Abstract
A binding enzyme-linked immunosorbent assay (ELISA) has been developed for measuring nanogram concentrations of semisynthetic pneumocandin antifungal agents in human plasma. Semisynthetic pneumocandin L-733,560 was conjugated to succinylated hemocyanin by water-soluble carbodiimide and was used as an immunogen to produce polyclonal antibodies in rabbits. Pneumocandins were used to directly coat the wells of a microtiter plate, and quantitation was achieved by using rabbit polyclonal antibodies to pneumocandin L-733,560 and goat anti-rabbit immunoglobulin G conjugated to either alkaline phosphatase or horseradish peroxidase. Maximum binding of L-733,560 and most related analogs to the wells of the microtiter plate was found to occur in the first 5 min of incubation at 4°C. Once bound to the plate, these pneumocandins could not be removed from the plate, either by treatment with 4.0 to 6.0 M urea or by treatment with 4.0 to 6.0 M guanidine hydrochloride for 24 h at 4°C. The binding ELISA is linear with drug concentration and can detect levels of L-733,560 as low as 5 ng/ml in human plasma. The assay is also useful for quantitating plasma levels of related semisynthetic pneumocandins including clinical candidate MK-0991.
The pneumocandins are a family of natural-product lipopeptides which exhibit antifungal and antipneumocystis activities (14). These compounds kill fungi by inhibiting the synthesis of β-1,3-glucan, a major component of the cell wall in certain genera of fungi including Candida, Aspergillus, and Pneumocystis carinii (8, 13, 14). Many of these organisms can cause life-threatening infections especially in immunocompromised patients. Due to the urgent need for novel therapeutic agents for these infections the pneumocandins have become attractive candidates for the development of novel antifungal agents. As a result of exploratory chemistry, semisynthetic pneumocandin MK-0991 (Fig. 1) was identified as a potential clinical candidate and is currently in clinical trials (11).
FIG. 1.
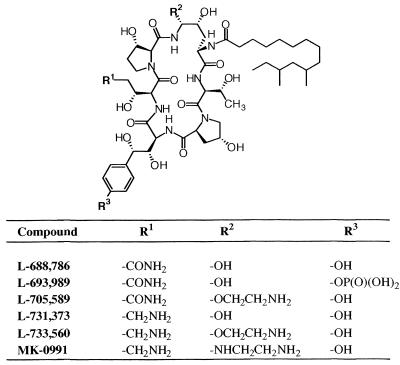
Structure of pneumocandin B0 (L-688,786) and its derivatives. All modifications were done on R1, R2, and R3.
Prior to the selection of MK-0991 as a clinical candidate, rabbit polyclonal antibodies had been raised against a closely related analog, L-733,560 (Fig. 1), for various studies including immunochemical localization of the drug, mechanism of action, and pharmacokinetics. Attempts to develop a standard competitive enzyme-linked immunosorbent assay (ELISA) were compromised by making use of the property of nonspecific binding of the semisynthetic pneumocandins to proteins and microtiter plates. This paper describes a binding immunoassay using rabbit polyclonal antibodies against L-733,560 which can measure nanogram quantities of L-733,560 and other related semisynthetic pneumocandins in human plasma. The assay employs the nonspecific binding of these charged compounds to microtiter plates as a method for immobilizing the compounds followed by the standard steps used for indirect ELISA.
The pneumocandin B0(L-688,786) was a natural product obtained from the fungus Zalerion arboricola (ATCC 20868). Other pneumocandins were prepared by the members of the Merck Medicinal Chemistry groups. All modifications were made on R1, R2, and R3. All these compounds are cyclic hexapeptides with a lipophilic side chain (Fig. 1). Antibodies were produced against L-733,560 in rabbits. MK-0991 is the Merck compound currently in clinical trials.
A succinylhemocyanin–carbodiimide–L-733,560 conjugate (SHCP-560) was synthesized as follows. Briefly, 5.0 mg of succinylated keyhole limpet hemocyanin (Calbiochem, La Jolla, Calif.) was dissolved in 1 ml of 3.0 mM KH2PO4–10% dimethyl sulfoxide (DMSO), pH 6.2. One milligram of L-733,560 was added, and the mixture was stirred for 1 min, followed by the addition of water-soluble carbodiimide [1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; 5.0 mg; Sigma Chemical Company, St. Louis, Mo.]. The mixture was stirred for 3 h at 25°C, after which an additional 5.0 mg of L-733,560 was made, and the mixture was stirred at 4°C overnight. The mixture was then dialyzed for 48 h at 4°C against 1 liter of phosphate-buffered saline (PBS) with one change after 24 h. The conjugate was a suspension, and therefore the ratio of L-733,560 coupled to hemocyanin could not be determined accurately. Radiolabeled L-733,560 was not available at the time of the experiment. This suspension was used as an immunogen to produce antibodies in rabbits.
A thyroglobulin–glutaraldehyde–L-733,560 coating antigen (TGP-560) was synthesized as follows. Five milligrams of bovine thyroglobulin (Sigma Chemical Company) was dissolved in 1 ml of PBS–10% DMSO. One milligram of L-733,560 dissolved in 1 ml of DMSO was added to a solution of bovine thyroglobulin, and the mixture was stirred for 5 min. Glutaraldehyde (1%, 200 μl; Aldrich Chemical Company, Milwaukee, Wis.) was added, and mixture was stirred for 2 h at 25°C. The reaction was stopped by the addition of 0.5 ml of 1.0 M glycine, and the stirring was continued for 1 h. The incubation mixture was dialyzed for 48 h against 1 liter of PBS at 4°C with one change after 24 h. The final product was a suspension.
A thyroglobulin-glutaraldehyde-thyroglobulin coating antigen (TGT) was synthesized as follows. To a solution of thyroglobulin, 5.0 mg/ml in PBS–10% DMSO, 200 μl of 1% glutaraldehyde was added, and the mixture was stirred at room temperature for 2 h. The remaining procedure was followed as described above.
Polyclonal antibodies were produced in rabbits by HRP, Inc., Denver, Pa. Briefly, two New Zealand White male rabbits were injected intradermally with 250 μg of the immunogen mixed with complete Freund’s adjuvant. After 3 weeks, rabbits were boosted subcutaneously with 125 mg of immunogen mixed with incomplete Freund’s adjuvant. A total of four boosts were given at 3-week intervals. Rabbits were bled 10 days after each boost, and the presence of serum antibodies was measured by an indirect ELISA (see below).
The presence of antibodies against L-733,560 in rabbit serum was detected by an indirect ELISA. Wells of microtiter plates (Immulon 4; Dynatech Laboratories, Chantilly, Va.) were coated overnight at 4°C with 100 μl of a 5-μg/ml solution of either TGP-560, TGT, or thyroglobulin, prepared in coating buffer (50 mM carbonate-bicarbonate, pH 9.6). The following day the wells were washed five times with 100 μl of 0.05% Tween 20 dissolved in PBS (PBS-Tween), and the remaining sites were blocked at 37°C for 1 h with 100 μl of 5% gelatin dissolved in coating buffer per well. After five washes with 100 μl of PBS-Tween, 100 μl of a serially diluted sample of test bleed or prebleed serum was added to each well. The plates were incubated at 37°C for 1 h and washed six times with 200 μl of PBS-Tween, and 100 μl of alkaline phosphatase-conjugated affinity-purified goat anti-rabbit immunoglobulin (IgG) (1:2,000 in PBS-Tween) was added to each well (H+L; Boehringer Mannheim, Indianapolis, Ind.). The plates were incubated at 37°C for 1 h and washed six times with 200 μl of PBS-Tween per well, followed by the addition of 100 μl of a 1-mg/ml solution of p-nitrophenylphosphate (Sigma), dissolved in diethanolamine buffer, pH 9.8, per well. The plate was incubated at 25°C for 30 min, and the reaction was stopped by the addition of 50 μl of 3.0 N NaOH. Absorbance was measured at 405 nm against appropriate blanks in a Microplate Twin Reader (ICN, Costa Mesa, Calif.).
The above indirect ELISA could not be used in form of a competition ELISA to determine cross-reactivity and amounts of pneumocandins in human plasma due to their nonspecific binding to proteins. Therefore, a binding ELISA which is described below was developed. All pneumocandins were dissolved in DMSO at the concentration of 1.0 mg/ml. They were serially diluted with PBS from 100 to 0.01 μg/ml. Wells of a microtiter plate were coated directly at 4°C overnight with 100 μl of pneumocandin solutions, and ELISA was carried out exactly as the indirect ELISA described above. Whenever greater sensitivity was desired, affinity-purified goat anti-rabbit IgG (H+L) conjugated to horseradish peroxidase (Boehringer Mannheim) was used as a second antibody. The substrate used was K-Blue (Elisa Technologies, Lexington, Ky.). The reaction was stopped after 10 min by the addition 50 μl of 3 N H2SO4, and the absorbance was read at 450 nm against appropriate blanks in a Microplate Twin Reader.
To determine coating kinetics, microtiter plates were coated with 100 μl of a 1.0-μg/ml solution of each pneumocandin in PBS per well. The plates were incubated at 4°C for the desired length of time, and the ELISA was continued as described above. The absorbance obtained was compared with that obtained when the plate was incubated at 4°C overnight. This reading was taken as 100% coating.
The strength of binding of semisynthetic pneumocandins to polystyrene plates wells was determined by coating the microtiter plate with each pneumocandin (100 μl/well) dissolved in PBS at a concentration of 1.0 μg/ml. The plates were incubated overnight at 4°C, and next day the coating solution was discarded. The wells were filled with 100 μl of denaturants such as 4.0 and 6.0 M urea and 4.0 and 6.0 M guanidine hydrochloride. For control, 100 μl of PBS was used. The plates were incubated at 4°C overnight. The next day the plates were washed five times with 100 μl of PBS-Tween. Following this, the ELISA was continued as described above. The optical density obtained from the control was taken as 100% binding.
To investigate the effects of different pHs on the binding to the microtiter plate, pneumocandins were dissolved, 1 μg/ml, in different buffers of different pHs containing 10% DMSO: 0.20 M citrate, pH 3.0; 0.10 M citrate-phosphate, pH 5.0; PBS, pH 7.2; 0.1 M NaHCO3, pH 9.0; or 0.1 M NaCO3, pH 11.0. Wells were coated with 100 μl of each solution, and ELISA was continued as described above.
Specificities of antibodies were determined by testing the abilities of two unrelated antibodies such as rabbit antihemocyanin (keyhole limpet) and antithyroglobulin (bovine) to react with L-733,560. The results were compared with those obtained with rabbit anti-L-733,560. Since hemocyanin was used as a carrier to develop antibodies against L-733,560, antibodies against hemocyanin were removed by affinity chromatography.
Cross-reactivities of the antibodies were determined from the titration curve of each pneumocandin at the antibody dilution of 1/1,600. The cross-reactivities of these compounds relative to those of L-733,560 were calculated from the amounts of these compounds required to obtain maximum binding with antibodies.
Human plasma (Biological Specialty Corporation, Colmar, Pa.) was centrifuged at 27,000 × g for 30 min, and the supernatant was used for the preparation of a standard solution. A 1.0-mg/ml solution of pneumocandin L-733,560 was prepared in DMSO. A 12-μl volume of this solution was added to 10.0 ml of human plasma. This was a stock solution (1,200 ng/ml). Several standard solutions of different concentrations ranging from 200 to 5 ng/ml were made from this stock solution by dilution with human plasma. To each standard solution, Triton X-100 was added (final concentration, 0.17%), and the mixture was thoroughly vortexed. Standards, 50 μl in volume, were added to the wells of the microtiter plate for coating overnight at 4°C. After five washes with 200 μl of PBS-Tween, the remaining sites of wells were blocked with 1% gelatin prepared in coating buffer (see above). The plate was then incubated at 37°C for 1 h, and the wells were washed five times with 200 μl of PBS-Tween. Antibody solution, 100 μl in volume diluted 1/800 in EIA buffer (PBS–Tween–0.50% gelatin) was added to each well, and the plate was incubated at 37°C for 1 h. Prior to their use, antibodies against L-733,560 were absorbed with an equal volume of human plasma at 4°C overnight and centrifuged in a microcentrifuge for 10 min. The plates were washed six times with 200 μl of PBS-Tween. One hundred microliters of affinity-purified goat anti-rabbit IgG (H+L) coupled to horseradish peroxidase (BM Biochemicals) was added (dilution, 1/10,000 with EIA buffer), and the plate was incubated at 37°C for 1 h. After six washes with 200 μl of PBS-Tween, 100 μl of the K-Blue substrate was added. The reaction was stopped after 10 min by the addition 50.0 μl of 3 N H2SO4. The absorbance was read at 450 nm against wells which contained human plasma without L-733,560.
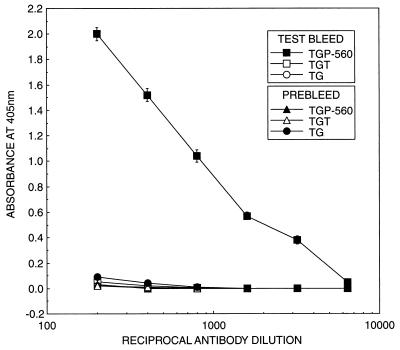
Figure 2 shows the presence of antibodies in rabbit serum against pneumocandin L-733,560 as determined by indirect ELISA. The coating antigen was TGP-560, which comprised a different carrier, a different linker, but the same hapten L-733,560. An optical density of 1.0 at 405 nm was obtained at an antibody dilution of 1:800; at an antibody dilution of 1:3,200 an optical density of 0.40 was obtained. When antibodies were tested against TGT and thyroglobulin as coating antigens, no reaction was observed. Prebleed serum failed to show reaction with TGP-560, TGT, and thyroglobulin as coating antigens, confirming the presence of antibodies in test bleed serum.
FIG. 2.
Relationship between dilution of antibodies and absorbance at 405 nm by an indirect ELISA. Antiserum used was the first bleed obtained after 1 month of immunization of rabbit with SHCP-560. Values are means ± standard deviations for six determinations.
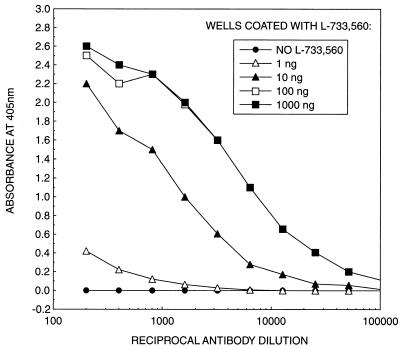
Figure 3 shows a checkerboard titration using L-733,560 as the coating antigen. The reaction was dependent on both antigen and antibody concentrations. A control without the antigen showed no reaction. At an antibody dilution of 1/800, maximum binding was observed at the antigen concentration of 100 ng/well. Other pneumocandins could also be titrated with this method (data not shown). L-733,560, MK-0991, and L-705,589 showed maximum binding to the wells after incubation of the plate for 5 min at 4°C, while L-731,373 required 1 h of incubation at 4°C to achieve maximum binding. No binding was observed with L-689,989 and L-688,786 even after overnight incubation at 4°C (data not shown).
FIG. 3.
Checkerboard titration of antibodies against L-733,560. Wells were coated overnight at 4°C with 100 μl of different amounts of L-733,560 dissolved in PBS. Antibodies were the third bleed obtained after 10 weeks of immunization of rabbit with SHCP-560. The data are means for three independent determinations each done in duplicate.
Effects of urea and guanidine hydrochloride on the binding of semisynthetic pneumocandin to the plate were studied. Even after incubation for 18 h at 4°C with either 4 to 6 M urea or 4 to 6 M guanidine hydrochloride, no dissociation of the pneumocandins from the microtiter plate was observed (data not shown).
Pneumocandins were coated at pH 3.0, 5.0, 7.2, 9.0, and 11.0. For most of the pneumocandins there was no difference in binding to antibodies when they were applied at pH 3.0, 5.0, 7.2, 9.0, and 11.0. However, pneumocandins L-688,736 and L-693,989, which showed negligible reaction at pH 3.0, 5.0, and 7.2, showed binding with antibodies at pH 9.0 and 11.0; pneumocandin L-688,786 showed 40 and 100% binding, respectively, while L-693,989 showed 100% reaction at pH 11.0 relative to that of L-733,560 (data not shown).
Table 1 shows the relative antibody cross-reactivities of pneumocandins compared to L-733,560. The semisynthetic pneumocandins MK-0991 and L-705,589 showed 25.0 and 50.0% cross-reactivities, respectively, while L-731,373 was only 12.5% as active as L-733,560; no cross-reactivity was observed with L-688,786 and its phosphoryl derivative L-693,989 when pneumocandins were applied in PBS at pH 7.2.
TABLE 1.
Cross-reactivities of the antibodies against L-733,560a
| Compound | Amt (ng) needed for maximum A450 [%] |
|---|---|
| L-733,560 | 50.0 [100] |
| MK-0991 | 200.0 [25.0] |
| L-705,589 | 100.0 [50.0] |
| L-731,373 | 400.0 [12.5] |
| L-693,989 | —b |
| L-688,786 | — |
Antibody dilution, 1/1,600.
—, no cross-reactivity observed.
Antibodies against L-733,560 were very specific since they reacted with only with L-733,560 and failed to show any reaction with keyhole limpet hemocyanin and thyroglobulin and vice versa. These antibodies showed only slight reaction with L-733,560: 4.1% for antibodies against hemocyanin and 5.5% for antibodies against bovine thyroglobulin (data not shown).
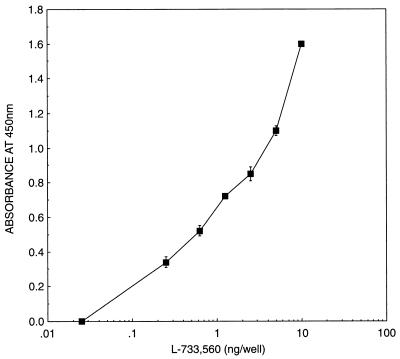
Standards of L-733,560 were prepared in human plasma, and the binding-ELISA approach was applied (Fig. 4). L-733,560 in human plasma could be detected with this method at concentrations as low as 5 ng/ml. Except for the addition of Triton X-100 to the plasma to dissociate L-733,560 from proteins, no other chemical modification of the plasma was needed.
FIG. 4.
Standard curve obtained from the addition of a range of concentrations of L-733,560 to human plasma. The data are means ± standard deviations for six independent determinations in which each concentration was tested in triplicate.
The precision and accuracy of the ELISA was determined by spiking human plasma with a range of concentrations of L-733,560 from 5 to 200 ng/ml. These spiked samples were assayed over a 4-week period. Intraassay (n = 10) and interassay (n = 9 or 10) coefficients of variation varied from 7.2 to 18.9% and 2.6 to 20.8%, respectively. Intraassay recovery was 92.2 to 106.0%, while interassay recovery was 96.0 to 102.2% (Table 2).
TABLE 2.
Intra- and interassay variations of L-733,560 added to human plasma
| Spiking concn (n) | Mean ng/ml | CVa (%) | Recovery (%) |
|---|---|---|---|
| Intraassay variation | |||
| 200.0 (10) | 192.5 | 7.2 | 96.3 |
| 100.0 (10) | 92.2 | 13.0 | 92.2 |
| 50.0 (10) | 49.0 | 8.6 | 98.0 |
| 25.0 (10) | 25.6 | 10.9 | 102.4 |
| 12.5 (10) | 12.1 | 9.0 | 96.8 |
| 5.0 (10) | 5.3 | 18.9 | 106.0 |
| Interassay variation | |||
| 200.0 (10) | 195.0 | 4.7 | 97.5 |
| 100.0 (9) | 102.2 | 2.6 | 102.2 |
| 50.0 (9) | 50.0 | 14.2 | 100.0 |
| 25.0 (10) | 26.0 | 8.8 | 104.0 |
| 12.5 (9) | 12.3 | 20.8 | 98.4 |
| 5.0 (9) | 4.8 | 8.3 | 96.0 |
CV, coefficient of variation.
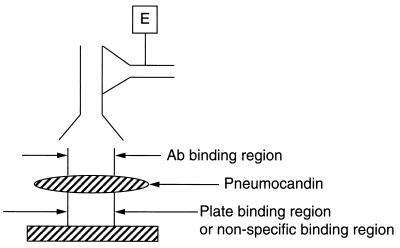
The semisynthetic pneumocandins are synthesized from natural-product lipopeptide B0 and exhibit antifungal activity by inhibiting synthesis of β-1,3-glucan, a critical enzyme of the fungal cell wall (8, 13–15). Members of this class of compounds promise to be potentially important agents in the treatment of life-threatening fungal infections. One of these compounds, MK-0991, is at present undergoing clinical trials (11). We developed rabbit polyclonal antibodies against a hemocyanin conjugate of a related analog, L-733,560, to study the mechanism of action of pneumocandins and their localization in the membranes of the organisms treated with the drug (11). Another objective was to use these antibodies to develop a quantitative ELISA to determine the levels of these compounds in plasma in animals and humans for pharmacokinetic studies. In our initial studies we could show the presence of antibodies in rabbit serum by a standard ELISA in which the wells of the microtiter plate were coated with a conjugate of thyroglobulin and L-733,560 using glutaraldehyde as a linker (Fig. 2). However, due to the nonspecific binding properties of these charged species of semisynthetic pneumocandins a standard competition ELISA could not be developed. Therefore, a binding ELISA was developed in which pneumocandins were applied directly to the microtiter plate and rabbit polyclonal antibody to L-733,560 and a second antibody coupled to either alkaline phosphatase or horseradish peroxidase were used to determine the amount of pneumocandin bound to the plate. Most of the semisynthetic pneumocandins bound to the plate within 1 h of incubation at 4°C and could not be removed by 6 M urea or 6 M guanidine hydrochloride after 24-h incubation at 4°C. A checkerboard titration showed that the antibody reaction was linear and depended on both pneumocandin concentration and antibody dilution (Fig. 3). Presumably, the nonspecific binding region of the pneumocandin anchors the drug to the plate, allowing antigenic determinants to bind to antibodies (Fig. 5). Other pneumocandins also showed binding with the L-733,560 antibodies, although to a lesser degree. The results obtained with this assay depend upon two factors: (i) binding of antibodies to pneumocandins and (ii) ability of pneumocandins to bind to the microtiter plate. Most of the results obtained could be interpreted in terms of binding of antibodies to pneumocandins. L-733,560 showed maximum binding. Both side chains R1 and R2 of pneumocandin L-733,560 appear to be involved in antibody binding (Fig. 1); the R2 side chain appears to play a major role. In pneumocandin MK-0991, the oxygen atom of the R2 chain is replaced by —NH—, which reduces the cross-reactivity to 25%. In L-705,589 the R2 side chain is the same but the R1 side chain is different, which reduces the cross-reactivity to only 50%. In L-731,373, the R2 side chain is absent and the compound shows very poor cross-reactivity with antibodies against L-733,560. Both L-688,786 and L-693,989 showed negligible reaction. Both have different R1 side chains and lack the R2 side chain. Their absence of cross-reactivity could arise due to their weak binding to the microtiter plate. The binding of antibodies to pneumocandins was tested by coating at different pHs from 3 to 11. No difference was observed with L-733,560, MK-0991, L-705,589, and L-731,373, but at alkaline pH both L-693,989 and L-688,786, which were inert at pH 3.0, 5.0, and 7.2, showed reaction. However, it is known that at alkaline pH pneumocandins undergo degradation (14). These data rule out the involvement of a charged species in binding to the plate but suggest such a role for the lipid side chain of the compound. To further explore this mechanism radiolabeled pneumocandins were not available to us. The specificity of these antibodies was confirmed by testing two different antibodies: anti-keyhole limpet hemocyanin and anti-bovine thyroglobulin. Both reacted strongly with their respective antigens but failed to react with L-733,560, and the affinity-purified antibodies against L-733,560 showed no cross-reactivity with the hemocyanin and thyroglobulin.
FIG. 5.
ELISA configuration.
The potential of these antibodies for pharmacokinetic studies was explored by adding known amounts of L-733,560 to human plasma and by determining their concentrations by the binding ELISA. As shown in Fig. 4, the lowest concentration which could be detected with confidence was 250 pg/well, or 5 ng/ml. Below this concentration it was not possible to determine the amounts of L-733,560 because of high background. The coefficients of variation within and between assays were found to range from 7.2 to 18.9% and from 2.6 to 20.8%, respectively. The within-assay recovery ranged from 92.2 to 106.0%, and between-assay recovery ranged from 96.0 to 102.2%, thus confirming the validity of the assay (Table 2). By using these antibodies, the amount of clinical candidate MK-0991 could be determined with confidence at the 100-ng/ml level in human plasma.
The binding ELISA described in this paper is unique since pneumocandins were directly applied to the wells of the microtiter plate. In a standard ELISA haptens are conjugated to high-molecular-weight carriers to facilitate their binding to the plate. In such a synthesis of hapten-carrier conjugate important epitopes on the haptens may get masked due to the large size of the carrier, which can result in weak binding with antibodies. In addition, due to the large size of the carrier, nonspecific binding of both first and second antibodies to the carrier can occur, producing erroneous results. In the present assay pneumocandins were directly bound to the plate, which allowed maximum binding between antigen and antibodies. There are several ELISA procedures in which peptides or haptens are directly bound to the microtiter plate; but these plates need preactivation with either glutaraldehyde, carbodiimide, or other chemical reagents (1, 3–7, 10, 12). Not only are these procedures lengthy, but very often they do not give consistent results (1, 9). Recently, adhesive polyphenolic protein purified from the mussel Aulacoma ater was shown to enhance the binding of antigens such as human chorionic gonadotropin to microtiter plates (2). The sensitivity of the assay increased 50- to 100-fold. We have shown in our method that pneumocandins bind to the plate strongly without preactivation. The binding is strong since, once bound to the plate, neither 6 M urea nor 6 M guanidine hydrochloride could dissociate the compounds from the plate. The binding assay reported in this paper is simple, reliable, and inexpensive. It eliminates the use of expensive instruments such as high-performance liquid chromatography, gas chromatography-mass spectrometry, or radioactivity counters routinely used for pharmacokinetic studies. It also avoids steps such as extraction of the compound with organic solvents, which can incur significant losses. Such losses are minimal in our assay because the samples are applied directly to polystyrene plates without preactivation. Except for the addition of 0.17% Triton X-100 to human plasma to dissociate pneumocandins from proteins, no other treatment is needed in our method.
Acknowledgments
We thank J. F. Dropinski and F. A. Bouffard of the Department of Medicinal Chemistry for providing pneumocandin derivatives.
REFERENCES
- 1.Boudet F, Theze J, Zouli M. UV-treated polystyrene microtitre plates for use in an ELISA to measure antibodies against synthetic peptides. J Immunol Methods. 1991;142:73–82. doi: 10.1016/0022-1759(91)90294-p. [DOI] [PubMed] [Google Scholar]
- 2.Burzio V A, Silva T, Pardo J, Pardo L O. Mussel adhesive enhances the immobilization of human chorionic gonadotropin to a solid support. Anal Biochem. 1996;241:190–194. doi: 10.1006/abio.1996.0398. [DOI] [PubMed] [Google Scholar]
- 3.Dagenais P, Desprez B, Albert J, Escher E. Direct covalent attachment of small peptide antigens to enzyme-linked immunosorbant assay plates using radiation and carbodiimide activation. Anal Biochem. 1994;222:149–155. doi: 10.1006/abio.1994.1466. [DOI] [PubMed] [Google Scholar]
- 4.Gegg C V, Eltzer M E. Directional coupling of synthetic peptides to poly-l-lysine and applications to the ELISA. Anal Biochem. 1993;210:309–313. doi: 10.1006/abio.1993.1200. [DOI] [PubMed] [Google Scholar]
- 5.Klasen E A, Rigutti A, Bos A, Bernini L F. Development of a screening system for detection of somatic mutation. I. Enzyme immunoassay for detection of antibodies against specific determinants. J Immunol Methods. 1982;54:241–250. doi: 10.1016/0022-1759(82)90065-5. [DOI] [PubMed] [Google Scholar]
- 6.Klasen E A, Rigutti A, Bos A, Bernini L F. Development of screening system for detection of somatic mutation. II. The use of peptides and insoluble protein fragments in a non-competetive solid-phase enzyme immunoassay. J Immunol Methods. 1983;59:281–287. doi: 10.1016/0022-1759(83)90189-8. [DOI] [PubMed] [Google Scholar]
- 7.Neurath A, Strick N. Enzyme-linked fluorescence immunoassays using β-galactosidase antibody covalently bound to polystyrene plates. J Virol Methods. 1981;3:155–165. doi: 10.1016/0166-0934(81)90050-1. [DOI] [PubMed] [Google Scholar]
- 8.Nollstadt K H, Powles M A, Fujioka H, Aikawa M, Schmatz D M. Use of β-1,3-glucan-specific antibody to study the cyst wall of Pneumocystis carinii and effects of pneumocandin B0 analog L-733,560. Antimicrob Agents Chemother. 1994;38:2258–2265. doi: 10.1128/aac.38.10.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordronneau P, Abdullah L H, Petrusch P. An efficient enzyme immunoassay for glutamate using glutaraldehyde coupling of of the hapten to microtiter plates. J Immunol Methods. 1991;142:169–176. doi: 10.1016/0022-1759(91)90103-m. [DOI] [PubMed] [Google Scholar]
- 10.Phillips D J, Reimer C B, Wells T W, Black C M. Quantitative characterization of specificity and potency of conjugated antibody with solid-phase, antigen bead standards. J Immunol Methods. 1980;34:315–327. doi: 10.1016/0022-1759(80)90104-0. [DOI] [PubMed] [Google Scholar]
- 11.Powles M A, Anderson J, Liberator P, Schmatz D M. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of semisynthetic pneumocandin analog L-743,872 against Pneumocystis carinii in murine models, abstr. F42; p. 107. [Google Scholar]
- 12.Rothmans J P, Delwel H R. Cross-linking of Schistosoma mansoni antigens and their covalent binding on the surface of polystyrene microtitration trays for use in the ELISA. J Immunol Methods. 1983;57:87–98. doi: 10.1016/0022-1759(83)90067-4. [DOI] [PubMed] [Google Scholar]
- 13.Schmatz D M, Romancheck M A, Pittarelli L A, Schwartz R F, Fromtling R A, Nollstadt K H, Vanmiddlesworth F L, Wilson K E, Turner M J. Treatment of Pneumocystis carinii pneumonia with β-1,3-glucan synthesis inhibitors. Proc Natl Acad Sci USA. 1990;87:5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmatz D M, Abruzzo G, Powles M A, McFadden D C, Balkovec J M, Black R M, Nollstadt K, Bartizal K. Pneumocandins from Zalerion arborica. IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumocystis carinii and Candida species. J Antibiot. 1992;45:1886–1891. doi: 10.7164/antibiotics.45.1886. [DOI] [PubMed] [Google Scholar]
- 15.Schmatz D M, Powles M A, McFadden D C, Pittarelli L, Balkovec J, Hammond M, Zambias R, Liberator P, Anderson J. Antipneumocystis activity of water-soluble lipopeptide L-693,989 in rats. Antimicrob Agents Chemother. 1992;36:1964–1970. doi: 10.1128/aac.36.9.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]