Abstract
Background: Limited data are available regarding marijuana smoking’s impact on the development or progression of chronic obstructive pulmonary disease (COPD) in middle-aged or older adults with a variable history of tobacco cigarette smoking.
Methods: We divided ever-tobacco smoking participants in the SubPopulations and InteRmediate Outcomes In COPD Study (SPIROMICS) into 3 groups based on self-reported marijuana use: current, former, or never marijuana smokers (CMSs, FMSs or NMSs, respectively). Longitudinal data were analyzed in participants with ≥2 visits over a period of ≥52 weeks.
Measurements: We compared CMSs, FMSs, and NMSs, and those with varying amounts of lifetime marijuana use. Mixed effects linear regression models were used to analyze changes in spirometry, symptoms, health status, and radiographic metrics; zero-inflated negative binomial models were used for exacerbation rates. All models were adjusted for age, sex, race, baseline tobacco smoking amount, and forced expiratory volume in 1 second (FEV1) %predicted.
Results: Most participants were followed for ≥4 years. Annual rates of change in FEV1, incident COPD, respiratory symptoms, health status, radiographic extent of emphysema or air trapping, and total or severe exacerbations were not different between CMSs or FMSs versus NMSs or between those with any lifetime amount of marijuana use versus NMSs.
Conclusions: Among SPIROMICS participants with or without COPD, neither former nor current marijuana smoking of any lifetime amount was associated with evidence of COPD progression or its development. Because of our study’s limitations, these findings underscore the need for further studies to better understand longer-term effects of marijuana smoking in COPD.
Keywords: COPD, exacerbations, spirometry, marijuana, high-resolution CT
Introduction
This article contains supplemental material.
The effect of marijuana use on lung health has not been extensively studied, with most data coming from cross-sectional1-11 and several longitudinal studies.12-17 While a significant association of marijuana smoking with symptoms of chronic bronchitis has been reported in most studies, associations with changes in lung function or other aspects of lung health over time, especially in those at risk of or with diagnosed chronic obstructive pulmonary disease (COPD), has been less studied. Three of the longitudinal analyses of lung function change found no association with marijuana use.12,16,17 One found a small forced expiratory volume in 1 second (FEV1) decrement over a 20-year period in the relatively small number (n=40) of heavy marijuana smokers, i.e., ≥20 joint-years (self-reported number of joints per day times the number of years smoked)13 and one found a decrement in FEV1 only in former but not current marijuana smokers.14 Analyzing a subgroup derived from the Canadian Cohort Obstructive Lung Disease (CanCOLD) study,18 Tan et al found that marijuana smoking was associated with worse FEV1 decline over a median of 5.9 years in comparison with tobacco-only smokers.15 While the latter finding mostly related to individuals with a heavy marijuana smoking history (hereafter defined as ≥20 joint years), the design of this study might have influenced the results.19,20
In a cross-sectional analysis of participants with COPD in the SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) with a tobacco smoking history of ≥20 pack years, Morris et al10 reported higher values for FEV1 percentage (%) predicted (pred) and a lower percentage of emphysema on HRCT images in both former marijuana smokers (FMSs) and current marijuana smokers (CMSs), compared with never marijuana smokers (NMSs) who smoked tobacco only, after adjustments for relevant variables. In a preliminary analysis focused on lung function change, we recently showed that ever marijuana smoking among SPIROMICS participants with ≥3 spirometry visits did not have a deleterious impact on FEV1 decline over time nor on the risk for developing spirometry-defined COPD in tobacco smokers without COPD at baseline.17 However, in order to examine the impact of marijuana smoking on the progression of respiratory symptoms, health status, HRCT metrics, or frequency of exacerbations in addition to the change in lung function, we analyzed a larger subgroup of SPIROMICS participants with ≥2 spirometry visits and an ever marijuana-smoking history as well as a heavy marijuana-smoking history (≥20 joint years) compared to those with ≥3 spirometry visits as previously reported.17 We aimed to determine whether SPIROMICS FMSs and CMSs exhibit higher rates of change in respiratory symptoms, HRCT metrics, and lung function over time (2–10 years) compared to NMSs and whether the reported cumulative lifetime exposure to marijuana would affect these changes. In addition, we evaluated whether self-reported marijuana smoking among SPIROMICS participants without spirometric evidence of COPD at baseline would affect the subsequent development of COPD.
Methods
Study Population
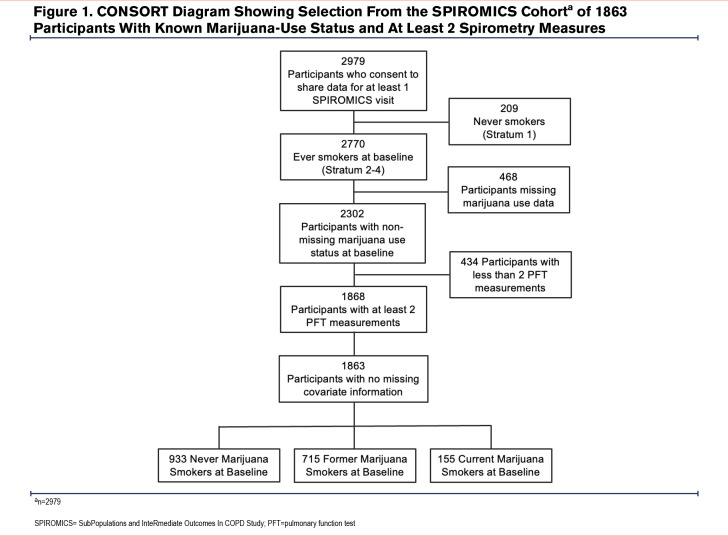
SPIROMICS is a prospective cohort study (N= 2979 participants) aiming to identify new COPD subgroups and intermediate markers of disease progression.21 Participants were followed annually over 3 years in SPIROMICS I and had an additional in-person visit in SPIROMICS II. Enrolled participants were 40–80 years old and had either normal spirometry and no tobacco-smoking history or had ≥20 pack years of tobacco smoking; the latter subgroup was further divided based on a post-bronchodilator FEV1 to forced vital capacity (FVC) ratio ≥0.70 or <0.70. Current asthma was an exclusionary criterion. SPIROMICS was approved by the institutional review boards of each individual site prior to the enrollment of participants. All participants provided informed consent. For the present analysis, data were obtained from ever tobacco-smoking SPIROMICS participants who had spirometry at the baseline visit and at least one follow-up visit, reported marijuana use or nonuse at the baseline visit, and had no missing covariate information (n=1863). These participants were divided into the following 3 groups based on their self-reported history of marijuana use: NMSs (n=933), FMSs, i.e., no marijuana smoking within the last 30 days (n=775), or CMSs, i.e., marijuana smoking within the last 30 days (n=155). Based on their baseline frequency and duration of marijuana use, participants were further categorized by their cumulative lifetime history of marijuana smoking defined in terms of joint years, calculated as the number of joints smoked per day times the number of years that marijuana was regularly smoked. Recognizing that marijuana is smoked using a variety of devices, we equated a bowlful of marijuana smoked via a pipe or a bong to one joint. Participants were not asked at the baseline visit about alternative modalities of inhaled marijuana such as vaping, hookah, and “dabbing.” Patients were also categorized into 4 joint-year groups as follows: 0 (n=933, i.e., NMSs); >0–<10 (n=314); 10–<20 (n=66); and ≥20 (n=137) joint years. Longitudinal data over a period of at least 52 weeks were compared between the 3 groups defined by marijuana-smoking status (NMSs, FMSs, CMSs) as well as between the 4 subgroups defined by the number of joint years.
Clinical Data
The study design of SPIROMICS has been described previously.21 The data included baseline demographic and clinical characteristics, post-bronchodilator spirometry following 2005 American Thoracic Society/European Respiratory Society criteria,22 St George’s Respiratory Questionnaire (SGRQ) scores,23 the modified Medical Research Council (mMRC) scale,24 COPD Assessment Test (CAT) scores,25 and exacerbations of COPD, ascertained at quarterly follow-up phone visits. HRCT metrics captured at enrollment and the 1-year follow-up visit included emphysema defined by percentage-voxels <-950 Hounsfield units (HU) at total lung capacity (% emphysema), air trapping using percentage-voxels <-856 HU at residual volume, airway wall thickness at an internal perimeter of 10 mm (Pi10)26 and parametric response mapping (PRM), a dynamic image registration technique assessing extent functional small airways disease (PRMfSAD) and emphysema (PRMEMPH).27-28
Data Analysis
We used linear mixed-effects models to assess changes in continuous outcomes over time: post-bronchodilator FEV1, post-bronchodilator FVC, SGRQ total score, CAT score, and HRCT metrics. Linear mixed-effects models, specifically proportional odds models, were used to assess changes in respiratory symptoms over time.
In assessing whether marijuana use among tobacco-smoking participants without COPD at baseline increased the risk of subsequent development of COPD, the primary outcome was time to development of airflow obstruction, defined by a post-bronchodilator FEV1/FVC<0.70. Survival curves and hazard ratios were computed from models fitted with and without covariates. We used zero-inflated negative binomial models to compare the rate of exacerbations between CMSs, FMSs, and NMSs. Exacerbations were classified as moderate (defined as an increase in symptoms of COPD requiring treatment with oral corticosteroids and/or antibiotics by a health care provider), severe (defined as an increase in symptoms of COPD requiring hospitalization or leading to death), and total (defined as moderate and/or severe exacerbations). To assess dose-response relationships, the same models were used with the primary predictor of interest being categorical joint-year history at baseline.
Results
Baseline Characteristics
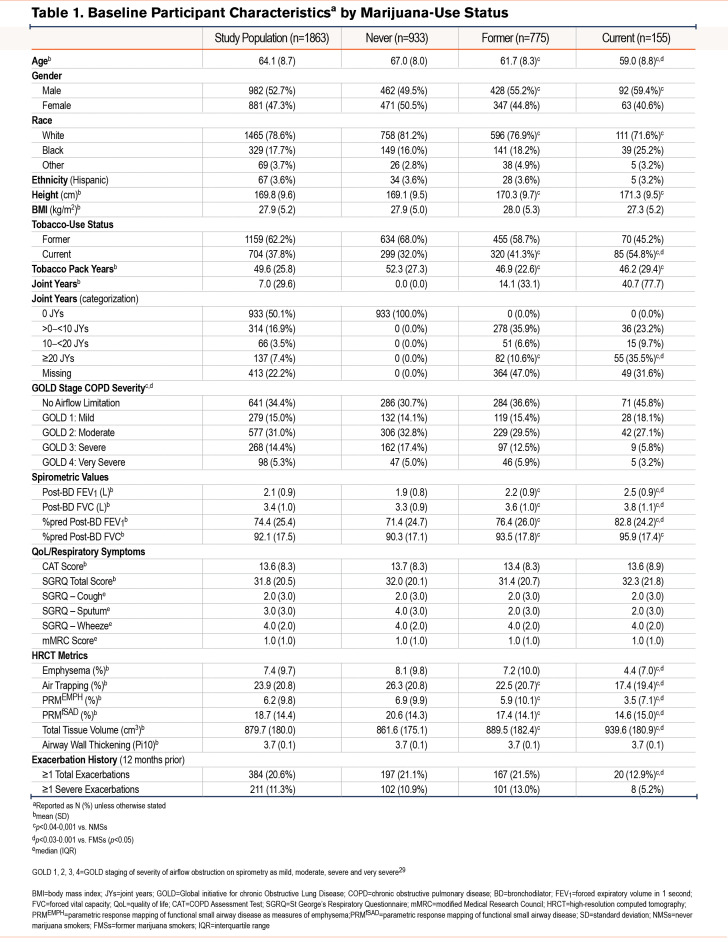
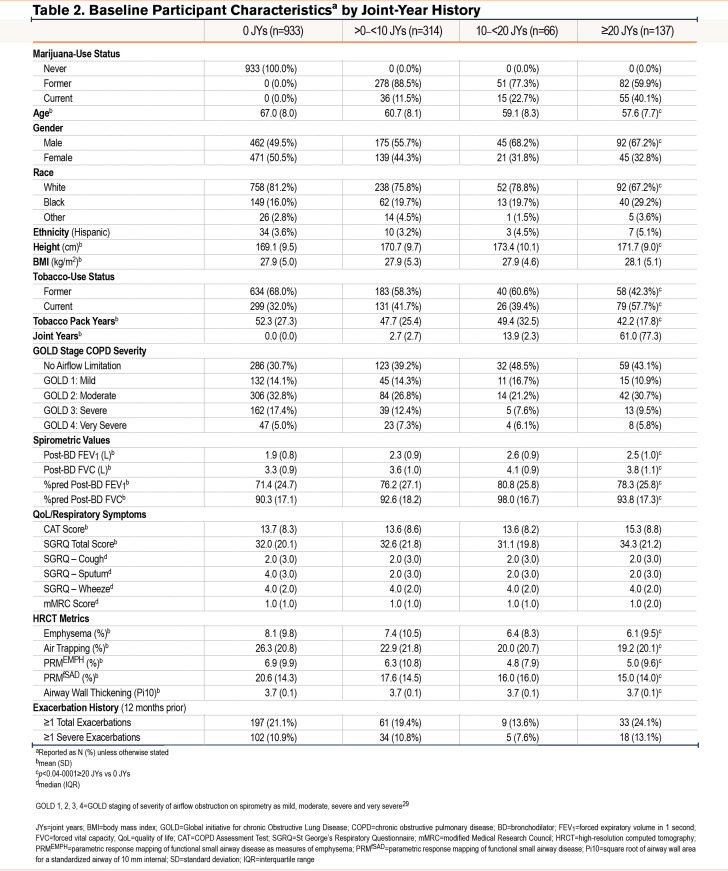
A consort diagram describing the derivation of the study cohort is shown in Figure 1. At enrollment, CMSs, when compared with NMSs, tended to be younger and more often current tobacco smokers, men, and Black (Table 1 and e-Table 1 in the online supplement).29 They also had fewer exacerbations during the year prior to enrollment, had a better FEV1, less frequent airflow obstruction, and less emphysema and air trapping, but had similar levels of respiratory symptoms. Similar findings were noted in comparison of FMSs with NMSs. Due to incomplete reporting, calculating the cumulative lifetime amount of marijuana use in joint years was not possible for all participants, so that the number of those classified by joint-year category (n=1450) is lower than that of the total analysis sample. Among those with the heaviest marijuana use (≥20 joint years), directionally similar baseline differences were noted in age, sex, the proportion of Black participants, and current tobacco-smoking status compared to those with 0 joint years, as were found in comparison between CMSs and FMSs with NMSs (Table 2 and e-Table 2 in the online supplement).
Follow-up Visits
All 3 marijuana use groups (NMSs, FMSs, and CMSs) had similar numbers of in-person clinic visits (median [interquartile range (IQR)] 3[1–2]) and follow-up time in years (mean [standard deviation (SD)] 4.2[2.2]) (e-Table 3 in the online supplement). All 4 joint-year groups also had similar numbers of follow-up clinic visits (median [IQR] 3[1–2]) and years of follow-up time (mean [SD] 4.2[2.1–2.2]) (e-Table 4 in the online supplement). E-Tables 5 and6 in the online supplement show the median (25th, 75th) number of non-missing follow-up data points for each outcome variable by marijuana-smoking status and joint-year category, respectively.
Marijuana-Smoking Effect on Change in Lung Function, Symptoms, and Health Status During Follow-Up
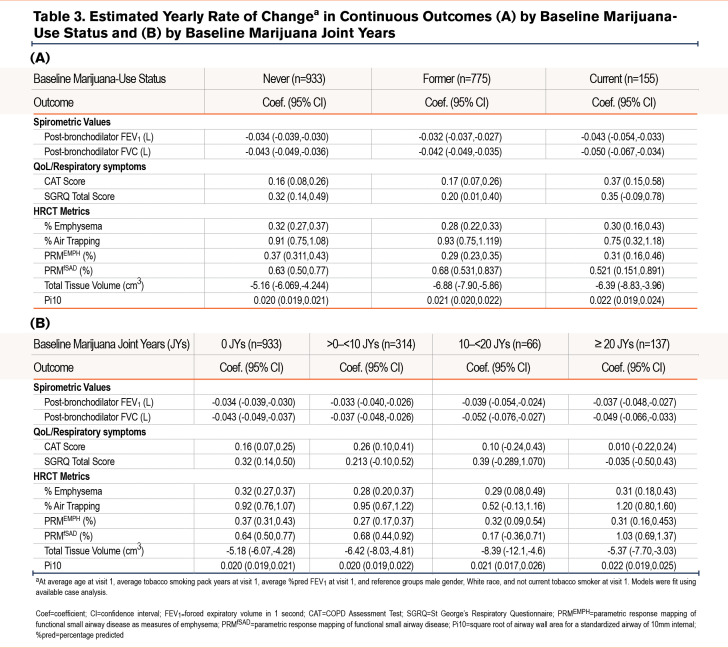
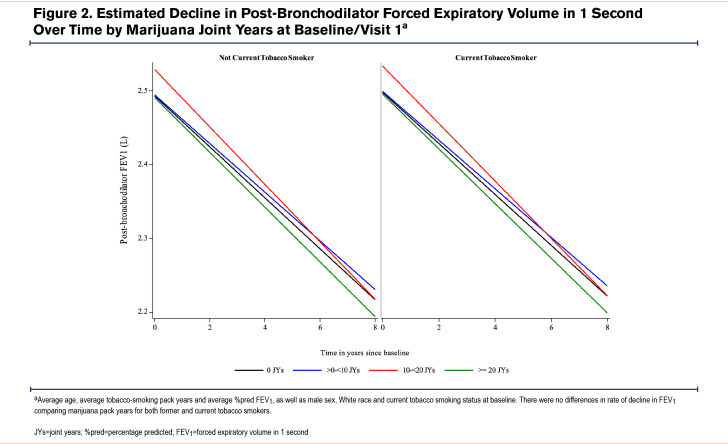
The estimated rates of change in continuous outcomes by baseline marijuana-smoking status are shown in Table 3A. While numerically higher annual rates of FEV1 and FVC decline and higher rates of worsening CAT and total SGRQ scores were found comparing CMSs (but not FMSs) with NMSs, these differences were neither clinically nor statistically significant (e-Table 7 in the online supplement). Similar rates of change in these parameters were found on comparison of FMSs with NMSs. Estimated rates of change in continuous outcomes between joint-year-based categories were similar across all joint-year groups (Table 3B) and between groups (e-Table 8 in the online supplement). Estimated annualized FEV1 decline during follow-up by marijuana joint years stratified by former and current tobacco-smoking history were similar, irrespective of tobacco-smoking status (Figure 2). Estimated participant-specific yearly changes in odds for worsening respiratory symptoms (cough, sputum, wheeze, and dyspnea) during follow-up compared to the baseline visit by baseline marijuana status and baseline joint years are shown in e-Table 9A and B and e-Figures 1 and 2 in the online supplement, respectively. The odds over time of more cough and sputum, but not more wheeze or dyspnea, were significantly higher in CMSs compared to FMSs or NMSs (e-Figure 1 in the online supplement), while no significant differences were found across the different joint-year categories that included both CMSs and FMSs (e-Figure 2 in the online supplement). Estimated yearly changes in CAT and SGRQ scores were not significantly different across marijuana-smoking status and joint-year categories as shown both in Table 3 A and B in the online supplement, respectively, and e-Tables 5 and 6 in the online supplement, respectively.
The Effect of Marijuana Smoking on Annual Rate of Change in High-Resolution Computed Tomography Metrics
Our analysis showed nominally less emphysema, air trapping, and functional small airways disease progression without statistical significance among CMSs compared to NMSs. Similarly, a comparison between NMSs, FMSs, and CMSs showed no significantly different changes in HRCT metrics, except for unadjusted increased total tissue volume loss among FMSs compared to NMSs (Table 3A and e-Table 7 in the online supplement). No difference in tissue volume loss between CMSs and NMSs was found.
Estimated rates of change in HRCT metrics were generally similar across all joint-year groups (Table 3B), except for a higher rate of increase in PRMfSAD on comparison of those with ≥20 joint years (coefficient 1.030; 95% confidence interval [CI]: 0.686,1.375) versus 0 joint years (coefficient 0.638; 95% CI: 0.504, 0.771), with a between-group difference 0.393 (95% C.I. 0.023, 0.762; p=0.037) when unadjusted for multiple testing (e-Table 8 in the online supplement).
Rate of Exacerbations
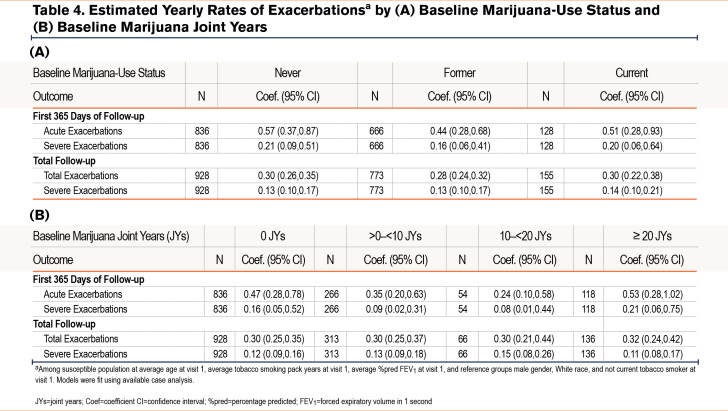
Estimated yearly rates of 1 or more total (moderate and severe) or severe exacerbations during the first 365 days or the total follow-up period by baseline marijuana-smoking status and marijuana joint years are shown in Table 4 A and B and e-Figures 3 and 4 in the online supplement. While rates of total and severe exacerbations were numerically lower among both CMSs and FMSs versus NMSs during the first follow-up year, and severe exacerbation rates were slightly higher among CMSs versus NMSs during the total follow-up period, none of these differences were statistically significant (e-Figure 3 in the online supplement). Estimated rates of total and severe exacerbations were numerically higher among those with ≥20 versus those with 0 joint years during the first follow-up year. During the total follow-up period, rates of total exacerbations, but not severe exacerbations, were slightly higher among those with ≥20 versus those with 0 joint years. However, none of these between-group differences were statistically significant (e-Figure 6 in the online supplement).
Longitudinal Effect of Marijuana Exposure on the Development of COPD
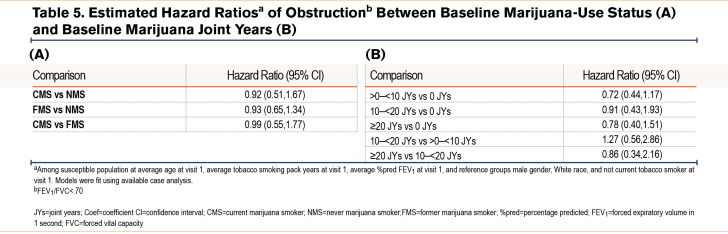
Estimated hazard ratios for the development of COPD during follow-up by baseline marijuana-smoking status and joint years among participants without spirometric evidence of COPD at baseline are shown in Table 5 and e-Figures 5 and 6 in the online supplement. The odds of developing COPD by spirometric criteria were lower among CMSs and FMSs versus NMSs, as well as among those with ≥20 versus those with 0 joint years, although these differences were not statistically significant.
Discussion
The increasing prevalence of marijuana smoking among adolescents and adults,30 including aging adults,31 in the wake of a growing number of states legalizing marijuana use underscores the need to better understand the impact of marijuana use on lung health. This need is particularly evident among adult tobacco smokers in their mid- and older life who have been understudied previously. The current analysis of the pulmonary consequences of marijuana smoking in the SPIROMICS cohort of current and former tobacco smokers with or at high risk of developing COPD is a longitudinal extension of a cross-sectional analysis of the baseline findings in the same cohort.10 While the latter cross-sectional study failed to identify deleterious effects of concomitant marijuana smoking on lung function or baseline structural radiographic abnormalities when compared with the effect of tobacco smoking alone, it could not answer the question of whether marijuana affects changes in these outcomes over 1 to several years of follow-up. In addition, the current study overlaps to some extent with a recent longitudinal analysis focused mainly on the trajectory of lung function in SPIROMICS participants limited to those with ≥ 3 spirometry visits.17 By including all those participants with ≥ 2, rather than only ≥ 3, spirometry visits at least 1 year apart, the current study has the advantage of including in the analysis larger numbers of CMSs and FMSs, most importantly of those heavy MSs with ≥ 20 joint years, in an effort to achieve greater statistical power in examining the influence of marijuana smoking on lung function decline. Furthermore, the current study examined changes in respiratory symptoms and HRCT metrics during follow-up that were not included in the previous report.
Our study revealed trends toward higher rates of decline in post-bronchodilator FEV1 and worsening CAT and SGRQ scores among CMSs (but not FMSs) compared with NMSs and contrastingly, smaller rates of change in percentage of emphysema and functional small airways disease. However, none of these differences were statistically significant. Similarly, when we compared different categories of lifetime cumulative amounts of marijuana smoking, no significant differences were noted in rates of change in lung function, CAT or SGRQ scores, or HRCT metrics, except for an increase in PRMfSAD among the heaviest marijuana-smoking category (≥20 joint years) in comparison to those with 0 joint years. It is noteworthy that significantly higher odds of worsening cough and sputum were noted among CMSs in comparison with both NMSs and FMSs, but not between FMSs and NMSs. The latter finding is consistent with previous data showing a significant reduction in symptoms of chronic bronchitis after cessation of marijuana smoking.32,33 Although some numerical differences were noted in rates of exacerbations across marijuana-use status and joint-year categories, none of the between-group differences were statistically significant. Finally, while the probability of subsequently developing COPD among tobacco smokers without COPD at baseline was lower among CMSs and FMSs compared with NMSs, as well as between the heaviest marijuana smokers versus those with no history of marijuana smoking, none of these differences reached statistical significance. Taken together, the aforementioned data failed to demonstrate that marijuana smoking of any lifetime cumulative amount had a demonstrable effect on changes over time in clinical outcomes relevant to COPD, including respiratory symptoms, health status, HRCT metrics, or frequency of exacerbations.
Our failure to find any impact of even heavy marijuana smoking (≥20 joint years) on lung function decline in ever-tobacco smokers with or at risk of COPD differs substantially from the findings of Tan et al.15 The authors demonstrated a dose-response effect of marijuana on lung function decline in the CanCOLD study subcohort with a significantly greater rate of decline in FEV1 only among those with ≥20 joint years (40.2 ml/year) compared to those who never used marijuana (10.7 ml/year). Surprisingly, in the same study, among those with ≥20 joint years of marijuana smoking, the rates of FEV1 decline were very similar for CMSs and FMSs, compared to NMSs. In contrast, the average rate of FEV1 decline among the heaviest former tobacco smokers was substantially lower than that of the current tobacco smokers. Since tobacco smokers with COPD have a substantial reduction in the rate of FEV1 decline after sustained smoking cessation,34 the disparate findings of Tan et al15 comparing the impact of quitting marijuana with that of quitting tobacco is surprising. The absence of a difference in the rates of decline between their current and former marijuana smoking participants, most of whom were dual smokers of marijuana and tobacco, may be a reflection of the impact of continuing tobacco smoking among those who had quit using marijuana rather than of an enduring effect of marijuana among the quitters. It is also noteworthy that the number of SPIROMICS participants who were particularly heavy marijuana smokers (≥20 joint years) (n=137) was almost 3 times higher than the number of CanCOLD participants with a heavy marijuana-smoking history (n=51), suggesting that our analysis of the impact of heavy marijuana use on lung function decline had greater statistical power. Finally, while the reference control group in our analysis of FEV1 decline in relation to marijuana smoking consisted of NMSs with a history of at least 20 pack years of tobacco smoking, the reference group in the analysis reported by Tan et al15 was comprised solely of never smokers of either substance. Thus, our aim was to examine whether marijuana smoking had an impact on the progression or development of COPD in current or former smokers of tobacco who already had COPD or were at increased risk of developing COPD, while Tan et al evaluated whether marijuana smoking led to an accelerated decline in lung function in a population of whom 43% were nonsmokers of tobacco.
Our findings are also at odds with the results of another recent study by Winhusen et al.1 Using data from electronic health records of patients treated in an integrated health care system located in Northeast Ohio, the authors reported a significantly greater risk for COPD, defined using International Classification of Diseases, 9th and 10th revisions’ codes, among persons with a diagnosis of cannabis use disorder compared to propensity-matched controls in a subgroup of patients with a diagnosis of tobacco use disorder (adjusted OR 1.44 C.I. 1.56–1.73; p=0.0001). These findings imply an additive effect of cannabis on top of tobacco use. However, limitations of the latter study include misclassification of COPD in the absence of spirometry data, suggested by the relatively young average age of the authors’ analysis population (42 years) versus ours (64 years), as well as the absence of data on the route of cannabis administration and the intensity and duration of its use. The marked disparity of these results with ours underscores the need for additional study.
The possibility of a dose-response impact of marijuana exposure is suggested by our finding of a significantly larger effect of ≥20 joint years on PRMfSAD in comparison with 0 joint years (i.e., NMSs), consistent with a deleterious effect of heavy marijuana use on small airways. The latter observation is consistent with the recently reported finding in a New Zealand birth cohort at age 45 years of an association of lifetime cannabis use, adjusted for tobacco pack years, with pre-bronchodilator peripheral airways resistance and reactance using impulse oscillometry.35 However, this finding was only significant in women and was weaker and no longer significant after bronchodilator use. In the same birth cohort at age 45, Hancox et al further reported a significant negative association of marijuana joint years with forced expiratory flow at 25% and 75% of the pulmonary volume, but this association was only significant in men.16 A dose-response effect could also be consistent with previous findings by Pletcher et al13 who followed a cohort of 5015 young adults with a high tobacco-smoking prevalence for over 20 years and found no evidence that increasing lifetime marijuana exposure adversely affected lung function except among those with very heavy lifetime exposure (>40 joint years).
The findings of the present study should be interpreted in the context of certain limitations. SPIROMICS was not specifically designed to examine the effects of marijuana smoking, and our analyses were conducted post hoc; therefore, this analysis may be underpowered due to a relatively small sample size and short duration of follow-up and our findings should be considered exploratory. SPIROMICS did not enroll a random sample, so that our results may not be generalizable. Marijuana is inhaled by various methods besides smoking a joint, including the use of a pipe or bong, hookah, a blunt, dabbing, vaping, or administered as edible cannabinoids,36 all of which information was not collected at the baseline visit. However, the most common mode of inhalation of marijuana is via smoking a joint,37 but the amount of marijuana actually delivered with each use is highly variable and difficult to quantitate so the method we used for quantitating the cumulative lifetime amount of marijuana smoked (joint years, or joint-equivalent years) is crude. Besides, marijuana use was self-reported and thus, prone to recall or reporting biases since marijuana use at some sites was illicit at the time of data collection. Our classification of the participants with respect to marijuana- and tobacco-use status and the lifetime amount of use was based on the information collected at baseline that did not take into account changes in marijuana or tobacco amount or use status during the follow-up period. Moreover, the groups we compared both by marijuana-smoking status (CMSs, FMSs, and NMSs) and by joint years were quite different, and we could not adequately control for all of the differences. Therefore, between-group differences in the true amount and modes of exposure to marijuana, as well as in socioeconomic differences, might explain any effects noted. Finally, since most participants with a history of marijuana smoking (n=314/547; 57%) were relatively light users (<10 joint years), it is possible that the cumulative amount of self-reported marijuana exposure was insufficient to have a detectably deleterious effect on lung health on top of the impact of a history of comparatively heavy tobacco smoking.
The present study also has several strengths. Participants were recruited and followed at 12 geographically varied sites nationwide, and women and African Americans were adequately represented, suggesting at least some measure of generalizability. All participants had extensive baseline and longitudinal characterization, allowing assessment of multiple clinical outcomes over several years. Spirometry and HRCT imaging were performed by strict adherence to recommended standards and protocols and were interpreted by dedicated reading centers. A relatively large number of participants were current marijuana smokers or reported a heavy lifetime exposure to marijuana, thereby, allowing for an assessment of dose-response relationship.
Conclusions
In a cohort of ever-tobacco smokers of ≥20 pack years with established COPD or at risk of developing COPD followed over an average of more than 4 years, a history of current and/or former smoking of marijuana of any cumulative lifetime amount was not found to be associated with a significantly deleterious impact on the progression of COPD. Among ever-tobacco smokers in the same cohort without COPD at enrollment, self-reported current and/or former concomitant marijuana smoking, including heavy marijuana smoking, was not found to be associated with an increased risk of subsequently developing COPD. However, in view of our study’s limitations and of previously published findings that conflict with our results, additional studies with larger sample sizes and longer duration of follow-up that are specifically designed to evaluate this issue are needed for a better understanding of potential long-term effects of marijuana smoking in persons with or at risk of developing COPD.
Abbreviations
Abbreviations: BD=bronchodilator; BMI=body mass index; CanCOLD=Canadian Cohort Obstructive Lung Disease; CAT=COPD Assessment Test; CI=confidence interval; CMSs=current marijuana smokers; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FMSs=former marijuana smokers; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; HRCT=high-resolution computed tomography; HU=Hounsfield unit; IQR=interquartile range; JYs=joint years; mMRC=modified Medical Research Council dyspnea scale; NMSs=never marijuana smokers; Pi10=square root of airway wall area for a standardized airway of 10 mm internal; %pred=percentage predicted; PRM=parametric response mapping; PRMEMPH =parametric response mapping of functional small airway disease as measures of emphysema; PRMfSAD=parametric response mapping of functional small airway disease; QoL=quality of life; SD=standard deviation; SGRQ=St George’s Respiratory Questionnaire; SPIROMICS=SubPopulations InteRmediate Outcome Measures In COPD Study
Funding Statement
SPIROMICS was supported by contracts from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute (NHLBI) (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan. This study was registered on ClinicalTrials.gov, NCT01969344.
References
- 1.Bloom JW,Kaltenborn WT,Paoletti P,Camili A,Lebowitz MD. Respiratory effects of non-tobacco cigarettes. Br Med J (Clin Res Ed). 1987;295(6612):1516-1518. doi: https://doi.org/10.1136/bmj.295.6612.1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tashkin DP,Coulson AH,Clark VA,et al. Respiratory symptoms and lung function in habitual heavy smokers of marijuana alone, smokers of marijuana and tobacco, smokers of tobacco alone, and nonsmokers. Am Rev Respir Dis. 1987;135(1):209-216. doi: https://doi.org/10.1164/arrd.1987.135.1.209 [DOI] [PubMed] [Google Scholar]
- 3.Taylor DR,Poulton R,Moffit TE,Ramankutty P,Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction. 2000;95(11):1669-1677. doi: https://doi.org/10.1046/j.1360-0443.2000.951116697.x [DOI] [PubMed] [Google Scholar]
- 4.Moore BA,Augustson EM,Moser RP,Budney AJ. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20(1):33-37. doi: https://doi.org/10.1111/j.1525-1497.2004.40081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldington S,Williams M,Nowitz M,et al. Effects of cannabis on pulmonary structure, function and symptoms. Thorax. 2007;62(12):1058-1063. doi: https://doi.org/10.1136/thx.2006.077081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan WC,Lo C,Jong A,et al. Marijuana and chronic obstructive lung disease: a population-based study. CMAJ. 2009;180(8):814-820. doi: https://doi.org/10.1503/cmaj.081040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hancox RJ,Poulton R,Ely M,et al. Effects of cannabis on lung function: a population-based cohort study. Eur Respir J. 2010;35(1):42-47. doi: https://doi.org/10.1183/09031936.00065009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macleod J,Robertson R,Copeland L,McKenzie J,Elton R,Reid P. Cannabis, tobacco smoking, and lung function: a cross-sectional observational study in a general practice population. Br J Gen Pract. 2015;65(631):e89-95. doi: https://doi.org/10.3399/bjgp15X683521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempker JA,Honig EG,Martin GS. The effects of marijuana exposure on expiratory airflow. A study of adults who participated in the U.S. National Health and Nutrition Examination Study. Ann Am Thorac Soc. 2015;12(2):135-141. doi: https://doi.org/10.1513/AnnalsATS.201407-333OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris MA,Jacobson SR,Tashkin DP,et al. Marijuana use associations with pulmonary symptoms and function in tobacco smokers enrolled in the Subpopulation and Intermediate Outcome Measures in COPD Study (SPIROMICS). Chronic Obstr Pulm Dis. 2018;5(1):46-56. doi: https://doi.org/10.15326/jcopdf.5.1.2017.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winhusen T,Theobold J,Kaelber DC,Lewis D. Regular cannabis use, with and without tobacco co-use, is associated with respiratory disease. Drug Alcohol Depend. 2019;204: 107557. doi: https://doi.org/10.1016/j.drugalcdep.2019.107557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tashkin DP,Simmons MS,Sherrill D,Coulson AH. Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age. Am J Respir Crit Care Med. 1997;155(1):141-148. doi: https://doi.org/10.1164/ajrccm.155.1.9001303 [DOI] [PubMed] [Google Scholar]
- 13.Pletcher MJ,Vittinghoff E,Kalhan R,et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307(2):173-181. doi: https://doi.org/10.1001/jama.2011.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherrill DL,Krzyzanowski M,Bloom JW,Lebowitz MD. Respiratory effects of non-tobacco cigarettes: a longitudinal study in general population. Int J Epidemiol. 1991;20(1):132-137. doi: https://doi.org/10.1093/ije/20.1.132 [DOI] [PubMed] [Google Scholar]
- 15.Tan WC,Bourbeau J,Aaron S,et al. The effects of marijuana smoking on lung function in older people. Eur Respir J. 2019;54(6):1900826. doi: https://doi.org/10.1183/13993003.00826-2019 [DOI] [PubMed] [Google Scholar]
- 16.Hancox R,Gray AR,Zhang X,et al. Differential effects of cannabis and tobacco on lung function in mid-adult life. Am J Respir Crit Care Med. 2022;205(10):1179-1185. doi: https://doi.org/10.1164/rccm.202109-2058OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barjaktarevic I,Cooper CD,Shing T,et al. Effect of marijuana smoking on lung function change in older ever tobacco smokers. Eur Respir J. 2022;60(3):2201133. doi: https://doi.org/10.1183/13993003.01133-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourbeau J,Tan WC,Benedetti A,et al. Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD. 2014; 11:125-132. doi: https://doi.org/10.3109/15412555.2012.665520 [DOI] [PubMed] [Google Scholar]
- 19.Hancox RJ,Sears R. The impact of marijuana smoking on lung function. Eur Respir J. 2019;54(6):1902065. doi: https://doi.org/10.1183/13993003.02065-2019 [DOI] [PubMed] [Google Scholar]
- 20.Tashkin DP,Roth MD. Impact of marijuana smoking on lung function in older persons. Eur Respir J. 2020;55(2):1902328. doi: https://doi.org/10.1183/13993003.02328-2019 [DOI] [PubMed] [Google Scholar]
- 21.Couper D,LaVange LM,Han M-L,et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014;69(5):491-494. doi: https://doi.org/10.1136/thoraxjnl-2013-203897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: https://doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 23.Jones PW,Quirk FH,Baveystock CM. The St George's Respiratory Questionnaire. Respir Med. 1991;85 Suppl B 25-31:discussion 33-37. doi: https://doi.org/10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 24.Fletcher CM. Standardized questionnaire on respiratory symptoms. BMJ. 1960;2(5213):1665. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2098438/pdf/brmedj03053-0087a.pdf13688719 [Google Scholar]
- 25.Gupta N,Pinto LM,Morogan A,Bourbeau J. The COPD assessment test: a systematic review. Eur Respir J. 2014;44(4):873-884. doi: https://doi.org/10.1183/09031936.00025214 [DOI] [PubMed] [Google Scholar]
- 26.Sieren JP,Newell JD,Barr RG,et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794-806. doi: https://doi.org/10.1183/09031936.00025214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano Y,Wong JC,de Jong PA,et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142-146. doi: https://doi.org/10.1164/rccm.200407-874OC [DOI] [PubMed] [Google Scholar]
- 28.Boes JL,Hoff BA,Bule M,et al. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD Study (SPIROMICS). Acad Radiol. 2015;22(2):186-194. doi: https://doi.org/10.1016/j.acra.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, 2022 report. GOLD website. Published 2022. Accessed August 2022. https://goldcopd.org/ [Google Scholar]
- 30.Department of Health and Human Services, Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey of Drug Use and Health (NSDUH), national releases. 2019 NSDUH release, 2020 NSDUH release. SAMHSA website. Published 2019-2020. Accessed April 10, 2022. https://www.samhsa.gov/data/nsduh/national-releases [Google Scholar]
- 31.Kaskie B,Ayyagari P,Milavetz G,Shane D,Arora K. The increasing use of cannabis among older Americans: a public health crisis or viable policy alternative. Gerontologist. 2017;57(6):1166-1172. doi: https://doi.org/10.1093/geront/gnw166 [DOI] [PubMed] [Google Scholar]
- 32.Tashkin DP,Simmons M,Tseng C-H. Impact of changes in regular use of marijuana and/or tobacco on chronic bronchitis. COPD. 2012;9(4):367-374. doi: https://doi.org/10.3109/15412555.2012.671868 [DOI] [PubMed] [Google Scholar]
- 33.Hancox RT,Shin HH,Gray AR,Poulton R,Sears MR. Effect of quitting cannabis on respiratory symptoms. Eur Respir J. 2015;46(1):80-87. doi: https://doi.org/10.1183/09031936.00228914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scanlon PD,Connett JE,Waller LA,et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease: The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381-390. doi: https://doi.org/10.1164/ajrccm.161.2.9901044 [DOI] [PubMed] [Google Scholar]
- 35.Tan HS,McAnally HM,Dummer J,Hancox RJ. Lifetime cannabis exposure and small airway function in a population-based cohort study. ERJ Open Research. 2022;8(2):00688-2021. doi: https://doi.org/10.1183/23120541.00688-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biehl JR,Burnham EL. Cannabis smoking in 2015: a concern for lung health? Chest. 2015;148(3):596-606. doi: https://doi.org/10.1378/chest.15-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steigerwald S,Wong PO,Cohen BE,et al. Smoking, vaping and use of edibles and other forms of marijuana among U.S. adults. Ann Intern Med. 2018;169(12):890-892. doi: https://doi.org/10.7326/M18-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.