Abstract
Multicystic dysplastic kidney (MCDK) is one of the most common fetal malformations, but its etiology remains unclear. Identification of the molecular etiology could provide a basis for prenatal diagnosis, consultation, and prognosis evaluation for MCDK fetuses. We used chromosome microarray analysis (CMA) and whole-exome sequencing (WES) to conduct genetic tests on MCDK fetuses and explore their genetic etiology. A total of 108 MCDK fetuses with or without other extrarenal abnormalities were selected. Karyotype analysis of 108 MCDK fetuses showed an abnormal karyotype in 4 (3.7%, 4/108) of the fetuses. However, CMA detected 15 abnormal copy number variations (CNVs) (14 pathogenic CNVs, and one variant of unknown significance [VUS] CNVs), in addition to four cases that were consistent with the results of karyotype analysis. Out of the 14 pathogenic CNVs cases, three were of 17q12 microdeletion, two of 22q11.21 microdeletion, 22q11.21 microduplication uniparental disomy (UPD), and one case of 4q31.3q32.2 microdeletion, 7q11.23 microduplication, 15q11.2 microdeletion, 16p11.2 microdeletion, and 17p12 microdeletion. Of the 89 MCDK fetuses with normal karyotype analysis and CMA, 15 were tested by WES. Two (13.3%, 2/15) fetuses were identified by WES as Bardet-Biedl syndrome (BBS) 1 and BBS2. Combined application of CMA-WES to detect MCDK fetuses can significantly improve the detection rate of genetic etiology, providing a basis for consultation, and prognosis evaluation.
Keywords: Multicystic dysplastic kidney, etiology, mutation, microdeletion, duplication, follow-up
Impact Statement
This study describes the importance of coupling the uses of chromosome microarray analysis (CMA) and whole-exome sequencing (WES) to genetically test multicystic dysplastic kidney (MCDK). MCDK is one of the most common fetal malformations, despite unclear etiology. A total of 108 MCDK fetuses with or without other extrarenal abnormalities were explored. We believe that our study makes a significant contribution to the literature because the combined application of CMA-WES to detect MCDK fetuses can significantly provide a basis for prenatal diagnosis, consultation, and prognosis evaluation of MCDK fetuses. Our study identified the novel microdeletions 4q33.1 q32.2, 15q11.2, 16p11.2, and 17p12 in MCDK cases, enriching the disease spectrum of the above microdeletions and microduplications.
Introduction
Multicystic dysplastic kidney (MCDK) is a congenital developmental abnormality with ureteral atresia, and is one of the most common birth defects, with an incidence of 1/4300 in newborns. 1 In the prenatal ultrasound phenotype, fetus with MCDK often presents with renal enlargement and enhanced echo of the renal parenchyma. When both kidneys are involved, oligohydramnios and inadequate bladder may also be present. 94.1% of MCDK cases are diagnosed using prenatal ultrasound. 2 The prognosis of fetuses with bilateral renal involvement was poor, while 39% of those with unilateral renal involvement still had contralateral renal malformations or other extrarenal dysplasia. 3
MCDK is one of the most common fetal malformations; however, its etiology remains unclear. Recent studies have shown that MCDK is associated with copy number variations (CNVs).4 –7 Chromosome microarray analysis (CMA) is a molecular technology that can cover whole-genome DNA with high-throughput, high-resolution, and fast-detection speed. This technology can detect not only microdeletion/microduplication (<5Mb), but also chimerism (>20%) and uniparental disomy (UPD).8,9 At present, CMA has been used in the detection of fetal malformations, as well as tumor genetic diagnosis and other fields.10,11 However, whole-exome sequencing (WES) can detect the relevant variation of most diseases in the exon region, and only needs to sequence approximately 1% of exons, and has quickly become an effective strategy for screening pathogenic genes susceptible to complex diseases.12 –14 WES has gradually been used in clinics, due to the low cost of testing. In this study, 108 fetuses with MCDK were analyzed using karyotype analysis and CMA, and 15 fetuses with normal karyotype analysis and CMA; results were analyzed using WES to explore the genetic etiology and provide a genetic basis for prenatal diagnosis and prognosis evaluation of MCDK fetuses.
Materials and methods
Patients
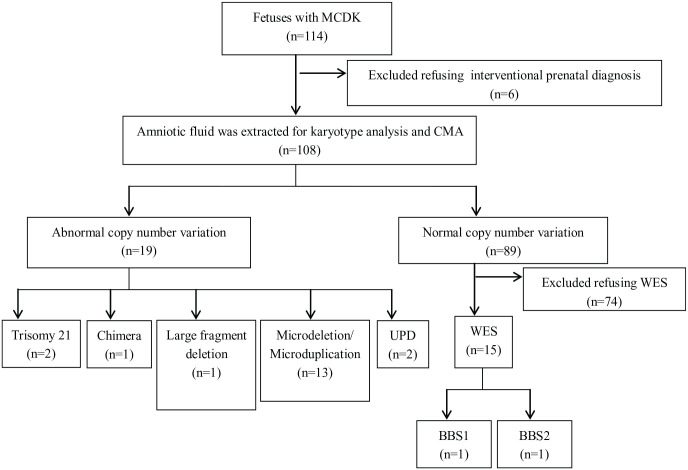
A total of 114 cases of MCDK fetuses identified by prenatal ultrasound at tertiary referral hospitals were included in the study between January 2016 and December 2021. Six patients refused interventional prenatal diagnosis after genetic counseling, and 108 subjects eventually provided informed consent for interventional prenatal diagnosis. Amniotic fluid was extracted from 108 cases for karyotype analysis and CMA, among which 15 MCDK fetuses with normal karyotypes and CMA were simultaneously analyzed using WES (Figures 1 and 2). The 108 cases ranged in age from 21 to 43 years and gestational age from 16 to 24 weeks. The cases were divided into isolated MCDK and non-isolated MCDK groups based on the fetal ultrasound phenotype. In this study, 64 cases (59.3%, 64/108) of isolated MCDK were identified as MCDK only, and no other ultrasound abnormalities were found. There were 44 cases (40.7%, 44/108) of non-isolated MCDK with other ultrasound abnormalities.
Figure 1.
Enrollment of pregnant women between January 2016 and December 2021.
Figure 2.

The intrauterine ultrasound image of a fetus with MCDK.
Karyotype analysis
Amniotic fluid (40 mL) was extracted from abdomen under ultrasound-guided extraction (20 mL for karyotype analysis, 10 mL for CMA, and 10 mL for WES). Under aseptic operation conditions, the extracted amniotic fluid was loaded into an aseptic centrifugal tube to collect amniotic fluid cells. The adherent cell culture method was used for 10–14 days. When the cells grew well, colchicine was added, and amniotic fluid cells were collected. Routine section, G banding (C and N banding if necessary), karyotype collection by GSL–120 automatic chromosome scanning platform, and karyotype analysis.
Chromosome microarray analysis
Amniotic fluid from 108 fetuses was collected by centrifugation. Genomic DNA was extracted from amniotic fluid cells using QIAamp DNA Blood Mini Kit. The procedure was performed according to the instructions provided by Affymetrix. The CMA results were analyzed using the supporting Chromosome Analysis Suite (ChAS) V3.2, and CNV properties were determined by analyzing the CMA results with related databases. Reference databases include internal and online public databases. Based on the corresponding criteria, 15 CNV properties were divided into pathogenic CNVs, likely pathogenic CNVs, variants of unknown significance (VUS), benign CNVs, and likely benign CNVs. For fetuses with VUS, it is recommended that parents’ peripheral blood samples be tested for CMA combined with pedigree analysis to further clarify the nature of CNV.
WES
In this study, 15 MCDK fetuses with normal karyotypes and CMA were further examined using WES. A Bioruptor was used to fragment DNA to approximately 200–250 bp. DNA library construction kit (Illumina, FC-121-2003), including terminal complementing, 3-terminal adenylation, splicing, fragment selection, and amplification. The target area was captured using NimbleGen magnetic beads and Hybridization and Wash Kit, followed by polymerase chain reaction (PCR) amplification with high-fidelity DNA polymerase (Roche). PE100 sequencing was performed in the HiSeq 2500 high-throughput mode. Mutations were annotated according to the American College of Medical Genetics and Genomics guidelines, and the effects of protein function and shear hazards were predicted. Finally, the obtained mutation sites were determined and divided into the following five categories: pathogenic, likely pathogenic, VUS, likely benign and benign. 16 Fetuses and parents with pathogenic and likely pathogenic mutants were verified using Sanger sequencing.
Follow-up of pregnancy outcome
All pregnant women were followed-up over telephonic call for pregnancy outcomes and postnatal conditions.
Statistical analysis
SPSS 20.0 (IBM) software was used for data processing. The chi-square test was applied to analyze the detection rate of pathogenic genome between groups, and P < 0.05 was considered to be statistically significant.
Results
Karyotype analysis results
Karyotype analysis detected abnormalities in four (3.7%, 4/108) of the 108 MCDK fetuses, including two cases of trisomy 21 syndrome, one case of chimerism (47, XX + 9[29]/46, XX[26]), and one case of 4p16.3p15.1 large fragment deletion (Table 1). After genetic counseling, the parents of four MCDK fetuses with chromosomal abnormalities were chosen to terminate pregnancy.
Table 1.
Abnormal karyotype results of fetuses with MCDK.
| Case | Karyotype | CMA results | Ultrasonic phenotype | Postnatal outcome |
|---|---|---|---|---|
| 1 | 47, XY,+21 | arr[hg19](21)x3 | Bilateral MCDK, Strong echo in left ventricle, Enhanced intestinal echo | TP |
| 2 | 47, XY,+21 | arr[hg19](21)x3 | Bilateral MCDK, FGR, CHD | TP |
| 3 | 47, XX + 9[29]/46, XX[26] | arr[hg19] (9)x2~3, (XX)x1 | Bilateral MCDK, Right kidney seeper, Right ureterectasia, Right foot deformity | TP |
| 4 | 46, XY,del(4)(p15) | arr[hg19]4p16.3p15.1(68,345-35,252,743)x1 | Bilateral MCDK, FGR, Nasal bone dysplasia | TP |
CMA: chromosomal microarray analysis; CHD: congenital heart disease; MCDK: Multicystic dysplastic kidney; FGR: growth restriction; TP: termination of pregnancy.
Chromosome microarray analysis
A total of 108 cases were tested by CMA simultaneously, and 19 abnormal CNVs were detected, including 18 pathogenic CNVs (16.7%, 18/108) and one VUS (0.9%, 1/108). Compared with karyotype analysis, 15 additional abnormal CNVs (including 14 pathogenic CNVs and one VUS) were detected by CMA, in addition to the four cases that were consistent with karyotype analysis. The 14 cases of pathogenic CNVs included three cases of 17q12 microdeletion, two cases of 22q11.21 microdeletion, 22q11.21 microduplication, UPD, and one case of 4q31.3q32.2, microdeletion, 7q11.23, 15q11.2, 16p11.2, and 17p12 microdeletions, respectively (Table 2). After genetic counseling, the parents of 18 fetuses with pathogenic CNV (including four cases of abnormal karyotype and 14 cases of microdeletion or microduplication) chose to terminate the pregnancy.
Table 2.
Abnormal CNVs in fetuses with MCDK.
| Case | CMA results | Size (Mb) | Ultrasonic phenotype | Interpretation | Inheritance | Postnatal outcome |
|---|---|---|---|---|---|---|
| 1 | arr[hg19] 22q11.21(18,916,842-21,800,471)x1 | 2.8 | Right MCDK | P | – | TP |
| 2 | arr[hg19] 22q11.21(20,730,143-21,800,471)x1 | 1.0 | Right MCDK, Polyhydramnios, Strephenopodia | P | – | TP |
| 3 | arr[hg19] 22q11.21(20,730,143-21,800,471)x3 | 1.0 | Right MCDK | P | de novo | TP |
| 4 | arr[hg19] 22q11.21(20,730,143-21,800,471)x3 | 1.0 | Left MCDK | P | de novo | TP |
| 5 | arr[hg19] 17q12(34,822,465-36,311,009)x1 | 1.4 | Bilateral MCDK, Mild tricuspid regurgitation | P | de novo | TP |
| 6 | arr[hg19] 17q12(34,822,465-36,243,365)x1 | 1.4 | Bilateral MCDK | P | - | TP |
| 7 | arr[hg19] 17q12(34,822,465-36,307,773)x1 | 1.48 | Bilateral MCDK | P | - | TP |
| 8 | arr[hg19] 4q31.3q32.2(155,463,038-162,158,990)x1 | 6.7 | Right MCDK, Enhanced intestinal echo | P | de novo | TP |
| 9 | arr[hg19] 7q11.23(72,701,098-74,069,645)x3 | 1.3 | Left MCDK, Ventricular septal defect | P | de novo | TP |
| 10 | arr[hg19] 15q11.2(22,770,421-23,277,436)x1 | 0.83 | Right MCDK | P | – | TP |
| 11 | arr[hg19] 16p11.2(29,428,531-30,177,916)x1 | 0.73 | Left MCDK, Ureterectasia, Single umbilical artery | P | – | TP |
| 12 | arr[hg19] 17p12(14,083,054-15,482,833)x1 | 1.4 | Left MCDK | P | Maternal | TP |
| 13 | arr[hg19] 16q23.2q24.3(79,800,878-90,146,366)hmz,16p13.3p12.3(94,807-19,302,326) hmz | – | Left MCDK, FGR, Ventricular septal defect, Aortarctia | P | UPD (Maternal) | TP |
| 14 | arr[hg19] 2p25.3p11.2(50,813-87,053,152) hmz, arr[hg19]2q11.1q37.3(95,550,957-242,773,583) hmz | – | Bilateral MCDK, FGR, CHD | P | UPD (Maternal) | TP |
| 15 | arr[hg19] 9q21.31q21.32(82,732,469-85,502,241)x1 | 2.7 | Left MCDK | VUS | – | TD |
CMA: chromosomal microarray analysis; CHD: congenital heart disease; MCDK: multicystic dysplastic kidney; FGR: growth restriction; P: pathogenic; VUS: variants of unknown significance; TP: termination of pregnancy; TD: term delivery.
WES
Fifteen MCDK fetuses with normal karyotypes and CMA results were simultaneously examined by WES. WES results showed that the BBS gene mutation occurred in two MCDK fetuses, and the abnormal detection rate was 13.3% (2/15) (Table 3). In one fetus, BBS1 gene mutation was derived from homozygous variation of the c.1177C>T locus of the mother and father (Figure 3). The mutation of C. 1177C>T leads to premature termination of the protein encoding at amino acid 393, which is predicted to produce truncated proteins and thus affect normal protein function. However, the other fetus was detected as a compound heterozygote with two pathogenic mutations, c.1814C>G(P.605*)/C.534+1G>T, in the BBS2 gene inherited from the father/mother, respectively (Figure 4). After genetic counseling, the parents of the two MCDK fetuses with genetic abnormalities chose to terminate the pregnancy.
Table 3.
WES detected in fetuses with MCDK.
| Case | WES | Ultrasonic phenotype | Interpretation | Inheritance | Postnatal outcome |
|---|---|---|---|---|---|
| 1 | BBS1, c.1177C>T(p. Arg393*) | Bilateral MCDK, oligoamnios | P | Homozygous variation | TP |
| 2 | BBS2, c.1814C>G(p.S605*) | Bilateral MCDK, cardiac enlargement, oligoamnios | P | Heterozygote variation | TP |
| BBS2, c.534+1G>T | – | P | Heterozygote variation | – |
MCDK: multicystic dysplastic kidney; P: pathogenic; TP: termination of pregnancy; WES: whole-exome sequencing.
Figure 3.
(A) The fetus had a homozygous mutation in exon 12 c.1177C>T. (B) The mother had a heterozygous mutation in exon 12 c.1177 C>T. (C) The father had a heterozygous mutation in exon 12 c.1177C>T.
Figure 4.

(A) The fetus has a complex heterozygous mutation in the BBS2 gene from the mother’s C. 534+1G>T site heterozygous mutation. (B) The father has a normal locus. (C) The mother had heterozygous variation in the C. 534+1G>T locus. (D) The fetus has a complex heterozygous mutation in the BBS2 gene from the father’s heterozygous mutation of C. 1814C>G. (E) The mother has normal loci. (F) The father had a heterozygous variation at the c.1814C>G locus.
Subgroup analysis based on whether MCDK fetuses were associated with other ultrasound abnormalities
In 64 cases with isolated MCDK fetuses, seven cases with pathogenic genomes were detected, with a positive rate of 10.9%. Thirteen pathogenic genomes were detected in 44 non-isolated MCDK fetuses, with a positive rate of 29.5%. The difference between the two groups was statistically significant (χ2 = 5.983, P = 0.014).
Subgroup analysis of unilateral and bilateral MCDK fetuses
The fetuses were divided into unilateral and bilateral MCDK groups according to whether unilateral or bilateral MCDK occurred. In 78 unilateral MCDK fetuses, 11 cases of pathogenic genome were detected, with a positive rate of 14.1%. In bilateral MCDK fetuses of 30 cases, nine cases of pathogenic genome were detected, with a positive rate of 30.0%. The difference between the two groups was statistically significant (χ2 = 3.629, P = 0.057).
Follow-up
In the follow-up of 108 pregnant women, 100 cases were successfully followed-up, eight cases were uncooperative or lost to follow-up, and the follow-up rate was 92.6% (8/108). Of the 100 cases successfully followed-up, 38 were terminated due to abnormal genomic or ultrasonic structure, and the remaining 62 fetuses (all with unilateral MCDK) had normal growth and development after birth.
Discussion
Fetal MCDK may be caused by an early renal pelvis, infundibular or ureteral atresia, and severe stricture, resulting in ipsilateral retrorenal degeneration and cystic dysplasia. MCDK is characterized by abnormal renal morphology on the affected side, which is replaced by multiple sacs of varying sizes separated by tissues containing the original dysplasia component. For fetuses with MCDK indicated by prenatal ultrasound, the resolution of currently used chromosome karyotype analysis can only reach 5Mb; thus, the detection rate of pathogenic fragments is low, and the source, size, nature, and genes contained in fragments cannot be accurately determined. Staebler et al. 17 conducted karyotype analysis on 73 MCDK fetuses and found chromosomal karyotype abnormalities in only two fetuses (2.7%). Hsu et al. 18 conducted karyotype analysis on 14 MCDK fetuses and found karyotype abnormalities in 46, XN, t(12,13)(p13q21.2) in one fetus. In this study, we conducted karyotyping analysis on 108 MCDK fetuses, and the results showed abnormal karyotyping in four fetuses, with a detection rate of 3.7%, is consistent with the above literature reports.
CMA can detect chromosomal abnormalities that cannot be detected by karyotype analysis. 19 In this study, 108 MCDK fetuses were examined simultaneously with CMA. Compared with karyotype analysis, 14 additional cases of pathogenic CNV were detected by CMA, in addition to four cases with the same results as karyotype analysis. Of the 14 cases of pathogenic CNVs found, three fetuses had 17q12 microdeletions. According to literature, the main clinical manifestations of 17q12 microdeletion syndrome include renal cysts and diabetes syndrome, autism and schizophrenia, learning difficulties, transient neonatal elevated blood calcium, and neonatal cholestasis. 20 The deletion fragment mainly contains HNF1B and LHX1 genes, and mutations inHNF1B and LHX1 are closely associated with urinary malformations.21 –23 In this study, there were two MCDK fetuses with 22q11.21 microdeletion and 22q11.21 microduplication. 22 q11.21 region contains HNF1B, SNAP29, and CRKL genes. By searching the literature and databases, it was found that changes in HNF1B, SNAP29 and CRKL genes can lead to abnormalities in the urinary system. 24 Changes in some chromosomal gene imprinting regions and UPD may also cause abnormalities in the fetal urinary system. In this study, UPD was found in two MCDK fetuses: maternal UPD on chromosome 2, and maternal UPD on chromosome 16. It has been reported that maternal UPD on chromosome 2 and maternal UPD on chromosome 16 may be associated with abnormal development of urinary system.25,26 In this study, there was also one MCDK fetus with 7q11.23 microduplication. 7q11.23 Microduplication can lead to multiple system involvements, including renal malformations. 27 In this study, one fetus had 4q31.3q32.2 microdeletions, including GRIA2 and LRAT genes. GRIA2 gene may be associated with neurodevelopmental disorders of speech and abnormal behavior, 28 and LRAT gene may be associated with early onset of severe retinal dystrophy, 29 but there is no literature reporting that the deletion of these two genes can cause malformation of the urinary system. One fetus had a microdeletion of 17p12, and it was found that deletion of this segment could lead to hereditary stress-susceptible peripheral neuropathy. 30 However, no studies have reported that deletion of this segment could cause abnormalities in the urinary system. One fetus had 16p11.2 microdeletion, which contained BP4-BP5 gene. Patients with 16p11.2 microdeletion have great differences in clinical phenotypes, including developmental delay, learning difficulties, language disorders, slight special facial features, epilepsy or EEG abnormalities, psychiatric disorders, obesity, heart malformations, and other abnormalities. 31 However, microdeletion of 16p11.2 has not been reported to cause urinary system abnormalities. One fetus had 15q11.2 microdeletions, including TUBGCP5, CYFIP1, NIPA2 and NIPA1. The clinical phenotype of 15q11.2 microdeletion varies widely and may be normal or abnormal, such as developmental delay, epilepsy, feeding difficulty, inattention, and autism. 32 However, no studies have reported that 15q11.2 microdeletion can cause urinary system abnormalities. Therefore, the relationship between 4q33.1 q32.2, 15q11.2, 16p11.2, and 17p12 microdeletions and MCDK was reported for the first time in this study. Whether these four types of microdeletions are related to kidney development needs to be further verified by increasing the number of samples.
Studies have shown that mutations in a single gene can affect renal development.33 –35 With the continuous progress in gene detection technology, an increasing number of studies are attempting to use WES for the detection of patients with renal dysplasia. Saisawat et al. 36 used WES to identify TRAP1 gene mutations in two families with isolated urological malformations. Vivante et al. 37 used a homozygous localization method combined with WES to identify nine pathogenic recessive mutations in 33 families. Humbert et al. 38 detected ITGA8 mutations in two families with bilateral renal absence using WES. In this study, we conducted WES on 15 MCDK fetuses with normal karyotypes and CMA results, and gene mutations were detected in two fetal samples, with a detection rate of 13.3% (2/15), indicating that the occurrence of MCDK is related to single gene variation to a certain extent. We detected c.1177C>T heterozygous mutations in BBS1in one fetal sample with bilateral polycystic kidney disease and oligohydramnios indicated by prenatal ultrasound, and c.1814C>G and C. 534+1G>T complex heterozygous mutations in BBS2 in one fetal sample with bilateral polycystic kidney disease, enlarged heart, and oligohydramnios, respectively. Both BBS1 and BBS2 are associated with kidney development and are pathogenic genes related to kidney development.39,40
In this study, the rates of pathogenic genome in the isolated MCDK fetuses and non-isolated MCDK fetuses were 10.9% and 29.5, respectively (P < 0.05), and the difference between the two groups was statistically significant. This indicates that non-isolated MCDK fetuses are at a significantly higher risk of developing genomic abnormalities than isolated MCDK fetuses are. Unilateral MCDK fetuses are more common; however, many cases are often accompanied by contralateral pyeloureteral junction obstruction, malrotation, or other types of dysplasia. In this study, the detection rate of the pathogenic genome in bilateral MCDK fetuses was higher than that in unilateral MCDK fetuses (14.1% versus 30.0%, P > 0.05), suggesting a stronger correlation between the bilateral MCDK and the pathogenic genome than that in unilateral MCDK, but the difference between the two was not statistically significant. Owing to the small sample size in this study, there may be bias in the detection rate of the pathogenic genome. Therefore, it is necessary to increase the sample size to obtain a more comprehensive and reliable assessment.
This study has some limitations. First, the follow-up of fetuses with MCDK in this study was incomplete. Our follow-up of MCDK fetuses after birth was telephonic, lacking imaging reports, and the results may be biased; therefore, our follow-up system needs to be further improved. Second, only 15 MCDK fetuses with normal karyotypes and CMA results were treated with WES. There may be missed diagnoses, so more cases need to be treated with WES in the next step.
Conclusions
In summary, we jointly used CMA-WES to detect fetuses with MCDK revealed by prenatal ultrasound, significantly improving the detection rate for genetic causes of MCDK fetuses. New candidate genes were also identified. It provides a basis for prenatal diagnosis, consultation, and prognosis evaluation of fetuses with MCDK. Microdeletions 4q33.1 q32.2, 15q11.2, 16p11.2, and 17p12 were first reported in MCDK cases. Enriches the disease spectrum of the above microdeletions and microduplications. WES results showed that the BBS gene mutation occurred in two MCDK fetuses.
Footnotes
Authors’ Contributions: MC drafted the manuscript. XW designed the experiment. XS collected the data. ML analyzed the data. HH conducted experiments. NL and CG interpreted data. LX supported the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The studies were approved by the ethics committee at the Fujian Provincial Maternal and Child Health Hospital (no. 2014042).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fujian Provincial Natural Science Foundation (2021J01407), the Fujian Provincial Health Technology Project (2020GGA020), the Fujian Provincial Health Technology Project (2020CXB008), the Fujian Provincial Natural Science Foundation (2019J01509), and Joint Funds for the Innovation of Science and Technology, Fujian Province (2020Y9159).
ORCID iD: Meiying Cai  https://orcid.org/0000-0002-7431-2475
https://orcid.org/0000-0002-7431-2475
References
- 1. Moralıoğlu S, Celayir AC, Bosnalı O, Pektaş OZ, Bulut IK. Single center experience in patients with unilateral multicystic dysplastic kidney. J Pediatr Urol 2014;10:763–8 [DOI] [PubMed] [Google Scholar]
- 2. Aslam M, Watson AR, Trent Anglia MCDK Study Group. Multicystic dysplastic kidney: long term outcomes. Arch Dis Child 2006;91:820–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon AC, Thomas DF, Arthur RJ, Irving HC. Multicystic dysplastic kidney: is nephrectomy still appropriate. J Urol 1988;140:1231–4 [DOI] [PubMed] [Google Scholar]
- 4. Toka HR, Toka O, Hariri A, Nguyen HT. Congenital anomalies of kidney and urinary tract. Semin Nephrol 2010;30:374–86 [DOI] [PubMed] [Google Scholar]
- 5. Schönfelder EM, Knüppel T, Tasic V, Miljkovic P, Konrad M, Wühl E, Antignac C, Bakkaloglu A, Schaefer F, Weber S, ESCAPE Trial Group. Mutations in Uroplakin IIIA are a rare cause of renal hypodysplasia in humans. Am J Kidney Dis 2006;47:1004–12 [DOI] [PubMed] [Google Scholar]
- 6. Iatropoulos P, Daina E, Mele C, Maranta R, Remuzzi G, Noris M. Discordant phenotype in monozygotic twins with renal coloboma syndrome and a PAX2 mutation. Pediatr Nephrol 2012;27:1989–93 [DOI] [PubMed] [Google Scholar]
- 7. Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiené A, Mir S, Montini G, Peco-Antic A, Wühl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol 2006;17:2864–70 [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Zhong M, Zheng D. Chromosomal mosaicism detected by karyotyping and chromosomal microarray analysis in prenatal diagnosis. J Cell Mol Med 2021;25:358–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brady PD, Vermeesch JR. Genomic microarrays: a technology overview. Prenat Diagn 2012;32:336–43 [DOI] [PubMed] [Google Scholar]
- 10. Srebniak MI, Van Opstal D, Joosten M, Diderich KE, de Vries FA, Riedijk S, Knapen MF, Go AT, Govaerts LC, Galjaard RJ. Whole-genome array as a first-line cytogenetic test in prenatal diagnosis. Ultrasound Obstet Gynecol 2015;45:363–72 [DOI] [PubMed] [Google Scholar]
- 11. Melanie Manning Louanne Hudgins . Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med 2010;12:742–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gahl WA, Markello TC, Toro C, Fajardo KF, Sincan M, Gill F, Carlson-Donohoe H, Gropman A, Pierson TM, Golas G, Wolfe L, Groden C, Godfrey R, Nehrebecky M, Wahl C, Landis DM, Yang S, Madeo A, Mullikin JC, Boerkoel CF, Tifft CJ, Adams D. The National Institutes of Health Undiagnosed Diseases Program: insights into rare diseases. Genet Med 2012;14:51–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wou K, DeBie I, Carroll J, Brock JA, Douglas Wilson R. Fetal exome sequencing on the horizon. J Obstet Gynaecol Can 2019;41:64–7 [DOI] [PubMed] [Google Scholar]
- 14. Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Hum Genet 2014;59:5–15 [DOI] [PubMed] [Google Scholar]
- 15. Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med 2020;22:245–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Staebler M, Donner C, Regemorter NV, Duprez L, Avni F. Should determination of the karyotype be systematic for all malformations detected by obstetrical ultrasound? Prenat Diagn 2010;25:567–73 [DOI] [PubMed] [Google Scholar]
- 18. Hsu PY, Yu CH, Lin K, Cheng YC, Chang CH, Chang FM. Prenatal diagnosis of fetal multicystic dysplastic kidney in the era of three-dimensional ultrasound: 10-year experience. Taiwan J Obstet Gynecol 2012;51:596–602 [DOI] [PubMed] [Google Scholar]
- 19. Jelin A, Sagaser K, Lawson C, Forster KR, Blakemore K. Chromosomal microarray analysis results from pregnancies with various ultrasonographic anomalies. Obstet Gynecol 2019;133:827–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dixit A, Patel C, Harrison R, Jarvis J, Hulton S, Smith N, Yates K, Silcock L, McMullan DJ, Suri M. 17q12 microdeletion syndrome: three patients illustrating the phenotypic spectrum. Am J Med Genet A 2012;158A:2317–21 [DOI] [PubMed] [Google Scholar]
- 21. Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, Aziza J, Bandin F, Schanstra JP, Bellanné-Chantelot C. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol 2007;18:923–33 [DOI] [PubMed] [Google Scholar]
- 22. Hendrix NW, Clemens M, Canavan TP, Surti U, Rajkovic A. Prenatally diagnosed 17q12 microdeletion syndrome with a novel association with congenital diaphragmatic hernia Fetal. Diagn Ther 2012;31:129–33 [DOI] [PubMed] [Google Scholar]
- 23. Hukriede NA, Tsang TE, Habas R, Khoo PL, Steiner K, Weeks DL, Tam PP, Dawid IB. Conserved requirement of Lim1 function for cell movements during gastrulation. Dev Cell 2003;4:83–94 [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Rivera E, Liu YP, Verbitsky M, Sanna-Cherchi S. Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med 2017;376:742–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu T, Li J, Li N, Liu R, Ding Y, Chang G, Chen Y, Shen Y, Wang X, Wang J. Obesity and developmental delay in a patient with uniparental disomy of chromosome 2. Int J Obes 2016;40:1935–41 [DOI] [PubMed] [Google Scholar]
- 26. Yingjun X, Zhiyang H, Linhua L, Fangming S, Linhuan H, Jinfeng T, Qianying P, Xiaofang S. Chromosomal uniparental disomy 16 and fetal intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol 2017;211:1–7 [DOI] [PubMed] [Google Scholar]
- 27. Earhart BA, Williams ME, Zamora I, Randolph LM, Votava-Smith JK, Marcy SN. Phenotype of 7q11.23 duplication: a family clinical series. Am J Med Genet A 2017;173:114–9 [DOI] [PubMed] [Google Scholar]
- 28. Zhou B, Zhang C, Zheng L, Wang Z, Chen X, Feng X, Zhang Q, Hao S, Wei L, Gu W, Hui L. Case report: a novel De Novo Missense mutation of the GRIA2 gene in a Chinese case of neurodevelopmental disorder with language impairment. Front Genet 2021;12:794766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramkumar S, Moon J, Golczak M, von Lintig J. LRAT coordinates the negative-feedback regulation of intestinal retinoid biosynthesis from β-carotene. J Lipid Res 2021;62:100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goh ES, Banwell B, Stavropoulos DJ, Shago M, Yoon G. Mosaic microdeletion of 17p11.2-p12 and duplication of 17q22-q24 in a girl with Smith-Magenis phenotype and peripheral neuropathy. Am J Med Genet A 2014;164A:748–52 [DOI] [PubMed] [Google Scholar]
- 31. Xie H, Liu F, Zhang Y, Chen Q, Shangguan S, Gao Z, Wu N, Wang J, Cui X, Wang L, Chen X. Neurodevelopmental trajectory and modifiers of 16p11.2 microdeletion: a follow-up study of four Chinese children carriers. Mol Genet Genomic Med 2020;8:e1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silva AI, Kirov G, Kendall KM, Bracher-Smith M, Wilkinson LS, Hall J. Analysis of diffusion tensor imaging data from the UK Biobank confirms dosage effect of 15q11.2 copy number variation on white matter and shows association with cognition. Biol Psychiatry 2021;90:307–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN. Mutation in hepatocyte nuclear factor|[ndash]|1|[beta]| gene (TCF2) associated with MODY. Nature Genetics 1997;17:384–5 [DOI] [PubMed] [Google Scholar]
- 34. Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 1995;9:358–64 [DOI] [PubMed] [Google Scholar]
- 35. Harshman LA, Brophy PD. PAX2 in human kidney malformations and disease. Pediatr Nephrol 2012;27:1265–75 [DOI] [PubMed] [Google Scholar]
- 36. Saisawat P, Kohl S, Hilger AC, Hwang DY, Yung Gee H, Dworschak GC, Tasic V, Pennimpede T, Natarajan S, Sperry E, Matassa DS, Stajić N, Bogdanovic R, de Blaauw I, Marcelis CL, Wijers CH, Bartels E, Schmiedeke E, Schmidt D, Märzheuser S, Grasshoff-Derr S, Holland-Cunz S, Ludwig M, Nöthen MM, Draaken M, Brosens E, Heij H, Tibboel D, Herrmann BG, Solomon BD, de Klein A, van Rooij IA, Esposito F, Reutter HM, Hildebrandt F. Whole-exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney Int 2014;85:1310–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vivante A, Hwang DY, Kohl S, Chen J, Shril S, Schulz J, van der Ven A, Daouk G, Soliman NA, Kumar AS, Senguttuvan P, Kehinde EO, Tasic V, Hildebrandt F. Exome sequencing discerns syndromes in patients from consanguineous families with congenital anomalies of the kidneys and urinary tract. J Am Soc Nephrol 2017;28:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Humbert C, Silbermann F, Morar B, Jeanpierre C. Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am J Hum Genet 2014;94:288294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mary L, Chennen K, Stoetzel C, Antin M, Leuvrey A, Nourisson E, Alanio-Detton E, Antal MC, Attié-Bitach T, Bouvagnet P, Bouvier R, Buenerd A, Clémenson A, Devisme L, Gasser B, Gilbert-Dussardier B, Guimiot F, Khau Van Kien P, Leroy B, Loget P, Martinovic J, Pelluard F, Perez MJ, Petit F, Pinson L, Rooryck-Thambo C, Poch O, Dollfus H, Schaefer E, Muller J. Bardet-Biedl syndrome: antenatal presentation of forty-five fetuses with biallelic pathogenic variants in known Bardet-Biedl syndrome genes. Clin Genet 2019;95:384–97 [DOI] [PubMed] [Google Scholar]
- 40. Esposito G, Testa F, Zacchia M, Crispo AA, Di Iorio V, Capolongo G. Genetic characterization of Italian patients with Bardet-Biedl syndrome and correlation to ocular, renal and audio-vestibular phenotype: identification of eleven novel pathogenic sequence variants. BMC Med Genet 2017;18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]