Abstract
We describe herein a recurrent catheter-related (Port-A-Cath; Smiths Industries Medical Systems [SIMS] Deltec, Inc., St. Paul, Minn.) infection caused by multidrug-resistant Mycobacterium chelonae with two colonial morphotypes in a 53-year-old woman with gastric adenocarcinoma. Four isolates recovered from this patient within a 3-month period were found to belong to a single clone on the basis of the isolates’ identical antibiotypes as determined by the E test and their identical random amplified polymorphic DNA patterns.
Mycobacterium chelonae, a rapidly growing mycobacterium, is an opportunistic pathogen which causes a wide variety of clinical syndromes (2, 3, 5, 8, 9, 12, 15, 18). Nosocomial infections associated with the use of various indwelling devices and diagnostic instruments have been reported (7, 16, 17). The propensity of the organism to adhere to the cardiac valve or vessel walls resulting in endocarditis or aortitis has also been described (3, 5, 9). However, none of the previous reports have described the use of molecular typing methods to document the long-term persistence of M. chelonae in the catheter and/or the walls of vessels, which contributed the recurrent nature of intravenous catheter-related infection caused by this organism (18).
Case report.
A 53-year-old woman with gastric adenocarcinoma underwent subtotal gastrectomy in 1993. She began to receive weekly intravenous infusions of fluorouracil (2,500 mg) in November 1996. The first catheter (Port-A-Cath; Smiths Industries Medical Systems [SIMS] Deltec, Inc., St. Paul, Minn.) was implanted via the right subclavian vein on 25 November 1996. She first developed fever and shaking chills several minutes after each manipulation of the Port-A-Cath device in June 1997. Two sets of cultures of blood aspirated via the catheter and cultured in a BACTEC 6A aerobic bottle (Becton Dickinson, Sparks, Md.) on 7 July 1997 both yielded M. chelonae with a smooth colonial morphotype (isolate A). The chest roentgenography was negative. She received lomefloxacin (200 mg every 12 h) for a total of 14 days, and the catheter was removed on 14 July. A second Port-A-Cath device was implanted via the left subclavian vein. The removed catheter was not sent for microbiologic studies. One month after the second catheter implantation, fever developed and tenderness, erythema, and pus discharge at the insertion site of the catheter were noticed. The wound culture was negative for M. chelonae or other organisms. The catheter was removed on 20 August, and a third catheter was implanted via the right subclavian vein. During the following month, the patient felt febrile after each infusion of the chemotherapy agent via the catheter, although the third catheter insertion site was absent of any signs of infection. However, the wound of the second insertion site did not heal satisfactorily and had persistent pus discharge.
The patient was admitted again on 13 October 1997. A wedge-shaped consolidation over the lower lobe of the right lung and a nodular patch over the right midlung field were found by chest roentenography and sonography. A skin biopsy of the soft tissue from the second insertion site revealed granulomatous inflammation and numerous acid-fast bacilli. The third catheter was removed on 15 October. A smear of the blood from the catheter showed many clusters of acid-fast bacilli. Culture of the biopsied skin tissue yielded M. chelonae with a smooth-colony morphotype (isolate B). However, a culture of the catheter tip and two sets of blood cultures collected immediately after the removal of the catheter all yielded M. chelonae with two colonial morphologies, i.e., small and smooth (isolate C) and large and rough (isolate D). Cultures of sputum, throat and nasal swabs, stool, urine, and skin were all negative for M. chelonae.
The patient started receiving clarithromycin (500 mg every 12 h), ciprofloxacin (300 mg every 12 h), and intravenous amikacin (750 mg every day) therapy on 15 October. Acid-fast stain and culture of an aspirate of the lung lesion, which were performed 7 days after the start of antibiotic treatment, were both negative for mycobacteria. Magnetic resonance imaging and magnetic resonance angiography of the chest revealed a focal stenosis of the right subclavian vein; the left subclavian, bilateral branchiocephalic, and internal jugular veins and the superior vena cava were patent. The antibiotics continued for 1 month, followed by clarithromycin and ciprofloxacin for an additional 1 month. A follow-up examination revealed that the lesions of the right lung and the right subclavian vein had disappeared.
Microbiology.
All isolates (isolates A to D) grew well on the Trypticase soy agar supplemented with 5% sheep blood agar (BBL Microbiology Systems, Cockeysville, Md.) and Middlebrook 7H11 agar (BBL Microbiology Systems) within 3 days of incubation. Two different colonial morphotypes were observed on both the blood agar plate (Fig. 1) and the Trypticase soy agar. Growth on MacConkey agar (BBL Microbiology Systems) of these isolates was evident on the fourth day of incubation. The isolates were differentiated from Mycobacterium fortuitum and Mycobacterium abscessus and identified as M. chelonae because they had all of the following characteristics: failure to grow in 5% NaCl; positive citrate and arylsulfatase reactions at 3 days; negative nitrate reduction and mannitol utilization reactions; negative for alkaline phosphatase, trypsin, and β-glucosidase (API ZYM system; bioMérieux Vitek, Inc., Hazelwood, Mo.); and resistance to polymyxin (300-U) and cephalothin (30-μg) disks (BBL Microbiology Systems) (8, 11, 16).
FIG. 1.
Colonial morphology of M. chelonae grown on the primary isolation plate (Trypticase soy agar supplemented with 5% sheep blood) from the Port-A-Cath tip after 3 days of incubation. Arrow, large, flat, rough, and wrinkled colony (isolate C); arrowhead, small and smooth colony (isolate D).
Cellular fatty acid analysis.
The procedure for the extraction and derivation of methyl esters of mycobacterial lipids was performed as described previously (4, 11). All isolates had major cellular fatty acid peaks (≥3% of total fatty acid) of 14:0 (tetradecanoic acid), 16:0 (hexadecanoic acid), 18:1 (octadecanoic acid), 18:0 (octadecanoic acid), and TBSA (tuberculostearic acid) and minor peaks of 16:1 (hexadecanoic acid), 17:0 (heptadecanoic acid), and 2-OH-20:0 (2-hydroxyeicosanoic acid). The cellular fatty acid profile was characteristic for the identification of the M. chelonae group (7).
Antimicrobial susceptibilities.
In vitro susceptibilities of these isolates, determined by the E test (PDM Epsilometer; AB Biodisk, Solna, Sweden), were measured on Mueller-Hinton agar supplemented with 5% sheep blood (BBL Microbiology Systems), and the results were read after 72 h of incubation (6). All isolates had identical antibiotypes (E test MICs of each agent for the four isolates were ≤2 gradient discrepancies): MICs of >256 μg/ml for cefoxitin, cefmetazole, tobramycin, minocycline, and erythromycin; MICs of >32 μg/ml for ampicillin-sulbactam, imipenem, ofloxacin, ciprofloxacin, trimethoprim-sulfamethoxazole, and rifampin; MICs of 16 to 32 μg/ml for amikacin; and MICs of 1.5 to 2 μg/ml for clarithromycin.
RAPD patterns.
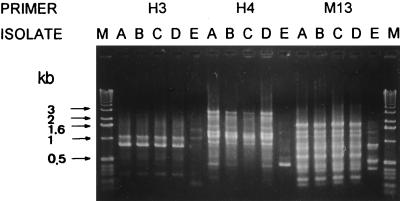
Random amplified polymorphic DNA (RAPD) patterns of these isolates were determined by means of arbitrarily primed PCR as described in our previous report (4). A total of six oligonucleotide primers were used: M13 (5′-TTATGTAAAACGACGGCCAGT-3′), H3 (5′-AGACGTCCAC-3′), H4 (5′-GGAAGTCGCC-3′), H9 (5′-TGTAGCTGGG-3′), ERIC1 (5′-GTGAATCCCCAGGAGCTTACAT-3′), and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) (Operon Technologies, Inc., Alameda, Calif.). For comparison, one clinical isolate of M. chelonae (isolate E) was also included in this study as a control strain. The four isolates (isolates A to D) had identical RAPD patterns (i.e., they shared every band), and these patterns were different from that of the control strain. Figure 2 shows the RAPD patterns for three of the primers (H3, H4, and M13).
FIG. 2.
RAPD patterns of the five isolates of M. chelonae obtained with three primers. Lanes M, molecular size markers (1-kb ladder; Gibco BRL, Gaithersburg, Md.); lanes A to E, patterns for isolates A to E, respectively. Molecular sizes in kilobase pairs are indicated to the left of the gel.
The finding in this report obtained by a molecular typing method confirms that M. chelonae caused recurrent central venous catheter-related septicemia, which further resulted in a pulmonary embolism and soft-tissue infection of the insertion site. The recurrent nature of the infection was probably caused by the presence of this organism in the thrombus along the vessel wall for a period of at least 3 months, although the thrombus was not removed for microbiological study.
Though two kinds of colonial morphology of M. chelonae on Middlebrook 7H11 agar are well known, i.e., one is round and smooth and the other is rough and wrinkled (8), infection caused by a single clone of this organism which simultaneously possessed these two obviously different colonial morphotypes has not been previously reported. The identity of RAPD patterns with six primers was clearly demonstrated for these two morphotypes, suggesting that they belonged to a single clone. However, we cannot explain why the rough morphotype was not seen in the first two positive cultures.
M. chelonae is resistant to numerous antimicrobial agents, showing variable susceptibilities to amikacin, tobramycin, doxycycline, erythromycin, cephalosporins, imipenem, and quinolones (1, 10, 13, 14). Clarithromycin is the most active drug against this organism, with 100% of isolates being susceptible at an MIC of ≤1 μg/ml (1). In contrast, our isolates were not susceptible to any of the antibiotics tested, including clarithromycin. However, after the removal of the infected catheter, our patient responded satisfactorily to treatment with clarithromycin, ciprofloxacin, and amikacin for 1 month, followed by 2 months of treatment with clarithromycin and ciprofloxacin. Combination therapy for infections due to this multidrug-resistant organism seems advisable, although some of the agents described had poor in vitro activity.
The findings in this case illustrate that M. chelonae should be included in the differential diagnosis of central venous catheter-related sepsis, particularly for immunocompromised hosts. And this organism has the propensity to adhere to the vascular wall. When recurrent infections occur at different sites of Port-A-Cath implantation, clinicians should make an effort to find any vascular lesions along the route of the catheter.
REFERENCES
- 1.Brown B A, Wallace R J, Jr, Onyi G O, De Rosas V, Wallace R J., III Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180–184. doi: 10.1128/aac.36.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper J F, Lichtenstein M J, Graham B S, Schaffner W. Mycobacterium chelonae: a cause of nodular skin lesions with a proclivity for renal transplant recipients. Am J Med. 1989;86:173–177. doi: 10.1016/0002-9343(89)90264-7. [DOI] [PubMed] [Google Scholar]
- 3.Galil K, Thurer R, Glatter K, Barlam T. Disseminated Mycobacterium chelonae infection resulting in endocarditis. Clin Infect Dis. 1996;23:1322–1323. doi: 10.1093/clinids/23.6.1322. [DOI] [PubMed] [Google Scholar]
- 4.Hsueh P R, Teng L J, Ho S W, Hsieh W C, Luh K T. Clinical and microbiological characteristics of Flavobacterium indologenes infections associated with indwelling devices. J Clin Microbiol. 1996;34:1908–1913. doi: 10.1128/jcm.34.8.1908-1913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram C W, Tanner D C, Durack D T, Kernodle G W, Jr, Corey G R. Disseminated infection with rapidly growing mycobacteria. Clin Infect Dis. 1993;16:463–471. doi: 10.1093/clind/16.4.463. [DOI] [PubMed] [Google Scholar]
- 6.Koonz F P, Erwin M E, Barrett M S, Jones R N. Etest for routine clinical antimicrobial susceptibility testing of rapid-growing mycobacteria isolates. Diagn Microbiol Infect Dis. 1994;19:183–186. doi: 10.1016/0732-8893(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 7.Lowry P W, Beck-Sague C M, Bland L A, Aguero S M, Arduino M J, Minuth A N, Murray R A, Swenson J M, Jarvis W R. Mycobacterium chelonae infection among patients receiving high-flux dialysis treatment at a clinic in California. J Infect Dis. 1990;161:85–90. doi: 10.1093/infdis/161.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Notle F S, Metchock B. Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 400–437. [Google Scholar]
- 9.Sran P K, Kansupada K, Whitcup S M. Mycobacterium chelonae infection mimicking cutaneous vasculitis: case report. Clin Infect Dis. 1996;23:1189–1191. doi: 10.1093/clinids/23.5.1189. [DOI] [PubMed] [Google Scholar]
- 10.Swenson J M, Wallace R J, Jr, Silcox V A, Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28:807–811. doi: 10.1128/aac.28.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng L J, Liaw S J, Hsueh P R, Fan J H, Luh K T, Ho S W. Constitutive fatty acid and enzyme profiles of Mycobacterium species. J Formos Med Assoc. 1997;96:336–345. [PubMed] [Google Scholar]
- 12.Wallace R J, Jr, Swenson J M, Silcox V A, Good R C, Tschen J A, Stone M S. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983;5:657–679. doi: 10.1093/clinids/5.4.657. [DOI] [PubMed] [Google Scholar]
- 13.Wallace R J, Jr, Swenson J M, Silcox V A, Bullen M G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonae on the basis of in vitro susceptibilities. J Infect Dis. 1985;152:500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- 14.Wallace R J, Jr, Brown B A, Onyi G. Susceptibilities of Mycobacterium fortuitum biovar. fortuitum and the two subgroups of Mycobacterium chelonae to imipenem, cefmetazole, cefoxitin, and amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1991;35:773–775. doi: 10.1128/aac.35.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace R J, Jr, Brown B A, Onyi G. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405–412. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- 16.Wallace R J, Jr, Silcox V A, Tsukamura M, Brown B A, Kilburn J O, Butler W R, Onyi G. Clinical significance, biochemical features, and susceptibility patterns of sporadic isolates of the Mycobacterium chelonae-like organism. J Clin Microbiol. 1993;31:3231–3239. doi: 10.1128/jcm.31.12.3231-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H C, Liaw Y S, Yang P C, Kuo S H, Luh K T. A pseudoepidemic of Mycobacterium chelonae infection caused by contamination of a fibreoptic bronchoscope suction channel. Eur Respir J. 1995;8:1259–1262. doi: 10.1183/09031936.95.08081259. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Rajagopalan M, Brown B A, Wallace R J., Jr Randomly amplified polymorphic DNA PCR for comparison of Mycobacterium abscessus strains from nosocomial outbreaks. J Clin Microbiol. 1997;35:3132–3139. doi: 10.1128/jcm.35.12.3132-3139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]