 | (met analy* or metanaly* or meta regression* or metaregression*).ti,ab,kf,kw. | 11,984 |
| 39 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 266,504 |
| 40 | (cochrane or systematic review?).jw. | 18,833 |
| 41 | or/31‐40 [SR filter‐Medline; based on CADTHhttps://www.cadth.ca/strings‐attached‐cadths‐database‐search‐filters] | 478,430 |
| 42 | 12 and 19 and 30 [RCT Results] | 2948 |
| 43 | 16 and 19 and 41 [SR Results] | 73 |
Appendix 3. Embase strategy 2021
| Embase (OVID) 1974 to 2021 October 29 | ||
| Search date: November 1, 2021 | ||
| # | Query | Results |
| 1 | (MH "Music Therapy") | 6269 |
| 2 | (MH "Music") | 10,960 |
| 3 | (MH "Singing") | 3542 |
| 4 | (MH "Voice+") OR (MH "Voice Perception") | 8256 |
| 5 | TI ( (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) ) OR AB ( (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) ) | 69,137 |
| 6 | S1 OR S2 OR S3 OR S4 OR S5 | 77,501 |
| 7 | AB (medline or cochrane or pubmed or medlars or embase OR CINAHL) | 104,870 |
| 8 | ( TI met analy* or metanaly* or meta regression* or metaregression*) ) OR ( AB met analy* or metanaly* or meta regression* or metaregression*) ) | 4256 |
| 9 | AB (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*) | 8724 |
| 10 | AB (hand search* or handsearch*) | 4686 |
| 11 | ( TI (data synthes* or data extraction* or data abstraction*) ) OR ( AB (data synthes* or data extraction* or data abstraction*) ) | 12,413 |
| 12 | ( TI ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) OR ( AB ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) | 124,389 |
| 13 | S7 OR S8 OR S8 OR S9 OR S10 OR S11 OR S12 | 178,941 |
| 14 | (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Randomized Controlled Trials+") OR (MH "Double‐Blind Studies") OR (MH "Clinical Trials+") | 326,454 |
| 15 | TI (quasirandom* or quasi‐random*) OR AB (quasirandom* or quasi‐random*) | 2144 |
| 16 | AB (trial OR placebo* or random*) OR SU random* | 570,644 |
| 17 | S14 OR S15 OR S16 | 672,944 |
| 18 | (MH "Animal Studies") NOT (MH "Human") | 117,833 |
| 19 | S17 NOT S18 | 656,117 |
| 20 | ( (MH "Infant, Newborn+") OR (MH "Infant, Large for Gestational Age") OR (MH "Infant, Low Birth Weight+") OR (MH "Infant, Postmature") OR (MH "Infant, Premature") ) OR ( (MH "Intensive Care, Neonatal+") OR (MH "Intensive Care Units, Neonatal") ) | 151,986 |
| 21 | TI ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) ) OR AB ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) ) OR SU ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) ) | 530,443 |
| 22 | S20 OR S21 | 530,546 |
| 23 | S6 AND S19 AND S22 | 900 |
| 24 | ( TI (music* or melod* or song* or sing or sings or singing or sung or singer* or chant* or whistl* or lullab*) ) OR ( AB (music* or melod* or song* or sing or sings or singing or sung or singer* or chant* or whistl* or lullab*) ) | 21,388 |
| 25 | S13 AND S22 AND S24 | 63 |
| 26 | S23 OR S25 | 937 |
Appendix 4. CINAHL strategy 2021
| CINAHL EbscoHost (1980‐) | ||
| Search date: November 12, 2021 | ||
| 1 | (MH "Music Therapy") | 6269 |
| 2 | (MH "Music") | 10,960 |
| 3 | (MH "Singing") | 3542 |
| 4 | (MH "Voice+") OR (MH "Voice Perception") | 8256 |
| 5 | TI ( (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) ) OR AB ( (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) ) | 69,137 |
| 6 | S1 OR S2 OR S3 OR S4 OR S5 | 77,501 |
| 7 | AB (medline or cochrane or pubmed or medlars or embase OR CINAHL) | 104,870 |
| 8 | ( TI met analy* or metanaly* or meta regression* or metaregression*) ) OR ( AB met analy* or metanaly* or meta regression* or metaregression*) ) | 4256 |
| 9 | AB (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*) | 8724 |
| 10 | AB (hand search* or handsearch*) | 4686 |
| 11 | ( TI (data synthes* or data extraction* or data abstraction*) ) OR ( AB (data synthes* or data extraction* or data abstraction*) ) | 12,413 |
| 12 | ( TI ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) OR ( AB ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) | 124,389 |
| 13 | S7 OR S8 OR S8 OR S9 OR S10 OR S11 OR S12 | 178,941 |
| 14 | (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Randomized Controlled Trials+") OR (MH "Double‐Blind Studies") OR (MH "Clinical Trials+") | 326,454 |
| 15 | TI (quasirandom* or quasi‐random*) OR AB (quasirandom* or quasi‐random*) | 2144 |
| 16 | AB (trial OR placebo* or random*) OR SU random* | 570,644 |
| 17 | S14 OR S15 OR S16 | 672,944 |
| 18 | (MH "Animal Studies") NOT (MH "Human") | 117,833 |
| 19 | S17 NOT S18 | 656,117 |
| 20 | ( (MH "Infant, Newborn+") OR (MH "Infant, Large for Gestational Age") OR (MH "Infant, Low Birth Weight+") OR (MH "Infant, Postmature") OR (MH "Infant, Premature") ) OR ( (MH "Intensive Care, Neonatal+") OR (MH "Intensive Care Units, Neonatal") ) | 151,986 |
| 21 | TI ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) ) OR AB ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) ) OR SU ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) ) | 530,443 |
| 22 | S20 OR S21 | 530,546 |
| 23 | S6 AND S19 AND S22 | 900 |
| 24 | ( TI (music* or melod* or song* or sing or sings or singing or sung or singer* or chant* or whistl* or lullab*) ) OR ( AB (music* or melod* or song* or sing or sings or singing or sung or singer* or chant* or whistl* or lullab*) ) | 21,388 |
| 25 | S13 AND S22 AND S24 | 63 |
| 26 | S23 OR S25 | 937 |
Appendix 5. PsycINFO strategy 2021
| APA PsycInfo (OVID) <1806 to November Week 2 2021> |
| Search date: November 12, 2021 |
| 1 exp Music/ or exp Music Therapy/ or exp Music Perception/ (25,833) |
| 2 singing/ (1401) |
| 3 rhythm/ or auditory stimulation/ or auditory perception/ (39670) |
| 4 voice/ (5025) |
| 5 (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or lullab*).ti,ab,id. (110,104) |
| 6 ((heartbeat* or (heart adj3 beat*)) adj4 (mother* or father* or parent* or feeding or rocking)).ti,ab,id. (20) |
| 7 ((speech or language or auditory) adj4 (perception or exposure)).ti,ab,id. (14,525) |
| 8 ((auditory or acoustic) adj4 (stimul* or cue? or cuing)).ti,ab,id. (18,412) |
| 9 ((intrauterine or womb or heart or heartbeat* or breathing or maternal or mother* or father? or parent*) adj4 sound?).ti,ab,id. (487) |
| 10 or/1‐9 [Music ] (161,110) |
| 11 (music* or singing or song or songs or sing? or lullab*).ti,ab,id. (48,691) |
| 12 ((vocal* or speaking or talking) adj2 (intervention? or parent* or father? or mother?)).ti,ab,id. (1312) |
| 13 or/1‐4,11‐12 [Music: less sensitive set to combine with SR Filter] (90,639) |
| 14 premature birth/ or birth/ or birth weight/ (16,009) |
| 15 neonatal development/ or neonatal period/ (3849) |
| 16 neonatal intensive care/ (1760) |
| 17 (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU).ti,ab,id. (137,836) |
| 18 or/14‐17 [Neonate] (143300) |
| 19 (randomized controlled trial or controlled clinical trial or randomized or randomised or placebo or clinical trial? or randomly).ti,ab,id. (202,439) |
| 20 randomized controlled trials/ or clinical trials/ or randomized clinical trials/ (13,006) |
| 21 or/19‐20 [RCT Filter] (203,414) |
| 22 "systematic review"/ (641) |
| 23 meta analysis/ (5084) |
| 24 "literature review"/ (22,928) |
| 25 "literature review"/ and (Medline or embase or cinahl or pubmed).ab. (336) |
| 26 ((systematic* adj3 (review* or overview*)) or ((methodologic* or scoping or overview?) adj3 (review* or overview*)) or (metaanal* or meta‐analy*)).ti,ab,id. (155,348) |
| 27 or/22‐26 [SR Filter] (176,414) |
| 28 10 and 18 and 21 [Music (sensitive) AND Neonate AND RCT Filter] (243) |
| 29 13 and 18 and 27 [Music (precise) AND Neonate AND SR Filter] (96) |
| 30 or/28‐29 [All results PsycINFO] (329) |
Appendix 6. Web of Science (WoS) strategy 2021
| Web of Science (WoS) Core Collection: Science Citation Index Expanded (1982‐); Social Sciences Citation Index (1982‐); Ars & Humanities Citation Indes (1982‐); Emerging Sources Citation Index (2015‐). Additonal WoS databases: KCI (Korean Journal Database (1980‐); Russian Science Citation Index (2005‐); SciELO Citation Index (2005‐) | Core Collection | Korean | Russian | Scielo | |
| Search date: November 12, 2021 | |||||
| # | Search Terms | ||||
| 1 | (((TI=((music* or melod* or voice* or song* or sing or sings or singing or sung or singer* or chant* or whistl* or "maternal speech"))) OR TI=(((heart or heartbeat or voice) NEAR/4 (mother* or father* or parent*)))) OR TI=(((womb or intrauterine) NEAR/4 (voice?)))) | 210,640 | 17,540 | 4153 | 8240 |
| 2 | TS=(music* or melod* or voice* or song* or sing or sings or singing or sung or singer* or chant* or whistl* or "maternal speech") OR AK=(music* or melod* or voice* or song* or sing or sings or singing or sung or singer* or chant* or whistl* or "maternal speech") | 353,922 | 57,832 | 4342 | 8482 |
| 3 | TS=((heart or heartbeat or voice) NEAR/4 (mother* or father* or parent*)) OR AK=((heart or heartbeat or voice) NEAR/4 (mother* or father* or parent*)) | 2275 | 218 | 27 | 67 |
| 4 | TS=((womb or intrauterine) NEAR/4 (voice OR voices)) | 5 | 0 | 0 | 0 |
| 5 | #1 OR #2 OR #3 OR #4 | 355,077 | 57,914 | 4363 | 8482 |
| 6 | TI= (neonate or neonates or baby or babies or preterm* or preemie or preemies or "new born" or "new borns" or newborn* or infancy or infant or infants) OR AK= (neonate or neonates or baby or babies or preterm* or preemie or preemies or "new born" or "new borns" or newborn* or infancy or infant or infants) | 321,654 | 7128 | 7508 | 15,402 |
| 7 | TS= (neonate or neonates or baby or babies or preterm* or preemie or preemies or "new born" or "new borns" or newborn* or infancy or infant or infants) | 654,144 | 14,682 | 7812 | |
| 8 | #6 OR #7 | 654,144 | 14,682 | 7812 | 15,402 |
| 9 | TI=(randomized or randomised or random or trial) OR TS=(randomised or randomized or trial) | 2,008,499 | 26,165 | 13,664 | 42,151 |
| 10 | #5 AND #8 AND #9 | 378 | 9 | 0 | 9 |
| 11 | TI=("systematic review" OR metaanaly* or "meta‐analy*") OR TS=("systematic review" OR metaanaly* or "meta‐analy*") OR AK=("systematic review" OR metaanaly* or "meta‐analy*") | 544,369 | 1234 | 6659 | |
| 12 | #5 AND #8 AND #11 | 101 | 2 | 0 | 2 |
| 13 | #10 OR #12 | 437 | 11 | 0 | 11 |
| Total: 449 | |||||
| Note: in KCI, Russian Science Citation Indexm and SciELO, tersm were searched in title, abstract and topic (ti,ab,ts) | |||||
Appendix 7. RILM strategy 2021
| RILM Abstracts of Music Literature (EBSCOhost) | ||
| Search date November 12, 2021 | ||
| # | Search terms | Results |
| 1 | TI ( (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) ) OR AB ( ((music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) NEAR/4 sound*) ) OR SW "therapy—music therapy" | 675,364 |
| 2 | (TI ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) ) OR AB ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) )) OR SU ( (baby* or babies or infant or infants or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or newborn* or new born or new borns or newly born or premature or prematures or prematurity or preterm or preterms or pre term or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) )) | 3612 |
| 3 | TI (randomized or randomised or trial or study) or AB (randomly) OR AB ((random* NEAR/3 (trial or controlled or study)) | 43,221 |
| 4 | (S1 AND S2 and S3) | 156 |
| 5 | systematic review or meta‐analysis | 293 |
| 6 | TI ((systematic or scoping or overview) NEAR/2 review) OR AB ((systematic or scoping or overview) NEAR/2 review) or TI (meta‐analy* or metaanaly*) OR AB (meta‐analy* or metaanly*) | 156 |
| 7 | SU systematic review | 7 |
| 8 | SU S6 OR S7 | 161 |
| 9 | SU S1 AND S2 AND S8 | 1 |
| 10 | S4 OR S9 | 157 |
Appendix 8. ERIC strategy 2021
| ERIC via ProQuest | |
| Search date: November 12, 2021 | |
| (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) AND (newborn* or "new born" or "new borns" or "newly born" or baby* or babies or premature or prematurity or preterm or "pre term" or "low birth weight" or "low birthweight" or VLBW or LBW or infant* or infancy or neonat*) AND (randomized controlled trial OR clinical trial OR randomized OR randomised OR placebo OR randomly) | 21 |
Appendix 9. Trial register search strategies 2021
| Date | Source | Search terms | # |
| 12‐Nov‐21 | ISRCTN | Music AND neonate (age group) | 3 |
| 12‐Nov‐21 | ICTRP | (music OR lullaby OR singing or song or songs) AND neonate | 17 |
| 12‐Nov‐21 | clinicaltrials.gov | (music AND (neonate or newborn) | 53 |
| Subtotal | 73 |
Appendix 10. Search strategies 2019
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2019). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
CENTRAL via CRS Web:
Date searched: 15 November 2019 Terms: 1MESH DESCRIPTOR Music Therapy EXPLODE ALL AND CENTRAL:TARGET 2MESH DESCRIPTOR Music EXPLODE ALL AND CENTRAL:TARGET 3MESH DESCRIPTOR Singing EXPLODE ALL AND CENTRAL:TARGET 4MESH DESCRIPTOR Acoustic Stimulation EXPLODE ALL AND CENTRAL:TARGET 5MESH DESCRIPTOR Auditory Perception EXPLODE ALL AND CENTRAL:TARGET 6MESH DESCRIPTOR Speech Perception EXPLODE ALL AND CENTRAL:TARGET 7MESH DESCRIPTOR Sound EXPLODE ALL AND CENTRAL:TARGET 8MESH DESCRIPTOR Voice EXPLODE ALL AND CENTRAL:TARGET 9(music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart ADJ3 beat*) or lullab*) AND CENTRAL:TARGET 10((auditory or acoustic) and (stimul* or cue*)) AND CENTRAL:TARGET 11((speech or language or auditory) and (perception or exposure)) AND CENTRAL:TARGET 12(acoustic and intrauterine) AND CENTRAL:TARGET 13((intrauterine or womb or heart* or breathing or maternal or mother* or parent*) and sound*) AND CENTRAL:TARGET 14#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 AND CENTRAL:TARGET 15MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 16infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 17#16 OR #15 AND CENTRAL:TARGET 18#14 AND #17 AND CENTRAL:TARGET
Results: 1633
MEDLINE via Ovid:
Date ranges: 1946 to 15 November 2019 Terms: 1. exp Music Therapy/ or exp Music/ 2. exp Singing/ 3. exp Acoustic Stimulation/ 4. exp Auditory Perception/ or exp Speech Perception/ 5. exp Sound/ 6. exp Voice/ 7. (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart adj3 beat*) or lullab*).mp. 8. ((auditory or acoustic) and (stimul* or cue*)).mp. 9. ((speech or language or auditory) and (perception or exposure)).mp. 10. (acoustic and intrauterine).mp. 11. ((intrauterine or womb or heart* or breathing or maternal or mother* or parent*) and sound*).mp. 12. or/1‐11 13. exp infant, newborn/ 14. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 15. 13 or 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomized.ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. exp animals/ not humans.sh. 26. 24 not 25 27. 15 and 26 28. randomi?ed.ti,ab. 29. randomly.ti,ab. 30. trial.ti,ab. 31. groups.ti,ab. 32. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 33. placebo*.ti,ab. 34. 28 or 29 or 30 or 31 or 32 or 33 35. 14 and 34 36. limit 35 to yr="2018 ‐Current" 37. 27 or 36 38. 12 and 37
Results: 2567
CINAHL via EBSCOhost:
Date ranges: 1981 to 15 November 2019 Terms: (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) AND (infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Results: 730
PsycINFO:
Date ranges: 1806 to 15 November 2019 Terms: (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) AND (newborn* or "new born" or "new borns" or "newly born" or baby* or babies or premature or prematurity or preterm or "pre term" or "low birth weight" or "low birthweight" or VLBW or LBW or infant* or infancy or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial)
Results: 562
Web of Science via Clarivate Analytics:
Date ranges: 1982 to 15 November 2019 Terms: (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) AND (newborn* or "new born" or "new borns" or "newly born" or baby* or babies or premature or prematurity or preterm or "pre term" or "low birth weight" or "low birthweight" or VLBW or LBW or infant* or infancy or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR randomly)
Results: 789
RILM Abstracts of Music Literature via EBSCOhost:
Date ranges: 1967 to 15 November 2019 Terms: (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) AND (newborn* or "new born" or "new borns" or "newly born" or baby* or babies or premature or prematurity or preterm or "pre term" or "low birth weight" or "low birthweight" or VLBW or LBW or infant* or infancy or neonat*) AND (randomized controlled trial OR clinical trial OR randomized OR randomised OR placebo OR randomly)
Results: 33
ProQuest Dissertations & Theses A&I via ProQuest:
Date ranges: 1637 to 15 November 2019 Terms: (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) AND (newborn* or "new born" or "new borns" or "newly born" or baby* or babies or premature or prematurity or preterm or "pre term" or "low birth weight" or "low birthweight" or VLBW or LBW or infant* or infancy or neonat*) AND (randomized controlled trial OR clinical trial OR randomized OR randomised OR placebo OR randomly)
Results: 100
ERIC via ProQuest:
Date ranges: 1966 to 15 November 2019 Terms: (music* or melod* or voice* or song* or vocal* or sing or sings or singing or sung or singer* or chant* or whistl* or heartbeat* or (heart and beat*) or lullab* or ((auditory or acoustic) and (stimul* or cue*)) or ((speech or language) and (perception or exposure) and (intrauterine OR womb)) or (acoustic and intrauterine) or ((intrauterine OR womb OR heart* OR breathing OR maternal OR mother* OR parent*) and sound*)) AND (newborn* or "new born" or "new borns" or "newly born" or baby* or babies or premature or prematurity or preterm or "pre term" or "low birth weight" or "low birthweight" or VLBW or LBW or infant* or infancy or neonat*) AND (randomized controlled trial OR clinical trial OR randomized OR randomised OR placebo OR randomly)
Results: 17
ISRCTN:
Date searched (if no date restrictions): Terms: Interventions: Music* AND Participant age range: Neonate Interventions: Auditory stimulation AND Participant age range: Neonate Interventions: Voice* AND Participant age range: Neonate Interventions: Song* AND Participant age range: Neonate Interventions: singing AND Participant age range: Neonate Interventions: Womb sounds AND Participant age range: Neonate
Results: 0
Appendix 11. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we sought information regarding the method of randomisation, blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as being at a low, high, or unclear risk of bias. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the Characteristics of included studies table. We evaluated the following issues and entered the findings into the Risk of bias tables.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Music versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Oxygen saturation during intervention | 10 | 958 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.33, 0.59] |
| 1.1.1 Oxygen saturation during intervention recorded music | 8 | 355 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.27, 0.68] |
| 1.1.2 Oxygen saturation during intervention live music | 3 | 603 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐3.17, 0.80] |
| 1.2 Oxygen saturation post‐intervention | 7 | 800 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.01, 1.26] |
| 1.2.1 Oxygen saturation postintervention recorded music | 6 | 232 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.08, 1.35] |
| 1.2.2 Oxygen saturation postintervention live music | 2 | 568 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.75, 1.97] |
| 1.3 Oxygen saturation after heel lance | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐0.02, 1.51] |
| 1.4 Infant Development: Bayley Scales of Infant and Toddler Development | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Bayley Scale cognitive composition score 24 months | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐4.85, 5.55] |
| 1.4.2 Bayley Scale motor composition score 24 months | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐5.45, 5.11] |
| 1.4.3 Bayley Scale language composition score 24 months | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐5.45, 6.21] |
| 1.5 Parental anxiety: STAI‐T | 4 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐3.20, 0.96] |
| 1.6 Heart rate during intervention | 11 | 1014 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐2.63, ‐0.12] |
| 1.6.1 Heart rate during intervention with frequency of intervention at least 8 times | 7 | 379 | Mean Difference (IV, Fixed, 95% CI) | ‐4.21 [‐6.48, ‐1.94] |
| 1.6.2 Heart rate during intervention with frequency of intervention less than 8 times | 4 | 635 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.63, 1.39] |
| 1.7 Heart rate post‐intervention period | 9 | 903 | Mean Difference (IV, Fixed, 95% CI) | ‐3.80 [‐5.05, ‐2.55] |
| 1.7.1 Heart rate postintervention recorded music | 7 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐6.36 [‐8.89, ‐3.83] |
| 1.7.2 Heart rate postintervention live music | 3 | 632 | Mean Difference (IV, Fixed, 95% CI) | ‐2.98 [‐4.42, ‐1.54] |
| 1.8 Heart rate after heel lance | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐3.45, 5.67] |
| 1.9 Respiratory rate during intervention | 7 | 750 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐1.05, 1.90] |
| 1.10 Respiratory rate post‐intervention | 5 | 636 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐1.57, 2.58] |
| 1.11 Behavioural outcomes: Behavioural state (Als) | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.52, 0.27] |
| 1.12 Hospitalisation in days | 3 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐7.64, 4.50] |
| 1.13 Weight gain | 4 | 137 | Mean Difference (IV, Random, 95% CI) | 3.88 [‐1.61, 9.38] |
| 1.14 Postnatal depression: EPDS | 2 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐1.80, 2.81] |
| 1.15 Parental state anxiety: STAI‐SKD | 3 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐2.72, 2.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Calabro 2003.
| Study characteristics | ||
| Methods | RCT, parallel group | |
| Participants | Preterm infants GA 34, diagnosed with chronic lung disorder or oxygen dependency, or both, availability of 4 consecutive days, no substance addiction Pretreatment:data not shown: only reported that groups were balanced for weight, type of oxygen, diagnosis, therapy, average heart, and oxygen saturation rates, sex N infants randomised:22 N infants analysed:17 Reasons for dropout:1 infant discharged Reasons for exclusion:4 infants showed hearing impairment Sample size calculation:sample size of 20 participants was calculated for the primary outcome of oxygen saturation level. Ten participants per group were required to detect a 5% difference in oxygen saturation (effect size of 1.3) at a significance level of 0.05 and a power of 80%. |
|
| Interventions |
Intervention characteristics Control: usual NICU environmental sounds Recorded sedative music
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
Organised behaviours
Disorganised behaviour
|
|
| Identification |
Sponsorship source: supported by Ronald McDonald House Declarations of interest: not stated Country: Australia Setting: Tertiary level NICU and special care nursery Comments: single‐centre study Authors name: Jacinta Calabro Institution: 1) Monash Medical Centre, Melbourne; 2) Royals Children's Hospital Melbourne Email: jacinta@musictherapyonline.org Address: 1) 246 Clayton Roas, Clayton VIC31682) 50 Flemington Rd, Parkville Vic 3052, Australia Website: 1) monashhealth.org; 2) www.rch.org.au; 3) http://tlcmusic.com.au/about |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation reported |
| Allocation concealment (selection bias) | Low risk | No information given for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reporting in the results were not shown in the table but delivered by the author later on. |
| Selective reporting (reporting bias) | Unclear risk | Selective reporting unclear |
| Other bias | Unclear risk | Other bias unclear |
Caparros‐Gonzalez 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Recorded sedative instrumental music
Control: silence
Overall
Inclusion criteria: the inclusion criteria were premature infants who had a satisfactory hearing screening test through a brain stem auditory evoked potentials test at 38 weeks and were in the incubator while in the NICU. Exclusion criteria: premature infants were excluded if they were being treated with sedatives, analgesia medications, caffeine, or steroids; if they were supported by ventilation, continuous positive airway pressure, or nasal cannula; if they had any auditory impairment, malformation, or disease; if they were exposed to any potentially central nervous system depressor prenatally; or if they were diagnosed with a heart, lung, or congenital disease. Pretreatment: there were no significant differences between groups. N infants analysed: 17 N infants randomised: 22 Reasons for dropouts: 5 participants were discharged from the incubator before finishing the intervention. These participants were excluded from the data analysis, as major protocol violations happened (they received only the adaptation session of the intervention). Reasons for exclusion: 5 participants were discharged from the incubator before finishing the intervention. These participants were excluded from the data analysis, as major protocol violations happened (they received only the adaptation session of the intervention). Sample size calculation: was conducted. We estimated that a sample size of 8 subjects was needed to obtain a good effect size of the relaxing music therapy intervention (f = 0.41; α = 0.05, 1 − β = 0.80), assuming a correlation between the levels of the within‐subjects variable = 0.50. |
|
| Interventions |
Intervention characteristics Recorded sedative instrumental music
Control: Silence
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
|
|
| Identification |
Sponsorship source: The study was funded by Spanish Ministry of Science and Innovation grant (INNPACTO IPT300000‐2010‐10). R.C.G. is supported by the I + D Project “PSI2015‐348 63494‐P” of the Spanish Ministry of Science and Innovation. A.T.‐L. is supported by an FPI grant from the Spanish Ministry and Competitiveness (BES‐2013‐064257). C.D.‐P. is supported by a UGR Postdoctoral Fellowship (2013 University of Granada. Declarations of interest: None of the authors has biomedical financial interests or potential conflicts of interest. Country: Spain Setting: NICU Comments: Authors name: Rafael A. Caparros‐Gonzalez Institution: Mind, Brain, and Behaviour Research Center (CIMCYC), Campus de Cartuja s/n, 18011, Universidad de Granada, Granada, Spain Email: rcg477@correo.ugr.es; rafawolfy@gmail.com Address: Mind, Brain, and Behavior Research Center (CIMCYC), Campus de Cartuja s/n, 18011, Universidad de Granada, Granada, Spain Website: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated |
| Allocation concealment (selection bias) | Low risk | To ensure allocation concealment, a 3rd researcher was responsible for this task. This researcher stored the experimental music tracks on a secure digital card, depending on the randomised matching. When the participant’s code was matched to the experimental condition, the relaxing music track was recorded; otherwise, the track consisted of silence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants were discharged from the incubator before finishing the intervention. These participants were excluded from the data analysis, as major protocol violations happened (they received only the adaptation session of the intervention). Therefore, results from 17 infants with gestational age at the time of delivery ranging from 32 to 36 weeks (M = 32.33; SD = 1.79) were analysed. According to an updated meta‐analysis, we estimated that a sample size of 8 subjects was needed to obtain a good effect size of the relaxing music therapy intervention (f = 0.41; α = 0.05, 1 − β = 0.80), assuming a correlation between the levels of the within‐subjects variable = 0.50. |
| Selective reporting (reporting bias) | Low risk | Low |
| Other bias | Unclear risk | Unclear |
Cevasco 2008.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Intervention A
Overall
Inclusion criteria: born prior to 36 weeks' corrected gestational age (CGA), and low birth weight infants (LBW), weighing less than 2500 grams at birth, hospitalised in a Level II (intermediate care for premature infants to grow and gain feeding skills) and Level III NICU (high‐risk care for infants needing advanced treatment to sustain life) infant born at or later than 28 and before 36 weeks' CGA, infant evinced toleration of auditory stimulation between 30‐32 weeks CGA. Exclusion criteria: severe abnormalities which affected the ability to listen to music (including significant neurological disorders such as periventricular leukomalacia or intraventricular haemorrhage), mother or infant tested positive for illegal drugs, the infant had disease necessitating quarantine, the infant on a ventilator or continuous positive airway pressure past 30 weeks' CGA. Pretreatment: analysis of demographic information for preterm infants revealed that there was no significant difference between groups and birth CGA, t (18) = ‐0.960, P > 0.05. The age range for infants in the experimental group was from 28 weeks to 34 weeks and 4 days CGA and 30 weeks to 34 weeks and 6 days in the control group. Also, there was no significant difference in birth weight between groups, t (18) = 0.214, P > 0.05. Birth weight ranged from 954 to 2080 grams for the experimental group and from 1200 to 2535 grams for the control group. N infants analysed: 20 (n = 10 experimental group, n = 10 control group) N infants randomised: 25 (n = 13 experimental group; n = 11 control group) Reasons for dropouts: 1 mother in the experimental and 1 in the control group did not complete a phone or mail survey. Reasons for exclusion: 2 mothers of twins in the experimental group were dropped from the study due to early discharge and only 1 or 2 days of music intervention and 1 infant in the control group became extremely sick and participation in the study was discontinued. N mothers randomised: 21 (n = 11 experimental group, n = 10 control group) Mothers analysed: 16 (n = 8 experimental group, n = 8 control group) |
|
| Interventions |
Intervention characteristics Control
Intervention A
|
|
| Outcomes |
Adapted Parental Perception Inventory
Weight gain
Hospitalisation
|
|
| Identification |
Sponsorship source: not mentioned Declarations of interest: not stated Country: USA Setting: Newborn Nursery, NICU Level I, II, III Comments: single centre Authors name: Andrea M. Cevasco Institution: University of Alabama Email: acevasco@ua.edu Address: Tuscaloosa, AL 35487, Vereinigte Staaten Website: https://music.ua.edu/people/andrea‐cevasco‐trotter/ |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All data only for the remaining participants after dropouts and exclusions |
| Selective reporting (reporting bias) | Unclear risk | Unclear selective reporting |
| Other bias | High risk | Per‐protocol analysis and not intention‐to‐treat Number of randomised PI reported was inconsistent/controversial: in abstract: was 20 and later intent was 25 (with 11 + 13 = 24 and not 25!) |
Epstein 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over |
|
| Participants |
Baseline characteristics SSC mother and infant
SSC & live mothers' singing in lullaby style (song chosen by mother) accompanied by a music therapist with voice and or guitar
Overall
Inclusion criteria: All preterm infants born before 32 weeks' gestation, admitted to the NICU during 2014–2018 who developed IVH grades 3 or 4 and/or PVL diagnosed by brain ultrasound. Inclusion after their clinical state stabilised, regardless of their corrected age at study entry. A clinically stable infant at the time of enrolment was defined as one without supplemental oxygen demands during enrolment, no significant apnoea requiring stimulation during the 3 days before enrolment, with or without methylxanthines, and no documented seizure episode or anticonvulsive treatment a week before the study enrolment. All infants had their hearing confirmed by distortion product otoacoustic emissions. Due to the national parental rights of birth and preterm birth in Israel, where fathers are entitled to very short paternity leave, only mothers who were able to attend all the sessions participated in the study. Exclusion criteria: if it became necessary for medical reasons to discontinue an infant’s participation in the study for a period shorter than 2 weeks, participation recommenced when the criteria for clinical stability were again met. Pretreatment: no group differences possibly emissions due to within‐subject cross‐over design N infants analysed: 35 N infants randomised: 40 Reasons for dropouts: no dropouts Reasons for exclusion: analysed (n = 35). Excluded from analysis (n = 5): did not complete all sessions = 3; prolonged illness between sessions = 2 Sample size calculation: the minimal clinically significant difference for LF/HF power in infants is unknown. The power calculation for the primary outcome was informed by a previous study [18] that found mean LF/HF power values of 1 (SD = 0.8) during MT combined with SSC, compared to 3.4 (SD = 1.6) during SSC alone. Therefore, the study was powered to detect a substantial effect on LF/HF (d = 0.80). In an individually randomised trial, with a 2‐sided 5% significance level and 80% power, a sample size of 35 infants was required. |
|
| Interventions |
Intervention characteristics SSC mother and infant
SSC & live mothers` singing in lullaby style (song chosen by mother) accompanied by a music therapist with voice and or guitar
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
State‐Trait‐Anxiety‐Inventory (STAI)
Heart rate mothers
Oxygen saturation mothers
HRV: low‐frequency power
HRV: high‐frequency power
HRV: LF/HF ratio
Behavioural state (Als)
|
|
| Identification |
Sponsorship source: not reported Declaration of interest: not stated Country: Israel Setting: Level III NICU Comments: Authors name: Shulamit Epstein Institution: School of Creative Arts Therapies, University of Haifa, Haifa, Israel Email: harnon@netvision.net.il Address: Department of Neonatology, Meir Medical Center, 59 Tchernichovsky St., 44281 Kfar Saba, Israel Website: https://hw.haifa.ac.il/en/departments/artschool |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation reported |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessor was blinded to treatment allocation and arranged physiological data of infants and mothers, STAI score, and behavioural scale according to the sequence of the intervention, without knowing whether the intervention was SSC or maternal singing with MT. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 did not complete all sessions, and 2 were excluded due to prolonged illnesses during their hospitalisations that interrupted the timing between study sessions for more than 2 weeks. Therefore, 35 preterm infants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | No selective reporting detected |
| Other bias | Low risk | No other bias detected |
Ettenberger 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Live music therapy with infants and parents in kangaroo‐care
Live music therapy with infant
Overall
Inclusion criteria: signed informed consent by the parents, medically stable infants born between the 30th and 37th week of gestation (without the requirement of inotropic support or mechanical ventilation and absence of frequent episodes of apnoea), and having initiated kangaroo‐care. Exclusion criteria: parents who declined to participate in the study, medically unstable infants, infants who suffered from congenital malformations, and if surgery was scheduled for the week after the initial music therapy session. In the case of eligible preterm infants who were the result of multiple births (twins, triplets, etc.), all neonates were allocated to the same intervention group. To avoid potential secondary effects and reduce the possibilities of over‐stimulation through the music therapy interventions, the following procedure was established: If a preterm infant showed signs of hypersensitivity towards the intervention (unusual agitated behaviour, excessive alteration of the vital signals), the music therapy intervention would be stopped. During a second intervention, a member of the medical staff would accompany the music therapist. If the same signs mentioned above occurred again and if the member of the medical staff interpreted these signs as a potential hypersensitivity towards the intervention, the preterm infant would be excluded from the study. Pretreatment: the 3 groups did not differ significantly in terms of sociodemographic or baseline data. Just one statistically significant association was found between the groups in terms of Social Security Status (paying or beneficiary) P = 0.01. N infants analysed: 19 (8 infants in IG1, 5 infants in IG2, and 6 infants in CG) N infants randomised: 30 Reasons for dropouts: no Reasons for exclusion: medical complications (n = 5), short hospitalisation (n = 5), or formal study requirements (n = 1) Sample size calculation: N sample size calculation due to the pilot study design Parents randomised: 27 Mothers analysed: 18 (8 mothers in intervention group 1, 5 mothers in intervention group 2, and 5 mothers in control group) |
|
| Interventions |
Intervention characteristics Control
Live music therapy with infants and parents in kangaroo‐care
Live music therapy with infant
|
|
| Outcomes |
Weight gain
Length of hospitalisation
State‐Trait‐Anxiety‐Inventory for Children (STAI‐C, Colombian adaption fromCastrillón 2005
Mother‐to‐infant bonding
Heart rate and oxygen saturation: were recorded before, during, and after the intervention. However, the lack of most of the data did not allow for analysis. |
|
| Identification |
Sponsorship source: not mentioned Declarations of interest: not stated Country: Colombia Setting: “Centro Policlínico del Olaya”, a hospital located in one of the poorer neighbourhoods of Bogotá, the capital of Colombia. The NICU offers 28 beds distributed on two floors for both intensive and acute care and intermediate care for the more stable infants. Comments: not available Authors name: Mark Ettenberger Institution: Anglia Ruskin University; Universidad National Colombia Email: mark.ettenberger@gmx.at Address: Not available Website: www.sono.la |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation took place in the form of assigning the participants according to a set of randomised numbers generated by computer software. |
| Allocation concealment (selection bias) | Low risk | A member of the administrative staff was the only person with this set of numbers and at the time of giving written consent to participate in the study, neither the music therapist nor the participants knew to which group the participants would be assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from just 8 out of 19 follow‐up participants |
| Selective reporting (reporting bias) | Low risk | Broad variety of outcome measures were used for this study to set the path for reliable outcome measures that will be used in a larger study that is planned to start soon. However, more outcome measures add also complexity to the data recollection and analysis process. |
| Other bias | Unclear risk | Unclear |
Farhat 2010.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded lullaby music
Inclusion criteria: Adjusted gestational age younger or equal to 34 weeks; birth weight between 1000–1500 grams; postnatal age of 4 days; and clinically stable neonates (not supported by ventilator assistance or oxygen therapy) Exclusion criteria: Diagnosed hearing impairment; congenital anomalies; maternal drug addiction; and use of oxygen Pretreatment: The two groups did not differ in demographic and clinical characteristics. N infants analysed: 44 (22/22) N infants randomised: 44 (22/22) Reasons for dropouts: not reported Reasons for exclusion: not reported Sample size calculation: Sample size was calculated by considering the mean and standard deviation (SD) of previous studies. The sample size was calculated for all variables including SPO2, heart rate (HR), respiratory rate (RR), and weight gain and a sample of 40 infants (20 in each group) was considered adequate. |
|
| Interventions |
Intervention characteristics Control
Recorded lullaby music
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
Weight gain
|
|
| Identification |
Sponsorship source: Mashhad University of Medical Science Declarations of interest: No financial interested to disclose Country: Iran Setting: NICU Comments: no comments Authors name: Rana Amiri Institution: Neonatal Research Center, NICU, Emamreza Hospital, Mashhad, Iran Email: amirir1@mums.ac.ir Address: Neonatal Research Center, NICU, Emamreza Hospital, Mashhad, Iran Website: Not available Tel: Tel.: +98 5112709006 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No sequence generation described |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealment happened |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Haslbeck 2021.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Control
Family‐centred live creative music therapy
Inclusion criteria: Infants were eligible if they were born prematurely (gestational age < 32 weeks), reached the chronological age ≥ seven days of life, and did not require invasive cardiovascular and respiratory support at recruitment. Exclusion criteria: Diagnosed with a major congenital anomaly/genetically defined syndrome, congenital malformation adversely affecting life expectancy/neurodevelopment, intraventricular haemorrhage (≥ Volpe grade 3), or when the infants were admitted for palliative care Pretreatment: Groups neither differed in neonatal parameters nor baseline demographic characteristics. Compared to infants lost to follow‐up, infants assessed at age 2 years had similar baseline characteristics except for lower gestational age (P < 0.001), longer supplemental oxygen therapy (P = 0.001), and longer hospital stay (P < 0.001). N infants analysed: 56 N infants randomised: 82 Reasons for dropouts: 26 dropouts (n = 13 in both the CMT and standard care groups) discontinuation resulted from moving abroad (n = 2) or for unknown reasons (n = 24). Reasons for exclusion: reasons for exclusion for per‐protocol analysis: not received CMT stimulation Sample size calculation: The sample size for this pilot trial was set at 60 participants per pilot trial design recommendations (40–42). |
|
| Interventions |
Intervention characteristics Control
Family‐centred live creative music therapy
|
|
| Outcomes |
Bayley Scale cognitive composition score
Bayley's language composition score
Bayley motor composition score
|
|
| Identification |
Sponsorship source: Foundation of Anna Müller Grocholski; Stiftung für Neonatologie; Vontobel Foundation Declaration of interest: The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Country: Switzerland Setting: NICU III level Comments: Authors name: Friederike B Haslbeck Institution: Department of Neonatology, Newborn Research Zurich, University Hospital Zurich, Zurich, Switzerland Email: friederike.haslbeck@usz.ch Address: Frauenklinikstrasse 10, 8091 Zurich Website: neonatologie.usz.ch |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation reported |
| Allocation concealment (selection bias) | Low risk | Allocation concealment reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No selective reporting detected |
| Other bias | Low risk | No other bias detected |
Jabraeili 2016.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group |
|
| Participants |
Baseline characteristics Control
Recorded mum’s lullaby
Recorded Brahms` lullaby
Inclusion criteria: 29‐34 weeks of gestation, postnatal age of 3 days, weight under 2800 grams Exclusion criteria: Congenital heart diseases and acute infections need for ventilators, receiving oxygen through continuous positive airway pressure, or receiving hypnotics as well as sedative drugs and traditional treatments during the intervention were excluded from the study. Pretreatment: No significant difference between the three groups in baseline data including infant gender, birth weight, weight in intervention days, gestational age, postnatal age, 1‐minute, and 5‐minute Apgar scores, mother’s age, and neonate’s race N infants analysed: 66 (Mom's Lullaby Group = 21 + Brahms Lullaby Group = 25 + Control Group = 20) N infants randomised: 75 Reasons for dropouts: Five infants in the mum's lullaby group, and 4 infants in the control group were excluded. Therefore, the study was completed with 66 premature infants. Reasons for exclusion: 3 infants were excluded because their parents refused to take part in the study. Sample size calculation: Reviewing a meta‐analysis and previously published studies that evaluated the effects of patterned sound in the NICU on preterm infants and utilising “Gpower” software, the sample size was determined considering quantitative variables based on (effect size: 0.42, α = 0.05, power: 0.8). This calculation referred to 20 neonates for each group. Considering an attrition rate of 10‐15% and also the Automated Auditory Brainstem Response results, a total of 25 subjects were included in each of the three groups. |
|
| Interventions |
Intervention characteristics Recorded mum’s lullaby
Recorded Brahms` lullaby
|
|
| Outcomes |
Oxygen saturation
|
|
| Identification |
Sponsorship source: Tabriz University of Medical Science (Project number: 359) Declaration of interest: The authors declared no conflict of interest in this study. Country: Iran Setting: NICU Comments: no comments Authors name: Tahmineh Sabet (MSc) Institution: Department of Pediatric Nursing, Faculty of Nursing and Midwifery, Tabriz University of Medical Sciences, Tabriz, Iran Email: sabet@sums.ac.ir Address: Department of Pediatrics, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran Website: not available |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mum's lullaby, and control groups. Randomised block design by a random assignment software was used for producing random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 infants in the mum's lullaby group, and 4 infants in the control group were excluded. Therefore, the study completed with 66 premature infants. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Johnston 2007.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over |
|
| Participants |
Baseline characteristics Control
Recorded mothers voice
Overall
Inclusion criteria: between 32 weeks 0 days and 35 weeks 6 days gestational age (GA) according to ultrasound or last menstrual period. All infants had Apgar scores at 5 minutes greater than 3. Exclusion criteria: congenital anomalies, intraventricular haemorrhage (IVH) greater than a grade 2, periventricular leukomalacia (PVL), requiring surgery, receiving analgesic therapy N infants analysed: 20 N infants randomised: 65 Reasons for dropouts: 19 had no blood work ordered and another 7 did not have a second session in the time they were eligible to actually participate in the study before being discharged from the NICU. There was equipment failure with either the camera or the pulse oximeter for 19, leaving a final sample of 20 with completed data sets at 2 points in time. Reasons for exclusion: not reported Sample size calculation: sample size estimates based on previous studies with differences in PIPP scores of 2 points and a standard deviation of 2 points, setting alpha at 0.05 and a power of 0.80, indicated 44 infants would be needed for separate groups, 22 for a repeated measures design; for facial actions of 22% and standard deviation of 30%, a sample size of 46 infants in a randomised controlled trial but only 20 in a repeated measures design would be indicated. Using heart rate differences of 15 with a standard deviation of 17 to 20 (from previous studies), a sample size of 27 was needed for a repeated measures design. Thus, a conservative sample of 27 was sought, allowing for a 20% loss. |
|
| Interventions |
Intervention characteristics Recorded mothers voice
|
|
| Outcomes |
Heart rate
Oxygen saturation
PIPP
|
|
| Identification |
Sponsorship source: Canadian Institutes of Health Research MOP‐38074 and in part by McGill University James McGill Chair Fund Declaration of interest: not reported Country: Canada Setting: 2 university‐affiliated NICUs were the sites for this study. Both were level 3 NICUs. Comments: no comment Authors name: Celeste Johnston Institution: School of Nursing, McGill University, Montreal, Quebec, Canada H3A 2A7 Email: celeste.johnston@mcgill.ca Address: School of Nursing, McGill University, 3506 University Street, Montreal, Quebec, Canada H3A 2A7 Website: http://childkindinternational.org/celeste‐johnston‐rn‐phd |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated list of random ordering for the 2 conditions of 20 infants was produced for each site before recruitment began. |
| Allocation concealment (selection bias) | Unclear risk | At the time of enrolment, that is, at the moment the mother gave consent, the infant was assigned the next sequenced study number on the list, which then determined the first session. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Faces were scored by research assistants who did not know the purpose of the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65 consented; 20 complete data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or trial registration found |
| Other bias | Low risk | A washout period of at least 24 hours lessened the possibility of a cumulative effect from one condition to the next. |
Kehl 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Qualitative data: parental interview on parental experience of music therapy |
|
| Participants |
Baseline characteristics Control
Live family‐centred Creative Music Therapy
Inclusion criteria: the participants were mothers and fathers of prematurely born infants recruited after birth at the Department of Neonatology of the University Hospital Zurich, Switzerland. Parents of clinically stable infants born before 32 weeks of gestation and at a chronological age of 7 days of life were eligible for study participation. Exclusion criteria: exclusion criteria were parents of premature infants with a genetically defined syndrome, severe congenital malformation adversely affecting life expectancy and/or neurodevelopment, high‐grade intraventricular haemorrhage, and/or cystic white matter lesions. Parents of premature infants admitted to palliative care were also excluded from the study. Lack of sufficient knowledge of the German language was a further defined exclusion criterion. Pretreatment: no significant differences in sociodemographic, pregnancy‐ and birth‐related characteristics (P > 0.05 for all variables) N parents analysed: 32 N parents randomised: 46 Reasons for dropouts: relocation to another hospital Reasons for exclusion: not reported Sample size calculation: no sample size calculation due to the pilot design |
|
| Interventions |
Intervention characteristics Control
Live family‐centred Creative Music Therapy
|
|
| Outcomes |
State‐Trait Anxiety Inventory (STAI‐t)
State‐Anxiety Inventory (STAI‐SKD)
Depressive symptoms (EPDS)
Parental Stressor Scale: Neonatal Intensive Care Unit (PSS: NICU)
Pictorial Representation of Attachment Measure (PRAM)
|
|
| Identification |
Sponsorship source: Anna Müller Grocholski Stiftung für Neonatologie; Vontobel Foundation Declaration of interest: the authors declared no conflict of interest. Country: Switzerland Setting: NICU Level III Comments: Authors name: Selina M. Kehl Institution: Department of Neonatology, University Hospital Zurich Email: Selina.kehl@kispi.uzh.ch, corresponding author: Friederike Haslbeck friederike.haslbeck@usz.ch Address: Frauenklinikstrasse 10, 8091 Zurich Website: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation reported |
| Allocation concealment (selection bias) | Low risk | Allocation concealment reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data detected |
| Selective reporting (reporting bias) | Low risk | No selective reporting detected |
| Other bias | Low risk | No other bias detected |
Kraft 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over |
|
| Participants |
Baseline characteristics Control
Live RBL music therapy
Inclusion criteria: parents of infants born with a gestational age of fewer than 30 weeks Exclusion criteria: infants whose parents did not speak or understand Dutch were not eligible for inclusion. Pretreatment: characteristics of the infants in both groups were comparable, except for a significantly higher number of infants with haemodynamically significant persistent ductus arteriosus in the waiting‐list group. 2 infants in the music therapy group died during the study period, including a female twin. N infants analysed: 21 N infants randomised: 59 Reasons for dropouts: not reported Reasons for exclusion: questionnaires not completed by parents Sample size calculation: not reported |
|
| Interventions |
Intervention characteristics Live music therapy
|
|
| Outcomes |
State‐Anxiety Inventory (STAI‐SKD)
State‐Trait Anxiety Inventory (STAI‐T)
Neonatal Infant Stressor Scale (NISS)
|
|
| Identification |
Sponsorship source: Junior Scientific Masterclass of the University of Groningen. Declaration of interest: the authors declared no conflict of interest. Country: the Netherlands Setting: NICU Level III Comments: Authors name: Karianne Kraft Institution: Division of Neonatology, Department of Pediatrics, Beatrix Children’s Hospital, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9713GZ Groningen, The Netherlands Email: k.e.kraft@umcg.nl Address: Beatrix Children’s Hospital, University MedicalCenter Groningen, University of Groningen, Hanzeplein 1, 9713GZ Groningen, The Netherlands Website: Tel: +31‐50‐361‐4215 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: assignment to treatment allocation was done through a web portal hosted by the study data centre at the TCC, at the UMCG. The randomisation schedule was computer‐generated, using the method of randomised blocks. This trial was thereby protected from selection bias by using concealed randomisation. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: assignment to treatment allocation was done through a web portal hosted by the study data centre at the TCC, at the UMCG. The randomisation schedule was computer‐generated, using the method of randomised blocks. This trial was thereby protected from selection bias by using concealed randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: outcome assessors were not blinded. They knew which children were in the music therapy group and which weren’t. Parents were not blinded as well. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The low response after randomisation could have caused bias. Stressed parents may not have felt the opportunity to complete the questionnaires, as they were occupied with anxiety and worries about their infant’s well‐being. |
| Selective reporting (reporting bias) | Low risk | No selective reporting detected |
| Other bias | High risk | We only collected limited background information on the mothers. It would have been interesting to investigate the relation of, e.g. age, maternal socioeconomic status, and marital status on anxiety as well. |
Kucuk Alemdar 2020.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded mothers spoken voice
Inclusion criteria: being born between the 30th and 34th week of gestation, having a birth weight > 1000 g, having a cardiorespiratory condition after admission to the NICU, having an Apgar mean score of > 6, having spontaneous respiration at birth, having no congenital malformation potentially causing asphyxia or otherwise affecting respiration, and having no cranial bleeding or hyperbilirubinemia that could have led to blood abnormalities Exclusion criteria: having chromosomal anomalies, necrotising enterocolitis, craniofacial malformation, bronchopulmonary dysplasia, respiratory distress syndrome, neonatal seizures, need for mechanical ventilation, periventricular leukomalacia, intracranial haemorrhage, or culture‐positive sepsis or meningitis at screening Pretreatment: no statistically significant difference was found between groups in terms of gender, gestational age, birth weight, birth height, head circumference, Apgar score at the first and fifth minutes. N infants analysed: 1) breast milk odour group; n = 30; 2) maternal voice group; n = 30; 3) incubator cloth group; n = 31; 4) control group; n = 32 N infants randomised: N = 136; 1) breast milk odour group; n = 33; 2) maternal voice group; n = 34; 3) incubator cloth group; n = 35; 4) control group; n = 34 Reasons for dropouts: clinically unstable; inadequate breast milk Reasons for exclusion: not meeting inclusion criteria Sample size calculation: power analysis was conducted, and the sample size was determined as 123 with a power of 0.90, a significance level of 0.05, and a medium effect size. |
|
| Interventions |
Intervention characteristics Control
Recorded mother's spoken voice
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
|
|
| Identification |
Sponsorship source: The Scientific Research Projects (BAP) of Management Unit of Giresun University (SAĞ‐BAP‐A‐200515‐23) Declaration of interest: the authors declared no conflict of interest. Country: Turkey Setting: level II NICU Authors name: Dilek Küçük Alemdar Institution: 1) Giresun University, Health Sciences Faculty, Department of Nursing, Giresun, Turkey; 2) Istanbul University, Health Sciences Faculty, Department of Midwifery, Istanbul, Turkey Email: dilek.alemdar@giresun.edu.tr; dilek.alemdar@giresun.edu.tr Address: Dilek Küçük Alemdar, PhD, RN, Assistant Professor Giresun University, Health Science Faculty, Department of Nursing Yenimahalle TR–28340 Piraziz‐Giresun (Turkey) Website: https://www.giresun.edu.tr/en |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program (www.randomization.org/block design) |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In every group, some infants (2‐4) had to be excluded due to being "clinically unstable". |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | No other bias detected |
Lafferty 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded Mozart's double piano sonata
Inclusion criteria: (1) neonates between 280/7 and 316/7 weeks of gestational age; (2) parents equal to or older than 18 years of age whose primary language was English; (3) and the intention of providing breast milk as primary source of nutrition Exclusion criteria: congenital anomalies affecting hearing or oral feeding and the need for high‐frequency ventilatory support. Demographic information was collected on each infant including gestational age, birth weight, sex, time to full enteral feeds, and highest mode of respiratory support at the time of the study. Pretreatment: no significant differences between the two groups N infants analysed: 40 N infants randomised: 40 Reasons for dropouts: no Reasons for exclusion: no Sample size calculation: the target enrolment number was calculated based on population data that the average time to regain birth weight for LBW infants is 10 days. To detect a 3‐day difference in time to regain birth weight, with 80% power and two‐sided α at 0.05, 16 babies were needed in each group. This number was rounded up to a target of 20 infants in each group to allow for dropouts. |
|
| Interventions |
Intervention characteristics Control
Recorded Mozart's double piano sonata
|
|
| Outcomes |
Weight gain per day
Adverse effects
|
|
| Identification |
Sponsorship source: none Declaration of interest: the authors declared no conflict of interest. Country: USA Setting: NICU Level III Authors name: Margaret A. Lafferty Institution: DepartmendDepartment of Pediatrics, Christiana Care Health System Email: margaret.lafferty@gmail.com Address: Margaret Lafferty, MD, Department of Pediatrics, Thomas Jefferson University Hospital, 833 Chestnut StreetSuite 1210, Philadelphia, PA 19107 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were randomised to music (intervention) or no music (control) exposure by using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | To accomplish the blinded nature of this study, a music player was present in each infant’s incubator in both groups (unclear if the nurse was blinded to group allocation since music player was placed in the incubator). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data reporting detected |
| Selective reporting (reporting bias) | Low risk | Compared with: https://clinicaltrials.gov/ct2/show/NCT02919358?term=NCT02919358&draw=2&rank=1, reported as planned |
| Other bias | Unclear risk | Unclear |
Lejeune 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded instrumental music
Inclusion criteria: gestational age at birth < 32 weeks Exclusion criteria: major brain lesions detected on early MRI, such as intraventricular haemorrhage grade III with or without apparent periventricular haemorrhagic infarction, or cystic periventricular leukomalacia Pretreatment: for the preterm group, there was only 1 significant difference for the family’s socioeconomic status (SES), t(35) = 2.760, P = 0.009. The family’s SES is a 12‐point scale based on paternal occupation and maternal education (range from 2 (the highest SES) to 12 (the lowest SES)). The SES of the families of preterm children who dropped out (mean = 3.20, SD = 0.9) was higher than that of the families of preterm children who participated in the follow‐up assessment (mean = 6.22, SD = 3.4) N infants analysed: 23 at 12 months (music: 13; control: 10), 17 at 24 months (music: 10; control: 7) N infants randomised: 39 (music: 20; control: 19) Reasons for dropouts: at 12 months: 2 refusals, 2 unreachable; at 24 months: 3 expired, 7 unreachable Reasons for exclusion: refusal 3, unreachable 3, cerebral anomaly 1, moved 5 Sample size calculation: not reported |
|
| Interventions |
Intervention characteristics Control
Recorded instrumental music
|
|
| Outcomes |
Bayley Scale: cognitive 24 months
Bayley Scale: motor at 24 months
Bayley Scale: language at 24 months
|
|
| Identification |
Sponsorship source: Swiss NationalScience Foundation n32473B_135817/1 and the Fondation Prim'enfance Declaration of interest: the authors declared no conflict of interest. Country: Switzerland Setting: Neonatal units of the University Hospital of Geneva Comments: no comments Authors name: Fleur Lejeune Institution: Child Clinical Neuropsychology Unit, Faculty of Psychology and Education Sciences, University of Geneva, Geneva, Switzerland Email: Fleur.Lejeune@unige.ch Address: Child Clinical Neuropsychology Unit, Faculty of Psychology and Education Sciences, University of Geneva, Geneva, Switzerland Website: https://www.unige.ch/fapse/en/recherche/groupes/psycho/clinique/upcne/ |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Preterm infants were randomised to either music intervention or control condition (without music). |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nurse determined the readiness for music exposure and chose the track based on a neonatal behavioural assessment scale. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subsequent analysis were done by trained psychologists or developmental paediatricians who were blind to the music group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost around half of randomised participants until analysis. |
| Selective reporting (reporting bias) | Low risk | No selective reporting found |
| Other bias | Low risk | 2 coders scored the episodes independently for 16% of the sample after thorough training on the scoring methods. Inter‐rater reliability was calculated using Pearson correlations. |
Liao 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded mothers singing voice
Inclusion criteria: a) gestational age < 37 weeks with a birth weight of ≤ 1800 g; (b) newborn Apgar score at 1 minute > 5 points; (c) length of NICU stay ≥ 1 week; (d) infant being cared for in an incubator; (e) spontaneous breathing and stable haemodynamics; (f) absence of severe craniocerebral diseases, genetic metabolic diseases, heart diseases, and/or other serious illnesses; and (g) no history of surgery Exclusion criteria: (a) presence of congenital hearing loss and (b) use of sedatives or muscle relaxants. The study termination criteria were as follows: (a) parent request for withdrawal; (b) serious adverse reactions; and, (c) medically instability or death or discharge from the incubator within the period of study. Staff members were asked to continue all usual routines and care practices. Pretreatment: no significant differences were found in sexes, types of delivery, use of dexamethasone, Apgar scores at 1 minute, gestational ages, birth weights, and body weights at the time of study amongst the 3 groups (P > 0.05). N infants analysed: 103 N infants randomised: 103 Reasons for dropouts: in the routine care group, 5 infants were withdrawn due to the worsening of their conditions (n = 2) and discharge during the study period (n = 3). In the white noise group, 4 infants were withdrawn due to the worsening of their condition (n = 3) and discharge (n = 1). In the mothers’ voice group, 3 infants were withdrawn due to the worsening of their condition (n = 3). Reasons for exclusion: Sample size calculation: the sample size was estimated using the free G‐power programme (3.1.9.7), which calculates the sample size using the mean difference and standard deviation. The preliminary experiment was conducted on 15 preterm infants with 5 infants in each group. The means (M) and standard deviations (SD) of the sleep efficiency of premature infants with the mothers’ voice, white noise, and routine care groups were as follows: M 1 = 0.456, M 2 = 0.578, M 3 = 0.400, SD 1 = 0.171, SD 2 = 0.173, and SD 3 = 0.174, respectively. With a calculated effect size of 0.43, a power of 0.90, an alpha of 0.05 (2‐sided), and an attrition rate of 20% in the present study, the minimum total sample of preterm infants was 90, and 103 premature infants were randomised finally. |
|
| Interventions |
Intervention characteristics Control
Recorded mothers' singing voice
|
|
| Outcomes |
Heart rate
Oxygen saturation
Weight Gain
Adverse effects
|
|
| Identification |
Sponsorship source: the study was supported by the Young Backbone Personnel Training Project that was funded by the Health Department of Fujian Province in China (Project No.:2014‐ZQN‐JC‐26). Declaration of interest: the authors declared no conflict of interest. Country: China Setting: NICU Level III Comments: Authors name: Jinhua Liao Institution: School of Nursing, Fujian Medical University, China Email: hulu2886@sina.com (R. Hu), nurhhg@nus.edu.sg (H.‐G. He) Address: School of Nursing, Fujian Medical University, 1 Xue Yuan Road, University Town, Fuzhou, Fujian, China Website: |
|
| Notes |
Primary outcome (comparison of sleep‐wake patterns) 1‐way ANOVA showed no significant differences in all sleep‐wake patterns amongst the 3 groups (P > 0.05). Paired‐Student t‐test showed no significant differences between pre‐ and post‐tests in all sleep‐wake patterns in both the white noise and mothers’ voice groups. Significant differences were only found between the pre‐test and post‐test evaluations in sleep efficiency, wake time after sleep onset, and average awakening time in the routine care group. Therefore, hypothesis 1 was partially supported. Comparison of cortisol levels No significant differences in cortisol levels were found in all the groups between the pre‐test and post‐test (P > 0.05). In addition, no significant differences were found amongst the 3 groups at pre‐test and post‐test evaluations (P = 0.218, P = 0.069, respectively) by the Kruskal–Wallis analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Divided into 3 by a 3rd‐party researcher who was blinded to the patients and did not participate in the clinical trial. Random sequence of 103 numbers was generated using Microsoft Excel |
| Allocation concealment (selection bias) | Low risk | The allocation was concealed using the closed‐envelope method and was kept by the 3rd researcher. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the routine care group, 5 infants were withdrawn due to the worsening of their condition (n = 2) and discharge during the study period (n = 3). In the white noise group, 4 infants were withdrawn due to the worsening of their condition (n = 3) and discharge (n = 1). In the mothers’ voice group, 3 infants were withdrawn due to the worsening of their condition (n = 3). Finally, 91 preterm infants completed the interventions and all the measurements. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if selected reporting happened |
| Other bias | Low risk | No other bias detected |
Loewy 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over |
|
| Participants |
Baseline characteristics Overall
Inclusion criteria: infants ≥ 32 weeks' premature with respiratory distress syndrome, clinical sepsis, and/or SGA (small for gestational age) Exclusion criteria: no specific exclusion criteria were mentioned. Pretreatment: not reported, since within‐subject N infants analysed: 272 N infants randomised: 284 Reasons for dropouts: early discharge (n = 4); data collection availability was not possible (n = 8) Reasons for exclusion: study was originally designed to take place at 8 hospitals for the duration of 2 years; 8 sites were approved by their hospitals' institutional review boards, but 2 sites were discontinued after the study mentioned year 1, due to music therapist position changes. Data collection took place at a total of 11 hospitals where the participation of new sites was co‐ordinated with the investigatory team. On average, each site maintained a total of 30 babies during the 2½ ‐year study period, excluding the discontinued site. Sample size calculation: an initial sample estimate revealed that 240 infants allowed for 80% power to detect a small size (Cohen) of 0.18. The final sample of 272 allowed for 84% power to detect an effect size of this magnitude. |
|
| Interventions |
Intervention characteristics Control
Lullaby song‐of‐kin
Ocean Disc
Gato Box
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
|
|
| Identification |
Sponsorship source: Heather on Earth Music Foundation Declaration of interest: the authors indicated they had no financial relationships relevant to this article to disclose. Country: USA Setting: multi‐centre study (11 hospitals/NICUs) Comments: the study was originally designed to take place at 8 hospitals for the duration of 2 years; 8 sites were approved by their hospitals' institutional review boards (IRBs), but 2 sites were discontinued after year 1, due to music therapist position changes. Data collection took place at a total of 11 hospitals where the participation of new sites was co‐ordinated with the investigatory team. On average, each site maintained a total of 30 babies during the 2½‐year study period, excluding the discontinued site. Authors name: Joanne Loewy Institution: The Louis Armstrong Center for music and Medicine, NICU, and Department of Biostatistics, Beth Israel Medical Center, New York, New York Email: jloewy@chpnet.org Address: The Louis Armstrong Center for music and Medicine, Beth Israel Medical Center, 6 Silver 21 1st Ave and 16th St, New York, NY 1003 Website: https://www.mountsinai.org/locations/music‐therapy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequencing was generated by the biostatistician, who had no contact with the participants. Randomisation of the sequence of presentation was generated by computer to allow for separate sequences for morning and afternoon. |
| Allocation concealment (selection bias) | Low risk | Allocation sequencing was generated by the biostatistician, who had no contact with the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some infants were omitted because of early discharge (n = 4) or data collection availability was not possible (n = 8). |
| Selective reporting (reporting bias) | Low risk | Selective outcome reporting bias not detected |
| Other bias | Unclear risk | Unclear |
Menke 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Live interactive improvised music therapy
Inclusion criteria: gestational age at birth younger or equal to 30 weeks, chronological age older or equal to 10 days of life, clinical stability (at the time of inclusion) as well as sufficient German language skills and informed consent of the parents Exclusion criteria: genetically defined syndrome, neurological diseases (e.g. high‐grade intraventricular haemorrhage, periventricular leukomalacia), severe sepsis (necrotising enterocolitis), clinically relevant malformations as well as malformations that potentially impaired the development of 2 children up to the age of 2 years. Preterm infants with the need for palliative care were also excluded from the study. Pretreatment: not reported N infants analysed: 50 N infants randomised: 65 Reasons for dropouts: Reasons for exclusion: change of study design during the course of recruiting. 2 infants had to be excluded from further analysis because of serious medical complications. 1 family withdrew from the study because of a misunderstanding regarding the procedure of the study. Sample size calculation: the target group of the study was premature infants and their parents or primary caregivers at the beginning and the end of the hospitalisation of the infant. According to a power analysis (single outcome, 2‐sample t‐test for independent samples), N = 104 parent‐infant pairs would have been needed to obtain a power of 80% assuming medium‐sized effects. However, this sample size was not feasible given the planned duration of the recruitment process of the pilot study of a maximum of 1 year. Based on the birth rate of the University Children’s Hospital in Heidelberg within the last 12 months, we aimed to initially recruit N = 65 parent‐infant pairs and, due to expected dropouts during the course of the study, to include N = 50 pairs in the final data analysis. |
|
| Interventions |
Intervention characteristics Live interactive improvised music therapy
|
|
| Outcomes |
Weight gain
Duration of hospitalisation in days
Parental Stress Questionnaire: Stress (PSQ)
State‐Anxiety Inventory (STAI‐SKD)
Edinburgh Postnatal Depression Scale (EPDS)
Parental Competences Questionnaire
State‐Trait Anxiety Inventory (STAI‐T)
Parental Stress Questionnaire: Resources (PSQ)
|
|
| Identification |
Sponsorship source: no funding Declaration of interest: not stated Country: Germany Setting: NICU Level III Comments: Authors name: Barbara Menke Institution: Department of Neonatology, University Children’s Hospital, Heidelberg, Germany Email: Barbara.Menke@srh.de Address: Im Neuenheimer Feld 43069120 Heidelberg Website: https://www.klinikum.uni‐heidelberg.de/zentrum‐fuer‐kinder‐und‐jugendmedizin/iv‐neonatologie |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a random sequence, participants were randomly assigned to the treatment or the control group. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: in the present study, blinding of the participating families could not be ensured because standard care with the music therapy intervention was tested against standard care without the music therapy intervention. It follows, as in almost all studies of the effectiveness of psychosocial interventions, that the music therapist was not blinded to group assignment. For organisational reasons, it was also not possible to ensure that the observers were fully blinded. The use of objective measurement data, such as body measurements at child discharge, reduces the risk of intentional bias in data collection. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data detected |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcomes were collected and reported as planned in the study protocol and approved by the Ethics Committee of the Medical Faculty, Heidelberg University (Study ID: S‐044/2016). At the beginning of the study, data were collected in a pre‐post follow‐up design with a fixed number of music therapy interventions, independent of the duration of hospitalisation. This initial design was not well accepted by many families who expected interventions throughout the entire hospital stay. Thus, the design was changed during the course of the study to a pure pre‐post design. The first 9 data sets were collected according to the initial design. These data sets were excluded from further analysis, as the 2 designs differed in schedule, rendering the data sets incomparable to the rest. Only complete data sets were included in data analysis, leading to the exclusion of 3 data sets containing missing data (1 data set in the treatment group and 2 in the control group). A total of 50 parent‐infant pairs remained as the final cohort to analyse physiological development at discharge. 47 mothers and 30 fathers completed the questionnaires on parental stress factors and were included in the respective analysis. |
| Other bias | Low risk | Judgement comment: both parents were invited to participate in music therapy. However, in practice, it was not always possible that both parents were present during music therapy interventions. For example, some families alternated their visits to the ward in order to be able to care for siblings at home. For this reason, it was not possible to ensure that both parents participated fully in the intervention in every case during the study period. This limits the conclusions that can be drawn from the music therapy intervention about the parental stress factors, especially for the fathers. A strict separation of experimental and control groups could not always be guaranteed despite the greatest efforts for organisational reasons in the course of the units. It could happen that families of the experimental and control groups temporarily shared a room. In this situation, which was unfavourable for the study, care was taken to ensure that the parents of the control group were not in the room during the music therapy interventions. However, this did not completely prevent the children in the control group from listening to the music. The music therapy intervention was investigated as a supplement to standard care. This was defined by an implemented care concept. However, a uniform definition of standard care is difficult. For further investigations, it should be examined whether further parameters, such as the use of psychological counselling, should also be recorded and thus controlled. |
Nakhwa 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control developmental programme
Developmental programme + recorded music
Overall
Inclusion criteria: preterm infants clinically diagnosed by a paediatrician Exclusion criteria: hypoxic ischaemic encephalopathy, any congenital anomalies Pretreatment: not reported N infants analysed: n = 36 (n = 18 controlled group; n = 18 experimental group) N infants randomised: 40 (20 + 20) Reasons for dropouts: There were 2 dropouts in each group because of early discharge. Reasons for exclusion: not reported Sample size calculation: not reported |
|
| Interventions |
Intervention characteristics Control developmental programme
Developmental programme + recorded music
|
|
| Outcomes |
Infant Motor Performance score
Infant Neurological International Battery Score
|
|
| Identification |
Sponsorship source: not reported Declaration of interest: not stated Country: India Setting: Single centre, NICU of Pravara Rural Hospital, Loni Comments: identification only partially reported Authors name: Priyanka K. Nakhwa Institution: Paediatric Physiotherapy, Pravara Rural Hospital, Loni (Bk) Address: Paediatric Physiotherapy, Pravara Rural Hospital, Loni. Website: https://www.pravara.com/rmc‐paediatrics.html |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Simple random sampling |
| Allocation concealment (selection bias) | High risk | Inadequate concealment of allocation prior to assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data detected |
| Selective reporting (reporting bias) | Unclear risk | Unclear if selective reporting happened |
| Other bias | Unclear risk | Unclear if other bias occurred |
Namjoo 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics
Recorded Persian lullaby
Live mother's lullaby
Inclusion criteria: infants aged over 28 weeks of gestation at birth Exclusion criteria: congenital malformations (heart disease, hydrocephalus, etc.), taking any sedatives for up to 3 hours before the intervention, mechanically ventilated during the intervention, hearing impairment (hearing health was confirmed by the startle reflex and the doctor’s opinion), suffering from hypoxic ischaemic encephalopathy Pretreatment: the results showed no statistically significant difference between the three groups in demographic characteristics of the mothers and infants (P > 0.05), and the 3 groups were homogeneous in terms of demographic variables N infants analysed: 90 N infants randomised: 90 Reasons for dropouts: no Reasons for exclusion: no Sample size calculation: the study sample size was 81 preterm infants (27 in each group) and was powered 90% using a pilot study. However, regarding the probability of dropouts and an increase in the power of our study, 96 samples were assigned to the intervention and control groups, respectively (total 96 samples). |
|
| Interventions |
Intervention characteristics Control
Recorded Persian lullaby
Live mother's lullaby
|
|
| Outcomes |
Heart rate
Oxygen saturation
|
|
| Identification |
Sponsorship source: Kerman University of Medical Sciences (IR.KMU.REC.97000409) Declaration of interest: the funding organisation(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication. Country: Iran Setting: NICU Level III Comments: Authors name: Razyeh Namjoo Institution: Nursing in Neonatal Intensive Care, Nursing Research Center, Kerman University of Medical Sciences, Kerman, Iran Email: m.nematolahi@kmu.ac.ir Address: Nursing Research Center, Kerman University of Medical Sciences, Kerman, Iran, Phone:+98 9132416944, Fax:+3431325218 Website: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The participants were assigned randomly to the groups. To this end, one infant was placed in the mother’s live lullaby group, one in the recorded lullaby group, and the third infant in the control group. The same procedure was repeated until all participants were assigned." |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealment happened |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data detected |
| Selective reporting (reporting bias) | Low risk | No selective reporting detected |
| Other bias | Low risk | No other bias detected |
Portugal 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded mother's voice and heartbeats
Inclusion criteria: newborns, admitted to the Neonatology Unit of the Hospital of São João, with gestational age between ≥ 26 and ≤ 33 weeks confirmed by ultrasound Exclusion criteria: newborn infants with major congenital or chromosomal abnormalities (Down syndrome, Turner syndrome, and Klinefelter syndrome), congenital infections, significant brain damage, with prenatal diagnosis (e.g. neonatal asphyxia), uncontrolled maternal disease, maternal history of tobacco, alcohol, and illicit drug consumption, history of significant maternal nutrient deprivation or malnutrition Pretreatment: no statistically significant differences were found between the groups regarding their characterisation. N infants analysed: 18 N infants randomised: 18 Reasons for dropouts: not available Reasons for exclusion: 7 of the parents declined to participate, and the remaining 67 were excluded for various reasons, from linguistic barriers, logistical difficulties (unavailability of monitors, unavailability of recording material, improvement works at the unit) Sample size calculation: not stated |
|
| Interventions |
Intervention characteristics Control
Recorded mother's voice and heartbeats
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
|
|
| Identification |
Sponsorship source: not stated Declaration of interest: not stated Country: Portugal Setting: NICU Comments: not available Authors name: Crisanta Maria Gomes da Silva Leopoldo Institution: Santa Maria Health School, Porto Email: crisanta‐portugal@sapo.pt Address: Praceta Fernão de Magalhães, 101, 10.º, Vila Nova de Gaia, 4049‐024, Porto, Portugal Website: not available |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation reported |
| Allocation concealment (selection bias) | Unclear risk | Unclear allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Unclear selective reporting |
| Other bias | Unclear risk | Unclear if there was other bias |
Tandoi 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded womb sounds
Inclusion criteria: 1. birth within the previous 24 hours; 2. gestational age greater than or equal to 32 weeks and less than 37 weeks; 3. birth weight greater than or equal to 1500 g; 4. written informed consent of a parent Exclusion criteria: major disease Pretreatment: 2 completely overlapping populations and therefore comparable. Both groups were comparable for the type of deliveries (50% versus 55% caesarean section) and type of feeding (60% versus 55% breastfeeding, respectively) N infants analysed: 34 N infants randomised: 34 Reasons for dropouts: no dropouts Reasons for exclusion: no exclusions Sample size calculation: This gave our study an 80% probability of detecting a difference of 15.0 bpm between the two groups with a significance of 5% two‐way, assuming a standard deviation of the response of 15.0 bpm. Similar sample sizes have been found to be consistent with the assessment of one standard deviation difference amongst groups, in a recent review on this topic. |
|
| Interventions |
Intervention characteristics Control
Recorded womb sounds
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
Behavioural scale (APIB)
|
|
| Identification |
Sponsorship source: not reported Declaration of interest: The authors reported no conflicts of interest. Country: Italy Setting: NICU Comments: no comments Authors name: Gaia Francescato Institution: Neonatology and NICU, Department of Clinical Sciences and Community Health, Fondazione IRCCS Ca ` Granda – Ospedale Maggiore Policlinico, University of Milan Email: gaia.francescato@mangiagalli.it or gaia.francescato@yahoo.it Address: Gaia Francescato, Neonatology, Department of Clinical Sciences and Community Health, Fondazione IRCCS Ca`Granda – OspedaleMaggiore Policlinico, University of Milan, via Della Commenda 1220122, Milano |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participant was assigned on the basis of a list of randomisation to 1 of the 2 groups in the study. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 participants enrolled and analysed |
| Selective reporting (reporting bias) | Unclear risk | 36 participants enrolled and analysed |
| Other bias | Unclear risk | Unclear |
Vastani 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Live mother's voice
Live strangers voice
Inclusion criteria: gestational age of 28 to less than 37 weeks, birth weight of between 1000 and 2500 g, at least 3 days passed from the birth, being kept in incubators, having no congenital anomalies, physiological stability, using no respiratory devices such as nasal cannula or positive‐pressure ventilation delivered through nasal/face mask and mechanical ventilator, receiving no muscle relaxants, undergoing no surgical operation, using no pacifier or feeding only with breast milk, healthy auditory system. Exclusion criteria: intracranial haemorrhage, periventricular leukomalacia, cerebral palsy, necrotising enterocolitis, patent ductus arteriosus, sepsis, chronic lung disease, anaemia, addiction to alcohol, narcotic drugs and psychotropic substances in mother. Occurrence of seizure during the intervention, the mother’s decision to withdraw from the study, the use of sedatives by the mother on the intervention day, the incidence of any problem in recording the physiological responses, and the infant’s need for any medical or nursing intervention or any contact during the study. Pretreatment: No significant differences were found between the three groups in terms of baseline demographics and clinical characteristics. N infants analysed: 60 N infants randomised: 66 Reasons for dropouts: not reported Reasons for exclusion: the data of 6 infants were omitted from analysis as they were outliers (the data showed excessive increase or decrease in heart rates insofar as it seemed that the pulse oximeter probes placed on the infant’s legs were dislocated). Sample size calculation: sample size was calculated using a pilot study on 2 groups of 5 infants. 1 group was the silent group and the other group received the mother’s voice stimulation similar to the main study. The post‐intervention mean of the neonatal heart rate was 1487.50 in the group that received the other’s voice and 1435.75 in the silent group. Then by using an online software (i.e. https://www.statstodo.com/SSizAOV_Pgm.php), and considering β = 0.20, and α = 0.05, the number of groups in the analysis = 3, the largest difference between the 2 means = 5 and the expected background standard deviation = 6, the sample size for each of the three groups was estimated to be 22 infants. |
|
| Interventions |
Intervention characteristics Control
Live mother's voice
Live strangers voice
|
|
| Outcomes |
Heart rate
|
|
| Identification |
Sponsorship source: not reported Declaration of interest: none declared Country: Iran Setting: NICU Comments: no comment Authors name: Seyedeh Roghayeh Jafarian Amiri, Institution: Department of Nursing, Babol University of Medical Sciences, Babol, IR Iran Email: Mozhganahmadivastani@gmail.com Address: Iran, Mazandaran, BabolGanjafrooz Street, University of Medical Sciences Website: http://en.mubabol.ac.ir/page/nuesing‐midwife.htm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The samples were randomly allocated into the 3 groups of 22 (silent, mother’s voice and stranger’s voice) by computer randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Moreover, the patient monitoring system was put in an automatic mode. The monitoring system and the digital scale used to measure the variables for all the infants were the same. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data detected |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | An inter‐rater reliability method was used to evaluate the reliability of the instruments (the digital scale and the patient monitoring system). To evaluate the reliability of the digital scale, 2 colleagues were trained and then were asked to weigh 10 infants and record the results in 2 distinct charts. Then the correlation coefficient was calculated, and the result was approximately equal to 1 (r = 0.97) for the digital scale. Moreover, the patient monitoring system was put in an automatic mode. The monitoring system and the digital scale used to measure the variables for all the infants were the same. |
White‐Traut 1988.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Routine care
Live talking mother
RISS mother
Inclusion criteria: maternal inclusion criteria included the ability to speak and understand English, vaginal delivery of the infant, and an age of at least 16 years. Infant inclusion criteria included gestational age between 28 and 35 weeks, birth weight appropriate for gestational age, and absence of ventilatory assistance by 24 hours after delivery. Exclusion criteria: infants suspected or diagnosed with chromosomal abnormalities were excluded. Women who experienced multiple births were not eligible to participate. Pretreatment: there were no significant differences amongst the groups on either maternal or infant variables prior to the intervention. N infants analysed: n = 33; n = 11 routine care, n =11 talking, n = 11 RISS N infants randomised: 33 mother‐infant pairs Reasons for dropouts: not reported Reasons for exclusion: not reported Sample size calculation: not reported |
|
| Interventions |
Intervention characteristics Routine care
Live talking mother
RISS mother
|
|
| Outcomes |
The Nursing Child Assessment Feeding Scale
Maternal behaviour
|
|
| Identification |
Sponsorship source: supported in part by a grant from the Schweppes Foundation Country: USA Setting: single centre; Special Care nursery, Department of Pediatrics, St. Luke's Medical Center, Chicago Comments: no comment Authors name: Rosemary C. White‐Traut Institution: College of Nursing at the University of Illinois‐Chicago Email: rwt@uic.edu Address: Dr. Rosemary White‐Traut, University of Illinois‐ Chicago, 845 S. Damen Street, Chicago, IL 60612 Website: https://today.uic.edu/nurse‐guides‐new‐moms‐of‐preemies & https://today.uic.edu/nurse‐guides‐new‐moms‐of‐preemies |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or exclusions reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | To obtain certification, an inter‐rater agreement score of 85% or greater had to be maintained for five observations of parent and infant behaviour scores. Reliability in scoring the feeding interactions was determined prior to assessments of feeding interactions and during the data collection period. |
Wirth 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control
Recorded lullabies
Recorded mothers voice
Inclusion criteria: preterm infants in a stable condition with a gestational age of 30 to 36 completed weeks and a postnatal age of fewer than 10 days Exclusion criteria: (a) lack of parental consent, (b) expected hospitalisation of fewer than 14 further days, (c) artificial ventilation, but no continuous positive airway pressure, (d) treatment with catecholamines, (e) cardiac anomalies or diseases, (f) congenital malformations, and (g) hearing loss, confirmed by auditory brainstem‐evoked potentials Pretreatment: the 3 groups did not differ significantly with respect to the infants’ baseline characteristics, except postnatal age at the start of the study. N infants analysed: 61 N infants randomised: 62 preterm infants, 20 were randomly assigned to the ‘Lullabies group', 20 to the ‘Maternal voice group’ and 22 to the ‘control group'. Reasons for dropouts: 9 infants dropped out: in 1 infant of the control group, parents withdrew consent, 1 infant died from necrotising enterocolitis and 7 infants were discharged before completion of the study period. Because of technical problems, data collection in 2 infants was incomplete. Reasons for exclusion: 46 infants were excluded for the following reasons: (a) lack of parental consent (n = 24), (b) expected short hospitalisation (n = 20), (c) admission at ⩾ 10 postnatal days (n = 1) and (d) congenital malformation (n = 1). Sample size calculation: no sample size calculation reported. For this pilot study, an empirical number of 20 infants per group was assessed. |
|
| Interventions |
Intervention characteristics Control
Recorded lullabies
Recorded mothers voice
|
|
| Outcomes |
Heart rate
Respiratory rate
|
|
| Identification |
Sponsorship source: KIM e.V., Marburg, Germany, financial support Country: Germany Setting: single centre, NICU or newborn special care unit, Children's Hospital, Philipps University Marburg, Germany Comments: no comments Authors name: Lara Wirth Institution: Children’s Hospital, Philipps University Marburg, Marburg, Germany Email: Lara.Wirth@gmx.de Address: L Wirth, Children’s Hospital, Philipps University Marburg, Baldingerstrasse, Marburg D‐35033, Germany Website: https://www.ukgm.de/ugm_2/deu/umr_lpl/27708.html |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of the randomisation program R 2.15.0. Block randomisation in a random block of 6 (2 lullabies, 2 maternal voice, 2 control). Safekeeping the randomisation results was done by sealed and consecutive numbered envelopes, together with the pseudonym code. Allocation of the included participants according to the numbering of the envelopes |
| Allocation concealment (selection bias) | Low risk | Infants were randomly assigned to 1 of 2 intervention groups or a control group by opening consecutive sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were performed on the intention‐to‐treat population. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Yu 2021.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Control heel stick procedure
Recorded maternal voice before, during & after heel stick procedure
Overall
Inclusion criteria: all infants with a gestational age of less than 37 weeks who received routine care every 8 hours to determine their blood glucose level through the heel sticks and who needed to be in an incubator on the 4th postnatal day were eligible to participate in this study. Exclusion criteria: congenital or chromosomal abnormalities, had congenital or acquired infections, were connected to a high‐frequency oscillatory ventilator, or were administered a sedative or inotropic agents. Premature infants were also excluded if their mothers smoked, consumed alcohol, or used illegal drugs during pregnancy or if the mothers were transferred to an intensive care unit rather than the postnatal ward (recordings were created only in the postnatal ward). Pretreatment: no significant differences were found between the control and intervention groups in terms of gender; length of gestation; birth body weight; Appearance, Pulse, Grimace, Activity, and Respiration score; Neonatal Medical Index; or mother's age, education level, or preterm birth experience (P > 0.05). These results indicated that the intervention and control groups were similar in terms of the studied variables. N infants analysed: 64 N infants randomised: 64 Reasons for dropouts: during the study, 1 infant in the intervention group was dressed and removed from the incubator on the 3rd day of the study, and 2 infants in the control group did not have to undergo the heel sticks on the third day. This data loss, however, had a negligible effect on the data analysis. All the infants passed the hearing test before discharge, and none of them had congenital deafness. The 2 groups, each consisting of 32 preterm infants, were included in the data analysis. Reasons for exclusion: no Sample size calculation: the sample size was calculated using G*Power analysis for repeated measures. The estimated median effect size was 0.15 with an α value of 0.05 and power of 0.8. |
|
| Interventions |
Intervention characteristics Control heel stick procedure
Recorded maternal voice before, during & after heel stick procedure
|
|
| Outcomes |
Heart rate
Respiratory rate
Oxygen saturation
Pain status control
Mother–Infant Bonding Inventory
|
|
| Identification |
Sponsorship source: Research Support Scheme of the Chang Gung Memorial Hospital, Linkou, Taiwan, grant no. CMRP20190320210 Country: Taiwan Setting: NICU Comments: Authors name: Wan‐Chen Yu Institution: Department of Nursing, Chang Gung Memorial Hospital, Taoyuan, Taiwan Email: chiwenchen@nycu.edu.tw (C.‐W. Chen), redmapleyu@gmail.com(W.‐C. Yu), newborntw@gmail.com (M.‐C. Chiang), kuanchia@nycu.edu.tw (K.‐C. Lin), m22096@cgmh.org.tw (C.‐C. Chang), d22038@cgmh.org.tw (K.‐H. Lin) Address: Department of Nursing, Chang Gung Memorial Hospital, Taoyuan, Taiwan Website: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Field block randomisation corresponding to individual preterm infants |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was used to randomly divide the enrolled infants into two groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment of the premature infants was double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data detected |
| Selective reporting (reporting bias) | Low risk | No selective reporting detected |
| Other bias | Unclear risk | The purpose of providing the mothers with the videos was to facilitate mother–infant bonding and to offer the mothers a visualisation of the study process. A Google questionnaire was administered to the mother on the 7th postnatal day. This questionnaire contained the Mother–Infant Bonding Inventory. Finally, the Mother–Infant Bonding Inventory data were collected by the researcher. |
ANOVA: analysis of variance; APIB: Assessment of Preterm Infants' Behavior; Bpm: beats per minute; BW: birth weight; CD: Compact Disc; CG: control group; CGA: corrected gestational age; CI: confidence interval; ; cm: centimetre; CMT: creative music therapy; CPAP: continuous positive airway pressure; dB level: decibel level; EPDS: Edinburgh Postnatal Depression Scale; g: gram; GA: gestational age; HF: high‐frequency; HR: heart rate; HRV: heart rate variability; IGI: intervention group 1; IG2: intervention group 2; IVH: intraventricular haemorrhage; kg: kilogram; LBW: low birth weight infants; LF: low‐frequency; M: means; Mdn: median; MIBS: mother‐to‐infant‐bonding scale; MRI: magnetic resonance imaging ; MT: music therapy; MTG: music therapy group; n: number; n.a.: not available; NICU: neonatal intensive care unit; NIDCAP: Newborn Individualised Developmental Care and Assessment Program; NISS: Neonatal Infant Stressor Scale; PCQ: Parental Competences Questionnaire; O2:Oxygen; PIPP: Premature Infant Pain Profile; PPI: Parental Perception Inventory; PRAM: Pictorial Representation of Attachment Measure; PSS: Parental Stressor Scale; PSQ: Parental Stress Questionnaire; PVL: periventricular leukomalacia; RBL: Rhythm Breath and Lullaby; RCT: randomised controlled trial; RISS: massage, talking, eye contact and rocking; RR: respiratory rate; SD: standard deviation; SES: socioeconomic status; SGA: Small for gestational age; SKD: State Scale; SPO2: oxygen saturation; SSC: skin‐to‐skin‐contact: STAI: State‐Trait Anxiety Inventory; STAI‐SKD: State‐Anxiety Inventor ; TOS: musical track recording obtained with several specific audio equipment; UMCG:University Medical Center Groningen ; URL: Uniform Resource Locator; wk: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alipour 2013 | Due to intervention (frequency of intervention only once) |
| Amini 2013 | Due to intervention (frequency of intervention only twice) |
| Arnon 2006 | Due to intervention (frequency of intervention only once) |
| Arnon 2014 | Due to intervention (frequency of intervention only once) |
| Auto 2013 | Due to comparator (no standard care comparison) |
| Badr 2017 | Due to intervention (frequency of intervention only once) |
| Barandouzi 2020 | Due to intervention (frequency of Intervention only once) |
| Bellieni 2001 | Due to comparator (additional intervention to music/voice provided; not in both compared arms) |
| Bergomi 2014 | Due to intervention (frequency of intervention only once) |
| Castano 2021 | Due to intervention (frequency of intervention only once) |
| Cavaiuolo 2015 | Due to intervention (frequency of intervention only once) |
| Cevasco 2005 | Due to intervention (no continuous music duration comprising at least 5 minutes) |
| Chirico 2017 | Due to study design |
| Chorna 2018 | Due to study design |
| Corrigan 2020 | Due to intervention (frequency of intervention only once) |
| Corrigan 2021 | Due to intervention (frequency of intervention only once) |
| Dalili 2020 | Due to intervention (combined with other interventions) |
| Dearn 2014 | Due to intervention (frequency of intervention only once) |
| Detmer 2020 | Due to study design |
| Doheny 2012 | Due to study design |
| Döra 2021 | Due to intervention (frequency of intervention only once) |
| Edraki 2017 | Due to intervention (freqeuncy of intervention only once) |
| Efendi 2018 | Due to intervention (frequency of intervention only twice) |
| Eskandari 2012 | Due to intervention (frequency of intervention only once) |
| Filippa 2021 | Due to intervention (frequency of intervention only once) |
| Garunkstiene 2014 | Due to intervention (frequency of intervention only once) |
| Holsti 2019 | Due to intervention (frequency of intervention only once) |
| Jie 2017 | Due to intervention (combined with other intervention) |
| Johnston 2009 | Due to intervention (frequency of intervention only once) |
| Kahraman 2020 | Due to intervention (frequency of intervention only once) |
| Kanagasabai 2013 | Due to intervention (combined with other interventions) |
| Karadag 2021 | Due to intervention (frequency of intervention only once) |
| Karimi 2020 | Due to patient population (music for mothers was not in relation to their infant) |
| Keith 2009 | Due to patient population |
| Kucuk Alemdar 2018 | Due to intervention (frequency of intervention only once) |
| Kurdahi Badr 2017 | Due to intervention (frequency of intervention only once) |
| Majidipour 2018 | Due to intervention (frequency of intervention only once) |
| Picciolini 2014 | Due to study design |
| Pouraboli 2019 | Due to intervention (frequency of intervention only once) |
| Qolizadeh 2019 | Due to intervention (frequency of intervention only once) |
| Ramezani 2020 | Due to intervention (frequency of intervention only once) |
| Ranger 2018 | Due to intervention (frequency of intervention only once) |
| Sarhangi 2021 | Due to patient population |
| Schlez 2011 | Due to intervention (frequency of intervention only once) |
| Shabani 2016 | Due to intervention (frequency of intervention only once) |
| Shah 2017 | Due to comparator (additional intervention to music/voice provided not in both compared arms) |
| Shukla 2018 | Due to intervention (frequency of intervention only once) |
| Standley 1995 | Due to study design |
| Standley 2000 | Due to study design |
| Standley 2003 | Relevant outcomes were not available because they had not been measured. |
| Standley 2010 | Due to intervention (no continuous music duration comprising at least 5 minutes) |
| Stokes 2018 | Due to intervention (frequency of intervention only twice) |
| Tang 2018 | Due to intervention (frequency of intervention only once) |
| Tekgunduz 2019 | Due to intervention (frequency of intervention only once) |
| Uematsu 2019 | Due to comparator (additional intervention to music/voice provided not in both compared arms) |
| Ullsten 2017 | Due to intervention (frequency of intervention only once) |
| Vahdati 2017 | Due to intervention (frequency of intervention only once) |
| Walworth 2012 | Due to comparator (additional intervention to music/voice provided not in both compared arms) |
| White‐Traut 2004 | Due to intervention (combined with other interventions) |
| Wu 2020 | Due to intervention (frequency of intervention only once) |
| Yildiz 2011 | Due to study design |
| Yilmaz 2021 | Due to patient population |
| Zeraati 2018 | Due to comparator (additional intervention to music/voice provided not in both compared arms) |
Characteristics of ongoing studies [ordered by study ID]
Aita 2021.
| Study name | Nurturing and quiet intervention on preterm infants' neurodevelopment and maternal stress and anxiety: protocol of a pilot randomised clinical trial |
| Methods | RCT |
| Participants | 26 weeks to 32 weeks |
| Interventions | SSC session lasting 2 hours during the day 4 times/week including a 15‐minute of auditory stimulation with maternal voice and controlled levels of NICU light and noise followed by a 1‐hour quiet period where infants will rest in their incubator/crib with olfactory stimulation and where the control of light and noise levels will be continued. |
| Outcomes | Feasibility and acceptability of the nurturing and quiet intervention and the study procedures as assessed by a self‐completed questionnaire (mothers) and a logbook (time frame: 1 year) Questionnaire completed by mothers ‐ each question treated separately (no total score) Log book completed by a research assistant |
| Starting date | 2020 |
| Contact information | Contact: Marilyn Aita, PhD 5143436111 ext 51473 marilyn.aita@umontreal.ca |
| Notes |
Bonjorn Juarez 2020.
| Study name | Reduction of visual and auditory stimuli to reduce pain during venipuncture in premature infants. A randomised controlled trial |
| Methods | RCT |
| Participants |
|
| Interventions |
Phototherapy goggles and earmuffs will be placed 3 minutes before the venipuncture (leaving the patient in resting state after the manipulation) and will be maintained during the procedure. Monitor alarms and devices will be silenced and will remain silenced and noise in the unit will be minimised during the procedure.
Babies in the control group will receive physical contention with administration of sucrose two minutes before carrying out the venipuncture procedure (usual care). Venipuncture will be performed with 22G extraction needles, or peripheric venous catheter. During the puncture, the eyes will not be covered, and monitor alarms and devices not be silenced. |
| Outcomes | Change in the Premature Infant Pain Profile score (time frame: from baseline pain determination just before venipuncture to 30 seconds after venipuncture) Calculation of pain response using a validated instrument (Premature Infant Pain Profile). It varies from "0" (no pain) to 21 (maximum pain response) |
| Starting date | 2019 |
| Contact information | Contact: Sergio Alonso‐Fernández, RN 0034934978437 salonso.germanstrias@gencat.cat Contact: Maria Bonjorn‐Juarez, RN 0034678116280 mariabonjornjuarez@gmail.com |
| Notes |
Brignoni‐Perez 2021.
| Study name | Listening to mom in the NICU: effects of increased maternal speech exposure on language outcomes and white matter development in infants born very preterm |
| Methods | RCT |
| Participants | 24‐31 weeks 6/7days' gestational age |
| Interventions | Increased maternal speech exposure, accomplished by playing audio recordings of each baby's own mother reading a children's book via an iPod placed in their crib/incubator |
| Outcomes | Measures of expressive and receptive language skills, obtained from a parent questionnaire collected at 12‐18 months |
| Starting date | |
| Contact information | |
| Notes |
ChiCTR1900026017.
| Study name | A randomised controlled trial for investigation of the associations between a music programme and neonatal health outcomes |
| Methods | Interventional study |
| Participants | 28–37 weeks |
| Interventions | 1: normal music; 2: soft music |
| Outcomes | Body weight gain |
| Starting date | 2019 |
| Contact information | Cheng Shuk Man Address: Ward 6HK, Department of Paediatrics, 6/F, Prince of Wales Hospital, 30‐32, Ngan Shing Street, Shatin No postcode Telephone: +852 35054179 Email: dickygabriel@yahoo.com.hk Affiliation: Prince of Wales Hospital, Chinese University of Hong Kong |
| Notes |
CTRI/2012/11/003117.
| Study name | A clinical trial to study the effectiveness of instrumental Indian classical music on serum cortisol concentrations of preterm infants on assisted ventilation admitted to a tertiary level neonatal intensive care unit (NICU) |
| Methods | RCT |
| Participants | Preterm infants |
| Interventions | Music refers to the playing of recorded Indian classical instrumental music, which has soft pitch (shruthi shuddi‐frequency) of sound waves or acoustical energy (Hz), set at a volume of 55‐60 dB (decibels) of sound pressure level which is the comfortable hearing threshold for preterm infants which will be checked and preset for the headphones, amplifiers and music player by using a Sound Level Meter (Hand Held Analyser type 2250, Bruel and Kjaer, Denmark) which is calibrated using a Sound Level Calibrator 4231 (Class 1) (Bruel and Kjaer, Denmark) using a weighted scale and played to the preterm neonate using headphones alternating with 30 minutes of quiet period for every 25 minutes 40 seconds in the first music session and 40 minutes 20 seconds in the second music session, i.e. a total of 1 hour/day during the late evening for a total of 2 weeks, starting from 3rd day of life after collecting basal level of serum cortisol |
| Outcomes | 1. The main outcome variable will be to determine the effectiveness of instrumental Indian classical music on serum cortisol concentrations of very preterm infants on assisted ventilation in the 1st and 2nd week of life. 2. To find the effectiveness on the developmental responses as measured by weight gain, head circumference and vital signs |
| Starting date | |
| Contact information | |
| Notes | Recruitment started in 2012 and was estimated for three years but no further publication found. |
CTRI/2016/06/007028.
| Study name | Comparison of music therapy with kangaroo mother care for pain reduction in premature babies |
| Methods | Randomised controlled trial |
| Participants | Premature infants from gestational age of 26‐36 weeks |
| Interventions | Intervention1: Music therapy: flute music Intervention 2: Kangaroo Mother Care: skin‐to‐skin contact between the mother and the baby during the procedure Control Intervention 1: Music therapy: soft flute music 55 dB during the procedure |
| Outcomes | Pain assessment by measuring Premature Infant pain Profile score time point: (1) Score the behavioural state before the potentially painful event by observing the infant for 15 seconds. (2) Record the baseline heart rate and oxygen saturation. (3) Observe the infant for 30 seconds immediately following the painful event. Score physiologic and facial changes seen during this time and record immediately. |
| Starting date | 2016 |
| Contact information | Somashekhar M Nimbalkar Address: Department of Paediatrics, Pramukhswami medical College, Karamsad, 388325 Anand, GUJARAT India Telephone: 09825087842 Email: somu_somu@yahoo.com Affiliation: Department of Paediatrics, Pramukh swami Medical College |
| Notes |
CTRI/2017/04/008395.
| Study name | Effect of music therapy on breast milk production in mothers practising Kangaroo Mother Care |
| Methods | Randomised controlled trial |
| Participants | Mothers who delivered between 28‐32 weeks of gestation |
| Interventions | Intervention 1: Music therapy: women in the Kangaroo Mother Care ward will listen to music prior to and during breast milk expression |
| Outcomes | Breast milk volume expressed Time point: Day 5 of intervention |
| Starting date | 2017 |
| Contact information | Dr Latha Sashi Address: 4‐1‐1230, Bogulkunta, Near Abids 500001 Hyderabad, ANDHRA PRADESH India Telephone: 9848135621 Email: drlatha_s@fernandezhospital.com Affiliation: Fernandez Hospital |
| Notes |
CTRI/2021/06/033970.
| Study name | A study to check whether music combined with standard Kangaroo Mother Care help in improving neurodevelopmental outcome of preterm infants |
| Methods | Randomised controlled trial |
| Participants | Preterm neonates (< 37 weeks) with birth weight between 2 and 2.5 kg |
| Interventions | Intervention 1: KMC plus instrumental music: mother infant dyads who will be practising KMC with instrumental music for 5 days with play time being a minimum of 90 minutes per day. Instrumental music selected is the Instrumental version of a popular lullaby song (Omaan Thingal Kidavo) Control Intervention 1: KMC: mother infant dyads who will be practising KMC alone for 5 days |
| Outcomes | Neurodevelopmental outcome using Developmental Assessment Scale in Indian Infants. Time point: Neurodevelopmental outcome will be measured at 6 months of age. |
| Starting date | 2021 |
| Contact information | Name: Dr Adhisivam B Address: Dept of Neonatology, JIPMER, Puducherry 605006 Pondicherry, PONDICHERRY India Telephone: 9488822113 Email: adhisivam1975@yahoo.vo.uk Affiliation: JIPMER Pondicherry |
| Notes |
CTRI/2021/07/035287.
| Study name | Effects of vestibular stimulation on neurodevelopment in preterm infants |
| Methods | Randomised controlled trial |
| Participants | 28‐36 weeks of gestation |
| Interventions | Intervention 1: (experimental): 1. Tactile stimulation daily, each session 5 minutes, for 4 weeks Intervention 2: Promotor stimulation daily, each session 5 minutes, for 4 weeks Intervention 3: Auditory stimulation: daily, each session 5 minutes, for 4 weeks Intervention 4: Vestibular stimulation: daily, each session 10 minutes, for 4 weeks Intervention 5: Positioning: daily each session 5 minutes, for 4 weeks Control Intervention 1: (control): 1. Tactile stimulation daily thrice, each session 7 minutes for 4 weeks Control Intervention 2: Promotor stimulation: daily, each session 7 minutes for 4 weeks Control Intervention 3: Auditory stimulation: daily, each session 8 minutes for 4 weeks Control Intervention 4: Positioning: daily, each session 8 minutes for 4 weeks Control Intervention 5: Tactile stimulation: daily, each session 7 minutes for 4 weeks |
| Outcomes | 1. Neurological maturity 2. Weight gain 3. Physiological parameters (oxygen saturation, heart rate, respiratory rate). Time point: 0 week and after 4 weeks |
| Starting date | 2021 |
| Contact information | Dr Kumaresan A Address: 5th floor Department of Neuro physiotherapy Saveetha college of physiotherapy, Saveetha Institute of Medical and Technical Sciences, Saveetha nagar Thandalam Chennai 602105 Kancheepuram, TAMIL NADU India Telephone: 07299934070 Email: kresh49@gmail.com Affiliation: Saveetha college of physiotherapy, Saveetha Institute of Medical and Technical Sciences |
| Notes |
D'Souza 2017.
| Study name | Effectiveness of Indian classical music on developmental responses of preterm infants ‐ a randomized controlled trial protocol |
| Methods | Randomised controlled trial |
| Participants | Preterm infants who are admitted to the NICU. A sample size of 132 preterm infants who are admitted to the NICU, will be assessed for eligibility to be included in the study. The parent(s) of the preterm infants will be approached for informed proxy consent. A study assistant will manage the allocation of participants and the provision of the intervention independently. The investigators who perform the outcome assessment will be blinded to the allocation of participants to the intervention and control arm of the study. |
| Interventions | Indian classical music |
| Outcomes | Outcome measures will be assessed at baseline (day 3 of life). Following the administration of the intervention in the experimental group, the outcome measures will be assessed after completion of 1 week of intervention (day 10 of life) and after completion of 2 weeks of intervention (day 17 of life). |
| Starting date | Not available |
| Contact information | D'Souza SRB: Department of Obstetrical and Gynaecological Nursing, Manipal College of Nursing, Manipal Department of Paediatrics, Kasturba Medical College, Manipal |
| Notes | Only abstract found, no trial entry |
DRKS00018806.
| Study name | Music and maternal voice: investigating the impact on premature babies outcomes |
| Methods | RCT |
| Participants | 27 + 0/7 and 34 + 6/7 gestation age |
| Interventions | Intervention 1: Lullabies sung by own mother Intervention 2: Lullabies sung by strange women Intervention Intervention 3: No intervention |
| Outcomes | Evaluation of the effect of the intervention (strange woman's voice and mothers voice) on vital parameters (heart frequency, blood pressure, oxygen saturation, breathing) of the premature babies ‐ evaluation of the effect of the intervention (strange woman's voice and mothers voice) on weight gain of the premature babies ‐ evaluation of the effect of the intervention (strange woman's voice and mothers voice) on cortisol levels of the premature babies (by means of saliva samples) |
| Starting date | 2019 |
| Contact information | Name: Nora K. Schaal Address: Universitätsstr. 1 40225 Düsseldorf Germany Telephone: 0211 8113863 Email: nora.schaal@hhu.de Affiliation: Institut für Experimentelle Psychologie Heinrich‐Heine‐Universität |
| Notes |
DRKS00025753.
| Study name | Does music therapy influence the neurological development of premature infants born less than 32 weeks of gestation? |
| Methods | RCT |
| Participants | Inclusion criteria: born with a gestational age less than 32 weeks |
| Interventions | Intervention 1: Music therapy for premature infants, born with a gestational age less than 32 weeks Intervention 2: No music therapy for premature infants, born with a gestational age less than 32 weeks |
| Outcomes | The end point of the investigations is the influence of music therapy on the neurological development of very premature infants. This parameter is checked by measuring the vital signs during the music therapy interventions and a questionnaire to evaluate the music therapy at discharge; re‐examination takes place when the child is 24 months old using the Bayley III scale and the questionnaire Austrian Communicative Developmental Inventory. |
| Starting date | 2018 |
| Contact information | Susann Kobus Address: Hufelandstraße 55 45147 Essen Germany Telephone: 020172386267 Email: susann.kobus@uk‐essen.de Affiliation: Universitätsklinikum Essen |
| Notes |
Filippa 2021a.
| Study name | Effects of early vocal contact in the neonatal intensive care unit: study protocol for a multi‐centre, randomised clinical trial |
| Methods | RCT |
| Participants | 25–32 weeks and 6 days' gestational age |
| Interventions | EVC will be performed by mothers 3 times per week for 2 weeks, more than 1 hour after afternoon feeding. It will begin when the newborns are in an active sleep state, in calm awake state or in active awake state, but not in deep sleep or crying. |
| Outcomes | During the interventions, time and frequency analysis of HRV will be applied. The change from baseline will be calculated across the 3 conditions (i.e. singing, speaking, and control). |
| Starting date | |
| Contact information | |
| Notes |
Ghetti 2019.
| Study name | Longitudinal study of music therapy's effectiveness for premature infants and their caregivers (LongSTEP): protocol for an international randomised trial |
| Methods | RCT |
| Participants | Preterm infants |
| Interventions | Music therapy focusing on parental singing specifically tailored to infant responses, will be delivered during NICU and/or during a post‐discharge 6‐month period. |
| Outcomes | Changes in mother‐infant bonding at 6‐month corrected age, as measured by the Postpartum Bonding Questionnaire |
| Starting date | |
| Contact information | |
| Notes |
IRCT2014101819566N2.
| Study name | Comparison of the effect of Koran (yasin) and lullaby music listening on physiological indicators of premature neonates admitted to Motahari hospital of Jahrom |
| Methods | RCT |
| Participants | Preterm newborns admitted to NICU |
| Interventions | 1) 20 minutes of koran 1‐83 Yasin‐Tartil male sound 2) 20 minutes of lullaby by male sound 3) ambient sound |
| Outcomes | O2 saturation, heart rate |
| Starting date | 2014 |
| Contact information | mohsenhojat@yahoo.com |
| Notes |
IRCT20170620034653N3.
| Study name | The effect of the Quran's voice on the formation of oral nutrition in premature infants admitted to the intensive care unit |
| Methods | RCT |
| Participants | Gestational age less than 32 weeks |
| Interventions | Intervention(s) Intervention 1: Intervention group: The Voice of the Holy Quran was performed at 45 dB for 20 minutes at three times before feeding at first, middle and end of the evening shift, and after that, the nutrition begins. This is done until the baby reaches her first full oral feeding. Intervention 2: Control group: Premature babies who do not hear the Quran's voice |
| Outcomes | Vital Signs ‐ duration of the passage from feeding to the tube to oral feeding‐weighing. Time point: Physiological responses including heart rate, respiratory rate, arterial oxygen saturation, mean arterial pressure and temperature were recorded in three stages, every 10 minutes; immediately before the intervention, during the intervention (minutes 10 and 20 after intervention) And 10 minutes after the end of the intervention. Method of measurement: using a monitoring device |
| Starting date | 2018 |
| Contact information | Zahra Tayebi Myaneh Address: Qazvin University of Medical Sciences, Shahid Bahonar Blvd, Qazvin. 00000000 Qazvin Iran Telephone: +98 28 3224 8614 Email: z.tayebi@qums.ac.ir Affiliation: Qazvin University of Medical Sciences |
| Notes |
IRCT20180526039844N1.
| Study name | Effect of voice and tune in pain of neonates |
| Methods | RCT |
| Participants | Baby has a foetal age of 34 weeks and older |
| Interventions | Intervention 1: In the 1st intervention (voice group), the baby is sleeping on an audio pillow and a sedative vocal with specific sound intensity (35‐40 dB) is distributed to the baby. Then, after 3 minutes, a study worker takes blood from the baby and is filmed by a researcher. The 2nd co‐worker examines films for the baby. Intervention 2: Intervention group (tune group): In the second intervention, the baby is sleeping on an audio pillow and a sedative tune with specific intensity (40‐35 dB) is distributed to the baby. Then, 3 minutes later, a worker takes blood from the newborn and is filmed by a researcher. The 2nd co‐worker examines films for the baby. Control Intervention: The baby is sleeping on an audio pillow without any intervention/ audio input; after 3 minutes, a worker takes blood from the newborn and is filmed by a researcher. The 2nd co‐worker examines films for the baby. |
| Outcomes | Intensity of pain. Time point: 3 minutes after start of intervention. Method of measurement: Neonatal Infants Pain Scale. |
| Starting date | 2018 |
| Contact information | Manijeh Rabiei Address: Esfahan University of Medical Sciences, Hezar Jerib street 8174673461 Isfahan Iran (Islamic Republic of)Telephone:+98 31 1668 7153Email:mrabiey1356@gmail.comAffiliation: Esfahan University of Medical Sciences |
| Notes |
NCT02471482.
| Study name | Effect of Mozart music on cerebral oxygenation and behavioural response of premature infants |
| Methods | RCT |
| Participants | Babies with corrected age of 28‐32 (28 + 0 to 32 + 0) weeks of gestation, more than 7 days old |
| Interventions | Experimental: music first group Mozart music lullaby for 30 minutes with recording of cerebral oxygenation (by using Near infrared spectroscopy) and vital signs (respiratory rate, heart rate, oxygen saturations and frequency of apnoeic episodes continuously) followed by 10 minutes of "washout" period and then period of no music for next 30 minutes (with same variables recorded as outlined for music session) This cycle is repeated every 6 hours for 24 hours. In addition, the behavioural response of the baby is observed during the study period by video recording which will be in 'mute' video mode and then reviewed by our developmental staff. |
| Outcomes | Change in cerebral oxygenation values with and without Mozart music as assessed by near infrared spectroscopy (time frame: 1 hour after feeding; period of music (assessed up to 30 minutes), washout period (assessed in next 10 minutes), period of no music (assessed in next 30 minutes)). Same cycle would be repeated every 6 hours for 24 hours only). |
| Starting date | 2015 |
| Contact information | Faiza Yasin Neonatal Department, Coombe Women and Infants University Hospital, Dublin‐8, Ireland |
| Notes |
NCT02948491.
| Study name | Effectiveness of music therapy on the development of preterm neonates (EMT‐DRNP) |
| Methods | Randomised parallel study |
| Participants | 90 participants |
| Interventions | 1. Recording of the voice of the mother singing the song to the child (to choose this option, the song must be sung to the child during pregnancy at least 2 to 3 times per week). 2. The mother's favourite song, obtained externally (to choose this option, the mother must bring the song that she listened to frequently, at least 5 times per week). 3. Pre‐determined lullaby (this option refers only to the lullaby melody or Brahms lullaby, edited to obtain stable volumes and normalisation of the audio, with the effect of progressively appearing and fading for the first and last 10 seconds of the intervention with music therapy, so there are no drastic acoustic changes in the intervention. Audacity editing software will be used.) |
| Outcomes | The Bayley‐III Cognitive Scale |
| Starting date | |
| Contact information | |
| Notes | Recruitment status unknown |
NCT03688386.
| Study name | A language intervention study of preterm infants |
| Methods | RCT |
| Participants | 23 weeks to 31 weeks |
| Interventions | Behavioural: reading intervention Written packet with 3 lessons. The 1st lesson includes how to begin to read and talk to their baby. The 2nd lesson includes reading or talking about the day using parent talk. The 3rd lesson includes continuing to engage with the baby through interactive reading. Behavioural: infant bonding Written packet with 3 lessons. The 1st lesson includes skin‐to‐skin and learning about their baby. The 2nd lesson includes learning readiness cues and participation in feeding times. The 3rd lesson includes developing routines and playing games. Behavioural: LENA recording The LENA device provides 16 hours of language recordings placed inside an infant vest. The recordings are uploaded to a computer which analyses total adult word counts, infant vocalisations, conversational turns, background noise, and silence. Behavioural: LENA linguistic feedback LENA recordings of adult word counts, infant vocalisations, and conversational turns will be provided in printed form after each recording with review of each recording and progress over time. |
| Outcomes |
Adult word counts, conversational turns, and infant vocalisations from each recording |
| Starting date | 2018 |
| Contact information | Betty Vohr, MDWomen and Infants Hospital of Rhode Island |
| Notes |
NCT03795454.
| Study name | Singing kangaroo‐infant care with combined auditory intervention and kangaroo care |
| Methods | Randomised, sequential assignment |
| Participants | 140 participants |
| Interventions | The parent is singing during the skin‐to‐skin sessions; music therapist gives the instructions. |
| Outcomes | Bayley Scales of Infant and Toddler Development, Third Edition (in Finnish in Finland and in Swedish in Sweden) |
| Starting date | May 3, 2013 |
| Contact information | Vineta Fellman, University of Helsinki |
| Notes |
NCT03830580.
| Study name | Benefit of singing in the care of premature children undergoing screening for retinopathy of prematurity in the neonatology and neonatal Resuscitation Unit of the Dijon University Hospital (Voix Chantée) |
| Methods | Clinical trial |
| Participants | Infant born prematurely before the end of 31 weeks of amenorrhoea or with a birth weight of less than 1250 g, or both |
| Interventions | A nurse or assistant trained in singing with an opera singer sings a lullaby She sits next to the incubator and sings from the time the sucrose is administered (2 minutes before the fundus exams is performed) until the end of the examination ‐ taking into account the child's reaction |
| Outcomes | Acute pain assessed by the Premature Infant Pain Profile scale (time frame: during the examination) The children's faces and bodies will be filmed ‐ without sound ‐ during the examination. The nurse who examines the fundus will start and finish the video recording. |
| Starting date | 2019 |
| Contact information | Contact: Anne‐Cécile CHARY‐TARDY 03.80.29.33.62 annececile.charytardy@chu‐dijon.fr |
| Notes |
NCT04314440.
| Study name | Cognitive processing in preterm infants and NICU music therapy |
| Methods | Randomised parallel group |
| Participants | 102 participants |
| Interventions | After the initial warm‐up session, music therapy will be delivered 3 times a week for 15‐20 minutes. All babies in the experimental group will be exposed to 3 weeks of music therapy sessions that are a total of 9 music therapy sessions prior to discharge from the hospital. The baby will be placed at the bassinet 15 minutes before music therapy begins. The baby will be in the bassinet during music therapy and 15 minutes post‐music therapy to allow measurement of physiological indicators. If parents are present during music therapy they will not engage in kangaroo care during the music therapy session or when physiological measurements are being taken pre‐ and post‐music therapy, but can do so at other times. |
| Outcomes |
Average difference in rate of habituation as measured by the time looking at a familiar stimulus in milliseconds between experimental and control groups Secondary outcome measures :
Average difference in heart rate before music therapy to after music therapy from before to during and from during to after music therapy
Average difference in breathing rate before music therapy to after music therapy from before to during and from during to after music therapy
Average difference in oxygen saturation before music therapy to after music therapy from before to during and from during to after music therapy
Difference in sleep between experimental and control group
Difference in apnoea between experimental and control group
Difference in bradycardia (number and severity) between experimental and control groups
Difference in de‐saturation degrees between experimental and control groups |
| Starting date | https://clinicaltrials.gov/ct2/show/NCT04314440 |
| Contact information | Saskatchewan Health Authority ‐ Regina Area |
| Notes |
NCT04335240.
| Study name | Premature family music therapy intervention: an Italian protocol to support parenting, attachment bond and preterm development |
| Methods | Prospective randomised controlled trial parallel group |
| Participants | 90 infants and their parents |
| Interventions | This protocol is a family‐centred care music therapy intervention. The methodologies will provide early intervention from the first days of hospitalisation in NICU and consist of music therapy sessions both "active" (parental chant, live music and lullaby) and "receptive" (listening to recorded tracks). The music therapy accompanies the newborn and the parents during the hospitalisation and focuses its attention on the emotional‐relational care, according to the different needs that they will develop over time. Therapy sessions will be performed starting from 3 times per week, on 3 different days of the week, during the entire hospitalisation of the enrolled infants. After discharge, music therapy treatment will be performed once a week until 12 months of corrected age. |
| Outcomes | Primary outcome measures :
Monitor oxygen saturation by medical monitor in Neonatal Intensive Care and Neonatology Secondary outcome measures :
Parenting Stress Index‐Short Form at 6‐12 months of corrected age, State‐Trait Anxiety Scale at 6‐12 months of corrected age, Beck Depression Inventory at 6‐12 months of corrected age
Temperament detected by the test Italian Questionnaires of Temperament.
General movements. Using digital recording and analysis
Griffiths Scale |
| Starting date | April 30, 2021 |
| Contact information | Barbara Sgobbi, Ospedale di Circolo ‐ Fondazione Macchi |
| Notes | https://clinicaltrials.gov/ct2/show/NCT04335240 |
NCT04559620.
| Study name | Mother providing recorded voice to her preterm infant in incubator improves her own grief and emotional status |
| Methods | Randomised parallel group |
| Participants | 40 infants and their mothers |
| Interventions | Maternal voice (song/words) played in the incubator for 30 minutess x 4/day x 7 days |
| Outcomes | Primary outcome measures :
Secondary outcome measures :
Frequency of these events is measured
(1‐10 questionnaire where higher score = better outcome) |
| Starting date | September 23, 2020 |
| Contact information | Drager |
| Notes | https://clinicaltrials.gov/ct2/show/NCT04559620 |
NCT04565210.
| Study name | Effects of oriental music on preterm infants: a randomised controlled trial |
| Methods | Randomised parallel group |
| Participants | 102 preterm infants |
| Interventions | Experimental: oriental music Infants assigned to this group will be exposed to oriental music. Active comparator: western music Infants assigned to this group will be exposed to western music. Placebo comparator: silence/control Infants assigned to this group will be exposed to the same protocol but using a track of silence. |
| Outcomes | Primary outcome measures:
It consists of changes in the time intervals between consecutive heartbeats called inter‐beat intervals. Secondary outcome measures:
The respiratory rate will be retrieved from bedside monitors.
The oxygen saturation will be retrieved from bedside monitors.
The behavioural score will be assessed using a 7‐point score by a certified nurse. |
| Starting date | September 25, 2020 |
| Contact information | American University of Beirut Medical Center |
| Notes | https://clinicaltrials.gov/ct2/show/NCT04565210 |
NCT04759170.
| Study name | The effects of recorded receptive music therapy on oral nutrition and the well‐being of the Italian premature baby: prospective randomised controlled study |
| Methods | Randomised parallel group |
| Participants | 40 |
| Interventions | 1. Mother recorded receptive music therapy intervention. The effects of recorded receptive music therapy on oral nutrition and the well‐being of the Italian premature baby Other: music therapy The effects of receptive music therapy on oral nutrition and the well‐being of the Italian premature baby 2. Father recorded receptive music therapy intervention. The effects of recorded receptive music therapy on oral nutrition and the well‐being of the Italian premature baby Other: music therapy The effects of receptive music therapy on oral nutrition and the well‐being of the Italian premature baby 3. Music therapist recorded receptive music therapy intervention The effects of recorded receptive music therapy on oral nutrition and the well‐being of the Italian premature baby |
| Outcomes | Primary outcome measures:
Evaluate and monitor the tolerance of recorded receptive music therapy on clinical stability parameter HRV Secondary outcome measures:
Evaluation of the achievement of exclusive oral sucking
Evaluation of the variation in the speed of meal intake between the beginning and the end of the intervention
Evaluation of the weight gain of the newborn in the 24 hours following stimulation
Assessment of the growth rate from the last day of treatment to discharge |
| Starting date | February 18, 2021 |
| Contact information | Barbara Sgobbi, Ospedale di Circolo ‐ Fondazione Macchi |
| Notes | https://clinicaltrials.gov/ct2/show/NCT04759170 |
NCT04759573.
| Study name | Effects of early vocal contact (EVC) in the neonatal intensive care unit: a multi‐centre, randomised clinical trial |
| Methods | Randomised parallel group |
| Participants | 80 infants and their parents |
| Interventions | Experimental: early vocal contact The EVC will take place in the hospital room while infants are in their individual incubators or open cribs. In the intervention group, mothers will be asked to speak and sing to their infants continuously over a 10‐minute period for each type of intervention (20 minutes in total). Mothers will be asked to talk in their native language and to sing familiar songs, while observing their infant's reactions. The order of the 2 vocalisations, speaking and singing, will be reversed in the next intervention. Early vocal contact will be performed by mothers three times a week for 2 weeks, more than 1 hour after afternoon feeding. It will begin when the newborns are in an active sleep state, in calm awake state or in active awake state, but not in deep sleep or crying. Preterm infants will be enrolled from 25 + 0 to 32 + 6 weeks of gestational age, following the established inclusion criteria. |
| Outcomes | Primary outcome measures:
Heart rate is the number of heartbeats per minute. Secondary outcome measures:
The general movement quality from video recording will be scored according to the Ferrari optimality score. 2 blinded coders will attribute a single final score for each infant at each time point. For each item, a description of optimal performance is given and scored with "2" (e.g. cramped components are absent). Less optimal performance is scored with "1" (e.g. cramped components are occasionally present); non‐optimal performance is scored with "0" (e.g. cramped components are predominately present). Adding the scores of each item within a category ("neck and trunk", "upper extremity" and "lower extremity") plus the score for "sequence" gives the GM optimality score with a minimum value of 5 and a maximum value of 42, indicating optimal performance. The minimum score (the worst performance) is 5.
The general movement quality from video recording will be scored according to the Ferrari optimality score. 2 blinded coders will attribute a single final score for each infant at each time point. For each item, a description of optimal performance is given and scored with "2" (e.g. cramped components are absent). Less optimal performance is scored with "1" (e.g. cramped components are occasionally present); non‐optimal performance is scored with "0" (e.g. cramped components are predominately present). Adding the scores of each item within a category ("neck and trunk", "upper extremity" and "lower extremity") plus the score for "sequence" gives the GM optimality score with a minimum value of 5 and a maximum value of 42, indicating optimal performance. The minimum score (the worst performance) is 5.
The general movement quality from video recording will be scored according to the Ferrari optimality score. 2 blinded coders will attribute a single final score for each infant at each time point. For each item a description of optimal performance is given and scored with "2" (e.g. cramped components are absent). Less optimal performance is scored with "1" (e.g. cramped components are occasionally present); non‐optimal performance is scored with "0" (e.g. cramped components are predominately present). Adding the scores of each item within a category ("neck and trunk", "upper extremity" and "lower extremity") plus the score for "sequence" gives the GM optimality score with a minimum value of 5 and a maximum value of 42, indicating optimal performance. The minimum score (the worst performance) is 5.
The GMDS will be assessed with mean values in 4 subscales (Locomotor, Per‐Social, Hear/Speech, Hand/Eye). A composite final Performance score will also be assessed for each participant at each time point. The mean values will be compared between the intervention and control groups. Scores range from 0 to 109, with better results with higher values.
The GMDS will be assessed with mean values in 4 subscales (Locomotor, Per‐Social, Hear/Speech, Hand/Eye). A composite final Performance score will also be assessed for each participant at each time point. The mean values will be compared between the intervention and control groups. Scores range from 0 to 109, with better results with higher values.
Each child, at each time point, will receive a final score for each questionnaire, measured as a discrete numeric value; the mean values will be compared in the intervention and control groups. The minimum score is 0 and the maximum is 429, with higher scores for better performance.
Each child, at each time point, will receive a final score for each questionnaire, measured as a discrete numeric value; the mean values will be compared in the intervention and control groups. The minimum score is 0 and the maximum is 429, with higher scores for better performance.
Each child, at each time point, will receive a final score for each questionnaire, measured as a discrete numeric value; the mean values will be compared in the intervention and control groups. The minimum score is 0 and the maximum is 429, with higher scores for better performance.
Linguistic test for assessing lexical comprehension and production for early childhood. The minimum score is 0 and the maximum is 60, with higher scores for better performance.
The PSS‐NICU aims at assessing the parental perception of stressors derived from the physical and psychosocial environment of the NICU across three domains: (i) their parental role, (ii) their infant's behaviour and appearance, and (iii) the sights and sounds in the NICU. For each domain, a mean score will be assessed, and a final composite stress score will be calculated from the mean values of the single scores. Each mother, at each time point, will receive a final score for the single questionnaire (range 0‐10). The minimum score is 0 and the maximum is 156, with lower scores for better mental health levels.
The PSS‐NICU aims at assessing the parental perception of stressors derived from the physical and psychosocial environment of the NICU across three domains: (i) their parental role, (ii) their infant's behaviour and appearance, and (iii) the sights and sounds in the NICU. For each domain, a mean score will be assessed, and a final composite stress score will be calculated from the mean values of the single scores. Each mother, at each time point, will receive a final score for the single questionnaire (range 0‐10). The minimum score is 0 and the maximum is 156, with lower scores for better mental health levels.
Time that the mothers spend in the NICU (hours) using maternal self‐report forms will be filled out after each visit to the NICU. |
| Starting date | February 18, 2021 |
| Contact information | Elisa Della Casa Muttini |
| Notes | https://clinicaltrials.gov/ct2/show/NCT04759573 |
NCT04886648.
| Study name | The effect of mother's voice and lullaby on preterm infants`physiological parameters, stress and sleeping‐waking state |
| Methods | Randomised parallel group |
| Participants | 90 |
| Interventions | Experimental: mother's voice Premature baby group with mother's voice application. Behavioural: music therapy Experimental: lullaby Premature baby group with lullaby application Behavioural: music therapy No intervention: control Premature baby group with no application |
| Outcomes | Primary outcome measures:
NSEF: no stress indicator '0', mild stress indicators '1', moderate stress indicators '2', and severe stress indicators scored as '3'. Secondary outcome measures:
The evaluation of the newborn status was carried out under the main headings of sleep and waking behaviours. Sleep behaviour; deep sleep, light sleep and drowsy. Waking behaviour is grouped under the headings of awake (extremely awake and eyelids awake), active awake and crying. As a result of the evaluation, it was decided that the behaviour of the newborn were in an organised or disorganised range, and the form was marked and scoring was created for the conditions starting from the deep sleep state to disorganised crying. In the evaluation of the data, the interpretation was made according to the state score of the newborn. It was interpreted that as the YUUDF score decreased, the sleep state of the newborns increased, and as the YUUDF score increased, the wakefulness and crying status of the newborns increased. Other outcome measures:
During the study, heart rate was recorded with the help of a pulse oximeter.
During the study, the number of respirations of preterm newborns was counted.
During the study, oxygen saturation was recorded with the help of a pulse oximeter. |
| Starting date | May 14, 2021 |
| Contact information | Dilek Deri̇nce Eryuruk, TC Erciyes University |
| Notes | https://clinicaltrials.gov/ct2/show/NCT04886648 |
Neel 2019.
| Study name | Randomised controlled trial protocol to improve multisensory neural processing, language and motor outcomes in preterm infants |
| Methods | RCT |
| Participants | Preterm infants |
| Interventions | 1) Exposure to recorded parent’s voice 2) Skin‐to‐skin holding |
| Outcomes | The primary outcome is multisensory response and the secondary outcome is neurodevelopmental outcomes, including sensory adaptation, motor, language, tactile processing, and speech sound differentiation. |
| Starting date | |
| Contact information | |
| Notes |
Pavel 2019.
| Study name | The effect of music therapy on the electroencephalogram (EEG) and heart rate variability (HRV) of premature infants during routine painful procedures |
| Methods | Randomised cross‐over study |
| Participants | Newborns delivered before 32 weeks' gestational age |
| Interventions | Either sucrose or sucrose and music therapy (Brahms’ lullaby) during routine venepuncture |
| Outcomes | EEG and HRV analysis |
| Starting date | |
| Contact information | |
| Notes |
Purdy 2011.
| Study name | A randomised controlled trial of NICU music: impact on preterm infant rest and growth |
| Methods | Randomised controlled blinded multi‐centre study |
| Participants | 28 to < 37 weeks' gestational age at birth |
| Interventions | Structured music intervention offered 2 x daily for 7 days |
| Outcomes | Rest, growth, serum insulin and insulin‐like growth factor‐1 |
| Starting date | |
| Contact information | |
| Notes |
Tosun 2014.
| Study name | The effect of aromatherapy, music therapy and vibration applications on neonatal stress and behaviours |
| Methods | RCT |
| Participants | Preterm infants |
| Interventions | Control, aromatherapy, music therapy and vibration application |
| Outcomes | Data were collected with a questionnaire form, Brazelton Newborn Behavioural Assessment Scale and Newborn Stress Evaluation Form: on the 1st, 3rd and 5th days, applied both pre‐ and post‐intervention; application continued one session/day for 5 days. |
| Starting date | |
| Contact information | |
| Notes |
dB: decibel level; EEG: electroencephalogram ; EVC: Early Vocal Contact; GMDS: Griffiths Mental Development Scales; HRV: Heart rate variability; KMC: Kangaroo‐mother care; LENA: language recordings: NICU: neonatal intensive care unit; NSEF: Newborn Stress Evaluation Form; NSWEF: Newborn Sleepıng‐Wakıng State Evaluation Form; O2: Oxygen; PSS: Parental Stressor Scale; RCT: Randomised controlled trial; SSC: Skin‐to‐skin contact
Differences between protocol and review
We made the following changes to the protocol (Haslbeck 2019).
Edited the search strategies;
Excluded studies published as only as abstracts.
Contributions of authors
The first author wrote the manuscript, and all other authors reviewed and edited the review. The first author led the data screening and extraction process. FH and KM screened abstracts and full texts. FH, KM, and TK contributed to data extraction. JL contributed to musical details in the data extraction of included studies. All analyses and write‐ups were done by the first author.
Sources of support
Internal sources
-
University Hospital Zurich and University Zurich, Switzerland
Paid the salary of Friederike Haslbeck. Funding from Vontobel‐Stitftung.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
FH works as a music therapist and senior researcher at the University Hospital Zurich, Switzerland. FH was the project leader of a study included in this review (Haslbeck 2021), and so did not determine the overall study inclusion and exclusion criteria; make study eligibility decisions about, extract data from, carry out the risk of bias assessment for, or perform GRADE assessments for that study. TK and KM assessed this study instead.
KM has no conflicts of interest to declare.
TK has no conflicts of interest to declare.
JL works as Director, Louis Armstrong Center for Music and Medicine, New York City, USA. She has published opinions in medical journals related to the subject of this review. JL was an author on a study included in this review (Loewy 2013), and so did not determine the overall study inclusion and exclusion criteria; make study eligibility decisions about, extract data from, carry out the risk of bias assessment for, or perform GRADE assessments for that study.
JM is Director of the German Cochrane Centre. JM determined the final overall inclusion and exclusion criteria.
DB works as the director of the Department of Neonatology at the University Hospital Zurich, Switzerland. DB was a co‐author on a study included in this review (Haslbeck 2021), and so did not determine the overall study inclusion and exclusion criteria; make study eligibility decisions about, extract data from, carry out the risk of bias assessment for, or perform GRADE assessments for that study. TK and KM assessed this study instead.
New
References
References to studies included in this review
Calabro 2003 {published and unpublished data}
- Calabro J, Wolfe R, Shoemark H. The effects of recorded sedative music on the physiology and behaviour of premature infants with a respiratory disorder. Australian Journal of Music Therapy 2003;14:19. [Google Scholar]
Caparros‐Gonzalez 2018 {published and unpublished data}
- Caparros-Gonzalez RA, la Torre-Luque A, Diaz-Piedra C, Vico FJ, Buela-Casal G. Listening to relaxing music improves physiological responses in premature infants: a randomized controlled trial. Advances in Neonatal Care 2018;18(1):58-69. [DOI: 10.1097/ANC.0000000000000448] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cevasco 2008 {published and unpublished data}
- Cevasco AM. The effects of mothers' singing on full-term and preterm infants and maternal emotional responses. Journal of Music Therapy 2008;45(3):273-306. [DOI: 10.1093/jmt/45.3.273] [PMID: ] [DOI] [PubMed] [Google Scholar]
Epstein 2021 {published and unpublished data}
- Epstein S, Bauer S, Levkovitz Stern O, Litmanovitz I, Elefant C, et al. Preterm infants with severe brain injury demonstrate unstable physiological responses during maternal singing with music therapy: a randomised controlled study. European Journal of Pediatrics 2021;180(5):1403-12. [DOI: 10.1007/s00431-020-03890-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ettenberger 2014 {published and unpublished data}
- Ettenberger M, Odell-Miller H, Rojas Cardenas C, Torres Serrano S, Parker M, Camargo Llanos SM. Music therapy with premature infants and their caregivers in Colombia: a mixed methods pilot study including a randomized trial. Voices: a world forum for music therapy 2014;14(2):26. [Google Scholar]
Farhat 2010 {published and unpublished data}
- Farhat A, Amiri R, Karbandi S, Esmaily H, Mohammadzadeh A. The effect of listening to lullaby music on physiologic response and weight gain of premature infants. Journal of Neonatal-perinatal Medicine 2010;3(2):103-7. [DOI: 10.3233/NPM-2010-0101] [DOI] [Google Scholar]
Haslbeck 2021 {published data only}
- Haslbeck FB, Bucher HU, Bassler D, Hagmann C, Natalucci G. Creative music therapy and neurodevelopmental outcomes in pre-term infants at 2 Years: a randomized controlled pilot trial. Frontiers in Pediatrics 2021;9:660393. [DOI: 10.3389/fped.2021.660393] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jabraeili 2016 {published data only}
- Jabraeili M, Sabet T, MustafaGharebaghi M, Asghari Jafarabadi M, Arshadi M. The effect of recorded mum's lullaby and Brahms' lullaby on oxygen saturation in preterm infants: a randomised double-blind clinical trial. Journal of Caring Sciences 2016;5(1):85-93. [DOI: 10.15171/jcs.2016.009] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2007 {published and unpublished data}
- Johnston CC, Filion F, Nuyt AM. Recorded maternal voice for preterm neonates undergoing heel lance. Advances in Neonatal Care 2007;7(5):258-66. [DOI: 10.1097/01.ANC.0000296634.26669.13] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kehl 2021 {published and unpublished data}
- Kehl SM, La Marca-Ghaemmaghami P, Haller M, Pichler-Stachl E, Bucher HU, Bassler D, et al. Creative music therapy with premature infants and their parents: a mixed-method pilot study on parents’ anxiety, stress and depressive symptoms and parent–infant attachment. International Journal of Environmental Research and Public Health 2021;18(1):1-20. [DOI: 10.3390/ijerph18010265] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraft 2021 {published and unpublished data}
- ISRCTN94562698. Live music for preterm infants [Musical intervention for extremely preterm infants during NICU stay: a feasibility pilot study]. isrctn.com/ISRCTN94562698 (first received 29 January 2019). [CENTRAL: CN-01950138]
- Kraft KE, Jaschke AC, Ravensbergen AG, Feenstra-Weeli A, Goor ME, Kroon ML, et al. Maternal anxiety, infant stress, and the role of live-performed music therapy during NICU stay in The Netherlands. International Journal of Environmental Research and Public Health 2021;18(13):02. [DOI: 10.3390/ijerph18137077] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kucuk Alemdar 2020 {published data only}
- Kucuk Alemdar D, Inal S. The effect of individualised developmental care practices in preterm infants. Journal of complementary Medicine Research 2020;27(2):97-104. [DOI: 10.1159/000504357] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lafferty 2021 {published data only}
- Lafferty MA, Mackley A, Green P, Ottenthal D, Locke R, Guillen U. Can Mozart improve weight gain and development of feeding skills in premature infants? A randomized trial. American Journal of Perinatology 2021;22:22. [DOI: 10.1055/s-0041-1731279] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lejeune 2019 {published data only}
- Lejeune F, Lordier L, Pittet MP, Schoenhals L, Grandjean, Huppi PS, et al. Effects of an early postnatal music intervention on cognitive and emotional development in preterm children at 12 and 24 months: preliminary findings. Frontiers in Psychology 2019;10(101550902):494. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2021 {published data only}
- Liao J, Liu G, Xie N, Wang S, Wu T, Lin Y, et al. Mothers' voices and white noise on premature infants' physiological reactions in a neonatal intensive care unit: A multi-arm randomized controlled trial. International Journal of Nursing Studies 2021;119:103934. [DOI: 10.1016/j.ijnurstu.2021.103934] [PMID: ] [DOI] [PubMed] [Google Scholar]
Loewy 2013 {published and unpublished data}
- Loewy J, Stewart K, Dassler AM, Telsey A, Homel P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics 2013;131(5):902-18. [DOI: 10.1542/peds.2012-1367] [PMID: ] [DOI] [PubMed] [Google Scholar]
Menke 2021 {published and unpublished data}
- Menke BM, Hass J, Diener C, Poschl J. Family-centered music therapy—empowering premature infants and their primary caregivers through music: results of a pilot study. PLOS One 2021;16(5):18. [DOI: 10.1371/journal.pone.0250071] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakhwa 2017 {published data only}
- Nakhwa PK, Mandar M, Shrikhande DY, Shrikhande S, Rokade P. Efficacy of music therapy in improvement of neuromotor development in preterm infants. Revista Romana de Kinetoterapie {Romanian Journal of Physical Therapy} 2017;23(40):5-11. [Google Scholar]
Namjoo 2021 {published data only}
- Namjoo R, Mehdipour-Rabori R, Bagherian B, Nematollahi M. Comparing the effectiveness of mother's live lullaby and recorded lullaby on physiological responses and sleep of preterm infants: a clinical trial study. Journal of Complementary and Integrative Medicine 2021;24:24. [DOI: 10.1515/jcim-2020-0507] [PMID: ] [DOI] [PubMed] [Google Scholar]
Portugal 2017 {published and unpublished data}
- Portugal CM, Sá LO, Guimarães MH. Cardiorespiratory effects of maternal sounds in infants bom between 26 and 33 weeks of gestation. Revista de Enfermagem Referencia 2017;4(12):55-63. [DOI: 10.12707/RIV16075] [DOI] [Google Scholar]
Tandoi 2015 {published data only}
- Tandoi F, Francescato G, Pagani A, Buzzetti G, Negri E, Agosti M. "The Original Sound": a new non-pharmacological approach to the postnatal stress management of preterm infants. Journal of Maternal-fetal & Neonatal Medicine 2015;28(16):1934-8. [DOI: 10.3109/14767058.2014.973392] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vastani 2017 {published data only}
- Vastani MA, Zahedpasha Y, Amiri SR, Khafri S, Farhadi R. The effect of experience on recognition of mother's voice in preterm infants. Nursing and Midwifery Studies 2017;6(1):7. [DOI: 10.17795/nmsjournal40964] [DOI] [Google Scholar]
White‐Traut 1988 {published data only}
- White-Traut RC, Nelson MN. Maternally administered tactile, auditory, visual, and vestibular stimulation: relationship to later interactions between mothers and premature infants. Research in Nursing & Health 1988;11(1):31-9. [DOI: 10.1002/nur.4770110106] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wirth 2016 {published and unpublished data}
- Wirth L, Dorn F, Wege M, Zemlin M, Lemmer B, Gorbey S, et al. Effects of standardized acoustic stimulation in premature infants: a randomized controlled trial. Journal of Perinatology 2016;36(6):486-92. [DOI: 10.1038/jp.2016.1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yu 2021 {published data only}
- Yu WC, Chiang MC, Lin KC, Chang CC, Lin KH, Chen CW. Effects of maternal voice on pain and mother-Infant bonding in premature infants in Taiwan: a randomized controlled trial. Journal of Pediatric Nursing 2021;30:30. [DOI: 10.1016/j.pedn.2021.09.022] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alipour 2013 {published data only}
- Alipour Z, Eskandari N, Ahmari Tehran H, Eshagh Hossaini SK, Sangi S. Effects of music on physiological and behavioral responses of premature infants: a randomized controlled trial. Complementary Therapies in Clinical Practice 2013;19(3):128-32. [DOI: 10.1016/j.ctcp.2013.02.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Amini 2013 {published data only}
- Amini E, Rafiei P, Zarei K, Gohari M, Hamidi M. Effect of lullaby and classical music on physiologic stability of hospitalized preterm infants: a randomized trial. Journal of Neonatal-Perinatal Medicine 2013;6(4):295-301. [DOI: 10.3233/NPM-1371313] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arnon 2006 {published data only}
- Arnon S, Shapsa A, Forman L, Regev R, Bauer S, Litmanovitz I, et al. Live music is beneficial to preterm infants in the neonatal intensive care unit environment. Birth (Berkeley, Calif.) 2006;33(2):131-6. [DOI: 10.1111/j.0730-7659.2006.00090.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arnon 2014 {published data only}
- Arnon S, Diamant C, Bauer S, Regev R, Sirota G, Litmanovitz I. Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatrica 2014;103(10):1039-44. [DOI: 10.1111/apa.12744] [PMID: ] [DOI] [PubMed] [Google Scholar]
Auto 2013 {published data only}
- Auto FM, Amancio OM, Córdoba Lanza F. The effect of music on weight gain of preterm infants older than 32 weeks: a randomized clinical trial. Revista Paulista de Pediatria 2013;33(4):e293-9. [DOI: 10.1590/0103-058231369512] [DOI] [Google Scholar]
Badr 2017 {published data only}
- Badr LK, Demerjian T, Daaboul T, Abbas H, Zeineddine MH, Charafeddine L. Preterm infants exhibited less pain during a heel stick when they were played the same music their mothers listened to during pregnancy. Acta Paediatrica 2017;106(3):438-45. [DOI: 10.1111/apa.13666] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barandouzi 2020 {published data only}
- Barandouzi ZA, Keshavarz M, Montazeri A, Ashayeri H, Rajaei Z. Comparison of the analgesic effect of oral sucrose and/or music in preterm neonates: a double-blind randomized clinical trial. Complementary Therapies in Medicine 2020;48:102271. [DOI: 10.1016/j.ctim.2019.102271] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bellieni 2001 {published data only}
- Bellieni CV, Buonocore G, Nenci A, Franci N, Cordelli DM, Bagnoli F. Sensorial saturation: an effective analgesic tool for heel-prick in preterm infants: a prospective randomized trial. Biology of the Neonate 2001;80(1):15-8. [DOI: 10.1159/000047113] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bergomi 2014 {published data only}
- Bergomi P, Chieppi M, Maini A, Mugnos T, Spotti D, Tzialla C, et al. Nonpharmacological techniques to reduce pain in preterm infants who receive heel-lance procedure: a randomized controlled trial. Research and Theory for Nursing Practice 2014;28(4):335-48. [DOI: 10.1891/1541-6577.28.4.335] [PMID: ] [DOI] [PubMed] [Google Scholar]
Castano 2021 {published data only}
- Castano D, Alzate EA, Murillo AF, Vera C, Villada MG, Giraldo SJ, et al. Effect of musical exposure on cerebral oxygen saturation,neurobehavior and sleep-wake cycle in premature infants hospitalized in a high complex institution. Randomized, triple-blind and controlled trial. Pediatrics 2021;147(3):676-7. [DOI: 10.1542/peds.147.3-MeetingAbstract.676] [DOI] [Google Scholar]
Cavaiuolo 2015 {published data only}
- Cavaiuolo C, Casani A, Di Manso G, Orfeo L. Effect of Mozart music on heel prick pain in preterm infants: a pilot randomized controlled trial. Journal of Pediatric and Neonatal individualized Medicine 2015;4(1):5. [Google Scholar]
Cevasco 2005 {published data only}
- Cevasco AM, Grant RE. Effects of the pacifier activated lullaby on weight gain of premature infants. Journal of Music Therapy 2005;42(2):123-39. [DOI: 10.1093/jmt/42.2.123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chirico 2017 {published data only}
- Chirico G, Cabano R, Villa G, Bigogno A, Ardesi M, Dioni E. Randomised study showed that recorded maternal voices reduced pain in preterm infants undergoing heel lance procedures in a neonatal intensive care unit. Acta Paediatrica 2017;106(10):1564-8. [DOI: 10.1111/apa.13944] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chorna 2018 {published data only}
- Chorna OD, Hamm EL, Shrivastava H, Maitre NL. Feasibility of event-related potential (ERP) biomarker use to study effects of mother's voice exposure on speech sound differentiation of preterm infants. Developmental Neuropsychology 2018;43(2):123-34. [DOI: 10.1080/87565641.2018.1433671] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corrigan 2020 {published data only}
- Corrigan MJ, Keeler JR, Miller HD, Ben Khallouq BA, Fowler SB. Music therapy and retinopathy of prematurity screening: using recorded maternal singing and heartbeat for post exam recovery. Journal of Perinatology 2020;40(12):1780-8. [DOI: 10.1038/s41372-020-0719-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Corrigan 2021 {published data only}
- Corrigan M, Keeler J, Miller H, Naylor C, Diaz A. Music therapy and family-integrated care in the NICU: using heartbeat-music interventions to promote mother-infant bonding. Advances in Neonatal Care 2021;16:16. [DOI: 10.1097/ANC.0000000000000910] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dalili 2020 {published data only}
- Dalili H, Zaker Z, Keihanidoust Z, Farahani Z, Shariat M. Comparison of neuro-developmental status in preterm neonates with and without family based interventions. World Journal of Advanced Research and Reviews 2020;08(02):56-63. [DOI: 10.30574/wjarr.2020.8.2.0357] [DOI] [Google Scholar]
- IRCT201705079568N17. Comparison of neuro-developmental status of preterm neonates with and without family based interventions [Influence of family based intervention on neuro-developmental status of preterm neonates]. en.irct.ir/trial/10142 (first received 02 October 2017). [CENTRAL: CN-01890699]
Dearn 2014 {published data only}
- Dearn T, Shoemark H. The effect of maternal presence on premature infant response to recorded music. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2014;43(3):341-50. [DOI: 10.1111/1552-6909.12303] [PMID: ] [DOI] [PubMed] [Google Scholar]
Detmer 2020 {published data only}
- Detmer MR, Evans K, Shina E, Walker K, DeLoach D, Malowitz JR. Multimodal neurologic enhancement improves preterm infants' developmental outcomes: a longitudinal pilot study. Neonatal Network 2020;39(1):16-23. [DOI: 10.1891/0730-0832.39.1.16] [PMID: ] [DOI] [PubMed] [Google Scholar]
Doheny 2012 {published data only}
- Doheny L, Hurwitz S, Insoft R, Ringer S, Lahav A. Exposure to biological maternal sounds improves cardiorespiratory regulation in extremely preterm infants. Journal of Maternal-Fetal & Neonatal Medicine 2012;25(9):1591-4. [DOI: 10.3109/14767058.2011.648237] [PMID: ] [DOI] [PubMed] [Google Scholar]
Döra 2021 {published data only}
- Döra Ö, Büyük ET. Effect of white noise and lullabies on pain and vital signs in invasive interventions applied to premature babies. Pain Management Nursing 2021;28:28. [DOI: 10.1016/j.pmn.2021.05.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Edraki 2017 {published data only}
- Edraki M, Beheshtipour N, Razavi SM, Zahadatpour Z. Studying the effect of lullaby on some physiological factors. Pharmacophore 2017;8(6S):e-1173552. [URL: pharmacophorejournal.com/article/studying-the-effect-of-lullaby-on-some-of-physiological-factors-due-to-endtracheal-tube-suction-of-preterm-infants-confined-to-bed-in-intensive-care-unit-icu]
Efendi 2018 {published data only}
- Efendi D, Caswini N, Rustina Y, Iskandar RA. Combination of mother therapeutic touch (MTT) and maternal voice stimulus (MVS) therapies stabilise sleep and physiological function in preterm infants receiving minor invasive procedures. Journal of Neonatal Nursing 2018;24(6):318-24. [DOI: 10.1016/j.jnn.2018.08.001] [DOI] [Google Scholar]
Eskandari 2012 {published data only}
- Eskandari N, Keshavars M, Ashayeri H, Jahdi F, Hosseini AF. Quran recitation: short-term effects and related factors in preterm newborns. Research Journal of Medical Sciences 2012;6(3):148-53. [HTTPS: www.medwelljournals.com/abstract/?doi=rjmsci.2012.148.153] [Google Scholar]
Filippa 2021 {published data only}
- Filippa M, Monaci MG, Spagnuolo C, Serravalle P, Daniele R, Grandjean D. Maternal speech decreases pain scores and increases oxytocin levels in preterm infants during painful procedures. Scientific Reports 2021;11(1):17301. [DOI: 10.1038/s41598-021-96840-4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garunkstiene 2014 {published data only}
- Garunkstiene R, Buinauskiene J, Uloziene I, Markuniene E. Controlled trial of live versus recorded lullabies in preterm infants. Nordic Journal of Music Therapy 2014;23(1):71-88. [DOI: 10.1080/08098131.2013.809783] [DOI] [Google Scholar]
Holsti 2019 {published data only}
- Holsti L, MacLean K, Oberlander T, Synnes A, Brant R. Calmer: a robot for managing acute pain effectively in preterm infants in the neonatal intensive care unit. Pain Reports 2019;4(2):e727. [DOI: 10.1097/PR9.0000000000000727] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jie 2017 {published data only}
- Jie Q, Yun-fei J, Fang L, Qian-hong T, Hui R, Rui C, et al. Effect of combined music and touch intervention on pain response and β-endorphin and cortisol concentrations in late preterm infants. BMC Pediatrics 2017;17:1-7. [DOI: 10.1186/s12887-016-0755-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2009 {published data only}
- Johnston CC, Filion F, Campbell-Yeo M, Goulet C, Bell L, McNaughton K, et al. Enhanced kangaroo mother care for heel lance in preterm neonates: a crossover trial. Journal of Perinatology 2009;29(1):51-6. [DOI: 10.1038/jp.2008.113] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kahraman 2020 {published data only}
- Kahraman A, Gumus M, Akar M, Sipahi M, Bal Yilmaz H, Basbakkal Z. The effects of auditory interventions on pain and comfort in premature newborns in the neonatal intensive care unit; a randomised controlled trial. Intensive and Critical Care Nursing 2020;61:102904. [DOI: 10.1016/j.iccn.2020.102904] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kanagasabai 2013 {published data only}
- Kanagasabai PS, Mohan D, Lewis LE, Kamath A, Rao BK. Effect of multisensory stimulation on neuromotor development in preterm infants. Indian Journal of Pediatrics 2013;80(6):460-4. [DOI: 10.1007/s12098-012-0945-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
Karadag 2021 {published data only}
- Karadag OE, Kerimoglu Yildiz G, Akdogan R, Yildiz S, Hakyemez Toptan H. The effect of simulative heartbeat nest used in preterm new-borns on vital signs, pain, and comfort in Turkey: a randomized controlled study. Journal of Pediatric Nursing 2021;23:23. [DOI: 10.1016/j.pedn.2021.10.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Karimi 2020 {published data only}
- Karimi A, Zabihi A, Zahed Pasha, Y, Jafarian Amiri SR, Hajian K, Moudi S. The effect of music on the anxiety of mothers of infants admitted to the neonatal ntensive Care Unit. Journal Babol Uniersity of Medical Sciences 2020;22:348–54. [DOI: 10.22088/jbums.22.1.348] [DOI] [Google Scholar]
Keith 2009 {published data only}
- Keith DR, Russell K, Weaver BS. The effects of music listening on inconsolable crying in premature infants. Journal of Music Therapy 2009;46(3):191-203. [DOI: 10.1093/jmt/46.3.191] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kucuk Alemdar 2018 {published data only}
- Kucuk Alemdar D, Guducu TufekcI F. Effects of maternal heart sounds on pain and comfort during aspiration in preterm infants. Japan Journal of Nursing Science 2018;15(4):330-9. [DOI: 10.1111/jjns.12202] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kurdahi Badr 2017 {published data only}
- Kurdahi Badr L, Demerjian T, Daaboul T, Abbas H, Hasan Zeineddine M, Charafeddine L. Preterm infants exhibited less pain during a heel stick when they were played the same music their mothers listened to during pregnancy. Acta Paediatrica 2017;106(3):438-45. [DOI: 10.1111/apa.13666] [PMID: ] [DOI] [PubMed] [Google Scholar]
Majidipour 2018 {published data only}
- Majidipour N, Nirouzad F, Madmoli Y, Sarrafzade S, Kalani L, Aghababaeian H, et al. The effect of Holy Quran recitation on the physiological responses of premature infants during phlebotomy: a randomized clinical trial. International Journal of Pediatrics-Masshad 2018;6(7):7869-81. [DOI: 10.22038/ijp.2017.24203.2038] [DOI] [Google Scholar]
Picciolini 2014 {published data only}
- Picciolini O, Porro M, Meazza A, Gianni ML, Rivoli C, Lucco G, et al. Early exposure to maternal voice: effects on preterm infants development. Early Human Development 2014;90(6):287-92. [DOI: 10.1016/j.earlhumdev.2014.03.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pouraboli 2019 {published data only}
- Pouraboli B, Rayyani M, Anari Mahlegha D, Hosseini F, Loghmani L. Lullaby effect with mother's voice on respiratory rate and the speed of its return to the pre-suction state in intubated preterm infants, during tracheal tube suction Kerman, Afzali Pour hospital 2016. Electronic Journal of General Medicine 2019;16(1):1-9. [DOI: 10.29333/ejgm/93471] [DOI] [Google Scholar]
Qolizadeh 2019 {published data only}
- Qolizadeh A, Myaneh ZT, Rashvand F. Investigating the effect of listening to the Holy Quran on the physiological responses of neonates admitted to neonatal intensive care units: a pilot study. Advances in Integrative Medicine 2019;6(4):159-62. [DOI: 10.1016/j.aimed.2018.08.004] [DOI] [Google Scholar]
Ramezani 2020 {published data only}
- Ramezani Z, Pasha YZ, Jafarian Amiri SR, Hajiahmadi M, Pirnia B. Comparison of the effectiveness of warm compress and music on the pain caused by heel blood sampling in neonates: a single-blind, prospective, randomized controlled trial. Crescent Journal of Medical and Biological Sciences 2020;7(1):138-9. [PDF: www.cjmb.org/uploads/pdf/pdf_CJMB_359.pdf] [Google Scholar]
Ranger 2018 {published data only}
- Ranger A, Helmert E, Bott TS, Ostermann T, Als H, Bassler D, et al. Physiological and emotional effects of pentatonic live music played for preterm neonates and their mothers in the newborn intensive care unit: a randomized controlled trial. Complementary Therapies in Medicine 2018;41:240-6. [DOI: 10.1016/j.ctim.2018.07.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sarhangi 2021 {published data only}
- Sarhangi F, Azarmnejad E, Javadi M, Tadrisi, SD, Rejeh N, Vaismoradi M. The effect of the mother's heartbeat sound on physiological parameters and pain intensity after blood sampling in neonates in the intensive care unit: a randomized controlled clinical trial. Journal of Neonatal Nursing 2021;27(2):123-8. [DOI: 10.1016/j.jnn.2020.07.006] [DOI] [Google Scholar]
Schlez 2011 {published data only}
- Schlez A, Litmanovitz I, Bauer S, Dolfin T, Regev R, Arnon S. Combining kangaroo care and live harp music therapy in the neonatal intensive care unit setting. Israel Medical Association Journal 2011;13(6):354-8. [PMID: ] [PubMed] [Google Scholar]
Shabani 2016 {published data only}
- Shabani F, Nayeri ND, Karimi R, Zarei K, Chehrazi M. Effects of music therapy on pain responses induced by blood sampling in premature infants: a randomized cross-over trial. Iranian Journal of Nursing and Midwifery Research 2016;21(4):391-6. [DOI: 10.4103/1735-9066.185581] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2017 {published data only}
- Shah SR, Kadage S, Sinn J. Trial of music, sucrose, and combination therapy for pain relief during heel prick procedures in neonates. Journal of Pediatrics 2017;190:153-8. [DOI: 10.1016/j.jpeds.2017.08.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shukla 2018 {published data only}
- Shukla VV, Bansal S, Nimbalkar A, Chapla A, Phatak A, Patel D, et al. Pain control interventions in preterm neonates: a randomized controlled trial. Indian Pediatrics 2018;55(4):292-6. [DOI: 10.1007/s13312-018-1270-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
Standley 1995 {published data only}
- Standley JM, Moore RS. Therapeutic effects of music and mother's voice on premature infants. Pediatric Nursing 1995;21(6):509-12, 574. [PMID: ] [PubMed] [Google Scholar]
Standley 2000 {published data only}
- Standley JM. The effect of contingent music to increase non-nutritive sucking of premature infants. Pediatric Nursing 2000;26(5):493-5, 498-9. [PMID: ] [PubMed] [Google Scholar]
Standley 2003 {published data only}
- Standley JM. The effect of music-reinforced nonnutritive sucking on feeding rate of premature infants. Journal of Pediatric Nursing 2003;18(3):169-73. [DOI: 10.1053/jpdn.2003.34] [PMID: ] [DOI] [PubMed] [Google Scholar]
Standley 2010 {published data only}
- Standley JM, Cassidy J, Grant R, Cevasco A, Szuch C, Nguyen J, et al. The effect of music reinforcement for non-nutritive sucking on nipple feeding of premature infants. Pediatric Nursing 2010;36(3):138-45. [PMID: ] [PubMed] [Google Scholar]
Stokes 2018 {published data only}
- Stokes A, Agthe AG, El Metwally D. Music exposure and maturation of late preterm sleep-wake cycles: a randomised crossover trial. Acta Paediatrica 2018;107(4):582-6. [DOI: 10.1111/apa.14079] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tang 2018 {published data only}
- Tang L, Wang H, Liu Q, Wang F, Wang M, Sun J, et al. Effect of music intervention on pain responses in premature infants undergoing placement procedures of peripherally inserted central venous catheter: a randomized controlled trial. European Journal of Integrative Medicine 2018;19:105-9. [DOI: 10.1016/j.eujim.2018.03.006] [DOI] [Google Scholar]
Tekgunduz 2019 {published data only}
- Tekgunduz KS, Polat S, Gurol A, Apay SE. Oral glucose and listening to lullaby to decrease pain in preterm infants supported with NCPAP: a randomized controlled trial. Pain Management Nursing 2019;20(1):54-61. [DOI: 10.1016/j.pmn.2018.04.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Uematsu 2019 {published data only}
- Uematsu H, Sobue I. Effect of music (Brahms lullaby) and non-nutritive sucking on heel lance in preterm infants: a randomized controlled crossover trial. Journal of Paediatrics and Child Health 2019;24(1):e33-9. [DOI: 10.1093/pch/pxy072] [PMID: ] [DOI] [PMC free article] [PubMed]
Ullsten 2017 {published data only}
- Ullsten A, Hugoson P, Forsberg M, Forzelius L, Klassbo M, Olsson E, et al. Efficacy of live lullaby singing during procedural pain in preterm and term neonates. Music and Medicine 2017;9(2):73-85. [DOI: 10.11124/jbisrir-2012-428] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vahdati 2017 {published data only}
- Vahdati M, Mohammadizadeh M, Talakoub S. Effect of kangaroo care combined with music on the mother-premature neonate attachment: a randomized controlled trial. Iranian Journal of Nursing and Midwifery Research 2017;22(5):403-7. [DOI: 10.4103/ijnmr.IJNMR_50_16] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walworth 2012 {published data only}
- Walworth D, Standley JM, Robertson A, Smith A, Swedberg O, Peyton JJ. Effects of neurodevelopmental stimulation on premature infants in neonatal intensive care: randomized controlled trial. Journal of Neonatal Nursing 2012;18(6):210-6. [DOI: 10.1016/j.jnn.2012.01.001] [DOI] [Google Scholar]
White‐Traut 2004 {published data only}
- White-Traut RC, Nelson MN, Silvestri JM, Patel M, Berbaum M, Gu GG, et al. Developmental patterns of physiological response to a multisensory intervention in extremely premature and high-risk infants. Journal of Obstetric, Gynecologic & Neonatal Nursing 2004;33(2):266-75. [DOI: 10.1177/0884217504263289] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2020 {published data only}
- Wu HP, Yang L, Lan HY, Peng HF, Chang YC, Jeng MJ, et al. Effects of combined use of mother's breast milk, heartbeat sounds, and non-nutritive sucking on preterm infants' behavioral stress during venipuncture: a randomized controlled trial. Journal of Nursing Scholarship 2020;52(5):467-75. [DOI: 10.1111/jnu.12571] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yildiz 2011 {published data only}
- Yildiz A, Arikan D. The effects of giving pacifiers to premature infants and making them listen to lullabies on their transition period for total oral feeding and sucking success. Journal of Clinical Nursing 2012;21(5-6):644-56. [DOI: 10.1111/j.1365-2702.2010.03634.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yilmaz 2021 {published data only}
- Yilmaz G, Kucuk Alemdar D. The effect of supportive nursing interventions on reducing stress levels of mothers of infants in the neonatal intensive care unit: a randomized controlled trial. Clinical Nursing Research 2021;31(5):941-51. [DOI: 10.1177/10547738211047359] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zeraati 2018 {published data only}
- Zeraati H, Nasimi F, Rezaeian A, Shahinfar J, Ghorban Zade M. Effect of multi-sensory stimulation on neuromuscular development of premature infants: a randomized clinical trial. Iranian Journal of Child Neurology 2018;12(3):32-9. [DOI: 10.22037/ijcn.v12i3.16134] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Aita 2021 {published data only}
- Aita M, Heon M, Lavallee A, De Clifford Faugere G, Altit G, Le May S, et al. Nurturing and quiet intervention (NeuroN-QI) on preterm infants' neurodevelopment and maternal stress and anxiety: a pilot randomized clinical trial protocol. Journal of Advanced Nursing 2021;77(7):3192-203. [DOI: 10.1111/jan.14819] [TRIAL REGISTRATION: NCT04593095] [DOI] [PubMed] [Google Scholar]
Bonjorn Juarez 2020 {published data only}
- Bonjorn Juarez M, Manrique Pons M, Grau Alcon L, Martinez-Momblan MA, Alonso-Fernandez S. Reduction of visual and auditory stimuli to reduce pain during venipuncture in premature infants. Study protocol for a randomized controlled trial. Journal of Advanced Nursing 2020;76(4):1077-81. [DOI: 10.1111/jan.14300] [TRIAL REGISTRATION: NCT04041635] [DOI] [PubMed] [Google Scholar]
Brignoni‐Perez 2021 {published data only}
- Brignoni-Perez E, Morales MC, Marchman VA, Scala M, Feldman HM, Yeom K, et al. Listening to Mom in the NICU: effects of increased maternal speech exposure on language outcomes and white matter development in infants born very preterm. Trials 2021;22(1):444. [DOI: 10.1186/s13063-021-05385-4] [TRIAL REGISTRATION: NCT04193579] [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR1900026017 {published data only}
- ChiCTR1900026017. A randomized controlled trial for investigation of the associations between a music program and neonatal health outcomes. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900026017 (first received 18 September 2019). [CENTRAL: CN-02065137]
CTRI/2012/11/003117 {published data only}
- CTRI/2012/11/003117. A clinical trial to study the effectiveness of instrumental Indian classical music on serum cortisol concentrations of preterm infants on assisted ventilation admitted to a tertiary level neonatal intensive care unit (NICU) [Effectiveness of Instrumental Indian Classical music on serum cortisol concentrations of preterm infants on assisted ventilation admitted to a tertiary level neonatal intensive care unit (NICU)‐ a prospective, randomized controlled trial]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2012/11/003117 (first received 16 November 2012). [CENTRAL: CN-01854686]
CTRI/2016/06/007028 {published data only}
- CTRI/2016/06/007028. Comparison of music therapy with kangaroo mother care for pain reduction in premature babies [Factorial design randomised control trial to compare music therapy with kangaroo mother care for pain in premature neonates]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2016/06/007028 (first received 22 June 1016). [CENTRAL: CN-01818320]
CTRI/2017/04/008395 {published data only}
- CTRI/2017/04/008395. Effect of music therapy on breast milk production in mothers practicing kangaroo mother care [Effect of music therapy on breast milk production in KMC mothers]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/04/008395 (first received 21 April 2017). [CENTRAL: CN-01894530]
CTRI/2021/06/033970 {published data only}
- CTRI/2021/06/033970. A study to check whether music combined with standard kangaroo mother care help in improving neurodevelopmental outcome of preterm infants [Does music combined with kangaroo mother care improve the neurodevelopmental outcome of preterm infants compared to standard kangaroo mother care?‐ A randomised controlled trial]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/06/033970 (first received 03 June 2021). [CENTRAL: CN-02327617]
CTRI/2021/07/035287 {published data only}
- CTRI/2021/07/035287. Vestibular stimulation for babies born before 34 weeks of gestational age [Effects of vestibular stimulation on neurodevelopment in preterm infants]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/07/035287 (first received 30 July 2021). [CENTRAL: CN-02328316]
D'Souza 2017 {published data only}
- D'Souza SR, Lewis LE, Kumar V, Ramesh BY, Purkayastha J, Kamath S. Effectiveness of Indian classical music on developmental responses of preterm infants - a randomized controlled trial protocol. Medico Legal Update 2017;17(2):243-7. [PDF: https://medicolegalupdate.org/scripts/MLU%20July-Decr%202017.pdf] [Google Scholar]
DRKS00018806 {published data only}
- DRKS00018806. Music and maternal voice: investigating the impact on premature babies outcomes [Music and maternal voice: investigating the impact on premature babies outcomes ‐ MAMBO study]. trialsearch.who.int/Trial2.aspx?TrialID=DRKS00018806 (first received 22 October 2019). [CENTRAL: CN-02067360]
DRKS00025753 {published data only}
- DRKS00025753. Does music therapy influence the neurological development of premature infants born less than 32 weeks of gestation? trialsearch.who.int/Trial2.aspx?TrialID=DRKS00025753 (first received 05 July 2021). [CENTRAL: CN-02328823]
Filippa 2021a {published data only}
- Filippa M, Della Casa E, D'Amico R, Picciolini O, Lunardi C, Sansavini A, et al. Effects of early vocal contact in the neonatal intensive care unit: study protocol for a multi-centre, randomised clinical trial. International Journal of Environmental Research and Public Health 2021;18(8):08. [DOI: 10.3390/ijerph18083915] [TRIAL REGISTRATION: NCT04759573] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghetti 2019 {published data only}
- Ghetti C, Bieleninik L, Hysing M, Kvestad I, Assmus J, Romeo R, et al. Longitudinal study of music therapy's effectiveness for premature infants and their caregivers (LongSTEP): protocol for an international randomised trial CNO. BMJ Open 2019;9(8):e025062. [DOI: 10.1136/bmjopen-2018-025062] [TRIAL REGISTRATION: NCT03564184] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2014101819566N2 {published data only}
- IRCT2014101819566N2. Comparison of the effect of Koran (yasin) and lullaby music listening on physiological indicators of premature neonates admitted in Motahari hospital of Jahrom. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2014101819566N2 (first received 8 November 2014). [CENTRAL: CN-01837693]
IRCT20170620034653N3 {published data only}
- IRCT20170620034653N3. The effect of the Quran's voice on the formation of oral nutrition in premature infants admitted to the intensive care unit [The effect Voice of the Holy Quran on the duration of transition from gavage to first oral feeding and vital signe at premature newborns hospitalized in neonatal intensive care unit]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20170620034653N3 (first received 19 June 2018). [CENTRAL: CN-01908773]
IRCT20180526039844N1 {published data only}
- IRCT20180526039844N1. Effect of voice and tune in pain of neonates [Comparison of the effectiveness of relaxing voice and tune voices using voice pillow on pain intensity of heel stick in hospitalized neonates]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20180526039844N1 (first received 02 July 2018). [CENTRAL: CN-01905961]
NCT02471482 {published data only}
- NCT02471482. Effect of Mozart music on cerebral oxygenation and behavioural response of premature infants [Effect of Mozart music on cerebral oxygenation and behavioural response of premature infants between 28‐32 weeks of corrected gestation]. clinicaltrials.gov/ct2/show/NCT02471482 (first received 07 November 2014). [CENTRAL: CN-01552873]
NCT02948491 {published data only}
- NCT02948491. Effectiveness of music therapy on the development of preterm neonates (EMT-DRNP) [Effectiveness of music therapy on the intra‐hospital evolution and neurodevelopment of preterm neonates treated in neonatal intensive care units in the Bucaramanga metropolitan area]. clinicaltrials.gov/ct2/show/NCT02948491 (first received 28 October 2016). [CENTRAL: CN-01588910]
NCT03688386 {published data only}
- NCT03688386. A language intervention study of preterm infants [Bridging connections: a language intervention study of preterm infants in the NICU]. clinicaltrials.gov/ct2/show/NCT03688386 (first received 18 June 2018). [CENTRAL: CN-01663692]
NCT03795454 {published data only}
- NCT03795454. Can singing kangaroo improve outcome of preterm Infants [Singing kangaroos ‐ infant care With combined auditory intervention and kangaroo care]. clinicaltrialsgov/show/nct03795454 (first received 30 November 2019). [CENTRAL: CN-01795746]
NCT03830580 {published data only}
- NCT03830580. Benefit of singing in the care of premature children undergoing screening for retinopathy of prematurity in the neonatology and neonatal resuscitation unit of the Dijon university hospital. clinicaltrialsgov/show/nct03830580 (first received 25 January 2019). [CENTRAL: CN-01796265]
NCT04314440 {published data only}
- NCT04314440. Cognitive processing in preterm infants and NICU music therapy. clinicaltrials.gov/show/NCT04314440 (first received 7 March 2016). [CENTRAL: CN-02089202]
NCT04335240 {published data only}
- NCT04335240. Premature family music therapy intervention (PFMI) [Premature family music therapy intervention (PFMI): an Italian protocol to support parenting, attachment bond and preterm development]. clinicaltrials.gov/show/NCT04335240 (first received 01 April 2020). [CENTRAL: CN-02091393]
NCT04559620 {published data only}
- NCT04559620. Mother's recorded voice for preterm infants. https://clinicaltrials.gov/show/NCT04559620 (first received 30 July 2020).
NCT04565210 {published data only}
- NCT04565210. Effects of oriental music on preterm infants [Effects of oriental music on preterm Infants: a randomized controlled trial. (OMNIA Trial)]. clinicaltrials.gov/show/NCT04565210 (first received 07 September 2020). [CENTRAL: CN-02181470]
NCT04759170 {published data only}
- NCT04759170. The effects of recorded receptive music therapy on oral nutrition and the well-being of the italian premature baby [The effects of recorded receptive music therapy on oral nutrition and the well‐being of the Italian premature baby: prospective randomized controlled study mom, dad and music therapist singing]. clinicaltrials.gov/show/NCT04759170 (first received 28 January 2021). [CENTRAL: CN-02249068]
NCT04759573 {published data only}
- NCT04759573. Effects of early vocal contact (EVC) in the neonatal intensive care unit [Effects of early vocal contact (EVC) in the neonatal intensive care unit: a multi‐centre, randomized clinical trial]. clinicaltrials.gov/show/NCT04759573 (first received 06 February 2021). [CENTRAL: CN-02236469]
NCT04886648 {published data only}
- NCT04886648. The effect of mother's voice and lullaby on preterm infants' physiological parameters, stress and sleeping-waking state. clinicaltrials.gov/show/NCT04886648 (first received 05 April 2021). [CENTRAL: CN-02257455]
Neel 2019 {published data only}
- Neel ML, Yoder P, Matusz PJ, Murray MM, Miller A, Burkhardt S, et al. Randomized controlled trial protocol to improve multisensory neural processing, language and motor outcomes in preterm infants. BMC Pediatrics 2019;19(1):81. [CENTRAL: NCT03232931] [DOI: 10.1186/s12887-019-1455-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavel 2019 {published data only}
- Pavel AM, Dhais FA, Howard C, O'Toole JM, Pavlidis E, Finn D, et al. The effect of music therapy on the electroencephalogram (EEG) and heart rate variability (HRV) of premature infants during routine painful procedures. Archives of Disease in Childhood 2019;104:A135. [DOI: 10.1136/archdischild-2019-epa.311] [DOI] [Google Scholar]
Purdy 2011 {published data only}
- Purdy IB, Anderson MS, Brown W, Kehoe P, Melwak MA, Garg M. A randomized controlled trial of NICU music: impact on preterm infant rest and growth. Communicating Nursing Research 2011;44:445. [Google Scholar]
Tosun 2014 {published data only}
- Tosun O, Erdem E, Elmali F, Kurtoglu S. The effect of aromatherapy, music therapy and vibration applications on neonatal stress and behaviours. Archives of Disease in Childhood 2014;99:A82-3. [DOI: 10.1136/archdischild-2014-307384.220] [DOI] [Google Scholar]
Additional references
Abbott 2002
- Abbott A. Music, maestro, please! Nature 2002;416(6876):12-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aita 2021
- Aita M, De Clifford FG, Lavallée A, Feeley N, Stremler R, Rioux É, et al. Effectiveness of interventions on early neurodevelopment of preterm infants: a systematic review and meta-analysis. BMC Pediatrics 2021;21(1):210. [DOI: 10.1186/s12887-021-02559-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Als 1986
- Als H, Lawhon G Brown E, Gibes R, Duffy FH, McAnulty G, et al. Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: neonatal intensive care unit and developmental outcome. Pediatrics 1985;78(6):1123-32. [PMID: ] [PubMed] [Google Scholar]
Als 2005
- Als H, Butler S, Kosta S, McAnulty G. The assessment of preterm infants' behavior (APIB): furthering the understanding and measurement of neurodevelopmental competence in preterm and full-term infants. JAMA 2005;11(1):94-102. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2018
- Anderson DE, Patel AD. Infants born preterm, stress, and neurodevelopment in the neonatal intensive care unit: might music have an impact? Developmental Medicine & Child Neurology 2018;60(3):256-66. [DOI: 10.1111/dmcn.13663] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bieleninik 2016
- Bieleninik L, Ghetti C, Gold C. Music therapy for preterm infants and their parents: a meta-analysis. Pediatrics 2016;138(3):e20160971. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bradt 2015
- Bradt J, Potvin N, Kesslick A, Shim M, Radl D, Schriver E, et al. The impact of music therapy versus music medicine on psychological outcomes and pain in cancer patients: a mixed methods study. Support Care Cancer 2015;23(5):1261-71. [DOI: 10.1007/s00520-014-2478-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Castrillón 2005
- Castrillón Moreno, DA, Borrero Copete, PE. Validación del inventario de ansiedad estado-rasgo (STAIC) en niñosescolarizados entre los 8 y 15 años. Acta Colombiana de Psicología 2005;13:79-90. [HTTPS://: actacolombianapsicologia.ucatolica.edu.co/article/view/433] [Google Scholar]
Chawanpaiboon 2019
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health 2019;7(1):e37-e46. [DOI: 10.1016/S2214-109X(18)30451-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chorna 2019
- Chorna O, Filippa M, De Almeida JS, Lordier L, Monaci MG, Hüppi P, et al. Review article neuroprocessing mechanisms of music during fetal and neonatal development: a role in neuroplasticity and neurodevelopment. Neural Plasticity 2019;2019(3972918):0.1155/2019/3972918. [DOI: 10.1155/2019/3972918] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark‐Gambelunghe 2015
- Clark-Gambelunghe MB, Clark DA. Sensory development. Pediatric Clinics of North America 2015;62(2):367-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed before November 2021. Melbourne, Australia: Veritas Health Innovation, assessed before November 2021. Available at covidence.org.
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. training.cochrane.org/handbook.
Dileo 1999
- Dileo C. A classification model for music and medicine. In: Applications of Music in Medicine. National Association of Music Therapy, 1999:1-6. [ISBN: 1884914004] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Filippa 2017
- Filippa M, Kuhn P, Westrup B. Early Vocal Contact and Preterm Infant Brain Development. Bridging the Gaps Between Research and Practice. Cham, Switzerland: Springer International Publishing, 2017. [DOI: 10.1007/978-3-319-65077-7] [DOI] [Google Scholar]
Flacking 2007
- Flacking R, Ewald U, Starrin B. "I wanted to do a good job": experiences of 'becoming a mother' and breastfeeding in mothers of very preterm infants after discharge from a neonatal unit. Social Science & Medicine 2007;64:2405-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Forcada‐Guex 2006
- Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics 2006;118(1):107-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gaden 2022
- Gaden TS, Ghetti C, Kvestad I, Bieleninik Ł, Stordal AS, Assmus J, et al. Short-term music therapy for families with preterm infants: a randomized trial. Pediatrics 2022;149(2):e2021052797. [DOI: 10.1542/peds.2021-052797] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghetti 2019
- Ghetti C, Bieleninik Ł, Hysing M, Kvestad I, Assmus J, Romeo R, et al. Longitudinal study of music therapy's effectiveness for premature infants and their caregivers (LongSTEP): protocol for an international randomised trial. BMJ Open 2019;9(8):e025062. [DOI: 10.1136/bmjopen-2018-025062] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- GRADEproGDT. Version accessed 9 June 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hanson‐Abromeit 2008
- Hanson-Abromeit D, Shoemark H, Loewy J. Music therapy with pediatric units: newborn intensive care unit (NICU). In: Hanson-Abromeit D, Colwell C, editors(s). Medical Music Therapy for Pediatrics in Hospital Settings. Using Music to Support Medical Interventions. Silver Spring (MD): American Music Therapy Association, 2008:15-69. [Google Scholar]
Hartling 2009
- Hartling L, Shaik MS, Tjosvold L, Leicht R, Liang Y, Kumar M. Music for medical indications in the neonatal period: a systematic review of randomised controlled trials. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94:F349-54. [DOI: 10.1136/adc.2008.148411] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haslbeck 2012
- Haslbeck FB. Music therapy for premature infants and their parents: an integrative review. Nordic Journal of Music Therapy 2012;21(3):203-26. [DOI: 10.1080/08098131.2011.648653] [PMID: 27763631]27763631 [DOI] [Google Scholar]
Haslbeck 2018
- Haslbeck FB, Stegemann T. The effect of music therapy on infants born preterm. Developmental Medicine & Child Neurology 2018;60(3):217. [DOI: 10.1111/dmcn.13677] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haslbeck 2020
Haslbeck 2020a
- Haslbeck FB, Jakab A, Held U, Bassler D, Bucher HU, Hagmann C. Creative music therapy to promote brain function and brain structure in preterm infants: a randomized controlled pilot study. NeuroImage: Clinical 2020;25(January):102171. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heim 1999
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biological Psychiatry 1999;46(11):1509-22. [DOI: 10.1016/S0006-3223(99)00224-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hepper 1994
- Hepper PG, Shahidullah BS. Development of fetal hearing. Archives of Disease in Childhood 1994;71(2):81-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. (available at www:training.cochrane.org/handbook).
Hodges 2010
- Hodges AL, Wilson LL. Effects of music therapy on preterm infants in the neonatal intensive care unit. Journal of Alternative Therapies in Health and Medicine 2010;16(5):72-3. [PMID: ] [PubMed] [Google Scholar]
Hoffenkamp 2015
- Hoffenkamp H, Tooten A, Hall R, Braeken J, Eliens M, Vingerhoets AD, et al. Effectiveness of hospital-based video interaction guidance on parental interactive behavior, bonding, and stress after preterm birth: a randomized controlled trial. Journal of Consulting and Clinical Psycholgy 2015;83(2):416-29. [DOI: 10.1037/a0038401] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hopewell 2008
- Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al, CONSORT Group. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet 2008;371(9609):281-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huotilainen 2010
- Huotilainen M, Näätänen R. Auditory perception and early brain development. www.child-encyclopedia.com/brain/according-experts/auditory-perception-and-early-brain-development (accessed prior to 27 June 2019).
Inder 2005
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005;115(2):286-94. [DOI: 10.1542/peds.2004-0326] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jobe 2014
- Jobe AH. Sensory deprivation in private rooms in the NICU. Journal of Pediatrics 2014;164(1):1-3. [DOI: 10.1016/j.jpeds.2013.10.049] [DOI] [Google Scholar]
Johnson 2008
- Johnson S, Wolke D, Marlow N. Developmental assessment of preterm infants at 2 years: validity of parent reports. Developmental Medicine and Child Neurology 2008;50(1):58-62. [DOI: 10.1111/j.1469-8749.2007.02010.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jotzo 2005
- Jotzo M, Poets CF. Helping parents cope with the trauma of premature birth: an evaluation of a trauma-preventive psychological intervention. Pediatrics 2005;115(4):915-9. [DOI: 10.1542/peds.2004-0370] [PMID: ] [DOI] [PubMed] [Google Scholar]
Koelsch 2014
- Koelsch S. Brain correlates of music-evoked emotions. Nature Reviews. Neuroscience 2014;15(3):170-80. [DOI: 10.1038/nrn3666] [PMID: ] [DOI] [PubMed] [Google Scholar]
Korja 2011
- Korja R, Latva R, Lehtonen L. The effects of preterm birth on mother–infant interaction and attachment during the infant’s first two years. Acta Obstetricia et Gynecologica Scandinavica 2011;91:164-73. [DOI: 10.1111/j.1600-0412.2011.01304.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krueger 2010
- Krueger C. Exposure to maternal voice in preterm infants: a review. Journal of Advances in Neonatal Care 2010;10(1):13-8. [DOI: 10.1097/ANC.0b013e3181cc3c69] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuhn 2013
- Kuhn P, Zores C, Langlet C, Escande B, Astruc D, Dufour A. Moderate acoustic changes can disrupt the sleep of very preterm infants in their incubators. Acta Paediatrica 2013;102(10):949-54. [DOI: 10.1111/apa.12330] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lahav 2014
- Lahav A, Skoe E. An acoustic gap between the NICU and womb: a potential risk for compromised neuroplasticity of the auditory system in preterm infants. Frontiers in Neuroscience 2014;8(DEC):1-8. [DOI: 10.3389/fnins.2014.00381] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakshmanan 2021
- Lakshmanan A, Song AY, Belfort MB, Yieh L, Dukhovny D, Friedlich PS, et al. The financial burden experienced by families of preterm infants after NICU discharge. Journal of Perinatology 2022;42(2):223–30. [DOI: 10.1038/s41372-021-01213-4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancet Child Adolescent Health 2019
Loewy 2013
- Loewy J, Stewart K, Dassler AM, Telsey A, Homel P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics 2013;31(5):902-18. [DOI: 10.1542/peds.2012-1367] [PMID: ] [DOI] [PubMed] [Google Scholar]
Loewy 2015
- Loewy, J. NICU music therapy: song of kin as critical lullaby in research and practice. Annals of the New York Academy of Sciences 2015;1337:178-85. [DOI: 10.1111/nyas.12648] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lordier 2019
- Lordier L, Meskaldji DE, Grouiller F, Pittet MP, Vollenweider A, Vasung L, et al. Music in premature infants enhances high-level cognitive brain networks. Proceedings of the National Academy of Sciences 2019;116(24):12103-8. [DOI: 10.1073/pnas.1817536116] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malouf 2022
- Malouf R, Harrison S, Burton HL, Gale C, Stein A, Franck LS, et al. Prevalence of anxiety and post-traumatic stress (PTS) among the parents of babies admitted to neonatal units: A systematic review and meta-analysis. EClinicalMedicine 2022;43:101233. [DOI: 10.1016/j.eclinm.2021.101233] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McMahon 2012
- McMahon E, Wintermark P, Lahav A. Auditory brain development in premature infants: the importance of early experience. Annals of the New York Academy of Sciences 2012;1252:17-24. [DOI: 10.1111/j.1749-6632.2012.06445.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Melchers 2009
- Melchers P, Preuss U. Kaufman Assessment Battery for Children. German edition. Frankfrut am Main (Germany): Pearson Assessment, 2009. [Google Scholar]
Ment 2008
- Ment LR, Vohr BR. Preterm birth and the developing brain. Lancet Neurology 2008;7(5):378-9. [DOI: 10.1016/S1474-4422(08)70073-5.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohan 2021
- Mohan A, Gokulakrishnan G, El-Saie A, Brickley A, Hagan J, Pammi M. Music therapy for preterm neonates in the neonatal intensive care unit: an overview of systematic reviews. Acta Paediatrica 2021;110(12):3180-200. [DOI: 10.1111/apa.16055] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mondanaro 2016
- Mondanaro J, Ettenberger M, Park L. Mars rising: music therapy and the increasing presence of fathers in the NICU. Music and Medicine 2016;8(3):96-107. [DOI: 10.47513/mmd.v8i3.440] [DOI] [Google Scholar]
Monson 2018
- Monson BB, Eaton-Rosen Z, Kapur K, Liebenthal E, Brownell A, Smyser CD, et al. Differential rates of perinatal maturation of human primary and nonprimary auditory cortex. eNeuro 2018;5(1):1-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moon 2013
- Moon CM, Lagercrantz H, Kuhl PK. Language experienced in utero affects vowel perception after birth: a two-country study. Acta Paediatrica, International Journal of Paediatrics 2013;102(2):156-60. [DOI: 10.1111/apa.12098] [EMBASE: 000313593700023] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mushtaq 2019
- Mushtaq A, Kazi F. Family-centered care in the NICU. Lancet Child & Adolescent Health 2019;3(5):295-6. [DOI: 10.1016/S2352-4642(19)30089-6] [DOI] [Google Scholar]
Park & Demakis 2017
- Park SE, Demakis GJ. Wechsler preschool and primary scale of intelligence. In: Zeigler-Hill V, Shackelford T, editors(s). Encyclopedia of Personality and Individual Differences. Cham: Springer International Publishing, 2017:1-4. [Google Scholar]
Park 2014
- Park M, Kohlrausch A, Bruijn W, Jager P, Simons K. Analysis of the soundscape in an intensive care unit based on the annotation of an audio recording. Journal of the Acoustical Society of America 2014;135(4):1875-86. [DOI: 10.1121/1.4868367] [PMID: ] [DOI] [PubMed] [Google Scholar]
Partanen 2013
- Partanen E, Kujala T, Tervaniemi M, Huotilainen M. Prenatal music exposure induces long-term neural effects. PLOS One 2013;8(10):e78946. [DOI: 10.1371/journal.pone.0078946] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perani 2010
- Perani D, Saccuman MC, Scifo P, Spada D, Andreolli G, Rovelli R, et al. Functional specializations for music processing in the human newborn brain. Proceedings of the Natlonal Academy of Sciences of the USA 2010;107(10):4758-63. [DOI: 10.1073/pnas.0909074107] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Philbin 2017
- Philbin MK. The sound environments and auditory perceptions of the fetus preterm newborn. In: Filippa M, Kuhn P, Westrup B, editors(s). Early Vocal Contact and Preterm Infant Brain Development. Cham, Switzerland: Springer International Publishing, 2017:91-112. [DOI: 10.1007/978-3-319-65077-7_6] [DOI] [Google Scholar]
Pierrat 2017
- Pierrat V, Marchand-Martin L, Arnaud C, Kaminski M, Resche-Rigon M, Lebeaux C, et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks' gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 2017;358:1-13. [DOI: 10.1136/bmj.j3448] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pineda 2014
- Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. Journal of Pediatrics 2014;164(1):52-60.e2. [DOI: 10.1016/j.jpeds.2013.08.047] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. Copenhagen: The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rickard 2005
- Rickard NS, Toukhsati SR, Field SE. The effect of music on cognitive performance: insight from neurobiological and animal studies. Behavioral Cognitive Neuroscience Reviews 2005;4(4):235-61. [DOI: 10.1177/1534582305285869] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roque 2017
- Roque AF, Lasiuk GC, Radünz V, Hegadoren K. Scoping review of the mental health of parents of infants in the NICU. Journal of Obstetric Gynecologic and Neonatal Nursing 2017;46(4):576-87. [DOI: 10.1016/j.jogn.2017.02.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rossetti 2013
- Rossetti A, Canga B. Environmental music therapy: rationale for ‘multi-individual’ music psychotherapy in modulation of the pain experience. In: Mondanaro JF, Sara AG, editors(s). Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press: The Louis Armstrong Center for Music and Medicine, 2013:468. [Google Scholar]
Ruiz 2018
- Ruiz N, Piskernik B, Witting A, Fuiko R, Ahnert L. Parent-child attachment in children born preterm and at term: a multigroup analysis. PLoS ONE 2018;13(8):1-14. [DOI: 10.1371/journal.pone.0202972] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2012
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3(4):312-24. [DOI: 10.1002/jrsm.1058] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rücker 2014
- Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in Medicine 2014;33(25):4353-69. [DOI: 10.1002/sim.6236] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rücker 2016
- Rücker G, Schwarzer G. Automated drawing of network plots in network meta-analysis. Research Synthesis Methods 2016;7(1):94-107. [DOI: 10.1002/jrsm.1143] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sacks 2007
- Sacks O. Tales of Music and the Brain. New York (NY): Vintage Books, 2007. [Google Scholar]
Schulz 1994
- Schulz KF, Chalmers I, Grimes DA, Altman DG. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA 1994;272(2):125-8. [PMID: ] [PubMed] [Google Scholar]
Schulz 2000
- Schulz KF. Assessing allocation concealment and blinding in randomised controlled trials: why bother? Evidence-based Nursing 2001;4(1):4-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shoemark 2015
- Shoemark H, Hanson-Abromeit D, Stewart L. Constructing optimal experience for the hospitalized newborn through neuro-based music therapy. Frontiers in Human Neuroscience 2015;9(487):1-5. [DOI: 10.3389/fnhum.2015.00487] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press, 1983. [Google Scholar]
Standley 2012
- Standley J. Music therapy research in the NICU: an updated meta-analysis. Neonatal Network 2012;31(5):311-6. [DOI: 10.1891/0730-0832.31.5.311] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2015
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314(10):1039-51. [DOI: 10.1001/jama.2015.10244] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Symington 2006
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD001814. [DOI: 10.1002/14651858.CD001814.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramo 2011
- Tramo MJ, Lense M, Ness C, Kagan J, Settle MD, Cronin JH. Effects of music on physiological and behavioral indices of acute pain and stress in premature infants: clinical trial and literature review. Music and Medicine 2011;3(2):72-83. [DOI: 10.1177/1943862111400613] [EMBASE: 69665973] [DOI] [Google Scholar]
Twilhaar 2018
- Twilhaar ES, De Kieviet JF, Aarnoudse-Moens CS, Van Elburg RM, Oosterlaan J. Academic performance of children born preterm: a meta-analysis and meta-regression. Archives of Disease in Childhood: Fetal and Neonatal Edition 2018;103(4):322-30. [DOI: 10.1136/archdischild-2017-312916] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Heijden 2016
- Van der Heijden MJ, Oliai Araghi S, Jeekel J, Reiss IK, Hunink MG, Van Dijk M. Do hospitalized premature infants benefit from music interventions? A systematic review of randomized controlled trials. PLOS One 2016;11(9):e0161848. [DOI: 10.1371/journal.pone.0161848] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dokkum 2021
- Van Dokkum NH, Kooi EM, Berhane B, Ravensbergen A-G, Hakvoort L, Jaschke AC, et al. Neonatal music therapy and cerebral oxygenation in extremely and very preterm infants: a pilot study. Music and Medicine 2021;13(2):91-8. [DOI: 10.47513/mmd.v13i2.813] [DOI] [Google Scholar]
Wachman 2011
- Wachman EM, Lahav A. The effects of noise on preterm infants in the NICU. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(4):F305-9. [DOI: 10.1136/adc.2009.182014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Woodward 2006
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine 2006;355(7):685-94. [DOI: 10.1056/NEJMoa053792] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yan 2003
- Yan J. Canadian Association of Neuroscience Review: development and plasticity of the auditory cortex. Canadian Journal of Neurological Sciences 2003;30(3):189-200. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yue 2021
- Yue W, Han X, Luo J, Zeng Z, Yang M. Effect of music therapy on preterm infants in neonatal intensive care unit: systematic review and meta-analysis of randomized controlled trials. Journal of Advanced Nursing 2021;77(2):635-52. [DOI: 10.1111/jan.14630] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Haslbeck 2019
- Haslbeck FB, Karen T, Loewy J, Meerpohl JJ, Bassler D. Musical and vocal interventions to improve neurodevelopmental outcomes for preterm infants (Protocol). Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013472. [DOI: 10.1002/14651858.CD013472] [DOI] [PMC free article] [PubMed] [Google Scholar]


