Abstract
Context
Ketogenic diet has recently made a comeback as a part of lifestyle and dietary modifications in patients with polycystic ovary syndrome (PCOS). Despite studies suggesting its beneficial effects in reversing hormonal imbalance in women with PCOS, evidence has been patchy and derived from small populations under varying conditions.
Objective
To pool evidence from clinical trials to study the effects of ketogenic diet on reproductive hormones (LH/FSH ratio, free testosterone, serum progesterone) and observe evidence of weight change.
Methods
PubMed, ScienceDirect, Scopus, and Web of Science core collection were searched for clinical trials evaluating the effects of ketogenic diet in established PCOS women consistent with the Rotterdam classification. Single- or double-arm studies that included an outcome of interest were included. Two investigators worked independently to screen potential articles and a designated investigator extracted data on study characteristics and evaluated the outcomes. Data were pooled using a random-effects model. The quality of selected studies was assessed using the Cochrane Risk of Bias Tool.
Results
Following ≥45 days of intervention with ketogenic diet among women with PCOS, significant improvement was observed in reproductive hormone levels, with reduced LH/FSH ratio (d −0.851; 95% CI −1.015, −0.686; P < .001), reduced serum free testosterone (d −0.223; 95% CI −0.328, −0.119; P< .001), and an increased in serum sex hormone binding globulin (SHBG) (d 9.086; 95% CI 3.379, 14.792; P = .002). Significant weight loss was unanimously observed in all included studies (d −11.56; 95% CI −14.97, −8.15; P < .001).
Conclusion
Short-term ketogenic diet potentially improved hormonal imbalances commonly associated with PCOS.
Keywords: polycystic ovary syndrome, ketogenic diet, lifestyle, clinical trial, meta-analysis
Polycystic ovary syndrome (PCOS) is a complex multisystem disorder that shares similar pathogenesis with other chronic diseases with genetic-environmental interaction [1]. The hallmark features of oligo-anovulation, hyperandrogenism, and polycystic ovaries (presence of 12 or more follicles measuring 2 to 9 mm in diameter and/or an ovarian volume of more than 10 mL in at least one ovary) [2] have been recently challenged, as the sonographic criteria from the Rotterdam Consensus may easily lead to misinterpretation, especially among the postmenarcheal teenagers and in women with regular, ovulatory cycles who happen to have strings of visible antral follicles and a dominant follicle or corpus luteum [3]. Therefore, apart from history of contraceptive hormones use or fertility treatment, the day of the menstrual cycle during assessment, together with its length and pattern should also be reviewed prior to making diagnosis [3]. Early diagnosis and treatment for PCOS patients are important as they are at increased risk of endometrial cancer [4], metabolic [1, 3], and cardiovascular complications [5].
PCOS remains the most common endocrine disorder in women [6], with colossal economic impact to the nation. In the year 2020, it is estimated to have cost approximately USD 3.7 billion to establish an initial diagnosis and manage related reproductive endocrine morbidities, excluding the costs of pregnancy-related sequelae and long-term sequelae [7]. Apart from the routine prescription of (combined) oral contraceptives, antiandrogen agents, insulin sensitizers, and/or ovulation inducers, the primary first-line therapy has always been lifestyle interventions that include dietary modification and physical activity to promote weight loss of at least 5% to 10% in women with PCOS [8, 9].
Since the mid-18th century, there was a shift in the global landscape, from agriculture to modern industry, which has led to changes in our normal daily food intake. Weight loss diets have become a fad among health enthusiasts. Recently, ketogenic diet has been making a comeback due to its potential to delay aging and as an effective fat burner [10]. It is seen as a potential dietary intervention to help women with PCOS to lose weight and maintain weight loss, improve sex hormones level (hence fertility), optimize cholesterol level, and normalize the menstrual cycle [11]. Ketogenic diet is characterized by low daily carbohydrate intake of below 50 g (the daily recommended carbohydrate allowance is 130 g/day) with varying amounts of allowable fat and protein [12], calculated based on ideal body weight. In PCOS, supplementation of ketogenic diets with fiber and nondigestible carbohydrates, such as raw cornstarch and sourdough bread, is advisable to counteract the effects of very low-carbohydrate diets on the gut microbiota [12] that may result in gastric upset and constipation.
Despite the suggested promising effects of ketogenic diet in women with PCOS, the evidence was relatively patchy, conducted in a heterogeneous setting in a small population and over a short time period. There have already been a handful of published reviews on the effects of a ketogenic diet in women with PCOS, but to the best of our knowledge, no meta-analysis has been performed to pool the evidence from clinical trials studying the effects of ketogenic diet on any specific outcome of interest.
Therefore, our study aimed to assess the effects of ketogenic diet on reproductive hormone levels among women with PCOS: luteinizing hormone to follicle-stimulating hormone (LH/FSH) ratio, sex hormone binding globulin (SHBG), progesterone, and free and total testosterone level, following at least 45 days of intervention with ketogenic diet. Effect on body weight is a secondary endpoint.
Methods
Search Strategy and Selection of Studies
This paper was prepared following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The study protocol was registered in the National Medical Research Register of the Ministry of Health Malaysia (NMRR ID-23-00295-9N5) and the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42023393762.
Systematic literature searches were performed in MEDLINE, ScienceDirect, Scopus, and Web of Science core collection databases, from inception until January 20, 2023. The keywords “ketogenic diet” or “KD” or “very-low-carbohydrate diet” were used in combination as medical subject heading (MeSH) terms and text words.
Eligible studies were included if they met the inclusion criteria for the study population (women diagnosed with PCOS by any established reference criteria), intervention (randomized or nonrandomized design with ketogenic diet as the intervention for at least 6 weeks), presence or absence of a reference group (control or other types of dietary modification), outcome (reproductive hormone levels—including serum LH, serum FSH, LH/FSH ratio, SHBG, progesterone, and free and/or total testosterone level), selected anthropometric parameters including weight, body mass index (BMI) and waist to hip ratio), study design (only interventional clinical trials) and statistics (sufficient data to allow calculation of differences within subjects subjected to ketogenic diet). Case–control studies were excluded to minimize bias in recall and selection. Review articles, letters to the editor, comments, and case reports were also excluded. The references of the included articles were also checked to ensure inclusivity. Only articles published in the English language were included. Single-arm interventional studies that reported a measure of association (with mean difference and SD, or sufficient data to calculate them) in the level of reproductive hormones of interest were considered eligible for inclusion. Selected data from double-arm studies were also included: a double-arm study looking at the effect of ketogenic diet in PCOS patients with and without hyperuricemia [13] only included data from the patients with non-hyperuricemia at baseline, while double-arm studies assessing effects from different weight loss regimen only included data from ketogenic diet arm [14, 15].
Missing data or additional information were requested via email from the corresponding authors of the articles as needed. Information with no clear inclusion/exclusion criteria for subject selection, and those with no clear mention of baseline parameters prior to intervention were excluded.
Two authors (I.L.M. and A.A.) independently assessed articles retrieved from the databases for eligibility. The decision to include the studies was based on the study title, abstract, and later, full-text screening. Findings from the 2 authors were collated using Mendeley Desktop 1.19.8. Discrepancies were resolved through consensus and discussion with a third investigator (K.K.) if consensus could not be reached.
Data Extraction
Data extraction using a standardized form was carried out by a single author (K.K.) whose primary interest lies in clinical endocrinology. The Cochrane Risk of Bias Tool for randomized trials was used for evaluation of bias (Supplementary Material) [16]. The following data were extracted from the original articles: first author, year of publication, country of study population, characteristics of the study cohort, number of participants included, length of follow-up (weeks), outcome of interest, and measures of effect size (mean difference and standard error).
Assessment of Methodological Quality
Two investigators (I.L.M. and A.A.) independently assessed the methodological quality of each included study using the Revised Cochrane risk of bias tool for randomized trials. This tool is structured into 5 domains and was identified based on both empirical evidence and theoretical considerations [16]. The domains were separated into 7 critical parts as follows: (i) preliminary considerations; (ii) risk of bias arising from randomization process; (iii) risk of bias due to deviations from the intended interventions (effect of assignment to intervention); (iv) risk of bias due to deviations from the intended interventions (effect of adhering to intervention); (v) risk of bias due to missing outcome data; (vi) risk of bias in measurement of the outcome; and (vii) risk of bias in selection of the reported result. The final results lead to an overall methodological evaluation of good, fair, or poor. Disagreements were resolved by consensus or by a third investigator (K.K.) if consensus could not be reached.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics for Windows, Version 28.0. A random-effects model was applied to combine the mean difference and SD of outcome of interest in relation to ketogenic diet intervention.
The pooled results were reported as mean difference and presented with 95% CI with two-sided P values. Meta-analysis was conducted if at least 2 studies were available for a particular outcome. The statistical heterogeneity between studies was not performed due to the small number of available studies.
The robustness of the results was established by eliminating each study one by one from the meta-analysis and recalculating the summary estimate (the “leave-one-out” approach). If ≥5 studies were available, the possibility of publication bias was explored by visual inspection of the funnel plot of the effect size against standard error. A probability value of <.05 was considered statistically significant.
Results
Search Results
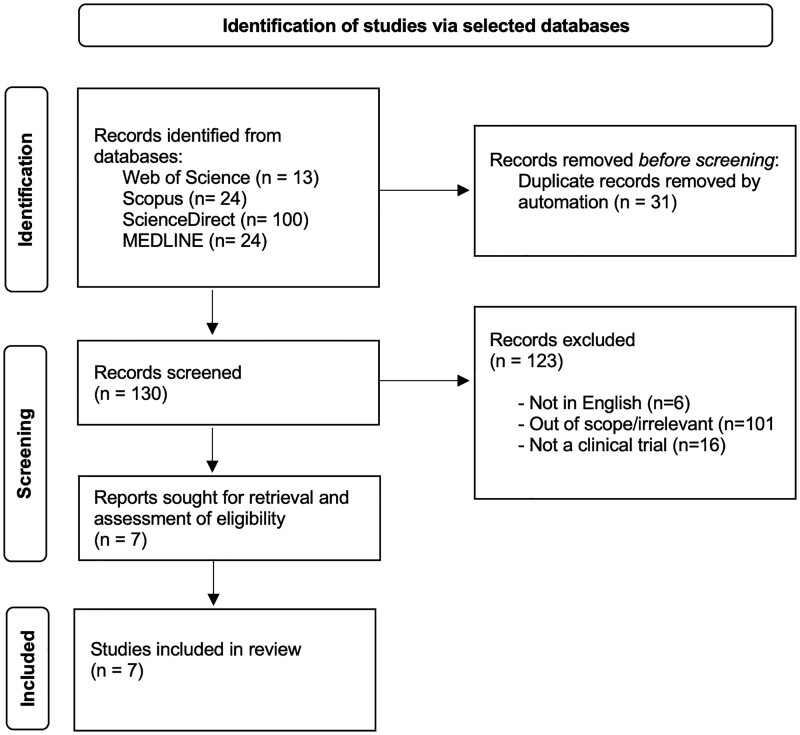
The selection process is shown in Fig. 1, following the recommendation by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Figure 1.
Flowchart of study selection.
The initial search from ScienceDirect database using the same search strategy produced 1928 articles. Hence, the search strategy for the database was further refined to include AND (“clinical trial” OR “clinical studies”) NOT (animal OR rats) to yield the final 100 articles.
The search produced a total of 161 articles from the 4 databases selected. After title and abstract screening, only 7 articles were selected for the evaluation of the full text. At the end of the selection process, all 7 articles were included in the quantitative analysis.
Table 1 presents the main characteristics of the 7 clinical trials included in the systematic review and meta-analysis. The overall analysis included 170 participants. Four studies were conducted in Italy [15, 17-19], 2 in China [13, 14] and 1 in the United States [20]. Assessment of dietary compliance was performed via evaluation of urine or blood ketosis in 4 studies [13, 14, 17, 20], counseling or phone reminder in 2 studies [14, 15], and food records [20]. The methodological quality score was fair in all included studies.
Table 1.
Characteristics of clinical studies evaluating the effects of ketogenic diet on reproductive hormones and selected anthropometric measurements
| Author, year | Title | Study design | Site | Total subjects, n | Duration of intervention | Results (reported as mean difference pre-post) |
|---|---|---|---|---|---|---|
| Mavropoulos, 2005 [20] | The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: A pilot study | Nonrandomized single-arm experimental design | Raleigh/Durham/Chapel Hill areas in North Carolina through a community PCOS support group | 5 (18-45 years) Mean: 34.5 years |
24 weeks |
|
| Paoli, 2020 [19] | Effects of a ketogenic diet in overweight women with polycystic ovary syndrome | Nonrandomized single-arm experimental design | Enrollment through public announcement in medical centers in Padova and Vicenza territory | 14 (18-45 years old) Mean: 27.5 years) |
12 weeks |
|
| Li, 2021 [14] | Ketogenic diet in women with polycystic ovary syndrome and liver dysfunction who are obese: a randomized, open-label, parallel-group, controlled pilot trial | Prospective, open-label, parallel-group, randomized controlled pilot trial | Beijing Shijitan Hospital, Capital Medical University | 8 (18-50 years old) Mean: 31.1 years |
12 weeks |
|
| Cincione, 2021 [18] | Effects of mixed of a ketogenic diet in overweight and obese women with polycystic ovary syndrome | Nonrandomized single-arm experimental design | University Medical Service of Dietetic and Metabolic Diseases of the Faculty of Medicine and Surgery of the University of Foggia | 17 (18-45 years old) Mean: 28.5 years |
45 days |
|
| Magagnini, 2022 [17] | Does the ketogenic diet improve the quality of ovarian function in obese women? | Nonrandomized single-arm experimental design | Division of Endocrinology, Metabolic Disease and Nutrition, University of Catania | 25 (Age: >18 years old) Mean: 25.4 years |
12 weeks |
|
| Yang, 2022 [13] | Effects of ketogenic diet in women with PCOS with different uric acid concentrations: a prospective cohort study | Double-blind, prospective interventional study in 2 study cohorts (hyperuricemia vs non-hyperuricemia group) | Beijing Shijitan Hospital, Capital Medical University | 28 (20-40 years old) Mean: 29.7 years |
12 weeks |
|
| Cincione, 2022 [15] | Short-term effects of ketogenic diet or modestly hypocaloric Mediterranean diet on overweight and obese women with polycystic ovary syndrome | Randomized controlled trial (KD vs Mediterranean diet) | University Medical Service of Dietetic and Metabolic Diseases of the Faculty of Medicine and Surgery of the University of Foggia | 73 (18-45 years) Mean: 33.4 years) |
45 days |
|
Abbreviations: BMI, body mass index; PCOS, polycystic ovary syndrome; FSH, follicle-stimulating hormone; HOMA-IR, homeostatic model assessment for insulin resistance; KD, ketogenic diet; LH, luteinizing hormone; SHBG, sex hormone binding globulin.
Ketogenic Diet Formulation
Table 2 describes the various formulations of ketogenic diet across included studies.
Table 2.
Different ketogenic diet composition across studies
| Author, year | Type of KD | Daily calorie allowance (kcal/day) | Carbohydrate | Protein | Fat | Herbal extracts | Supplements | Replacement meal |
|---|---|---|---|---|---|---|---|---|
| Mavropoulos, 2005 [20] | LCKD | NA | <20 g/day (including low-carbohydrate vegetables 1 cupful/day) | Unlimited consumption of animal-based foods (fish, meat, chicken) | NA | NA | Encouraged daily multivitamin | No |
| Paoli, 2020 [19] | CKD | 1600-1700 | 20.3 ± 5.2 g/day | 100.8 ± 8.6 g/day | 132.4 ± 11.7 g/day | Allowed (selective) | Allowed (selective)—high protein (19 g/portion) with low-carbohydrate (3.5 g/portion) with dry phytoextracts | No |
| Cincione, 2021 [18] | VLCKD | 600 | ≤30 g/day | 1.1-1.2 g/kg ideal body weight | 30 g/day | Not allowed | Multivitamin and multimineral supplements | Isolated whey protein powders during breakfast and lunch |
| Li, 2021 [14] | CKD | 1300-1500 | ≤50 g/day (5-10% of daily calories) |
18-27% of daily calories | 70-75% of daily calories | NA | NA | No |
| Yang, 2022 [13] | CKD | NA | ≤50 g/day (5-10% of daily calories) |
18-27% of daily calories | 70-75% of daily calories | NA | NA | No |
| Cincione, 2022 [15] | VLCKD | 600 | ≤30 g/day | 1.1-1.2 g/kg/day | 30 g/day | NA | Multivitamin and multimineral supplements | Isolated whey protein powders |
| Magagnini, 2022 [17] | VLCKD | First phase (4 weeks) 600-800 Second phase (4 weeks) 1200-1500 Third phase (4 weeks) 1500-2000 |
The use of high-biological protein replacement containing 18 g of protein, 4 g carbohydrate and 3-4 g of fat in varying stages | Replacement of natural protein with protein preparation at lunch and dinner during the first phase | ||||
Abbreviations: CKD, classical ketogenic diet; KD, ketogenic diet; LCKD, low-carbohydrate, ketogenic diet; NA, information not available; VLCKD, very low-calorie ketogenic diet.
Reproductive Hormones
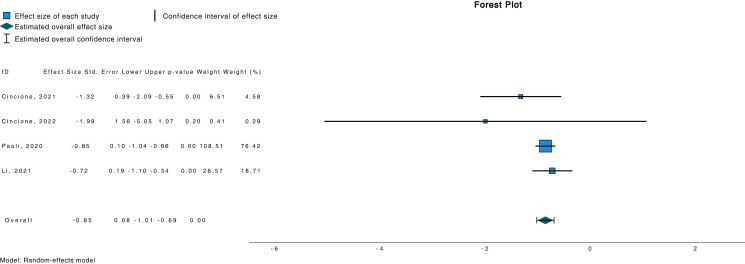
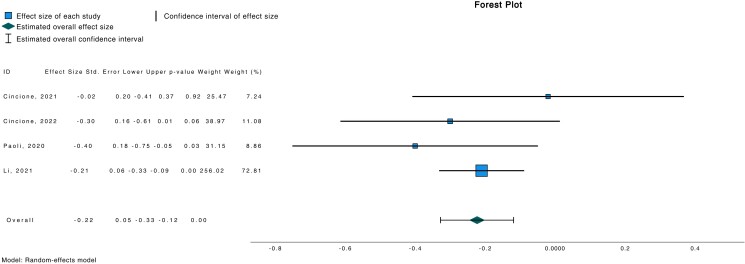
Meta-analytic pooling under a random-effects model indicated a significant association between ketogenic diet and reduced LH/FSH ratio (d −0.851; 95% CI −1.015, −0.686; P < .001) (Fig. 2) in a population of 112 subjects. Similarly, a reduced free testosterone (d −0.223; 95% CI −0.328, −0.119; P < .001) was also observed in the same study cohort (Fig. 3).
Figure 2.
Forest plot investigating association between LH/FSH and ketogenic diet.
Figure 3.
Forest plot investigating association between free testosterone and ketogenic diet.
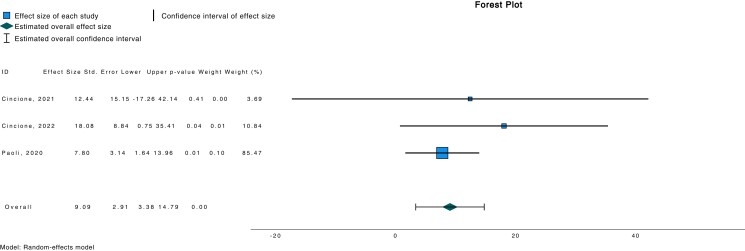
We also found a statistically significant increment in serum SHBG following ketogenic diet intervention (d 9.086; 95% CI 3.379, 14.792; P = .002) (Fig. 4). On the other hand, serum progesterone remained equivocal following intervention with ketogenic diet [11, 16] (d 4.28; 95% CI −4.75, 13.30; P = .353).
Figure 4.
Forest plot investigating association between SHBG and ketogenic diet.
Anthropometric Measurements
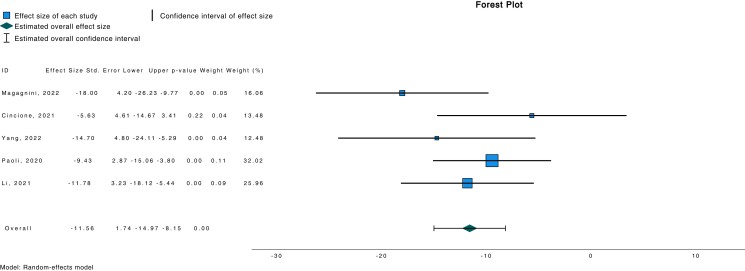
There was also a significant weight loss observed with a ketogenic diet (d −11.56; 95% CI −14.97, −8.15; P < .001) (Fig. 5).
Figure 5.
Forest plot investigating association between weight loss and ketogenic diet.
Sensitivity Analysis and Publication Bias
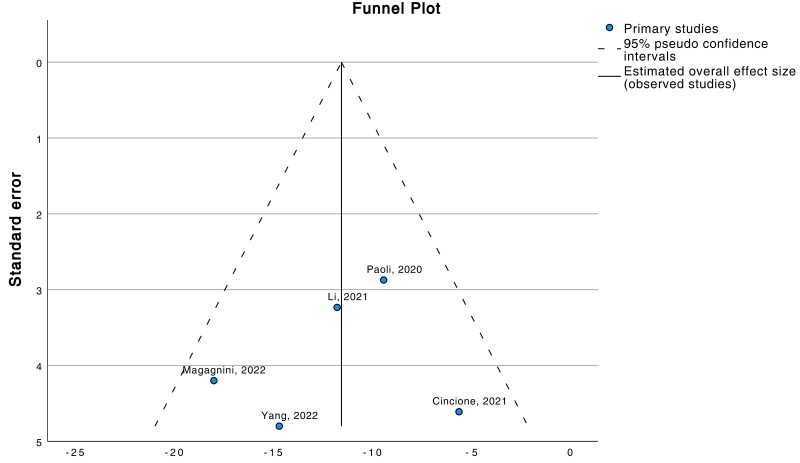
A leave-one-out sensitivity analysis was performed by iteratively removing one study at a time to confirm that our results were not determined by a single study. There was no change in the result of the main analysis for each of the outcomes tested. Publication bias was also assessed with a funnel plot (Fig. 6).
Figure 6.
The funnel plot shows symmetrical distribution of the study distribution (ketogenic diet vs weight loss), suggesting the absence of potential publication biases.
Discussion
The present study is the first systematic review with meta-analysis that evaluated evidence of association between ketogenic diet and reproductive hormone levels among women with PCOS in a pool of 170 subjects. The pooled analysis of eligible clinical trials, each carried out in a limited number of subjects, showed a possible improvement in LH/FSH ratio, serum free testosterone and serum SHBG. These results, although interesting and clinically relevant, must be carefully interpreted due to the small number of subjects and studies analyzed.
PCOS is a complex trait arising from diverse genetic (ie, insulin resistance, insulin secretory defects, hyperandrogenemia, polycystic ovarian morphology) and environmental interaction (ie, acquired obesity) [21]. The complex pathogenesis of the disorder attests to the syndrome’s multifactorial nature and heterogenous manifestation [21].
The pathophysiology of PCOS closely relates to an abnormality in the hypothalamic-pituitary-ovarian or adrenal axis with disturbance in the secretory pattern of the gonadotropin-releasing hormone (GnRH) that results in the relative increase in LH to FSH ratio [22]. In healthy women, the LH to FSH ratio usually lies between 1 and 2, whereas this ratio becomes reversed in women with PCOS, reaching as high as 2 or 3 [22]. An excess in serum LH stimulates ovarian androgen production, whereas a relative deficit in FSH further impairs follicular development. Hence, as a result of raised LH/FSH ratio, ovulation does not occur in women with PCOS. In this study, we found that a ketogenic diet significantly reduced the LH/FSH ratio [14, 15, 18, 19]. The mechanism on which carbohydrate restrictions from the ketogenic diet was able to exert this effect is suggested to be indicative of endocrine re-normalization due to improved insulin sensitivity [20].
Apart from reducing LH/FSH ratio, our study also found that a ketogenic diet reduced the level of free testosterone in women with PCOS [14, 15, 18, 19]. In PCOS, hyperandrogenism is attributable to increasing levels of circulating free testosterone due to increased steroidogenesis from proliferation of ovarian theca cells resulting from an imbalance in LH/FSH ratio [23]. Hyperandrogenism plays a critical role in the occurrence and development of PCOS-related complications, such as obesity, type 2 diabetes mellitus, hypertension and atherosclerosis, cardiac hypertrophy and coronary heart disease, and kidney diseases [24].
On a related note, serum SHBG is a glycated homodimeric plasma transport protein that positively correlates with the total number of follicles in women [25] and is currently considered a biomarker for metabolic disease [18]. Specifically, women with PCOS who are overweight or obese characteristically have a decreased serum SHBG concentration and increased serum total testosterone and free androgen index [25]. Dietary modification with ketogenic diet was found to increase the level of circulating SHBG [15, 18, 19] and hence improved metabolic and ovulatory dysfunction in women with PCOS. The low-carbohydrate ketogenic diet was speculated to result in reduction in hyperinsulinemia and therefore decreased stimulation of ovarian androgen production as well as increased SHBG levels, synergistically limiting the circulating free androgens [20]. Despite the known clinical importance of SHBG assessment in the diagnosis and management of PCOS, a recent study in a cohort of 120 patients with PCOS undergoing in vitro fertilization reported that serum SHBG concentration was positively correlated with the ovarian response in non-PCOS patients, but not in PCOS patients [25], suggesting its limited clinical value in monitoring controlled ovarian hyperstimulation in women with PCOS.
Our pooled analysis did not find any changes in serum progesterone level following intervention with ketogenic diet. However, the number of subjects available for pooled analysis were small; hence, this finding needs careful interpretation and reassessment when more studies become available. Serum progesterone is initially produced by corpus luteum upon successful ovulation to prepare the uterine lining for implantation. In general, women with PCOS are anovulatory so serum progesterone levels are low [26]. We were only able to perform pooled analysis on selected reproductive hormones due to dissimilar parameters studied across the included papers and the lack of response from the authors for their raw data, limiting our expansion of analysis to include other relevant hormonal markers (ie, free androgen index, individual serum LH and FSH level).
Our secondary outcome measurement was evidence of weight changes with a ketogenic diet. All included studies noted significant weight loss following very low-carbohydrate, ketogenic diets. Nutritional ketosis formulation induces weight loss through the following hypothesized mechanisms [27]: reduced appetite due to higher satiety effect of proteins in ketogenic diet formulation, a possible direct appetite-suppressant property of the ketone bodies, reduced lipogenesis and increased lipolysis, reduced resting respiratory quotient and, therefore, better metabolic efficiency when consuming fats, and increased in the metabolic costs of gluconeogenesis, together with the thermic effect of high dietary proteins. Therefore, this finding supports more robust research directed toward long-term evaluation of carbohydrate restriction and PCOS.
Despite the known positive effects of ketogenic diet in improving insulin resistance [17, 19], improving sex hormone imbalances and metabolic health [14, 15, 18, 19], and improving body composition [13, 17], it is important to look at the baseline characteristics of the study participants when evaluating the effects of ketogenic diet, as they can have a significant impact on the transferability and applicability of the results [28]. Demographic and baseline clinical parameters, such as the gender, weight, BMI, age, and health status (such as comorbid liver or kidney derangements) will impact the risks and benefits of a ketogenic diet. It is also worth highlighting that not all keto studies can be generalized for PCOS due to these potential confounding factors [28]. Furthermore, there is no specific formulation that is tailored for PCOS; in addition, induction of nutritional ketosis may be hard on gut health and some might consider the diet as too demanding, eventually affecting long-term compliance. The impact of ketogenic diet on fertility is still unclear and the long-term implications are not adequately studied and still not well understood.
To the best of our knowledge, the present study is the first systematic review with meta-analysis that evaluated all available clinical studies on the effects of ketogenic diet on the reproductive hormone levels among women with PCOS. The pooled analysis of clinical studies also showed a possible improvement in selected anthropometric measures, such as weight and BMI. These results, although interesting and useful to formulate a hypothesis, must be interpreted with caution due to the small number of subjects investigated.
Conclusion
In conclusion, herein we reported the possible association between ketogenic diet and improvement in reproductive hormone levels in women with PCOS via meta-analysis of the available and usable studies. The accessible literature still has several limitations necessitating careful review, reducing the applicability and transferability of these results to the general PCOS population. However, these findings have important clinical implications, especially for endocrinologists, gynecologists, and dieticians, who should carefully plan and customize individual diet recommendations for women with PCOS.
Acknowledgment
The authors would like to thank the Director General of Health Malaysia for his permission to publish the paper.
Abbreviations
- BMI
body mass index
- PCOS
polycystic ovary syndrome
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- SHBG
sex hormone binding globulin
Contributor Information
Karniza Khalid, Email: karniza.khalid@moh.gov.my, Endocrine Unit, Specialised Diagnostic Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, 50588 Kuala Lumpur, Malaysia.
Saraswathy Apparow, Endocrine Unit, Specialised Diagnostic Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, 50588 Kuala Lumpur, Malaysia.
Irma Liyana Mushaddik, Clinical Research Centre, Hospital Tuanku Fauziah, Ministry of Health Malaysia, 01000 Perlis, Malaysia.
Amalina Anuar, Clinical Research Centre, Hospital Tuanku Fauziah, Ministry of Health Malaysia, 01000 Perlis, Malaysia.
Syed A A Rizvi, College of Biomedical Sciences, Larkin University, Miami, FL 18301, USA.
Anasufiza Habib, Biochemistry Unit, Specialised Diagnostic Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, 50588 Kuala Lumpur, Malaysia.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Disclosures
The authors declare no conflict of interest.
Data Availability
Original data generated and analyzed during this study are included in this published article.
References
- 1. Parker J, O’Brien C, Hawrelak J, Gersh FL. Polycystic ovary syndrome: an evolutionary adaptation to lifestyle and the environment. Int J Environ Res Public Health. 2022;19(3):1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum Reprod. 2004;19(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 3. Smet M, McLennan A. Rotterdam criteria, the end. Australas J Ultrasound Med. 2018;21(2):59‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lobo RA, Carmina E. The importance of diagnosing the polycystic ovary syndrome. Ann Intern Med. 2000;132(12):989‐993. [DOI] [PubMed] [Google Scholar]
- 5. Hyderali BN, Mala K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2015;191:15‐22. [DOI] [PubMed] [Google Scholar]
- 6. Fong S L, Douma A, Verhaeghe J. Implementing the international evidence-based guideline of assessment and management of polycystic ovary syndrome (PCOS): how to achieve weight loss in overweight and obese women with PCOS? J Gynecol Obstet Hum Reprod. 2021;50(6):101894. [DOI] [PubMed] [Google Scholar]
- 7. Riestenberg C, Jagasia A, Markovic D, Buyalos RP, Azziz R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J Clin Endocrinol Metab. 2022;107(2):575‐585. [DOI] [PubMed] [Google Scholar]
- 8. Sadeghi HM, Adeli I, Calina D, et al. . Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mole Sci. 2022;23(2):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2020;11:2042018820938305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovács Z, Brunner B, Ari C. Beneficial effects of exogenous ketogenic supplements on aging processes and age-related neurodegenerative diseases. Nutrients. 2021;13(7):2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batch JT, Lamsal SP, Adkins M, Sultan S, Ramirez MN. Advantages and disadvantages of the ketogenic diet: a review article. Cureus. 2020;12(8):e9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crosby L, Davis B, Joshi S, et al. . Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr. 2021;8:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang M, Bai W, Jiang B, et al. . Effects of a ketogenic diet in women with PCOS with different uric acid concentrations: a prospective cohort study. Reprod Biomed Online. 2022;45(2):391‐400. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Bai W-P, Jiang B, et al. . Ketogenic diet in women with polycystic ovary syndrome and liver dysfunction who are obese: a randomized, open-label, parallel-group, controlled pilot trial. J Obstet Gynaecol Res. 2021;47(3):1145‐1152. [DOI] [PubMed] [Google Scholar]
- 15. Cincione IR, Graziadio C, Marino F, et al. . Short-time effects of ketogenic diet or modestly hypocaloric Mediterranean diet on overweight and obese women with polycystic ovary syndrome. J Endocrinol Invest. 2023;46(4):769‐777. [DOI] [PubMed] [Google Scholar]
- 16. Cochrane Collaboration . . RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. Cochrane Bias [Internet]. 2022. 1‐24. Accessed January 31, 2023. Available from:https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 17. Magagnini MC, Condorelli RA, Cimino L, et al. . Does the ketogenic diet improve the quality of ovarian function in obese women? Nutrients. 2022;14(19):4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cincione RI, Losavio F, Ciolli F, et al. . Effects of mixed of a ketogenic diet in overweight and obese women with polycystic ovary syndrome. Int J Environ Res Public Health. 2021;18(23):12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond). 2005;2(35):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saadia Z. Follicle stimulating hormone (LH: FSH) ratio in polycystic ovary syndrome (PCOS)—obese vs. non- obese women. Med Arch. 2020;74(4):289‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashraf S, Nabi M, Rashid F, Amin S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egypt J Med Hum Genet. 2019;20(1):1‐10. [Google Scholar]
- 24. Ye W, Xie T, Song Y, Zhou L. The role of androgen and its related signals in PCOS. J Cell Mol Med. 2021;25(4):1825‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhai J, Li S, Zhu Y, Sun Y, Chen ZJ, Du Y. Serum sex hormone binding globulin concentration as a predictor of ovarian response during controlled ovarian hyperstimulation. Front Med (Lausanne). 2021;8:719818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luan YY, Zhang L, Peng YQ, Li YY, Liu RX, Yin CH. Immune regulation in polycystic ovary syndrome. Clin Chim Acta. 2022;531:265‐272. [DOI] [PubMed] [Google Scholar]
- 27. Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barrea L, Verde L, Camajani E, et al. . Ketogenic diet as medical prescription in women with polycystic ovary syndrome (PCOS). Curr Nutr Rep. 2023;12(1):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.