Abstract
Background
Evidence suggests that sebum content is important in skin disorders such as acne. However, sebum levels change depending on the external environment, and quantifying skin sebum levels is challenging. Here, we propose an optimal method for quantifying the facial sebum level.
Materials and methods
Four hundred and sixty participants (160 males and 300 females) aged 20–40 were enrolled in this study. A Sebumeter SM 810 was used to measure the sebum level at five facial locations: the forehead, the chin, the left cheek, the right cheek, and the nose. The participants were divided into two groups; one group underwent a one‐time measurement (n = 390, male: female = 120: 270), and the other underwent three consecutive measurements (n = 70, male: female = 40: 30). The casual sebum level (CSL) was measured in all patients after a 30‐min acclimatization; subsequently, the sebum removal process was conducted, followed by a resting period of 1 h to determine the sebum excretion rate (SER). Spearman's correlation analysis and the Wilcoxon signed‐rank test were used to compare the sebum level consistency and differences between the groups.
Results
Although three consecutive measurements better reflected the sebum content, the one‐time measurement also represented the relative sebum level. One hour after sebum removal, the sebum level recovered to 70%–90%; thus, this method was applicable for use in SER quantification. Of the five testing points, the sebum content was highest in the nose and lowest in the cheeks (both left and right). In addition, the cheeks were the most stable sites in terms of testing points, testing times, and CSL/SER values. A one‐time measurement of the CSL could represent the SER 1 h after the sebum removal. In our cohort, the sebum level of males with oily skin was decreased at age 32–35, and that of males with non‐oily skin increased at 28–35. The opposite trend was observed in female participants.
Conclusion
Sebum measurement methods were assessed, including testing times, indices (interval of time) and sites in a conditioned external environment. A one‐time measurement of the CSL 1 h after sebum removal was sufficient to determine the sebum level and SER, and the cheeks are recommended as the testing site. Sex and skin type differences were observed in sebum level changes with age.
Keywords: casual sebum level (CSL), facial sebum features, sebum excretion rate (SER), sebum measurement
Abbreviations
- CSL
casual sebum level
- FDR
false discovery rate
- SC
stratum corneum
- SER
sebum excretion rate
- TEWL
transepidermal water loss
- UV
ultraviolet
1. INTRODUCTION
Human skin is the largest organ of the integumentary system, covering almost the entire body. The skin comprises the epidermis, dermis, and subcutaneous tissue. 1 , 2 , 3 Numerous studies have confirmed that skin, especially the epidermis, functions as a physical barrier to prevent the invasion of foreign pathogens and antigens and regulate the skin barrier. 4 , 5 The biophysical parameters that reflect the status of skin conditions include stratum corneum (SC) hydration, the skin surface pH, epidermal permeability, transepidermal water loss (TEWL), and sebum content. 6 , 7 , 8 , 9
Among these biophysical parameters, the skin sebum level was reported to be significantly associated with skin disorders such as acne, atopic dermatitis, psoriasis, and rosacea. 10 , 11 , 12 , 13 One study revealed that the skin sebum content is higher in dermatitis patients with a history of contact with sulfur mustard than in healthy people. 14 A comparative study demonstrated that increased sebum levels correlate with facial pore development in males. 15 Additionally, increased sebum secretion can induce acne, and changes in the sebum composition also highly correlate with acne. 16 The skin sebum is an important component of the skin barrier and plays a critical role in maintaining skin moisture, reducing TEWL, protecting the skin against the external environment (e.g., ultraviolet (UV) light), and sustaining skin barrier function. 17 , 18 , 19 , 20 Additionally, the sebum content influences the skin microbiome, which could adversely affect skin conditions and lead to inflammation and skin disorders. 21 Therefore, the sebum level is an important parameter widely used in clinical diagnostics and cosmetic applications. 22 , 23 , 24 However, the skin sebum level is susceptible to changes in response to the external environment and challenging to measure, various skin parameters including sebum production can rapidly change by even minor environmental variation. 25 For example, people who exercise or are exposed to sunlight outdoors might excrete more skin oils. Additionally, wiping sweat and cleaning the skin surface can dramatically decrease the lipids on the skin, the excretion of which varies even throughout a single day.
The sebum level is a necessary index for evaluating cosmetic or clinical outcomes in the cosmetics industry and clinical practice. Numerous techniques have recently been developed to measure skin sebum levels, 26 , 27 including visual grading, lipid‐sensitive tape, solvent extraction, 28 absorbent paper pads, and bentonite clay. 29 Among these methods, the Sebumeter developed by Courage and Khazaka GmbH is a photometric technique widely used due to the ease of operation, accuracy of results, and low‐cost. 30 In brief, oils on the skin surface are absorbed onto lipid‐absorbent films, causing the films to become increasingly transparent as more oils are absorbed; sebum levels are then quantified by measuring the changes in the film. However, the lipid absorbent film cannot absorb all the sebum on the skin surface within 30 s of skin contact performed during device application, and the testing sites that best represent the facial sebum level remain controversial. In addition, the sebum excretion rate (SER) measurement process is lengthy and complicated, leading to poor participant compliance and considerable difficulties in sebum‐level investigations, especially in large cohorts. Thus, a simplified and easy‐to‐use method for sebum level measurement could save time and resources when the sample size is large.
In this study, we aimed to optimize facial sebum measurement methods in terms of the measurement times, sites, and indices (interval of time) in a controlled external environment to explore the sebum level and excretion rate under certain conditions. We also assessed the skin sebum levels of different facial features to simplify the measurement procedures. Finally, we used the optimized measurement value and facial features to define the age‐ and sex‐related characteristics of sebum dynamics at the initial stage of aging in individuals in Shanghai.
2. MATERIALS AND METHODS
2.1. The participants
Four hundred and sixty healthy individuals from Shanghai were enrolled in this study at the Skin and Cosmetic Research Department, Shanghai Skin Disease Hospital. There were 300 female participants (20–35 years of age) and 160 male participants (20–40 years of age). The female participants were divided into three groups by age (20–25, 26–30, and 31–35 years; 100 individuals per group). The male participants were divided into four groups by age (20‐25, 26–30, 31–35, and 36–40 years; 40 individuals per group). The grouped participants were then further divided by skin type (oily vs. non‐oily; 50 female and 20 male participants per group) according to an 18‐item Chinese version of the Oily Skin Self‐Image Questionnaire (OSSIQ). 31 , 32 The participants’ details and demographic data are shown in Table S1.
2.2. Inclusion and exclusion criteria
All the participants met the following criteria: (1) healthy male subjects aged 20–40 years or healthy female subjects aged 20–35 years; (2) self‐reported oily or non‐oily skin type; and (3) had lived in Shanghai for at least 5 years. Those who met the following criteria were excluded: (1) rosacea, seborrheic dermatitis, or moderate or severe acne; (2) a systematic disease or another disease that affected the ability to think and make judgments; (3) retinoic acid or benzoyl peroxide use within 2 weeks; and (4) isotretinoin or other sebaceous ligand function–affecting drug use within 6 months.
2.3. Procedures
The study procedures comprised an initial measurement of the casual sebum level (CSL) and the subsequent measurement of the sebum excretion rate (SER) 1 h after sebum removal (Shown in Figure 1).
FIGURE 1.
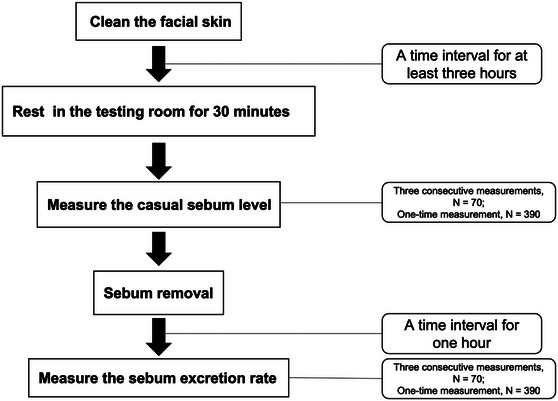
Flow chart of the measuring procedures.
First, the participants cleaned their faces by themselves using a certain facial cleanser at least 3 h before arriving at the testing room and did not apply any skin care products or cosmetics afterward. The measurements were conducted after a 30‐min acclimatization to the testing room (constant temperature of 20°C ± 2°C; humidity of 50% ± 5%) to ensure that any sweating had dissipated.
A Sebumeter810 (Courage & Khazaka Electronic GmbH, Cologne, Germany) was used to measure the participants’ CSL at five facial locations: the middle of the forehead, the left cheek, the right cheek, the nose, and the chin. Among the 460 participants, 70 (10 from each age and sex group) underwent three consecutive measurements at the same facial location, the relocation of the second and third measurements was precisely within the area of the first time by using a 3M transparent film to ensure that the tape was not exposed to a fresh part of the skin.
After the first measurement, the participants cleaned their faces to remove the total sebum and any remaining impurities using 30% ethanol on cotton pieces, gently wiping the sebum from the skin until the Sebumeter reading was below 10 a.u. The participants then rested in the testing room for 1 h before the second measurement was conducted. The 70 participants who underwent three measurements in the first round also underwent the same three measurements in the second round. The remaining 390 participants underwent a one‐time measurement in the first round, and the second measurement represented the cumulative sebum excreted during the 1 h after the sebum removal process. The value was divided by the time to yield the SER value in a.u./min.
2.4. Determination of the real CSL, real SER, one‐time CSL, and one‐time SER values
The sum of the three measurements in the first measurement was considered the “real CSL” value. The sum of the three measurements in the second‐time measurement was used to determine the sebum excretion rate (“real SER”). Correspondingly, the value of the one‐time first measurement was considered the “one‐time CSL” value, the second‐time measurement was used to calculate the sebum excretion rate (“one‐time SER” value).
2.5. Statistical analysis
An unpaired sample t‐test was used to assess differences in the measurement data from the different sex and age groups. Spearman's correlation analysis was used to analyze the correlation among the different testing time groups. Spearman's correlation analysis was used to analyze the correlation between the CSL and SER values. Paired‐matched Wilcoxon signed‐rank test analyses were performed to compare different groups in terms of the testing site. A false discovery rate (FDR) was used for multiple testing corrections. A p value < 0.05 was considered to represent significance. The statistical analysis results and the plots were all generated by R software and the R package ggplot2.
3. RESULTS
3.1. Evaluating the testing times and controlling the external confounding factors in measuring the sebum level
In clinical practice, dermatological clinicians typically take one‐time measurements to simplify the measuring procedures. Three measurements are recommended to more precisely measure the sebum levels; however, there is no evidence that three measurements represent the total value of the sebum level. Thus, we compared the three measurements to investigate the decreasing rate of the sebum value. The results showed sebum values decreased 40%−50% after each test at all locations apart from the nose (Figure S1a, S1b). The fourth measurement of the sebum value represented less than 10% of the sum of the four measurements according to the decrease rate, with a value typically below 20 a.u.; thus, we believe that it is reasonable to take three measurements within specific location to determine the relative sebum level of that site.
Another confounding factor in measuring the sebum level is the external environment the participants are exposed to. Therefore, acclimatization in a constant temperature and humidity room for 1 h is ideal for controlling the external environment. We tested the CSL after acclimatization and compared it to the CSL value of the first‐time measurement. The results indicated that in both the one‐time and three‐time measurements, the sebum level recovered 70%–90% 1 h after sebum removal (Figure S1c, S1d). Therefore, 1 h is reasonable for sebum recovery after the sebum removal. This approach is simple and is not time‐consuming and the corresponding CSL and SER values of the considered groups were shown in Table 1.
TABLE 1.
The CSL and SER values (measurement taken 1 h after sebum removal) of the groups.
| Gender (N) | Age group (N) Range | Skin type *(N) | CSL (a.u.) Mean ± SD | SER (a.u./min)Mean ± SD |
|---|---|---|---|---|
| Male (160) | 20–25 year (40) | Oily (20) | 133.13 ± 97.92 | 1.87 ± 1.64 |
| Non‐oily (20) | 75.94 ± 49.97 | 1.09 ± 1.22 | ||
| 26–30 year (40) | Oily (20) | 127.27 ± 61.92 | 1.59 ± 0.89 | |
| Non‐oily (20) | 60.73 ± 29.36 | 0.61 ± 0.43 | ||
| 31–35 year (40) | Oily (20) | 143.35 ± 57.64 | 1.67 ± 1.10 | |
| Non‐oily (20) | 93.74 ± 71.21 | 1.49 ± 1.41 | ||
| 36–40 year (40) | Oily (20) | 136.30 ± 85.71 | 1.69 ± 1.21 | |
| Non‐oily (20) | 80.60 ± 54.09 | 0.69 ± 0.63 | ||
| Female (300) | 20–25 year (100) | Oily (50) | 92.29 ± 54.66 | 1.05 ± 0.86 |
| Non‐oily (50) | 49.96 ± 47.67 | 0.68 ± 0.85 | ||
| 26–30 year (100) | Oily (50) | 96.74 ± 62.03 | 0.95 ± 0.88 | |
| Non‐oily (50) | 59.14 ± 51.22 | 0.63 ± 0.79 | ||
| 31–35 year (100) | Oily (50) | 83.14 ± 51.25 | 0.95 ± 0.89 | |
| Non‐oily (50) | 35.58 ± 38.77 | 0.33 ± 0.59 |
Note: *, skin type was defined by OSSIQ. SD, standard deviation. Non‐oily skin included dry skin and neutral skin.
3.2. The best testing area for sebum level quantification
To find the best testing area that represents the sebum level of the whole face, sebum samples were taken from five facial areas and the stability was compared using a paired‐matched Wilcoxon signed rank test (Tables 2 and 3). The nose significantly excreted higher sebum levels than the other tested locations, and the sebum levels of the forehead were higher than those of the remaining three locations. The left and right cheek showed no significant differences and had the lowest sebum levels (Figure 2A, B). The results suggest that the conventional U zone (the left and right cheeks) can accurately reflect the sebum level of the facial skin with stable, lower, and sustainable sebum content, while the sebum level in the nose varied dramatically; thus, the nose is not recommended as a testing site. At this stage, we hypothesized that the sebum levels of the cheeks (both left and right sides) may represent those of the face overall. However, further evaluation of the performance of the cheeks is required when we correlate the value of one‐time and three‐time measurements and the value of CSL and SER.
TABLE 2.
Pairwise Wilcoxon signed‐rank test of the five testing points using the value of one‐time CSL.
| Median1 | Median2 | Mean1 | Mean2 | p‐value | Significance | |
|---|---|---|---|---|---|---|
| Chin‐Forehead | 83 | 95 | 100.67 ± 69.62 | 109.47 ± 65.88 | 8.50E−06 | **** |
| Chin‐Leftcheek | 83 | 70 | 100.67 ± 69.62 | 85.21 ± 66.60 | 7.80E−13 | **** |
| Chin‐Rightcheek | 83 | 70 | 100.67 ± 69.62 | 86.27 ± 65.34 | 1.60E−11 | **** |
| Chin‐Nose | 83 | 203 | 100.67 ± 69.62 | 190.08 ± 87.05 | < 2.00E−16 | **** |
| Forehead‐Leftcheek | 95 | 70 | 109.47 ± 65.88 | 85.21 ± 66.60 | < 2.00E−16 | **** |
| Forehead‐Rightcheek | 95 | 70 | 109.47 ± 65.88 | 86.27 ± 65.34 | < 2.00E−16 | **** |
| Forehead‐Nose | 95 | 203 | 109.47 ± 65.88 | 190.08 ± 87.05 | < 2.00E−16 | **** |
| Leftcheek‐Rightcheek | 70 | 70 | 85.21 ± 66.60 | 86.27 ± 65.34 | 6.00E−02 | ns |
| Leftcheek‐Nose | 70 | 203 | 85.21 ± 66.60 | 190.08 ± 87.05 | < 2.00E−16 | **** |
| Rightcheek‐Nose | 70 | 203 | 86.27 ± 65.34 | 190.08 ± 87.05 | < 2.00E−16 | **** |
Note: ns, not significant. ****, p value < 0.0001. The p value was adjusted by the false discovery rate.
TABLE 3.
Pairwise Wilcoxon signed‐rank test of the five testing points using the value of one‐time SER.
| Median1 | Median2 | Mean1 | Mean2 | p‐value | Significance | |
|---|---|---|---|---|---|---|
| Chin‐Forehead | 0.98 | 1.05 | 1.19 ± 1.00 | 1.39 ± 1.15 | 1.50E−07 | **** |
| Chin‐Leftcheek | 0.98 | 0.68 | 1.19 ± 1.00 | 1.01 ± 1.04 | 1.20E−10 | **** |
| Chin‐Rightcheek | 0.98 | 0.68 | 1.19 ± 1.00 | 1.19 ± 1.00 | 4.00E−09 | **** |
| Chin‐Nose | 0.98 | 2.03 | 1.19 ± 1.00 | 2.29 ± 1.49 | < 2.00E−16 | **** |
| Forehead‐Leftcheek | 1.05 | 0.68 | 1.39 ± 1.15 | 1.01 ± 1.04 | < 2.00E−16 | **** |
| Forehead‐Rightcheek | 1.05 | 0.68 | 1.39 ± 1.15 | 1.19 ± 1.00 | < 2.00E−16 | **** |
| Forehead‐Nose | 1.05 | 2.03 | 1.39 ± 1.15 | 2.29 ± 1.49 | < 2.00E−16 | **** |
| Leftcheek‐Rightcheek | 0.68 | 0.68 | 1.01 ± 1.04 | 1.19 ± 1.00 | 2.00E−01 | ns |
| Leftcheek‐Nose | 0.68 | 2.03 | 1.01 ± 1.04 | 2.29 ± 1.49 | < 2.00E−16 | **** |
| Rightcheek‐Nose | 0.68 | 2.03 | 1.19 ± 1.00 | 2.29 ± 1.49 | < 2.00E−16 | **** |
Note: ns, not significant. ****, p value < 0.0001. The p value was adjusted by the false discovery rate.
FIGURE 2.
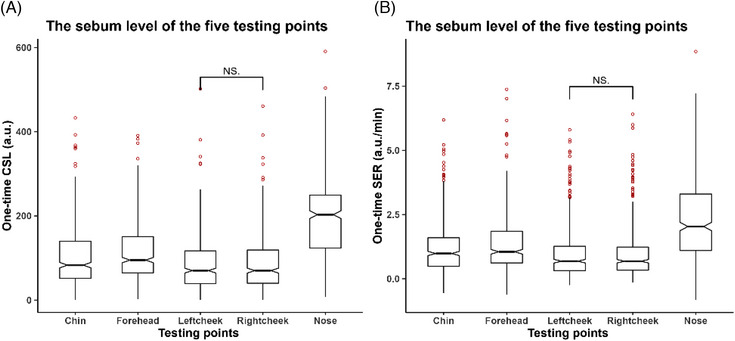
Evaluating the five testing points. (A) The casual sebum level of the five testing points (one‐time CSL). (B) The sebum excretion rate of the five testing points (one‐time SER).
3.3. One‐time and three‐time measurements equally represent the sebum level
In clinical practice, dermatological clinicians typically take one‐time measurements to simplify the procedure. We investigated whether a one‐time measurement can substitute three measurements in sebum level determination due to the ease of performing a single measurement and the resource‐saving nature of this approach. To evaluate the possibility of substituting these measurement procedures, a Spearman correlation analysis was performed using the one‐time value (one‐time CSL) and three‐time value (real CSL). The results showed that the one‐time CSL and real CSL values significantly correlated in the left cheek (Figure 3A, R2 = 0.989) and the one‐time SER and real SER (Figure 3B, R2 = 0.985) values also correlated and demonstrated consistent results within the other four testing sites (shown in Figure S2). Thus, a one‐time measurement can represent the sebum level as accurately as performing three measurements. In addition, when comparing the correlations among different testing points, it was found that the measurements taken on the cheeks exhibited a stronger correlation compared to the other testing points. As a result, using a single measurement on the cheek could be a more suitable alternative when considering the value of three‐time measurement.
FIGURE 3.
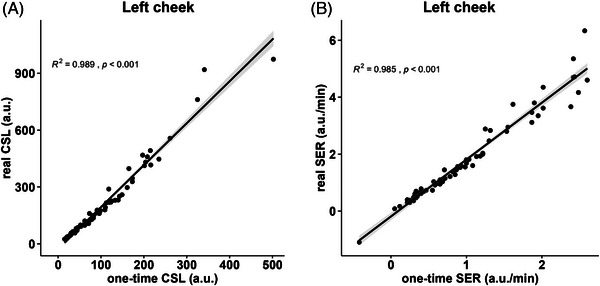
Scatter plots of the one‐time and three consecutive measurements, showing Spearman's correlation coefficient (R2) and the p value of the left cheek testing point. (A) One‐time CSL and real SCL (B) One‐time SER and real SER.
3.4. One‐time CSL represented the real SER after sebum removal
The real SER was clinically considered closer to the sebum excretion ability. We further performed a Spearman correlation analysis of the one‐time CSL before sebum removal and the real SER and one‐time CSL after sebum removal to investigate the representative reliability of the two one‐time CSL values in relation to the sebum excretion rate. The one‐time CSL after sebum removal (Figure 4B, R2 = 0.988) performed better than the one‐time CSL before sebum removal (Figure 4A, R2 = 0.803), and consistency was observed across the other four testing sites (shown in Figure S3). Thus, the one‐time CSL value after sebum removal can represent the true sebum level as accurately as the real SER, which was in line with the results previously reported. 27 , 33 Additionally, when comparing the correlations among different testing points, we observed that measurements taken on the cheeks exhibited a stronger correlation compared to other testing points. Therefore, when using a one‐time CSL measurement taken 1 h after sebum removal to reflect the overall sebum level of the face, the cheeks (both left and right sides) could be the most suitable testing points to consider.
FIGURE 4.
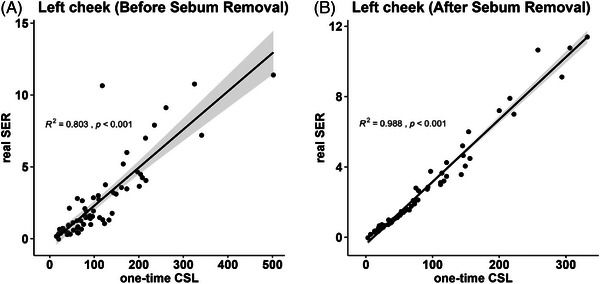
Scatter plots of the one‐time CSL and real SER, showing Spearman's correlation coefficient (R2) and the p value of the left cheek testing point. (A) Before sebum removal. (B) After sebum removal.
3.5. Sebum‐level dynamics in the initial stage of aging
Skin aging is an irreversible process that contributes to the loss of sebum excretion, and the characteristic sebum excretion rate in the initial stage of aging (aged 20–35) is an important factor for understanding, preventing and delaying sebaceous gland alterations during the skin aging process. To this end, a locally weighted regression (loess) analysis was performed to investigate the sebum level dynamics with age and sex using the one‐time CSL (after sebum removal) value from the left cheek. The results showed that the sebum level of men was higher than that of women, both in the oily skin and non‐oily skin groups. Among those with non‐oily skin, the sebum level of men increased with aging and was higher at age 28 than at younger ages, while this trend was reversed in women (Figure 5A). Regarding those with oily skin, in men, the sebum showed a decreasing trend at age 32, while that of women showed a slightly increasing trend (Figure 5B).
FIGURE 5.
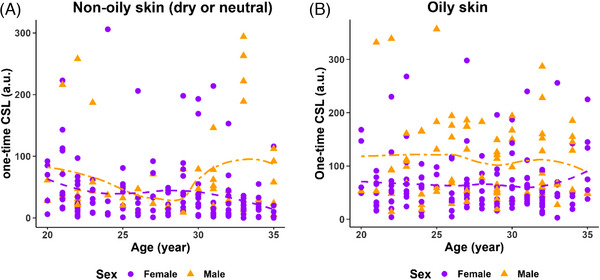
Sebum‐level dynamics in the initial stage of aging (in youth) using the left cheek value. (A) In subjectively non‐oily skin (dry skin or neutral skin). (B) In subjectively oily skin.
4. DISCUSSION
Lipids on human skin surfaces are critical components of the skin barrier. 34 , 35 Among many sebum level measurement methods, the Sebumeter has been widely used due to the advantages of simple operation, accuracy, and good reproducibility. However, in practice, the 30 s of skin contact applied with the Sebumeter tape is insufficient to fully absorb all of the sebum on the skin. In fact, the first application of the lipid absorption tape absorbs approximately 34%−60% of the total sebum. 30 Thus, lipid absorption tapes need to be applied multiple times to absorb all the sebum in a given skin area, and multiple test values at this location are added to obtain the sebum content value. The repeated use of lipid absorption tapes undoubtedly increases the difficulty of measurement, especially in large‐sample epidemiological surveys. Although the current clinical measurement methods usually use one‐time tests instead of multiple tests, the feasibility and accuracy of one‐time tests remain to be verified. Additionally, the selection of a suitable testing points to reflect the sebum level of the overall face in a simplified measurement procedure has not been well discussed thus far.
In this study, the facial sebum level was systematically investigated using Sebumeter by fully measuring the five testing points and thoroughly analyzing the correlation between the one‐time and three‐time values, and the CSL and SER. Taking the results together, we concluded that the one‐time value taken 1 h after sebum removal correlated highly with the real level of sebum excretion rate. Thus, a one‐time measurement is suitable for large biosample investigations in epidemiology studies.
In addition to the testing times, the testing areas representing the overall facial sebum level remain controversial. Facial sebum levels are the most intuitive and convenient to measure and are directly related to facial disorders. However, sebum levels vary greatly across facial locations, and multisite testing is needed to obtain the full picture of facial sebum production. Common sites for measurement include the middle of the forehead, the left and right cheeks, the nose and the chin. Although multisite measurement can more objectively reflect the facial sebum level, it complicates the measurement procedure.
Another problem is the measurement of the sebum excretion rate (SER). Different from the casual sebum level (CSL), SER reflects the amount of sebum excreted over time, measures individuals’ sebum‐producing ability, and is closely related to the physiological activity of sebaceous glands, which is important information to determine when investigating the pathogenesis of sebaceous gland diseases, such as seborrheic dermatitis and acne. 36 , 37 , 38 , 39 SER determination involves three steps: cleaning, wiping, and measuring. Cleaning removes sebum and other substances on the surface of the skin that could interfere with the measurements. After cleaning and wiping, sebum production is measured over a period of time (usually 1 h) to determine the SER. The three steps of SER measurement are complicated, time‐consuming, and are associated with poor volunteer compliance; thus, they are suitable only for measuring a small number of samples tested in the laboratory/clinic. To this end, we established a strictly controlled sebum removal process and evaluate an appropriate and shorter time period for sebum content recovery. In our 1‐h setting, clinicians in the testing room utilized cotton pieces soaked in 30% ethanol to conduct the sebum removal procedure. The sebum was gently wiped from the skin until the Sebumeter reading was below 10 arbitrary units (a.u.). This rigorous procedure ensured strict control over the remaining sebum content and was time‐saving compared to the 3‐h time interval for the uncontrolled removal of facial sebum. Furthermore, it is mentioned that in both the one‐time and three‐time measurements, the sebum level demonstrated a recovery of 70%–90% 1 h after sebum removal, which is consistent with a previous study showing that the sebum level demonstrated a recovery of 90% 1 h after face washing. 40 Based on this observation, it is suggested that a 1‐h time period after sebum removal is an ideal interval for sebum measurement.
In this study, we recruited 460 healthy participants from Shanghai and measured five testing sites to investigate the correlation between SER and CSL: the forehead, left cheek, right cheek, chin, and nose. All five testing locations demonstrated a relatively high correlation between CSL and SER, and CSL could represent the SER value to some extent. In clinical practice, we could measure the CSL as the initial test and select interested participants for SER measurement according to CSL values. Among the five testing locations, the correlation coefficient value between the CSL and SER value was lowest at the nose, while the cheeks outperformed other testing points. Furthermore, it was observed that the cheeks exhibited a higher correlation with the mean value of the five testing points compared to the other three testing points (Figure S4). This finding indicates that the measurements taken on the cheeks may be more closely associated with the overall value of the face. We speculated that this phenomenon resulted from more active sebum secretion at the nose and that the CSL value of the nose may exceed the normal measurement range of the Sebumeter (0−300 a.u.), contributing to the inaccuracy of the measurement of the nose sebum, thus causing the relatively lower correlation between CSL and SER, while the values of the other sites were more stable and reproducible.
Due to the difference in sebaceous gland density, physiological activity and contact with the external environment, different body parts greatly differ in sebum excretion abilities. 19 , 20 , 41 Facial sebum level measurement typically includes the forehead, left cheek, right cheek, nose, and chin. To simplify the procedure of sebum measurement in terms of the testing site, we measured the sebum levels of the five parts of the face and analyzed the differences between the CSL and SER values. There was no significant difference in the CSL and SER values between the left and right cheeks. This result suggested that when determining the CSL and SER values, measuring the left and right cheeks could provide stable values for facial sebum level quantification. The sebum level of the nose was significantly higher than that of the other tested sites and varied differently among individuals; thus, the nose did not represent the average sebum level of the face.
Numerous studies have focused on skin type classification, and measurement methods for the biophysical properties of the skin have been developed. 42 , 43 , 44 , 45 The methods for sebum level quantification meet the substantial demand for clinical applications; however, discrepancies remain. 46 Regarding the cosmetics industry, people need personalized skin care products to achieve better clinical outcomes. In our study, we investigated the sebum level dynamics of young people (aged 20–35) and found that the sebum level of youth varied differently by sex and skin type, although the results need further validation in larger cohorts. These results provide novel insights for guiding the development of cosmetic products in consideration of sebum levels. However, large‐scale validation is needed to strengthen our findings.
5. CONCLUSION
We optimized the methods and procedures used for facial sebum measurement from the following three aspects: testing times, indices (interval of time) and sites. We constructed a complete set of facial sebum measurement schemes through large‐sample validation. In large‐scale facial sebum measurement studies, a one‐time measurement of the forehead (or chin) and left cheek (or right cheek) CSL values can be used to characterize individual facial sebum levels. Specifically, when using a one‐time CSL measurement taken 1 h after sebum removal to reflect the overall sebum level of the face, the cheeks (both left and right sides) were the most suitable testing points to consider. The establishment of this method is of great significance for conducting large‐scale investigations into facial sebum phenotypes and furthering our understanding of the population characteristics regarding facial sebum. This one‐time measurement method is also simple to perform and is less time‐consuming for clinical applications.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
This study complied with the Declaration of Helsinki Principles and was approved by the institutional review board of Shanghai Skin Disease Hospital and Fudan University (FE222411), and written informed consent was obtained from all participants.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We thank all participants involved in this study and all authors and coworkers for their efforts in completing this paper. The study was supported by research grants from CAMS Innovation Fund for Medical Sciences (2019‐I2M‐5‐066), and Shanghai Municipal Science and Technology Major Project (2017SHZDZX01).
Liu Y, Jiang W, Tang Y, Zhang Q, Zhen Y, Wang X, et al. An optimal method for quantifying the facial sebum level and characterizing facial sebum features. Skin Res Technol. 2023;29:e13454. 10.1111/srt.13454
Yujie Liu and Wencai Jiang contributed equally to this work.
Contributor Information
Yanyun Ma, Email: yanyunma@fudan.edu.cn.
Yimei Tan, Email: ameit@163.com.
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cundell AM, microbial ecology of the human skin microbial ecology of the human skin. Microb Ecol. 2018;76:113–120. [DOI] [PubMed] [Google Scholar]
- 2. Richardson M, Understanding the structure and function of the skin. Nurs Times. 2003;99:46–48. [PubMed] [Google Scholar]
- 3. Selwyn S, Microbiology and ecology of human skin. Practitioner. 1980;224:1059–1062. [PubMed] [Google Scholar]
- 4. Lee HJ, Kim M, Skin Barrier Function and the Microbiome. Int J Mol Sci. 2022;23(21):13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trompette A, Ubags ND, Skin barrier immunology from early life to adulthood. Mucosal Immunol. 2023;16:194–207. [DOI] [PubMed] [Google Scholar]
- 6. Boer M, Duchnik E, Maleszka R, Marchlewicz M, Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol Alergol. 2016;33, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Firooz A, Zartab H, Sadr B, et al., Daytime changes of skin biophysical characteristics: a study of hydration, transepidermal water loss, pH, sebum, elasticity, erythema, and color index on middle Eastern skin. Indian J Dermatol. 2016;61, 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fitzpatrick TB, Breathnach AS, The epidermal melanin unit system. Dermatol Wochenschr. 1963;147, 481–489. [PubMed] [Google Scholar]
- 9. Mukherjee S, Mitra R, Maitra A, et al. Sebum and hydration levels in specific regions of human face significantly predict the nature and diversity of facial skin microbiome. Sci Rep. 2016;6, 36062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Addor FAS, Aoki V, Skin barrier in atopic dermatitis. An Bras Dermatol. 2010;85:184–194. [DOI] [PubMed] [Google Scholar]
- 11. Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:Sf3595563‐sf35955610 . [DOI] [PubMed] [Google Scholar]
- 12. Damiani G, Finelli R, Kridin K, et al. Masks trigger facial seborrheic dermatitis and psoriasis: evidence from a multicenter, case‐control study during COVID‐19 pandemic. Ital J Dermatol Venerol. 2022;157:419–423. [DOI] [PubMed] [Google Scholar]
- 13. Goh CL, Wu Y, Welsh B, et al. Expert consensus on holistic skin care routine: focus on acne, rosacea, atopic dermatitis, and sensitive skin syndrome. J Cosmet Dermatol. 2022;21(1):45–54. [DOI] [PubMed] [Google Scholar]
- 14. Davoudi SM, Sadr B, Hayatbakhsh MR, et al. Comparative study of skin sebum and elasticity level in patients with sulfur mustard‐induced dermatitis and healthy controls. Skin Res Technol. 2010;16, 237–242. [DOI] [PubMed] [Google Scholar]
- 15. Kim By, Choi Jw, Park Kc, Youn Sw, Sebum, acne, skin elasticity, and gender difference—which is the major influencing factor for facial pores? Skin Res Technol. 2013;19:e45‐e53. [DOI] [PubMed] [Google Scholar]
- 16. Li X, He C, Chen Z, Zhou C, Gan Y, Jia Y, A review of the role of sebum in the mechanism of acne pathogenesis. J Cosmet Dermatol. 2017;16:168–173. [DOI] [PubMed] [Google Scholar]
- 17. Bouwstra J, Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 2003;42:1–36. [DOI] [PubMed] [Google Scholar]
- 18. Imokawa G, Kuno H, Kawai M, Stratum corneum lipids serve as a bound‐water modulator. J Invest Dermatol. 1991;96:845–851. [DOI] [PubMed] [Google Scholar]
- 19. Montagna W, An introduction to sebaceous glands. J Invest Dermatol. 1974;62:120–123. [DOI] [PubMed] [Google Scholar]
- 20. Thody AJ, Shuster S, Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416. [DOI] [PubMed] [Google Scholar]
- 21. Akaza N, , Nishiyama E, et al. The microbiome in comedonal contents of inflammatory acne vulgaris is composed of an overgrowth of Cutibacterium Spp. and other cutaneous microorganisms. Clin Cosmet Investig Dermatol. 2022;15:2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Capitanio B, Lora V, Ludovici M, et al. Modulation of sebum oxidation and interleukin‐1α levels associates with clinical improvement of mild comedonal acne. J Eur Acad Dermatol Venereol. 2014;28:1792–1797. [DOI] [PubMed] [Google Scholar]
- 23. Sirithanabadeekul P, Leetrakulwanna V, Suwanchinda A, A novel technique in reducing sebum production and improving atrophic acne scars. J Cosmet Dermatol. 2022:5872‐5879. [DOI] [PubMed] [Google Scholar]
- 24. Thadanipon K, Kitsongsermthon J, Comparative study into facial sebum level, pore size, and skin hydration between oily‐skinned and dry‐skinned Thai women. Skin Res Technol. 2020;26, 163–168. [DOI] [PubMed] [Google Scholar]
- 25. Park EH, Jo DaJ, Jeon HW, Na SJ, Effects of winter indoor environment on the skin: unveiling skin condition changes in Korea. Skin Res Technol. 2023;29, e13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blanc D, Agache P. Methods of measurement and influence of physical factors. Int J Cosmet Sci. 1980;2:243–250. [DOI] [PubMed] [Google Scholar]
- 27. Piérard GE, Piérard‐Franchimont C, Marks R, Paye M, Rogiers V, EEMCO guidance for the in vivo assessment of skin greasiness. The EEMCO Group. Skin Pharmacol Appl Skin Physiol. 2000;13:372–389. [DOI] [PubMed] [Google Scholar]
- 28. Greene RS, Downing DT, Pochi PE, Strauss JS, Anatomical variation in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970;54:240–247. [DOI] [PubMed] [Google Scholar]
- 29. Downing DT, Stranieri AM, Strauss JS, The effect of accumulated lipids on measurements of sebum secretion in human skin. J Invest Dermatol. 1982;79:226–228. [DOI] [PubMed] [Google Scholar]
- 30. Crowther JM, Method for quantification of oils and sebum levels on skin using the Sebumeter(®). Int J Cosmet Sci. 2016;38:210–216. [DOI] [PubMed] [Google Scholar]
- 31. Segot‐Chicq E, Compan‐Zaouati D, Wolkenstein P, et al. Development and validation of a questionnaire to evaluate how a cosmetic product for oily skin is able to improve well‐being in women. J Eur Acad Dermatol Venereol. 2007;21:1181–1186. [DOI] [PubMed] [Google Scholar]
- 32. Wu Y, Niu Y, Zhong S, et al. A preliminary investigation of the impact of oily skin on quality of life and concordance of self‐perceived skin oiliness and skin surface lipids (sebum). Int J Cosmet Sci. 2013;35:442–447 [DOI] [PubMed] [Google Scholar]
- 33. Saint‐Léger D, Skin surface lipids in man. Evaluation and perspectives of research. Ann Dermatol Venereol. 1982;109, 379–392 [PubMed] [Google Scholar]
- 34. Cui Le, Jia Y, Cheng Z‐W, et al. Advancements in the maintenance of skin barrier/skin lipid composition and the involvement of metabolic enzymes. J Cosmet Dermatol. 2016;15:549–558. [DOI] [PubMed] [Google Scholar]
- 35. Schurer NY, Elias PM, The biochemistry and function of stratum corneum lipids. Adv Lipid Res. 1991;24:27–56. [DOI] [PubMed] [Google Scholar]
- 36. Foolad N, Shi VY, Prakash N, Kamangar F, Sivamani RK, The association of the sebum excretion rate with melasma, erythematotelangiectatic rosacea, and rhytides. Dermatol Online J. 2015;21. [PubMed] [Google Scholar]
- 37. Herane MI, Ando I, Acne in infancy and acne genetics. Dermatology. 2003;206:24–28. [DOI] [PubMed] [Google Scholar]
- 38. Kwon SH, Jeong MY, Park KC, Youn SW, Huh CH, Na JI, A new therapeutic option for facial seborrhoeic dermatitis: indole‐3‐acetic acid photodynamic therapy. J Eur Acad Dermatol Venereol. 2014;28:94–99. [DOI] [PubMed] [Google Scholar]
- 39. Pan J, Wang Q, Tu P, A topical medication of all‐trans retinoic acid reduces sebum excretion rate in patients with forehead acne. Am J Ther. 2017;24:e207‐e212 [DOI] [PubMed] [Google Scholar]
- 40. Eo J, Seo YK, Baek JH, Choi AR, Shin MK, Koh JS, Facial skin physiology recovery kinetics during 180 min post‐washing with a cleanser. Skin Res Technol. 2016;22:148–151. [DOI] [PubMed] [Google Scholar]
- 41. Kim M‐K, Choi S‐Y, Byun H‐J, et al. Comparison of sebum secretion, skin type, pH in humans with and without acne. Arch Dermatol Res. 2006;298:113–119 [DOI] [PubMed] [Google Scholar]
- 42. Man MQ, Xin SJ, Song SP, Cho SY, Zhang XJ, Tu CX, Feingold KR, Elias PM, Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22:190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qin J, Qiao L, Hu J, et al. New method for large‐scale facial skin sebum quantification and skin type classification. J Cosmet Dermatol. 2021;20:677–683. [DOI] [PubMed] [Google Scholar]
- 44. Seo JI, Ham HI, Baek JH, Shin MK, An objective skin‐type classification based on non‐invasive biophysical parameters. J Eur Acad Dermatol Venereol. 2022;36:444–452. [DOI] [PubMed] [Google Scholar]
- 45. Zhang GJ, Papillon A, Ruvolo E, Bargo PR, Kollias N, In Conference on Photonic Therapeutics and Diagnostics VI . San Francisco, CA, 2010;7548. [Google Scholar]
- 46. Youn SW, Kim SJ, Hwang InA, Park KC, Evaluation of facial skin type by sebum secretion: discrepancies between subjective descriptions and sebum secretion. Skin Res Technol. 2002;8:168–172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.


