Abstract
Background: Previous studies have reported mixed associations between inhaled corticosteroids (ICSs) and cardiovascular disease (CVD) in people with chronic obstructive pulmonary disease (COPD). Using updated literature, we investigated the association between ICS-containing medications and CVD in COPD patients, stratified by study-related factors.
Methods: We searched MEDLINE and EMBASE for studies that reported effect estimates for the association between ICS-containing medications and the risk of CVD in COPD patients. CVD outcomes specifically included heart failure, myocardial infarction, and stroke-related events. We conducted a random-effects meta-analysis and a meta-regression to identify effect-modifying study-related factors.
Results: Fifteen studies met inclusion criteria and investigated the association between ICS-containing medications and the risk of CVD. Pooled results from our meta-analysis showed a significant association between ICS-containing medication and reduced risk of CVD (hazard ratio 0.87, 95% confidence intervals 0.78 to 0.97). Study follow-up time, non-ICS comparator, and exclusion of patients with previous CVD modified the association between ICS use and risk of CVD.
Conclusions: Overall, we found an association between ICS-containing medications and reduced risk of CVD in COPD patients. Results from the meta-regression suggest that subgroups of COPD patients may benefit from ICS use more than others and further work is needed to determine this.
Keywords: COPD, inhaled corticosteroids, cardiovascular disease
Introduction
This article contains supplemental material.
Inhaled corticosteroids (ICSs) have been shown to slow lung function decline, reduce the risk of exacerbations, and improve health status among people with chronic obstructive pulmonary disease (COPD), however, they have also been associated with an increased risk of pneumonia and recent guidelines suggest that use of dual bronchodilators may reduce the risk of mortality in symptomatic COPD patients with a history of frequent or severe exacerbations.1-3 Previous studies have suggested that ICSs may have a protective effect on the development of cardiovascular disease (CVD) in people with COPD. Observational studies have shown that COPD patients initiating ICSs have a reduced risk of developing coronary heart disease (CHD) compared with non-ICS users.4 In addition, a study of COPD patients in Canada found that the risk of myocardial infarction (MI) was lower for patients on ICSs at a dose of 50–200 micrograms per day compared with patients not on ICS.5
Many randomized control trials (RCTs) have not found an association between ICSs and CVD. A meta-analysis of 31 RCTs, with at least 4 weeks of follow-up, found no association between ICSs (in any combination) and CVD (relative risk 0.99, 95% confidence interval [CI] 0.90 to 1.90).6 An additional systematic review published in 2009 included both RCTs and observational studies and found no association between ICSs and CVD in RCTs (relative risk 1.02, 95% CI 0.81 to 1.27), however, in 2 observational studies a protective effect of ICSs was seen on CVD mortality (relative risk 0.79, 95% CI 0.72 to 0.86).7 This systematic review, however, was published in 2009 and there have been many more observational studies and RCTs published since then. Furthermore, no previous studies have investigated study-related factors that may modify the relationship between ICS use and CVD risk in people with COPD. Given the mixed results for the association between ICS use and risk of CVD from previous studies, it is possible that subgroups of COPD patients exist in which an ICS has a more protective effect compared with other subgroups of COPD.
We aimed to investigate the association between the use of ICSs or ICS-containing medications and CVD (both fatal and non-fatal events) compared with non-ICS-containing medications in COPD patients. We also aimed to investigate study-related factors that modify the relationship between ICSs and CVD risk and stratify by subgroups of COPD patients.
Methods
Data Sources and Searches
We systematically searched MEDLINE and EMBASE (up until the June 1, 2022) using the following search terms: (1) COPD, chronic obstructive lung disease; (2) ICS, inhaled corticosteroid, budesonide, fluticasone, beclomethasone, mometasone; (3) CVD, cardiovascular disease, cardiovascular mortality, myocardial infarction, heart failure, stroke. The Boolean operator or was used to combine search terms within a concept and the operator and was used to combine all 3 concepts. Table E1 in the online supplement reports the full list of search terms used.
Study Selection
Study exposures included any ICS-containing medication and any non-ICS-containing medication as the comparison. ICS-containing medications could include any ICS monotherapy, dual therapy with a long-acting beta2-agonist (LABA), or triple therapy with a LABA and a long-acting muscarinic antagonist (LAMA). Non-ICS-containing medications could include placebo, short-acting bronchodilator therapies, and any long-acting bronchodilator therapies. Studies were included if the outcome of interest was any CVD- related outcome. This included primary and secondary care events as well as fatal events. CVD events specifically included heart failure, MI, stroke, and CVD mortality. Studies were included if they specifically investigated the association between ICSs and CVD and reported effect estimates for this relationship. We did not exclude studies based on cardiovascular history in order to investigate the association between ICSs and future CVD in different types of COPD populations.
Two groups of 2 reviewers (KG and JC, MK and DR) independently reviewed titles and abstracts. Any inconsistencies were discussed with the wider group and with a third party (HW) if needed. After identifying initial studies based on titles and abstract, full article texts were reviewed. Studies were excluded if they were not available in English, were conference abstracts, review articles, or protocols. References from previous systematic reviews and meta-analyses were also searched.
Data Extraction and Quality Assessment
Study elements that were extracted included: study name, study population size, follow-up time, inclusion and exclusion criteria, the types of medication in each ICS and non-ICS-containing exposure group, definition of CVD outcome, and effect estimates. Two reviewers extracted the information, and any inconsistencies were discussed.
The quality of studies was assessed using the Cochrane Risk of Bias Tool for RCTs.8 This tool was used to assess selection bias, reporting bias, performance bias, detection bias, attrition bias, and any other bias not reported within these categories.8 The Risk of Bias in Non-Randomized Studies—of Interventions tool9 was used to assess the quality of observational studies, specifically non-randomized studies of interventions. Broadly this tool assessed bias due to confounding, selection bias, misclassification bias, missing data bias, and bias in reporting of results.9
Meta-analysis and Meta-regression
We performed a 2-stage meta-analysis whereby first, a fixed-effects meta-analysis for individual studies with more than one comparator was performed to estimate the summary effect estimate. Second, a random-effects meta-analysis was performed for all studies, including the summary effect estimates for the studies with more than one comparator. To investigate whether study-related factors modified the relationship between ICSs and the risk of CVD we performed a meta-regression. We specifically investigated follow-up time (defined as 3 years or less and greater than 3 years), study design (observational or RCT), CVD outcome severity (fatal or non-fatal), non-ICS comparator (bronchodilators versus placebo), and previous history of CVD (whether studies excluded people with a history of CVD or not). The meta-regression outputs a ratio of the hazard ratios (HRs) under investigation. Factors that significantly modified the relationship between ICS use and the risk of CVD were used to stratify meta-analyses.
Results
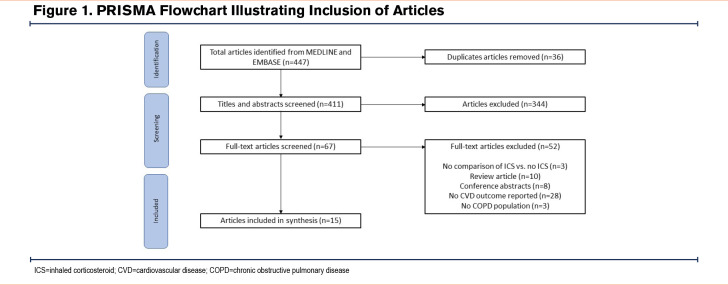
A total of 447 studies were identified. After removing duplicates, 415 articles were screened by their titles and abstracts. A total of 72 articles were included for the full-text screening, and a total of 15 were included in the final analysis. The main reason for the exclusion of full texts was due to the lack of studies including CVD outcomes (Figure 1). These 15 studies represented up to 240,903 people with COPD, however, it is possible for people with COPD to be included in more than one study.
Study Characteristics
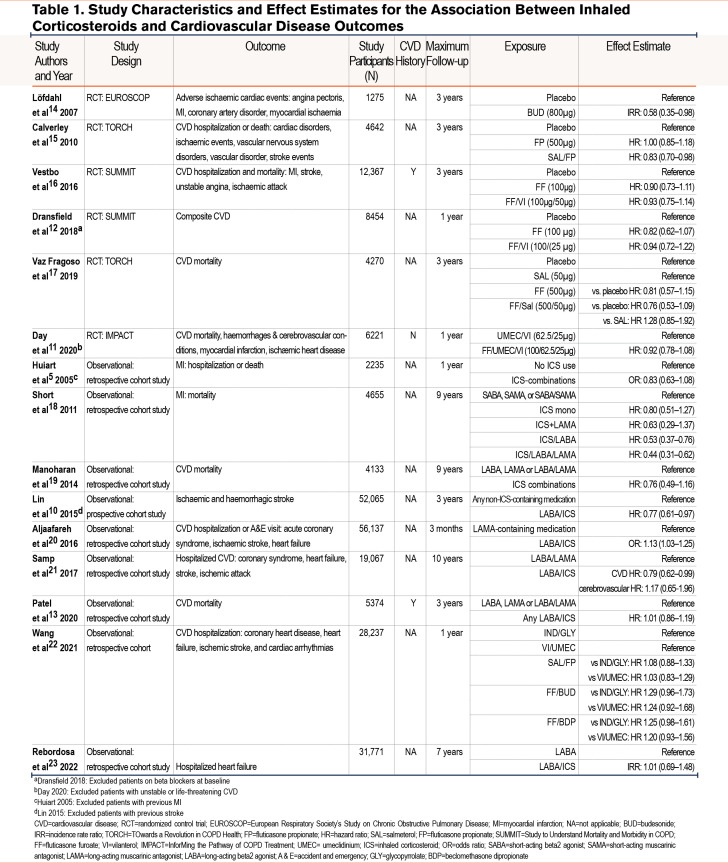
Table 1 reports study characteristics.5,10-23 A total of 9 studies were observational which included data from North American insurance databases, Canadian insurance databases, Taiwanese insurance databases, electronic health care records from the United Kingdom (Clinical Practice Research Datalink), COPD cohort data from Scotland, and Taiwanese hospital data. The remaining 6 studies were RCTs consisting of the TOwards a Revolution in COPD Health (TORCH) study, InforMing the Pathway for COPD Treatment (IMPACT) study, Study to Understand Mortality and Morbidity in COPD (SUMMIT), and the European Respiratory Society’s Study on Chronic Obstructive Pulmonary Disease (EUROSCOP )study.
A total of 11 studies had follow-ups for 3 years or less and 4 had follow-ups of greater than 3 years. Four studies excluded patients with a history of CVD.5,10-12 Two studies required patients to have a history of CVD.12,13 The remaining studies did not specify inclusion or exclusion criteria around a history of CVD. The number of patients included in the studies ranged from 1275 to 61,651. In total, 29 ICS compared with no ICS comparisons were extracted from the 15 articles. From the 15 articles, a total of 29 individual comparisons were made between people on ICSs and people not on ICSs, depending on the type of ICS- and non-ICS-containing medication within the studies.
Overall, of the 29 comparisons, 1 study found a higher risk of CVD in patients on LABA/ICS combinations compared with LAMA-containing medications. Six comparisons from 5 studies found that patients on ICSs had a lower risk of CVD compared with patients not on ICS combinations. These comparisons included 2 which compared ICS/LABA combinations with any non-ICS combinations or LABAs/LAMAs and 4 which compared ICS-containing combinations with placebo or short-acting medications. A remaining 22 comparisons found no significant association between ICS-containing medications and CVD. Of these comparisons, 9 compared ICS combinations with placebo or short-acting medications, and 13 compared ICS combinations with any non-ICS-containing medications or LABA/LAMA combinations.
Meta-analysis
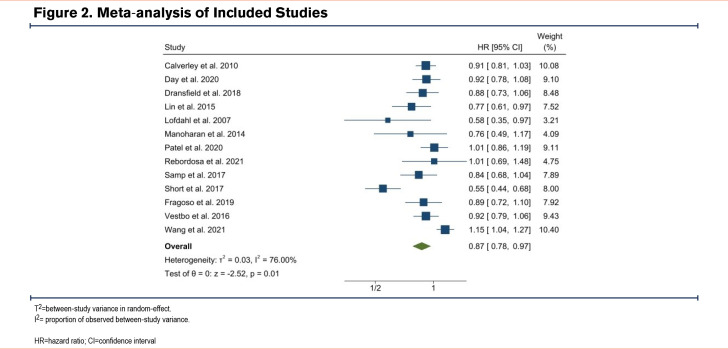
Figure 2 illustrates a meta-analysis of all studies that reported survival or event rate effect estimates. All studies except 2 were included in the meta-analysis. Overall, there was a significant association between ICS-containing medications and reduced risk of CVD (summary HR: 0.87, 95% CI 0.78 to 0.97; p=0.01). Between-study heterogeneity was 76%.
Meta-regression
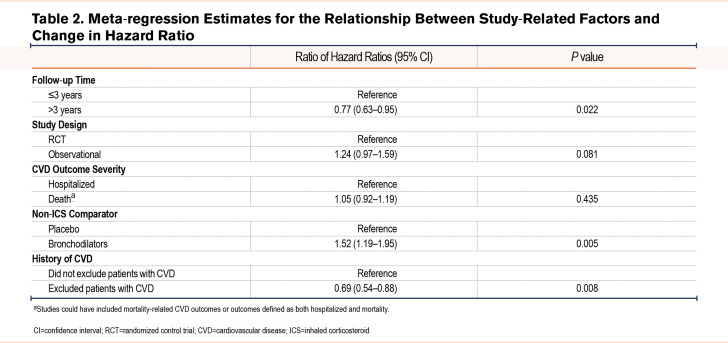
Table 2 reports the differences in HRs for the association between study-related factors and changes in the summary HR. Studies with follow-ups greater than 3 years were significantly more likely to show a reduced risk of CVD compared with studies with follow-ups of 3 years or less (ratio of HRs 0.77, 95% CI 0.62 to 0.95). In addition, studies that compared ICSs with bronchodilators were significantly more likely to report an increased risk of CVD compared with studies that compared ICSs with placebos (ratio of HRs 1.54, 95% CI 1.20 to 1.98; p=0.005). Studies that excluded patients with a history of CVD were significantly more likely to report a reduced risk of CVD compared with studies that did not exclude patients with a previous history (ratio of HRs 0.68, 95% CI 0.54 to 0.86; p=0.006). There was no difference in the association between ICS use and CVD risk between RCTs and observational studies or between studies that included hospitalized CVD outcomes and death-related outcomes.
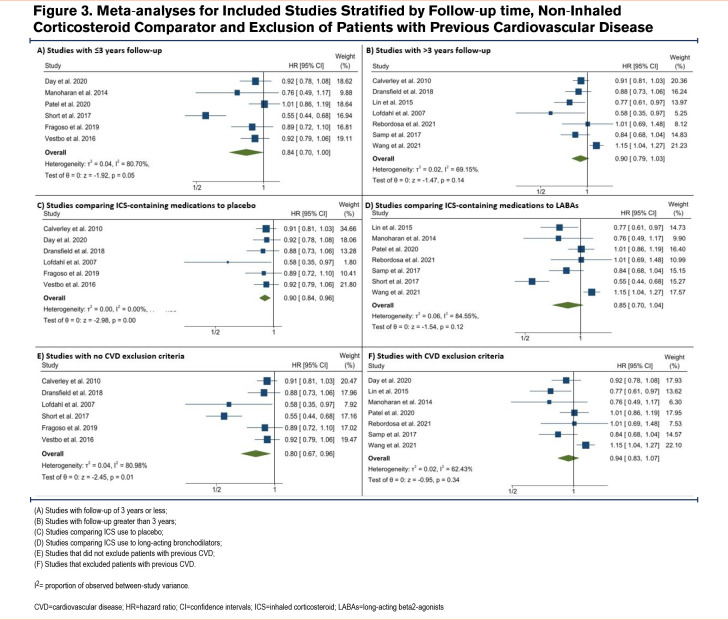
Figure 3 shows pooled estimates separately for studies stratified by study follow-up (panels A and B), non-ICS comparator (panels C and D), and exclusion of patients with previous CVD (panels E and F). The pooled HR for studies with follow-ups of 3 years or less was 0.93 (95% CI 0.85 to 1.02, panel A), whereas the pooled HR for studies with follow-ups greater than 3 years was 0.75 (95% CI 0.57 to 0.99, panel B). The pooled HR for studies that compared ICS use to placebo or short-acting bronchodilators was 0.80 (95% CI 0.67 to 0.96, panel C), whereas the pooled HR for studies that compared ICS use to long-acting bronchodilators was 0.94 (95% CI 0.83 to1.07, panel D). Finally, the pooled HR for studies that did not exclude patients based on previous CVD was 0.87 (95% CI 0.77 to 0.99, panel E), whereas the pooled HR for studies that did exclude patients with previous CVD was 0.86 (95% CI 0.72 to1.02, panel F) albeit for only 2 studies.
Risk of Bias
For RCT studies, the majority of domains had low bias, however, the study by Dransfield et al12 was a posthoc analysis of the SUMMIT trial, and the association between ICS use and time to first CVD event was stratified by baseline beta-blocker use. Beta-blockers were not used to randomize patients in the original trial and stratification could have led to selection bias as only estimates for patients who were not on beta-blockers were included. Moderate bias was also seen in the study by Calverley et al due to possible self-reporting of outcome events.15 In addition, of the total number of patients included in the TORCH study, Fragoso et al excluded 424 patients based on a reclassification of airflow obstruction using the global lung function initiative17 ( Table E2 in the online supplement).
Most bias domains for the observational studies were also low bias, however, the most common bias in these studies was potential residual confounding or lack of adequate adjustment for clinically important confounders. For example, smoking status, ethnicity, and additional COPD severity markers were not adjusted for in some studies as well as time-varying confounders5,10,13,20-22. Selection bias was moderate for studies that included hospitalized COPD patients, patients with private health care insurance, or patients with complete data.20,21,23 Lastly, studies were given moderate bias for deviation from intended intervention if it was possible that patients were not taking their prescribed medication adequately22 (Table E3 in the online supplement).
Discussion
Overall, we found there to be a significant pooled risk reduction in CVD with use of ICS- containing medications. In our meta-regression, we found that study follow-up, non-ICS comparator, and exclusion criteria based on CVD history were significant effect modifiers for the association between ICS use and risk of CVD. Specifically, the pooled estimate for studies with follow-ups greater than 3 years showed a significant association between ICS use and reduced risk of CVD but this was not seen in studies with follow-ups of 3 years or less. In addition, the pooled estimate for studies comparing ICSs with placebos showed a significant association between ICS use and reduced risk of CVD but this was not seen in studies comparing ICS use to long-acting bronchodilators. Lastly, the pooled estimate for studies that did not exclude patients with previous CVD showed a significant association between ICS use and reduced risk of CVD, but this was not seen for studies that did exclude patients with previous CVD, however, this only included 2 studies.
Our findings contradict the findings of a previous meta-analysis by Xia Jing et al.6 The previous meta-analysis found no increased risk of cardiovascular events in COPD patients on ICS-containing medications compared with controls. Comparisons specifically included ICS compared with a placebo and ICS in combination with a LABA compared with LABA monotherapy or a LABA/LAMA combination. This differed from our meta-analysis that did not exclude studies based on the ICS and non-ICS comparator. Additionally, the previous meta-analysis included only RCTs, whereas we expanded the search criteria to include both RCTs and observational studies. Interestingly, while we found no association between ICSs and CVD overall and in observational studies, we did find a protective association between ICSs and CVD in RCTs, whereas the previous meta-analysis of RCTs found no association. This might be due to differences in the CVD outcome inclusion criteria. Our meta-analysis included heart failure, stroke, and MI, whereas the previous study included a broader definition of CVD from MI to cardiac disorder symptoms and hypertension but did not include stroke.6
We found that for studies with follow-ups greater than 3 years, there was a significant association between ICS use and reduced risk of CVD. These studies specifically included 4observational studies alone. While we did not find that study design modified the association between ICS use and risk of CVD, it is possible that other factors such as study follow-up are proxies for study design in general, and as seen in our study, many RCTs have follow-up for a maximum of 3 years. This might explain why a significant association between ICSs and reduced risk of CVD was seen for observational studies in a previous meta-analysis but not for RCTs.7 The long-term risk reduction of CVD seen in our study could be explained by the continued use of ICSs, an anti-inflammatory medication, which over time might provide a protective effect.
Furthermore, as aforementioned, our study found an association in comparisons between ICSs and placebos or short-acting medications but not in ICS versus LABA/LAMA combination medications. It is well reported that treatment with long-acting bronchodilators is the mainstay of treatment for COPD and studies have shown that their combination is associated with reduced frequency of exacerbations and an overall decline in forced expiratory volume in 1 second as compared with placebo or monotherapy LABA and LAMA.24,25 Lower lung function and a reduced exacerbation rate have also been associated with ICS-containing medications as well as reduced risk of CVD.26-29 It is possible that both groups of patients treated with ICSs and LABA/LAMA combinations showed an improvement in lung function and exacerbation rate which in turn reduced the risk of developing CVD or having a CVD event in both groups making it difficult to detect any differences between the groups.
The exact etiology for the association between COPD and CVD remains unclear. The prevalence of CVD in COPD patients is estimated30,31 to lie between 14% to over 60%. People with COPD and CVD share common risk factors such as smoking, age, and air pollution.32-34 In terms of smoking, it is thought that patients who smoke are exposed to harmful particles that increase systemic inflammation, a characteristic of both COPD and CVD.35,36 Other mechanisms such as hypoxia and oxidative stress may be involved in the relationship between COPD and CVD.31 ICSs have been shown to reduce biomarkers such as lymphocytes and neutrophils and in turn improve lung function and reduce exacerbations.26,37,38 This reduced inflammation could, therefore, lead to a reduced risk of CVD in COPD patients taking ICS medications.
Limitations
Our study updated the literature on the association between ICSs and CVD, specifically heart failure, MI, and stroke-related CVD, and included both RCTs and observational studies. While RCTs are important due to their methodological design, they can have strict inclusion and exclusion criteria reducing their generalizability. For this reason, we did not restrict our search criteria by study design and included both RCTs and observational studies. In addition, we included studies that investigated differences in CVD outcomes between people on ICSs compared with placebo as well as ICSs compared with bronchodilators. By performing a meta-regression, we were able to determine whether characteristics such as the non-ICS-comparator led to differences in study findings. Despite this, there were limitations to our study.
First, previous meta-analyses included a greater number of studies compared with those included in our meta-analysis. This was because we only searched for studies using the terms heart failure, MI, and stroke, as well as the term cardiovascular disease. Previous studies included broader CVD events such as hypertension and CVD-related symptoms, however, by limiting the types of CVD included in our study we were able to reduce further heterogeneity between outcome definitions. It is important to note that our review included studies with effect estimates for the association between ICSs and CVD.
Second, some studies included in our analysis did not have CVD as their primary outcome. This is particularly common among the RCTs included in our study. It is, therefore, possible then that these studies did not prioritize ensuring the CVD outcomes were recorded as accurately and fully as the other outcomes for which the study was specifically designed. For example, one study highlighted that adverse cardiac events were not validated and were based on self-report. We did not exclude studies based on this; however, we did perform a risk of bias review for each included study and reported potential biases where appropriate. In addition, the proportion of patients who adhered to their COPD-related medications is unknown. It is possible that patients prescribed ICS medications may adhere to all their medications, including possible cardiovascular-related medications, compared with patients not taking ICS medications. In this scenario, it may appear that an ICS is protective and biased our results. We have had to assume in this work that people prescribed ICSs actually took them, and, therefore, what we are seeing is a true effect. We are also conscious that adherence is likely to differ between observational studies undertaken using data from routine clinical practice and data from trials. This may also explain some of the differences seen in the results between observational studies and trial data.
This is particularly important for observational studies because they do not all have prospective data collection for research purposes. Observational studies can be significantly biased especially when investigating the use of medications as confounding by indication is one of the main biases associated with these types of studies. While some observational studies used statistical methods to overcome this bias such as matching, not all studies did this, and residual bias could still exist. Therefore, our results on whether study design was a modifier of the relationship between ICSs and the risk of CVD should be interpreted with caution. Further prospective RCTs would help better understand this relationship and reduce possible study design bias. Specifically, an RCT of people diagnosed with COPD who are prescribed and not prescribed with a primary endpoint of any major adverse cardiac events over a year of follow-up is needed.
Lastly, only 1 study reported baseline ethnicity and 2 studies reported baseline social deprivation. While the mean age of COPD patients included was representative of COPD patients in the United Kingdom, we cannot comment on the generalizability of the included studies in terms of other patient characteristics.
Conclusion
Our systematic review and meta-analysis found an association between ICS-containing medication and decreased risk of CVD in people with COPD and results suggest that subgroups of COPD patients may benefit from ICS use more than others, such as those on long-term ICSs. Further studies are needed to better understand this relationship and whether this association varies by COPD phenotype and patient characteristics.
Abbreviations
Abbreviations: A & E=accident and emergency; BDP=beclomethasone dipropionate; BUD=budesonide; CHD=coronary heart disease;COPD=chronic obstructive pulmonary disease; CVD=cardiovascular disease; EUROSCOP=European Respiratory Society’s Study on Chronic Obstructive Pulmonary Disease; FF=fluticasone furoate; FP=fluticasone propionate; GLY=glycopyrrolate; HR=hazard ratio; ICSs=inhaled corticosteroids; IMPACT=InforMing the Pathway of COPD Treatment; IRR=incidence rate ratio; LABA=long-acting beta2 agonist; LAMA=long-acting muscarinic antagonist; MI=myocardial infarction; OR=odds ratio; RCTs=randomized controlled trials; SABA=short-acting beta2 agonist; SAL=salmeterol; SAMA=short-acting muscarinic antagonist; SUMMIT=Study to Understand Mortality and Morbidity in COPD; TORCH=TOwards a Revolution in COPD Health; UMEC= umeclidinium; VI=vilanterol
Funding Statement
No funding is associated with this study.
References
- 1.Calverley PMA,Anderson JA,Brook RD,et al. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am J Respir Crit Care Med. 2018;197(1):47-55. doi: https://doi.org/10.1164/rccm.201610-2086OC [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2022 report. GOLD website. Published 2022. Accessed December 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf [Google Scholar]
- 3.National Institute for Heath and Care Excellence (NICE). Chronic obstructive pulmonary disease in over 16s: diagnosis and management. NICE website. Published December 5, 2018. Updated July 26, 2019. Accessed December 2022. https://www.nice.org.uk/guidance/ng115 [PubMed] [Google Scholar]
- 4.Shin J,Yoon H-Y,Lee YM,Ha E,Lee JH. Inhaled corticosteroids in COPD and the risk for coronary heart disease: a nationwide cohort study. Sci Rep. 2020;10(1):18973. doi: https://doi.org/10.1038/s41598-020-74854-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huiart L,Ernst P,Ranouil X,Suissa S. Low-dose inhaled corticosteroids and the risk of acute myocardial infarction in COPD. Eur Respir J. 2005;25(4):634-639. doi: https://doi.org/10.1183/09031936.05.00079004 [DOI] [PubMed] [Google Scholar]
- 6.Jing X,Li Y,Xu J. Risk of cardiovascular events associated with inhaled corticosteroid treatment in patients with chronic obstructive pulmonary disease: a meta-analysis. Can Respir J. 2018;2018:7097540. doi: https://doi.org/10.1155/2018/7097540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loke YK,Kwok CS,Singh S. Risk of myocardial infarction and cardiovascular death associated with inhaled corticosteroids in COPD. Eur Respir J. 2010;35(5):1003-1021. doi: https://doi.org/10.1183/09031936.00095909 [DOI] [PubMed] [Google Scholar]
- 8.Cochrane. Cochrane methods bias. RoB2: a revised Cochrane risk-of-bias tool for randomized trials. Cochrane website. Published August 22, 2019. Accessed December 2022. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials [Google Scholar]
- 9.Cochrane. Cochrane methods bias. Risk of bias in non-randomized studies - of interventions (ROBINS-I). Cochrane website. Published 2016. Accessed December 2022. https://methods.cochrane.org/bias/risk-bias-non-randomized-studies-interventions [Google Scholar]
- 10.Lin SH,Perng DW,Chen CP,et al. Increased risk of community-acquired pneumonia in COPD patients with comorbid cardiovascular disease. Int J Chron Obstruct Dis. 2016;11:3051-3058. doi: https://doi.org/10.2147/COPD.S115137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day NC,Kumar S,Criner G,et al. Single-inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: results on cardiovascular safety from the IMPACT trial. Respir Res. 2020;21(1):139. doi: https://doi.org/10.1186/s12931-020-01398-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dransfield MT,McAllister DA,Anderson JA,et al. β-blocker therapy and clinical outcomes in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. An observational substudy of SUMMIT. Ann Am Thorac Soc. 2018;15(5):608-614. doi: https://doi.org/10.1513/AnnalsATS.201708-626OC [DOI] [PubMed] [Google Scholar]
- 13.Patel HC,Hayward C,Patel KS,Claggett B,Vazir A,Cowie MR. Impact on survival of combination inhalers in patients with COPD at high risk of cardiovascular events. Int J Cardiol. 2020;300:237-244. doi: https://doi.org/10.1016/j.ijcard.2019.11.138 [DOI] [PubMed] [Google Scholar]
- 14.Löfdahl CG,Postma DS,Pride NB,Boe J,Thorén A. Possible protection by inhaled budesonide against ischaemic cardiac events in mild COPD. Eur Respir J. 2007;29(6):1115-1119. doi: https://doi.org/10.1183/09031936.00128806 [DOI] [PubMed] [Google Scholar]
- 15.Calverley PM,Anderson JA,Celli B,et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010;65(8):719-725. doi: https://doi.org/10.1136/thx.2010.136077 [DOI] [PubMed] [Google Scholar]
- 16.Vestbo J,Anderson JA,Brook RD,et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817-1826. doi: https://doi.org/10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 17.Vaz Fragoso CA,Gill TM,Leo-Summers LS,Van Ness PH. Re-evaluation of combination therapy in chronic obstructive pulmonary disease (COPD). Respir Med. 2019;151:27-34. doi: https://doi.org/10.1016/j.rmed.2019.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short PM,Lipworth SIW,Elder DHJ,Schembri S,Lipworth BJ. Effect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ. 2011;342:d2549. doi: https://doi.org/10.1136/bmj.d2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manoharan A,Short PM,Anderson WJ,Lipworth BJ. Impact of long-acting bronchodilators and exposure to inhaled corticosteroids on mortality in COPD: a real-life retrospective cohort study. Lung. 2014;192(5):649-652. doi: https://doi.org/10.1007/s00408-014-9611-8 [DOI] [PubMed] [Google Scholar]
- 20.Aljaafareh A,Valle JR,Lin YL,Kuo YF,Sharma G. Risk of cardiovascular events after initiation of long-acting bronchodilators in patients with chronic obstructive lung disease: a population-based study. SAGE Open Med. 2016;4:2050312116671337. doi: https://doi.org/10.1177/2050312116671337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samp JC,Joo MJ,Schumock GT,Calip GS,Pickard AS,Lee TA. Risk of cardiovascular and cerebrovascular events in COPD patients treated with long-acting β2-agonist combined with a long-acting muscarinic or inhaled corticosteroid. Ann Pharmacother. 2017;51(11):945-953. doi: https://doi.org/10.1177/1060028017719716 [DOI] [PubMed] [Google Scholar]
- 22.Wang MT,Lai JH,Huang YL,et al. Comparative effectiveness and safety of different types of inhaled long-acting β(2)-agonist plus inhaled long-acting muscarinic antagonist vs inhaled long-acting β(2)-agonist plus inhaled corticosteroid fixed-dose combinations in COPD a propensity score-inverse probability of treatment weighting cohort study. Chest. 2021;160(4):1255-1270. doi: https://doi.org/10.1016/j.chest.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 23.Rebordosa C,Plana E,Rubino A,et al. Risk assessment of acute myocardial infarction and stroke associated with long-acting muscarinic antagonists, alone or in combination, versus long-acting beta2-agonists. Int J Chron Obstruct Pulmon Dis. 2022;17:1715-1733. doi: https://doi.org/10.2147/COPD.S363997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mammen MJ,Pai V,Aaron SD,Nici L,Alhazzani W,Alexander PE. Dual LABA/LAMA therapy versus LABA or LAMA monotherapy for chronic obstructive pulmonary disease. A systematic review and meta-analysis in support of the American Thoracic Society Clinical Practice Guideline. Ann Am Thorac Soc. 2020;17(9):1133-1143. doi: https://doi.org/10.1513/AnnalsATS.201912-915OC [DOI] [PubMed] [Google Scholar]
- 25.Rodrigo GJ,Price D,Anzueto A,et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Dis. 2017;12:907-922. doi: https://doi.org/10.2147/COPD.S130482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whittaker HR,Jarvis D,Sheikh MR,Kiddle SJ,Quint JK. Inhaled corticosteroids and FEV(1) decline in chronic obstructive pulmonary disease: a systematic review. Respir Res. 2019;20(1):277. doi: https://doi.org/10.1186/s12931-019-1249-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst P,Saad N,Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. EurRespir J. 2015;45(2):525-537. doi: https://doi.org/10.1183/09031936.00128914 [DOI] [PubMed] [Google Scholar]
- 28.Whittaker HR,Bloom C,Morgan A,Jarvis D,Kiddle SJ,Quint JK. Accelerated FEV1decline and risk of cardiovascular disease and mortality in a primary care population of COPD patients. Eur Respir J. 2021;57(3):2000918. doi: https://doi.org/10.1183/13993003.00918-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvestre OM,Nadruz W,Querejeta Roca G,et al. Declining lung function and cardiovascular risk: the ARIC study. J Am Coll Cardiol. 2018;72(10):1109-1122. doi: https://doi.org/10.1016/j.jacc.2018.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannino DM,Thorn D,Swensen A,Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962-969. doi: https://doi.org/10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 31.Morgan AD,Zakeri R,Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis. 2018;12:1753465817750524. doi: https://doi.org/10.1177/1753465817750524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabe KF,Hurst JR,Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27(149):180057. doi: https://doi.org/10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin V,Crack PJ,Bozinovski S,Miller AA,Vlahos R. COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond). 2016;130(13):1039-1050. doi: https://doi.org/10.1042/CS20160043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutlu GM,Green D,Bellmeyer,et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117(10):2952-2961. doi: https://doi.org/10.1172/JCI30639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Çolak Y,Afzal S,Lange P,Nordestgaard BG. Smoking, systemic inflammation, and airflow limitation: a Mendelian randomization analysis of 98 085 individuals from the general population. Nicotine Tob Res. 2019;21(8):1036-1044. doi: https://doi.org/10.1093/ntr/nty077 [DOI] [PubMed] [Google Scholar]
- 36.Kalkhoran S,Chang Y,Rigotti NA. Electronic cigarette use and cigarette abstinence over 2 years among U.S. smokers in the Population Assessment of Tobacco and Health study. NicotineTob Res. 2020;22(5):728-733. doi: https://doi.org/10.1093/ntr/ntz114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jen R,Rennard S,Sin DD. Effects of inhaled corticosteroids on airway inflammation in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J ChronObstruct Pulmon Dis. 2012;7:587-595. doi: https://doi.org/10.2147/COPD.S32765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janson C. Treatment with inhaled corticosteroids in chronic obstructive pulmonary disease. J Thorac Dis. 2020;12(4):1561-1569. doi: https://doi.org/10.21037/jtd.2020.02.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.