Abstract
Objective
Given the importance of sleep in maintaining neurocognitive health, both sleep duration and quality might be component causes of dementia. However, the possible role of insomnia symptoms as risk factors for dementia remain uncertain.
Methods
We prospectively studied 22,078 participants in the Swedish National March Cohort who were free from dementia and stroke at baseline. Occurrence of dementia was documented by national registers during a median follow-up period of 19.2 years. Insomnia symptoms and sleep duration were ascertained by Karolinska Sleep Questionnaire. Multivariable Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI).
Results
Compared to participants without insomnia at baseline, those who reported any insomnia symptom experienced a greater incidence of dementia during follow-up (HR 1.08, 95% CI: 1.03, 1.35). Difficulty initiating sleep versus non-insomnia (HR 1.24, 95% CI: 1.02, 1.52), but not difficulty maintaining sleep or early morning awakening was associated with an increased risk of dementia. Short sleep duration was associated with increased risk of dementia (6 h vs. 8 h, HR 1.29, 95% CI: 1.11–1.51; 5 h vs. 8 h, HR 1.26, 95% CI: 1.00–1.57). Stratified analyses suggested that insomnia symptoms increased the risk of dementia only amongst participants with ≥7 h sleep (vs. non-insomnia HR 1.24, 95% CI: 1.00–1.54, P = 0.05), but not amongst short sleepers (<7 h). Short sleep duration also did not further inflate the risk of dementia amongst insomniacs.
Conclusion
Insomnia and short sleep duration increase the risk of dementia amongst middle-aged to older adults.
Keywords: insomnia, sleep duration, dementia, national cohort, longitudinal study, older people
Key Points
Frequent complaint of insomnia is associated with increased dementia incidence.
Difficulty initiating sleep, but not other symptoms of insomnia is linked to the increased risk of dementia.
The link between insomnia symptoms and dementia is moderated by self-reported sleep duration.
Introduction
The role of healthy sleep in maintaining cognitive function has been increasingly recognised when several studies found a link between short sleep duration (typically defined by subjective sleep duration of <7 h/day) and both impaired cognitive function and dementia amongst populations with different ethnic backgrounds [1–5]. Short sleep duration in middle-aged adults has also been associated with an elevated ratio of white matter hyperintensity—a sign of small vessel cerebrovascular disease and a risk factor for Alzheimer’s disease (AD) [6]. In addition to the duration of sleep, sleep quality including nocturnal awakenings and sleep onset latency may also influence risk of cognitive decline and dementia [7]. Low-quality sleep characterised by arousals and insufficient slow-wave sleep may hinder the glymphatic system which filters toxins from the brain during sleep and is a probable key mechanism in the pathogenesis of AD and dementia [8, 9].
Compromised sleep quality due to untreated sleep disorders are highly prevalent. Manifested by frequent (≥3 nights/week), chronic (≥3 months) difficulties to initiate or maintain sleep and subsequent daytime dysfunctions, insomnia is the most common sleep disorder with an estimated prevalence between 10% and 20% in the general population [10, 11]; its nocturnal symptoms can affect as much as 50% of adults [12]. A recent cohort study amongst middle-aged adults suggested a cumulative incidence of new-onset insomnia of 13.9% during a 5-year follow-up; nearly half of the individuals that developed insomnia had persisting symptoms for a year or longer [13].
Despite evidence of a link between insomnia and cognitive impairment [14], prospective studies investigating the possible association with dementia are scarce. They are often limited by small sample size, and results between studies are heterogeneous [15, 16]. Because insomnia combined with short sleep duration appears more detrimental for both overall and cognitive health [17, 18], a synergistic relation between insomnia and sleep duration in the aetiology of dementia also deserves investigation. To this end, we prospectively investigated the possible relationship between insomnia, sleep duration and dementia amongst community dwelling adults from Sweden during a 19-year follow-up period. We hypothesised that these symptoms were associated with an increased risk of dementia, and might act synergistically.
Methods
Study population
All participants were enrolled in the Swedish National March Cohort which was designed to investigate associations between lifestyle factors and chronic diseases as described previously in detail [19]. The baseline assessment was carried out in September 1997 during a 4-day nationwide fund-raising event ‘National March’ for the Swedish Cancer Society, which took place in nearly 3,600 sites throughout Sweden. All participants were invited to fill out a questionnaire regarding demographic, lifestyle and medical information. Participants also provided their individually unique national registration number, which enabled follow-up by linkage to multiple nationwide, continuously updated and essentially complete databases. The study was approved by the Regional Ethics Review Board at Karolinska Institutet in Stockholm. All participants provided informed consent.
In total 42,059 individuals aged between 18 and 94 completed the baseline questionnaire. For the purpose of the present study, individuals aged ≥46 years at baseline (~65 years or older by the end of follow-up), without dementia or history of stroke [20] were selected (n = 27,634). Complete report of the main exposure variables was available from 25,511 individuals. We further excluded individuals with an incomplete answer to any covariate, thus leaving 22,078 participants for the final analysis.
Assessments of sleep characteristics
Insomnia symptoms and sleep duration were assessed by the Karolinska Sleep Questionnaire as part of the baseline questionnaire of the Swedish National March Cohort [21]. Questions of insomnia symptoms referred to the past 12 months. Difficulty initiating sleep (DIS), difficulty maintaining sleep (DMS) and early morning awakenings (EMA) were asked as follows: ‘Have you had problems to fall asleep?’; ‘Have you woken up and have had problem to fall asleep again?’; ‘Have you woken up too early?’, with optional answers of ‘never’; ‘seldom (sometimes per year)’; ‘sometimes (several times per month)’; ‘fairly often (3-4 times per week)’; ‘always (5 or more times per week)’ to each of the three questions. Having insomnia symptoms was defined by an answer of ‘fairly often’ or ‘always’ to at least one of the three questions [22].
Sleep duration was assessed by two questions: ‘How many hours, approximately, per night do you usually sleep during a weekday night?’; ‘How many hours, approximately, per night do you usually sleep when you are off-duty?’ The response alternatives were: <5, 5, 6, 7, 8 or ≥ 9 h to each question. We calculated the weighted average sleep duration of each participant based on five weekday nights and two off-duty nights per week, where <5 h and ≥9 h were treated as 4 h and 9 h, respectively. Decimals in the weighted average sleep duration were rounded to the nearest whole number of hours. The average sleep duration was categorised into <5, 5, 6, 7, 8 or ≥9 h per night. The reference category was set to 8 h.
Ascertainment of dementia
Using participants’ national registration number, information regarding the first date when dementia was ascertained was obtained from the Swedish National Patient Registers or the Swedish Cause of Death Register. The National Patient Register covers discharge following all in-patient care in Sweden since 1987. Since 2001 outpatient doctor visits from both private and public caregivers are also covered. Mortality data were obtained by linkage to the Swedish Cause of Death Register. Individuals with dementia as the underlying cause of death were identified. All events were coded using the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10). We used the following codes to identify dementia: 290.0, 290.1, 290.4, 294.1, 290.8, 290.9, 331.0, 331.1, 331.2, 331.9 (ICD-9) and F00, F01, F02, F03, G30, G31.1, G31.8, F05.1 (ICD-10). In the Swedish healthcare system, dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders criteria, Fourth Edition (DSM-IV) [23]. AD was diagnosed according to the National Institute of Neurological and Communicative Diseases and Stroke and the AD and Related Disorders Association criteria [24]. Cases of dementia without neuroimaging and without sufficient clinical details in the medical records to set a specific dementia subtype diagnosis were classified as unspecified dementia.
Covariates
Selection of potential confounding factors was based on the Lancet Commission’s 2020 report on dementia prevention, intervention and care [25], as well as subject matter knowledge guided by directed acyclic graphs. Details are given in Supplement (Appendix 1).
Statistical analyses
To investigate the association between baseline sleep characteristics and dementia, Cox proportional hazards regression model was used to estimate the hazard ratios (HRs) with 95% confidence intervals (95% CI), where insomnia symptoms or sleep duration was used as the exposure variable. Time to event was calculated from baseline on October 1, 1997 until date of dementia diagnosis, emigration, death, or December 31, 2016, whichever occurred first. Dates of emigration and death were obtained from data held by the National Board of Health and Welfare and the Cause of Death Register [26]. Ties in the failure times were handled using the Breslow method. In Cox regression analyses Model 1 we adjusted for age and sex, and in Model 2 we additionally adjusted for education level, depression, social isolation, body mass index, level of physical activity, smoking status, alcohol consumption, hypertension and diabetes. In additions, we assessed the multiplicative interaction between insomnia symptoms and sleep duration and use the likelihood ratio test for statistical significance. We also mutually adjusted for insomnia symptoms and sleep duration in the test for interaction. Violations of the proportional hazards assumption in Cox regression was assessed by testing the potential interactions between age in time scale and main exposure variables regarding risk of dementia, where a non-significant interaction term indicated no violation, Schoenfeld residuals were used for this purpose.
We conducted two sensitivity analyses. First, Fine-Grey proportional hazards regression was performed to investigate the association between the exposure variables and dementia after controlling for all confounders, considering the competing risk of death. Second, to minimise the possible effect of reverse causation, individuals diagnosed with dementia during the first 2 years of follow-up were excluded [27, 28].
All analyses were performed with complete data. A two-sided P-value of ˂0.05 was regarded as statistically significant. All statistical analyses were performed using Stata version 15.1 (Stata Corp LLC, College Station, TX, USA).
Results
Baseline characteristics
Baseline characteristics of the study cohort are summarised in Table 1, followed with Chi-squared test or one-way analysis of variance for detecting the between-group differences In total 3,647 participants (16.5% of the cohort) were classified as having insomnia symptoms. The prevalence of insomnia symptoms was higher amongst women than men. Compared to the participants without any insomnia symptom, those who suffered from the symptoms were on average older, more likely to only have compulsory education, more often feeling depressed and socially isolated, less physically active and had a higher prevalence of hypertension and diabetes. Short sleepers (<7 h) were more prone to insomnia symptoms than those who reported sufficient sleep duration (≥7 h).
Table 1.
Baseline characteristics of the study sample by status of insomnia.
| Symptoms of Insomnia | |||
|---|---|---|---|
| Characteristics | Total (N = 22,078) | No (N = 18,431) | Yes (N = 3,647) |
| Age, years | 59.9 ± 8.8 | 59.8 ± 8.8 | 60.6 ± 8.7 |
| Women | 13,648 (61.8) | 11,153 (60.5) | 2,495 (68.4) |
| Education | |||
| Compulsory school or below | 5,604 (25.4) | 4,575 (24.8) | 1,029 (28.2) |
| High school | 8,859 (40.1) | 7,398 (40.1) | 1,461 (40.1) |
| University or above | 7,615 (34.5) | 6,458 (35.0) | 1,157 (31.7) |
| Depression | |||
| Seldom/never | 10,078 (45.7) | 9,096 (49.4) | 982 (26.9) |
| Sometimes | 10,889 (49.3) | 8,725 (47.3) | 2,164 (59.3) |
| Often/always | 1,111 (5.0) | 610 (3.3) | 501 (13.7) |
| Social isolation | |||
| Seldom/never | 14,205 (64.3) | 12,353 (67.0) | 1852 (50.8) |
| Sometimes | 6,763 (30.6) | 5,416 (29.4) | 1,347 (36.9) |
| Often/always | 1,110 (5.0) | 662 (3.6) | 448 (12.3) |
| Body mass index, kg/m 2 | 25.0 ± 3.4 | 25.0 ± 3.3 | 25.1 ± 3.5 |
| Physical activity, hours/week | |||
| ≤2 | 2,822 (12.8) | 2,303 (12.5) | 519 (14.2) |
| 3–4 | 6,289 (28.5) | 5,249 (28.5) | 1,040 (28.5) |
| 5–6 | 5,031 (22.8) | 4,229 (23.0) | 802 (22.0) |
| >6 | 7,936 (36.0) | 6,650 (36.1) | 1,286 (35.3) |
| Smoking | |||
| Never | 12,533 (56.8) | 10,478 (56.9) | 2055 (56.4) |
| Former | 8,162 (37.0) | 6,810 (37.0) | 1,352 (37.1) |
| Current | 1,383 (6.3) | 1,143 (6.2) | 240 (6.6) |
| Alcohol consumption, g/week | |||
| ≤168 | 18,947 (85.8) | 15,808 (85.8) | 3,139 (86.1) |
| >168 | 3,131 (14.2) | 2,623 (14.2) | 508 (13.9) |
| Hypertension | 3,695 (16.7) | 2,973 (16.1) | 722 (19.8) |
| Diabetes | 672 (3.0) | 540 (2.9) | 132 (3.6) |
| Sleep duration, hours/day | |||
| < 5 | 646 (2.9) | 336 (1.8) | 310 (8.5) |
| 5 | 1,061 (4.8) | 455 (2.5) | 606 (16.6) |
| 6 | 3,957 (17.9) | 2,813 (15.3) | 1,144 (31.4) |
| 7 | 9,835 (44.6) | 8,728 (47.4) | 1,107 (30.4) |
| 8 | 6,119 (27.7) | 5,679 (30.8) | 440 (12.1) |
| ≥ 9 | 460 (2.1) | 420 (2.3) | 40 (1.1) |
Data presented as mean ± standard deviation or N (% in column).
Insomnia symptoms and risk of dementia
A total of 1,387 participants were found to be diagnosed with dementia during a median follow-up of 19.2 years, which generated 382,242 person-years at risk. Table 2 displays the HRs and 95% CIs for dementia according to symptoms of insomnia. With non-insomnia participants as the reference group, participants with insomnia symptoms had an 18% higher risk of developing dementia during follow-up (95% CI: 3–35%, P = 0.02), after adjustment for age, sex, education level, depression, social isolation, body mass index, level of physical activity, smoking status, alcohol consumption, hypertension and diabetes. When we analysed specific insomnia symptoms, DIS was associated with a 24% increased risk of dementia (2–52%). We found no association between DMS or EMA and risk of dementia.
Table 2.
Insomnia symptoms and incidence rate of dementia.
| Exposure | N of dementia incidence (% in group) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Status of insomnia symptoms | |||||
| Non-insomnia | 1,100 (6.0) | 1 | 1 | ||
| Have insomnia symptom | 287 (7.9) | 1.28 (1.12, 1.46) | <0.001 | 1.18 (1.03, 1.35) | 0.018 |
| Symptom of insomnia | |||||
| Non-insomnia | 1,100 (6.0) | 1 | 1 | ||
| Other symptoms than DIS | 172 (7.0) | 1.20 (1.02, 1.41) | 0.028 | 1.12 (0.95, 1.32) | 0.173 |
| DIS | 115 (9.5) | 1.40 (1.15, 1.70) | 0.001 | 1.24 (1.02, 1.52) | 0.031 |
| Non-insomnia | 1,100 (6.0) | 1 | 1 | ||
| Other symptoms than DMS | 132 (7.6) | 1.33 (1.11, 1.59) | 0.002 | 1.25 (1.04, 1.50) | 0.017 |
| DMS | 155 (8.1) | 1.24 (1.05, 1.47) | 0.013 | 1.11 (0.94, 1.33) | 0.221 |
| Non-insomnia | 1,100 (6.0) | 1 | 1 | ||
| Other symptoms than EMA | 133 (8.9) | 1.36 (1.14, 1.63) | 0.001 | 1.25 (1.04, 1.51) | 0.016 |
| EMA | 154 (7.1) | 1.21 (1.02, 1.43) | 0.030 | 1.11 (0.93, 1.32) | 0.248 |
Analysed by Cox proportional hazards regression. Model 1, adjusted for age and sex; Model 2, adjusted for Model 1 and education level, depression, social isolation, body mass index, level of physical activity, smoking status, alcohol consumption, hypertension and diabetes.
Sleep duration and risk of dementia
The association between self-reported sleep duration and risk of dementia is shown in Figure 1. In general, sleep duration was inversely associated with risk of dementia (HR 0.93, 95% CI: 0.89–0.98), P for trend <0.01). When we used sleep duration of 8 h as a reference, shorter sleep duration was associated with an increased risk of dementia (6 h vs. 8 h, HR 1.29, 95% CI: 1.11–1.51, P < 0.01; 5 h vs. 8 h, HR 1.26, 95% CI: 1.00–1.57, P < 0.05; 4 h vs. 8 h, HR 1.28, 95% CI: 1.00–1.64, P = 0.05). However, compared to 8 h, a sleep duration of 9 h or longer was not significantly associated with risk of dementia (HR 1.18, 95% CI: 0.84–1.66, P = 0.33).
Figure 1.
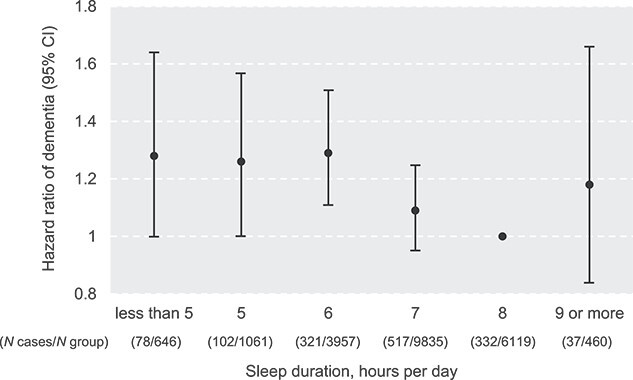
Self-reported sleep duration and risk of incident dementia in the study cohort (N = 22,078). Analysed by Cox proportional hazards regression, adjusted for age, sex, education level, depression, social isolation, body mass index, level of physical activity, smoking status, alcohol consumption, hypertension and diabetes.
Interaction between insomnia and sleep duration for the risk of dementia
An interaction between status of insomnia symptoms and self-reported sleep duration regarding the risk of dementia was detected in the fully adjusted Cox regression model (HR 1.15, 95% CI: 1.02–1.28, P < 0.05). Since the elevated risk of incident dementia was only observed amongst sleep duration categories <7 h, we further stratified the cohort into normal (≥7 h, n = 16,414) and short (< 7 h, n = 5,664) sleep duration groups. Cox regression analysis in the data stratified by sleep duration further suggested an association between insomnia symptoms and risk of dementia amongst normal sleepers (having insomnia symptom vs. not, HR 1.24, 95% CI: 1.00–1.54, P < 0.05), whilst insomnia did not further increase the risk of dementia amongst short sleepers (insomniacs vs. non-insomniacs, HR 1.03, 95% CI: 0.85–1.24, P = 0.76). In analyses stratified by status of insomnia symptoms, sleep duration (as continuous variable) was negatively associated with risk of dementia amongst participants with no insomnia (HR 0.91, 95% CI: 0.86–0.96, P for trend<0.01) with no overall association between sleep duration and risk of dementia amongst insomniacs (HR 1.06, 95% CI: 0.95–1.17, P for trend = 0.30, Model 2). Figure 2 shows the HRs and 95% CIs for dementia by sleep duration categories in participants with and without insomnia symptoms, respectively. Amongst non-insomnia individuals, sleep duration of <7 h were associated with a higher subsequent risk for dementia (6 h vs. 8 h, HR 1.25, 95% CI: 1.05–1.49, P < 0.05; 5 h vs. 8 h, HR 1.70, 95% CI; 1.27–2.27, P < 0.001). Whereas in insomniacs, we found a significant association between long sleep duration (≥ 9 vs. 8 h) and dementia (HR 2.84, 95% CI: 1.23–6.55, P < 0.05).
Figure 2.
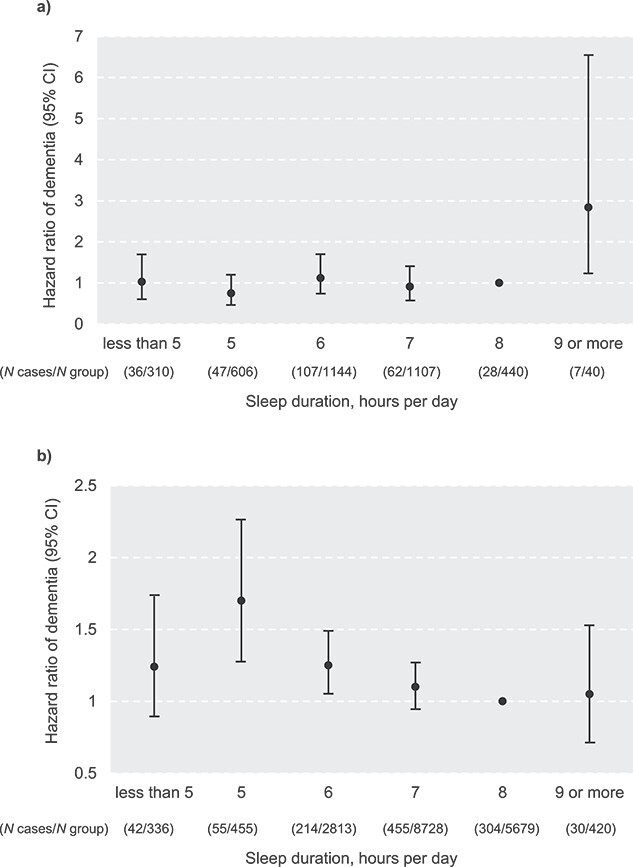
Self-reported sleep duration and risk of incident dementia in (a) insomniacs (N = 3,647); (b) non-insomniacs (N = 18,431). Analysed by Cox proportional hazards regression, adjusted for age, sex, education level, depression, social isolation, body mass index, level of physical activity, smoking status, alcohol consumption, hypertension and diabetes.
Sensitivity analyses
When we excluded dementia ascertained during the first 2 years of the follow-up (n = 10), the association between insomnia symptoms and risk of dementia remained unchanged (Supplement Table S1). Associations between sleep duration and risk of dementia were also similar (HR 0.93, 95% CI, 0.88–0.97, P for trend<0.01, Model 2). The interaction between insomnia and sleep duration regarding the risk of dementia also remained (HR 1.14, 95% CI: 1.02–1.28, P < 0.05, Model 2). In addition, survival analysis taking death (n = 5,115, 23.2% of the total cohort) as a competing risk for dementia did not change our main findings. Sub-distribution hazard ratios and 95% CIs for dementia in Fine-Grey proportional hazards regression regarding insomnia symptoms and sleep duration are listed in Supplement Table S2.
Discussion
In this prospective study amongst 22,078 middle- to older aged adults from Sweden, we found that both insomnia and short sleep duration were independently associated with an increased risk of dementia. We also found evidence of a complex interaction between insomnia symptoms and sleep duration regarding the subsequent risk of dementia.
In spite of the high prevalence of insomnia and its detrimental impact on nocturnal sleep, evidence associating insomnia symptoms with incident dementia is scarce. Data collected from the U.S. National Health and Ageing Trends Study suggested that routinely reported insomnia symptoms including DIS and DMS were associated with increased risk of dementia during an 8-year follow-up [29]. In contrast, in a recent study from the UK Biobank, individuals who had insomnia complaints had a lower risk of dementia during a median of 10.8 years follow-up [30]. However, unlike our study, frequency of insomnia complaints was not defined by nights per week. Hence, participants reporting more frequent insomnia symptoms might be defined as non-insomniacs according to the diagnostic criteria [22]. In addition, several important confounding factors for the development of dementia, including depression, hypertension and diabetes were not considered in the UK Biobank study [30].
DIS is of particular concern regarding the development of dementia. This finding further supports earlier studies where an association between sleep initiating difficulty and dementia/impaired cognitive function has been suggested [31–33]. For instance, a prospective cohort study amongst Korean elderlies found that individuals with a sleep latency of over 30 min had an ~40% higher risk of cognitive decline at the 4-year follow-up, compared to individuals with a normal sleep latency [31]. In a prospective study, actigraphy-determined longer sleep latency was associated with increased risk of dementia, especially AD [33].
Mechanisms underlying the association between frequent DIS and dementia are unclear. The association is unlikely to be driven solely by short sleep duration, because the proportion of short sleepers amongst DIS was lower than amongst those who reported DMS in our population. Evidence from a recent study involving over 30,000 adults with a mean age of 63 years specifically linked sleep-onset insomnia with lower heart rate variability [34]. Impaired cardiac autonomic function hallmarked by reduced heart rate variability has been reported as a risk factor for developing cognitive impairment and dementia in later life [35]. Interestingly, in that study, worse performance in verbal fluency and prospective memory tests only occurred in those with sleep-onset insomnia, but not amongst those with sleep maintenance insomnia [34]. On the other hand, in an older population with a mean age of 75 years, cognitive decline was more pronounced in individuals with DMS compared to those with no DMS [36]. Hence DIS may represent an age-specific predictor for cognitive decline and dementia of relevance, particularly in youngest-old individuals.
Our study confirms previously observed association between short sleep duration and dementia in a British population, in which self-reported sleep duration of ˂7 h per night posed an increased risk for dementia [5]. We found that this association is modulated by insomnia symptoms. For those who reported sufficient sleep, insomnia symptoms appeared to be an independent sleep-related risk factor for dementia, suggesting an additional manageable factor for the prevention of cognitive decline. This finding can be explained by different potential mechanisms. First, it is well known that self-reported sleep duration is in low agreement with objectively determined sleep duration. In a US study, self-reported sleep duration was overestimated by 49–73 min per night compared to polysomnography [37]. In addition, reductions in slow wave sleep and slow wave activity, both closely linked to the function of glymphatic system, have been reported amongst patients with insomnia [38]. Moreover, a sufficient sleep duration according to self-estimation does not protect the individuals with insomnia from the risk of dementia. In fact, we saw an increased risk of dementia amongst participants who reported both insomnia symptoms and a sleep duration of 9 h or longer. A link between long sleep duration and dementia has been observed in earlier studies. One prospective study suggested that compared to a self-reported sleep duration of 7 h per night, women who reported ≥8 h of sleep showed an ~35% higher risk of dementia during the 13-years of follow-up [1]. In another prospective study, extended time in bed and delayed rising—both of which hallmark long sleep duration, but poor sleep quality [39, 40]—predicted increased dementia risk during a 17-year follow-up period [41].
Strengths of our study include the prospective design, large size and virtually complete long-term follow-up through linkage to high-quality national registers. Use of the validated Karolinska Sleep Questionnaire allowed ascertainment of insomnia symptoms and sleep duration in greater detail than most previous studies whilst control of confounding was facilitated by extensive recording of co-variates. Our study also has limitations. Firstly, sleep characteristics were self-reported only at baseline and might have changed during follow-up. Because such misclassification is likely non-differential it most likely entails underestimation of any true causal association. Nonetheless, induction time for dementia is most likely long and may warrant follow-up during a time span of two decades to capture causal effects. In a previous study with repeated assessment of sleep duration, changes of habitual sleep duration between age 50 and 70 were not associated with a higher incidence of dementia [5]. Studies are nevertheless warranted to examine whether persistent insomnia over time poses an elevated risk for dementia. Secondly, due to the insidious onset of dementia, correct ascertainment of incidence is in most settings not feasible. Hence, information from discharge diagnosis and death certificates reflects prevalence rather than incidence. Because such misclassification is unlikely to be associated with sleep characteristics, it may not materially bias our risk estimates. Thirdly, because over 50% of all dementia diagnosis were labelled as unspecific, we were unable to accurately estimate HRs separately for AD and other types of dementia. Finally, although the study is large and included both men and women at different ages from all over Sweden, our findings warrant further verification due to the relatively small samples when stratifying our population by sleep duration categories. Also, all the participants were invited to take part in a fund-raising event, which for example could have made them more prone to healthy volunteer bias. It should be kept in mind though, that population-based cohorts often have problems with loss to follow-up and poor response rate, whilst the register-based nature of the study helped minimising these potential limitations [42].
In conclusion, our study highlights the significance of different sleep factors in the prevention of cognitive decline and dementia. Our findings suggest that in middle-aged to older population, both insomnia symptoms and self-reported sleep duration may predict future risk of dementia. A midlife assessment of sleep, including both sleep duration and frequency of nocturnal insomnia symptoms, may be recommended in public health practice to be able to intervene and as a corollary improve long-term health.
Supplementary Material
Contributor Information
Xiao Tan, Department of Big Data in Health Science, Zhejiang University School of Public Health and Department of Psychiatry, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China; The Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province, Hangzhou, China; Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
Torbjörn Åkerstedt, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Stress Research Institute, Department of Psychology, Stockholm University, Stockholm, Sweden.
Ylva Trolle Lagerros, Clinical Epidemiology Unit, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; Center for Obesity, Academic Specialist Center, Stockholm Health Services, Stockholm, Sweden.
Anna Miley Åkerstedt, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Division of Medical Psychology, Karolinska University Hospital, Stockholm, Sweden.
Rino Bellocco, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Department of Statistics and Quantitative Methods, University of Milano-Bicocca, Milan, Italy.
Hans-Olov Adami, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Clinical Effectiveness Group, Institute of Health and Society, University of Oslo, Oslo, Norway.
Weimin Ye, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Jin-Jing Pei, Stress Research Institute, Department of Psychology, Stockholm University, Stockholm, Sweden.
Hui-Xin Wang, Stress Research Institute, Department of Psychology, Stockholm University, Stockholm, Sweden.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This research was supported by ZJU 100 Young Professor Project (X.T.), Åke Wiberg Foundation (X.T., M19–0266), Fredrik and Ingrid Thuring Foundation (X.T., 2019–00488) and Region Stockholm Clinical Research Appointment (Y.T.L.), the Swedish Research Council (HX.W., 2018–02998), and the Swedish Research Council for Health, Working life, and Welfare (HX.W., 2019–01120, 2020–00313). The funders had no role in the design and conduct of the study, nor the decision to prepare and submit the manuscript for publication.
References
- 1. Chen JC, Espeland MA, Brunner RLet al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement 2016; 12: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winer JR, Deters KD, Kennedy Get al. Association of Short and Long Sleep Duration with amyloid-β burden and cognition in aging. JAMA Neurol 2021; 78: 1187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma Y, Liang L, Zheng F, Shi L, Zhong B, Xie W. Association between sleep duration and cognitive decline. JAMA Netw Open 2020; 3: e2013573. 10.1001/jamanetworkopen.2020.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry A, Katsoulis M, Masi Set al. The relationship between sleep duration, cognition and dementia: a Mendelian randomization study. Int J Epidemiol 2019; 48: 849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabia S, Fayosse A, Dumurgier Jet al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun 2021; 12: 2289. 10.1038/s41467-021-22354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yaffe K, Nasrallah I, Hoang TDet al. Sleep duration and white matter quality in middle-aged adults. Sleep 2016; 39: 1743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer's disease dementia. Lancet Neurol 2019; 18: 296–306. [DOI] [PubMed] [Google Scholar]
- 8. Xie L, Kang H, Xu Qet al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Da Mesquita S, Louveau A, Vaccari Aet al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature 2018; 560: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep 2006; 29: 85–93. [DOI] [PubMed] [Google Scholar]
- 11. Buysse DJ. Insomnia. JAMA 2013; 309: 706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Appleton SL, Reynolds AC, Gill TK, Melaku YA, Adams RJ. Insomnia prevalence varies with symptom criteria used with implications for epidemiological studies: role of anthropometrics, sleep habit, and comorbidities. Nat Sci Sleep 2022; 14: 775–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morin CM, Jarrin DC, Ivers H, Mérette C, LeBlanc M, Savard J. Incidence, persistence, and remission rates of insomnia over 5 years. JAMA Netw Open 2020; 3: e2018782. 10.1001/jamanetworkopen.2020.18782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fortier-Brochu E, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep 2014; 37: 1787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi L, Chen SJ, Ma MYet al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev 2018; 40: 4–16. [DOI] [PubMed] [Google Scholar]
- 16. Almondes KM, Costa MV, Malloy-Diniz LF, Diniz BS. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiatr Res 2016; 77: 109–15. [DOI] [PubMed] [Google Scholar]
- 17. Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 2013; 17: 241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernandez-Mendoza J, He F, Puzino Ket al. Insomnia with objective short sleep duration is associated with cognitive impairment: a first look at cardiometabolic contributors to brain health. Sleep 2021; 44: zsaa150. 10.1093/sleep/zsaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trolle Lagerros Y, Hantikainen E, Mariosa Det al. Cohort profile: the Swedish National March Cohort. Int J Epidemiol 2017; 46: dyw193–795e. 10.1093/ije/dyw193. [DOI] [PubMed] [Google Scholar]
- 20. Wolters FJ, Chibnik LB, Waziry Ret al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer cohorts consortium. Neurology 2020; 95: e519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akerstedt T, Ingre M, Broman JE, Kecklund G. Disturbed sleep in shift workers, day workers, and insomniacs. Chronobiol Int 2008; 25: 333–48. [DOI] [PubMed] [Google Scholar]
- 22. American Academy of Sleep Medicine International Classification of Sleep Disorders. 3rd edition. Darien, IL: American Academy of Sleep Medicine, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: Text Revision, 2000. [Google Scholar]
- 24. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34: 939–44. [DOI] [PubMed] [Google Scholar]
- 25. Livingston G, Huntley J, Sommerlad Aet al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brooke HL, Talbäck M, Hörnblad Jet al. The Swedish cause of death register. Eur J Epidemiol 2017; 32: 765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun Y, Chen C, Yu Yet al. Replacement of leisure-time sedentary behavior with various physical activities and the risk of dementia incidence and mortality: a prospective cohort study. J Sport Health Sci 2022; S2095-2546: 00112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petermann-Rocha F, Lyall DM, Gray SRet al. Associations between physical frailty and dementia incidence: a prospective study from UK biobank. Lancet Healthy Longev 2020; 1: e58–68. [DOI] [PubMed] [Google Scholar]
- 29. Robbins R, Weaver MD, Barger LK, Wang W, Quan SF, Czeisler CA. Sleep difficulties, incident dementia and all-cause mortality among older adults across 8 years: findings from the National Health and aging trends study. J Sleep Res 2021; 30: e13395. 10.1111/jsr.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palpatzis E, Bass N, Jones R, Mukadam N. Longitudinal association of apolipoprotein E and sleep with incident dementia. Alzheimers Dement 2022; 18: 888–98. [DOI] [PubMed] [Google Scholar]
- 31. Suh SW, Han JW, Lee JRet al. Sleep and cognitive decline: a prospective nondemented elderly cohort study. Ann Neurol 2018; 83: 472–82. [DOI] [PubMed] [Google Scholar]
- 32. Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord 2006; 20: 41–8. [DOI] [PubMed] [Google Scholar]
- 33. Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement 2020; 16: 1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yao CW, Pelletier A, Fereshtehnejad SM, Cross N, Dang-Vu T, Postuma RB. Insomnia symptom subtypes and manifestations of prodromal neurodegeneration: a population-based study in the Canadian longitudinal study on aging. J Clin Sleep Med 2022; 18: 345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forte G, Favieri F, Casagrande M. Heart rate variability and cognitive function: a systematic review. Front Neurosci 2019; 13: 710. 10.3389/fnins.2019.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johar H, Kawan R, Emeny RT, Ladwig KH. Impaired sleep predicts cognitive decline in old people: findings from the prospective KORA age study. Sleep 2016; 39: 217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jackson CL, Patel SR, Jackson WB 2nd, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: multi-ethnic study of atherosclerosis. Sleep 2018; 41: zsy057. 10.1093/sleep/zsy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dijk DJ. Slow-wave sleep deficiency and enhancement: implications for insomnia and its management. World J Biol Psychiatry 2010; 11: 22–8. [DOI] [PubMed] [Google Scholar]
- 39. Andreasson A, Axelsson J, Bosch JA, Balter LJ. Poor sleep quality is associated with worse self-rated health in long sleep duration but not short sleep duration. Sleep Med 2021; 88: 262–6. [DOI] [PubMed] [Google Scholar]
- 40. Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: a review of possible mechanisms. Sleep Med Rev 2018; 40: 127–34. [DOI] [PubMed] [Google Scholar]
- 41. Bokenberger K, Ström P, Dahl Aslan AKet al. Association between sleep characteristics and incident dementia accounting for baseline cognitive status: a prospective population-based study. J Gerontol A Biol Sci Med Sci 2017; 72: 134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thygesen LC, Ersbøll AK. When the entire population is the sample: strengths and limitations in register-based epidemiology. Eur J Epidemiol 2014; 29: 551–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


