ABSTRACT.
The forcibly displaced Myanmar nationals (FDMNs) known as Rohingya refugees are the largest group of stateless individuals globally. According to the emergencies humanitarian actors at the United Nations Office for the Coordination of Humanitarian Affairs, the worldwide refugee crisis involving FDMNs is intensifying at the fastest rate in history. Growing public health demands are being exacerbated by current difficulties in addressing poor access to health services, severe food shortages, and a lack of adequate housing. Infectious diseases constitute a major public health emergency in this vulnerable population. A study was carried out in FDMN children to investigate common soil-transmitted helminth (STH) infection at the time of enrollment and prospectively followed-up to 12 months after 2 doses albendazole treatment. At baseline, the prevalence of STH infection with at least one species was found to be 91.7% and 87.3% for Kato–Katz (KK) and quantitative polymerase chain reaction (qPCR) methods, respectively. Similarly, for follow-up children, the overall infection rate was 95.3% and 91.5%, respectively. Trichuris trichiura was the most predominant STH infection by both KK (baseline 87%, follow-up 89.1%) and qPCR (baseline 77.5%, follow-up 82.9%). The overall prevalence of stunting in the children was 37.8% at baseline and rose to 51.3% at 12 months. Alpha-1 antitrypsin (r = 0.13, P = 0.01) and myeloperoxidase (r = 0.12, P = 0.01) levels showed a positive correlation with Aascaris lumbricoides egg count per gram at baseline. An in-depth investigation is urgently needed to identify the underlying protective measures and the root cause of STH infections to improve the health of FDMN children.
INTRODUCTION
According to the WHO, approximately 25% of population across different age groups worldwide may harbor helminth infections, which are transmitted through the soil and cause at least 12,000 fatalities annually.1 Ascaris lumbricoides, Trichuris trichiura, and two hookworms—Ancylostoma duodenale and Necator americanus—are most common soil-transmitted helminth (STH) infections worldwide. WHO recognized STH infections as the most prominent neglected tropical diseases.2,3 In accordance with the evidence from the most recent report, Asia accounts for more than 70% of STH infections.4 Poverty, insufficient sanitary condition and hygiene, a paucity of adequate drinking water, restricted healthcare accessibility, and precautionary measures are all intimately associated to STH infections.5–7 STH infections has been predicted to be responsible for 5.18 million disability-adjusted life years.8 Although STH infection causes relatively few deaths, persistent infections during childhood can negatively impact nutritional status, cognitive function, exacerbating anemia, trigger a range of adverse tissue reactions and even cause intestinal obstruction particularly among school-aged children (SAC).9,10 Over the past decade, the WHO has increasing recommended routine mass medication administration as a main strategy for reducing STH burden.11 The present approach involves administering the anthelminthic medications albendazole or mebendazole to preschoolers (pre-SAC, ages 2–4 years) and SAC (ages 5–14 years), regardless of their infection status.12
As an ethnic minority group, the Rohingya have spent centuries residing in Myanmar’s Rakhine state. Rohingya refugees have been fleeing to Bangladesh from Myanmar since the 1970 s. However, in recent years, approximately one million Rohingya people were forced to flee into Bangladesh as a result of violence in Myanmar’s Rakhine state, which started on August 25, 2017, and refugees are continuing coming into Bangladesh across its borders. The refugees, who came in Bangladesh with limited resources, are now dependent on humanitarian aid for all types of life-sustaining necessities, including shelter, food, health, clothing, water, and sanitation facilities. More than 80% of the forcibly displaced Myanmar nationals (FDMNs) population comprises women and children,13 and 0.3 million of those children are between the ages of 5 and 17 years.14 In Bangladesh’s Cox’s Bazar district, 34 refugee camps provided housing for up to a million people.15 An enormous number of health issues, including diarrhea, hepatitis, cholera, and typhoid, are experienced by the Rohingyas living in camps as a result of inadequate water, sanitation and hygiene, and land shortages; 40,000 Rohingya refugees reside in a single square kilometer in the camp sites.16 Their health is at risk due to the overcrowding and inadequate sanitation conditions in the Rohingya refugee camps,17 and intestinal parasitic infection is a common concern. Of all the countries in the world, Myanmar has the eighth-largest frequency of STH infections, with a calculated 40.8 million individuals at risk and demanding preventative treatment, including more than 13 million children who live in endemic areas.18 Healthcare support is thus an issue of dire need for the FDMNs. However, the health status of Rohingya children is not well understood, especially the prevailing frequency of helminth infection, which is unknown. Henceforth, this study was conducted with the objective of determining the frequency of common STH infections among Rohingya children. Furthermore, we conducted an exploratory analysis on associations between STH infection and child growth as well as inflammatory biomarkers; in particular, fecal alpha-1 antitrypsin (A1AT) and myeloperoxidase (MPO) have been investigated.
MATERIALS AND METHODS
Study site, population, and sampling.
This study was conducted between October 20, 2019 and December 13, 2020 among Rohingya refugees residing in FDMN camp number 24 in Teknaf subdistrict of Cox’s Bazar district. This camp accommodates ∼26,435 people who were forcefully displaced from their homes in Myanmar. Teknaf is located in the subtropical monsoon climate zone, which has significant seasonal variations in rainfall, high temperatures, and humidity.
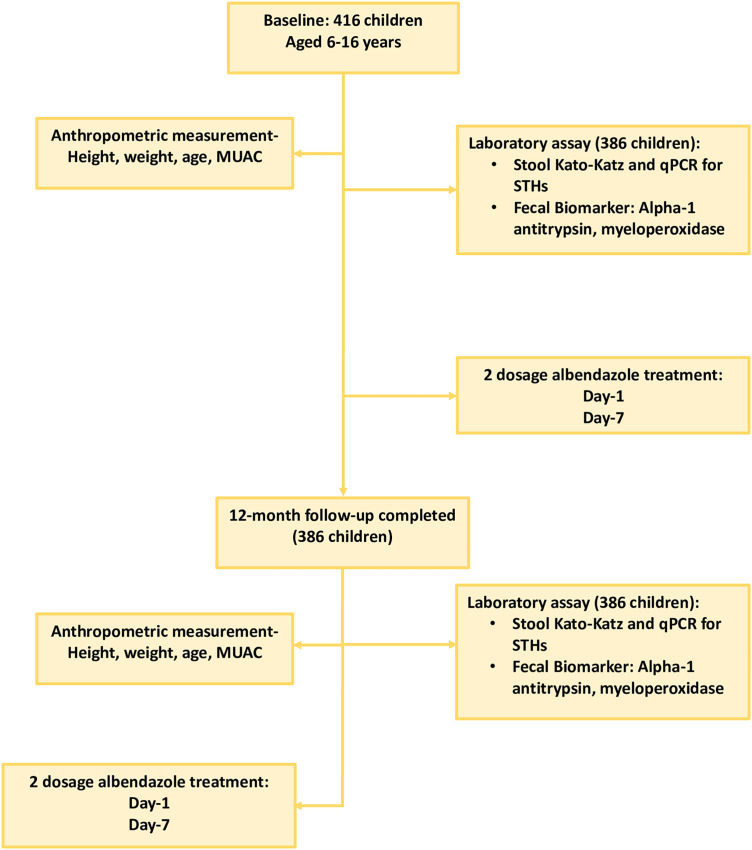
This was a longitudinal cross-sectional study, conducted among children aged between 6 and 16 years with baseline and end assessments with a 12-month follow-up after two doses of albendazole treatment. The aim of the study and methodology were briefed to participants or legal guardians before enrollment of participants into the study. Children who met the following inclusion criteria were included in the study: displaced Myanmar children of either sex, aged 6 to 16 years, no deworming therapy within past 6 months, and 6 months of residency in the selected cluster. The single smears Kato–Katz (KK) microscopy method was used to examine STH infection and intensity from participating children. After baseline assessment, a 400-mg albendazole treatment was given to each child after stool sample collection and a second dose was given 7 days after the first dose under the supervision of field and clinical staff supervision. Under unforeseen circumstances imposed by the COVID-19 shutdown in Bangladesh in March 2020, the 6-month follow-up visit was extended to 12-months. After 12 months of enrollment, a follow-up visit was done, and another stool sample was collected to assess the effects of albendazole treatment on helminth status, inflammatory biomarkers, and nutritional status (Figure 1). During this follow-up visit after stool collection, each child received two 400-mg doses of albendazole treatment, 1 week apart.
Figure 1.
Study design flow diagram. MUAC = mid-upper arm circumference; qPCR = quantitative polymerase chain reaction; STH = soil-transmitted helminth.
Anthropometric measurements of children.
The height, weight, and mid-upper arm circumference (MUAC) of the children were measured s study at baseline and 12-month follow-up before anthelmintic treatment. Height was measured by a length board (Shorr Board) with a plastic tape extended between a footplate and a head bar, with a precision of 0.1 cm. The mean of three consecutive measurements was considered the observed value. The standing weight of the child was measured by an electronic scale (TANITA, HD-314, Tsimshatsui East Kowloon, Hong Kong), which measures accurately to the nearest 100 g. The MUAC of a child was measured at the midpoint between the shoulder and elbow of the bare left arm using tricolored insertion MUAC tape to the nearest centimeter. Three consecutive readings were taken, and the averaged value was used during data analysis. Height-for-age z score was determined by using WHO Anthro software (version 3.2.2, Informer Technologies, Inc. Los Angeles, CA).
Laboratory analysis: Stool microscopy and ova counts for STH species.
At enrollment and at follow-up visits, study staff from collected stool samples all enrolled participants, which were then transported to the study site field laboratory for diagnosis of STH infection by using KK technique (VESTGAARD FRANDSEN GROUP, Lausanne, Switzerland).19 In summary, a thin layer of stool was smeared on a piece of paper, and then a nylon screen was placed above it. To gather the sieved stool, the top portion of the surface was scraped using a flat-sided spatula. Then the sieved stool samples were placed with the spatula after a template had been set on the slide. After carefully removing the template, a cylinder of stools remained on the slide. The slide was then reversed, the stool sample was pressed hard against the hydrophilic cellophane strip to distribute it equally, and the stool material was covered with a presoaked cellophane strip. Lastly, the slide was positioned on the bench with the cellophane facing upward to allow for the drying of water as glycerol clears the stools. Before microscopic examination, the slides were maintained at room temperature for ≥ 1 hours to remove any fecal matter from all STHs, except for hookworm eggs. Ten percent of slides were examined separately by two technologists for quality control, and 5% were reviewed by the same skilled parasitologist who conducted KK training.
Extraction of DNA from stool specimens.
A modified Qiagen stool DNA extraction protocol (QIAamp Fast DNA Stool Mini kit) was performed to extract DNA from stool sample,20 which included a 95°C incubation step and a 3-minute bead-beating step. Briefly, ∼200 mg of faces was suspended in 1 mL InhibitEX buffer containing ∼370 mg of acid washed glass beads (Sigma) and mixed well by vortexing. After vortexing, bead beating was done at maximum speed for 3 minutes. Heating for 5 minutes at 95°C, then the suspension were treated with AL buffer containing proteinase K for 10 minutes at 70°C. During extraction, we used phocine herpesvirus (PhHV) as an internal control, to detect the inhibition of each sample and then quantified by quantitative polymerase chain reaction (qPCR) reaction. In each batch, we used InhibitEX buffer instead of stool as extraction blank (24 samples usually) to detect possible contamination.
Multiplex real-time qPCR.
The STHs species real-time qPCR assay was performed using real-time TaqMan PCR primer and probe, as described previously.21,22 In brief, to detect STHs we used the forward primer, the reverse primer and the probe for the A. lumbricoides ITS1, T. trichiura 18 s, A. duodenale ITS2, N. americanus ITS2, and PhHV gB target genes (Table 1). To assess the qPCR efficiency and inhibition of the sample, an internal control was included in the qPCR run. Both the negative and positive controls were used each time. Amplification consisted of 3 minutes at 95°C followed by 40 cycles of 10 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C. Samples that had a quantification cycle (Cq) value ≤ 36 were considered positive. The CFX96 Real-Time Detection System (Bio-Rad, Bio-Rad Laboratories, Hercules, CA) was used to amplify, detect, and analyze data. Annealing step (60°C) of each amplification cycle was measured the fluorescence.
Table 1.
Primers and probes sequences used in this study
| STH panel primers and probes | Reference | |||
|---|---|---|---|---|
| Pathogen | Sequences | Target | ||
| A. lumbricoides | F | 5′-TGCACATAAGTACTATTTGCGCGTAT-3′ | ITS1 | 23 |
| R | 5′-CCGCCGACTGCTATTACATCA-3′ | |||
| P | SUN-5′-GAGCCACATAGTAAATT-3′-3MGB-NFQ | |||
| A. duodenale | F | 5′-GAA TGA CAG CAA ACT CGT TGT TG-3′ | ITS2 | 23 |
| R | 5′-ATA CTA GCC ACT GCC GAA ACG T-3′ | |||
| P | AminoC6 + TxRed-5′-ATC GTT TAC CGA CTT TAG-3′ -BHQ2a∼Q | |||
| N. americanus | F | 5′-CTG TTT GTC GAA CGG TAC TTG C-3′ | ITS2 | 23 |
| R | 5′-ATA ACA GCG TGC ACA TGT TGC-3′ | |||
| P | FAM-5′-CTG TAC TAC GCA TTG TAT AC-3′ -MGBE∼Q | |||
| T. trichiura | F | 5′-TTGAAACGACTTGCTCATCAACTT-3′ | 18 s | 24 |
| R | 5′-CTGATTCTCCGTTAACCGTTGTC-3′ | |||
| P | Cy5.5-5′-CGATGGTACGCTACGTGCTTACCATGG-3′- BHQ2a∼Q | |||
| PhHV | F | 5′-GGGCGAATCACAGATTGAATC-3′ | gB |
25 Modified |
| R | 5′-GCGGTTCCAAACGTACCAA-3′ | |||
| P | Quasar 705-5′-CGATGGTACGCTACGTGCTTACCATGG-3′- BHQ2a∼Q | |||
F = forward primer; P = probe; PhHV = phocine herpesvirus; R = reverse primer; STH = soil-transmitted helminth.
Fecal biomarkers measurements.
Fecal A1AT was measured using ELISA from ImmuChrom GmbH (BioVendor R&D, Brno, Czech Republic) and MPO was measured using commercial ELISA kits from Immunodiagnostic AG (Bernsheim, Germany) according to the manufacturer’s instructions.
Statistical analysis.
Questionnaires were reviewed for accuracy and completeness. For statistical analysis, data were entered into MS Excel, cleaned, and exported into SPSS version 20, GraphPad Prism version 5 (SPSS Inc., Chicago, IL), and R programming version 4.2.1 (Posit Software, Boston, MA). The demographic details of the study participants were illustrated using a descriptive analysis that included frequency, mean, and percentage. Cohen’s kappa test was analyzed to assess agreement between KK and qPCR methods. The level of agreement was graded using the following system: perfect agreement (0.81–1.0), substantial agreement (0.61–0.80), moderate agreement (0.41–0.60), fair agreement (0.21–0.40), poor agreement (0.00–0.20), and no agreement (< 0). χ2 tests were performed to calculate difference between groups of categorical data. The samples that were categorized as positive by both PCR and KK were correlated using Spearman rank correlation test. Independent-samples t tests were used to compare scores on the continuous variable. Statistical significance was defined as a P value of 0.05 or less.
RESULTS
Sociodemographic characteristics of the study participants.
In this study, 416 FDMN children were enrolled at baseline, and 386 of those children completed a 12-month follow-up. We used the KK and multiplex qPCR methods to investigate stool samples from 386 children at both baseline and at the 12-month follow-up. The percentage of females was comparatively lower than that of males (45%, N = 174). The mean (± SE) age of the children was 10.06 (± 0.14) years at baseline. The average age of males and females were comparable (9.95 years for males, 10.06 years for females).
Prevalence and egg intensity of STH infection at baseline and 12-month follow-up after albendazole treatment.
The overall prevalence of infection with at least one species of STH was found to be 91.7% (354/386) and 87.3% (337/386) children at baseline using the KK and multiplex qPCR methods, respectively. The most predominant STH was T. trichiura, with prevalence rates of 87% children at baseline, followed by A. lumbricoides 49.2% and hookworm (N. americanus or A. duodenale) 8.3% by the KK, respectively.
Similarly, for follow-up children, 12 months after antihelminthic treatment, the overall infection rate with at least one species of STH was 95.3% for the KK and 91.5% for the multiplex qPCR method. As at baseline, T. trichiura was the most predominant STH, with prevalence rates of 89.1% in follow-up children, followed by A. lumbricoides (52.1%) and hookworm (N. americanus or A. duodenale) (4.1%) by KK, respectively. However, results of the for qPCR analysis showed that the prevalence of T. trichiura was 82.9%, A. lumbricoides was 60.1%, and N. americanus hookworm was 2.3% for the follow-up children. Using multiplex qPCR, A. duodenale was not detected for at baseline of follow-up.
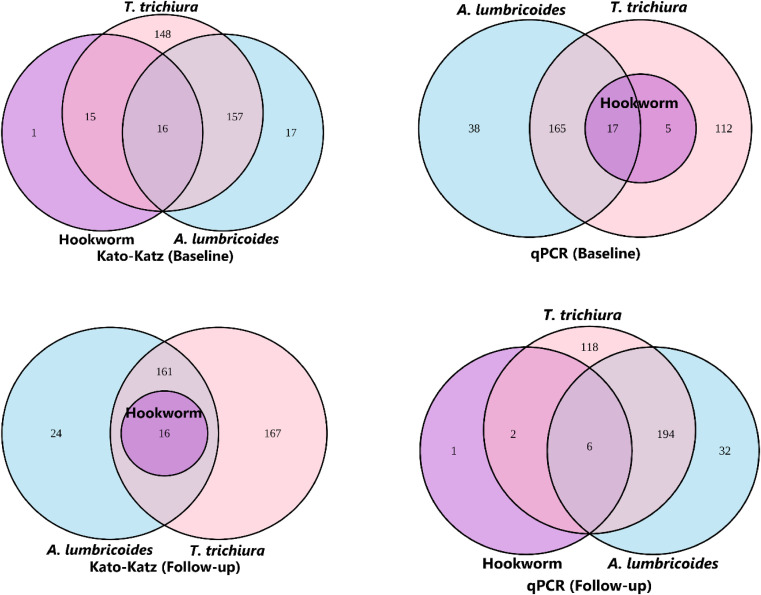
At baseline, co-infection rate of A. lumbricoides with T. trichiura was 44.8% and 47.1% using KK and multiplex qPCR, respectively. During the follow-up, the co-infection rate of A. lumbricoides with T. trichiura was 45.9% and 51.8% using KK and multiplex qPCR, respectively. Consequently, co-infection rate of A. lumbricoides with hookworm was 8% and 5.7% using KK and multiplex qPCR, respectively, at baseline. Subsequently during follow-up, the co-infection rate of A. lumbricoides with hookworm was 4.1% and 2.1% using KK and multiplex qPCR, respectively. Moreover, using the KK method, T. trichiura co-infection with hookworm was 8.0% and 4.1% at baseline and follow-up, respectively, whereas it was 5.7% and 2.1% at baseline and follow-up using the multiplex qPCR method. The co-infection of all these three helminths together, such as A. lumbricoides with both T. trichiura and hookworm was 4.1% and 4.4% at baseline and 4.1% and 1.6% at follow-up using KK and multiplex qPCR, respectively. The number of STH infections and co-infections are illustrated in Figure 2. However, only hookworm species showed a significant difference in prevalence rate between baseline and follow-up for both the KK (χ2 = 9.59, P = 0.002) and multiplex qPCR (χ2 = 5.68, P = 0.01).
Figure 2.
Euler diagram of co-infections detected by Kato–Katz (KK) and quantitative polymerase chain reaction (qPCR) methods. Soil-transmitted helminth egg or DNA were detected in stool samples collected from forcibly displaced Myanmar national children in Bangladesh using KK or multiplex qPCR for both baseline and follow-up (N = 386).
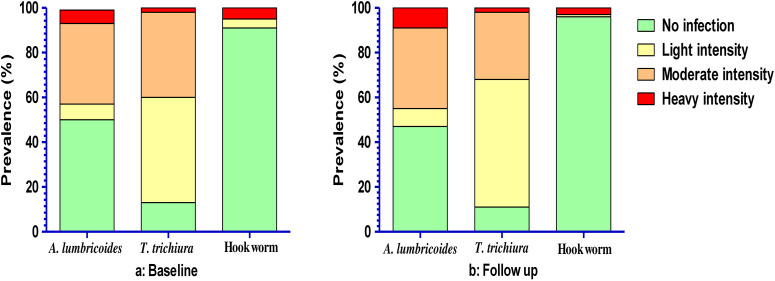
The egg intensity of each species of STHs infection has been represented in Figure 3. Among the infected children, A. lumbricoides was mostly of moderate intensity at both baseline (36%, N = 139) and follow-up (35.5%, N = 137). However, both at baseline (46.9%, N = 181) and at follow-up (56.7%, N = 219), the majority of the children with T. trichiura had light egg intensity. For hookworm, heavy egg intensity was found at both baseline and follow-up.
Figure 3.
Observed soil-transmitted helminth (STH) infection intensity prevalence by Kato–Katz as per WHO STHs eggs count guidelines. Prevalence was estimated from stool samples collected from forcibly displaced Myanmar national children for both baseline and follow-up.
Agreement between KK and multiplex real-time qPCR.
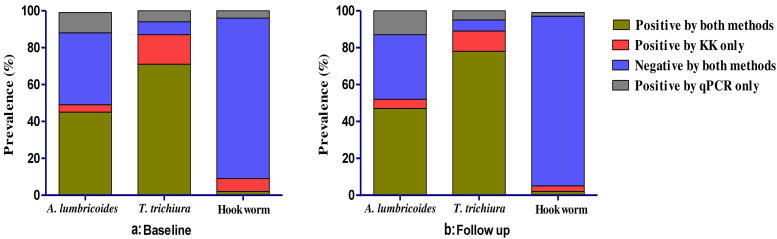
As shown in Table 2, there was substantial agreement between KK and qPCR for detection of A. lumbricoides at both baseline (κ = 0.68, P < 0.001) and follow-up (κ = 0.64, P < 0.001). Of the 190 children at baseline who were positive by KK, only 14 were negative using multiplex qPCR; 44 of 220 multiplex qPCR-positive children were negative for KK. Similarly, for children at follow-up, out of 201 KK-positive children, 19 were negative by multiplex qPCR and 50 of 232 multiplex qPCR-positive children were negative using KK.
Table 2.
Agreement calculation between egg count per gram using KK and frequency matched categories for qPCR DNA Ct values
| STH | Time point | Kappa | 95% CI | Observed agreements | P value |
|---|---|---|---|---|---|
| A. lumbricoides | Baseline | 0.68 | 0.63 to 0.77 | 84.97% | < 0.001 |
| Follow-up | 0.64 | 0.56 to 0.72 | 82.12% | < 0.001 | |
| T. trichiura | Baseline | 0.28 | 0.16 to 0.39 | 78.50% | < 0.001 |
| Follow-up | 0.32 | 0.19 to 0.44 | 83.42% | < 0.001 | |
| Hookworm | Baseline | 0.13 | −0.02 to 0.27 | 88.60% | 0.01 |
| Follow-up | 0.22 | −0.01 to 0.44 | 95.08% | < 0.001 |
Ct = cycle threshold; KK = Kato–Katz; qPCR = quantitative polymerase chain reaction; STH = soil-transmitted helminth. The number of observed agreements was 386 for all STH; interpreted kappa < 0 = no agreement, 0.00 to 0.20 = slight agreement, 0.21 to 0.40 = fair agreement, 0.41 to 0.60 = moderate agreement, 0.61 to 0.80 = substantial agreement, and 0.81 to 1.00 = perfect agreement. P value for the difference was determined by the Cohen’s kappa agreements.
For T. trichiura, there was fair agreement between KK and multiplex qPCR at both baseline (κ = 0.28, P < 0.001) and follow-up (κ = 0.32, P < 0.001); at baseline, of the 336 children who were positive using the KK method, 60 were negative for multiplex qPCR, whereas 23 of 299 qPCR-positive children were negative by the KK method. During the follow-up period, 344 children who were positive for KK, 44 children were negative using the multiplex qPCR method and 20 of 320 multiplex qPCR-positive children were negative for KK method.
For hookworm, there was a slight agreement between KK and multiplex qPCR at baseline (κ = 0.13, P = 0.01), and there was fair agreement at follow-up (κ = 0.22, P < 0.001). At baseline, of the 32 children who were positive using the KK method, 27 children were negative for multiplex qPCR whereas 17 of 22 multiplex qPCR-positive children were negative for KK method. During the follow-up period, of the 16 children who were positive for KK, 13 were negative using the multiplex qPCR method and six of nine multiplex qPCR-positive children were negative for KK method (Figure 4).
Figure 4.
Overview of diagnostic performance of soil-transmitted helminth (STH) species determined by KK and multiplex real-time qPCR methods. The bar graph illustrates the % prevalence of STH infestation in individuals as determined by two methods. KK = Kato–Katz; qPCR = quantitative polymerase chain reaction.
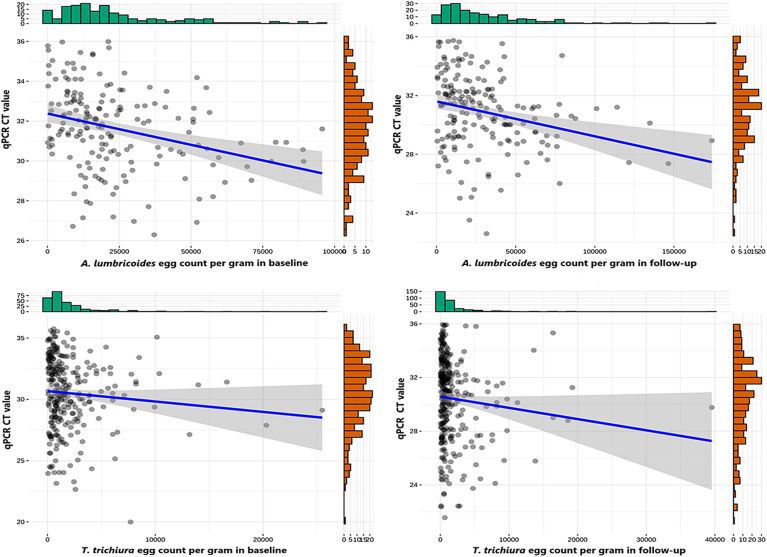
Upon correlating multiplex qPCR with KK for A. lumbricoides, the cycle threshold value (Ct value) was negatively correlated with egg count per gram (EPG) values at both baseline (r = –0.35, P < 0.001) and follow-up (r = –0.31, P < 0.001). Similarly, for the T. trichiura, Ct values were also negatively correlated with EPG values obtained using KK (r = –0.15, P = 0.01) and qPCR (r = –0.17, P = 0.003) for both the baseline and follow-up samples, respectively. Scatter plots of infection intensity for A. lumbricoides and T. trichiura are shown in Figure 5. Due to the low number of positive samples, comparisons of infection intensity were not performed for hookworm species. For A. lumbricoides, the mean egg count of KK-positive samples that were multiplex qPCR-positive was 23,189.52 EPG, whereas the mean egg count of KK-positive samples that were multiplex qPCR-negative was 6,560.57 EGP at baseline (P = 0.002) and for follow-up samples, the mean egg count of KK-positive samples that were qPCR-positive was 29,649.49 EPG, whereas the mean egg count of KK-positive samples that were multiplex qPCR-negative was 20,306.53 EPG (P = 0.18). Similarly, for T. trichiura, the mean egg count of KK-positive samples was higher (2,055.01 EPG) for multiplex qPCR-positive samples compared with the multiplex qPCR-negative samples (778 EPG) in baseline (P = 0.001). For the follow-up samples, the mean egg count of T. trichiura for both KK- and multiplex qPCR-positive samples was 1,897.54 EPG compared with multiplex qPCR-negative samples, which was 400.91 EPG (P = 0.006). In addition, the mean egg count of hookworm KK-positive samples was 10,710 EPG for multiplex qPCR-positive samples, whereas it was 14,950 EPG for multiplex qPCR-negative sample at baseline (P = 0.62); however, during the follow-up, the KK-positive samples’ mean egg count was 9,408 EPG for multiplex qPCR-positive samples and 12,729.83 EPG for qPCR-negative sample (P = 0.70).
Figure 5.
Relationship between cycle threshold (Ct) values measured by multiplex quantitative polymerase chain reaction (qPCR) and eggs per gram detected using Kato–Katz (KK). Soil-transmitted helminth egg or DNA was detected by KK and multiplex qPCR in stool samples collected from forcibly displaced Myanmar national children aged 6–16 years in Bangladesh. The Spearman correlation between eggs per gram and Ct values was –0.35 in baseline and –0.31 in follow-up for Ascaris lumbricoides (P < 0.001), and for Trichuris trichiura, it was –0.15 (P = 0.01) at baseline and –0.17 (P = 0.003) at follow-up.
STHs infections and nutritional status.
At the time of enrolment, stunting was shown to be prevalent in 37.8% of children, rising to 51.3% at 12-month follow-up visits. Compared with the baseline period (–1.49 ± 1.34), the mean ± SD height-for-age z-score during the follow-up period was significantly lower (–1.95 ± 1.40) but weight, MUAC, and height increased with age. There was no difference between groups with and without stunting at baseline for any STH infection. Infection with T. trichiura showed a statistical difference between the stunted and the nonstunted groups only during follow-up (χ2 = 5.43, P = 0.02) (Table 3). Moreover, children with T. trichiura infections had significantly lower Z scores for height-for-age z-score (–1.55 ± 1.33), weight (26.81 ± 10.10 kg), height (128.62 ± 16.53 cm), and MUAC (18.14 ± 2.89 cm) compared with those without infections (–1.16 ± 1.36, 32.66 ± 12.17, 137.36 ± 17.91, and 19.52 ± 3.28, respectively) for both baseline and follow-up (–2.02 ± 1.43 versus –1.42 ± 1.01, 28.47 ± 10.44 versus 35.62 ± 11.38, 130.60 ± 17.09 versus 141.57 ± 14.61, and 19.34 ± 3.00 versus 21.11 ± 3.05, respectively).
Table 3.
Comparison of infection rate between stunted and nonstunted groups in baseline and follow-up children
| Time point | Child status, n (%) | A. lumbricoides | P value | T. trichiura | P value | Hookworm | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive, n (%) | Negative, n (%) | Positive, n (%) | Negative, n (%) | Positive, n (%) | Negative, n (%) | |||||
| Baseline | Stunted, 146 (37.8) | 70 (47.9) | 76 (52.1) | 0.75 | 130 (89.0) | 16 (11.0) | 0.44 | 14 (9.6) | 132 (90.4) | 0.57 |
| Nonstunted, 240 (62.2) | 120 (50.0) | 120 (50.0) | 206 (85.8) | 34 (14.2) | 18 (7.5) | 222 (92.5) | ||||
| Follow-up | Stunted, 194 (51.3) | 102 (52.6) | 92 (47.4) | 0.84 | 180 (92.8) | 14 (7.2) | 0.02 | 8 (4.1) | 186 (95.9) | 0.79 |
| Nonstunted, 184 (48.7) | 94 (51.1) | 90 (48.9) | 157 (85.3) | 27 (14.7) | 6 (3.3) | 178 (96.7) | ||||
P value for difference was determined by the chi-square (χ2) statistical test for categorical data.
Association between STH infection and fecal inflammatory biomarkers.
Fecal A1AT and MPO concentrations were higher in children with STH infection compared with uninfected children. Only A1AT was found to be significantly associated with A. lumbricoides infection in baseline (P = 0.02). The fecal biomarker levels of the study participants (concentrations, µg/mL) by STH infection is shown in Table 4.
Table 4.
Comparison of fecal biomarker A1AT and MPO concentrations (µg/mL) between STH infected and noninfected children
| Time point | STHs | A1AT, µg/mL ± SD | P value | MPO, µg/mL ± SD | P value | ||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||
| Baseline | A. lumbricoides | 898.36 ± 767.26 | 729.09 ± 718.66 | 0.02 | 1.84 ± 3.66 | 1.79 ± 3.97 | 0.77 |
| T. trichiura | 838.74 ± 760.50 | 635.48 ± 625.84 | 0.07 | 1.75 ± 3.62 | 2.00 ± 4.94 | 0.66 | |
| Hookworm | 875.56 ± 743.31 | 779.23 ± 652.76 | 0.28 | 2.50 ± 4.01 | 1.72 ± 3.80 | 0.15 | |
| Follow-up | A. lumbricoides | 880.74 ± 778.99 | 786.80 ± 674.61 | 0.21 | 1.60 ± 2.62 | 1.49 ± 2.78 | 0.70 |
| T. trichiura | 845.15 ± 750.84 | 758.41 ± 547.74 | 0.47 | 1.56 ± 2.53 | 1.44 ± 3.84 | 0.79 | |
| Hookworm | 856.41 ± 699.60 | 834.82 ± 733.64 | 0.91 | 2.64 ± 3.53 | 1.50 ± 2.65 | 0.09 | |
A1AT = alpha-1 antitrypsin; MPO = myeloperoxidase; STH = soil-transmitted helminth. P value for difference was determined by the independent sample t test for continuous data. Values are expressed as mean ± SD.
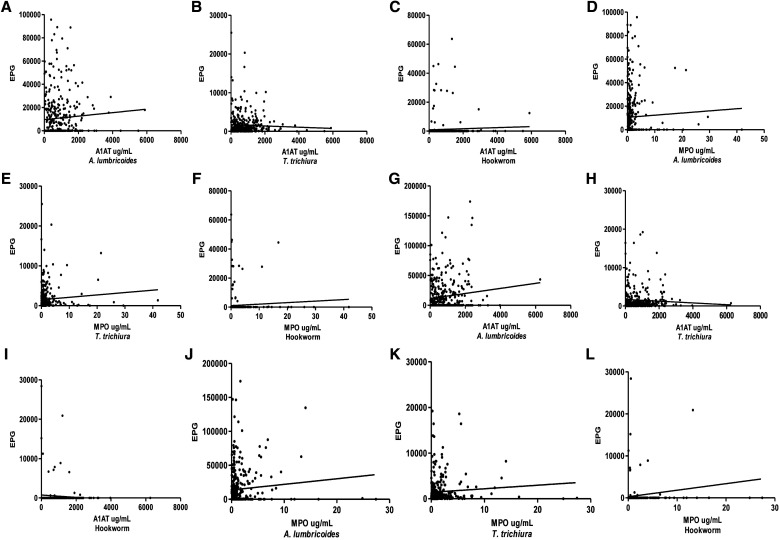
In addition, A1AT (r = 0.13, P = 0.01) and MPO (r = 0.12, P = 0.01) expression levels for A. lumbricoides at baseline were positively correlated with EPG, but no correlation was found between EPG and fecal biomarkers for follow-up children. On the other hand, only MPO expressions levels were found to be positively correlated with T. trichiura EPG (r = 0.13, P = 0.01) for follow-up children. For hookworm infection, no correlation was found between EPG and fecal biomarkers (Figure 6).
Figure 6.
Correlation analyses between levels of fecal biomarkers (A1AT and MPO) with soil-transmitted helminth species egg count per gram for both baseline (A–F) and follow-up (G–L) samples. Spearman’s correlation coefficient was calculated for P values. A1AT = alpha-1 antitrypsin; MPO = myeloperoxidase.
DISCUSSION
In this study, we found that the infestation of common helminths (A. lumbricoides, T. trichiura, and N. americanus) was high among the FDMN children. In developing nations, intestinal parasites, particularly STH infections, are a significant cause for public health concern, with SAC being more susceptible.26 Our study suggests that both the KK and multiplex qPCR methods show a high overall detection rate for STH infection in stool samples from FDMN children, which had similar high levels to the 69% found among Burmese children in a previous report using the KK technique.27 In addition, infection by hookworm was frequently reported among refugees and asylum seekers from Myanmar.28 According to a cross-sectional study conducted in 2016 in Phyu Township, Bago Region, Myanmar, 78.8% of SAC had at least one STH infection using qPCR, which is similar to our study findings.29 According to the Integrated Community-based Survey for Program Monitoring, which was conducted in 12 districts of Bangladesh between 2017 and 2020, the prevalence of any STH species has decreased significantly, from 79.8% in 2005 to 14%, whereas the effect has been consistent for all age groups.30 However, STHs and other neglected tropical diseases are common in underprivileged areas. In general, mild infections have no symptoms, whereas severe infections can have severe clinical effects. These infections may in turn spread to the Bangladeshi host community and thereby exert a negative effect on the nation’s healthcare system if they are not controlled. To sustain the health of the refugee community and host community, it is necessary to guarantee that the Rohingya population has access to preventative, promotional, and curative health services.
During follow-up, both KK microscopy and qPCR showed a higher proportion of A. lumbricoides and T. trichiura infections compared with baseline, which may be attributed to possible reinfection due to a longer duration of follow-up. This was expected because the study’s follow-up term was increased from 6 months to 12 months, and the COVID-19 shutdown in Bangladesh caused the follow-up period to be interrupted. However, hookworm reinfection occurs at a slower rate,31 and the incidence of hookworm infection was found to be significantly decreased in our study. Moreover, this study revealed that A. lumbricoides prevalence as assessed by the KK approach was considerably less than that detected by qPCR. In contrast, T. trichiura and hookworm infection were more prevalent by KK microscopy compared with qPCR, which showed similar patterns to other studies on STHs.32–34 Omitted infections by qPCR may be caused by an absence of ova in the 200-mg stool specimens used for DNA isolation or by errors in the DNA isolation process, notably for T. trichiura and hookworm, for which it is necessary to lyse the eggs to release DNA and successfully amplify by qPCR. Another aspect that should be taken into account is the existence of inhibitors in the stool specimen, which differs between individuals. However, the KK and qPCR results showed a substantially greater incidence of STH infection overall in FDMN children, but relatively heavy intensity infections were found less frequently by the KK method. These ratios for STHs are probably understated; for instance, the KK method frequently overlooks unfertilized A. lumbricoides eggs.35 We also assessed the diagnostic agreement between qPCR and KK microscopy for the detection of STHs. The investigation showed that the qPCR and Kato Katz techniques for the diagnosis of STH were in agreement. The reason KK performs poorly, especially for hookworm, is probably because hookworm ova are quickly degraded after being collected from stools.36,37 This study did not find any evidence that could indicate a potential association between childhood helminth infections and physical retardation in children; only T. trichiura infections at follow-up showed a difference between the stunted and the nonstunted groups. Four cross-sectional studies conducted in urban slums conducted in Indonesia, Ujung Pandang, rural Brazil, and urban Zaire have also demonstrated a link between T. trichiura infection and stunted child growth.38–41 In this context, various investigations revealed that severe T. trichiura infections are linked to protein energy malnutrition in children, which results in stunting.42,43 Our results corroborate with the most recent systematic review on the benefits of deworming for children,44 but they differ from a prior systematic study examining the impact of STHs on child nutrition and growth in contexts where STH prevalence was in more than 50% of children.45 However, a recent assessment found differences between children with good nutritional status and those who were stunted in terms of the incidence rates of STH infections.46 Additionally, persistent infections and/or high-intensity infections can lead to poor iron absorption, malnutrition, growth retardation, intestinal obstruction, and anemia due to iron shortage.10,47–49 On the other hand, our study found that the majority of children had mild to moderate infections as indicated by intensity as per stool EPG. Furthermore, our study found that the prevalence of stunted children increased after a year, which may have been a result of the COVID-19 pandemic. The prevalence of moderate or severe wasting among younger children in low- and middle-income countries was forecasted to rise by 14.3% as a result of the socioeconomic consequences of COVID-19.50 According to another projection, the COVID-19 pandemic was expected to lead to 1.6 million more children worldwide experiencing stunting between 2020 and 2022.51 We used A1AT and MPO tests to investigate the relationship between intestinal inflammation and STH infections. We found a significant association between A. lumbricoides and fecal A1AT concentrations in the children at baseline. Although there is little evidence linking local inflammation to STH infections, major issues with much of the literature include the use of inconsistent biomarkers for intestinal inflammation and that studies are frequently done with limited sample sizes.52 MPO and A1AT have already been identified as potential indicators of gut inflammation and increased gut permeability brought on by environmental enteric dysfunction (EED).22,53,54 A1AT represents enteric protein loss, which is linked to EED, and MPO are precise indicators of intestinal inflammation.54–56 The tendency for elevated fecal biomarker levels in STH infections in our study may indicate the presence of a local inflammatory response that results in epithelium injury and protein loss. A greater proportion of stunted children in the study’s follow-up population could be another potential cause of such protein loss.
Intrinsic host-related factors could influence the susceptibility of infection in one sex more than the other.57 We have analyzed the data to find out whether there are any differences in males or females in regard to infection prevalence or stunting or A1AT or MPO levels. However, there were no differences in infection prevalence, stunting, and A1AT or MPO levels in male and female children (Supplemental Table 1).
The main limitation of our study is that we were unable to assess the anthelminthic/albendazole effect after 6 months due to COVID-19 restrictions and we had to do so after 12 months. Another limitation of our study is that only one slide from a single stool specimen per children was examined for STHs.
CONCLUSION
Our study demonstrates that FDMN children had notably high prevalence rates of STH infection, especially T. trichiura and A. lumbricoides. However, albendazole treatment shows low efficacy after 1 year against both A. lumbricoides and T. trichiura. In addition, stunting was found to be significantly associated with T. trichiura infection. Furthermore, a significant number of reinfections occurred within a year of the posttreatment period, which may suggest that a variety of issues make it difficult to control STHs in vulnerable children. A thorough investigation of the protective effects and actual cause of STH infection is urgently needed to effectively improve FDMN children’s health. Furthermore, to avoid the detrimental effects of worm infection on children, regular anthelmintic treatment recommended.
Supplemental Materials
ACKNOWLEDGMENTS
We are grateful to all of the participants for their valuable contribution in this study. We also acknowledge the Refugee Relief and Repatriation Commissioner of Bangladesh for support to conduct this study in the forcibly displaced Myanmar nationals camps. icddr,b is grateful to the governments of Bangladesh, Canada, Sweden, and the United Kingdome for providing core/unrestricted support. We also grateful to Parag Palit for his help in writing the manuscript.
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. WHO , 2017. Soil-Transmitted Helminth Infections: Fact Sheet. Geneva, Switzerland: World Health Organization Media Centre. [Google Scholar]
- 2. Dunn JC, Turner HC, Tun A, Anderson RM, 2016. Epidemiological surveys of, and research on, soil-transmitted helminths in Southeast Asia: a systematic review. Parasit Vectors 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moser W, Labhardt ND, Cheleboi M, Muhairwe J, Keiser J, 2017. Unexpected low soil-transmitted helminth prevalence in the Butha-Buthe district in Lesotho, results from a cross-sectional survey. Parasit Vectors 10: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silver ZA, Kaliappan SP, Samuel P, Venugopal S, Kang G, Sarkar R, Ajjampur SS, 2018. Geographical distribution of soil transmitted helminths and the effects of community type in South Asia and South East Asia – a systematic review. PLoS Negl Trop Dis 12: e0006153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hotez PJ, 2009. Mass drug administration and integrated control for the world’s high-prevalence neglected tropical diseases. Clin Pharmacol Ther 85: 659–664. [DOI] [PubMed] [Google Scholar]
- 6. Asaolu S, Ofoezie I, 2003. The role of health education and sanitation in the control of helminth infections. Acta Trop 86: 283–294. [DOI] [PubMed] [Google Scholar]
- 7. Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M, 2013. Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis 7: e2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pullan RL, Smith JL, Jasrasaria R, Brooker SJ, 2014. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sayasone S, Utzinger J, Akkhavong K, Odermatt P, 2015. Multiparasitism and intensity of helminth infections in relation to symptoms and nutritional status among children: a cross-sectional study in southern Lao People’s Democratic Republic. Acta Trop 141: 322–331. [DOI] [PubMed] [Google Scholar]
- 10. Crompton DW, Nesheim MC, 2002. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr 22: 35–59. [DOI] [PubMed] [Google Scholar]
- 11. WHO , 2006. Preventive Chemotherapy in Human Helminthiasis. Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 12. Makoge VD, Edward LNN, Moyou SRS, 2012. Falciparum malaria, helminth infection, and anaemia in asymptomatic pupils in four villages in Cameroon. Eur Zool J 1: 54–59. [Google Scholar]
- 13. Guglielmi S, Jones N, Muz J, Baird S, Mitu K, Ala Uddin M, 2020. Age-and gender-based violence risks facing Rohingya and Bangladeshi adolescents in Cox’s Bazar. Policy Brief. London, United Kingdom: Gender and Adolescence: Global Evidence.
- 14. Khan HT, Rahman MA, Molla MH, Shahjahan M, Abdullah RB, 2022. Humanitarian emergencies of Rohingya older people in Bangladesh: a qualitative study on hopes and reality. Ageing Int 47: 20–37. [Google Scholar]
- 15. Hsan K, Naher S, Griffiths MD, Shamol HH, Rahman MA, 2019. Factors associated with the practice of water, sanitation, and hygiene (WASH) among the Rohingya refugees in Bangladesh. J Water Sanit Hyg Dev 9: 794–800. [Google Scholar]
- 16. Banerjee S, 2019. The Rohingya Crisis: A Health Situation Analysis of Refugee Camps in Bangladesh. Observer Research Foundation. Available at: https://www.orfonline.org/research/the-rohingya-crisis-a-health-situation-analysis-of-refugee-camps-in-bangladesh-53011/.
- 17. Islam MM, Nuzhath T, 2018. Health risks of Rohingya refugee population in Bangladesh: a call for global attention. J Glob Health 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mondiale de la Santé O, Organization WH, 2016. Preventive chemotherapy for helminth diseases: progress report, 2014. Wkly Epidemiol Rec 91: 93–104. [PubMed] [Google Scholar]
- 19. Tarafder M, Carabin H, Joseph L, Balolong E, Jr, Olveda R, McGarvey S, 2010. Estimating the sensitivity and specificity of Kato–Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘gold standard’. Int J Parasitol 40: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steiner KL, Ahmed S, Gilchrist CA, Burkey C, Cook H, Ma JZ, Korpe PS, Ahmed E, Alam M, Kabir M, 2018. Species of cryptosporidia causing subclinical infection associated with growth faltering in rural and urban Bangladesh: a birth cohort study. Clin Infect Dis 67: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J. et al. , 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosek M. et al. , 2013. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 88: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blackburn CC, Yan SM, McCormick D, Herrera LN, Iordanov RB, Bailey MD, Bottazzi ME, Hotez PJ, Mejia R, 2023. Poverty associated with the environmental contamination of gastrointestinal parasites in the southern United States. medRxiv 2023.01. 10.23284404.
- 24. Liu J. et al. , 2013. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rolfe K, Parmar S, Mururi D, Wreghitt T, Jalal H, Zhang H, Curran M, 2007. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. J Clin Virol 39: 318–321. [DOI] [PubMed] [Google Scholar]
- 26. WHO , 2017. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 27. Montresor A, Zin TT, Padmasiri E, Allen H, Savioli L, 2004. Soil-transmitted helminthiasis in Myanmar and approximate costs for countrywide control. Trop Med Int Health 9: 1012–1015. [DOI] [PubMed] [Google Scholar]
- 28. Mohd Hanapi I, Sahimin N, Lewis J, Lau Y, Othman J, Tedong P, Mohd Zain S, 2021. Public health status of Myanmar refugees in South East Asia: a Malaysian case study. Trop Biomed 38: 594–604. [DOI] [PubMed] [Google Scholar]
- 29. Aung E. et al. , 2022. High prevalence of soil-transmitted helminth infections in Myanmar schoolchildren. Infect Dis Poverty 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhakal S, Karim MJ, Al Kawsar A, Irish J, Rahman M, Tupps C, Kabir A, Imtiaz R, 2020. Post-intervention epidemiology of STH in Bangladesh: data to sustain the gains. PLoS Negl Trop Dis 14: e0008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Appleton C, Mosala T, Levin J, Olsen A, 2009. Geohelminth infection and re-infection after chemotherapy among slum-dwelling children in Durban, South Africa. Ann Trop Med Parasitol 103: 249–261. [DOI] [PubMed] [Google Scholar]
- 32. Mationg MLS. et al. , 2017. Status of soil-transmitted helminth infections in schoolchildren in Laguna Province, the Philippines: determined by parasitological and molecular diagnostic techniques. PLoS Negl Trop Dis 11: e0006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gordon CA, McManus DP, Acosta LP, Olveda RM, Williams GM, Ross AG, Gray DJ, Gobert GN, 2015. Multiplex real-time PCR monitoring of intestinal helminths in humans reveals widespread polyparasitism in northern Samar, the Philippines. Int J Parasitol 45: 477–483. [DOI] [PubMed] [Google Scholar]
- 34. Barda B, Schindler C, Wampfler R, Ame S, Ali SM, Keiser J, 2020. Comparison of real-time PCR and the Kato–Katz method for the diagnosis of soil-transmitted helminthiasis and assessment of cure in a randomized controlled trial. BMC Microbiol 20: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCarthy JS, Lustigman S, Yang G-J, Barakat RM, García HH, Sripa B, Willingham AL, Prichard RK, Basáñez M-G, 2012. A research agenda for helminth diseases of humans: diagnostics for control and elimination programmes. PLoS Negl Trop Dis 6: e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Connell EM, Nutman TB, 2016. Molecular diagnostics for soil-transmitted helminths. Am J Trop Med Hyg 95: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dacombe R, Crampin A, Floyd S, Randall A, Ndhlovu R, Bickle Q, Fine P, 2007. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans R Soc Trop Med Hyg 101: 140–145. [DOI] [PubMed] [Google Scholar]
- 38. Hadju V, Stephenson L, Mohammed H, Bowman D, Parker R, 1998. Improvements of growth. Appetite, and physical activity in helminth-infected schoolboys 6 months after single dose of albendazole. Asia Pac J Clin Nutr 7: 170–176. [PubMed] [Google Scholar]
- 39. Tshikuka JG, Gray-Donald K, Scott M, Olela KN, 1997. Relationship of childhood protein-energy malnutrition and parasite infections in an urban African setting. Trop Med Int Health 2: 374–382. [DOI] [PubMed] [Google Scholar]
- 40. Silva D, 1999. Ascaris–Trichuris association and malnutrition in Brazilian children. Paediatr Perinat Epidemiol 13: 89–98. [DOI] [PubMed] [Google Scholar]
- 41. Parraga IM, Assiss AM, Prado MS, Barreto ML, Reis MG, King CH, Blanton RE, 1996. Gender differences in growth of school-aged children with schistosomiasis and geohelminth infection. Am J Trop Dis 55: 150–156. [DOI] [PubMed] [Google Scholar]
- 42. Getz L, 1945. Massive infection with Trichuris trichiura in children: report of four cases, with autopsy. Am J Dis Child 70: 19–24. [Google Scholar]
- 43. Cooper ES, Bundy D, Whyte-Alleng C, Venugopal S, Spencer J, Whitney P, Cromwell O, Haynes B, MacDonald TT, 1991. Immediate hypersensitivity in colon of children with chronic Trichuris trichiura dysentery. Lancet 338: 1104–1107. [DOI] [PubMed] [Google Scholar]
- 44. Taylor-Robinson DC, Maayan N, Donegan S, Chaplin M, Garner P, 2019. Public health deworming programmes for soil‐transmitted helminths in children living in endemic areas. Cochrane Database Syst Rev 9: CD000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hall A, Hewitt G, Tuffrey V, De Silva N, 2008. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr 4: 118–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fauziah N, Ar-Rizqi MA, Hana S, Patahuddin NM, Diptyanusa A, 2022. Stunting as a risk factor of soil-transmitted helminthiasis in children: a literature review. Interdiscip Perspect Infect Dis 2022: 8929025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ, 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 48. Lwanga F, Kirunda BE, Orach CG, 2012. Intestinal helminth infections and nutritional status of children attending primary schools in Wakiso District, Central Uganda. Int J Environ Res Public Health 9: 2910–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stephenson LS, Latham MC, Ottesen E, 2000. Malnutrition and parasitic helminth infections. Parasitology 121: S23–S38. [DOI] [PubMed] [Google Scholar]
- 50. Headey D. et al. , 2020. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet 396: 519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verhagen W, Bohl D, Cannon M, Pulido A, Pirzadeh A, Nott I, Moyer JD, 2021. The Future of Food Security in the Wake of the COVID-19 Pandemic. Available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4006474. Accessed July 6, 2023.
- 52. De Gier B. et al. , 2018. Soil-transmitted helminth infections and intestinal and systemic inflammation in schoolchildren. Acta Trop 182: 124–127. [DOI] [PubMed] [Google Scholar]
- 53. George CM. et al. , 2015. Fecal markers of environmental enteropathy are associated with animal exposure and caregiver hygiene in Bangladesh. Am J Trop Med Hyg 93: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Campbell RK. et al. , 2017. Biomarkers of environmental enteric dysfunction among children in rural Bangladesh. J Pediatr Gastroenterol Nutr 65: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crossley JR, Elliott RB, 1977. Simple method for diagnosing protein-losing enteropathies. BMJ 1: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Däbritz J, Musci J, Foell D, 2014. Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J Gastroenterol 20: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruggieri A, Anticoli S, D’Ambrosio A, Giordani L, Viora M, 2016. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita 52: 198–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.