ABSTRACT.
A 10-year-old boy with sickle cell disease (SCD) type SC presented with fever and abdominal pain after travel to Ghana and was diagnosed with Plasmodium falciparum infection. Despite adequate antimalarial treatment, he developed evidence of hyperinflammation with marked elevated ferritin, C-reactive protein, and triglycerides and subsequent bone marrow necrosis, characterized by elevated nucleated red blood cells and significant bone pain. This case report highlights the possible association between malaria and bone marrow necrosis in patients with SCD. Important considerations in treatment and workup of patients presenting with malaria and hyperinflammation are discussed.
CASE DESCRIPTION
A 10-year-old boy with sickle cell disease (SCD) type SC (HbSC), diagnosed by high-performance liquid chromatography hemoglobin variant analysis, presented with fever and abdominal pain 11 days after returning from Ghana (Accra and a nearby village). He did not receive malaria prophylaxis before or during travel. The family reported multiple mosquito bites during the trip.
Initial bloodwork was positive for Plasmodium falciparum (0.7% parasitemia) by Giemsa stained thick and thin blood smears. There was mild anemia (hemoglobin 104 g/L) and evidence of hemolysis (increased reticulocytes of 141.5 × 109/L, elevated lactate dehydrogenase of 10,279 U/L, increased unconjugated bilirubin of 39 μmol/L, and low haptoglobin of < 0.08 g/L). As per institutional practice for malaria management, the patient was admitted to the pediatric medicine ward with infectious diseases and hematology consultations. On exam, he was clinically stable with a mildly tender but nonacute abdomen and a normal neurological exam. The patient was started on intravenous (IV) fluids (dextrose 5% and sodium chloride), ceftriaxone, and intravenous artesunate (2.4 mg/kg/dose) because he was unable to tolerate oral atovaquone-proguanil due to nausea.
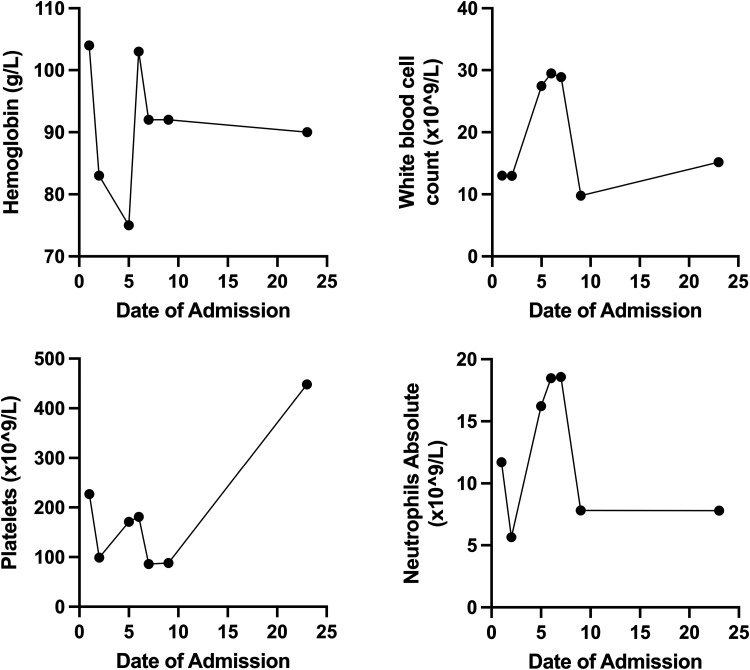
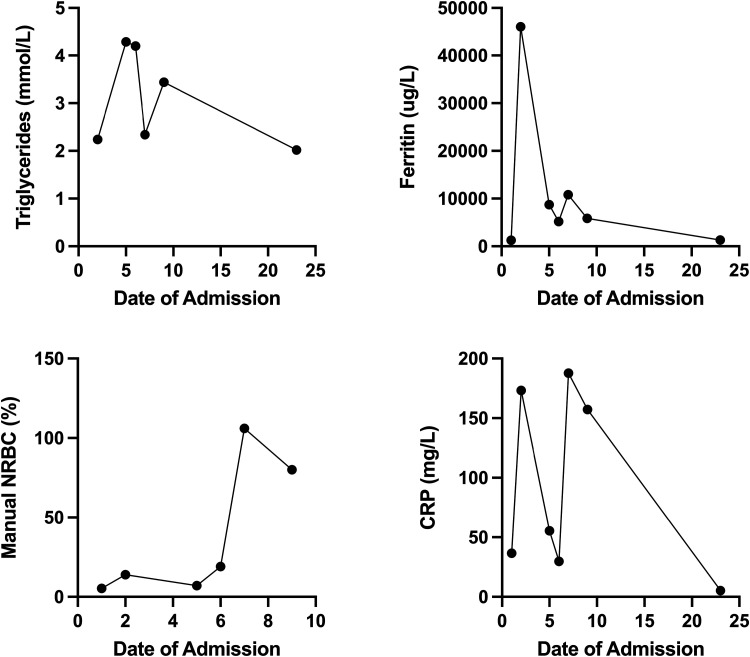
On day of admission (DOA) 2 to the hospital, the patient remained febrile and developed worsening anemia (hemoglobin 83 g/L) and new thrombocytopenia (platelets 99 × 109/L) (Figure 1). Biochemical markers suggested hyperinflammation including a marked increase in ferritin from 1,257 to 31,609 (Figure 2). The diagnosis of macrophage activation syndrome (MAS) in the context of P. falciparum infection, also known as secondary hemophagocytic lymphohistiocytosis (sHLH), was considered, and rheumatology was consulted. As the benefits of treating hyperinflammation outweighed the risks of worsening malaria while on treatment, a 2-day course of IV pulse methylprednisolone (1 g) was initiated on DOA 3 with subsequent steroid taper. After the first dose of methylprednisolone, the patient defervesced, his inflammatory markers started to trend down, and ceftriaxone was discontinued.
Figure 1.
The patient’s day of admission is plotted on the x-axis, and the components of the complete blood cell count are plotted on the y-axis. The corresponding units are denoted in the y-axis of each panel. The line graph shows the trend of each component throughout the patient’s course of disease.
Figure 2.
The patient’s day of admission (DOA) is plotted on the x-axis, and markers of bone marrow necrosis (BMN)/inflammation are plotted on the y-axis. The corresponding units are denoted in the y-axis of each panel. Manual nucleated red blood cells were not calculated after DOA 10. The line graph shows the trend of BMN/inflammatory markers throughout the patient’s course of disease.
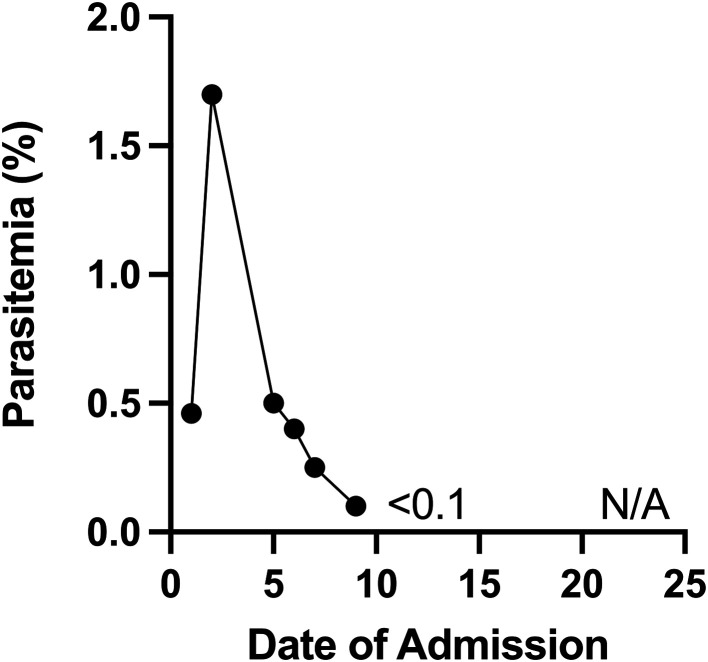
A switch from artesunate to atovaquone-proguanil was attempted on DOA 4, but the patient remained nauseous, and thus he was continued on IV artesunate. On DOA 5, the patient experienced acute hip, leg, and abdominal pain; had a new oxygen requirement (heated high flow at 15 L/minute, FiO2 40%); and had recurrence of fever to 39.4 °C. The neurological exam was pertinent for confusion but no focal deficits. Ceftriaxone was reinitiated for presumed acute chest syndrome, and a morphine infusion was started. Bloodwork was significant for a worsening anemia, reticulocytopenia, and thrombocytopenia; a leucoerythroblastic smear (increased nucleated red blood cells and an immature shift in white blood cells); and elevated lactate dehydrogenase, ferritin, and triglycerides (Figures 1 and 2). The clinical and laboratory findings were suggestive of bone marrow necrosis and fat embolism syndrome (BMN/FES). On DOA 7, the patient was admitted to the pediatric intensive care unit for treatment of BMN/FES by double volume exchange transfusion and plasma exchange. Given his clinical deterioration, further daily doses of IV pulse methylprednisolone over a total of 5 days were given for treatment of HLH, with subsequent improvement and transfer back to the pediatric medicine ward on DOA 9. Notably, the patient received a total of 13 days of antimalarial treatment (10 days IV artesunate and 3 days of oral atovaquone-proguanil) due to fluctuating parasitemia levels. His parasitemia level was highest on DOA 1 at up to 2% and 1.7% on DOA 2, including few immature schizont stages. He then had down-trending parasitemia levels, including 1% on DOA 5, 0.3% on DOA 7, and few to no parasites detectable thereafter (Figure 3 and Table 1). The patient required ongoing hospitalization for rehabilitation and monitoring of end organ function. On DOA 23, the patient was discharged home to complete a 3-week oral corticosteroid taper. He has remained well since discharge.
Figure 3.
The patient’s day of admission (DOA) is plotted on the x-axis, and the parasitemia index level is plotted on the y-axis. Parasitemia measurements were discontinued on DOA16.
Table 1.
Timeline of microbiological investigations for malaria
| doa | Thick and thin smear microscopy | BinaxNOW RDT | PCR Ct value | ||||
|---|---|---|---|---|---|---|---|
| Ring forms | Trophozoite forms | Schizont forms | Parasitemia (%) | T1 band (PfHRP2) | T2 band (Aldolase) | ||
| 1 | Moderate | Rare | None | 0.3 | Very strong | Very strong | 22 |
| 2 | Many | Rare | Rare | 0.5 | Very strong | Very strong | NP |
| Many | Rare | Rare | 2.0 | NP | NP | NP | |
| 3 | Numerous | Few | Rare | 1.7 | Very strong | Very strong | NP |
| 4 | Numerous | Few | Rare | 1.1 | NP | NP | NP |
| Numerous | None | None | 1.2 | Very strong | Moderate | NP | |
| 5 | Many | Few | None | 1.0 | Strong | Faint | NP |
| Numerous | None | None | 0.4 | Very strong | Strong | NP | |
| 6 | Numerous | None | None | 0.5 | Very strong | Moderate | NP |
| Numerous | None | None | 0.5 | Strong | Faint | NP | |
| 7 | Many | None | None | 0.4 | NP | NP | NP |
| 8 | Many | None | None | 0.3 | Strong | Faint | NP |
| 11 | Few | None | None | <0.1 | Very faint | None | NP |
| Few | None | None | <0.1 | Very faint | None | NP | |
| 12 | Few | None | None | <0.1 | Very faint | None | NP |
| 13 | None | None | None | Neg | Very faint | None | 25.5 |
| 14 | Rare | None | None | <0.1 | None | None | 28.6 |
| 15 | Rare (thick only) | None | None | <0.1 | None | None | NP |
| 16 | Three rings (thick only) | None | None | <0.1 | None | None | NP |
Ct = cycle threshold; DOA = date of admission; NP = not performed; PCR = polymerase chain reaction; PfHRP2 = Plasmodium falciparum histidine-rich protein 2; RDT = rapid diagnostic test. Thick and thin smear microscopy was performed by the hospital hematology laboratory and confirmed by the provincial reference laboratory, which confirms 200 to 300 positive malaria specimens per year. Parasitemia was calculated from thin smears as a percentage of infected red blood cells, with a minimum of 2,000 red blood cells assessed across randomly obtained fields of view and excluding gametocytes. Subjective quantities of each Plasmodium stage are also described. Immunochromatographic testing was performed by the BinaxNOW RDT (Abbott, Chicago, IL). Subjective band intensity is also described. Real-time PCR was conducted as previously clinically validated for academic purposes.21 Bolded text refers to positive results.
DISCUSSION
Endemic in many tropical and subtropical countries, malaria is one of the most important travel-acquired infections that can present and be diagnosed in nonendemic regions. Typical symptoms range from mild flulike illness to severe malaria with cerebral disease and end organ failure.1 In rare cases, malaria can also trigger secondary inflammatory conditions, leading to life-threatening complications.2 Hemoglobinopathies have been shown to variably reduce the risk of developing severe malaria.3–7 Literature suggests that different hemoglobin variants have been naturally selected among the population due to the protection they confer from malaria. For example, a meta-analysis of case–control and prospective studies looking at hemoglobinopathies and malaria showed that HbAS is consistently associated with large reductions in the risk of developing severe malaria.4 Both homozygous and heterozygous alpha-thalassemia has also been shown to protect against severe malaria.4,5 Various mechanisms for protection have been proposed, including enhancement of IgG responses to the parasites major cytoadherence ligand and virulence factor P. falciparum erythrocyte membrane protein, impaired parasite growth, enhanced immune clearance of parasitized erythrocytes, and induction of protective immunomodulatory mechanisms.6,7 These proposed mechanisms do not appear to prevent parasitemia but rather reduce the likelihood of developing severe disease.4 In contrast, in patients with the homozygous recessive form of SCD (HbSS), a higher mortality has been reported in children hospitalized with malaria than in controls.8,9 It has been suggested that mortality is associated with effects of infection such as hypovolemia, acidosis, severe anemia, and malaria-associated bacteremia, rather than with malaria infection itself.8 Type SC is thought to be more protective of malaria than HbSS, with a slightly reduced overall incidence of malaria in patients with HbSC.8 However, similar to HbSS, due to the underlying clinical hematology disease, the HbSC population is at higher risk of increased severity of morbidity and mortality if they do contract malaria.9
Macrophage activation syndrome is a life-threatening clinical syndrome caused by excessive proliferation and activation of T lymphocytes and well-differentiated macrophages. Infections are a common trigger of MAS, and at least two case reports describe P. falciparum malaria as a rare cause of MAS/sHLH.2,10 Identification of hyperinflammation in the context of malaria is important because MAS is primarily treated with systemic corticosteroids, which would otherwise not be recommended in patients with malaria.11 In our patient, MAS was suspected due to the significant hyperinflammation (hyperferritinemia, elevated C-reactive protein and triglycerides) and bicytopenia associated with recurrent fever on appropriate antimalarial treatment. Recent literature has suggested clinical suspicion as a key component to making the diagnosis of MAS.12
Notably, our patient had initial defervescence on DOA 3 but quantifiable parasitemia levels up to DOA 7, meeting WHO classification for late parasitological failure.13 Laboratory malaria examination findings are reported in Table 1. Although both rapid diagnostic testing and polymerase chain reaction can remain positive for weeks after therapy due to residual post-parasiticidal products and should not be used to monitor response, the prolonged detection of P. falciparum ring-stage parasites while on parenteral artesunate monotherapy and atovaquone–proguanil is noteworthy. Artemisinin partial resistance, defined by the WHO as delayed parasite clearance by day 3 of artesunate monotherapy or artemisinin-based combination therapy, is well described in the greater Mekong subregion and increasingly recognized in some African countries such as Eritrea, Rwanda, and Uganda, but is not yet documented in Ghana.14 Artemisinin-induced ring-stage dormancy is also reported regardless of resistance phenotype, although it is usually associated with either submicroscopic parasitemia levels or less typical ring-stage morphology.15 Subtherapeutic dosing could theoretically lead to delayed parasitological clearance, however the patient received the recommended WHO dosing to accommodate interindividual variability in parenteral artesunate plasma concentrations.16 Unfortunately, neither drug resistance testing nor plasma level monitoring were available for our patient at the time of publication.
Another potential explanation for the late parasitological failure could be the relative severity of infection seen in our patient in the context of ongoing inflammation with subsequent MAS and BMN/FES, which may lead to a hyperstimulated but ineffective immune response. That said, in previous case reports describing P. falciparum malaria as a rare cause of MAS, the parasitemia cleared within 2 to 4 days after initiation of antimalarial treatment.2,10 Alternatively, aspects of SC disease may have impaired the host response in clearing the parasite.13
There was initial improvement with systemic steroids, but our patient subsequently developed BMN/FES as suggested by his clinical presentation, fever, cytopenias, leucoerythroblastic smear, and hyperinflammatory markers. Although no definitive diagnostic criteria exist for BMN/FES, the constellation of HbSC with clinical findings of unusually severe pain, neurological symptoms, and respiratory deterioration, as well as the laboratory findings of leukoerythroblastosis, anemia, thrombocytopenia, and elevated LDH and ferritin are strongly suggestive of BMN/FES in this patient. The patient did not have a bone marrow biopsy to evidence necrosis or histological evidence of fat or necrotic marrow emboli. However, bone marrow biopsy and histology are not required because the diagnosis is made clinically.15 A rare but severe complication seen in patients with SCD, especially HbSC, BMN/FES is associated with high rates of morbidity and mortality. Bone marrow necrosis results from vaso-occlusive crisis within the bone marrow, which releases necrotic marrow, hematopoietic cells, fat globules, and phospholipids into the venous circulation. This leads to mechanical obstruction from lipids and inflammation secondary to phospholipids, which can present as respiratory failure, stroke, end organ dysfunction, and even death. Prompt identification and treatment is critical for patient outcomes, and red cell exchange with or without plasma exchange should be initiated immediately in all patients for whom the condition is suspected. We hypothesize that the patient’s malaria infection alongside the evident hyperinflammation may have triggered the BMN and FES because it has previously and similarly been reported for parvovirus B19, herpes simplex virus, and dengue virus. To our knowledge, this is the first FES/BMN case associated with a malaria infection.
Interestingly, elevated ferritin, lactate dehydrogenase, and cytopenias are overlapping features of MAS and BMN/FES.12,17 Hemophagocytosis, a key feature of MAS, has also been identified on bone marrow biopsies from BMN/FES patients.18 We cannot definitively confirm whether the patient’s presentation was due to both MAS and BMN/FES. However, given the two peaks in inflammatory markers and slightly different clinical presentations between DOA 2 and DOA 5, we hypothesize that the malaria infection triggered a hyperinflammatory state, and the BMN/FES of SCD evolved secondarily as his bone pain, leukoerythroblastosis, and confusion, which are BMN/FES features, developed a few days after the MAS. Moreover, inflammatory markers decreased after corticosteroid initiation and subsequently increased at the time of onset of bone pain and confusion. Although blood and plasma exchange transfusion was largely targeted at the BMN/FES, it can also be used to treat MAS and might have therefore led to improvement of both clinical syndromes. Exchange transfusion is no longer estimated to benefit most cases of severe or complicated malaria; therefore, its direct impact on the patient’s underlying infection is uncertain.19 Although malaria and the subsequent hyperinflammation are the most likely triggers for the patient’s BMN/FES, steroids have also been described to trigger BMN/FES and therefore cannot be ruled out as a contributing cause of his BMN/FES presentation.20
Prompt diagnosis and treatment of both MAS and BMN/FES is critical for a favorable outcome because they each require distinct management. This case reinforces the importance for clinicians to investigate actively for both MAS and BMN/FES based on patients’ comorbidities, particularly for patients presenting with persistent fever, cytopenias, and markers of inflammation, despite appropriate antimalarial therapy.
REFERENCES
- 1. World Health Organization , 2022. Malaria Fact Sheet. Available at: https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed July 26, 2022.
- 2. Dass R, Barman H, Duwarah SG, Choudhury V, Jain P, Deka NM, Murari M, 2009. Macrophage activation syndrome in malaria. Rheumatol Int 30: 1099–1101. [DOI] [PubMed] [Google Scholar]
- 3. Rosário do Sambo M, Penha-Gonçalves C, Trovoada MJ, Costa J, Lardoeyt R, Coutinho A, 2015. Quantitative trait locus analysis of parasite density reveals that HbS gene carriage protects severe malaria patients against Plasmodium falciparum hyperparasitaemia. Malar J 14: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor SM, Parobek CM, Fairhurst RM, 2012. Impact of haemoglobinopathies on the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis 12: 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams TM, Wambua S, Uyoga S, Macharia A, Mwacharo JK, Newton CRJC, Maitland K, 2005. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood 106: 368–371. [DOI] [PubMed] [Google Scholar]
- 6. Travassos MA. et al. , 2015. Hemoglobin C trait provides protection from clinical falciparum malaria in Malian children. J Infect 212: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López C, Saravia C, Gomez A, Hoebeke J, Patarroyo MA, 2010. Mechanisms of genetically-based resistance to malaria. Gene 467: 1–12. [DOI] [PubMed] [Google Scholar]
- 8. McAuley CF. et al. , 2010. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Clin Trials J 116: 1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makani J. et al. , 2010. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood 115: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trapani S, Canessa C, Fedi A, Giusti G, Barni S, Montagnani C, Galli L, Resti M, De Martino M, 2013. Macrophage activation syndrome in a child affected by malaria: the choice of steroid. Int J Immunopathol Pharmacol 26: 535–539. [DOI] [PubMed] [Google Scholar]
- 11. Varo R, Crowley VM, Sitoe A, Madrid L, Serghides L, Kain KC, Bassat Q, 2018. Adjunctive therapy for severe malaria: a review and critical appraisal. Malar J 17: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henderson LA, Cron RQ, 2019. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: diagnosis and management. Paediatr Drugs 22: 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization , 2010. Methods for Surveillance of Antimalarial Drug Efficacy. Available at: https://www.who.int/docs/default-source/documents/publications/gmp/methods-for-surveillance-of-antimalarial-drug-efficacy.pdf. Accessed May 2, 2023.
- 14. Ahorhorlu SY. et al. , 2023. Assessment of artemisinin tolerance in Plasmodium falciparum clinical isolates in children with uncomplicated malaria in Ghana. Malar J 22: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peatey C, Chen N, Gresty K, Anderson K, Pickering P, Watts R, Gatton ML, McCarthy J, Cheng Q, 2021. Dormant Plasmodium falciparum parasites in human infections following artesunate therapy. J Infect Dis 223: 1631–1638. [DOI] [PubMed] [Google Scholar]
- 16. Byakika-Kibwika P. et al. , 2012. Pharmacokinetics and pharmacodynamics of intravenous artesunate during severe malaria treatment in Ugandan adults. Malar J 11: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsitsikas DA, Bristowe J, Abukar J, 2020. Fat embolism syndrome in sickle cell disease. J Clin Med 9: 3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosch A, Salmas W, Willmott S, McKinnon N, Malcolmson C, Kirby M, 2022. Identifying and treating severe bone marrow necrosis and fat embolism syndrome in pediatric patients with sickle cell disease: a case report. J Pediatr Hematol Oncol 44: e884–e887. [DOI] [PubMed] [Google Scholar]
- 19. Tan KR, Weigand RE, Arguin PM, 2013. Exchange transfusion for severe malaria: evidence base and literature review. Clin Infect Dis 57: 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang Jun C, Gay R, Khella Sami L, 1994. Sickling crisis, fat embolism, and coma after steroids. Lancet 344: 951–952. [DOI] [PubMed] [Google Scholar]
- 21. Phuong M, Lau R, Ralevski F, Boggild AK, 2015. Survival analysis of diagnostic assays in Plasmodium falciparum malaria. Malar J 14: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]