ABSTRACT.
Plasmodium vivax is the second-most common malaria pathogen globally, but is considered very rare in the predominantly Duffy-negative sub-Saharan African population. In 259 malaria patients from highland southern Rwanda, we assessed Plasmodium species and Duffy blood group status by polymerase chain reaction (PCR). Plasmodium falciparum, P. vivax, Plasmodium malariae, and Plasmodium ovale were seen in 90.7%, 8.1%, 11.6%, and 5.0%, respectively. Plasmodium vivax occurred more frequently as a monoinfection than in combination with P. falciparum. All P. vivax–infected individuals showed heterozygous Duffy positivity, whereas this was the case for only 3.1% of patients with P. falciparum monoinfection and malaria-negative control subjects (P < 0.01). Based on PCR diagnosis, P. vivax is not rare in southern Rwanda. All episodes of P. vivax were observed in heterozygous Duffy-positive patients, whereas elsewhere in Africa, P. vivax is also reported in Duffy-negative individuals. Refined mapping of Plasmodium species is required to establish control and elimination strategies including all malaria species.
Plasmodium vivax causes only a fraction of global malaria episodes, but is the second-most common malaria pathogen after the dominant Plasmodium falciparum. The proportion of vivax malaria among all reported malaria cases varies widely, from 71.5% in the Americas and 40% in Southeast Asia to only 0.3% in sub-Saharan Africa (SSA).1 Long considered rather benign, increasing evidence shows P. vivax to contribute substantially to the disease burden, including severe malaria and adverse pregnancy outcomes.2–4 The low frequency of P. vivax in SSA is attributed to the virtual absence in African populations of the parasite’s erythrocyte invasion receptor—that is, the Duffy blood group antigen (encoded by the Duffy-associated receptor chemokine [DARC]).5 However, recent data show P. vivax infection among Duffy-negative individuals across Africa, suggesting alternative invasion pathways.6 This points to a significant underestimation of vivax malaria on the African continent,7 which is a result, in part, of the comparatively low sensitivity of microscopy in P. vivax detection, particularly in mixed infections.8
As for Rwanda, East Africa, cases resulting exclusively from P. falciparum have been reported to the WHO during the past decade.1 Although P. vivax has been reported occasionally,9,10 the actual epidemiology of that parasite in Rwanda is largely obscure. Rwanda has achieved a remarkable decline in the malaria burden during the past few years,1 which can be attributed largely to the scale-up of vector control measures and widely available artemisinin combination therapy. Eliminating the P. vivax reservoir—that is, hepatic hypnozoites—requires the administration of primaquine, which, however, is not included in the national drug policy for P. falciparum malaria. Moreover, primaquine can cause dose-dependent hemolysis in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency, which is common in Africa, and respective pretreatment testing would be needed.
The main objective of this study was to assess P. vivax prevalence among patients with uncomplicated malaria in 2018 and 2019 in Huye, southern Rwanda. In addition, we explored Duffy antigen genotypes among P. vivax patients, P. falciparum patients, and healthy control subjects.
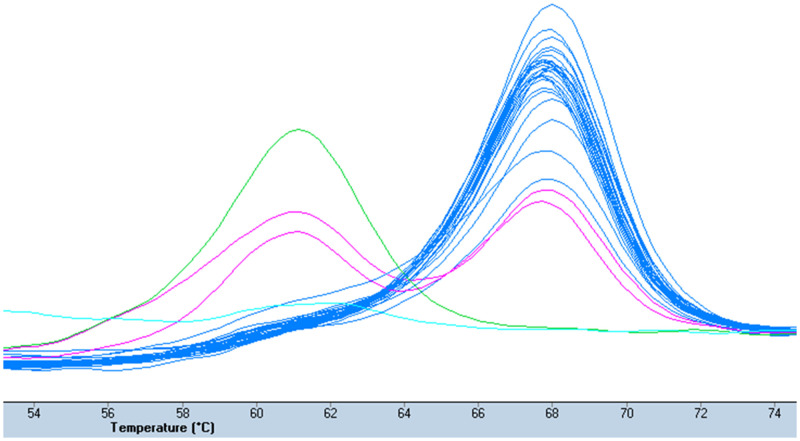
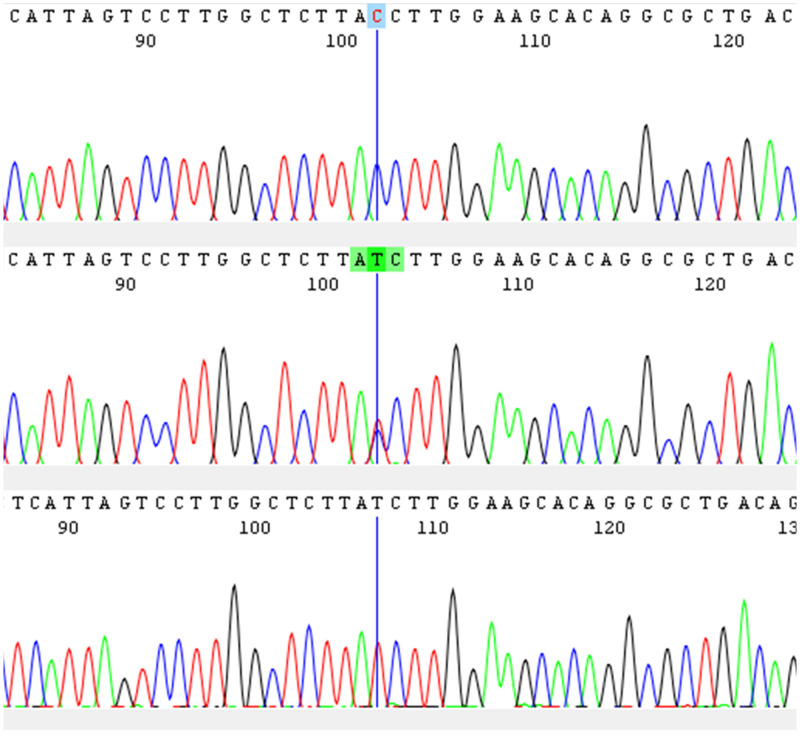
In March through June 2018 and September through December 2019, we recruited consenting malaria patients (> 1 year of age) at Sovu Health Center and Kabutare District Hospital, Huye District, southern Rwanda (population, ∼390,000; average altitude, 1,700 m; yearly rainfall, 1,200 mm; mean temperature, 19°C). Detailed study procedures are reported elsewhere.10,11 In short, patients were febrile (≥ 37.5°C, axillary) or reported fever in the preceding 48 hours, and tested positive for Plasmodium infection by a rapid diagnostic test (RDT) (SD Bioline Malaria Ag Pf/Pan; Abbott Global Point of Care, Chicago, IL). Malaria was confirmed by thick blood film microscopy and treated with artemether–lumefantrine. Aliquots of blood were preserved as dried blood spots (Whatman 3MM chromatography paper, Cytiva, Marlborough, MA). Healthy control subjects (negative RDT, no fever) were recruited in 2019—preselected by age, gender, and village—to match respective parameters in malaria patients. DNA was extracted from whole blood (patients) and dried blood spots (patients and healthy control subjects) using the QIAamp blood mini kit (Qiagen, Hilden, Germany). Plasmodium infection and species were confirmed by nested PCR in 2018)12 and real-time PCR assays in 2019 (commercial primers and probes; TIB MOLBIOL, Berlin, Germany).13 The nested PCR targets the 18S ribosomal RNA (rRNA) gene in the Plasmodium spp. genome. The real-time assay targets the CytB gene for P. falciparum, the 18S rRNA gene for P. vivax and Plasmodium ovale, and the MSP1 gene for P. malariae. To exclude potential P. vivax DNA contamination of blood samples, we confirmed P. vivax positivity in dried blood spot extracts. We assessed the Duffy genotype (DARC) in all P. vivax cases, in 100 P. falciparum cases (in 2018, n = 36; in 2019, n = 64), and in 98 control subjects (in 2019) by high-resolution melting curve assays on a Roche LightCycler 480 (Figure 1).14,15 In addition, we assessed 20 samples randomly for the Duffy genotype by Sanger sequencing (forward primer, 5′-CAGGAAGACCCAAGGCCAG-3′; reverse primer, 5′-CCATGGCACCGTTTGGTTCAGG-3′) (Figure 2). Primers and probes as well as sequencing were provided by Eurofins Genomics, Ebersberg, Germany.
Figure 1.
High-resolution melting curve of Duffy genotyping assay. Melting peaks of homozygous Duffy-positive (one peak at 61°C), homozygous Duffy-negative (-33T > C; one peak at 68°C), and heterozygous (two peaks) samples, and a negative control.
Figure 2.
Alignment of a homozygous Duffy-negative (upper), a heterozygous (middle), and a homozygous Duffy-positive (lower) sample.
In 2018 and 2019, we recruited 259 treatment-seeking malaria patients with PCR-confirmed Plasmodium infection. Plasmodium falciparum, P. vivax, P. malariae, and P. ovale were observed in 90.7%, 8.1%, 11.6%, and 5.0%, respectively (Table 1). One in seven falciparum malaria patients showed a mixed-species infection. Plasmodium vivax occurred more frequently as a monoinfection than in combination with P. falciparum.
Table 1.
Plasmodium species detected by PCR in patients in Huye, Rwanda, 2018 and 2019
| Plasmodium species | 2018 (n = 183), n (%) | 2019 (n = 76), n (%) | Total (N = 259), n (%) |
|---|---|---|---|
| Plasmodium falciparum | 168 (91.8) | 67 (88.2) | 235 (90.7) |
| Plasmodium vivax | 15 (8.2) | 6 (7.9) | 21 (8.1) |
| Plasmodium malariae | 24 (13.1) | 6 (7.9) | 30 (11.6) |
| Plasmodium ovale | 12 (6.6) | 1 (1.3) | 13 (5.0) |
| Monoinfection | |||
| P. falciparum | 135 (73.8) | 64 (84.2) | 199 (76.8) |
| P. vivax | 7 (3.8) | 5 (6.6) | 12 (4.6) |
| P. malariae | 2 (1.1) | 4 (5.3) | 6 (2.3) |
| P. ovale | 2 (1.1) | 0 (0) | 2 (0.8) |
| Mixed-species infection with P. falciparum | |||
| P. vivax | 6 (3.3) | 1 (1.3) | 7 (2.7) |
| P. malariae | 20 (10.9) | 2 (2.6) | 22 (8.5) |
| P. ovale | 8 (4.4) | 1 (1.3) | 9 (3.5) |
| Mixed-species infection without P. falciparum | |||
| P. malariae + P. vivax | 1 (0.5) | 0 (0) | 1 (0.4) |
| P. malariae + P. ovale | 1 (0.5) | 0 (0) | 1 (0.4) |
PCR = polymerase chain reaction.
All 21 P. vivax–infected individuals were heterozygous for the Duffy antigen (DARC -33T > C)—that is, they were Duffy positive. Among the P. falciparum cases (monoinfection), 98 were genotyped successfully for Duffy. Heterozygosity was 3.1% (3 of 98; allele frequency, 1.5%), and no Duffy-positive homozygosity was seen. Identically, among 98 successfully genotyped healthy control subjects, Duffy heterozygosity was 3.1% (3 of 98; allele frequency, 1.5%), with no Duffy-positive homozygous individuals. Duffy positivity was associated significantly with P. vivax infection compared with control subjects without Plasmodium infection, and with malaria patients with P. falciparum infection (for both, P < 0.01).
Among 259 PCR-confirmed malaria cases from southern Rwanda, 8.1% had a P. vivax infection, including 4.6% as a monoinfection. This contrasts with the absence of reported vivax cases in Rwanda and has implications for malaria control. All vivax cases were Duffy positive (heterozygous carriage of DARC -33T > C), whereas 97% of healthy control subjects and patients with P. falciparum monoinfection were Duffy negative. To the best of our knowledge, this is the first report on Duffy genotypes in a Rwandan population.
Plasmodium vivax is considered to have more potential for geographic spreading in the Rwandan highlands compared with P. falciparum because of its broader temperature tolerance.16 Also, P. vivax has a transmission advantage over P. falciparum because of its earlier onset of gametocyte development.17 For the treatment of vivax malaria, chloroquine as well as 2 weeks of primaquine for relapse prevention are recommended in Rwanda.1 Such treatment would eventually eliminate the P. vivax reservoir if cases were diagnosed. However, primaquine can cause dose-dependent acute hemolysis in individuals with G6PD deficiency. This trait is common but generally mild in SSA; in the study population, it is present in ∼10% of the general population that overlaps with our study population.18 Radical primaquine treatment thus requires G6PD deficiency testing in place, for example, by available rapid tests, and cautious primaquine administration in G6PD deficiency. The WHO recommends giving primaquine at 0.75 mg base/kg body weight once a week for 8 weeks in people with G6PD deficiency, with close supervision for hemolysis (instead of daily 0.25–0.5 mg base/kg body weight for 2 weeks).19
The occurrence of P. vivax infection exclusively in Duffy-positive hosts in our study should be interpreted with care. In nearby P. vivax–endemic Ethiopia, Duffy negativity among vivax carriers reached > 10%.20 Our sample size of 21 is too small to rule out with confidence the presence of 10% Duffy-negative P. vivax cases (e.g., at 2 of 21, 95% CI, 0.01–30.3). Elsewhere, P. vivax parasitemia has been found to be lower in Duffy-negative than in Duffy-positive hosts.20,21 We may have missed low-density P. vivax infections at recruitment as a result of species-dependent sensitivity limitations of RDTs and microscopy.8 Notwithstanding these limitations, our data do reveal a reservoir of P. vivax in a largely Duffy-negative population. When both Duffy alleles are present in a population, the Duffy-positive reservoir might facilitate P. vivax strains to develop mechanisms to invade Duffy-negative erythrocytes.21
The paradigm of P. vivax being benign has been challenged repeatedly,2–4 and the use of molecular tools in diagnosis reveals its underestimation. Particularly in regions of Africa where malaria elimination is in reach, control and elimination strategies will require the inclusion of all malaria species. Refined mapping of Plasmodium species distribution is required.
ACKNOWLEDGMENTS
We are grateful to the staff of Sovu Health Centre and Kabutare District Hospital for their collaboration and help.
REFERENCES
- 1. World Health Organization , 2022. World Malaria Report 2022. Geneva, Switzerland: WHO. [Google Scholar]
- 2. Baird JK, 2013. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev 26: 36–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI, 2017. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob Health 5: e1101–e1112. [DOI] [PubMed] [Google Scholar]
- 4. Drysdale M, Tan L, Martin A, Fuhrer IB, Duparc S, Sharma H, 2023. Plasmodium vivax in children: hidden burden and conspicuous challenges, a narrative review. Infect Dis Ther 12: 33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller LH, Mason SJ, Clyde DF, McGinniss MH, 1976. The resistance factor to Plasmodium vivax in blacks: the Duffy-blood-group genotype, FyFy. N Engl J Med 295: 302–304. [DOI] [PubMed] [Google Scholar]
- 6. Wilairatana P, Masangkay FR, Kotepui KU, De Jesus Milanez G, Kotepui M, 2022. Prevalence and risk of Plasmodium vivax infection among Duffy-negative individuals: a systematic review and meta-analysis. Sci Rep 12: 3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Battle KE, Baird JK, 2021. The global burden of Plasmodium vivax malaria is obscure and insidious. PLoS Med 18: e1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. malERA Consultative Group on Diagnoses and Diagnostics , 2011. A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med 8: e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCaffery JN, Munyaneza T, Uwimana A, Nace D, Lucchi N, Halsey ES, Rogier E, 2022. Symptomatic Plasmodium vivax infection in Rwanda. Open Forum Infect Dis 9: ofac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergmann C. et al. , 2021. Increase in Kelch 13 polymorphisms in Plasmodium falciparum, southern Rwanda. Emerg Infect Dis 27: 294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Loon W, Oliveira R, Bergmann C, Habarugira F, Ndoli J, Sendegeya A, Bayingana C, Mockenhaupt FP, 2022. In vitro confirmation of artemisinin resistance in Plasmodium falciparum from patient isolates, southern Rwanda, 2019. Emerg Infect Dis 28: 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN, 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61: 315–320. [DOI] [PubMed] [Google Scholar]
- 13. Abidha CA, Amoako YA, Nyamekye RK, Bedu-Addo G, Grziwotz F, Mockenhaupt FP, Telschow A, Danquah I, 2022. Fasting blood glucose in a Ghanaian adult is causally affected by malaria parasite load: a mechanistic case study using convergent cross mapping. Malar J 18: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Araújo F, Pereira C, Aleixo A, Henriques I, Monteiro F, Meireles E, Lacerda P, Cunha-Ribeiro LM, 2001. Rapid genotyping of the major alleles at the Duffy (FY) blood group locus using real-time fluorescence polymerase chain reaction. Immunohematology 17: 42–44. [PubMed] [Google Scholar]
- 15. Kasehagen LJ. et al. , 2007. Reduced Plasmodium vivax erythrocyte infection in PNG Duffy-negative heterozygotes. PLoS One 2: e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Battle KE. et al. , 2019. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet 394: 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balasubramanian S. et al. , 2020. Efficient transmission of mixed Plasmodium falciparum/vivax infections from humans to mosquitoes. J Infect Dis 221: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gahutu JB, Musemakweri A, Harms G, Mockenhaupt FP, 2012. Prevalence of classic erythrocyte polymorphisms among 749 children in southern highland Rwanda. Trans R Soc Trop Med Hyg 106: 63–65. [DOI] [PubMed] [Google Scholar]
- 19. Recht J, Ashley EA, White NJ, 2018. Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: divergent policies and practices in malaria endemic countries. PLoS Negl Trop Dis 12: e0006230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo E. et al. , 2021. Contrasting epidemiology and genetic variation of Plasmodium vivax infecting Duffy-negative individuals across Africa. Int J Infect Dis 108: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ménard D. et al. , 2010. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci USA 107: 5967–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]