Abstract
Purpose
To investigate the differences in peripapillary vessel density (VD) between compressive optic neuropathy (CON) and normal-tension glaucoma (NTG).
Methods
We compared patients with chronic CON and NTG, particularly after strictly controlling the mean extent of macular damage by the area of the ganglion cell–inner plexiform layer (GCIPL) loss in optical coherence tomography (OCT). We compared retinal nerve fiber layer (RNFL) and GCIPL thickness from OCT and peripapillary and macular VD from OCT angiography (OCTA) between the CON and NTG groups.
Results
From the initial 184 patients with CON and 443 patients with OAG, we included 41 patients with CON (57 eyes) and 64 patients with NTG (75 eyes) with a comparable extent of macular GCIPL thinning. Under similar mean macular involvement, the peripapillary VD was significantly lower in the CON group than in the NTG group after considering the effects of age, spherical equivalent, visual field sensitivity, peripapillary RNFL (pRNFL) thickness, GCIPL thickness, and image quality scores (P < 0.001). Marked loss of VD in the temporal and nasal sectors in CON was notable, attributing to the significantly lower peripapillary VD compared to NTG.
Conclusions
Patients with CON had a significantly lower peripapillary VD than those with NTG under similar mean degrees of pRNFL thickness and GCIPL damage. Our results reveal the potential utility of OCTA VD besides OCT pRNFL thickness, in relation to different topographic patterns of pRNFL loss, and possible differences in the pathogenesis of microvascular compromise between CON and NTG.
Keywords: vessel density, glaucoma, compressive optic neuropathy, OCT angiography, OCT
Optical coherence tomography angiography (OCTA) has revealed microvascular compromise in various optic neuropathies, including glaucoma,1–4 which seems to be closely linked to damage to the retinal nerve fiber layer (RNFL). For example, a decrease in vessel density (VD) is topographically correlated with RNFL and visual field (VF) loss.2,3,5 However, the pattern and extent of VD loss in different optic neuropathies are not well understood. For example, compressive optic neuropathy (CON) in chiasmal compression leads to RNFL and ganglion cell complex (GCC) loss and corresponding VF defect.6 Similarly, glaucoma is characterized by RNFL and VF defects. OCTA has revealed impaired peripapillary and macular retinal microvasculatures in both diseases,2–4 but the possible difference in VD changes remains largely unknown with regard to the different pathogeneses of the disease and microvascular compromise,2 different preferential locations of RNFL thinning,7 and differences in the longitudinal progression and recovery of visual loss.6,8
Moreover, an accurate comparison between CON and glaucoma requires a careful selection of cases with a highly controlled degree of damage because of distinct longitudinal and topographic patterns of RNFL and VF losses. In this study, we aimed to compare the peripapillary VDs in CON and normal-tension glaucoma (NTG) with a similar mean macular ganglion cell–inner plexiform layer (GCIPL) and peripapillary RNFL (pRNFL) thicknesses. Our results demonstrate the difference in clinical utility between OCT and OCTA, and also illustrate the difference between CON and glaucoma.
Methods
This cross-sectional study reviewed the medical records of patients who visited the Neuro-ophthalmology and Glaucoma Department of Samsung Medical Center (Seoul, South Korea) between July 2018 and February 2023. This study followed all guidelines for experimental investigation in human subjects, was approved by the Samsung Medical Center Institutional Review Board, and adhered to the tenets of the Declaration of Helsinki.
Patient Inclusion/Exclusion
We included patients ≥ 20 years of age with CON in chiasmal compression and those with NTG. The clinical diagnosis of chiasmal compression was based on preoperative VF defects and/or decreased visual acuity and magnetic resonance imaging (MRI) evidence of mass compression of the optic chiasm. All of the patients underwent transsphenoidal resection. We only included patients with CON who underwent OCTA at the chronic stage, over 6 months after their surgery. The purpose of this study was to compare the different relationships of VD and RNFL thinning between CON and NTG, rather than to identify a method for early differential diagnosis. Thus, we specifically included eyes with a disease onset > 6 months, as progressive thinning of the GCIPL has been reported for up to 1 year in patients with CON.8
NTG was defined as an open angle on gonioscopy, signs of glaucomatous optic nerve damage (i.e., neuroretinal rim notching, thinning, or localized RNFL defect), a glaucomatous VF defect, and untreated baseline intraocular pressure (IOP) ≤ 21 mmHg. A glaucomatous VF defect was defined as a defect conforming to one or more of the following criteria: (1) results outside normal limits on a glaucoma hemifield test; (2) at least three adjacent abnormal points with a P < 0.05 probability of being normal and at least one of these being abnormal with a P < 0.01 probability by pattern deviation; or (3) a pattern standard deviation of P < 0.05, as confirmed on two consecutive reliable tests.
We particularly selected patients with a similar mean degree of macular GCIPL loss, determined by the area of the abnormal macular GCIPL thinning in thickness maps of < 75 µm. For patients with CON, we included eyes with vertical GCIPL losses of ≥50% of the area. For patients with NTG, we included eyes with horizontal GCIPL losses of complete or near-complete 50% of the area due to the characteristic fovea-sparing of glaucomatous RNFL damage. These criteria were chosen over VF or pRNFL because of the subjectivity of VF, considerable interindividual variability of VF and pRNFL, and a closer association with central VF damage of clinical importance than with peripapillary RNFL loss.
The exclusion criteria were as follows: (1) patients with coexistent glaucoma; (2) patients with any other ocular (retinal diseases and optic neuropathies other than glaucoma or chiasmal compression), neurological, or systemic diseases, or previous retinal surgery that might affect OCT or OCTA images; and (3) patients with poor-quality OCT or OCTA images.
All participants underwent visual acuity assessments, refraction tests, slit-lamp biomicroscopy, tonometry, dilated stereoscopic examinations of the optic disc, color fundus photography (TRC-50DX mydriatic retinal camera; Topcon Healthcare, Oakland, NJ, USA), spectral-domain OCT with the CIRRUS HD-OCT (Carl Zeiss Meditec, Jena, Germany), OCTA (DRI OCT Triton Plus; Topcon, Tokyo, Japan), and standard automated perimetry (Humphrey Field Analyzer 640, with 30-2 or 24-2 Swedish interactive threshold algorithm; Carl-Zeiss Meditec, Dublin, CA, USA).
OCTA for Peripapillary and Macular Vessel Density
The peripapillary and macular areas were imaged using a commercially available swept-source OCTA device (Topcon DRI OCT Triton Plus) with a central wavelength of 1050 nm, an acquisition speed of 100,000 A-scans per second, and axial and transverse resolutions of 7 µm and 20 µm, respectively, in tissues. En face projections of volumetric scans allowed visualization of structural and vascular details within the segmented layers. An active eye tracker that follows eye movements detected blinking and adjusted the scan position accordingly, thereby reducing motion artifacts during the acquisition of OCTA images. OCTA images were excluded if they had (1) low image quality score of <40, or (2) image artifacts such as decentration, segmentation, motion, blinking, and shadows.9 Artifacts were defined according to previous reports9,10: Segmentation errors were defined as errors in the segmentation of the retinal layers leading to a deviation of the slab; motion artifacts referred to vertical or horizontal white lines, interruption, displacement, or doubling of vessels; blink artifacts were defined as a signal void black band composed of multiple adjacent B-scans on the en face OCTA due to closure of the eye during image capture; and shadow artifacts referred to localized, decreased intensity of the retinal layers.
Each patient underwent two imaging sessions consisting of a peripapillary scan (4.5 mm × 4.5 mm) centered on the optic disc and a 3.0 mm × 3.0 mm perifoveal scan centered on the macula (both resolution of 320 × 320 pixels). Peripapillary VD was calculated from en face OCTA images of the radial peripapillary capillary (RPC) layer generated by automated layer segmentation from the internal limiting membrane (ILM) to the posterior boundary of the RNFL. The software automatically fitted a 3-mm Early Treatment Diabetic Retinopathy Study (ETDRS) circle at the disk center and generated the vessel density for each sector. The location of the center of the optic disk was manually corrected when necessary. Macular VD was calculated from en face OCTA images of the superficial retinal capillary plexus extending from 3 µm below the ILM to 15 µm below the IPL. The same 3-mm ETDRS circle fitted at the foveal center automatically generated the VD. The ETDRS circle provides five VD areas (central, nasal, temporal, superior, and inferior). The RPC and superficial retinal capillary plexuses were automatically separated via layer segmentation using the OCT instrument software (IMAGEnet 6 V.1.14.8538).
Clock-Hour Involvement
We also evaluated the clock-hour involvement in CON and NTG using OCT 12 clock-hour measurements. Clock-hour involvement was defined as a thickness measurement below the fifth percentile (yellow color), which corresponded to other examinations (fundus photo, OCT, VF, and OCTA) to ensure meaningful findings.
Structure–Function and Vasculature–Function Correlations
We compared the structure–function and vasculature–function correlations by comparing the pRNFL thickness, peripapillary VD, and VF sensitivity.
Statistical Analysis
Both eyes were included if they satisfied the inclusion criteria. To compare the CON and NTG groups, we used a generalized estimation equation (GEE) to consider the possible relationship between both eyes of each patient. In the correlation analysis, the decibel (dB) values for the VF sensitivity were anti-logged to obtain the sensitivity on a linear scale (1/Lambert = 100.1×dB value).11 Correlation was assessed by calculating Pearson's correlation coefficient. The Steiger test was performed to determine whether there was a significant difference in the correlation coefficients among the VD, pRNFL thickness, and VF mean deviation (MD) relationships. Univariate and multivariate logistic regression analyses were performed using the GEE. Factors with P < 0.2 in the univariate analysis were included in the multivariate analysis. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS Statistics 23.0 (IBM, Chicago, IL, USA).
Results
The database included 184 patients with chiasmal compression who underwent surgery and were followed up for 6 months postoperatively, and 439 patients with NTG. We excluded CON patients with (1) no residual optic nerve damage (97 patients), or (2) mild damage that did not meet the inclusion criteria (33 patients). We excluded NTG patients with (1) mild RNFL defects that did not approach the horizontal raphe and fovea (287 patients), (2) substantial superotemporal defects (51 patients), or (3) far-advanced disease (36 patients).
Initially, 54 patients with CON and 64 patients with NTG had comparable average areas of GCIPL thinning in the vertical and horizontal directions, respectively. Of the 152 eyes of the 123 patients, we excluded one eye with retinoschisis, eight eyes of seven patients with OCTA artifacts, six eyes of six patients with OCT artifacts, three eyes of three patients with media opacities, and four eyes of three patients with coexisting CON and glaucoma. Finally, we included 56 eyes from 40 patients with CON and 71 eyes from 60 patients with NTG.
The baseline clinical characteristics are shown in Table 1. Age, refractive error, IOP at examination, average pRNFL thickness, average macular GCIPL thickness, and VF MD were all comparable between the two groups. Intracranial lesions in the CON group included pituitary adenoma (37 eyes in 27 patients), craniopharyngioma (12 eyes in eight patients), meningioma (four eyes in four patients), Rathke's cleft cyst (two eyes in one patient), and hypophysitis (two eyes in one patient). The interval between surgery and OCTA examination was 2.1 ± 1.4 years (range, 0.5–5.3).
Table 1.
Characteristics of Patients With CON and NTG
| CON | NTG | P * | |
|---|---|---|---|
| Eyes, n | 56 | 71 | N/A |
| Age (y), mean ± SD | 52.7 ± 11.5 | 53.2 ± 8.4 | 0.827 |
| Gender (male/female), n | 31/25 | 32/38 | 0.372 |
| SE (D), mean ± SD | −1.49 ± 2.45 | −2.38 ± 2.19 | 0.177 |
| IOP at examination (mmHg), mean ± SD | 16.0 ± 5.3 | 14.0 ± 2.9 | 0.013 |
| MD (dB), mean ± SD | −4.60 ± 5.13 | −6.78 ± 4.81 | 0.063 |
| PSD (dB), mean ± SD | 6.11 ± 5.25 | 10.83 ± 5.04 | <0.001 |
| Hemifield involvement, n (%) | N/A | Inferior, 43 (61.4) | N/A |
| Superior, 4 (5.7) | |||
| Both, 23 (32.9) | |||
| OCT, Mean ± SD | |||
| Average peripapillary RNFL thickness (µm) | 77.4 ± 9.4 | 74.5 ± 8.3 | 0.092 |
| Superior | 97.6 ± 15.1 | 97.3 ± 17.9 | 0.919 |
| Temporal | 51.8 ± 10.1 | 62.1 ± 10.0 | 0.001 |
| Inferior | 101.8 ± 16.6 | 72.5 ± 14.6 | <0.001 |
| Nasal | 59.0 ± 8.5 | 64.4 ± 9.1 | 0.018 |
| Average GCIPL thickness (µm) | 67.0 ± 5.5 | 68.0 ± 4.6 | 0.359 |
| Minimum GCIPL thickness (µm) | 55.6 ± 8.9 | 51.6 ± 4.8 | 0.015 |
| Sectoral GCIPL thickness (µm) | |||
| Superior | 64.8 ± 7.0 | 76.6 ± 8.7 | <0.001 |
| Superotemporal | 76.3 ± 7.6 | 73.2 ± 7.2 | 0.032 |
| Inferotemporal | 79.6 ± 8.0 | 55.3 ± 6.8 | <0.001 |
| Inferior | 66.8 ± 6.7 | 56.0 ± 6.0 | <0.001 |
| Inferonasal | 55.7 ± 8.0 | 67.2 ± 7.7 | <0.001 |
| Superonasal | 57.8 ± 8.7 | 79.9 ± 8.1 | <0.001 |
| OCTA, Mean ± SD | |||
| Average peripapillary VD (%) | 51.6 ± 4.6 | 55.1 ± 3.8 | <0.001 |
| Superior | 58.9 ± 8.1 | 62.5 ± 6.5 | 0.012 |
| Temporal | 38.7 ± 5.7 | 52.7 ± 8.1 | <0.001 |
| Inferior | 65.6 ± 8.5 | 51.3 ± 9.7 | <0.001 |
| Nasal | 43.4 ± 6.4 | 53.8 ± 5.5 | <0.001 |
| Average macular VD (%) | 46.5 ± 2.6 | 47.3 ± 2.8 | 0.233 |
| Superior | 47.4 ± 2.9 | 49.4 ± 4.9 | 0.174 |
| Temporal | 46.9 ± 2.8 | 46.4 ± 3.5 | 0.341 |
| Inferior | 47.3 ± 3.5 | 45.6 ± 4.6 | 0.032 |
| Nasal | 44.4 ± 3.8 | 47.7 ± 6.1 | 0.147 |
CON, compressive optic neuropathy; NTG, normal-tension glaucoma; N/A, not applicable; SE, spherical equivalent; IOP, intraocular pressure; MD, mean deviation; PSD, pattern standard deviation; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer; GCIPL, ganglion cell inner plexiform layer; OCTA, optical coherence tomography angiography; VD, vessel density. Bold text denotes statistical significance.
P values were calculated using a generalized estimation equation (GEE).
Peripapillary and Macular Vessel Density
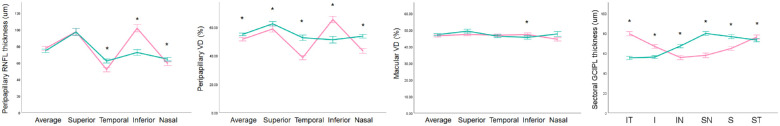
The CON group showed a significantly lower average peripapillary VD than the NTG group despite a similar GCIPL thickness, as well as average pRNFL thickness (Table 1). However, there were significant differences in the sectoral distribution of GCIPL thicknesses, as expected.
The difference in average peripapillary VD was significant after adjusting for age, spherical equivalent, minimum GCIPL thickness, pRNFL thickness, and MD, as well as image quality scores (odds ratio [OR] = 0.649; 95% confidence interval [CI], 0.541–0.779; P < 0.001), as scan quality can significantly influence VD measurements on OCTA.12–14 Peripapillary VD differed in all four sectors and was particularly different in the nasal and temporal sectors (Fig. 1). In contrast, the macular VD thickness did not differ between the two groups, as expected, except in the inferior sector. Minimum GCIPL thickness was lower in the NTG group than in the CON group.
Figure 1.
Distribution of VD and RNFL thickness in CON and NTG. Average peripapillary RNFL thickness was similar between the CON and NTG groups. In contrast, peripapillary VD differed in all four sectors, especially in the nasal and temporal sectors. On the other hand, the macular VD and average GCIPL thicknesses did not differ between the two groups, except in the inferior sector. The sectoral distributions of GCIPL thicknesses were significantly different, as expected The asterisks indicate statistical significance. Error bars indicate 95% confidence intervals. Note that the peripapillary VD measurements reveal a more prominent and obvious intergroup difference compared to OCT-based RNFL thickness measurements. I, inferior; IN, inferonasal; IT, inferotemporal; S, superior; SN, superonasal; ST, superotemporal.
In addition, between CON eyes with clock-hour involvement ≤6 hours and NTG eyes (all ≤6 hours), the difference in average peripapillary VD was also significant after adjusting for age, spherical equivalent, minimum GCIPL thickness, pRNFL thickness, MD, and image quality score (OR = 0.611; 95% CI, 0.485–0.769; P < 0.001). Also, between eyes with clock-hour involvement ≤3 hours, the difference in average peripapillary VD was significant after adjusting for age, spherical equivalent, minimum GCIPL thickness, pRNFL thickness, MD, and image quality score (OR = 0.655; 95% CI, 0.487–0.881; P = 0.005). The results between eyes with clock-hour involvement >3 hours could not be calculated because of the small number of eyes. Different characteristics between CON and NTG subgroups by clock-hour involvement are shown in Supplementary Tables S1 and S2. None of the patients in our study had optic discs that were extremely large or small in size such that the ETDRS inner circle seemed inappropriate to provide reliable measurements for VD calculation.
Structure–Function and Vasculature–Function Correlations
The correlation coefficients between OCT thickness, OCTA VD, and VF sensitivity are shown in Table 2. In the CON group, the average pRNFL and GCIPL thicknesses, average peripapillary VD, and average macular VD were significantly associated with VF MD. In the NTG group, only the average peripapillary and macular VDs were significantly associated with VF MD. The correlation between average macular GCIPL thickness and MD in the two groups was statistically significant (P = 0.012). In the CON group, the average pRNFL and GCIPL thicknesses were significantly associated with VF pattern standard deviation (PSD), whereas in the NTG group only the average pRNFL was significantly associated with VF PSD. Figure 2 shows representative cases.
Table 2.
Structure–Function and Vasculature–Function Correlations
| Average Peripapillary RNFL Thickness | Average Macular GCIPL Thickness | Average Peripapillary VD | Average Macular VD | |
|---|---|---|---|---|
| MD (1/Lambert) | ||||
| CON | 0.419 (0.001) | 0.488 (<0.001) | 0.349 (0.008) | 0.295 (0.028) |
| NTG | 0.211 (0.079) | 0.118 (0.332) | 0.243 (0.043) | 0.254 (0.034) |
| P* | 0.102 | 0.012 | 0.263 | 0.404 |
| PSD (1/Lambert) | ||||
| CON | −0.357 (0.007) | −0.297 (0.026) | −0.220 (0.104) | −0.234 (0.082) |
| NTG | −0.267 (0.025) | −0.095 (0.436) | −0.185 (0.125) | −0.021 (0.863) |
| P* | 0.293 | 0.125 | 0.421 | 0.118 |
Values are presented as Pearson's correlation coefficient (p-value). MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; VD, vessel density; CON, compressive optic neuropathy; NTG, normal-tension glaucoma. Bold text denotes statistical significance.
P values were calculated using Steiger's Z test between the CON and NTG groups.
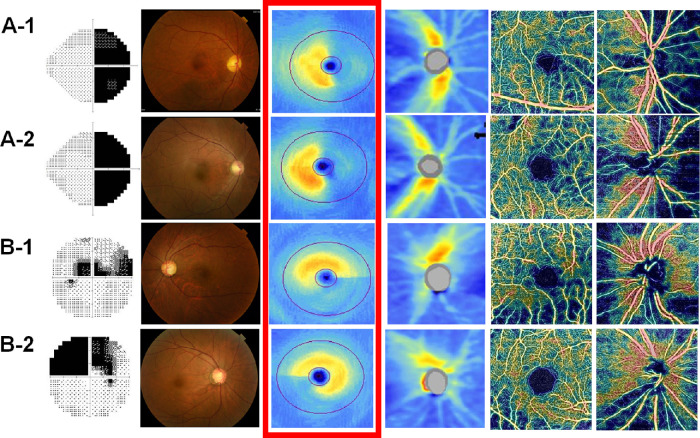
Figure 2.
Representative cases of CON and NTG. (A-1) A 60-year-old female patient 3.4 years after undergoing surgery for craniopharyngioma. The average GCIPL thickness and peripapillary RNFL thickness and the average peripapillary VD and macular VD were 64 µm, 69 µm, 51.5%, and 44.8%, respectively. (A-2) A 48-year-old male patient 3.2 years after surgery for a pituitary adenoma. The average GCIPL thickness and peripapillary RNFL thickness and the average peripapillary VD and macular VD were 64 µm, 64 µm, 49.7%, and 44.1%, respectively. (B-1) A 56-year-old female patient with NTG. The average GCIPL thickness and peripapillary RNFL thickness and the average peripapillary VD and macular VD were 64 µm, 68 µm, 56.6%, and 44.4%, respectively. (B-2) A 46-year-old female patient with NTG. The average GCIPL thickness and peripapillary RNFL thickness and the average peripapillary VD and macular VD were 64 µm, 65 µm, 56.4%, and 50.7%, respectively. The images are presented as automated VF test grayscale maps, color fundus photographs, macular GCIPL thickness maps, peripapillary RNFL thickness maps, macular en face superficial retinal capillary plexus VD maps, and peripapillary en face radial peripapillary capillary VD maps from left to right.
Discussion
OCTA is a novel imaging technique that demonstrates microvascular changes in various optic neuropathies, such as glaucoma and ischemic and inflammatory optic neuropathies.1,15–18 OCTA has shown a high correlation with OCT RNFL thickness measurements19,20 and even better sensitivity.4 However, more information is needed to better understand OCTA in comparison to OCT, in terms of clinical utility regarding the mechanistic differences, applied in different diseases.
In this cross-sectional study, we observed that VD measured in the peripapillary area was significantly lower in the CON group than in the NTG group, under similar average thickness measurements of peripapillary and macular areas. Our result is the first to compare VD between CON and NTG under a highly matched severity of damage by average thickness and highlights different characteristics between OCTA VD and OCT RNFL thickness measurements in the evaluation of optic neuropathies.
Decreased VD has been reported in the peripapillary and macular areas in both CON and glaucoma, seperately.3,15,17,18,21 However, few studies have directly compared OCTA VD between eyes with CON and those with glaucoma. One study compared patients with CON and those with open-angle glaucoma who had similar average pRNFL thickness.2 They reported that peripapillary retinal VD was significantly higher globally in CON (37.34% ± 3.38%) than in open-angle glaucoma (35.91% ± 3.27%) and normal controls (40.24% ± 2.62%),2 a finding that was contrary to the findings of the present study on NTG. However, the previous study used en face slabs to measure the inner retinal layer, extending from the internal limiting membrane to 130 µm below it,2 which differs from the technique used in our study. In addition, the average pRNFL thickness was similar in both groups (76.6 ± 19.8 µm in CON vs. 74.3 ± 10.2 µm in glaucoma); however, the VF MD was significantly worse in the CON group than in the glaucoma group (–7.40 ± 8.52 dB vs. –3.26 ± 2.42 dB), and it is unclear whether the severity of damage was highly matched between the two groups.2 Lei et al.22 reported comparable peripapillary VD and lower superficial macular VD in the glaucoma group than in the CON group. However, they used different devices and different scan areas and included only 12 eyes of patients with CON and 15 eyes of patients with glaucoma. Moreover, if macula damage was controlled, the peripapillary VD would have been greater in the glaucoma group, as per our results. In addition, we studied NTG rather than open-angle glaucoma because differential diagnoses can be particularly challenging under normal IOP; no other study, to the best of our knowledge, has compared CON and NTG. As both of the two studies included patients with high-IOP primary open-angle glaucoma, the different patient group characteristics may also explain the different results from our study.
In our study, the mean degree of macular GCIPL severity was highly controlled in the two groups. The CON group consisted of eyes with complete or near-complete vertical half-loss of the macular GCIPL by the area. The glaucoma group consisted of eyes with complete or near-complete horizontal half-loss of the macular GCIPL. In addition, only eyes with chronic CON were included in our study (on average, 2.1 years) because delayed RNFL thinning is possible up to 1 year postoperatively.8 This comparative design is new. As there was no reason in the design to diminish the validity of our results, we speculated that such design led to successful revealing of subtle difference in peripapillary VD indicative of the high sensitivity of OCTA.
The potential utility of OCTA in addition to OCT arises from several possibilities. First, the floor effect in OCT is considered a candidate. It is caused by the thickness of remaining non-axonal components, such as vasculature and gliosis,23 not allowing OCT thickness under 30 µm even with severe axonal degeneration.24 In contrast, this floor effect is absent in OCTA measurements.25 Second, topographic difference in detection sensitivity is also possible, regarding the physiologic thickness and structure of the RNFL. Specifically, the intergroup difference was most marked in the nasal sector in our study. The nasal sector is physiologically thinner than other sectors, a condition known to be difficult to detect noticeable thinning with OCT.26,27
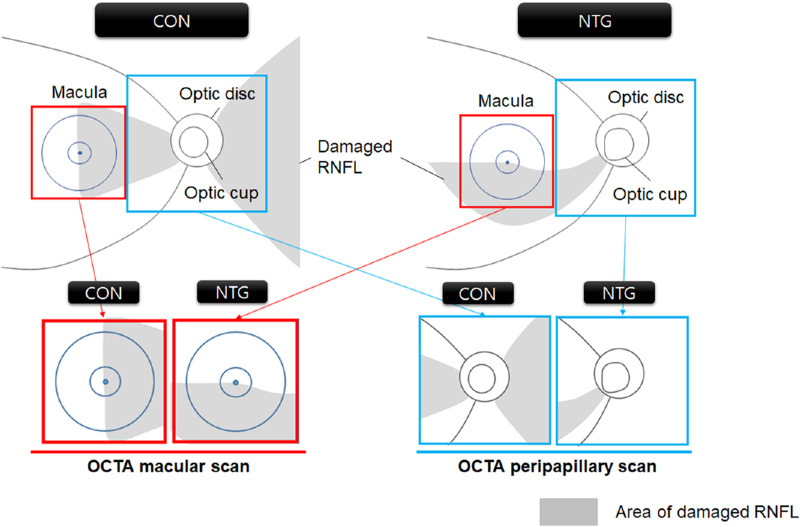
In line with these characteristics, the marked dropout of the microvasculature was easily recognizable in the nasal sector (Fig. 2). In other words, the OCTA en face map provided an intuitive understanding of the different topographic patterns between CON and glaucoma (Fig. 3).
Figure 3.
Different distribution of RNFL damage in CON and NTG detected by macular and peripapillary OCTA scans. The OCTA macula scans detected a 3 × 3 mm2 area centered on the macula (red boxes), and the peripapillary scans detected a 4.5 × 4.5 mm2 area centered on the optic disc (blue boxes). The proportional area of the damaged RNFL (gray shadows) is distributed differently in the macular (red boxes) and peripapillary areas (blue boxes), similarly in the macular area, and markedly differently in the peripapillary area. Note that the diagram, albeit not encompassing all cases, reflects the general conditions.
Several other factors may also contribute to the different outcomes of OCT and OCTA. The VD was calculated as the percentage area of the pixels with binarized signals. In contrast, RNFL thickness measured by OCT is continuous and exhibits interindividual variations; therefore, VD may be less affected by interindividual variations in RNFL thickness, the reflectance of the RNFL, or optic disc tilt. This technique may enable an amplification effect focused on abnormality, which allows for sensitive detection of subtle changes that can be missed due to inherent technical limitations of OCT.28–31 In addition, different “dynamic ranges” between neural structural changes and vessel density changes are also possible, as suggested by Shoji et al.17 Finally, the mechanism of VD loss may be diverse.2,19 Reduced metabolic demand due to axonal atrophy is one possible mechanism.20,32 However, differences between the pathogenesis of the focal VD dropout in the peripapillary choroid in cases of CON and glaucoma have been suggested.2 Further investigations are warranted to better understand the detailed mechanisms.
Nevertheless, several limitations must be acknowledged, particularly those stemming from the potential weaknesses inherent in the use of OCTA to calculate VD data. One limitation may be that the results from OCTA may only replicate those from OCT and may be more susceptible to bias. For example, topographical differences in RNFL thickness between the CON and NTG groups have already been demonstrated using OCT.33 In addition, the long-term reproducibility of OCTA is less than that of OCT-measured thickness,34 and OCTA may show image artifacts.9,35,36 Moreover, technical issues exist in the calculation of VD, such as the exclusion of large vessels. Nevertheless, we believe that OCTA still has several merits for the following reasons. First, OCTA VD findings corroborated the pattern of differences observed between the CON and NTG in OCT while augmenting the differences more markedly, as shown in Figure 1. Second, the more pronounced presentation was not a transient artifact but rather a representation of true pathologic changes, as we not only analyzed the numerical data but also confirmed the correlation to fundus photographs, red-free photographs, VF, and OCT. Third, OCTA has the potential to provide valuable insights in locations where OCT may be limited. For example, the physiologically thin nasal sector, in which OCT could not detect a significant deviation from the normal population, showed decreased vascularity in many eyes in the CON group. Finally, it was recently shown that OCTA-measured macular and ONH vascular parameters have good long-term reproducibility.34 Therefore, we consider that VD may facilitate easier recognition of pathological changes in certain circumstances and could be a helpful ancillary parameter for understanding the different characteristics of microvascular responses in different optic neuropathies through different devices. Similarly, Lee et al.21 have also postulated that the intraretinal vascular changes might be amplified, resulting in increased sensitivity of detection compared to that of pRNFL thickness.
The main purpose of this study was not to identify an OCTA-based parameter for the differential diagnosis between CON and NTG but rather to understand the differential microvascular responses between the diseases and the varying outcomes reported between the devices. Therefore, the results of our study would not indicate that the VD parameter in OCTA may serve as a discriminator between CON and NTG. Instead, the results would illustrate the potential for sensitive detection of abnormalities in areas where conventional OCT may demonstrate limited performance. Nevertheless, considering the potential benefits of OCTA, we believe that further research aimed at differentiating between patients with early-stage CON, including those in the acute phase, and patients with glaucoma would also be immensely valuable.
The peripapillary RNFL (ppRNFL) thickness tended to be thinner in the CON group than in the NTG group in our study. The statistically insignificant difference in average RNFL thickness, despite the significant difference in VD between the two diseases, may be attributed to the difference in the OCT device. Nevertheless, the current OCT ppRNFL thickness measurements successfully revealed topographic inter-group differences as well as highly indifferent macular GCIPL thicknesses, which are inevitably strongly correlated with ppRNFL thickness. Moreover, the VD difference between the groups was much more pronounced than those in the OCT measurements, which suggests that the disparity in OCT devices alone may not fully explain the observed results. Thus, we speculate that the milder difference in ppRNFL thickness compared to peripapillary vessel density (ppVD) may persist regardless of the OCT device used.
During the examination, IOP was lower in the NTG group than in the CON group, which may be attributed to the use of IOP-lowering medications in patients with NTG. However, the effect of the IOP on VD outcomes is unclear. Although Chen et al.37 reported that, in patients with ocular hypertension, a reduction in IOP by 6.5 mmHg could led to an 1.2% increase in peripapillary VD, the IOP difference was smaller (2.0 mmHg) in our study and the VD difference was larger (3.5%). The multivariate analysis also did not reveal a significant effect of IOP.
In addition, our study supports the stronger correlation of IOP with VF4,17,38–44 shown across different optic neuropathies. We suggest that differences in MD, PSD, and minimum GCIPL thickness indicate distinct patterns of RNFL damage between the CON and NTG groups. CON appears to involve a wider area of the nasal half of the RGC, whereas glaucoma is characterized by focal RGC loss exclusively around the macula. As expected, eyes with NTG demonstrated deeper VF loss, higher PSD, and lower minimum GCIPL thickness. Because the worst MD was in the NTG group than in the CON group, we speculated that the dramatic VF recovery following surgical decompression in the CON group could be related.
We would like to address that the pattern of RNFL loss was the most obvious difference between the two diseases and should be primarily considered when distinguishing between CON and NTG. In CON, the optic atrophy occupies a horizontal band across the disk, is generally wider nasally than temporally, and is known as band atrophy or bowtie atrophy in chiasmal compression.6 In contrast, vertical elongation of the cup with thinning of the neuroretinal rim (NRR) and RNFL in the superior and inferior poles is characteristic of glaucoma. This difference has also been reported in several studies of ischemic optic neuropathy.45–47 Our results also corroborate this different RNFL loss pattern. At the same time, the different involvement patterns and degrees of RNFL loss may also affect comparisons between diseases. Therefore, for the differential diagnosis between CON and NTG, we suggest first examining the topographic pattern of RNFL loss using all available modalities, including VF tests, photographs, and OCT. In addition, OCTA may provide useful additional data for visualizing local abnormalities, especially when analyzed as a whole en face image, which could be intuitively correlated with other tests in addition to the numerical results of the VD calculation.
Our study has several limitations. First, the sample size was small. Nevertheless, it was challenging to recruit CON patients with a chronic follow-up status and glaucoma patients who had damage approaching the macular. Moreover, the results obtained from highly controlled comparisons may be more valuable than those obtained from uncontrolled studies involving a larger number of patients. Second, we used an arbitrary cut-off for the extent of damage in the macular GCIPL thickness maps. However, we also compared the average thickness, and the distinct halved pattern of vertical or horizontal loss would be sufficient for the purpose of our study. Third, the OCTA scan area was limited, resulting in restricted information. Although we used 3 × 3 mm2 scans, larger 6 × 6 mm2 or 9 × 9 mm2 scans are available, which could potentially provide better insight into the changes. Nevertheless, as the current OCTA successfully demonstrated marked nasal VD loss in the CON group, the scan size may not impair the validity of the key findings.
In conclusion, we observed a significantly lower peripapillary VD in patients in the CON group than in those in the NTG group using OCTA, under similar mean OCT thicknesses of pRNFL and macular GCIPL. These findings illustrate the potential utility of OCTA that may successfully complement OCT in relation to different topographic patterns of optic neuropathy and may enhance our understanding of the possible mechanisms of microvascular damage in different optic neuropathies.
Supplementary Material
Acknowledgments
Disclosure: E.J. Lee, None; J.C. Han, None; C. Kee, None; K.-A. Park, None; D.-S. Kong, None; S.D. Hong, None
References
- 1. Pujari A, Bhaskaran K, Sharma P, et al.. Optical coherence tomography angiography in neuro-ophthalmology: current clinical role and future perspectives. Surv Ophthalmol . 2021; 66(3): 471–481. [DOI] [PubMed] [Google Scholar]
- 2. Lee EJ, Kim JA, Kim TW, Kim H, Yang HK, Hwang JM.. Glaucoma-like parapapillary choroidal microvasculature dropout in patients with compressive optic neuropathy. Ophthalmology . 2020; 127(12): 1652–1662. [DOI] [PubMed] [Google Scholar]
- 3. Lee GI, Park KA, Oh SY, Kong DS.. Changes in parafoveal and peripapillary perfusion after decompression surgery in chiasmal compression due to pituitary tumors. Sci Rep . 2021; 11(1): 3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hou H, Moghimi S, Proudfoot JA, et al.. Ganglion cell complex thickness and macular vessel density loss in primary open-angle glaucoma. Ophthalmology . 2020; 127(8): 1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee EJ, Kim S, Hwang S, Han JC, Kee C.. Microvascular compromise develops following nerve fiber layer damage in normal-tension glaucoma without choroidal vasculature involvement. J Glaucoma . 2017; 26(3): 216–222. [DOI] [PubMed] [Google Scholar]
- 6. Danesh-Meyer HV, Yoon JJ, Lawlor M, Savino PJ.. Visual loss and recovery in chiasmal compression. Prog Retin Eye Res . 2019; 73: 100765. [DOI] [PubMed] [Google Scholar]
- 7. Lee EJ, Yang HK, Kim TW, Hwang JM, Kim YH, Kim CY.. Comparison of the pattern of retinal ganglion cell damage between patients with compressive and glaucomatous optic neuropathies. Invest Ophthalmol Vis Sci . 2015; 56(12): 7012–7020. [DOI] [PubMed] [Google Scholar]
- 8. Lee GI, Son KY, Park KA, Kong DS, Oh SY.. Longitudinal changes in the retinal microstructures of eyes with chiasmal compression. Neurology . 2021; 96(1): e131–e140. [DOI] [PubMed] [Google Scholar]
- 9. Holmen IC, Konda SM, Pak JW, et al.. Prevalence and severity of artifacts in optical coherence tomographic angiograms. JAMA Ophthalmol . 2020; 138(2): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghasemi Falavarjani K, Al-Sheikh M, Akil H, Sadda SR. Image artefacts in swept-source optical coherence tomography angiography. Br J Ophthalmol . 2017; 101(5): 564–568. [DOI] [PubMed] [Google Scholar]
- 11. Hood DC, Kardon RH.. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res . 2007; 26(6): 688–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rao HL, Pradhan ZS, Weinreb RN, et al.. Determinants of peripapillary and macular vessel densities measured by optical coherence tomography angiography in normal eyes. J Glaucoma . 2017; 26(5): 491–497. [DOI] [PubMed] [Google Scholar]
- 13. Yu JJ, Camino A, Liu L, et al.. Signal strength reduction effects in OCT angiography. Ophthalmol Retina . 2019; 3(10): 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. You QS, Tan O, Pi S, et al.. Effect of algorithms and covariates in glaucoma diagnosis with optical coherence tomography angiography. Br J Ophthalmol . 2022; 106(12): 1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou H, Moghimi S, Zangwill LM, et al.. Macula vessel density and thickness in early primary open-angle glaucoma. Am J Ophthalmol . 2019; 199: 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rao HL, Pradhan ZS, Weinreb RN, et al.. Regional comparisons of optical coherence tomography angiography vessel density in primary open-angle glaucoma. Am J Ophthalmol . 2016; 171: 75–83. [DOI] [PubMed] [Google Scholar]
- 17. Shoji T, Zangwill LM, Akagi T, et al.. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol . 2017; 182: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yarmohammadi A, Zangwill LM, Manalastas PIC, et al.. Peripapillary and macular vessel density in patients with primary open-angle glaucoma and unilateral visual field loss. Ophthalmology . 2018; 125(4): 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee GI, Park KA, Oh SY, Kong DS.. Analysis of optic chiasmal compression caused by brain tumors using optical coherence tomography angiography. Sci Rep . 2020; 10(1): 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghasemi Falavarjani K, Tian JJ, Akil H, Garcia GA, Sadda SR, Sadun AA. Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina . 2016; 36(suppl 1): S168–S177. [DOI] [PubMed] [Google Scholar]
- 21. Lee GI, Park KA, Oh SY, Kong DS.. Parafoveal and peripapillary perfusion predict visual field recovery in chiasmal compression due to pituitary tumors. J Clin Med . 2020; 9(3): 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lei K, Qu Y, Tang Y, et al.. Discriminating between compressive optic neuropathy with glaucoma-like cupping and glaucomatous optic neuropathy using OCT and OCTA. Transl Vis Sci Technol . 2023; 12(3): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hood DC, Anderson SC, Wall M, Kardon RH.. Structure versus function in glaucoma: an application of a linear model. Invest Ophthalmol Vis Sci . 2007; 48(8): 3662–3668. [DOI] [PubMed] [Google Scholar]
- 24. Asrani S, Essaid L, Alder BD, Santiago-Turla C.. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol . 2014; 132(4): 396–402. [DOI] [PubMed] [Google Scholar]
- 25. Moghimi S, Bowd C, Zangwill LM, et al.. Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology . 2019; 126(7): 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bayer A, Akman A.. Artifacts and anatomic variations in optical coherence tomography. Turk J Ophthalmol . 2020; 50(2): 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen JJ, Kardon RH.. Avoiding clinical misinterpretation and artifacts of optical coherence tomography analysis of the optic nerve, retinal nerve fiber layer, and ganglion cell layer. J Neuroophthalmol . 2016; 36(4): 417–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soliman MA, Van Den Berg TJ, Ismaeil AA, De Jong LA, De Smet MD. Retinal nerve fiber layer analysis: relationship between optical coherence tomography and red-free photography. Am J Ophthalmol . 2002; 133(2): 187–195. [DOI] [PubMed] [Google Scholar]
- 29. Kim TW, Park UC, Park KH, Kim DM.. Ability of Stratus OCT to identify localized retinal nerve fiber layer defects in patients with normal standard automated perimetry results. Invest Ophthalmol Vis Sci . 2007; 48(4): 1635–1641. [DOI] [PubMed] [Google Scholar]
- 30. Teesalu P, Tuulonen A, Airaksinen PJ.. Optical coherence tomography and localized defects of the retinal nerve fiber layer. Acta Ophthalmol Scand . 2000; 78(1): 49–52. [DOI] [PubMed] [Google Scholar]
- 31. Hood DC, Fortune B, Mavrommatis MA, et al.. Details of glaucomatous damage are better seen on OCT en face images than on OCT retinal nerve fiber layer thickness maps. Invest Ophthalmol Vis Sci . 2015; 56(11): 6208–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feucht N, Maier M, Lepennetier G, et al.. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult Scler . 2019; 25(2): 224–234. [DOI] [PubMed] [Google Scholar]
- 33. Danesh-Meyer HV, Yap J, Frampton C, Savino PJ.. Differentiation of compressive from glaucomatous optic neuropathy with spectral-domain optical coherence tomography. Ophthalmology . 2014; 121(8): 1516–1523. [DOI] [PubMed] [Google Scholar]
- 34. Nishida T, Moghimi S, Hou H, et al.. Long-term reproducibility of optical coherence tomography angiography in healthy and stable glaucomatous eyes. Br J Ophthalmol . 2023; 107(5): 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Enders C, Lang GE, Dreyhaupt J, Loidl M, Lang GK, Werner JU.. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS One . 2019; 14(1): e0210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spaide RF, Fujimoto JG, Waheed NK.. Image artifacts in optical coherence tomography angiography. Retina . 2015; 35(11): 2163–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen X, Hong Y, Di H, Wu Q, Zhang D, Zhang C.. Change of retinal vessel density after lowering intraocular pressure in ocular hypertension. Front Med (Lausanne) . 2021; 8: 730327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bowd C, Zangwill LM, Weinreb RN, Medeiros FA, Belghith A.. Estimating optical coherence tomography structural measurement floors to improve detection of progression in advanced glaucoma. Am J Ophthalmol . 2017; 175: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mwanza JC, Kim HY, Budenz DL, et al.. Residual and dynamic range of retinal nerve fiber layer thickness in glaucoma: comparison of three OCT Platforms. Invest Ophthalmol Vis Sci . 2015; 56(11): 6344–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin YH, Huang SM, Yeung L, et al.. Correlation of visual field with peripapillary vessel density through optical coherence tomography angiography in normal-tension glaucoma. Transl Vis Sci Technol . 2020; 9(13): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al.. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology . 2016; 123(12): 2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen HS, Liu CH, Wu WC, Tseng HJ, Lee YS.. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci . 2017; 58(9): 3637–3645. [DOI] [PubMed] [Google Scholar]
- 43. Shin JW, Lee J, Kwon J, Choi J, Kook MS.. Regional vascular density-visual field sensitivity relationship in glaucoma according to disease severity. Br J Ophthalmol . 2017; 101(12): 1666–1672. [DOI] [PubMed] [Google Scholar]
- 44. Jia Y, Wei E, Wang X, et al.. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology . 2014; 121(7): 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fard MA, Suwan Y, Moghimi S, et al.. Pattern of peripapillary capillary density loss in ischemic optic neuropathy compared to that in primary open-angle glaucoma. PLoS One . 2018; 13(1): e0189237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fard MA, Fakhraee G, Ghahvechian H, Sahraian A, Moghimi S, Ritch R. Macular vascularity in ischemic optic neuropathy compared to glaucoma by projection-resolved optical coherence tomography angiography. Am J Ophthalmol . 2020; 209: 27–34. [DOI] [PubMed] [Google Scholar]
- 47. Fard MA, Afzali M, Abdi P, Yasseri M, Ebrahimi KB, Moghimi S.. Comparison of the pattern of macular ganglion cell-inner plexiform layer defect between ischemic optic neuropathy and open-angle glaucoma. Invest Ophthalmol Vis Sci . 2016; 57(3): 1011–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.