Abstract
Aims
Dapagliflozin reduced the combined risk of worsening heart failure or cardiovascular death among patients with heart failure with mildly reduced or preserved ejection fraction. In this study, the safety and efficacy of dapagliflozin according to background diuretic therapy and the influence of dapagliflozin on longitudinal diuretic use were evaluated.
Methods and results
In this pre-specified analysis of the Dapagliflozin Evaluation to Improve the LIVEs of Patients With Preserved Ejection Fraction Heart Failure (DELIVER) trial, the effects of dapagliflozin vs. placebo were assessed in the following subgroups: no diuretic, non-loop diuretic, and loop diuretic furosemide equivalent doses of <40, 40, and >40 mg, respectively. Of the 6263 randomized patients, 683 (10.9%) were on no diuretic, 769 (12.3%) were on a non-loop diuretic, and 4811 (76.8%) were on a loop diuretic at baseline. Treatment benefits of dapagliflozin on the primary composite outcome were consistent by diuretic use categories (P interaction = 0.64) or loop diuretic dose (P interaction = 0.57). Serious adverse events were similar between dapagliflozin and placebo arms, irrespective of diuretic use or dosing. Dapagliflozin reduced new initiation of loop diuretics by 32% [hazard ratio (HR) 0.68; 95% confidence interval (CI): 0.55–0.84, P < 0.001] but did not influence discontinuations/disruptions (HR 0.98; 95% CI: 0.86–1.13, P = 0.83) in follow-up. First sustained loop diuretic dose increases were less frequent, and sustained dose decreases were more frequent in patients treated with dapagliflozin: net difference of −6.5% (95% CI: −9.4 to −3.6; P < 0.001). The mean dose of loop diuretic increased over time in the placebo arm, a longitudinal increase that was significantly attenuated with treatment with dapagliflozin (placebo-corrected treatment effect of −2.5 mg/year; 95% CI: −1.5, −3.7, P < 0.001).
Conclusion
In patients with heart failure with mildly reduced or preserved ejection fraction, the clinical benefits of dapagliflozin relative to placebo were consistent across a wide range of diuretic categories and doses with a similar safety profile. Treatment with dapagliflozin significantly reduced new loop diuretic requirement over time.
Keywords: heart failure with mildly reduced ejection fraction, heart failure with preserved ejection fraction, diuretics, SGLT2i
Structured Graphical Abstract
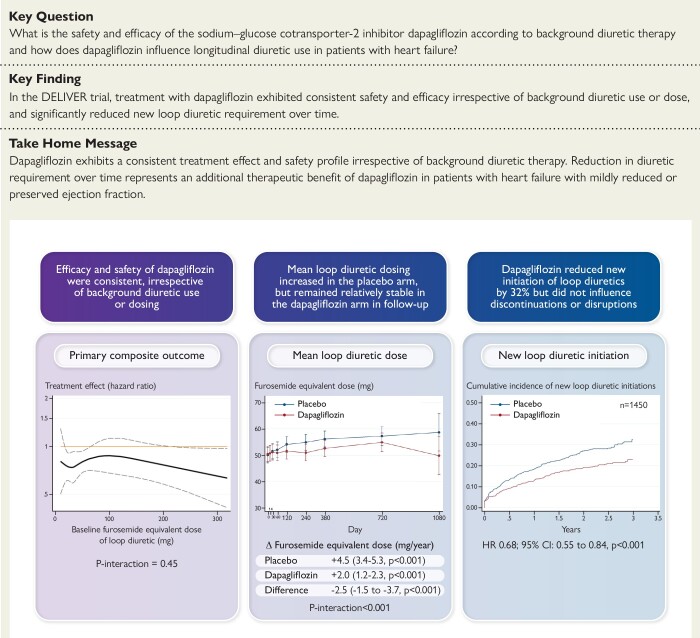
Structured Graphical Abstract.
The clinical benefits of dapagliflozin vs. placebo exhibit consistent safety and efficacy across a wide range of background diuretic use. Treatment with dapagliflozin reduces loop diuretic requirement over time. CI, confidence interval; HR, hazard ratio.
See the editorial comment for this article ‘SGLT2 inhibitors and diuretics in heart failure: clicking reset on the renal volume setpoint?’, by B.A. Borlaug and J.M. Testani, https://doi.org/10.1093/eurheartj/ehad345.
Introduction
Sodium–glucose co-transporter 2 (SGLT2) inhibitors now represent an important therapeutic pillar in the management of patients with chronic heart failure across the spectrum of left ventricular ejection fraction (LVEF),1–3 demonstrating a significant and sustained reduction in cardiovascular death or worsening heart failure events.4,5 Among other effects, SGLT2 inhibitors promote early natriuresis and diuresis, which may contribute to their clinical benefits in heart failure.6 Diuretic therapy is a cornerstone of the management of patients with heart failure,7 and their concomitant use with SGLT2 inhibitors will be a frequent occurrence. Understanding the interplay between SGLT2 inhibitors and conventional diuretics is therefore of great importance.
While loop diuretics are an essential facet of management of congestion in patients with heart failure, prolonged use, especially at higher doses, may result in neurohormonal activation, electrolyte abnormalities, and kidney dysfunction.8 The use of loop diuretics has been associated with worse clinical outcomes in heart failure9 in a dose-dependent manner.10,11 However, whether loop diuretic use is a mediator of risk or is merely a marker of more advanced disease remains uncertain. SGLT2 inhibitors may enhance diuretic efficiency, with a considerably more favourable neurohormonal, electrolyte, and safety profile than conventional diuretics.12 Whether SGLT2 inhibitors may translate to long-term diuretic sparing is thus important to characterize in the context of heart failure with mildly reduced or preserved ejection fraction. In this pre-specified analysis of the Dapagliflozin Evaluation to Improve the LIVEs of Patients With Preserved Ejection Fraction Heart Failure (DELIVER) trial, we assessed the efficacy and safety of dapagliflozin by background diuretic use and dosing and examined the effect of dapagliflozin on post-randomization changes in diuretic use.
Methods
Study design
The DELIVER trial study design and primary results have been previously reported.3 Briefly, DELIVER was a double-blind, randomized controlled trial which evaluated the effect of dapagliflozin 10 mg once daily vs. placebo among ambulatory or hospitalized patients age ≥40 years with symptomatic heart failure (New York Heart Association class II–IV) and at least intermittent diuretic requirement, LVEF >40%, evidence of structural heart disease, and elevated natriuretic peptides. Background diuretic use and dosing according to local standards of care were maintained throughout the study period. Patients were excluded if they received an SGLT2 inhibitor in the 4 weeks prior to randomization, experienced prior intolerance to SGLT2 inhibitor, had a history of Type 1 diabetes, screening estimated glomerular filtration rate (eGFR) <25 mL/min/1.73 m2, or systolic blood pressure <95 mmHg. The study protocol was approved by the ethics committees at each study site and participants provided written informed consent.
Clinical endpoints
The primary endpoint was a composite of cardiovascular death or worsening heart failure event including hospitalization or urgent visits (requiring intravenous heart failure therapies). Secondary endpoints included total (first and recurrent) heart failure events and cardiovascular death, change in Kansas City Cardiomyopathy Questionnaire (KCCQ) total symptom score at 8 months, cardiovascular death, and all-cause death. The following safety outcomes were also evaluated: any serious adverse event, any adverse event leading to drug discontinuation or interruption, and select other adverse events of interest (including lower limb amputation, volume depletion, and renal adverse events).
Ascertainment of diuretic dose information and diuretic categorization
All patients with information on diuretic treatment were included in this analysis. Only records relating oral administration recorded in ‘g’, ‘μg’, ‘mg’ with standard dose frequency were included. Intermittent, as-needed diuretic therapy was not considered. Of the 13 357 diuretic records identified in DELIVER, 3586 records (27%) were excluded leaving 9771 records (73%) for analysis. For all patients on loop diuretic, a total daily furosemide dose equivalent was calculated. Bumetanide 1 mg, torsemide 20 mg, azosemide 60 mg, and ethacrynic acid 100 mg were considered equivalent to 80 mg of oral furosemide.13,14 Information on diuretic start and stop dates for any diuretic type or dose was collected throughout the study in an effort to capture between visit changes. In analyses of post-baseline changes in diuretic use, patients were excluded at time points with missing or insufficient diuretic dose data and included again at the next available time point. In the analysis of mean loop diuretic dose over time, only patients on a non-zero dose of loop diuretic were included. Where patients were on combination diuretic therapy, they were considered part of the group of the most potent diuretic in the combination for the purpose of analysis (i.e. analysed in the ‘loop diuretic’ subgroup when treated with a combination of loop and thiazide diuretic). Patients treated with a mineralocorticoid receptor antagonist (MRA) alone were included in the ‘no diuretic’ category. The non-loop diuretic category most frequently included patients on thiazide and thiazide-like diuretics.
Statistical analysis
Baseline characteristics were compared according to the following categories defining baseline diuretic use: no diuretic, non-loop diuretic, and furosemide dose equivalents of >40, 40, and <40 mg, respectively. Data are reported as mean ± standard deviation, median (interquartile range) for non-normal distributions, and frequency (percentage) for categorical variables. Student’s t-test, Pearson χ2 test, and ANOVA were used where appropriate.
The treatment effect of dapagliflozin on the primary composite outcome, its components, and all-cause death were evaluated across diuretic categories in time-to-first event analyses using Cox regression and Kaplan–Meier curves. Treatment effects on the KCCQ total symptom score were assessed using repeated measures mixed-effect models, adjusted for baseline value, at 1 and 8 months. Total worsening heart failure events and cardiovascular death were analysed using semi-parametric proportional rates methods of Lin et al. Effects on safety outcomes were assessed with Cox regression in an on-treatment analysis. Interaction testing was carried out to evaluate the interaction between treatment and diuretic type as well as loop diuretic dose.
A repeated measures mixed-effect model was employed to assess temporal changes in mean furosemide equivalent dose over trial time among patients on loop diuretics. Treatment, time, and the interaction between assigned treatment and time were included as fixed effects. Cox-proportional hazards models were used to assess the time to new loop diuretic initiation (among baseline loop diuretic non-users) and discontinuation or disruption (among baseline loop diuretic users). A disruption in loop diuretic use was considered as any interruption in diuretic treatment ≥30 days. Results were displayed with Kaplan–Meier curves. The percentage of all patients experiencing a first sustained change (≥30 days) in loop diuretic dose was compared between treatment groups with regression analysis. All analyses were carried out using STATA version 17.0 (Stata Corp., College Station, TX, USA). A P-value of <0.05 was considered to be statistically significant.
Results
Baseline diuretic use in DELIVER
In DELIVER, of 6263 patients, 10.9% (n = 683) were on no diuretic, 12.3% (n = 769) were on a non-loop diuretic, and 76.8% (n = 4811) were on a loop diuretic at the time of randomization (Table 1). Among those on loop diuretics at baseline, 37.7% (n = 1811) were receiving a furosemide equivalent dose of <40 mg, 39.5% (n = 1902) were on a dose equivalent 40 mg, and 22.8% (n = 1098) were on a dose equivalent >40 mg (Table 1).
Table 1.
Baseline characteristics according to diuretic use
| No diuretic (n = 683) |
Non-loop diuretic (n = 769) |
<40 mga,b (n = 1811) |
40 mgb (n = 1902) |
>40 mgb (n = 1098) |
P-valuec | |
|---|---|---|---|---|---|---|
| Randomized to dapagliflozin | 338 (49.5) | 390 (50.7) | 912 (50.4) | 939 (49.4) | 552 (50.3) | 0.96 |
| Mean furosemide equivalent dose (mg) | 17 ± 6 | 40 ± 3 | 116 ± 95 | |||
| Age (years) | 70.4 ± 10.3 | 71.5 ± 9.2 | 71.8 ± 9.5 | 72.2 ± 9.4 | 71.5 ± 9.7 | <0.001 |
| Men | 377 (55.2) | 391 (50.8) | 1061 (58.6) | 1035 (54.4) | 652 (59.4) | <0.001 |
| Race | <0.001 | |||||
| White | 377 (55.2) | 523 (68.0) | 1162 (64.2) | 1450 (76.2) | 927 (84.4) | |
| Asian | 232 (34.0) | 159 (20.7) | 559 (30.9) | 222 (11.7) | 102 (9.3) | |
| Black or African American |
9 (1.3) | 25 (3.3) | 24 (1.3) | 57 (3.0) | 44 (4.0) | |
| Indian or Alaska Native | 39 (5.7) | 24 (3.1) | 48 (2.7) | 69 (3.6) | 9 (0.8) | |
| Other | 26 (3.8) | 38 (4.9) | 18 (1.0) | 104 (5.5) | 16 (1.5) | |
| Region | <0.001 | |||||
| Europe and Saudi Arabia | 249 (36.5) | 353 (45.9) | 864 (47.7) | 929 (48.8) | 610 (55.6) | |
| Asia | 230 (33.7) | 154 (20.0) | 542 (29.9) | 210 (11.0) | 90 (8.2) | |
| Latin America | 154 (22.5) | 214 (27.8) | 165 (9.1) | 519 (27.3) | 129 (11.7) | |
| North America | 50 (7.3) | 48 (6.2) | 240 (13.3) | 244 (12.8) | 269 (24.5) | |
| Medical history | ||||||
| Atrial fibrillation/flutter | 323 (47.3) | 315 (41.0) | 1043 (57.6) | 1121 (58.9) | 750 (68.3) | <0.001 |
| Prior stroke | 61 (8.9) | 61 (7.9) | 180 (9.9) | 178 (9.4) | 117 (10.7) | 0.33 |
| Diabetes Mellitus | 269 (39.4) | 336 (43.7) | 738 (40.8) | 895 (47.1) | 568 (51.7) | <0.001 |
| Prior MI | 218 (31.9) | 274 (25.0) | 218 (31.9) | 511 (28.2) | 465 (24.4) | <0.001 |
| Hypertension | 591 (86.5) | 982 (89.4) | 591 (86.5) | 1543 (85.2) | 1723 (90.6) | <0.001 |
| Prior HFH | 216 (31.6) | 615 (56.0) | 216 (31.6) | 775 (42.8) | 797 (41.9) | <0.001 |
| Body mass index (kg/m2) | 27.9 ± 5.2 | 29.2 ± 5.4 | 28.7 ± 5.8 | 30.5 ± 6.2 | 32.1 ± 6.6 | <0.001 |
| Smoking status | <0.001 | |||||
| Current | 67 (9.8) | 64 (8.3) | 138 (7.6) | 140 (7.4) | 75 (6.8) | |
| Former | 211 (30.9) | 241 (31.3) | 647 (35.7) | 679 (35.7) | 483 (44.0) | |
| Never | 405 (59.3) | 464 (60.3) | 1026 (56.7) | 1083 (56.9) | 540 (49.2) | |
| NYHA class | <0.001 | |||||
| II | 566 (82.9) | 665 (86.5) | 1415 (78.1) | 1385 (72.8) | 682 (62.1) | |
| III | 116 (17.0) | 101 (13.1) | 392 (21.6) | 513 (27.0) | 409 (37.2) | |
| IV | 1 (0.1) | 3 (0.4) | 3 (0.2) | 4 (0.2) | 7 (0.6) | |
| LVEF (%) | 5 ± 9 | 57 ± 9 | 54 ± 9 | 54 ± 9 | 54 ± 9 | <0.001 |
| NT-proBNP (ng/L) | 875 (579–1503] | 790 (497–1252) | 993 (616–1665) | 1084 (646–1942) | 1252 (748–2227) | <0.001 |
| Systolic blood pressure (mmHg) | 127 ± 15 | 132 ± 15 | 128 ± 15 | 128 ± 15 | 126 ± 16 | <0.001 |
| Heart rate (bpm) | 72 ± 12 | 71 ± 11 | 72 ± 12 | 72 ± 12 | 72 ± 12 | 0.14 |
| Creatinine (µmol/L) | 95 ± 27 | 96 ± 29 | 100 ± 28 | 104 ± 32 | 115 ± 34 | <0.001 |
| eGFR (mL/min/1.73 m2) | 66 ± 19 | 65 ± 19 | 63 ± 19 | 60 ± 19 | 55 ± 19 | <0.001 |
| ACEi | 223 (32.7) | 249 (32.4) | 718 (39.6) | 728 (38.3) | 377 (34.3) | <0.001 |
| ARB | 245 (36.0) | 389 (50.6) | 586 (32.4) | 679 (35.7) | 373 (34.0) | <0.001 |
| ARNI | 55 (8.1) | 11 (1.4) | 105 (5.8) | 77 (4.0) | 53 (4.8) | <0.001 |
| Beta blocker | 549 (80.6) | 613 (79.7) | 1508 (83.3) | 1576 (82.9) | 931 (84.8) | 0.031 |
| MRA | 543 (79.7) | 160 (20.8) | 714 (39.4) | 741 (39.0) | 509 (46.4) | <0.001 |
Data are presented as n (%), mean ± SD, or median (interquartile range).
A small number of patients (n = 185) on loop diuretic at baseline with missing or inadequate dose information were included in this group.
Dose in furosemide equivalents.
P-value for the comparison of no-diuretic, non-loop diuretic, and loop diuretic furosemide dose of <40, 40, and >40 mg.
HFH, heart failure hospitalization; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; NT-proBNP, N-terminal prohormone of B-type natriuretic peptide; MRA, mineralocorticoid receptor antagonist; eGFR, estimated glomerular filtration rate; MI, myocardial infarction.
Patient profiles
Patients on the highest furosemide equivalent doses (>40 mg) at baseline were more often men, with a higher burden of comorbidities, higher body mass index, more severe heart failure symptoms, and more frequently had a prior history of heart failure hospitalization. These patients also had lower baseline eGFR and were more likely to be treated with an MRA but less likely to be treated with sacubitril/valsartan (Table 1).
Efficacy and safety of dapagliflozin according to baseline diuretic use
The cumulative incidence of the primary composite outcome and its components as well as all-cause death were observed to be lowest in patients on non-loop diuretic or no diuretic and highest in patients on furosemide equivalent doses >40 mg (all P < 0.001; Supplementary data online, Figure S1). These findings were consistent when total daily furosemide equivalent dose was analysed as a continuous variable with increasing risk observed at higher furosemide equivalent doses for all evaluated outcomes (Supplementary data online, Figure S2).
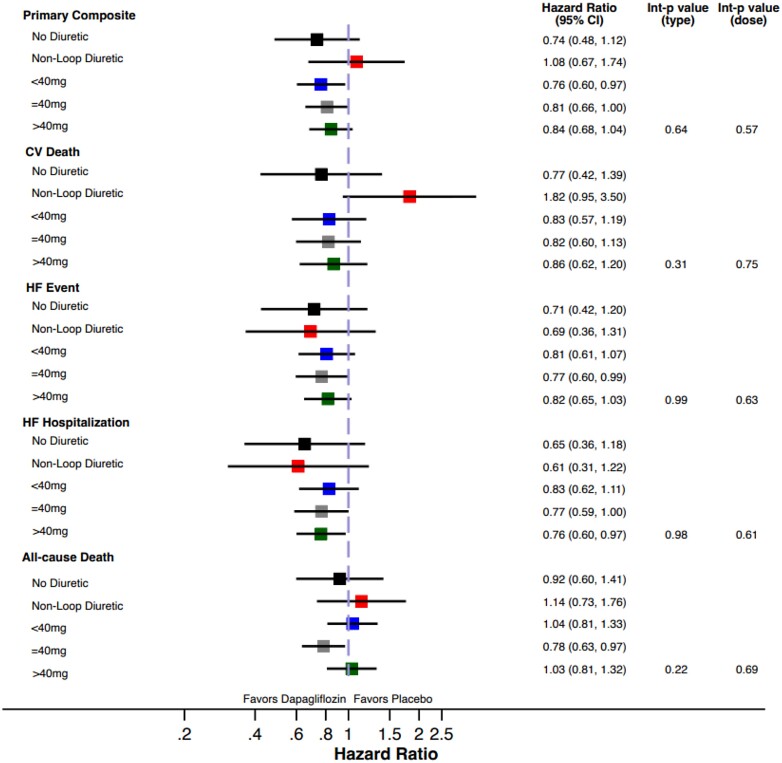
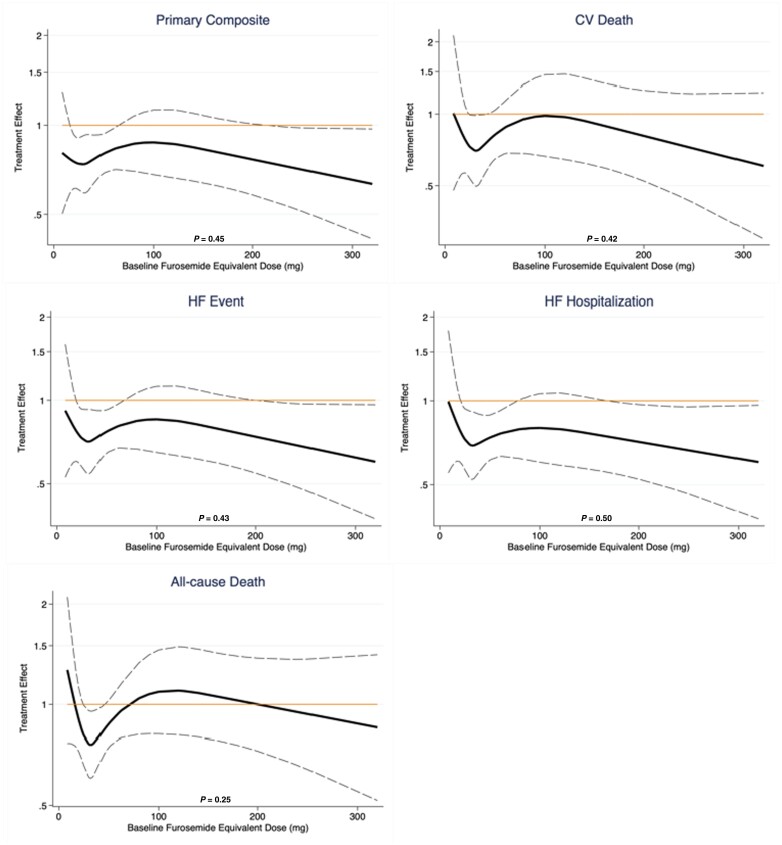
The treatment benefit of dapagliflozin compared to placebo on the primary composite outcome did not significantly vary by baseline diuretic use/type (P interaction = 0.64) or loop diuretic dose (P interaction = 0.57, Figure 1, Table 2). Similarly, treatment effects on the components of the primary composite endpoint, all-cause death, and change in KCCQ total symptom score at 1 and 8 months was consistent across diuretic use categories (Table 2). Moreover, when baseline loop diuretic dose was analysed as a continuous variable, the treatment effect of dapagliflozin remained consistent across a broad range of doses for all evaluated outcomes (Figure 2). The safety profile of dapagliflozin was consistent across diuretic categories with similar risk of drug discontinuation or interruption due to adverse events between treatment groups. The occurrence of any serious adverse events or study drug discontinuation due to an adverse event suggestive of volume depletion or renal events were also similar between treatment groups, irrespective of diuretic use/type or dose (Table 3).
Figure 1.
Forest plot of efficacy outcomes according to categories of background diuretic therapy at randomization.
Table 2.
Treatment effect on efficacy outcomes according to background diuretic therapy
| Primary composite | No diuretic (n = 683) |
Non-loop diuretic (n = 769) |
<40 mga (n = 1811) |
40 mga (n = 1902) |
>40 mga (n = 1098) |
P interaction (diuretic use/type) | P interaction (diuretic dose) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | |||
| Event rate | 7.3 | 5.3 | 3.9 | 4.2 | 8.3 | 6.4 | 9.8 | 7.9 | 18.1 | 15.3 | ||
| HR (95% CI) | 0.74 (0.48–1.12) | 1.08 (0.67–1.74) | 0.76 (0.60–0.97) | 0.81 (0.66–1.00) | 0.84 (0.68, 1.04) | 0.64 | 0.57 | |||||
| CV death | ||||||||||||
| Event rate | 3.3 | 2.6 | 1.6 | 2.8 | 3.2 | 2.6 | 4.0 | 3.3 | 6.3 | 5.5 | ||
| HR (95% CI) | 0.77 (0.42–1.39) | 1.82 (0.95–3.50) | 0.83 (0.57–1.19) | 0.82 (0.60–1.13) | HR = 0.86 (0.62, 1.20) | 0.31 | 0.75 | |||||
| HF event | ||||||||||||
| Event rate | 4.7 | 3.4 | 2.7 | 1.9 | 5.9 | 4.8 | 6.9 | 5.3 | 15.4 | 12.6 | ||
| HR (95% CI) | 0.71 (0.42–1.20) | 0.69 (0.36–1.31) | 0.81 (0.61–1.07) | 0.77 (0.60–0.99) | 0.82 (0.65, 1.03) | 0.99 | 0.63 | |||||
| HFH | ||||||||||||
| Event rate | 3.8 | 2.5 | 2.5 | 1.5 | 5.2 | 4.3 | 6.3 | 4.8 | 14.7 | 11.2 | ||
| HR (95% CI) | 0.65 (0.36–1.18) | 0.61 (0.31–1.22) | 0.83 (0.62–1.11) | 0.77 (0.59–1.00) | 0.76 (0.60, 0.97) | 0.61 | 0.91 | |||||
| All-cause death | ||||||||||||
| Event rate | 5.8 | 5.4 | 4.3 | 4.9 | 6.2 | 6.4 | 9.2 | 7.2 | 10.7 | 11.1 | ||
| HR (95% CI) | 0.92 (0.60–1.41) | 1.14 (0.73–1.76) | 1.04 (0.81–1.33) | 0.78 (0.63–0.97) | 1.03 (0.81, 1.32) | 0.22 | 0.69 | |||||
| KCCQ-TSS 1 month |
78.7 ± 20.2 | 77.5 ± 19.6 | 80.2 ± 17.9 | 80.4 ± 18.1 | 77.9 ± 19.0 | 80.5 ± 16.9 | 74.4 ± 19.7 | 75.2 ± 20.5 | 69.3 ± 22.5 | 71.9 ± 22.4 | ||
| Difference | 1.7 (−0.7 TO 4.1) | 1.4 (−0.6 to 3.5) | 2.0 (0.7–3.3) | 0.6 (−1.0 to 2.2) | 4.0 (1.7, 6.3) | 0.08 | 0.13 | |||||
| KCCQ-TSS 8 months |
80.1 ± 18.8 | 82.0 ± 17.4 | 79.6 ± 19.7 | 81.5 ± 17.6 | 78.8 ± 19.7 | 81.6 ± 16.8 | 75.0 ± 21.3 | 77.1 ± 20.0 | 72.5 ± 21.8 | 74.7 ± 21.1 | ||
| Difference | 3.5 (0.9–6.2) | 3.5 (0.9–6.1) | 2.0 (0.4–3.6) | 1.7 (−0.3 to 3.7) | 2.9 (0.1, 5.7) | 0.88 | 0.78 | |||||
Dose in furosemide equivalents.
CI, confidence interval; CV, cardiovascular; HF, heart failure, HFH, heart failure hospitalization; HR, hazard ratio; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire total symptom score.
Figure 2.
Treatment effect by baseline furosemide equivalent diuretic dose. The P-values displayed represent the P interaction of treatment effects of dapagliflozin across a range of furosemide dose equivalents, evaluated as a continuous function. CV, cardiovascular; HF, heart failure.
Table 3.
Safety outcomes in follow-up by treatment assignment according to background diuretic therapy
| Safety outcome | No diuretic (n = 683) |
Non-loop diuretic (n = 769) |
<40 mga (n = 1811) |
40 mga (n = 1902) |
>40 mga (n = 1098) |
P interaction (diuretic use/type) | P interaction (diuretic dose) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | |||
| Any SAE | 143 (41.6%) |
126 (37.4%) |
135 (35.8%) | 140 (36.0%) |
380 (42.3%) |
367 (40.3%) |
457 (47.6%) | 423 (45.1%) |
308 (56.4%) | 305 (55.4%) |
0.84 | 0.51 |
| Any AE leading to discontinuation of IP | 15 (4.4%) |
19 (5.6%) |
11 (2.9%) |
23 (5.9%) |
57 (6.3%) |
57 (6.3%) |
54 (5.6%) |
41 (4.4%) |
44 (8.1%) |
42 (7.6%) |
0.65 | 0.43 |
| Any AE leading to interruption of IP | 50 (14.5%) |
38 (11.3%) |
54 (14.3%) | 48 (12.3%) |
131 (14.6%) |
122 (13.4%) |
148 (15.4%) |
125 (13.3%) |
111 (20.3%) | 103 (18.7%) |
0.96 | 0.50 |
| Any AE that potentially placed a patient at increased risk for a lower limb amputationb | 14 (4.1%) |
11 (3.3%) |
11 (2.9%) |
8 (2.1%) |
48 (5.3%) |
46 (5.0%) |
57 (5.9%) |
60 (6.4%) |
69 (12.6%) |
63 (11.4%) |
0.90 | 0.80 |
| Any SAE or DAE suggestive of volume depletion | 3 (0.9%) |
1 (0.3%) |
1 (0.3%) |
2 (0.5%) |
11 (1.2%) |
13 (1.4%) |
10 (1.0%) |
11 (1.2%) |
7 (1.3%) |
15 (2.7%) |
0.50 | 0.14 |
| Any renal SAE or DAE | 6 (1.7%) |
5 (1.5%) |
4 (1.1%) |
6 (1.5%) |
18 (2.0%) |
15 (1.6%) |
18 (1.9%) |
20 (2.1%) |
33 (6.0%) |
27 (4.9%) |
0.76 | 0.93 |
Dose in furosemide equivalents.
Preceeding events were identified based on a predefined list of the European Medicines Agency’s Pharmacovigilance Risk Assessment Committee preferred terms.
SAE, serious adverse event; AE, adverse event; IP, investigational product; DAE, adverse events leading to discontinuations.
Effects of dapagliflozin on diuretic use over time
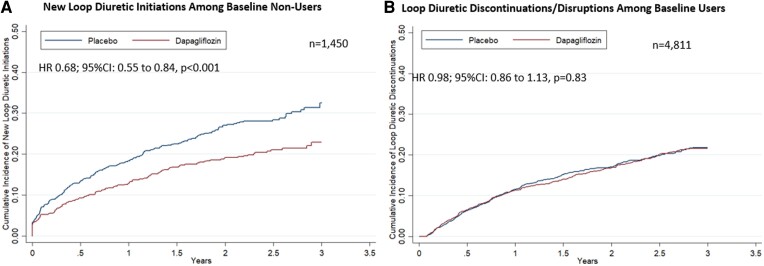
Among the 1450 patients not treated with a loop diuretic at baseline, new initiations occurred in 346 patients. Dapagliflozin reduced new initiations of loop diuretics by 32% [hazard ratio (HR) 0.68; 95% confidence interval (CI): 0.55–0.84, P < 0.001] (Figure 3). Among the 4811 participants on baseline loop diuretic, there was no significant difference in new loop diuretic discontinuations or disruptions (HR 0.98; 95% CI: 0.86–1.13, P = 0.83) in follow-up.
Figure 3.
Treatment effect on loop diuretic use in study follow-up. Kaplan–Meier curves displaying the (A) time to loop diuretic initiation among patients not on loop diuretic at baseline and (B) time to discontinuation or disruption among patients being treated with loop diuretic at baseline are presented. Disruption was defined as any interruption in diuretic use of ≥30 days. CI, confidence interval; HR, hazard ratio.
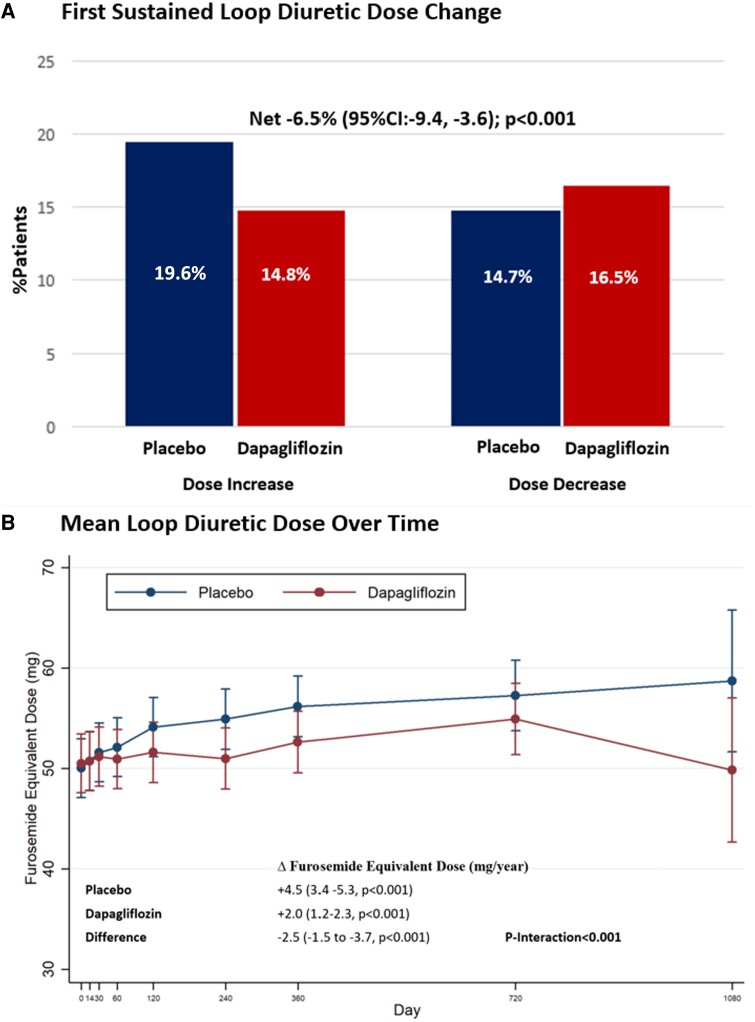
The mean baseline furosemide equivalent dose, calculated among all patients on loop diuretics, was similar between treatment groups: 51 ± 59 mg in the dapagliflozin arm and 50 ± 61 mg in the placebo arm. Patients randomized to dapagliflozin compared to placebo less frequently experienced a loop diuretic initiation or dose increase (14.8% vs. 19.6%, P < 0.001) and more frequently experienced a loop diuretic discontinuation or dose decrease (14.7% vs. 16.5%, P < 0.001), with a significant net reduction with dapagliflozin of −6.5% (95% CI: −9.4, −3.6, P < 0.001) (Figure 4). These results were consistent when considering dosing changes only among baseline loop diuretic users (Supplementary data online, Table S1). In follow-up, mean loop diuretic dose increased at a rate of 4.5 mg/year (95% CI: 3.4–5.3, P < 0.001) in the placebo group and a rate of 2.0 mg/year (95% CI: 1.2–2.3, P < 0.001) in the dapagliflozin group. Treatment with dapagliflozin significantly attenuated the rate of rise in loop diuretic dose relative to placebo resulting in a mean dose reduction over time of 2.5 mg/year (95% CI: −1.5, −3.7, P < 0.001). The difference in loop diuretic dose requirement between treatment groups emerged after day 60 and increased over time (P interaction < 0.001) (Figure 4).
Figure 4.
Effect of dapagliflozin on (A) first sustained loop diuretic dose change and (B) mean loop diuretic dose over time. The interaction between mean diuretic dosing, treatment allocation, and time was assessed.
Effect of dapagliflozin on diuretic use according to baseline kidney function
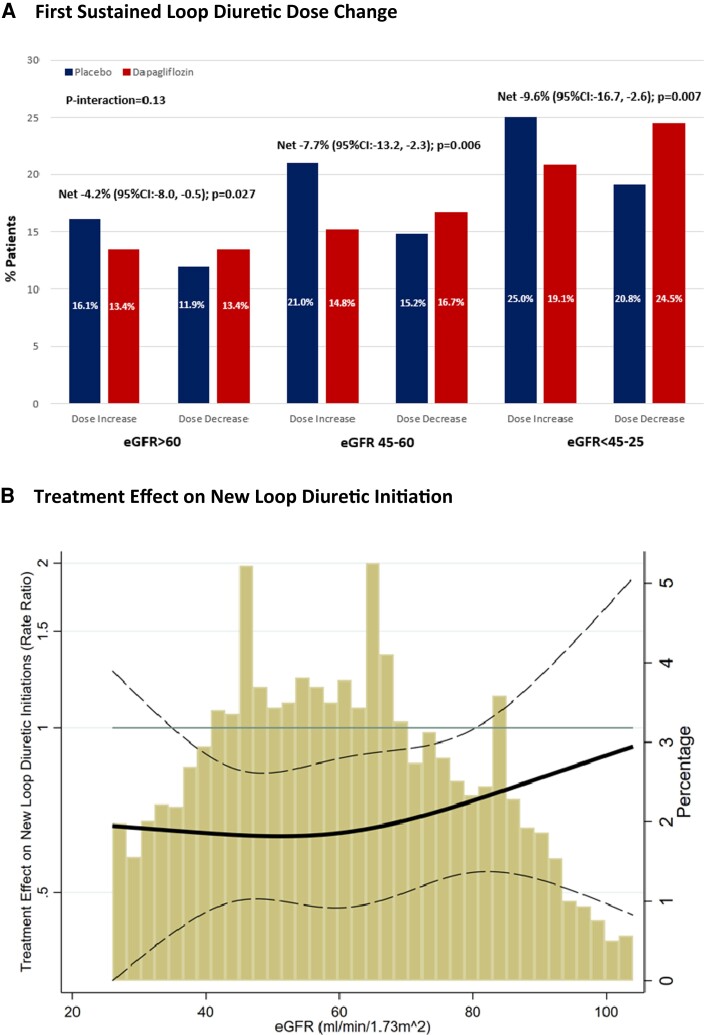
There was a significant net reduction in loop diuretic dose with dapagliflozin irrespective of baseline kidney function [eGFR >60 mL/min/1.73 m2: −4.2% (95% CI: −8.0, −0.5, P = 0.027); eGFR 45–60 mL/min/1.73 m2: −7.7% (95% CI: −13.2, −2.3, P = 0.006); eGFR <45–25 mL/min/1.73 m2: −9.6% [−16.7, −2.6, P = 0.007], P interaction = 0.13] (Figure 5A). When eGFR was analysed as a continuous variable, relative to placebo, dapagliflozin consistently reduced new loop diuretic initiations across the spectrum of baseline kidney function (Figure 5B).
Figure 5.
Effect of dapagliflozin on (A) first sustained loop diuretic dose change and (B) new loop diuretic initiation according to baseline kidney function.
Discussion
In a pre-specified analysis of the DELIVER trial of patients with heart failure with mildly reduced or preserved ejection fraction, treatment with dapagliflozin compared to placebo resulted in consistent clinical benefits and tolerability across a broad range of diuretic use categories and doses. Dapagliflozin significantly reduced new loop diuretic initiations, dose increases, and mean loop diuretic dose over time, compared to placebo (Structured Graphical Abstract).
Participants in DELIVER with the greatest diuretic requirement expectedly experienced the highest clinical risk across all evaluated outcomes, a finding that is well established in both patients with heart failure with preserved ejection fraction (HFpEF)10,11 and heart failure with reduced ejection fraction (HFrEF).15,16 Nevertheless, the treatment effect of dapagliflozin vs. placebo remained consistent irrespective of background diuretic therapy. These observations are consistent with data on the use of SGLT2 inhibitors in patients with HFrEF in the DAPA-HF trial, where dapagliflozin resulted in similar improvement in clinical outcomes across diuretic subgroups.13 Similarly, in the EMPEROR-Reduced trial, the benefits of empagliflozin did not differ among patients with and without recent volume overload.17 Taken together, these data from DELIVER add to the growing body of literature suggesting the ease of use and practical implementation of SGLT2 inhibitors among patients with a broad spectrum of diuretic requirements, including among those not receiving a diuretic.
In DELIVER, dapagliflozin significantly reduced mean loop diuretic requirement over time in a population of well characterized patients with heart failure with mildly reduced or preserved ejection fraction. Similarly, other SGLT2i including empagliflozin have also been shown to reduce the need for diuretic intensification.4 In contrast, among patients with HFrEF in DAPA-HF, this longitudinal effect on diuretic requirement was less apparent. Notably, a higher proportion of patients were on background diuretic therapy in DELIVER compared to DAPA-HF (89% vs. 81%). Unlike DAPA-HF, DELIVER allowed enrolment of stabilized (no longer requiring intravenous diuretics) patients actively or recently hospitalized for heart failure, which may suggest greater degree of baseline congestion in the DELIVER population. The potential diuretic effect of SGLT2 inhibitors has been postulated to be more marked in the presence of greater volume overload.18,19
Patients with worse kidney function incur greater levels of diuretic resistance and therefore require higher rates and doses of diuretics. Importantly, in this analysis, the diuretic sparing effects of dapagliflozin were consistent across the spectrum of baseline kidney function. These data suggest that such agents may reduce diuretic requirements over time, even in patients with significant kidney disease.
The reduction in mean loop diuretic dose with dapagliflozin appears to be driven by a reduction in new loop diuretic initiations and less need for sustained diuretic dose increase. Interestingly, rates of loop diuretic discontinuation or temporary interruption did not differ significantly between treatment groups. The effects of dapagliflozin to prevent diuretic intensification to a greater degree than effects on diuretic dose reduction or discontinuation is similar to previous observations from adjacent trials.4,5 The need for diuretic intensification in the ambulatory or inpatient setting represents an element of worsening heart failure and carries prognostic significance.20 Given that the reduced need for diuretic up-titration occurred in the context of an overall reduction in loop diuretic requirement that emerged late (day 60) and increased over time, this may reflect dapagliflozin’s combined effect to prevent the progression of heart failure beyond initial decongestion.
Other contributing explanations for these observations should be noted. It is possible that the lack of impact on diuretic discontinuation may reflect clinical inertia to make alterations to diuretic regimens; thereby, resulting in fewer discontinuations even where volume status may have improved.21 The more favourable neurohormonal, renal, and electrolyte profile of these drugs may also result in fewer clinical triggers for diuretic modification with improved volume status.12
In DELIVER, we observed a similar safety profile of dapagliflozin compared to placebo across a range of diuretic doses. Notably, no between-arm differences in the occurrence of renal adverse events or those related to volume depletion were detected even at the highest doses of diuretic. In a similar analysis of dapagliflozin in patients with HFrEF,13 volume depletion was slightly more frequently observed in dapagliflozin treated patients on higher doses of diuretic. Traditionally, the concern for volume depletion has been of greater consequence in patients with HFpEF given the tendency to greater preload dependence in the setting of diastolic dysfunction.22 In this light, the results of the present study are reassuring. Moreover, rates of study drug discontinuation were relatively low and did not differ between arms, highlighting the tolerability of the concomitant use of SGLT2 inhibitors and conventional diuretics.
Certain important limitations of this analysis should be noted. First, missing or inadequate dose information precluded analysis of all patients at certain time points. Second, specific clinical rationale motivating modifications to diuretic regimens was not available and may reflect issues other than volume status alone. Third, while information on diuretic regimens and start and stop dates were collected at study visits, these data were not cross-referenced against pharmacy claims data or other objective sources. Finally, DELIVER did not collect measures of diuresis and natriuresis such as urinary volumes or urinary electrolyte profiles.
In conclusion, in this pre-specified analysis of DELIVER in patients with heart failure and mildly reduced or preserved ejection fraction, dapagliflozin safely improved clinical outcomes across a wide range of diuretic use and doses. Treatment with dapagliflozin significantly reduced loop diuretic requirement over time, which appears to be driven by lower needs for diuretic intensification and underscores dapagliflozin’s effect to prevent worsening heart failure. However, there were limited differences in the necessity for loop diuretic discontinuation or dose reduction between treatment arms. As such, these data may argue against anticipatory loop diuretic dose reduction at the time of SGLT2 inhibitor initiation.
Supplementary data
Supplementary data is available at European Heart Journal online.
Pre-registered clinical trial number
The pre-registered clinical trial number is NCT03619213.
Ethical approval
The study protocol was approved by the ethics committees at each study site and participants provided written informed consent.
Supplementary Material
Contributor Information
Safia Chatur, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Muthiah Vaduganathan, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Brian Claggett, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Orly Vardeny, Minneapolis VA Center for Care Delivery and Outcomes Research, University of Minnesota, Minneapolis, MN, USA.
Akshay S Desai, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Pardeep S Jhund, BHF Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Rudolf A de Boer, Department of Cardiology, Erasmus Medical Center, Rotterdam, the Netherlands.
Carolyn S P Lam, National Heart Centre Singapore & Duke-National University of Singapore, Singapore, Singapore.
Mikhail N Kosiborod, Saint Luke’s Mid America Heart Institute and University of Missouri-Kansas City, Kansas City, MO, USA.
Sanjiv J Shah, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Felipe Martinez, Universidad Nacional de Córdoba, Córdoba, Argentina.
Silvio E Inzucchi, Yale School of Medicine, New Haven, CT, USA.
Adrian F Hernandez, Duke University Medical Center, Durham, NC, USA.
Tariq Haddad, Department of Cardiology, Virginia Heart, Falls Church, VA, USA.
Sumeet S Mitter, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Zi Michael Miao, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Magnus Petersson, Late-Stage Development, Cardiovascular, Renal, and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Anna Maria Langkilde, Late-Stage Development, Cardiovascular, Renal, and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
John J V McMurray, BHF Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Scott D Solomon, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Data availability
The trial sponsor is committed to Responsible Data Sharing Principles, including sharing of anonymized individual patient-level data and clinical documents related to DELIVER with qualified researchers. The trial data availability is according to the criteria and processes described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Funding
The DELIVER trial was funded by AstraZeneca.
References
- 1. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 2. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 3. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–1098. 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 4. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation 2021;144:1284–1294. 10.1161/CIRCULATIONAHA.121.056824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-reduced trial. Circulation 2021;143:326–336. 10.1161/CIRCULATIONAHA.120.051783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang J, Ye L, Yan Q, Zhang X, Wang L. Effects of sodium-glucose cotransporter 2 inhibitors on water and sodium metabolism. Front Pharmacol 2022;13:800490. 10.3389/fphar.2022.800490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 8. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:137–155. 10.1002/ejhf.1369 [DOI] [PubMed] [Google Scholar]
- 9. Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 2006;27:1431–1439. 10.1093/eurheartj/ehi890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalogeropoulos AP, Thankachen J, Butler J, Fang JC. Diuretic and renal effects of spironolactone and heart failure hospitalizations: a TOPCAT Americas analysis. Eur J Heart Fail 2020;22:1600–1610. 10.1002/ejhf.1917 [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Qadir HM, Tu JV, Yun L, Austin PC, Newton GE, Lee DS. Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. Am Heart J 2010;160:264–271.e1. 10.1016/j.ahj.2010.05.032 [DOI] [PubMed] [Google Scholar]
- 12. Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020;142:1028–1039. 10.1161/CIRCULATIONAHA.120.045691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, de Boer RA, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation 2020;142:1040–1054. 10.1161/CIRCULATIONAHA.120.047077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014;7:261–270. 10.1161/CIRCHEARTFAILURE.113.000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006;97:1759–1764. 10.1016/j.amjcard.2005.12.072 [DOI] [PubMed] [Google Scholar]
- 16. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O’Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007;9:1064–1069. 10.1016/j.ejheart.2007.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-reduced trial. J Am Coll Cardiol 2021;77:1381–1392. 10.1016/j.jacc.2021.01.033 [DOI] [PubMed] [Google Scholar]
- 18. Ohara K, Masuda T, Morinari M, Okada M, Miki A, Nakagawa S, et al. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol Metab Syndr 2020;12:37. 10.1186/s13098-020-00545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukuoka S, Dohi K, Takeuchi T, Moriwaki K, Ishiyama M, Omori T, et al. Mechanisms and prediction of short-term natriuretic effect of sodium-glucose cotransporter 2 inhibitor in heart failure patients coexisting type 2 diabetes mellitus. Heart Vessels 2020;35:1218–1226. 10.1007/s00380-020-01597-x [DOI] [PubMed] [Google Scholar]
- 20. Mallick A, Gandhi PU, Gaggin HK, Ibrahim N, Januzzi JL. The importance of worsening heart failure in ambulatory patients: definition, characteristics, and effects of amino-terminal pro-B-type natriuretic peptide guided therapy. JACC Heart Fail 2016;4:749–755. 10.1016/j.jchf.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 21. Weeda ER, Cassarly C, Brinton DL, Shirley DW, Simpson KN. Loop diuretic use among patients with heart failure and type 2 diabetes treated with sodium glucose cotransporter-2 inhibitors. J Diabetes Complications 2019;33:567–571. 10.1016/j.jdiacomp.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Kraaij DJ, Jansen RW, Sweep FC, Hoefnagels WH. Neurohormonal effects of furosemide withdrawal in elderly heart failure patients with normal systolic function. Eur J Heart Fail 2003;5:47–53. 10.1016/S1388-9842(02)00205-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The trial sponsor is committed to Responsible Data Sharing Principles, including sharing of anonymized individual patient-level data and clinical documents related to DELIVER with qualified researchers. The trial data availability is according to the criteria and processes described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.