Abstract
Introduction
The role of sex compared to comorbidities and other prognostic variables in patients with coronavirus disease (COVID-19) is unclear.
Methods
This is a retrospective observational study on patients with COVID-19 infection, referred to 13 cardiology units. The primary objective was to assess the difference in risk of death between the sexes. The secondary objective was to explore sex-based heterogeneity in the association between demographic, clinical and laboratory variables, and patients’ risk of death.
Results
Seven hundred and one patients were included: 214 (30.5%) women and 487 (69.5%) men. During a median follow-up of 15 days, deaths occurred in 39 (18.2%) women and 126 (25.9%) men. In a multivariable Cox regression model, men had a nonsignificantly higher risk of death vs. women (P = 0.07).
The risk of death was more than double in men with a low lymphocytes count as compared with men with a high lymphocytes count [overall survival hazard ratio (OS-HR) 2.56, 95% confidence interval (CI) 1.72–3.81]. In contrast, lymphocytes count was not related to death in women (P = 0.03).
Platelets count was associated with better outcome in men (OS-HR for increase of 50 × 103 units: 0.88 95% CI 0.78–1.00) but not in women. The strength of association between higher PaO2/FiO2 ratio and lower risk of death was larger in women (OS-HR for increase of 50 mmHg/%: 0.72, 95% CI 0.59–0.89) vs. men (OS-HR: 0.88, 95% CI 0.80–0.98; P = 0.05).
Conclusions
Patients’ sex is a relevant variable that should be taken into account when evaluating risk of death from COVID-19. There is a sex-based heterogeneity in the association between baseline variables and patients’ risk of death.
Keywords: coronavirus study, inflammation, outcome, sex differences
Introduction
Sex and sex-based differences in prevalence and/or severity of a number of infectious diseases are largely known.1
On average, women have stronger innate and adaptive immune responses than males, and this results in faster clearance of pathogens and greater vaccine efficacy.2
Growing evidence suggests that sex-related differences also affect coronavirus disease (COVID-19).3–6
According to data available, it seems that women and men had similar susceptibility to get infected by COVID-19; however, there are relevant differences in the course of infection, risk of developing complications and mortality with an almost two-fold risk of death in males compared with women.3,7
However, the role of sex in Caucasian patients with COVID-19 is still unclear as no analysis has been done regarding its independent role compared with cardiac and noncardiac comorbidities.3
To our knowledge, only one study has investigated sex differences in the association with severity and mortality of COVID-19 in Chinese people.8
In our study, we assessed the independent role of sex compared to comorbidities and other prognostic variables in patients with COVID-19. This is the first study on Caucasian subjects investigating the role of sex as a determinant of outcomes and its interaction with different prognostic variables in a large cohort of patients with COVID-19.
Methods
Study design and participants
This a multicenter observational study on a retrospective cohort of consecutive adult patients with laboratory-confirmed COVID-19 infection, referred to 13 Italian Cardiology Units from 1 March to 9 April 2020. A confirmed case of COVID-19 was defined by a positive result on reverse-transcriptase-polymerase chain reaction (RT-PCR) assay of a nasopharyngeal swab. Patients hospitalized for cardiovascular reasons without a confirmed COVID-19 diagnosis were excluded.
Patients were followed up after the hospital admission and all-cause in-hospital mortality or discharge was ascertained until 23 April 2020.
The primary objective of this study was to assess the difference in risk of death between women and men. The secondary objective was to explore sex-based heterogeneity in the association between demographic, clinical or laboratory prognostic variables and patients’ risk of death.
This study complied with the edicts of the Declaration of Helsinki and was approved by the ethical committee of Spedali Civili di Brescia, Brescia, Italy (no. NP 4105).
Data collection
Epidemiological, clinical and laboratory data of all patients were obtained from the electronic medical records of each designated hospital.
Detailed demographics information, comorbidities, symptoms, and disease severity of all patients were recorded or diagnosed on hospital admission. Laboratory examinations including routine blood tests; lymphocyte subsets; inflammatory or infection-related biomarkers; and cardiac, renal, liver, and coagulation function tests were obtained at initial diagnosis. Data regarding clinical treatment included COVID-19 specific therapy (oxygen therapy, nonmechanical and mechanical ventilation, antiviral agents, hydroxychloroquine, Tolicizumab, corticosteroids, antibiotic, anticoagulants) and background treatment. Coexisting comorbidities, chronic concomitant medications, as well as complications onset during the infection course were ascertained from medical records.
There were no cases lost to follow-up in this study.
Statistical analyses
Data were presented stratified by sex. Continuous variables were shown as means and standard deviations, skewed variables as medians and interquartile ranges (IQR), and dichotomous variables as counts and percentages. Comparisons between two independent groups were made, respectively, using Student's t-test for means, Wilcoxon test for medians, and chi-squared test for proportions. For all variables with at least one expected count of less than 5, a Fisher's exact test instead of a chi-squared test was used.
Cumulative incidence function (CIF) of death was computed taking into account hospital discharge as a competing event. Comparison of CIFs among subgroups was performed by means of the Gray test. Variables clinically relevant or significantly associated with the risk of death at the univariable analysis were tested in a multiple Cox regression model to identify independent risk factors. The hazard ratios (HRs), 95% confidence intervals (CIs) and P-values from a Wald test were reported. Heterogeneity between HRs calculated for males and females was evaluated including in a Cox regression model of the interaction term between sex and the risk factor of interest. Models were adjusted for age, smoking and comorbidities.
A two-tailed P-value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R version 3.6.1 (R Core Team 2019, Vienna, Austria).
Results
Between 1 March and 9 April 2020, 701 patients with confirmed COVID -19 infection were admitted to the 13 hospitals included in our study; 214 (30.5%) were women and 487 (69.5%) men.
The demographic and clinical characteristics of the patients stratified by sex are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study population at admission stratified by gender (N = 701)
| Female (N = 214) | Male (N = 487) | ||||
| N | N | P-value | |||
| Age (years) | 214 | 68.4 ± 14.0 | 487 | 66.7 ± 12.8 | 0.121 |
| Body mass index ≥30 (kg/m2) | 162 | 31 (19.1) | 378 | 81 (21.4) | 0.627 |
| Smoker (ever) | 182 | 39 (21.4) | 410 | 123 (30.0) | 0.040 |
| Hypertension | 211 | 120 (56.9) | 485 | 278 (57.3) | 0.979 |
| Dyslipidaemia | 211 | 53 (25.1) | 484 | 140 (28.9) | 0.348 |
| Diabetes | 211 | 42 (19.9) | 485 | 120 (24.7) | 0.197 |
| Heart failure | 211 | 24 (11.4) | 485 | 69 (14.2) | 0.371 |
| Atrial fibrillation | 211 | 35 (16.6) | 485 | 71 (14.6) | 0.587 |
| Coronary artery disease | 211 | 39 (18.5) | 485 | 109 (22.5) | 0.279 |
| Prior cardiac surgery or percutaneous valve treatment | 211 | 20 (9.5) | 485 | 51 (10.5) | 0.780 |
| Prior heart transplantation/LVAD | 211 | 0 (0.0) | 485 | 4 (0.8) | 0.320 |
| Chronic obstructive pulmonary disease | 211 | 23 (10.9) | 485 | 45 (9.3) | 0.601 |
| Chronic kidney disease (eGFR <60 ml/min/m2) | 211 | 45 (21.3) | 485 | 83 (17.1) | 0.225 |
| Prior ACEi/ARB therapy | 199 | 70 (35.2) | 459 | 184 (40.1) | 0.271 |
| Prior BB therapy | 198 | 70 (35.4) | 458 | 180 (39.3) | 0.385 |
| Prior anticoagulant therapy | 198 | 28 (14.1) | 452 | 64 (14.2) | 1.000 |
| Prior statin therapy | 198 | 54 (27.3) | 460 | 127 (27.6) | 1.000 |
| Prior calcium antagonist therapy | 200 | 52 (26.0) | 460 | 116 (25.2) | 0.909 |
| Temperature (°C) | 210 | 37.2 ± 0.9 | 478 | 37.3 ± 1.0 | 0.409 |
| Fever (≥37.5°C) | 210 | 85 (40.5) | 478 | 211 (44.1) | 0.417 |
| Respiratory rate ≥22 (bpm) | 182 | 0.0 (0.0–1.0) | 357 | 1.0 (0.0–1.0) | 0.151 |
| SBP (mmHg) | 212 | 129 ± 22 | 476 | 130 ± 22 | 0.383 |
| DBP (mmHg) | 212 | 73 ± 13 | 476 | 75 ± 13 | 0.065 |
| Heart rate (bpm) | 210 | 87 ± 20 | 477 | 87 ± 17 | 0.869 |
| Oxygen saturation (ambient air, %) | 209 | 94 (88–96) | 478 | 92 (87–96) | 0.071 |
| PaO2/FiO2 (mmHg/%) | 184 | 252 (153–326) | 424 | 232 (119–314) | 0.044 |
| PaO2/FiO2 <300 (mmHg/%) | 184 | 124 (67.4) | 424 | 302 (71.2) | 0.394 |
| SOFA score | 153 | 2 (1–3) | 305 | 2 (2–3) | 0.086 |
| COVID score peak | 39 | 5.0 (1.0–10.0) | 132 | 9.0 (3.0–14.0) | 0.012 |
| LV ejection fraction (%) | 82 | 56 (53–60) | 183 | 55 (45–60) | 0.010 |
Data shown as mean ± standard deviation, median (IQR) or count (%).
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; COVID, coronavirus disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FiO2, fraction of inspired oxygen; LV, left ventricular; LVAD, left ventricular assist device; PaO2, oxygen partial pressure at arterial gas analysis; SBP, systolic blood pressure; SOFA, sequential organ failure assessment.
When comparing women and men, we found no significant differences in patients’ age, body mass index and prevalence of comorbidities. The prevalence of smokers was slightly higher in men.
No differences were also found with respect to clinical characteristics at the time of hospitalization except for a lower PaO2/FiO2 ratio in men (median value: 232 mmHg/%, IQR: 119–314) as compared with women (median value: 252 mmHg/%, IQR: 153–326; P = 0.04).
Significant sex-based differences were observed in a number of laboratory analyses performed at the time of hospitalization (Table 2).
Table 2.
Laboratory findings of the study population at admission stratified by gender (N = 701)
| Female (N = 214) | Male (N = 487) | |||||
| Reference range | N | N | P-value | |||
| Red blood cell count (×106/μl) | 4.0–5.2 | 212 | 4.28 (3.77–4.69) | 482 | 4.55 (4.10–5.93) | <0.001 |
| Haemoglobin (g/dl) | 12.0–16.0 | 211 | 12.4 (10.8–13.7) | 480 | 13.6 (12.2–14.7) | <0.001 |
| Haematocrit (%) | 37.0–47.0 | 211 | 37.5 (32.9–40.3) | 479 | 39.8 (36.1–43.2) | <0.001 |
| White blood cell count (per μl) | 4000–10 800 | 212 | 6610 (4715–9003) | 482 | 6945 (5303–9598) | 0.062 |
| Lymphocytes absolute (per μl) | 900–4000 | 189 | 1000 (710–1510) | 436 | 900 (598–1183) | <0.001 |
| Platelets count- (×103/μL) | 130–400 | 211 | 214 (168–294) | 480 | 201 (151–262) | 0.021 |
| Serum creatinine (mg/dl) | 0.60–1.00 | 207 | 0.80 (0.68–1.08) | 478 | 1.02 (0.88–1.38) | <0.001 |
| eGFR (CKD-EPI) ml/min | >80 | 207 | 74 (49–91) | 478 | 76 (52–90) | 0.931 |
| Serum sodium (mEq/l) | 136–145 | 209 | 138 (136–141) | 475 | 138 (135–140) | 0.069 |
| Serum potassium (mEq/l) | 3.4–4.5 | 209 | 3.9 (3.5–4.3) | 472 | 4.0 (3.6–4.4) | 0.047 |
| Serum chloride (mEq/l) | 98–107 | 154 | 101 (99–104) | 356 | 100 (97–103) | 0.071 |
| CRP (mg/dl) | <5.0 | 198 | 36 (8–92) | 455 | 67 (18–150) | <0.001 |
| Procalcitonin (ng/ml) | <0.5 | 98 | 0.10 (0.05–0.26) | 206 | 0.21 (0.09–0.85) | <0.001 |
| Ferritin (μg/l) | 30–400 | 105 | 396 (221–640) | 231 | 915 (504–1729) | <0.001 |
| D-dimer (ng/ml) | <232 | 144 | 871 (445–1864) | 324 | 924 (468–2055) | 0.746 |
| Interleukin-6 (pg/ml) | <7.00 | 47 | 27 (11–54) | 98 | 50 (19–98) | 0.024 |
| Troponin (elevated) | 179 | 77 (43.0) | 435 | 201 (46.2) | 0.527 | |
| NT-proBNP (pg/ml) | <93 | 59 | 341 (98–875) | 168 | 313 (99–1232) | 0.680 |
| Bilirubin (mg/dl) | <1.2 | 186 | 0.5 (0.3–0.6) | 434 | 0.6 (0.5–0.8) | <0.001 |
| Aspartate transaminase (U/l) | 18–39 | 204 | 31 (21–47) | 469 | 44 (30–69) | <0.001 |
| Alanine transaminase (U/l) | 10–50 | 206 | 23 (16–36) | 470 | 37 (25–59) | <0.001 |
| LDH (U/l) | 135–225 | 163 | 305 (225–455) | 403 | 377 (263–545) | <0.001 |
| Creatine phosphokinase (U/l) | 39–308 | 119 | 79 (41–158) | 258 | 144 (65–355) | <0.001 |
| Serum albumin (g/l) | 45–52 | 110 | 3.2 (2.7–3.6) | 272 | 3.2 (2.8–3.6) | 0.980 |
| INR | 0.9–1.2 | 192 | 1.1 (1.0–1.2) | 439 | 1.1 (1.0–1.2) | 0.330 |
| ABG test pH | 7.37–7.45 | 190 | 7.46 (7.43–7.49) | 428 | 7.47 (7.43–7.50) | 0.339 |
| ABG test lactate (mmol/l) | 0.5–2.2 | 156 | 1.1 (0.8–1.4) | 353 | 1.3 (0.9–1.7) | <0.001 |
Data shown as median (IQR) or count (%).
ABG, arterial blood gas; CKD-EPI, chronic kidney disease epidemiology collaboration formula; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; INR, international normalized ratio; LDH, lactate dehydrogenase; NT-proBNP, N-terminal fragment of the prohormone brain natriuretic peptide.
As compared with men, women had higher levels of lymphocytes [median values (IQR) in women: 1000/μl (710–1510) vs. 900/μl (598–1183) in men; P-value <0.001], and platelets [214 000/μl (168 000–293 500) in women vs. 200 500/μl (151 000–262 250) in men; P = 0.02].
On the contrary, men had significantly higher levels of all the laboratory markers associated with systemic inflammation, including C-reactive protein (CRP, median value: 67 mg/dl vs. 36 mg/dl, P < 0.001), procalcitonin (median value: 0.21 ng/ml vs. 0.1 ng/ml; P < 0.001), Ferritin (median value: 915 μg/l vs. 396 μg/l; P < 0.001) and Interleukin-6 (median value: 50 pg/ml vs. 27 pg/ml; P = 0.02)
Table 3 reports details on in-hospital patients’ management and outcome according to sex. Regarding treatment, no differences were found between sexes, with the only exception being tocilizumab, which was administered more often in men than in women (13.5% vs. 7%; P = 0.02).
Table 3.
In-hospital management and outcomes of the study population stratified by gender (N = 701)
| Female (N = 214) | Male (N = 487) | ||||
| N | N | P-value | |||
| Hospital length of stay (days) | 214 | 14.0 (9.0–23.0) | 487 | 15.0 (9.0–25.0) | 0.436 |
| Pharmacological treatment | |||||
| Lopivanir/Ritonavir | 213 | 51 (23.9) | 483 | 138 (28.6) | 0.241 |
| Darunavir/Ritonavir | 213 | 40 (18.8) | 483 | 135 (28.0) | 0.013 |
| Remdesivir | 213 | 1 (0.5) | 483 | 4 (0.8) | 1.000 |
| Corticosteroid | 213 | 101 (47.4) | 483 | 244 (50.5) | 0.502 |
| Tocilizumab | 213 | 15 (7.0) | 483 | 65 (13.5) | 0.021 |
| Hydroxychloroquine | 213 | 170 (79.8) | 483 | 415 (85.9) | 0.055 |
| Antibiotics | 213 | 179 (84.0) | 483 | 432 (89.4) | 0.060 |
| Ventilatory support | |||||
| Oxygen support with FiO2 <50% | 211 | 82 (38.9) | 482 | 220 (45.6) | 0.116 |
| Oxygen support with FiO2 ≥50% | 209 | 101 (48.3) | 475 | 281 (59.2) | 0.011 |
| Noninvasive ventilation | 211 | 76 (36.0) | 484 | 226 (46.7) | 0.011 |
| Intubation | 211 | 21 (10.0) | 486 | 87 (17.9) | 0.011 |
| Complication | |||||
| ARDS | 185 | 38 (20.5) | 429 | 132 (30.8) | 0.012 |
| Sepsis | 211 | 21 (10.0) | 469 | 47 (10.0) | 1.000 |
| Acute renal insufficiency | 151 | 14 (9.3) | 342 | 58 (17.0) | 0.037 |
| Multiorgan failure | 150 | 7 (4.7) | 334 | 27 (8.1) | 0.243 |
| STEMI | 211 | 2 (0.9) | 477 | 9 (1.9) | 0.518 |
| NSTEMI | 181 | 5 (2.8) | 378 | 12 (3.2) | 0.998 |
| Heart failure | 181 | 12 (6.6) | 378 | 40 (10.6) | 0.177 |
| Ventricular arrhythmia | 211 | 2 (0.9) | 477 | 6 (1.3) | 1.000 |
| Pulmonary embolism | 211 | 11 (5.2) | 478 | 38 (7.9) | 0.260 |
| Other embolism | 211 | 2 (0.9) | 478 | 14 (2.9) | 0.168 |
| Stroke | 211 | 2 (0.9) | 478 | 1 (0.2) | 0.224 |
| Major bleeding | 182 | 10 (5.5) | 378 | 22 (5.8) | 1.000 |
| Delirium | 149 | 6 (4.0) | 334 | 12 (3.6) | 1.000 |
| Outcome | |||||
| Death | 214 | 39 (18.2) | 487 | 126 (25.9) | 0.036 |
| Cause of deatha | |||||
| Respiratory insufficiency | 39 | 28 (71.8) | 123 | 98 (79.7) | 0.418 |
| Myocardial infarction | 39 | 3 (7.7) | 123 | 2 (1.6) | 0.091 |
| Pulmonary embolism | 39 | 3 (7.7) | 123 | 10 (8.1) | 1.000 |
| Stroke | 39 | 0 (0.0) | 123 | 4 (3.3) | 0.573 |
| Multiorgan failure | 39 | 10 (25.6) | 123 | 36 (29.3) | 0.815 |
| Bleeding | 39 | 1 (2.6) | 123 | 4 (3.3) | 1.000 |
Data shown as median (IQR) or count (%).
Multiple causes of death allowed.
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction.
Despite similar demographic characteristics and treatments, major sex-based differences in outcomes were observed. As compared with women, a larger number of men required noninvasive ventilation (46.7% vs. 36%; P = 0.01) or intubation (17.9% vs. 10%; P = 0.01). Furthermore, a larger number of men developed major complications including ARDS (30.8% vs. 20.5%; P = 0.01) and acute kidney failure (17.3% and 9.3%, P = 0.04). Overall, 39 (18.2%) and 126 (25.9%) deaths occurred in women and in men respectively (P = 0.036).
Among 452 discharged patients, the median time from hospital admission to discharge was 14.5 days (IQR 9.0–23.0), whereas among 165 patients deceased during hospitalization, the median time to death was 10.0 days (IQR 6.0–17.0).
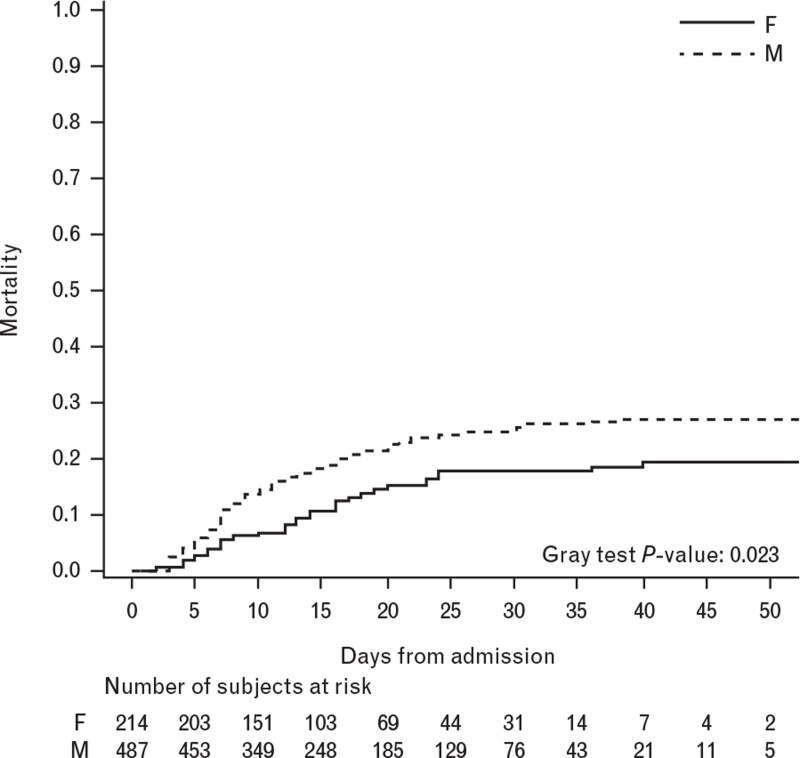
During a median follow-up time of 15 days, the cumulative incidence of death, computed taking into account hospital discharge as a competing event, was higher among males than females during the follow-up (Gray test P-value: 0.023) (Fig. 1).
Fig. 1.
Cumulative incidence of death during hospitalization stratified by gender (N = 701).
At univariate Cox regression analysis, a significantly higher risk of death was associated with demographic factors, including elderly age and smoking; laboratory variables, including lower levels of lymphocytes, red blood cells and oxygen saturation and higher levels of CRP, troponin and lactate dehydrogenase; and presence of several comorbidities, including hypertension, cardiovascular (CV) diseases, chronic obstructive pulmonary disease and chronic kidney disease. Using the multivariable Cox regression model, including all these variables, male patients had a nonsignificantly higher risk of death as compared with women [overall survival hazard ratio (OS-HR) 1.59, 95% CI 0.96–2.64 P = 0.07, Table 4].
Table 4.
Univariable and multivariable Cox regression model for death
| Univariable | Multivariable (N = 459) | |||||
| Level/units | N | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Baseline variables | ||||||
| Age | +5 years | 701 | 1.36 (1.27–1.46) | <0.001 | 1.27 (1.13–1.42) | <0.001 |
| Sex | M vs. F | 701 | 1.35 (0.94–1.94) | 0.099 | 1.59 (0.96–2.64) | 0.074 |
| Body mass index | ≥30 vs. <30 (kg/m2) | 540 | 1.22 (0.79–1.87) | 0.376 | ||
| Smoker (ever) | Yes vs. No | 592 | 1.45 (1.02–2.07) | 0.040 | ||
| Respiratory rate | ≥22 vs. <22 | 539 | 1.68 (1.16–2.43) | 0.006 | ||
| SBP | +10 mmHg | 688 | 0.94 (0.87–1.01) | 0.084 | ||
| Oxygen saturation | +5% | 687 | 0.83 (0.77–0.89) | <0.001 | 0.84 (0.75–0.95) | 0.004 |
| PaO2/FiO2 | +50 mmHg/% | 608 | 0.87 (0.81–0.94) | <0.001 | 0.90 (0.80–1.01) | 0.060 |
| SOFA | +1 point | 458 | 1.39 (1.29–1.50) | <0.001 | ||
| Red blood cell count | +0.5 ×106/μl | 694 | 0.84 (0.75–0.94) | 0.002 | ||
| White blood cell count | +1000 U/μl | 694 | 1.03 (1.00–1.06) | 0.026 | ||
| Lymphocytes | <900 vs. ≥900 | 625 | 2.12 (1.51–2.97) | <0.001 | 1.60 (1.04–2.47) | 0.032 |
| Platelets | +50 ×103/μl | 691 | 0.91 (0.83–0.99) | 0.030 | 0.86 (0.76–0.98) | 0.021 |
| Creatinine | +1 mg/dl | 685 | 1.13 (1.06–1.21) | <0.001 | ||
| eGFR (CKD-EPI) | +10 ml/min | 685 | 0.82 (0.78–0.86) | <0.001 | 0.93 (0.84–1.03) | 0.136 |
| CRP | +10 mg/l | 653 | 1.02 (1.01–1.04) | 0.001 | 1.03 (1.01–1.05) | 0.016 |
| Procalcitonin | +0.5 ng/ml | 304 | 1.00 (0.99–1.01) | 0.666 | ||
| Ferritin | +100 μg/l | 336 | 1.01 (1.00–1.02) | 0.123 | ||
| D-dimer | +1000 ng/ml | 468 | 1.02 (0.99–1.05) | 0.167 | ||
| Interleukin-6 | +10 pg/ml | 145 | 1.00 (1.00–1.01) | 0.215 | ||
| Troponin | Elevated vs. normal | 614 | 3.22 (2.26–4.59) | <0.001 | 1.63 (1.06–2.50) | 0.026 |
| NT-proBNP | +1000 ng/l | 227 | 1.03 (1.00–1.05) | 0.036 | ||
| Bilirubin | +0.3 mg/dl | 620 | 1.07 (0.97–1.19) | 0.167 | ||
| LDH | +1000 mg/dl | 566 | 1.12 (1.05–1.19) | <0.001 | ||
| Bilirubin | +0.3 mg/dl | 620 | 1.07 (0.97–1.19) | 0.167 | ||
| INR | +1 | 631 | 1.22 (1.03–1.44) | 0.024 | ||
| ABG test lactate | +1 mmol/l | 509 | 1.22 (1.14–1.30) | <0.001 | ||
| Comorbidities | ||||||
| Hypertension | Yes vs. no | 696 | 1.91 (1.37–2.67) | <0.001 | 1.11 (0.70–1.75) | 0.657 |
| Diabetes | Yes vs. no | 696 | 1.34 (0.95–1.87) | 0.096 | ||
| Heart failure | Yes vs. no | 696 | 2.45 (1.70–3.52) | <0.001 | 1.97 (1.16–3.36) | 0.013 |
| Atrial fibrillation | Yes vs. no | 696 | 2.48 (1.74–3.53) | <0.001 | 1.27 (0.76–2.12) | 0.361 |
| Coronary artery disease | Yes vs. no | 696 | 2.28 (1.65–3.16) | <0.001 | 1.09 (0.69–1.72) | 0.722 |
| Chronic obstructive pulmonary disease | Yes vs. no | 696 | 1.76 (1.14–2.71) | 0.011 | 1.50 (0.86–2.63) | 0.154 |
| Chronic kidney disease | Yes vs. no | 696 | 2.80 (2.03–3.87) | <0.001 | 0.89 (0.51–1.56) | 0.687 |
| Medication history | ||||||
| Prior ACEi-ARBS therapy | Yes vs. no | 658 | 1.56 (1.14–2.13) | 0.006 | ||
| Prior BB therapy | Yes vs. no | 656 | 1.99 (1.45–2.72) | <0.001 | ||
| Prior statin therapy | Yes vs. no | 658 | 1.88 (1.36–2.61) | <0.001 | ||
| Prior calcium antagonists therapy | Yes vs. no | 660 | 1.39 (0.99–1.95) | 0.055 | ||
ABG, arterial blood gas; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; CKD-EPI, chronic kidney disease epidemiology collaboration formula; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FiO2, fraction of inspired oxygen; INR, international normalized ratio; LDH, lactate dehydrogenase; NT-proBNP, N-terminal fragment of the prohormone brain natriuretic peptide; PaO2, oxygen partial pressure at arterial gas analysis; SBP, systolic blood pressure; SOFA, sequential organ failure assessment.
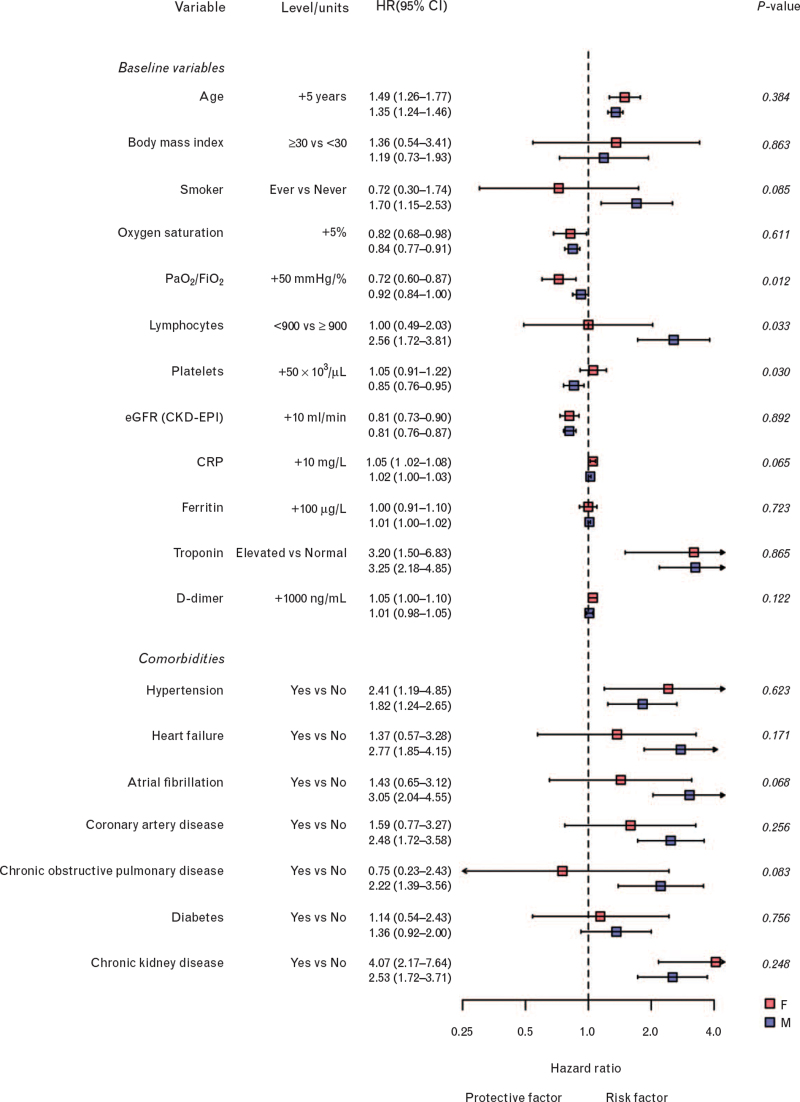
A significant interaction with sex was found in the association between patients’ risk of death and some laboratory variables, namely lymphocytes count, platelets count and PaO2/FiO2 ratio (Fig. 2).
Fig. 2.
Forest plot comparing association of baseline variables and comorbidities with risk of death between females and males.
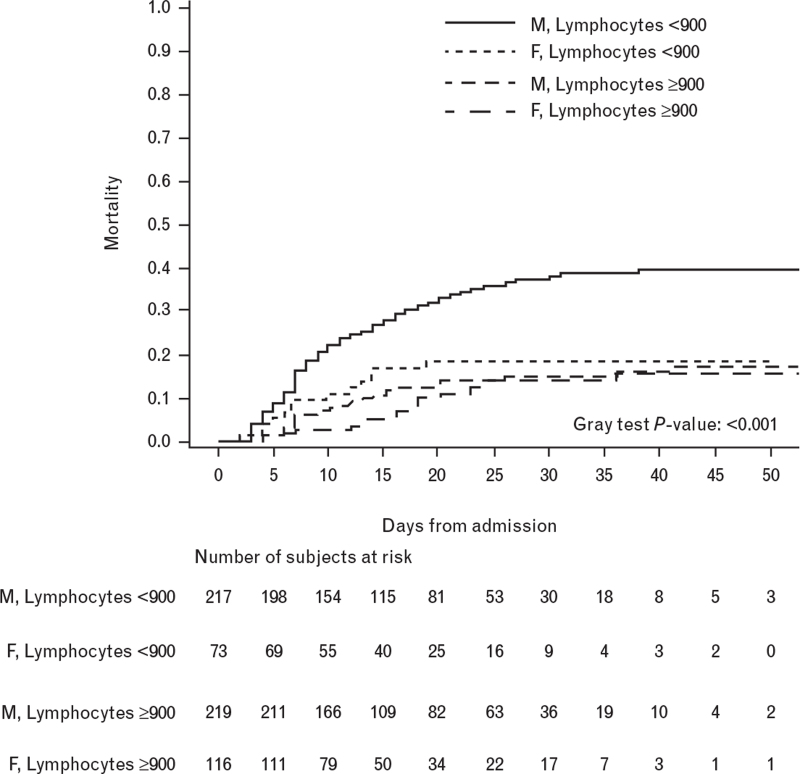
Fig. 3.
Cumulative incidence of death during hospitalization stratified by gender and lymphocytes at admission (N = 625).
The risk of death was more than double in male patients with a low lymphocytes count (i.e. below the median value of 900/μl) as compared with men with a high lymphocytes count (i.e. above 900/μl; OS-HR 2.56, 95% CI 1.72–3.81), whereas it was not different in women with low or high lymphocytes counts (OS-HR 1.00, 95% CI 0.49–2.03; P for heterogeneity = 0.03).
Such interaction remained significant on multivariable Cox regression analysis after adjusting, respectively, for patients’ age (P for heterogeneity = 0.04) or age and comorbidities (P for heterogeneity = 0.03) or age, comorbidities and patients’ smoking habitus (P for heterogeneity = 0.05) (Table 5).
Table 5.
Unadjusted and adjusted Cox regression HRs (95% CIs) for death stratified by sex, and P-values for heterogeneity between strata
| Unadjusted | Adjusted for age | Adjusted for age and comorbiditiesa | Adjusted for age, smoke and comorbiditiesa | ||||||
| Sex | HR (95%CI) | P-value | HR (95% CI) | P-value | HR (95%CI) | P-value | HR (95% CI) | P-value | |
| Platelets | F | 1.05 (0.91–1.22) | 0.030 | 1.05 (0.91–1.22) | 0.029 | 1.05 (0.91–1.22) | 0.031 | 1.07 (0.92–1.25) | 0.091 |
| M | 0.85 (0.76–0.95) | 0.86 (0.77–0.96) | 0.86 (0.77–0.96) | 0.88 (0.78–1.00) | |||||
| Lymphocytes (<900 vs. ≥900) | F | 1.00 (0.49–2.03) | 0.033 | 1.00 (0.49–2.05) | 0.039 | 1.01 (0.49–2.06) | 0.033 | 1.18 (0.56–2.49) | 0.051 |
| M | 2.56 (1.72–3.81) | 2.44 (1.64–3.63) | 2.49 (1.66–3.73) | 2.60 (1.65–4.10) | |||||
| PaO2/FiO2 | F | 0.72 (0.60–0.87) | 0.012 | 0.69 (0.56–0.85) | 0.024 | 0.69 (0.56–0.85) | 0.022 | 0.72 (0.59–0.89) | 0.053 |
| M | 0.92 (0.84–1.00) | 0.88 (0.80–0.96) | 0.88 (0.80–0.97) | 0.88 (0.80–0.98) | |||||
CI, confidence interval; HR, hazard ratio.
At least one of: obesity, hypertension, diabetes, heart failure, atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, or chronic kidney disease.
Similarly, a higher platelets count was associated with a reduction in the risk of death in men (OS-HR for increase of 50 × 103 units: 0.88, 95% CI 0.78–1.00) but not in women (1.07, 95% CI 0.92–1.25) although this interaction did not reach statistical significance (adjusted P for heterogeneity = 0.09). The association between a higher PaO2/FiO2 ratio and better survival was larger in women (OS-HR for increase of 50 mmHg/%: 0.72, 95% CI 0.59–0.89) as compared with men (OS-HR 0.88, 95% CI 0.80–0.98; adjusted P for heterogeneity = 0.05).
There were also trends for a different impact of cardiovascular comorbidities on patients’ outcome according to sex, although no significant difference was found (Fig. 2).
Discussion
In this large multicenter retrospective cohort analysis, the risk of death from COVID-19 was numerically higher in men as compared with women. Men also had an increased rate of untoward events or complications, including invasive ventilation, ARDS and acute renal failure. These differences in outcomes were found despite similar demographic and clinical characteristics, including comorbidities and treatment between the two sexes.
Different mortality rates between men and women affected by COVID-19 have been reported.3–7 Several explanations have been postulated to account for such a difference, ranging from sex differences in immune responses to mere association with a different prevalence of chronic comorbidities or different behaviors in men and women.3
In this analysis, we have not found differences between men and women regarding the prevalence of major cardiovascular and respiratory comorbidities, and also the pharmacological management of COVID-19 was similar in the two groups.
This finding is in keeping with a recent national Italian registry showing no significant differences in these relevant clinical variables according to sex, but a worse prognosis for male patients.7
The statistically significant and clinically meaningful difference in mortality was also confirmed after adjusting for other factors not balanced between the two groups, and potentially affecting patients’ prognosis, such us smoking habitus.
In addition, we identified differences in a number of relevant factors associated with innate and adaptive immune responses between men and women, including different degrees of lymphocytopenia and blood levels of markers of systemic inflammation and showed a significant sex-based heterogeneity in their prognostic role.
Our results support the hypothesis of the importance of biological intrinsic factors, such as differences in immune responses, in the pathogenesis of sex-based dimorphism of the course and severity of COVID-19 infection.
New findings recently published in the Nature journal, further support such a hypothesis, revealing several molecular differences in immune responses during the disease course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in male and female patients. It has been shown that several key elements of innate response are greater in men, such as higher plasma levels of IL-8 and IL-18 immune cytokines, whereas women mounted significantly more robust T cell activation during SARS-CoV-2 infection.9
Relevant differences of both innate and adaptive immune responses between men and women explain the different prevalence and mortality from infectious and autoimmune diseases and from several types of cancers.1,2
These sex-based differences of immune responses reflect complex interactions among genes, hormones, and the environment.1,2
The X chromosome contains a large number of immune-related genes.10
Immune-related genes encoded on the X chromosome may escape X inactivation, resulting in higher expression levels in women than men.1,2
Sex hormones modulate the development and function of multiple immune cell populations.1,2 Putative androgen response elements (AREs) and estrogen response elements (EREs) are present in the promoters of several innate and adaptive immune genes, suggesting that sex steroids may directly regulate their expression.1,2
In-vivo studies showed that male mice were more susceptible to COVID-19 infection. Hormonal suppression through an oestrogen receptor antagonist or ovariectomy increased mortality in female mice, demonstrating the protective role of oestrogens against COVID-19.11
The COVID-19 spike protein binds to the ACEII receptor on human cells, and the human protease TMPRSS2 activates the spike protein and allows the viral entry, being paramount for viral spread and pathogenesis in the infected host.12
The human TMPRSS2 gene promoter has an ARE and androgens are positive regulators of its transcription.13 An allelic variant predicted to induce higher levels of TMPRSS2 has been recently found as being more frequent in the Italian than in the East Asian population.14
Although its relation with tissue levels may vary, plasma levels of the COVID-19 receptor in human cells, ACE2, were significantly higher in males compared with females in a recent study of patients with heart failure, consistently with the increased susceptibility and more severe clinical course of COVID-19 in men.15 Taken together all these intrinsic biological factors could explain the higher virulence of COVID-19 in men.13
Obviously, a different prevalence of comorbidities could further contribute to the worsening of the prognosis for men. Indeed, in our cohort of patients, chronic CV comorbidities had a significant association with worse prognosis, particularly in male patients.
Limitations
The retrospective nature of our analyses is the major limitation of the study.
However, the availability of individual data of a large cohort of patients allowed us to adjust analyses for sex-related differences in the most relevant variables associated with patients’ prognosis, including age, comorbidities and smoking status.
Furthermore, we did not have data on the menopausal status of women as well as on hormonal replacement therapy in postmenopausal women, preventing the possibility to assess the association between sex-hormonal status and the prognosis of female patients with COVID-19.16
Conclusion
In conclusion, our results demonstrate that sex is a variable that may influence patients’ prognosis in COVID-19 independently from other known factors, particularly comorbidities and smoking habitus.
Furthermore, they also highlight the need to take patients’ sex into account when evaluating the risk of death from COVID-19, not only because it is a meaningful independent prognostic factor, but also because there is a relevant sex-based heterogeneity in the association between several other factors and patients’ risk of death. Future research should investigate the biological mechanisms that drive the pathogenesis of the sex-based dimorphism of COVID-19 virulence.
Indeed, to identify the molecular mechanisms underlying the different prognosis between men and women could have relevant implications, including the possibility to tailor specific preventive strategies and therapeutic approaches for women and men, in order to improve outcomes for both.
Acknowledgements
Author contributions: Marco Metra, Carlo Lombardi and Claudia Specchia had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Carlo Lombardi and Claudia Specchia contributed equally to this work and are co-first authors.
Author contributions: V.C. received consulting honoraria from CVie Therapeutics Limited, Servier, and Windtree Therapeutics.
P.A. received speaker and advisor honoraria from Novartis, AstraZeneca, Vifor, Daiichi-Sankyo, Boehringer Ingelheim, Pfizer, GSK and MSD.
A.M. reports personal consulting honoraria from Novartis, Servier, Astra Zeneca for participation in advisory board meetings and receives grants from Novartis and Niccomo for research trials.
M.P. received a research grant and speaking fees from Novartis, Servier, Vifor.
M.M. reports personal consulting honoraria from Bayer, Novartis, Fresenius, Servier, and Windtree Therapeutics for participation to advisory board meetings and executive committees of clinical trials.
Funding: This research was not supported by any public or private funding.
Conflicts of interest
There are no conflicts of interest.
Additional information: List of centers and collaborators: Additional Supporting Information may be found in Appendix S1.
Footnotes
Drs Carlo Mario Lombardi and Claudia Specchia contributed equally to this study.
References
- 1.Klein S, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–638. [DOI] [PubMed] [Google Scholar]
- 2.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol 2014; 35:97–104. [DOI] [PubMed] [Google Scholar]
- 3.Wenham C, Smith J, Morgan R, et al. COVID-19: the gendered impacts of the outbreak. Lancet 2020; 395:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith J. Overcoming the ‘tyranny of the urgent’: integrating gender into disease outbreak preparedness and response. Gender Dev 2019; 27:355–369. [Google Scholar]
- 5.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19). China CDC Weekly 2020; 2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The COVID-19 Task force of the Department of Infectious Diseases and the IT Service Istituto Superiore di Sanità. Integrated surveillance of COVID-19 in Italy. Available at: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_16-aprile-2020.pdf. [Accessed 16 April 2020 update] [Google Scholar]
- 8.Qin L, Li X, Shi J, et al. Gendered effects on inflammation reaction and outcome of COVID-19 patients in Wuhan. J Med Virol 2020; 92:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; 588:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010; 10:594–604. [DOI] [PubMed] [Google Scholar]
- 11.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol 2017; 198:4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pala L, Conforti F. Sex-based dimorphism in the SARS-CoV2 virulence. J Intern Med 2020; 288:477–478. [DOI] [PubMed] [Google Scholar]
- 14.Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020; 12:10087–10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 2020; 41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anna Vittoria Mattioli, Susanna Sciomer, Federica Moscucci, et al. Cardiovascular prevention in women: a narrative review from the Italian Society of Cardiology working groups on ‘cardiovascular prevention, hypertension and peripheral circulation’ and on ‘women disease’. J Cardiovasc Med (Hagerstown) 2019; 20:575–583. [DOI] [PubMed] [Google Scholar]